Abstract
We reported the selective transport of classical 2-amino-4-oxo-6-substituted pyrrolo[2,3-d]pyrimidines with a thienoyl-for-benzoyl-substituted side chain and a 3- (3a) and 4-carbon (3b) bridge. Compound 3a was more potent than 3b against tumor cells; While 3b was completely selective for transport by folate receptors (FRs) and the proton-coupled folate transporter (PCFT) over reduced folate carrier (RFC), 3a was not. To determine if decreasing the distance between the bicyclic scaffold and L-glutamate in 3b would preserve transport selectivity and potency against human tumor cells, 3b regioisomers with [1,3] (7 and 8) and [1,2] (4, 5 and 6) substitutions on the thienoyl ring, and with acetylenic insertions in the 4-atom bridge, were synthesized and evaluated. Compounds 7 and 8 were potent nanomolar inhibitors of KB and IGROV1 human tumor cells with complete selectivity for FRα and PCFT over RFC.
INTRODUCTION
Membrane transport of antifolate therapeutics such as methotrexate MTX is integral to chemotherapy efficacy in treating a variety of malignancies and non-malignant diseases.1 The major membrane transporters include the reduced folate carrier (RFC), the proton-coupled folate transporter (PCFT) and the high affinity folate receptors (FRs) α and β.1–4 While all systems transport folate cofactors and classical antifolates such as MTX, raltitrexed (RTX) and pemetrexed (PMX), they differ in terms of mechanism and substrate specificities. Whereas both RFC and PCFT are integral membrane proteins that act as facilitative transporters, FRs are glycosyl phosphatidylinositol-modified proteins that mediate cellular uptake of (anti)folates by receptor-mediated endocytosis.
The major folate transporters also differ in terms of their tissue distributions. For instance, RFC is ubiquitously expressed in tumors and tissues and is the primary uptake mechanism for folate cofactors.1 FRs are expressed abundantly in certain malignancies, most notably as the FRα isoform in ovarian carcinomas, and in a limited number of normal epithelial tissues such as renal tubules.2 Major sites of PCFT expression include the upper small intestine (e.g., jejunum), and the liver and kidney.3,5,6 In solid tumors such as hepatomas, ovarian carcinomas, and non-small cell lung carcinomas, PCFT is highly expressed.7 PCFT exhibits an acidic pH optimum4,6, which is compatible with the low pH microenvironments of the small intestine and many solid tumors. While PCFT is modestly expressed in most other normal tissues, for those in which PCFT is expressed they are unlikely to present the low pH conditions optimal for membrane transport by this mechanism.3
Membrane transport of classical antifolates by RFC in itself would likely result in limited drug selectivity toward tumors over normal proliferative tissues such as bone marrow since these normal tissues all express RFC. The concept of selectively targeting FR-expressing tumors with folate-based antitumor agents has been tested. Examples of folate-based therapeutics include 1 [(2R)-2-((4R)-4-carboxy-5-(4-((2-(hydroxymethyl)-4-oxo-4,6,7,8-tetrahydro-3H-cyclopenta[g]quinazolin-6-yl)(prop-2-yn-1-yl)amino)phenyl)-5-oxopentanamido)pentanedioic acid] [ONX0801 (BGC945)], a small molecule antifolate licensed by Onyx Pharmaceuticals which is selectively transported by FRs and inhibits thymidylate synthase8, and vincaleukoblastin-23-oic acid, O4-deacetyl-, 2-[(2-mercaptoethoxy)carbonyl]hydrazide, disulfide with N-[4-[[(2-amino-1, 4-dihydro-4-oxo-6pteridinyl)methyl]amino]benzoyl]-L-(-glutamyl-L-∀-aspartyl-L-∀-aspartyl-L-cysteine 2 [EC-145 (Endocyte)], a desacetylvinblastine monohydrazide–folic acid complex.9 Compound 1 is in clinical trials in the UK. A randomized Phase II trial is near completion in which 2 with doxorubicin was compared to doxorubicin alone for the treatment of platinum-resistant ovarian cancer (ClinicalTrials.gov Identifier: NCT00722592).
We recently identified 6-substituted pyrrolo- and thieno[2,3-d]pyrimidine antifolates with selective membrane transport by PCFT and/or FRs over RFC, thus selectively inhibiting proliferation of cells expressing these systems including human tumors.7,10–14 FR targeting to tumors is based on patterns of expression basolateral membrane localization unique to tumor cells. In terms of PCFT, the notion of using this transport mechanism to selectively deliver chemotherapy drugs is appealing given the low pH microenvironments of many solid tumors which approximate the pH optimum for PCFT.15–17 The pyrrolo[2,3-d]pyrimidine thienoyl analogs 3a and 3b (Figure 1) with 3- and 4-carbons, respectively, in the bridge between the pyrrolo[2,3-d]pyrimidine ring and the thiophene ring were the most potent of this series against cells expressing FRs and/or PCFT.12,13 Cytotoxicity was attributed to the potent inhibition of β-glycinamide ribonucleotide (GAR) formyltransferase (GARFTase), which catalyzes the first folate-dependent reaction in de novo purine nucleotide biosynthesis.
Figure 1.

Structures of 6-Substituted pyrrolo[2,3-d]pyrimidines 3a and 3b and the thienoyl regioisomers 4–13.
Our finding that compound 3a was more potent than 3b toward FR and PCFT-expressing cells suggested that the distance between the bicyclic scaffold and the L-glutamate may be a critical determinant of drug activity.13 However, in engineered hamster cells, transport selectivity for FRα and PCFT over RFC with compound 3a was less complete than for 3b. We hypothesized that structurally engineered substitutions on the thiophene ring of 3a would afford distances between the bicyclic scaffold and the L-glutamate midway between those for the 3-atom bridge 3a and the 4-atom bridge 3b. We predicted that this would preserve the potency of 3a along with the selectivity for FRα and PCFT as in 3b. We synthesized 3b regioisomers, differing in positions of substitution of the 4 atom carbon bridge and the L-glutamic acid on the thiophene ring (compounds 4–8), and in the presence of a linear, shorter acetylenic linkage (compounds 9–13 and 20) in the carbon bridge region (Figure 1). These modifications decreased the distances between the bicyclic pyrrolo[2,3-d]pyrimidine and the L-glutamate moieties, in order to systematically determine their impact on drug selectivity for FRs and PCFT over RFC, and on potency against human tumor cells. In this report, we describe the synthesis and biologic properties of compounds 4–13 (Figure 1), and 20 (Scheme 3).
Scheme 3.
aReagents and conditions: (a) i. 1N NaOH; ii. 1N HCl
CHEMISTRY
The synthesis of target compounds 4–8 (Scheme 1) started from the reported intermediate 16.12 A Sonogashira coupling of 16 with various bromo-substituted thiophene carboxylates 15a–e (Scheme 2) afforded 17a–e in 50–56% yield. Subsequent hydrogenation and saponification of 17a–e afforded target compounds 4–8. Direct hydrolysis of intermediates 17a–e provided conformationally restricted analogs 9–13. Compounds 15a–e (Scheme 2) required in Scheme 1 were synthesized by a peptide coupling of the commercially available bromo-substituted thiophene carboxylic acids 14a–e (Scheme 2) with L–glutamate diethyl ester hydrochloride in 89–91% yield.
Scheme 1.
aReagents and conditions: (a) CuI, Pd(0)(PPh3)4, Et3N, DMF, RT, 12 h; (b) i. 1N NaOH, MeOH, CHCl3, RT, 6 h; ii. 1N HCl; (c) 10% Pd/C, H2, 55 psi, 2 h.
Scheme 2.
aReagents and conditions: (a) N-methylmorpholine, 2 chloro 4,6 dimethoxy 1,3,5-triazine, L-glutamate diethyl ester hydrochloride, DMF, RT, 12 h.
Compound 19 (Scheme 3) was previously reported as a precursor to 3b (Figure 1).12 For comparison to 3b, it was also of interest to synthesize and evaluate the classical analog 20 derived from 19. Synthesis of 20 from 19 is shown in Scheme 3.
BIOLOGICAL EVALUATION AND DISCUSSION
6-Substituted pyrrolo[2,3-d]pyrimidine thienoyl regioisomers as inhibitors of cell proliferation
The distance between the bicyclic scaffold and L-glutamate portions of a series of pyrrolo- and thieno[2,3-d]pyrimidine antifolates seems to dictate both selectivity for cellular uptake by FRs and PCFT over RFC, as well as cytotoxic potencies against tumor cells.10–14 Thus, decreasing the bridge lengths from 8-carbons to 4-carbons was accompanied by substantially increased antiproliferative activities, reflecting transport by PCFT and/or FRs and inhibition of intracellular GARFTase. Decreasing the bridge length from 4- to 3-carbons further increased drug potencies. However, for the pyrrolo[2,3-d]pyrimidine thienoyl antifolate series, this appeared to be accompanied by some loss of absolute transporter specificity for FRs and/or PCFT over RFC.13 Finally, for all series, analogs with bridge lengths less than 3-carbons showed substantially decreased drug activities.
