Abstract
Cytotoxic T cells that are present in tumors and capable of recognizing tumor epitopes are nevertheless generally impotent in eliciting tumor rejection. Thus, identifying the immune escape mechanisms responsible for inducing tumor-specific CD8+ T cell dysfunction may reveal effective strategies for immune therapy. The inhibitory receptors PD-1 and Tim-3 are known to negatively regulate CD8+ T cell responses directed against the well-characterized tumor antigen NY-ESO-1. Here, we report that the upregulation of the inhibitory molecule BTLA also plays a critical role in restricting NY-ESO-1-specific CD8+ T cell expansion and function in melanoma. BTLA-expressing PD-1+Tim-3− CD8+ T cells represented the largest subset of NY-ESO-1-specific CD8+ T cells in melanoma patients. These cells were partially dysfunctional, producing less IFN-γ than BTLA− T cells, but more IFN-γ, TNF and IL-2 than the highly dysfunctional subset expressing all three receptors. Expression of BTLA did not increase with higher T cell dysfunction or upon cognate antigen stimulation, as it does with PD-1, suggesting that BTLA upregulation occurs independently of functional exhaustion driven by high antigen load. Added with PD-1 and Tim-3 blockades, BTLA blockade enhanced the expansion, proliferation and cytokine production of NY-ESO-1-specific CD8+ T cells. Collectively, our findings indicate that targeting BTLA along with the PD-1 and Tim-3 pathways is critical to reverse an important mechanism of immune escape in patients with advanced melanoma.
Keywords: BTLA, PD-1, CD8+ T cells, Melanoma, tumor-induced T cell dysfunction
Introduction
T cell exhaustion represents a progressive loss of T cell function occurring upon chronic exposure to high antigen load and has first been reported in mice chronically infected with LCMV (1). Exhausted CD8+ T cells upregulate multiple inhibitory receptors, including PD-1, 2B4, CTLA-4, CD160 and LAG-3 (2). The expression of multiple inhibitory receptors by T cells is associated with greater exhaustion and more severe infections. Several lines of evidence support the role of inhibitory pathways in impeding effective anti-tumor T cell immune responses. First, tumor antigen (TA)-specific CTLs present in PBLs or at tumor sites have been shown to upregulate PD-1 expression (3, 4). Second, a highly dysfunctional subset of TA-specific T cells present at the periphery or at tumor sites, co-upregulates PD-1 and T cell immunoglobulin and mucin-domain-containing molecule 3 (Tim-3) expression (5, 6). PD-1 and Tim-3 pathway blockades act in synergy to enhance TA-specific CD8+ T cell numbers and functions in patients with advanced melanoma and induce tumor regression in animals (5, 6).
Whether distinct TA-specific CD8+ T cell subsets exhibiting variable levels of dysfunction and expressing different sets of inhibitory molecules exist has not been determined yet. Such information may provide novel therapeutic targets to reverse tumor-induced T cell dysfunction in patients with advanced cancers. To address this question, we have investigated the expression of the B and T cell lymphocyte attenuator (BTLA; CD272) in combination with PD-1 and Tim-3 on spontaneous NY-ESO-1-specific CD8+ T cells from patients with advanced melanoma. BTLA, an immunoglobulin-like molecule that belongs to the CD28 family, is expressed by a range of cells, including T cells, B cells, NK cells and APCs (7-9). BTLA is an inhibitory receptor involved in negative regulation of T cell function. BTLA deficient mice exhibit severe experimental autoimmune encephalomyelitis, prolonged airway allergic inflammation and higher rejection of minor mismatched allografts (8, 10, 11). BTLA inhibits T cell proliferation and cytokine production in vitro and mediates the negative regulation of CD8+ T cell homeostasis and memory cell generation in vivo (12). In addition, BTLA plays a critical role in the induction of peripheral T cell tolerance in vivo (13). BTLA binds to herpes virus entry mediator (HVEM), which is expressed by a wide range of cells including T cells, B cells, NKs, monocytes and dendritic cells (9). In addition, BTLA is also expressed by melanoma cells (14). In cancer immunology, BTLA expression has been observed on MART-1-specific CD8+ T cells isolated from PBMCs of melanoma patients and contributed to limiting T cell expansion and IFN-γ production upon cross-linking with HVEM expressed by melanoma cells (14). In the present study, we show that BTLA expression is upregulated on spontaneous NY-ESO-1-specific CD8+ T cells that are detectable ex vivo in PBLs of melanoma patients. BTLA+PD-1+Tim-3− cells, which represent the largest subset of NY-ESO-1-specific CD8+ T cells that are detectable ex vivo in PBLs of melanoma patients, exhibit partial T cell dysfunction, producing more IFN-γ, TNF and IL-2 than BTLA+PD-1+Tim-3+ but less IFN-γ than BTLA−PD-1+Tim-3− and BTLA−PD-1−Tim-3− NY-ESO-1-specific CD8+ T cells. Finally, BTLA blockade acts in combination with PD-1 and Tim-3 blockades to further enhance NY-ESO-1 specific CD8+ T cell expansion and function.
Materials and Methods
Study subjects
Blood samples were obtained under the University of Pittsburgh Cancer Institute (UPCI) IRB-approved protocols 00-079, 96-099 and 05-140 from eleven HLA-A2+ patients with NY-ESO-1-expressing stage IV melanoma who exhibited spontaneous NY-ESO-1-specific CD8+ T cell responses assessed ex vivo by flow cytometry using APC-labeled HLA-A2/NY-ESO-1 157-165 tetramers. The percentages of ex vivo detectable NY-ESO-1 157-165-specific CD8+ T cells isolated from patients’ PBMCs ranged from 0.015% to 2.7% of total CD8+ T cells (median 0.03%). PBMCs used in this study were obtained from patients with no prior immunotherapy.
Phenotypic analysis
CD8+ T lymphocytes were purified from PBMCs of patients using MACS Column Technology (Miltenyi Biotec) and incubated with APC-labeled HLA-A2/NY-ESO-1 157-165, HLA-A2/CMV 495-503, HLA-A2/EBV-BMLF-1 280-288, HLA-A2/Flu-M 58-66, HLA-A2/MART-1 26-35 or HLA-A2/HIVpol 476-484 tetramers as control. The purity of CD8+ T cells was always greater than 95%. Tetramers were provided by the Ludwig Cancer Institute for Cancer Research, Lausanne branch. Next, cells were incubated with CD8-FITC (Beckman Coulter) or CD8-V500 (BD Biosciences), Tim-3-PE (R&D Systems) or IgG2a-PE (BD Biosciences), BTLA-biotin or IgG2a-biotin (eBioscience), PD-1-PE-Cy7 or IgG1-PE-Cy7 (BioLegend), CD57-FITC, HLA-DR-PerCp-Cy5.5, CD38-PerCp-Cy5.5 (BD Pharmingen) and streptavidin-ECD (Invitrogen) conjugated antibodies or reagent. A violet amine reactive dye (Invitrogen) was used to assess the viability of the cells. Two million five hundred thousand events were collected during flow cytometric analysis on a FACSAria machine (BD Biosciences) and analyzed using Flowjo software (Tree Star).
Intracellular cytokine staining assay
For ex vivo cytokine production assays, two million five hundred thousand purified CD8+ T cells were incubated for 6 hours in 10% human serum DMEM-Iscove medium with the same number of non-CD3 autologous cells pulsed with HLA-A2-restricted peptides NY-ESO-1 157-165 or HIVpol 476-484 (10 μg/ml). For in vitro stimulation (IVS) assays, five million PBMCs were incubated for six days in culture medium containing 50 IU/ml rhIL-2 (PeproTech) with peptide NY-ESO-1 157-165 or peptide HIVpol 476-484 (10 μg/ml) in the presence of 10 μg/ml anti-BTLA (clone 8.2; gift from Dr. Daniel Olive) and/or anti-PD-1 (clone EH12.2H7; Biolegend) and/or anti-Tim-3 (clone 2E2; gift from Dr. Vijay Kuchroo) blocking mAbs or isotype control antibodies. On day 6, cells were restimulated for 6 hours with peptide NY-ESO-1 157-165 or HIVpol 476-484 as control (10 μg/ml). After one hour of incubation, Brefeldin A (Sigma-Aldrich) was added to the culture medium (10 μg/ml). After tetramer labeling, cells were surface stained with CD8-PE, CD14-ECD, CD19-ECD, CD56-biotin, CD4-PE-Cy7 (Beckman Coulter), streptavidin-ECD and intracellularly stained with IFN-γ-FITC (Miltenyi Biotec), IL-2-PerCp-Cy5.5 (Biolegend) and TNF-Alexa700 (BD Pharmingen) antibodies. Two million five hundred thousand events were collected during flow cytometric analysis.
CFSE proliferation assay
Five million CFSE-labeled PBMCs were incubated for six days in culture medium containing 50 IU/ml rhIL-2 with peptide NY-ESO-1 157-165 or HIVpol 476-484 (10 μg/ml), in the presence of 10 μg/ml anti-BTLA and/or anti-PD-1 and/or anti-Tim-3 blocking mAbs or isotype control antibodies. On day 6, cells were stained with APC-labeled HLA-A2/NY-ESO-1 157-165 tetramers, CD14-ECD, CD19-ECD, CD56-biotin, streptavidin-ECD, CD8-PE-Cy7 and CD4-PerCp-Cy5.5 (Biolegend) conjugated antibodies and reagents. Two million events were collected during flow cytometric analysis.
Statistics
Statistical hypotheses were tested with the Wilcoxon signed rank test (for paired results from the same patient) using SAS v. 9.1 (Cary, NC). Tests were two-sided and considered significant for p<=0.05. Because rank tests are not sensitive to the actual values in a comparison, only to their ranks, differing sets of values can produce identical p-values.