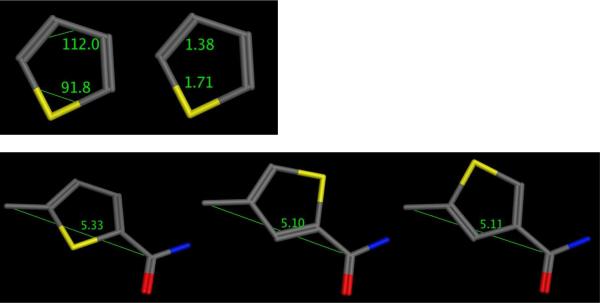
In the present study, we used the pyrrolo[2,3-d]pyrimidine thienoyl platform with a 4-carbon bridge. We systematically assessed the effects on cell proliferation for an expanded series of pyrrolo[2,3-d]pyrimidine thienoyl regioisomers of 3b with a 4-carbon bridge and thienoyl ring substitutions, 2',3' (4), 4',5' (5), 3',4' (6), 3',5' (7), and 2',4' (8) (Figure 1), as an alternative approach for decreasing the distance between the bicyclic pyrrolo[2,3-d]pyrimidine and the L-glutamate portions. Hence, in analogs 4–6, the [1,2] substitution pattern on the thiophene forces the bicyclic scaffold and L-glutamate closer together than in the parent 3-atom bridge compound 3b which includes a [1,3] pattern on the thiophene ring. Compounds 7 and 8, also [1,3] substitutions on the thiophene ring, have an intervening smaller carbon compared to the larger sulfur in 3b. Further, the C-C-C (112°) bond angle of both 7 and 8 is larger than the C-S-C (91.8°) bond angle of 3b, and the lengths of the C-S (1.71 Å) and C-C (1.38 Å) bonds are also different (Figure 2, MOE 2008.10)18. Molecular modeling (Figure 2, MOE 2008.10) shows that the distances from the α-carbon of the bridge to the amide carbonyl are shorter in compounds 7 and 8 (5.10 Å and 5.11 Å, respectively) than that of 3b (5.33 Å). The distances from the bicyclic scaffold to the L-glutamate for compounds 7 and 8 are designed to be intermediate between those for 3b and 3a.
Figure 2.
Molecular modeling of the thiophene ring (MOE 2008.10) and angles and distances of the regioisomers of 3b.
Compounds 9–13 and 20 are analogs of 3b-8 and contain a single conformational restriction in the 4-carbon atom bridge, in the form of a linear acetylenic linkage instead of the saturated C-C linkage of 3b-8. This also provides some conformational rigidity in the four-carbon atom chain and decreases the length of the carbon bridge (3.52 Å), compared to the saturated analogs (3.87 Å) 3b-8.
Compounds 4–13 and 20 were initially screened for growth inhibition in a series of cell lines with defined expression patterns of the major (anti)folate transporters for comparison with compound 3b. The experimental models were a panel of isogenic Chinese hamster ovary (CHO) sublines derived from RFC-, FR- and PCFT-null MTXRIIOuaR2–4 CHO cells engineered to express FRα (RT16),10 human RFC (PC43-10)18, or human PCFT (R2/hPCFT4)5,11. Additional growth inhibition studies were performed with KB and IGROV1 human tumor cells that express FRα, RFC, and PCFT. We previously showed that during cell outgrowth, the pH of the culture media decreased from pH 7.2 to ~6.814 such that all 3 transporters would be active in drug transport.
For measurements of inhibitory effects on cell proliferation, the cell lines were cultured with the antifolates over a range of concentrations, as described in the Experimental Section. With RT16 CHO, KB and IGROV1 cells, FRα-mediated drug uptake was established with a parallel culture treated with the cytotoxic drugs and excess (200 nM) folic acid to block FRs. For the PC43-10 and R2/hPCFT4 CHO sublines, results were compared to those for parental R2 cells or to vector control R2 cells transfected with empty pcDNA3.1 vector [designated R2(VC)]. Patterns of drug sensitivity or resistance for the novel pyrrolo[2,3-d]pyrimidine analogs were compared to those for compounds 3a and 3b, and to those previously reported for classic antifolates (e.g., MTX, PMX, LMTX, RTX).10–14 Results of the cell proliferation assays are summarized in Table 1.
Table 1.
IC50s (in nM) for 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl regioisomers 3b, 4–13, and 20, compared to IC50s for 3a and classical antifolates in RFC, PCFT, and FR-expressing cell lines.
| Antifolate | RFC | hFRα | PCFT | RFC/ FRα/PCFT | RFC/ FRα/PCFT | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PC43-10 | R2 | RT16 | RT16 (+FA) | R2/hPCFT4 | R2(VC) | KB | KB (+FA) | IGROV1 | IGROV1 (+FA) | |
| 3a | 101.0(16.6) | 273.5(49.1) | 0.31 (0.14) | >1000 | 3.34 (0.26) | 288 (12) | 0.26(0.03) | >1000 | 0.55(0.10) | >1000 |
| 3b | >1000 | >1000 | 1.82(0.28) | >1000 | 43.4(4.1) | >1000 | 0.55(0.10) | >1000 | 0.97(0.12) | >1000 |
| 4 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | 171(36) | >1000 | ND | ND |
| 5 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | 267(33) | >1000 | ND | ND |
| 6 | >1000 | >1000 | 147(53) | >1000 | >1000 | >1000 | 35.3(4.4) | >1000 | 102.9(25.9) | >1000 |
| 7 | >1000 | >1000 | 2.54(0.52) | >1000 | 41.54 (13.09) | >1000 | 0.17(0.02) | >1000 | 0.48(0.08) | >1000 |
| 8 | >1000 | >1000 | 2.60(0.59) | >1000 | 63.82 (16.23) | >1000 | 0.27(0.07) | >1000 | 1.4 (0.5) | >1000 |
| 9 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | ND | ND |
| 10 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | ND | ND |
| 11 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | ND | ND |
| 12 | >1000 | >1000 | 13.48(2.42) | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| 13 | >1000 | >1000 | 8.26 (1.86) | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| 20 | >1000 | >1000 | 12.07(5.02) | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| MTX | 12(1.1) | 216(8.7) | 114(31) | 461(62) | 120.5 (16.8) | >1000 | 6.0(0.6) | 20(2.4) | 21(3.4) | 22(2.1) |
| PMX | 138(13) | 894(93) | 42(9) | 388(68) | 13.2(2.4) | 974.0(18.1) | 68(12) | 327(103) | 102(25) | 200(18) |
| RTX | 6.3(1.3) | >1000 | 15(5) | >1000 | 99.5(11.4) | >1000 | 5.9(2.2) | 22(5) | 12.6(3.3) | 20(4.3) |
| LMTX | 12(2.3) | >1000 | 12(8) | 188(41) | 38.0(5.3) | >1000 | 1.2(0.6) | 31(7) | 3.1(0.9) | 16(6) |
ND: Not determined
Growth inhibition assays were performed for CHO sublines engineered to express RFC (PC43-10), FRα (RT16), or PCFT (R2/hPCFT4), for comparison with transporter-null [R2, R2(VC)] CHO cells, and for the KB and IGROV1 tumor sublines (express RFC, FRα, and PCFT), as described in the Experimental Section. For the FR experiments, growth inhibition assays were performed in the presence and the absence of 200 nM folic acid (FA). The data shown are mean values from 2–10 experiments (plus/minus SEM in parentheses). Results are presented as IC50 values (in nmolar concntrations), corresponding to the concentrations that inhibit growth by 50% relative to cells incubated without drug. Data for MTX, PMX, RTX, and LMTX were previously published.10–14
MTX, PMX, RTX, and LMTX were all active (albeit variably) toward RFC-, PCFT-, or FRα-expressing CHO cells. The 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl analogs with contiguous thiophene substitutions [2'3' (4 and 10); 4'5' (5 and 11); 3'4'(6 and 12)] were completely inactive toward the RFC- and PCFT-expressing CHO sublines. Compounds 7, 8, 13, and 20 were likewise inert toward PC43-10 cells, although both compounds 7 and 8 (but not conformationally rigid 13 and 20) inhibited proliferation of R2/hPCFT4 cells (IC50s of 41.5 and 63.8 nM for 7 and 8, respectively) essentially equivalent to compound 3b (IC50 of 43.4 nM). This establishes cellular uptake of compounds 7 and 8 by PCFT. Drug sensitivities for 7 and 8 with R2/hPCFT4 cells exceeded those for MTX and RTX, and were comparable to those for LMTX. However, 7 and 8 were less potent than PMX (~3- and ~5-fold, respectively) and 3a (~12- and ~19-fold, respectively). None of the 6-substituted pyrrolo[2,3-d]pyrimidine regioisomers (3b, 4–13 and 20) had any impact on proliferation of R2 or R2(VC) cells.
For FRα-expressing RT16 cells, compounds 4, 5, 10, and 11 were all inert at drug concentrations up to 1000 nM. Compounds 6, 7, 8, 12, 13, and 20, like 3b, all showed some level of antiproliferative activity toward RT16 cells. The active drugs followed an order of potency, 7 ~ 8 ~ 3b > 12 ~ 13 > 20 >> 6. FR mediated growth inhibitions for the most active analogs (i.e., 3b, 7, and 8) exceeded those for standard antifolates (Table 1) and for all the active analogs, inhibitions were abolished with 100-fold excess folic acid.
KB and IGROV1 human tumor cells express all three transport systems7 and in these models, 7 and 8 were the most potent drugs in our series (Table 1). Inhibitory activities for 7 and 8 were similar to those for 3a and 3b in both KB and IGROV1 cells. In KB cells, compounds 4, 5, and 6 were all substantially less active than 7 and 8, in order of decreasing potency, 6 >> 5 ~ 4. For the active analogs, growth inhibition was again reversed by excess folic acid, establishing that like compound 3b, FRα was the major mechanism of uptake in these cells despite the presence of RFC and PCFT. Thus, for the 7 and 8 regioisomers of 3b, we confirmed our hypothesis that by varying the distance between the bicyclic scaffold and the L-glutamate we preserved both the absolute selectivity of 3b and the high antiproliferative potencies of 3a and 3b.
Interestingly, none of the acetylenic compounds (9–13 and 20) were inhibitory toward FRα-expressing human KB cells, in striking contrast to the results with RT16 CHO cells with compounds 12, 13, and 20. This suggests that for 12, 13, and 20, there must be differences in cellular metabolism, drug binding to intracellular targets, and/or substrate activity for folylpolyglutamate synthetase between the human and hamster cells. When several of these analogs (3b, 6, 12, 13, and 20, compared to 7 and 8) were tested in IGROV1 cells, analogous results to those with KB cells were obtained (Table 1).
Additional experiments used colony-forming assays with KB tumor cells continuously exposed to compounds 3b, 7, or 8 for up to 14 days, after which colonies were stained with 1% methylene blue and counted. Compounds 7 and 8 inhibited KB cell colony formation (Supplement, Figure 1S) with similar IC50s (0.39 and 0.16 nM, respectively) to that for compound 3b (IC50 = 0.20 nM) and substantially lower than that for MTX (IC50 = 8.5 nM).
These results establish that (i) none of the regioisomers 3b, 4–13, and 20 are RFC substrates. (ii) For the saturated bridge analogs (3b, 4–8), a [1,3] relationship between the carbon bridge domain and the L-glutamate moiety is an important determinant of transport substrate activity with FRα or PCFT. (iii) Compounds 7 and 8, like 3b, were substantially less inhibitory toward PCFT-expressing cells than compound 3a. However, this difference was much reduced in human tumor cells for which FRα was the primary mode of drug delivery. The results with compounds 4, 5, and 6 and FRα-expressing cells suggest that decreasing distance between the bicyclic ring and the L-glutamate beyond a point has adverse effects on drug activity, analogous to those resulting from decreasing bridge lengths to less than 3-atoms. (iv) None of the conformationally restricted acetylenic analogs (9–13 and 20) were substrates for PCFT. (v) For this series, the [1,3] acetylenic analogs (12, 13, and 20) are substrates for FRα, although other factors unrelated to membrane transport seem likely to account for the marked differences in their antiproliferative activities between the human and hamster sublines.
Impact of elevated leucovorin and nucleosides on anti-tumor activity of compounds 7 and 8
Since our proliferation assays with FR-expressing cells routinely use limiting (2 nM) concentrations of extracellular leucovorin [(6R,S)5-formyl tetrahydrofolate, a chemically stable reduced folate] (LCV), additional experiments were performed with KB cells to assess the impact of elevated (50 nM) LCV [for which the active (6S) stereoisomer approximates the concentration of 5-methyl tetrahydrofolate in serum] on the growth inhibitory effects of the most potent analogs (compounds 7 and 8) of our series compared to compound 3b (Figure 3). In KB cells, the impact of 50 nM LCV was uneven and ranged from ~7-fold for 3b to ~20-fold for 7 and >38-fold for 8. For the data in Figure 3, IC50s in the presence of 50 nM LCV were 2.2 nM for 3b, 3.4 nM for 7 and >20 nM for 8. Thus, both compounds 3b and 7 maintained substantial antitumor potencies even in the presence of elevated physiologic levels of reduced folate.
Figure 3. Growth inhibition of KB cells by the 6-Substituted pyrrolo[2,3-d]pyrimidine thienoyl regioisomers 3b, 7, and 8 in the presence of physiologic concentrations of reduced folate.
Cell proliferation inhibition was measured over a range of concentrations of the antifolates in complete folate free RPMI1640 in the presence of LCV at 2 and 50 nM. Cell densities were measured as in the experimental with a fluorescence dye and a fluorescence plate reader. Results were normalized to cell density in the absence of drug. Results shown are representative data of experiments performed in duplicate.
As previously reported for 3b,12 growth inhibitory effects of 7 and 8 toward KB (Figure 4) and IGROV1 cells (not shown) were completely reversed when drug exposures were performed in the presence of adenosine (60 μM) but not thymidine (10 μM). This established that the effects of 7 and 8 on cell proliferation were the consequence of inhibiting the de novo purine nucleotide biosynthetic pathway. For both 7 and 8, 5-amino-4-imidazolcarboxamide (AICA) (320 μM) was also protective. Since AICA is metabolized to AICA ribonucleotide (AICAR), the substrate for AICAR formyltransferase (AICARFTase; the 2nd folate-dependent reaction after GARFTase),10,197 and 8 must derive their growth inhibitory effects by inhibiting GARFTase. This was further tested below.
Figure 4. Protection of KB cells from growth inhibition by the 6-Substituted pyrrolo[2,3-d]pyrimidine thienoyl regioisomers 7 and 8 in the presence of nucleosides and 5-amino-4-imidazole (AICA).
Proliferation inhibition was measured for KB cells over a range of concentrations of compounds 7 and 8, as shown, in complete folate-free RPMI1640 with 2 nM LCV in the absence of other additions (labeled “No additions”), or in the presence of 200 nM folic acid (labeled “Folate-added”), adenosine (60 μM), thymidine (10 μM), or AICA (320 μM). Cell densities were measured as in the experimental with a fluorescence dye and a fluorescence plate reader. Results were normalized to cell densities in the absence of drug. Results shown are representative data of experiments performed in triplicate. Analogous results were obtained with IGROV1 cells (not shown).
Cellular pharmacology of cytotoxic 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl regioisomers
Results of our cell proliferation assays in engineered CHO and human tumor cells suggested that 7 and 8 are bona fide transport substrates for FRα and PCFT. From results of nucleoside and AICA protection studies, 7 and 8, like 3b, following internalization, inhibit de novo purine nucleotide biosynthesis, apparently at the level of GARFTase. Interestingly, whereas the acetylenic bridge analogs 12, 13 and 20 inhibited proliferation of FRα-expressing RT16 CHO cells, these compounds were inactive toward human tumor cells.
Using inhibition of human tumor cell proliferation as a primary metric, we selected compounds 3b and 4–8 for testing FRα binding, reflected in competitive inhibition of [3H]folic acid binding to FRα in RT16 CHO cells. For these experiments, RT16 cells were washed with acid buffered saline (pH 3.5), treated (at neutral pH) with 50 nM [3H]folic acid, along with a range of concentrations of the pyrrolo[2,3-d]pyrimidines 3b and 4–8, folic acid, LMTX, PMX, or MTX. After additional washes (neutral pH), cells were solubilized (in 0.5 N NaOH). FRα-bound [3H]folic acid was measured, normalized to cellular protein, and relative inhibitor affinities were calculated as inverse molar ratios of the concentrations of unlabeled ligands which decreased [3H]folic acid binding by 50%.
In these experiments, compounds 7 and 8 were all high affinity ligands (relative affinities ≥ 1) for FRα, essentially equivalent to 3b and folic acid, and substantially better than LMTX (Figure 5). Like MTX, compounds 4–6 did not bind to FRα in this assay, in spite of detectable (albeit lower) levels of FRα-directed activity in our cell proliferation experiments (with KB cells; Table 1). The detectable antiproliferative activities with 4, 5, and 6 in KB cells (but not RT16 CHO cells) likely reflect markedly elevated levels of FRα in this human tumor cell line, as previously reported.10
Figure 5. Binding of 6-Substituted pyrrolo[2,3-d]pyrimidine thienoyl regioisomers (3b, 4–8) to FRα.

Data are shown for the effects of the unlabeled ligands with FRα-expressing RT16 CHO cells. Relative binding affinities for assorted folate/antifolate substrates were determined over a range of ligand concentrations and were calculated as the inverse molar ratios of unlabeled ligands required to inhibit [3H]folic acid binding by 50%. By definition, the relative affinity of folic acid is 1. Details for the binding assays are provided in the Experimental Section. Results are presented as average values and ranges from 2–3 experiments. Undefined abbreviations: FA, folic acid.
Since compounds 7 and 8 inhibited proliferation of R2/hPCFT4 cells, these analogs were tested as competitive inhibitors of PCFT transport of [3H]MTX in R2/hPCFT4 cells. Experiments were performed at pH 5.5, approximating the pH optimum for PCFT, and at pH 6.8, which is commonly achieved during cell outgrowth.14 Kis were calculated over a range of inhibitor concentrations from Dixon plots, and results were compared to those for 3b and PMX (Table 2). At pH 5.5, Ki values for 3b, 7, and 8 were all similar (within a factor of ~2) and approached the Ki for PMX. At pH 6.8, Kis for PMX and 3b were likewise similar (~27- and 31-fold higher, respectively, than the values at pH 5.5). In comparison, Kis for 7 and 8 were further increased at pH 6.8 (~70-fold greater) over the Kis recorded at pH 5.5.