Results
BTLA and PD-1 upregulation defines a large subset of NY-ESO-1-specific CD8+ T cells in PBLs of patients with advanced melanoma
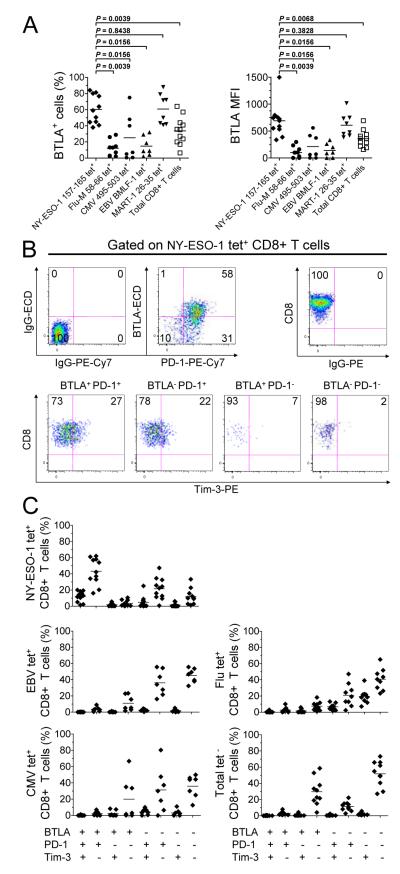
We first investigated the expression of BTLA, PD-1 and Tim-3 on spontaneous ex vivo detectable NY-ESO-1-specific, virus-specific (Flu, CMV and EBV) and MART-1-specific CD8+ T cells isolated from PBMCs of eleven HLA-A*0201+ (HLA-A2+) stage IV melanoma patients using HLA-A2 (A2) tetramers. In all patients, the frequencies of BTLA+ cells among NY-ESO-1-specific CD8+ T cells (mean 60.4% ± SD 17%) were significantly higher than those of Flu-specific (12.2% ± 10%), CMV-specific (25.2% ± 29.3%), EBV-specific CD8+ T cells (14.8% ± 12%) and total CD8+ T cells (33.8% ± 17.3%) (Fig. 1A and Supplemental Fig. 1). As previously shown, BTLA expression was upregulated on MART-1-specific CD8+ T cells (14). Similar observations were made in terms of mean fluorescence intensity (MFI) (Fig. 1A, right panel). In line with one previous report (14), naïve (CCR7+CD45RA+) and central memory (CCR7+CD45RA−) CD8+ T cells displayed higher percentages of BTLA+ cells than effector memory (CCR7−CD45RA−) and effector cells (CCR7−CD45RA+). Strikingly, the upregulation of BTLA expression by NY-ESO-1-specific CD8+ T cells both in terms of percentages and MFI was much higher than in any of these CD8+ T cell subsets (Supplemental Fig. 2, A and B). We further observed that BTLA+PD-1+Tim-3− (42.7% ± 16.4%) represented the largest NY-ESO-1-specific CD8+ T cell subset as compared to BTLA+PD-1+Tim-3+ (12.4% ± 6.1%), BTLA−PD-1+Tim-3− (21.5% ± 13.3%), BTLA+PD-1−Tim-3− (3.5% ± 3.6%) and BTLA−PD-1−Tim-3− (11.8% ± 9.5%) CD8+ T cells (Fig. 1, B and C). In striking contrast with NY-ESO-1-specific CD8+ T cells, EBV-, Flu- and CMV-specific CD8+ T cells were in their large majority either BTLA−PD-1−Tim-3− or BTLA−PD-1+Tim-3− and displayed very low frequencies of BTLA+PD-1+Tim-3+ and BTLA+PD-1+Tim-3− cells (Fig. 1C). The variability in the frequencies of BTLA+PD-1−Tim-3− and BTLA−PD-1−Tim-3− cells within total tetramer − (tet −) CD8+ T cells between patients appeared to correlate with their maturation/differentiation status. We observed a positive correlation between the expression of BTLA on CD8+ T cells and the expression of CCR7, CD45RA and CD27 (Supplemental Fig. 2C). The expression and distribution of BTLA, PD-1 and Tim-3 on virus-specific and total CD8+ T cells in PBMCs from ten healthy donors, were similar to those of melanoma patients (Supplemental Fig. 3).
Figure 1.
BTLA is upregulated and co-expressed with PD-1 and Tim-3 on NY-ESO-1-specific CD8+ T cells. (A) Pooled data from eleven melanoma patients showing the percentage (%) and MFI of BTLA expression on NY-ESO-1-specific, MART-1-specific, virus-specific and total CD8+ T cells. (B) Dot plots from one representative melanoma patient showing ex vivo BTLA, PD-1 and Tim-3 expression on NY-ESO-1-specific CD8+ T cells. Values indicate the percentage of CD8+ T cells that express PD-1 and/or BTLA among tet+ CD8+ T cells and express Tim-3 within different subsets of tet+ CD8+ T cells defined by BTLA and PD-1 expression. (C) Pooled data from eleven melanoma patients showing the distribution of NY-ESO-1-specific, virus-specific and total CD8+ T cells according to BTLA, PD-1 and Tim-3 expression. Horizontal bars depict the mean percentage or MFI of BTLA and/or PD-1 and/or Tim-3 expression on tet+ CD8+ T cells. Data shown are representative of two independent experiments.
Collectively, our results demonstrate that BTLA expression is upregulated on tumor-induced NY-ESO-1-specific CD8+ T cells isolated from PBMCs of patients with advanced melanoma. In contrast to virus-specific and total CD8+ T cells, the vast majority of BTLA+NY-ESO-1-specific CD8+ T cells upregulate PD-1 expression. BTLA+PD-1+Tim-3− cells represent the largest subset of NY-ESO-1-specific CD8+ T cells.
BTLA+PD-1+Tim-3− and BTLA+PD-1+Tim-3+ NY-ESO-1-specific CD8+ T cells represent two dysfunctional T cell populations
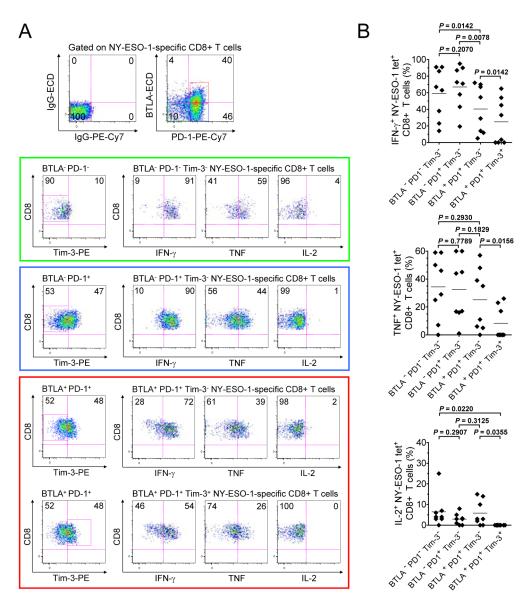
We examined the functional status of NY-ESO-1-specific CD8+ T cells from melanoma patients according to BTLA, PD-1 and Tim-3 expression. After short ex vivo stimulation (6 hr) with cognate peptide, percentages of cytokine-producing NY-ESO-1-specific CD8+ T cells were assessed among BTLA− PD-1−Tim-3−, BTLA−PD-1+Tim-3−, BTLA+PD-1+Tim-3− and BTLA+PD-1+Tim-3+ cells, which represent the main subsets of NY-ESO-1-specific CD8+ T cells (Fig. 1C). BTLA+PD-1+Tim-3− and BTLA+PD-1+Tim-3+ NY-ESO-1-specific CD8+ T cells produced significantly less IFN-γ than BTLA−PD-1+Tim-3− (p = 0.0078) and BTLA−PD-1− Tim-3− (p = 0.0078) (Fig. 2, A and B). In addition, BTLA+PD-1+Tim-3+ NY-ESO-1-specific CD8+ T cells, but not BTLA+PD-1+Tim-3− cells, produced significantly less TNF and IL-2 than BTLA−PD-1+Tim-3− (p = 0.0078 and p = 0.0355, respectively) and BTLA− PD-1−Tim-3− cells (p = 0.0156 and p = 0.0220, respectively). Interestingly, BTLA+PD-1+Tim-3+ NY-ESO-1-specific CD8+ T cells produced significantly less IFN-γ (p = 0.0142), TNF (p = 0.0156) and IL-2 (p = 0.0355) than BTLA+PD-1+Tim-3− NY-ESO-1-specific CD8+ T cells. We did not observe any significant differences in cytokine production between BTLA−PD-1−Tim-3− and BTLA−PD-1+Tim-3− NY-ESO-1-specific CD8+ T cells (Fig. 2, A and B). These observations were confirmed in a second set of independent experiments (Supplemental Fig. 4).
Figure 2.
BTLA+PD-1+Tim-3− and BTLA+PD-1+Tim-3+ NY-ESO-1-specific CD8+ T cells represent two dysfunctional T cell populations. Dot plots from one representative patient (A) and summary data for 8 melanoma patients (B) showing the percentages of cytokine-producing A2/NY-ESO-1 157-165 tet+ CD8+ T cells according to BTLA, PD-1 and Tim-3 expression. Values indicate the percentages of cytokine-producing CD8+ T cells among Tim-3+ and/or Tim-3− fractions of BTLA−PD-1− (green gate and frame), BTLA−PD-1+ (blue gate and frame) and BTLA+PD-1+ (red gate and frame) NY-ESO-1-specific CD8+ T cells. The P values were calculated using the Wilcoxon signed rank test. Data shown are representative of two independent experiments performed in duplicate.
Collectively, our findings demonstrate that BTLA+PD-1+Tim-3− cells, which represent the largest subset of NY-ESO-1-specific CD8+ T cells, exhibit partial T cell dysfunction, producing more IFN-γ, TNF and IL-2 than BTLA+PD-1+Tim-3+ but less IFN-γ than BTLA−PD-1+Tim-3− and BTLA−PD-1−Tim-3− NY-ESO-1-specific CD8+ T cells.
PD-1 and Tim-3 but not BTLA expression correlates with increased levels of NY-ESO-1-specific CD8+ T cell dysfunction and activation
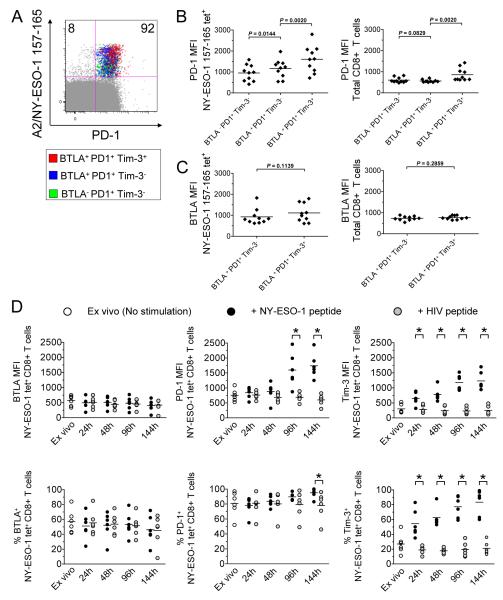
Because high PD-1 levels correlate with increased CD8+ T cell exhaustion in chronic viral infections (15, 16), we next defined whether the levels of PD-1 and BTLA expression by NY-ESO-1-specific CD8+ T cells correlated with T cell dysfunction. In line with previous findings in chronic viral infections, we observed that PD-1 expression (MFI) was higher on partially dysfunctional BTLA+PD-1+Tim-3− NY-ESO-1-specific CD8+ T cells than on BTLA−PD-1+Tim-3− cells but lower than on highly dysfunctional BTLA+PD-1+Tim-3+ cells (Fig. 3, A and B). However, and in sharp contrast with PD-1 expression, similar levels of BTLA (MFI) were expressed by BTLA+PD-1+Tim-3− and BTLA+PD-1+Tim-3+ NY-ESO-1-specific CD8+ T cells, arguing against a correlation between BTLA expression and the severity of NY-ESO-1-specific CD8+ T cell dysfunction (Fig. 3C). We next evaluated BTLA, PD-1 and Tim-3 expression on NY-ESO-1-specific CD8+ T cells over a 6-day IVS in the presence of cognate or irrelevant peptide. We observed a significant and progressive increase in PD-1 and Tim-3 but not BTLA expression by NY-ESO-1-specific CD8+ T cells upon stimulation with cognate but not with irrelevant peptide (Fig. 3D).