Table 2.
Ki values for PCFT substrates.
| Substrate | Parameter | pH 5.5 | pH 6.8 |
|---|---|---|---|
| 3b | Ki (micromolar) | 0.23 ± 0.06 (n=7) | 7.23 ± 2.23 (n=7) |
| 7 | Ki (micromolar) | 0.39 + 0.10 (n=4) | 28.1 ± 5.3 (n=4) |
| 8 | Ki (micromolar) | 0.42 ± 0.11 (n=4) | 30.7 ± 8.5 (n=4) |
| PMX | Ki (micromolar) | 0.094 ± 0.006 (n=8) | 2.54 ± 0.31 (n=8) |
Ki values for PCFT substrates were determined by Dixon plots with [3H]MTX as substrate and a range of inhibitor concentrations in R2/hPCFT4 cells. KiS were calculated from the slopes of the Dixon plots and kinetic constants (Kt and Vmax) for MTX. Results are presented as mean values ± standard errors from multiple experiments (numbers shown in parentheses).
Collectively, these experiments confirm the high affinity binding for the [1,3] thienoyl regioisomers 3b, 7, and 8 to FRα and PCFT, suggested from their potent anti-proliferative and cytotoxic effects toward FRα- and PCFT-expressing CHO and human tumor cells. While compounds 7 and 8, like 3b, are potent inhibitors of PCFT at pH 5.5, binding of 7 and 8 to PCFT (as reflected in Ki values) appears to be more sensitive to pH than for either 3b or PMX.
Results of nucleoside/AICA protection experiments with KB (Figure 4) and IGROV1 tumor cells (not shown) suggested that GARFTase in the de novo purine nucleotide biosynthetic pathway was the primary intracellular target of 7 and 8, analogous to 3b12, thus explaining the potent anti-proliferative activities of these agents. To confirm GARFTase inhibition with compounds 7 and 8, we used an in situ GARFTase activity assay in which intact cells were incubated with the antifolate drugs along with [14C]glycine as a radiotracer. In the presence of azaserine, [14C]glycine is incorporated into GAR, accumulating [14C]formyl GAR which can be isolated by ion exchange chromatography and radioactivity determined.10–13,19
Data are shown in Figure 6 for the effects of compounds 3b, 7, and 8 on in situ GARFTase activity in IGROV1 cells, although completely analogous results were obtained with KB cells (not shown). In this assay, 3b, 7, and 8, like LMTX, were all potent inhibitors at low nanomolar concentrations.
Figure 6. In situ GARFTase inhibition assay.

For the in situ assays, incorporation of [14C]glycine into [14C]formyl GAR was measured in IGROV1 tumor cells cultured for 15 h in complete folate-free RPMI plus 2 nM LCV. Details are described in the Experimental Section. Results are presented as a percent of controls treated without drugs for IGROV1 cells treated with nM concentrations of 3b, 7, 8, or LMTX. LMTX was not tested at 5 nM (labeled “ND” for not determined). Results are presented as average values plus/minus ranges. Mean IC50s were calculated as 3.46 nM for 3b, 1.79 nM for 7, 2.03 nM for 8, and 5.77 nM for LMTX.
Conclusions
A lack of selectivity for folate transporters is a major cause of toxicity and chemotherapy failure with clinically used antifolates. We previously reported potent inhibition of tumor cell proliferation by a classical 2-amino-4-oxo-6-substituted pyrrolo[2,3-d]pyrimidine with a thienoyl-for-benzoyl-substituted side chain and a 4-carbon bridge (compound 3b).7,12 Compound 3b was selective for transport by FRs and PCFT over RFC and inhibited GARFTase in de novo purine biosynthesis.7,12 An analog of this series with a 3-carbon bridge (compound 3a) was even more active, suggesting that the distance between the bicyclic scaffold and L-glutamate could be an important determinant of drug activity, although this may be partly at the expense of absolute transport selectivity.13 Accordingly, we pursued other approaches for altering this feature, without appreciably impacting drug potency toward PCFT- and FRα-expressing cells while preserving selectivity for transport by non-RFC uptake mechanisms over RFC.
We now describe the synthesis and biological characterization of regioisomers of 3b (compounds 4–8), differing in positions of thienoyl ring substitution so as to vary the distance and orientation between the bicyclic scaffold and the L-glutamate. Additional conformationally restricted analogs of 3b and 4–8 (designated 20 and 9–13, respectively) were also studied in which a linear acetylenic linkage was inserted into the bridge region. Of this expanded series, analogs with a saturated bridge and with a [1,3] substitution pattern on the thienoyl ring (compounds 3b, 7, and 8) were the best inhibitors of KB and IGROV1 human tumor cell growth and clonogenicity. This was associated with a potent inhibition of GARFTase in de novo purine nucleotide biosynthesis. Whereas compounds 3b, 7 and 8 all showed FRα- and PCFT-mediated effects on cell proliferation, neither 3b nor compounds 4–8 showed activity toward CHO cells expressing only RFC. Further, none of the conformationally restricted linear acetylenic bridge analogs 9–13 and 20 inhibited human tumor cell growth, although the [1,3] thiophene-substituted compounds 12, 13 and 20 were distinctly (and selectively) inhibitory toward FRα-expressing RT16 CHO cells. This further supports our conclusion that only the [1,3] substituted thiophene is amenable to high affinity FRα binding and cellular uptake, and that species differences in intracellular drug metabolism and/or target enzyme binding must account for the lack of activity of these acetylenic analogs with human tumor cells.
Thus, our present and published results establish that the pyrrolo[2,3-d]pyrimidine scaffold, along with a thienoyl side chain, is particularly amenable to FR and PCFT transport in the absence of RFC transport, and to potent GARFTase inhibition. Our new finding that [1,3] substituted regioisomers of this series are preferred over the [1,2] analogs is consistent with the notion of an optimal distance between the bicyclic ring system and L-glutamate moiety for maximal anti-proliferative effects. However, among [1,3] compounds 3b, 7 and 8, or even including 3a, there was no consistent relationship between relative drug sensitivities toward FRα- and PCFT-expressing cells and these intramolecular distances. Indeed, compounds 7 and 8 showed in vitro potencies that were generally comparable to those for compound 3b or even 3a, although this was cell line-dependent. This may reflect differential impacts of extra- and/or intracellular reduced folates on efficacies of the various analogs, combined with cell-specific differences in folate transport (i.e., RFC levels) or metabolism, and, for PCFT, differences in relative binding affinities between drugs at pH ~6.8.
Of course, the most distinguishing feature of the three active regioisomers, 3b, 7, and 8, is their complete lack of RFC-mediated growth inhibition (presumably reflecting their lack of uptake) which is key to their potential therapeutic utility, as this would obviate toxicity to normal susceptible tissues for which drug transport by FRs or PCFT should be nominal. Clearly, the profound selectivity of these novel 6 substituted pyrrolo[2,3-d]pyrimidine [1,3] thienoyl antifolates for FRs and PCFT over RFC offers a paradigm for selective tumor targeting, based on established patterns of FR and PCFT expression in human tumors7 and optimal PCFT membrane transport under acidic conditions characteristic of the solid tumor microenvironment.
EXPERIMENTAL SECTION
All evaporations were carried out in vacuum with a rotary evaporator. Analytical samples were dried in vacuo (0.2 mmHg) in a CHEM-DRY drying apparatus over P2O5 at 60 °C. Melting points were determined on a MEL-TEMP II melting point apparatus with FLUKE 51 K/J electronic thermometer and are uncorrected. Nuclear magnetic resonance spectra for proton (1H NMR) were recorded on Bruker Avance II 400 (400 MHz) and 500 (500 MHz) spectrometer. The chemical shift values are expressed in ppm (parts per million) relative to tetramethylsilane as an internal standard: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad singlet. Thin-layer chromatography (TLC) was performed on Whatman Sil G/UV254 silica gel plates with a fluorescent indicator, and the spots were visualized under 254 and 366 nm illumination. Proportions of solvents used for TLC are by volume. Column chromatography was performed on a 230–400 mesh silica gel (Fisher, Somerville, NJ) column. The amount (weight) of silica gel for column chromatography was in the range of 50–100 times the amount (weight) of the crude compounds being separated. Columns were dry-packed unless specified otherwise. Elemental analyses were performed by Atlantic Microlab, Inc., Norcross, GA. Element compositions are within ±0.4% of the calculated values. Fractional moles of water or organic solvents frequently found in some analytical samples of antifolates could not be prevented despite 24–48 h of drying in vacuo and were confirmed where possible by their presence in the 1H NMR spectra. Elemental analysis was used to determine the purity of the final compounds 4–13. All solvents and chemicals were purchased from Aldrich Chemical Co. and Fisher Scientific and were used as received. Purity of the final compounds 4–13 were >95% and were determined by elemental (C, H, N, S) analysis.