Figure 3.
The levels of PD-1 and Tim-3 but not BTLA expression are correlated with the levels of NY-ESO-1-specific CD8+ T cell dysfunction and activation. (A) Representative dot plot from one melanoma patient showing ex vivo PD-1 expression within BTLA−PD-1+Tim-3−, BTLA+PD-1+Tim-3− and BTLA+PD-1+Tim-3+ A2/NY-ESO-1 157-165 tet+ CD8+ T cell subsets. Pooled data from ten melanoma patients showing the MFI of PD-1 (B) and BTLA (C) expression on different subsets of NY-ESO-1 157-165 tet+ and total CD8+ T cells according to BTLA, PD-1 and Tim-3 expression. Horizontal bars depict the mean MFI of PD-1 or BTLA expression. Data shown are representative of at least two independent experiments. (D) BTLA, PD-1 and Tim-3 expression on NY-ESO-1 tet+ CD8+ T cells assessed ex vivo and after indicated hours following in vitro stimulation with cognate peptide (NY-ESO-1 peptide) or irrelevant peptide (HIV peptide). Data shown are representative of at least two independent experiments. The P values were calculated using the Wilcoxon signed rank test.*, p < 0.05 was considered significant.
Altogether, our findings suggest that in contrast to PD-1 and Tim-3, BTLA expression by NY-ESO-1-specific CD8+ T cells does not augment upon stimulation with cognate antigen. Unlike PD-1, BTLA expression is not further increased in highly dysfunctional BTLA+PD-1+Tim-3+ NY-ESO-1-specific CD8+ T cells as compared to BTLA+PD-1+Tim-3− NY-ESO-1-specific CD8+ T cells.
BTLA blockade increases the frequency of cytokine-producing NY-ESO-1-specific CD8+ T cells upon prolonged antigen stimulation and adds to PD-1 and Tim-3 blockades
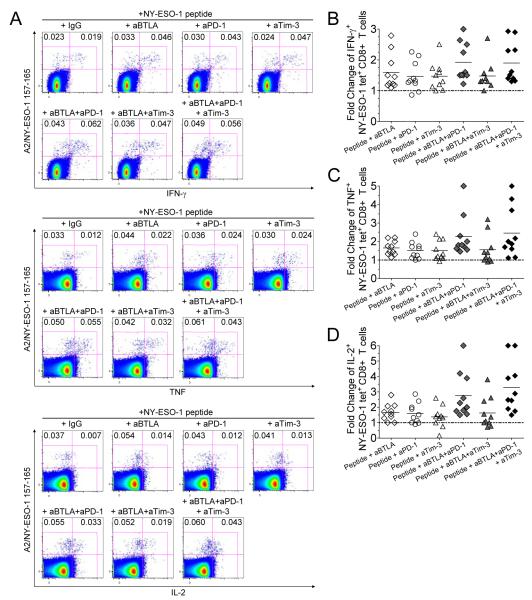
PBMCs from ten melanoma patients with spontaneous NY-ESO-1-specific CD8+ T cells were incubated for 6 days with NY-ESO-1 157-165 peptide in the presence of blocking mAbs against BTLA and/or PD-1 and/or Tim-3 or IgG control antibodies. After 6 days, cells were briefly restimulated (6 hr) with NY-ESO-1 or HIV peptide, before evaluating cytokine production by A2/NY-ESO-1 157-165 tet+ CD8+ T cells. In agreement with previous findings (9), HVEM, the ligand for BTLA, was expressed by APCs (monocytes, B cells and mDCs) in PBMCs of melanoma patients used for this assay (Supplemental Fig. 5) We noted a significant increase in the frequencies of NY-ESO-1-specific CD8+ T cells that produced IFN-γ (p = 0.0020), TNF (p = 0.0059) and IL-2 (p = 0.0142) in the presence of cognate peptide and anti-BTLA mAbs, as compared to cognate peptide and IgG control (Fig. 4A and Supplemental Fig. 6), resulting in a 1.6-fold, 1.7-fold and 1.7-fold change in the frequencies of IFN-γ-, TNF- and IL-2-producing NY-ESO-1-specific CD8+ T cells, respectively (Fig. 4, B-D). The frequencies of IFN-γ-, TNF- and IL-2-producing NY-ESO-1-specific CD8+ T cells further increased in the presence of both anti-BTLA and anti-PD-1 mAbs when compared to either IgG control antibodies (p = 0.0059), anti-BTLA mAbs alone (p = 0.0208, p = 0.0438 and p = 0.0466, respectively) and anti-PD-1 mAbs alone (p = 0.0080, p = 0.0142 and p = 0.0144, respectively) (Fig. 4A and Supplemental Fig. 6), resulting in a 1.9-fold, 2.3-fold and 2.8-fold change in the frequencies of IFN-γ-, TNF- and IL-2-producing NY-ESO-1-specific CD8+ T cells, respectively (Fig. 4, B-D). No additive effect was noted in the presence of both anti-BTLA and anti-Tim-3 mAbs. The triple blockade further augmented the frequencies of NY-ESO-1-specific CD8+ T cells that produced IL-2, but not IFN-γ or TNF, as compared to all other conditions including BTLA/PD-1 blockade (p = 0.0408) (Fig. 4, A and D and Supplemental Fig. 6). Notably, the effects of BTLA, PD-1 and Tim-3 blockades were observed only in the presence of the NY-ESO-1 peptide but not an irrelevant peptide, underlining the need for prolonged stimulation with cognate antigen (data not shown).
Figure 4.
BTLA blockade increases the frequencies of cytokine-producing NY-ESO-1-specific CD8+ T cells and adds to PD-1 and Tim-3 blockades. (A) Representative flow cytometry analysis from one melanoma patient showing percentages of IFN-γ-, TNF and IL-2-producing A2/NY-ESO-1 157-165 tet+ CD8+ T cells among total CD8+ T cells. PBMCs were incubated for 6 days with NY-ESO-1 157-165 peptide or with HIVpol 476-484 peptide and blocking mAbs against BTLA (aBTLA) and/or PD-1 (aPD-1) and/or Tim-3 (aTim-3) or an isotype control antibody (IgG), prior to evaluating intracellular cytokine production of A2/NY-ESO-1 157-165 tet+ CD8+ T cells in response to cognate peptide. Fold changes of the frequencies of IFN-γ- (B), TNF- (C) and IL-2-producing (D) A2/NY-ESO-1 157-165 tet+ CD8+ T cells after 6-day IVS with cognate peptide and blocking anti-BTLA and/or anti-PD-1 and/or anti-Tim-3 mAbs. The ratio of the frequency of cytokine-producing tet+ CD8+ T cells from melanoma patients (n = 10) in the presence of indicated antibody treatment and isotype control antibody is shown. Data shown are representative of two independent experiments performed in duplicate.
In contrast to BTLA blockade alone, BTLA blockade in combination with PD-1 blockade or PD-1 and Tim-3 blockades augmented the percentages of cells that produced TNF and IL-2 among total NY-ESO-1-specific CD8+ T cells as compared to incubation with IgG control antibody (p = 0.0020 and p = 0.0098, respectively), PD-1 blockade alone (p = 0.0020 and p = 0.0020) or BTLA blockade alone (p = 0.0195 and p = 0.0039), suggesting that BTLA blockade in combination with PD-1 blockade increases NY-ESO-1-specific CD8+ T cell capacity to produce cytokines on a cell-per-cell basis (Supplemental Fig. 7).
Collectively, our findings demonstrate that BTLA blockade enhances the expansion of cytokine-producing NY-ESO-1-specific CD8+ T cells and adds to the effect of PD-1 blockade to further increase the expansion of cytokine-producing NY-ESO-1-specific CD8+ T cells and to partially restore their ability to produce cytokines. In addition, BTLA blockade acts in combination with PD-1 and Tim-3 blockades to further increase the frequencies of IL-2-producing NY-ESO-1-specific CD8+ T cells.
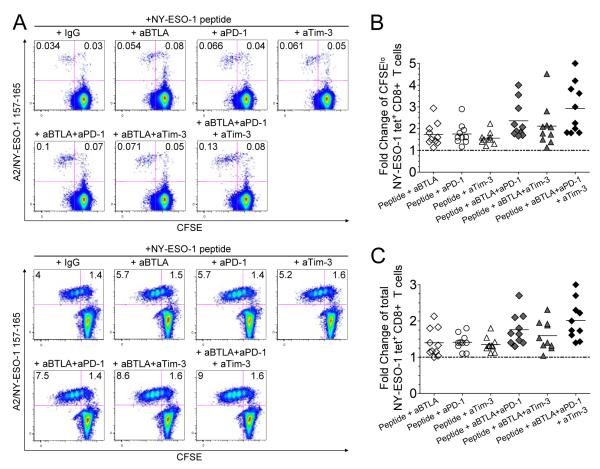
BTLA blockade increases proliferation of NY-ESO-1-specific CD8+ T cells upon prolonged antigen stimulation and adds to PD-1 and Tim-3 blockades
Finally, we evaluated the effect of BTLA pathway blockade alone or in combination with PD-1 and/or Tim-3 pathway blockades on the proliferative capacity and expansion of NY-ESO-1-specific CD8+ T cells in response to cognate antigen. CFSE-labeled PBMCs from ten melanoma patients with spontaneous NY-ESO-1-specific CD8+ T cell response were stimulated for 6 days with NY-ESO-1 157-165 peptide in the presence of mAbs against BTLA, PD-1 and/or Tim-3 or IgG control antibodies. BTLA blockade increased the frequencies of CFSElo and total A2/NY-ESO-1 157-165 tet+ CD8+ T cells as compared to stimulation with peptide and IgG control antibody (Fig. 5A and Supplemental Fig. 8), resulting in a 1.7-fold and a 1.4-fold change in the frequencies of CFSElo and total A2/NY-ESO-1 157-165 tet+ CD8+ T cells, respectively (Fig. 5, B and C). BTLA blockade in combination with PD-1 blockade further increased the frequencies of CFSElo and total A2/NY-ESO-1 157-165 tet+ CD8+ T cells as compared to BTLA blockade alone (p = 0.0137 and p = 0.0020, respectively) or PD-1 blockade alone (p = 0.0243 and p = 0.0371, respectively), resulting in 2.4-fold and a 1.8-fold change in the frequencies of CFSElo and total A2/NY-ESO-1 157-165 tet+ CD8+ T cells, respectively. The triple BTLA/PD-1/Tim-3 blockade further augmented the frequencies of CFSElo and total A2/NY-ESO-1 157-165 tet+ CD8+ T cells as compared to either BTLA/PD-1 (p = 0.0128 and p = 0.0151, respectively) or BTLA/Tim-3 (p = 0.0098 and p = 0.0195, respectively) blockade (Fig. 5A and Supplemental Fig. 8). This resulted in the highest increases in the frequencies of proliferating and total A2/NY-ESO-1 157-165 tet+ CD8+ T cells (2.9-fold and 2.0-fold, respectively) as compared to all other experimental conditions (Fig. 5, B and C). No significant proliferation of NY-ESO-1 157-165-specific CD8+ T cells was observed in the presence of an irrelevant peptide with or without blockade (data not shown).