General procedure for the synthesis of compounds 15a–e
To a 250 mL round-bottomed flask, was added a mixture of bromo-substituted thiophene-carboxylic acid 14a–e (1.04 g, 5 mmol), N-methylmorpholine (909 mg, 9 mmol), 2-chloro-4,6-dimethoxy-1,3,5-triazine (1.58 g, 9 mmol) and anhydrous DMF (20 mL). The mixture was stirred for 1.5 h at room temperature. N-methylmorpholine (909 mg, 9 mmol) and L-glutamic acid diethylester hydrochloride (1.8 g, 7.5 mmol) were added to the flask, and the reaction mixture was then stirred at room temperature for 12 h. After evaporation of solvent under reduced pressure, MeOH (20 mL) was added followed by silica gel (2.5 g). The resulting plug was loaded on to a silica gel column (2.5 × 12 cm) and eluted with 50% EtOAc in hexane. Fractions with desired Rf (TLC) were pooled and evaporated to afford 15a–e as colorless liquid.
(S)-2-[(2-Bromo-thiophene-3-carbonyl)-amino]-pentanedioic acid diethyl ester (15a)
Compound 15a was prepared using the general method described for the preparation of 15a–e, from 2-bromo-thiophene-3-carboxylic acid, 14a (1.04 g, 5 mmol) to give 1.76 g (90%) of 15a as a colorless liquid. TLC Rf 0.44 (hexane/EtOAc 1:1); 1H NMR (CDCl3-d1): δ 1.24–1.35 (m, 6H, COOCH2CH3), 2.09–2.56 (m, 4H, β-CH2, γ-CH2), 4.10–4.17 (m, 2H, COOCH2CH3), 4.23–4.29 (m, 2H, COOCH2CH3), 4.78–4.86 (m, 1H, α-CH), 7.20–7.22 (d, J = 7.2 Hz, 1H, CONH, exch), 7.26–7.27 (d, J = 5.6 Hz, 1H, Ar), 7.36–7.37 (d, J = 5.6 Hz, 1H, Ar).
(S)-2-[(3-Bromo-thiophene-2-carbonyl)-amino]-pentanedioic acid diethyl ester (15b)
Compound 15b was prepared using the general method described for the preparation of 15a–e, from 3-bromo-thiophene-2-carboxylic acid, 14b (1.04 g, 5 mmol) to give 1.8 g (91%) of 15b as a colorless liquid. TLC Rf 0.45 (hexane/EtOAc 1:1); 1H NMR (CDCl3-d1): δ 1.24–1.36 (m, 6H, COOCH2CH3), 2.13–2.55 (m, 4H, β-CH2, γ-CH2), 4.10–4.16 (m, 2H, COOCH2CH3), 4.25–4.30 (m, 2H, COOCH2CH3), 4.83–4.88 (m, 1H, α-CH), 7.07–7.08 (d, J = 5.6 Hz, 1H, Ar), 7.48–7.49 (d, J = 5.6 Hz, 1H, Ar), 7.73–7.75 (d, J = 7.2 Hz, 1H, CONH, exch).
(S)-2-[(4-Bromo-thiophene-3-carbonyl)-amino]-pentanedioic acid diethyl ester (15c)
Compound 15c was prepared using the general method described for the preparation of 15a–e, from 4-bromo-thiophene-3-carboxylic acid, 14c (1.04 g, 5 mmol) to give 1.74 g (89%) of 15c as a colorless liquid. TLC Rf 0.45 (hexane/EtOAc 1:1); 1H NMR (CDCl3-d1): δ 1.24–1.35 (m, 6H, COOCH2CH3), 2.07–2.56 (m, 4H, β-CH2, γ-CH2), 4.11–4.17 (m, 2H, COOCH2CH3), 4.23–4.30 (m, 2H, COOCH2CH3), 4.78–4.87 (m, 1H, α-CH), 7.35–7.36 (d, J = 3.6 Hz, 1H, Ar), 7.36–7.38 (d, J = 7.2 Hz, 1H, CONH, exch), 8.07–8.08 (d, J = 3.6 Hz, 1H, Ar).
(S)-2-[(4-Bromo-thiophene-2-carbonyl)-amino]-pentanedioic acid diethyl ester (15d)
Compound 15d was prepared using the general method described for the preparation of 15a–e, from 4-bromo-thiophene-2-carboxylic acid, 14d (1.04 g, 5 mmol) to give 1.8 g (91%) of 15d as a colorless liquid. TLC Rf 0.44 (hexane/EtOAc 1:1); 1H NMR (CDCl3-d1): δ 1.25–1.34 (m, 6H, COOCH2CH3), 2.13–2.51 (m, 4H, β-CH2, γ-CH2), 4.14–4.17 (m, 2H, COOCH2CH3), 4.23–4.28 (m, 2H, COOCH2CH3), 4.71–4.76 (m, 1H, α-CH), 7.04–7.06 (d, J = 7.2 Hz, 1H, CONH, exch), 7.42–7.43 (d, J = 1.6 Hz, 1H, Ar), 7.16–7.47 (d, J = 1.6 Hz, 1H, Ar).
(S)-2-[(5-Bromo-thiophene-3-carbonyl)-amino]-pentanedioic acid diethyl ester (15e)
Compound 15e was prepared using the general method described for the preparation of 15a–e, from 5-bromo-thiophene-3-carboxylic acid, 14e (1.04 g, 5 mmol) to give 1.76 g (90%) of 15e as a colorless liquid. TLC Rf 0.45 (hexane/EtOAc 1:1); 1H NMR (CDCl3-d1): δ 1.25–1.34 (m, 6H, COOCH2CH3), 2.12–2.53 (m, 4H, β-CH2, γ-CH2), 4.12–4.18 (m, 2H, COOCH2CH3), 4.23–4.28 (m, 2H, COOCH2CH3), 4.70–4.75 (m, 1H, α-CH), 6.96–6.98 (d, J = 7.2 Hz, 1H, CONH, exch), 7.40–7.41 (d, J = 1.6 Hz, 1H, Ar), 7.83–7.84 (d, J = 1.6 Hz, 1H, Ar).
General procedure for the synthesis of compounds 17a–e
To a 250-mL round-bottomed flask, equipped with a magnetic stirrer and gas inlet, was added a mixture of tetrakis(triphenylphosphine)palladium(0) (277 mg, 0.24 mmol), triethylamine (1.52 g, 15 mmol), 15a–e (882 mg, 2.25 mmol) and anhydrous DMF (20 mL). To the stirred mixture, under N2, were added copper(I) iodide (46 mg, 0.24 mmol) and 2-amino-6-but-3-ynyl-3,7-dihydro-pyrrolo[2,3-d]pyrimidin-4-one, 16 (303 mg, 1.5 mmol). The reaction mixture was stirred at room temperature overnight (17–18 h). Silica gel (0.5 g) was then added, and the solvent was evaporated under reduced pressure. The resulting plug was loaded on to a silica gel column (1.5 × 12 cm) and eluted with CHCl3 followed by 3% MeOH in CHCl3 and then 5% MeOH in CHCl3. Fractions with desired Rf (TLC) were pooled and evaporated to afford 17a–e.
(S)-2-({2-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-but-1-ynyl]-thiophene-3-carbonyl}-amino)-pentanedioic acid diethyl ester (17a)
Compound 17a was prepared using the general method described for the preparation of 17a–e, from 16 (303 mg, 1.5 mmol) and 12a (882 mg, 2.25 mmol) to give 412 mg (53%) of 17a as a brown powder. mp 81–82 °C; TLC Rf 0.53 (CHCl3/MeOH 5:1); 1H NMR (DMSO-d6): δ 1.16–1.22 (m, 6H, COOCH2CH3), 1.92–2.14 (m, 2H, β-CH2), 2.43–2.46 (t, J = 8 Hz, 2H, γ-CH2), 2.80 (m, 4H, CH2CH2), 4.01–4.52 (q, J = 7 Hz, 2H, COOCH2CH3), 4.10–4.16 (m, 2H, COOCH2CH3), 4.43–4.48 (m, 1H, α-CH), 5.99 (s, 1H, C5-CH), 6.01 (s, 2H, 2-NH2, exch), 7.35–7.36 (d, J = 5.5 Hz, 1H, Ar), 7.53–7.54 (d, J = 5.5 Hz, 1H, Ar), 8.34–8.36 (d, J = 7.5 Hz, 1H, CONH, exch), 10.16 (s, 1H, 3 NH, exch), 10.88 (s, 1H, 7-NH, exch).
(S)-2-({3-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-but-1-ynyl]-thiophene-2-carbonyl}-amino)-pentanedioic acid diethyl ester (17b)
Compound 17b was prepared using the general method described for the preparation of 17a–e, from 16 (303 mg, 1.5 mmol) and 15b (882 mg, 2.25 mmol) to give 423 mg (55%) of 17b as a brown powder. mp 77–78 °C; TLC Rf 0.52 (CHCl3/MeOH 5:1); 1H NMR (DMSO-d6): δ 1.16–1.22 (m, 6H, COOCH2CH3), 1.92–2.18 (m, 2H, β-CH2), 2.39–2.43 (m, 2H, γ-CH2), 2.84 (m, 4H, CH2CH2), 3.99–4.03 (q, J = 7 Hz, 2H, COOCH2CH3), 4.14–4.18 (q, J = 7 Hz, 2H, COOCH2CH3), 4.53–4.57 (m, 1H, α-CH), 6.00 (s, 1H, C5-CH), 6.01 (s, 2H, 2-NH2, exch), 7.16–7.17 (d, J = 5.0 Hz, 1H, Ar), 7.81–7.82 (d, J = 5.0 Hz, 1H, Ar), 8.19–8.20 (d, J = 7.5 Hz, 1H, CONH, exch), 10.16 (s, 1H, 3-NH, exch), 10.91 (s, 1H, 7-NH, exch).