Figure 5.
BTLA blockade increases the frequencies of proliferating and total NY-ESO-1-specific CD8+ T cells and adds to PD-1 and Tim-3 blockades. (A) Representative flow cytometry analysis from two melanoma patients showing percentages of CFSElo A2/NY-ESO-1 157-165 tet+ CD8+ T cells among total CD8+ T cells. CFSE-labeled PBMCs were incubated for 6 days with NY-ESO-1 157-165 peptide or HIVpol 476-484 peptide and blocking mAbs against BTLA (aBTLA) and/or PD-1 (aPD-1) and/or Tim-3 (aTim-3) or an isotype control antibody (IgG). Fold changes of the frequencies of CFSElo (B) and total (C) A2/NY-ESO-1 157-165 tet+ CD8+ T cells after 6-day IVS with cognate peptide and blocking anti-BTLA and/or anti-PD-1 and/or anti-Tim-3 mAbs (n = 10). The ratio of the percentages of CFSElo and total A2/NY-ESO-1 157-165 tet+ CD8+ T cells in the presence of indicated antibody treatment and isotype control antibody is shown. Data shown are representative of two independent experiments performed in duplicate.
Collectively, our findings demonstrate that BTLA blockade enhances the proliferation and expansion of NY-ESO-1-specific CD8+ T cells. In addition, BTLA blockade acts in combination with PD-1 and Tim-3 blockades to further increase the frequencies of proliferating and total NY-ESO-1-specific CD8+ T cells.
Discussion
In this study, we report a novel subset of dysfunctional TA-specific CD8+ T cells in patients with advanced melanoma. We show that NY-ESO-1-specific PD-1+BTLA+Tim-3− CD8+ T cells exhibit partial dysfunction, producing more IFN-γ, TNF and IL-2 than BTLA+PD-1+Tim-3+ cells but less IFN-γ than BTLA−PD-1+Tim-3− and BTLA−PD-1−Tim-3− NY-ESO-1-specific CD8+ T cells. PD-1+BTLA+Tim-3− NY-ESO-1-specific CD8+ T cells represent the largest molecularly-defined dysfunctional T cell subset among circulating NY-ESO-1-specific CD8+ T cells.
In patients with advanced melanoma, we have shown that the large majority of circulating NY-ESO-1-specific CD8+ T cells upregulate PD-1 expression and that PD-1 regulates the expansion of NY-ESO-1-specific CD8+ T cells (3). Along with previous studies of HIV-specific CD8+ T cells which have shown that PD-1 blockade fails to completely restore virus-specific CD8+ T cell dysfunction (17), we observed that PD-1 blockade did not increase TA-specific T cell function on a cell-per-cell basis. Several hypotheses have been proposed to explain the failure of PD-1 blockade to completely reverse T cell dysfunction of PD-1+ exhausted T cells. First, virus-specific exhausted T cells express variable levels of PD-1, and PD-1high T cell subsets appeared to be less responsive to PD-1 blockade than PD-1low/int CD8+ T cells (15, 16). In line with this observation, we have found that PD-1 expression was higher on partially dysfunctional PD-1+BTLA+Tim-3− TA-specific CD8+ T cells than on BTLA−PD-1+Tim-3− cells but lower than on highly dysfunctional BTLA+PD-1+Tim-3+ cells. Second, exhausted T cells in chronic viral infections have been shown to upregulate multiple inhibitory receptors, including PD-1, 2B4, CD160 and LAG-3 (2). The co-expression of these receptors was associated with lower T cell function and more severe infection, and the blockade of multiple inhibitory pathways appeared to better reverse T cell dysfunction. Consistent with these observations, we have shown that a subset of highly dysfunctional NY-ESO-1-specific CD8+ T cells upregulate both PD-1 and Tim-3 in patients with advanced melanoma (5). In the present study, we further demonstrate that PD-1+BTLA+Tim-3− and PD-1+BTLA+Tim-3+ TA-specific CD8+ T cells represent two distinct subsets of TA-specific CD8+ T cells with increased T cell dysfunction, illustrating the hierarchical loss of T cell function in the context of chronic antigen simulation in patients with advanced melanoma. Such information may be useful to further the studies of the gene programs driving T cell exhaustion in cancer patients and provide potential novel targets to reverse T cell exhaustion (18, 19).