(S)-2-({4-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-but-1-ynyl]-thiophene-3-carbonyl}-amino)-pentanedioic acid diethyl ester (17c)
Compound 17c was prepared using the general method described for the preparation of 17a–e, from 16 (303 mg, 1.5 mmol) and 15c (882 mg, 2.25 mmol) to give 433 mg (56%) of 17c as a brown powder. mp 80–81 °C; TLC Rf 0.53 (CHCl3/MeOH 5:1); 1H NMR (DMSO-d6): δ 1.16–1.22 (m, 6H, COOCH2CH3), 1.92–2.14 (m, 2H, β-CH2), 2.42–2.46 (m, 2H, γ-CH2), 2.69–2.78 (m, 4H, CH2CH2), 4.01–4.05 (q, J = 7 Hz, 2H, COOCH2CH3), 4.11–4.16 (m, 2H, COOCH2CH3), 4.45–4.50 (m, 1H, α-CH), 5.99 (s, 1H, C5-CH), 6.00 (s, 2H, 2-NH2, exch), 7.16–7.17 (d, J = 3.5 Hz, 1H, Ar), 8.08–8.09 (d, J = 3.5 Hz, 1H, Ar), 8.40–8.41 (d, J = 7.5 Hz, 1H, CONH, exch), 10.16 (s, 1H, 3-NH, exch), 10.88 (s, 1H, 7-NH, exch).
(S)-2-({4-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-but-1-ynyl]-thiophene-2-carbonyl}-amino)-pentanedioic acid diethyl ester (17d)
Compound 17d was prepared using the general method described for the preparation of 17a–e, from 16 (303 mg, 1.5 mmol) and 15d (882 mg, 2.25 mmol) to give 406 mg (53%) of 17d as a brown powder. mp 79–80 °C; TLC Rf 0.53 (CHCl3/MeOH 5:1); 1H NMR (DMSO-d6): δ 1.16–1.22 (m, 6H, COOCH2CH3), 1.92–2.12 (m, 2H, β-CH2), 2.42–2.45 (t, J = 7.5 Hz, 2H, γ-CH2), 2.71–2.78 (m, 4H, CH2CH2), 4.03–4.07 (q, J = 7 Hz, 2H, COOCH2CH3), 4.09–4.13 (q, J = 6.5 Hz, 2H, COOCH2CH3), 4.36–4.40 (m, 1H, α-CH), 6.00 (s, 1H, C5-CH), 6.02 (s, 2H, 2-NH2, exch), 7.87–7.88 (m, 2H, Ar), 8.76–8.77 (d, J = 7.5 Hz, 1H, CONH, exch), 10.17 (s, 1H, 3-NH, exch), 10.90 (s, 1H, 7-NH, exch).
(S)-2-({5-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-but-1-ynyl]-thiophene-3-carbonyl}-amino)-pentanedioic acid diethyl ester (17e)
Compound 17e was prepared using the general method described for the preparation of 17a–e, from 16 (303 mg, 1.5 mmol) and 15e (882 mg, 2.25 mmol) to give 387 mg (50%) of 17e as a brown powder. mp 81–82 °C; TLC Rf 0.53 (CHCl3/MeOH 5:1); 1H NMR (DMSO-d6): δ 1.16–1.22 (m, 6H, COOCH2CH3), 1.92–2.12 (m, 2H, β-CH2), 2.41–2.44 (t, J = 7.5 Hz, 2H, γ-CH2), 2.78 (m, 4H, CH2CH2), 4.03–4.07 (q, J = 7 Hz, 2H, COOCH2CH3), 4.09–4.13 (m, 2H, COOCH2CH3), 4.36–4.41 (m, 1H, α-CH), 6.00 (s, 1H, C5-CH), 6.01 (s, 2H, 2-NH2, exch), 7.58–7.59 (d, J = 1.5 Hz, 1H, Ar), 8.12–8.13 (d, J = 1.5 Hz, 1H, Ar), 8.56–8.58 (d, J = 7.5 Hz, 1H, CONH, exch), 10.16 (s, 1H, 3-NH, exch), 10.88 (s, 1H, 7-NH, exch).
General procedure for the synthesis of compounds 18a–e
To a Parr hydrogenation flask was added 17a–e (200 mg, 0.39 mmol), 10% palladium on activated carbon (100 mg), and MeOH (50 mL). Hydrogenation was carried out at 55 psi of H2 for 4 h. The reaction mixture was filtered through Celite, washed with MeOH (100 mL) and concentrated under reduced pressure to give 18a–e.
(S)-2-({2-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-butyl]-thiophene-3-carbonyl}-amino)-pentanedioic acid diethyl ester (18a)
Compound 18a was prepared using the general method described for the preparation of 18a–e, from 17a (200 mg, 0.39 mmol) to give 191 mg (95%) of 18a as a light yellow powder. mp 81–82 °C; TLC Rf 0.54 (CHCl3/MeOH 5:1); 1H NMR (DMSO-d6): δ 1.15-1.17 (m, 6H, COOCH2CH3), 1.61 (m, 4H, CH2CH2), 1.91–2.12 (m, 2H, β-CH2), 2.41–2.44 (t, J = 7.0 Hz, 2H, γ-CH2), 2.51–2.53 (m, 4H, CH2CH2), 4.03–4.07 (q, J = 7 Hz, 2H, COOCH2CH3), 4.08–4.13 (m, 2H, COOCH2CH3), 4.35–4.40 (m, 1H, α-CH), 5.96 (s, 3H, C5-CH, 2-NH2, exch), 7.35 (s, 2H, Ar), 8.40–8.42 (d, J = 7.5 Hz, 1H, CONH, exch), 10.99 (s, 1H, 3-NH, exch), 11.35 (s, 1H, 7-NH, exch).
(S)-2({3 [4-(2 Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-butyl]-thiophene-2-carbonyl}-amino)-pentanedioic acid diethyl ester (18b)
Compound 18b was prepared using the general method described for the preparation of 18a–e, from 17b (200 mg, 0.39 mmol) to give 187 mg (93%) of 18b as a light yellow powder. mp 80–81 °C; TLC Rf 0.53 (CHCl3/MeOH 5:1); 1H NMR (DMSO-d6): δ 1.15–1.20 (m, 6H, COOCH2CH3), 1.57 (m, 4H, CH2CH2), 1.93–2.12 (m, 2H, β-CH2), 2.40–2.43 (t, J = 8.0 Hz, 2H, γ-CH2), 2.51 (m, 2H, CH2), 2.87 (m, 2H, CH2), 4.03–4.07 (q, J = 7.0 Hz, 2H, COOCH2CH3), 4.08–4.14 (m, 2H, COOCH2CH3), 4.34–4.39 (m, 1H, α CH), 5.96 (m, 3H, C5-CH, 2-NH2, exch), 7.02–7.03 (d, J = 5.0 Hz, 1H, Ar), 7.59–7.60 (d, J = 5.0 Hz, 1H, Ar), 8.39–8.40 (d, J = 7.5 Hz, 1H, CONH, exch), 11.09 (s, 1H, 3 NH, exch), 11.41 (s, 1H, 7-NH, exch).
(S)-2-({4-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-butyl]-thiophene-3-carbonyl}-amino)-pentanedioic acid diethyl ester (18c)
Compound 18c was prepared using the general method described for the preparation of 18a–e, from 17c (200 mg, 0.39 mmol) to give 193 mg (96%) of 18c as a light yellow powder. mp 79–80 °C; TLC Rf 0.54 (CHCl3/MeOH 5:1); 1H NMR (DMSO-d6):δ 1.17–1.18 (m, 6H, COOCH2CH3), 1.56 (m, 4H, CH2CH2), 1.91–2.11 (m, 2H, β-CH2), 2.43–2.46 (t, J = 7.5 Hz, 2H, γ-CH2), 2.46–2.49 (m, 2H, CH2), 2.75–2.77 (m, 2H, CH2), 4.03–4.08 (q, J = 7.0 Hz, 2H, COOCH2CH3), 4.08–4.13 (m, 2H, COOCH2CH3), 4.35–4.39 (m, 1H, α CH), 5.83 (s, 1H, C5-CH), 5.98 (s, 2H, 2-NH2, exch), 7.20–7.21 (d, J = 3.5 Hz, 1H, Ar), 7.92–7.93 (d, J = 3.5 Hz, 1H, Ar), 8.53–8.54 (d, J = 7.5 Hz, 1H, CONH, exch), 10.13 (s, 1H, 3-NH, exch), 10.78 (s, 1H, 7-NH, exch).
(S)-2-({4-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3d]pyrimidin-6-yl)-butyl]-thiophene-2-carbonyl}-amino)-pentanedioic acid diethyl ester (18d)
Compound 18d was prepared using the general method described for the preparation of 18a–e, from 17d (200 mg, 0.39 mmol) to give 185 mg (92%) of 18d as a light yellow powder. mp 81–82 °C; TLC Rf 0.55 (CHCl3/MeOH 5:1); 1H NMR (DMSO-d6): δ 1.17–1.19 (m, 6H, COOCH2CH3), 1.62 (m, 4H, CH2CH2), 1.95–2.12 (m, 2H, β-CH2), 2.41–2.45 (t, J = 7.5 Hz, 2H, γ-CH2), 2.54–2.62 (m, 4H, CH2CH2), 4.03–4.07 (q, J = 7.0 Hz, 2H, COOCH2CH3), 4.09–4.13 (m, 2H, COOCH2CH3), 4.36–4.40 (m, 1H, α-CH), 5.99 (s, 3H, C5 CH, 2-NH2, exch), 7.41 (s, 1H, Ar), 7.76 (s, 1H, Ar), 8.67–8.68 (d, J = 7.5 Hz, 1H, CONH, exch), 11.00 (s, 1H, 3-NH, exch), 11.39 (s, 1H, 7-NH, exch).
(S)-2-({5-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-butyl]-thiophene-3-carbonyl}-amino)-pentanedioic acid diethyl ester (18e)
Compound 18e was prepared using the general method described for the preparation of 18a–e, from 17e (200 mg, 0.39 mmol) to give 183 mg (91%) of 18e as a light yellow powder. mp 82–83 °C; TLC Rf 0.54 (CHCl3/MeOH 5:1); 1H NMR (DMSO-d6): δ 1.15–1.18 (m, 6H, COOCH2CH3), 1.64 (m, 4H, CH2CH2), 1.93–2.12 (m, 2H, β-CH2), 2.41–2.44 (t, J = 7.5 Hz, 2H, γ CH2), 2.55 (m, 2H, CH2), 2.81 (m, 2H, CH2), 4.03–4.07 (q, J = 7.0 Hz, 2H, COOCH2CH3), 4.08–4.12 (q, J = 7.0 Hz, 2H, COOCH2CH3), 4.36–4.40 (m, 1H, α CH), 5.96 (s, 3H, C5-CH, 2-NH2, exch), 7.26 (s, 1H, Ar), 7.98 (s, 1H, Ar), 8.46–8.48 (d, J = 8.0 Hz, 1H, CONH, exch), 10.76 (s, 1H, 3-NH, exch), 11.22 (s, 1H, 7-NH, exch).
General procedure for the synthesis of compounds 4–13
To a solution of 18a–e (100 mg, 0.19 mmol) or 17a–e (100 mg, 0.19 mmol) in 10 mL of MeOH/CHCl3 (1:1) or MeOH/CH2Cl2 (1:1) was added 1 N NaOH (5 mL) and the mixture was stirred under N2 at room temperature for 16 h. TLC showed the disappearance of the starting material and one major spot at the origin. The reaction mixture was evaporated to dryness under reduced pressure. The residue was dissolved in water (10 mL), the resulting solution was cooled in an ice bath, and the pH was adjusted to 3–4 with dropwise addition of 1 N HCl. The resulting suspension was frozen in a dry ice-acetone bath, thawed to 4–5 °C in the refrigerator, and filtered. The residue was washed with a small amount of cold water and dried in vacuum using P2O5 to afford 4–13.
(S)-2-({2-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-butyl]-thiophene-3-carbonyl}-amino)-pentanedioic acid (4)
Compound 4 was prepared using the general method described for the preparation of 4–13, from 15a (100 mg, 0.19 mmol) to give 83 mg (95%) of 4 as a light yellow powder. mp 176–177 °C; 1H NMR (DMSO-d6): δ 1.61 (m, 4H, CH2CH2), 1.87–2.10 (m, 2H, β-CH2), 2.34–2.37 (t, J = 7.5 Hz, 2H, γ CH2), 2.47 (m, 2H, CH2), 3.09–3.11 (m, 2H, CH2), 4.32–4.37 (m, 1H, α CH), 5.86 (s, 1H, C5-CH), 5.98 (s, 2H, 2-NH2, exch), 7.33–7.34 (d, J = 5.0 Hz, 1H, Ar), 7.35–7.36 (d, J = 5.0 Hz, 1H, Ar), 8.28–8.29 (d, J = 7.5 Hz, 1H, CONH, exch), 10.16 (s, 1H, 3-NH, exch), 10.80 (s, 1H, 7-NH, exch), 12.36 (br, 2H, COOH, exch). Anal. (C20H23N5O6S × 1.85 H2O) C, H, N, S.
(S)-2-({3-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-butyl]-thiophene-2-carbonyl}-amino)-pentanedioic acid (5)
Compound 5 was prepared using the general method described for the preparation of 4–13, from 18b (100 mg, 0.19 mmol) to give 83 mg (95%) of 5 as a light yellow powder. mp 159–160 °C; 1H NMR (DMSO-d6): δ 1.56 (m, 4H, CH2CH2), 1.88–2.11 (m, 2H, β-CH2), 2.33–2.36 (t, J = 7.5 Hz, 2H, γ CH2), 2.48 (m, 2H, CH2), 2.86–2.88 (m, 2H, CH2), 4.31–4.36 (m, 1H, α CH), 5.85 (s, 1H, C5-CH), 5.98 (s, 2H, 2-NH2, exch), 7.01–7.02 (d, J = 5.0 Hz, 1H, Ar), 7.58–7.59 (d, J = 5.0 Hz, 1H, Ar), 8.24–8.26 (d, J = 7.5 Hz, 1H, CONH, exch), 10.16 (s, 1H, 3-NH, exch), 10.79 (s, 1H, 7-NH, exch), 12.38 (br, 2H, COOH, exch). Anal. (C20H23N5O6S × 1.0 H2O) C, H, N, S.
(S)-2-({4-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-butyl]-thiophene-3-carbonyl}-amino)-pentanedioic acid (6)
Compound 6 was prepared using the general method described for the preparation of 4–13, from 18c (100 mg, 0.19 mmol) to give 83 mg (95%) of 6 as a light yellow powder. mp 195–196 °C; 1H NMR (DMSO-d6): δ 1.56 (m, 4H, CH2CH2), 1.87–2.10 (m, 2H, β-CH2), 2.35–2.38 (t, J = 8.0 Hz, 2H, γ CH2), 2.47–2.48 (m, 2H, CH2), 2.75–2.81 (m, 2H, CH2), 4.32–4.36 (m, 1H, α CH), 5.85 (s, 1H, C5-CH), 5.96 (s, 2H, 2-NH2, exch), 7.19–7.20 (d, J = 3.0 Hz, 1H, Ar), 7.92–7.93 (d, J = 3.0 Hz, 1H, Ar), 8.40–8.41 (d, J = 8.0 Hz, 1H, CONH, exch), 10.14 (s, 1H, 3-NH, exch), 10.78 (s, 1H, 7-NH, exch), 12.31 (br, 2H, COOH, exch). Anal. (C20H23N5O6S × 0.4 CHCl3) C, H, N, S.
(S)-2-({4-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-butyl]-thiophene-2-carbonyl}-amino)-pentanedioic acid (7)
Compound 7 was prepared using the general method described for the preparation of 4–13, from 18d (100 mg, 0.19 mmol) to give 83 mg (95%) of 7 as a light yellow powder. mp 174–175 °C; 1H NMR (DMSO-d6): δ 1.61 (m, 4H, CH2CH2), 1.88–2.11 (m, 2H, β-CH2), 2.33–2.36 (t, J = 7.5 Hz, 2H, γ-CH2), 2.56 (m, 4H, CH2CH2), 4.32–4.37 (m, 1H, α-CH), 5.88 (s, 1H, C5-CH), 5.96 (s, 2H, 2-NH2, exch), 7.39 (s, 1H, Ar), 7.75 (s, 1H, Ar), 8.54–8.55 (d, J = 8.0 Hz, 1H, CONH, exch), 10.13 (s, 1H, 3-NH, exch), 10.81 (s, 1H, 7-NH, exch), 12.45 (br, 2H, COOH, exch). Anal. (C20H23N5O6S × 0.31 CHCl3) C, H, N, S.
(S)-2-({5-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-butyl]-thiophene-3-carbonyl}-amino)-pentanedioic acid (8)
Compound 8 was prepared using the general method described for the preparation of 4–13, from 18e (100 mg, 0.19 mmol) to give 83 mg (95%) of 8 as a light yellow powder. mp 175–176 °C; 1H NMR (DMSO-d6): δ 1.64 (m, 4H, CH2CH2), 1.87–2.10 (m, 2H, β-CH2), 2.32–2.35 (t, J = 7.5 Hz, 2H, γ-CH2), 2.55 (m, 2H, CH2), 2.80–2.82 (m, 2H, CH2), 4.33–4.37 (m, 1H, α CH), 5.88 (s, 1H, C5-CH), 5.96 (s, 2H, 2-NH2, exch), 7.27 (s, 1H, Ar), 7.96 (s, 1H, Ar), 8.31–8.33 (d, J = 8.0 Hz, 1H, CONH, exch), 10.12 (s, 1H, 3-NH, exch), 10.80 (s, 1H, 7-NH, exch), 12.43 (br, 2H, COOH, exch). Anal. (C20H23N5O6S × 1.5 H2O) C, H, N, S.