As previously reported and in sharp contrast to PD-1 and Tim-3, we observed a positive correlation between the upregulation of BTLA and the upregulation of CCR7, CD45RA and CD27 by total CD8+ T cells, supporting that BTLA upregulation is inversely correlated with CD8+ T cell maturation (14, 20). Several lines of evidence suggest that the factors controlling BTLA upregulation by TA-specific dysfunctional T cells are distinct from those controlling PD-1 and Tim-3 expression. First, as opposed to PD-1+ exhausted LCMV-specific CD8+ T cells (2), the large majority of the PD-1+ NY-ESO-1-specific CD8+ T cells in patients with advanced melanoma upregulate BTLA. Second, and in striking contrast with PD-1 expression, we observed no significant upregulation of BTLA expression between PD-1+BTLA+Tim-3− and PD-1+BTLA+Tim-3+ TA-specific CD8+ T cells, ruling against a correlation between BTLA expression and the severity of T cell dysfunction in patients with advanced melanoma. Third, NY-ESO-1-specific CD8+ T cells upregulated PD-1 and Tim-3 but not BTLA expression upon prolonged stimulation with cognate antigen. Collectively, these findings are consistent with the idea that the upregulation of BTLA by dysfunctional TA-specific CD8+ T occurs independently of functional exhaustion upon high antigen load. The molecular mechanisms driving BTLA upregulation by antigen-specific CD8+ T cells in patients with advanced melanoma but not in chronic viral infections remain to be identified. Adaptive T cell immune responses to chronic viral infections and cancers are both shaped by high antigen load and the immunosuppressive environment. However, one major difference is the occurrence of T cell anergy, which appears to be an early event in the course of tumor progression possibly due to poor antigen presentation at tumor sites (21, 22). In this perspective, it is conceivable that upon chronic antigen expression at tumor sites, subsets of TA-specific T cells become dysfunctional through both exhaustion upon high antigen load and anergy upon suboptimal priming. In contrast, T cells directed against exogenous antigens in chronic viral infections may become dysfunctional mainly by functional exhaustion. In support of this hypothesis, exhausted T cells in chronic LCMV infections do not upregulate anergy-associated genes used to define molecular signatures of anergy in vitro and in vivo such as grail, Egr-2 and Egr-3 (23, 24). In addition, high levels of BTLA expression have been reported on anergic T cells directed against an antigen expressed by somatic tissues (13). Therefore and together with these studies, our findings of BTLA upregulation by PD-1+ TA-specific CD8+ T cells indicate major differences in the profile of inhibitory receptors upregulated by dysfunctional antigen-specific T cells in cancer versus chronic viral infections.
One critical finding is that BTLA blockade adds to PD-1 blockade to enhance NY-ESO-1-specific CD8+ T cell expansion and function. BTLA blockade, in combination with PD-1 and Tim-3 blockades further augment the frequencies of IL-2-producing, proliferating and total NY-ESO-1-specific CD8+ T cells among total CD8+ T cells upon prolonged antigen stimulation.
In summary, we report a novel major subset of dysfunctional TA-specific CD8+ T cells, which upregulate BTLA and PD-1 in patients with advanced melanoma. The precise mechanisms driving BTLA upregulation by TA-specific CD8+ T cells appear to be independent of functional exhaustion and need to be further investigated. Our findings support the targeting of BTLA, PD-1 and Tim-3 pathways to reverse tumor-induced T cell dysfunction in patients with advanced melanoma.
Supplementary Material
Acknowledgements
We thank Dr. Lisa Borghesi and Mr. Dewayne Falkner of the Flow Facility of the University of Pittsburgh, Department of Immunology for their technical support. We also thank Ms. Lisa Spano for editorial assistance.
Grant Support: This work was supported by the National Institutes of Health/National Cancer Institute grants R01CA90360 and R01CA157467 (to H.M. Zarour), an award from the Melanoma Skin Spore grant NCI P50CA121973 (to H.M. Zarour) and a grant from the Cancer Research Institute (to H.M. Zarour).
Footnotes
Conflict of interest: The authors have no conflicting financial interests.
References
- 1.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–13. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fourcade J, Kudela P, Sun Z, Shen H, Land SR, Lenzner D, et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J Immunol. 2009;182:5240–9. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy KM, Nelson CA, Sedy JR. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev Immunol. 2006;6:671–81. doi: 10.1038/nri1917. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD 1. Nat Immunol. 2003;4:670–9. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 9.Murphy TL, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- 10.Deppong C, Juehne TI, Hurchla M, Friend LD, Shah DD, Rose CM, et al. Cutting edge: B and T lymphocyte attenuator and programmed death receptor-1 inhibitory receptors are required for termination of acute allergic airway inflammation. J Immunol. 2006;176:3909–13. doi: 10.4049/jimmunol.176.7.3909. [DOI] [PubMed] [Google Scholar]
- 11.Tao R, Wang L, Han R, Wang T, Ye Q, Honjo T, et al. Differential effects of B and T lymphocyte attenuator and programmed death-1 on acceptance of partially versus fully MHC-mismatched cardiac allografts. Journal of immunology. 2005;175:5774–82. doi: 10.4049/jimmunol.175.9.5774. [DOI] [PubMed] [Google Scholar]
- 12.Krieg C, Boyman O, Fu YX, Kaye J. B and T lymphocyte attenuator regulates CD8+ T cell-intrinsic homeostasis and memory cell generation. Nature immunology. 2007;8:162–71. doi: 10.1038/ni1418. [DOI] [PubMed] [Google Scholar]
- 13.Hurchla MA, Sedy JR, Gavrieli M, Drake CG, Murphy TL, Murphy KM. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly Induced in anergic CD4+ T cells. J Immunol. 2005;174:3377–85. doi: 10.4049/jimmunol.174.6.3377. [DOI] [PubMed] [Google Scholar]
- 14.Derre L, Rivals JP, Jandus C, Pastor S, Rimoldi D, Romero P, et al. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest. 2010;120:157–67. doi: 10.1172/JCI40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A. 2008;105:15016–21. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–37. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–92. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, et al. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–20. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nature medicine. 2010;16:1147–51. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serriari NE, Gondois-Rey F, Guillaume Y, Remmerswaal EB, Pastor S, Messal N, et al. B and T lymphocyte attenuator is highly expressed on CMV-specific T cells during infection and regulates their function. Journal of immunology. 2010;185:3140–8. doi: 10.4049/jimmunol.0902487. [DOI] [PubMed] [Google Scholar]
- 21.Staveley-O’Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, et al. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1178–83. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Current opinion in immunology. 2010;22:223–30. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–31. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 24.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–84. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.