(S)-2-({2-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-butyl]-thiophene-3-carbonyl}-amino)-pentanedioic acid (9)
Compound 9 was prepared using the general method described for the preparation of 4–13, from 17a (100 mg, 0.19 mmol, dissolved in 10 mL of MeOH/CH3COOH (9:1)) to give 84 mg (95%) of 9 as a light yellow powder. mp 196–197 °C; 1H NMR (DMSO-d6): δ 1.91–2.13 (m, 2H, β-CH2), 2.34–2.37 (t, J = 7 Hz, 2H, γ-CH2), 2.81 (m, 4H, CH2CH2), 4.41–4.45 (m, 1H, α-CH), 6.01 (s, 3H, C5 CH, 2-NH2, exch), 7.37–7.38 (d, J = 5.5 Hz, 1H, Ar), 7.53–7.54 (d, J = 5.5 Hz, 1H, Ar), 8.25–8.26 (d, J = 7.5 Hz, 1H, CONH, exch), 10.19 (s, 1H, 3-NH, exch), 10.88 (s, 1H, 7-NH, exch), 12.47 (br, 2H, COOH, exch). Anal. (C20H19N5O6S × 1.0 CH3COOH) C, H, N, S.
(S)-2-({3-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-butyl]-thiophene-3-carbonyl}-amino)-pentanedioic acid (10)
Compound 10 was prepared using the general method described for the preparation of 4–13, from 17b (100 mg, 0.19 mmol) to give 83 mg (94%) of 10 as a light yellow powder. mp 179–180 °C; 1H NMR (DMSO-d6): δ 1.96–2.18 (m, 2H, β-CH2), 2.31–2.34 (t, J = 7.5 Hz, 2H, γ-CH2), 2.84 (m, 4H, CH2CH2), 4.41–4.53 (m, 1H, α-CH), 6.01 (s, 3H, C5-CH, 2-NH2, exch), 7.16–7.17 (d, J = 5.0 Hz, 1H, Ar), 7.80–7.81 (d, J = 5.0 Hz, 1H, Ar), 8.22–8.23 (d, J = 7.5 Hz, 1H, CONH, exch), 10.20 (s, 1H, 3-NH, exch), 10.89 (s, 1H, 7-NH, exch), 12.30 (br, 2H, COOH, exch). Anal. (C20H19N5O6S × 0.3 CHCl3) C, H, N, S.
(S)-2-({4-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-butyl]-thiophene-3-carbonyl}-amino)-pentanedioic acid (11)
Compound 11 was prepared using the general method described for the preparation of 4–13, from 17c (100 mg, 0.19 mmol) to give 84 mg (95%) of 11 as a light yellow powder. mp 211–212 °C; 1H NMR (DMSO-d6): δ 1.92 –2.14 (m, 2H, β-CH2), 2.33 2.37 (t, J = 8.0 Hz, 2H, γ-CH2), 2.70–2.80 (m, 4H, CH2CH2), 4.45–4.50 (m, 1H, α-CH), 6.00 (s, 3H, C5-CH, 2-NH2, exch), 7.74–7.75 (d, J = 3.0 Hz, 1H, Ar), 8.10–8.11 (d, J = 3.0 Hz, 1H, Ar), 8.30–8.32 (d, J = 8.0 Hz, 1H, CONH, exch), 10.17 (s, 1H, 3-NH, exch), 10.86 (s, 1H, 7-NH, exch), 12.44 (br, 2H, COOH, exch). Anal. (C20H19N5O6S × 0.3 CHCl3) C, H, N, S.
(S)-2-({4-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-but-1-ynyl]-thiophene-2-carbonyl}-amino)-pentanedioic acid (12)
Compound 12 was prepared using the general method described for the preparation of 4–13, from 17d (100 mg, 0.19 mmol) to give 85 mg (96%) of 12 as a light yellow powder. mp 195–196 °C; 1H NMR (DMSO-d6): δ 1.91–2.11 (m, 2H, β-CH2), 2.34–2.37 (t, J = 7.5 Hz, 2H, γ-CH2), 2.71–2.79 (m, 4H, CH2CH2), 4.32–4.36 (m, 1H, α-CH), 6.01 (s, 3H, C5-CH, 2-NH2, exch), 7.86–7.88 (m, 2H, Ar), 8.64–8.65 (d, J = 7.5 Hz, 1H, CONH, exch), 10.16 (s, 1H, 3-NH, exch), 10.88 (s, 1H, 7-NH, exch), 12.46 (br, 2H, COOH, exch). Anal. (C20H19N5O6S × 0.37 CH2Cl2) C, H, N, S.
(S)-2-({5-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-but-1-ynyl]-thiophene-3-carbonyl}-amino)-pentanedioic acid (13)
Compound 13 was prepared using the general method described for the preparation of 4–13, from 17e (100 mg, 0.19 mmol) to give 84 mg (95%) of 13 as a light yellow powder. mp 197–198 °C; 1H NMR (DMSO-d6): δ 1.92–2.14 (m, 2H, β-CH2), 2.33–2.37 (t, J = 8.0 Hz, 2H, γ-CH2), 2.70–2.80 (m, 4H, CH2CH2), 4.45–4.50 (m, 1H, α-CH), 6.00 (s, 3H, C5–CH, 2-NH2, exch), 7.74–7.75 (d, J = 3.0 Hz, 1H, Ar), 8.10–8.11 (d, J = 3.0 Hz, 1H, Ar), 8.30–8.32 (d, J = 8.0 Hz, 1H, CONH, exch), 10.17 (s, 1H, 3-NH, exch), 10.86 (s, 1H, 7-NH, exch), 12.44 (br, 2H, COOH, exch). Anal. (C20H19N5O6S × 0.41 CH2Cl2) C, H, N, S.
(S)-2-({5-[4-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)-but-1-ynyl]-thiophene-2-carbonyl}-amino)-pentanedioic acid (20)
To a solution of 19 (50 mg, 0.1 mmol) in MeOH (10 mL) was added 1 N NaOH (5 mL) and the mixture was stirred under N2 at room temperature for 16 h. TLC showed the disappearance of the starting material and one major spot at the origin. The reaction mixture was evaporated to dryness under reduced pressure. The residue was dissolved in water (10 mL), the resulting solution was cooled in an ice bath, and the pH was adjusted to 3–4 with dropwise addition of 1 N HCl. The resulting suspension was frozen in a dry ice-acetone bath, thawed to 4–5 °C in the refrigerator, and filtered. The residue was washed with a small amount of cold water and dried in vacuum using P2O5 to afford 43 mg (95%) of 20 as a light yellow powder. mp 189–190 °C; 1H NMR (DMSO-d6): δ 1.89–2.05 (m, 2H, β-CH2), 2.30–2.34 (t, J = 7.6 Hz, 2H, γ-CH2), 2.76 (m, 4H, CH2CH2), 4.33 (m, 1H, α-CH), 5.92 (s, 2H, 2-NH2, exch), 5.98–5.99 (d, J = 4 Hz, 1H, C5-CH), 7.19–7.20 (d, J = 4 Hz, 1H, Ar), 7.74–7.75 (d, J = 4 Hz, 1H, Ar), 8.69–8.71 (d, J = 8 Hz, 1H, CONH, exch), 10.15 (s, 1H, 3-NH, exch), 10.86 (s, 1H, 7-NH, exch), 12.47 (br, 2H, COOH, exch). HRMS calcd for C20H19N5O6S (M+H)+, 458.1134; found: 458.1155.
Reagents for biological studies
[3', 5', 7-3H]MTX (20 Ci/mmol) and [3', 5', 7, 9-3H] folic acid (25 Ci/mmol), [14C(U)] glycine (87mCi/mmol) were purchased from Moravek Biochemicals (Brea, CA). Unlabeled folic acid was purchased from Sigma Chemical Co. (St. Louis, MO). LCV [(6R,S) 5-formyl tetrahydrofolate] was provided by the Drug Development Branch, National Cancer Institute, Bethesda, MD. The sources of the classical antifolate drugs were as follows: MTX, Drug Development Branch, National Cancer Institute (Bethesda, MD); RTX [N-(5-[N-(3,4-dihydro-2-methyl-4-oxyquinazolin-6-ylmethyl)-N-methyl-amino]-2-thienoyl)-L-glutamic acid], AstraZeneca Pharmaceuticals (Maccesfield, Cheshire, England); and LMTX (5,10-dideaza 5,6,7,8-tetrahydrofolate) and PMX [N-{4-[2-(2 amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-L-glutamic acid] (Alimta), Eli Lilly and Co. (Indianapolis, IN). Other chemicals were obtained from commercial sources in the highest available purity.
Cell lines and assays of antitumor drug activities
The origin of the engineered CHO sublines including RFC- and FRα-null MTXRIIOuaR2–4 (R2), and RFC- (pC43-10), PCFT- (R2/PCFT4), or FRα- (RT16) expressing CHO sublines were previously described.10,11,14,18 Likewise, pDNA3.1 vector control CHO cells (R2/VC) were reported. The CHO cells were cultured in α-minimal essential medium (MEM) supplemented with 10% bovine calf serum (Invitrogen, Carlsbad, CA), 100 units/ml penicillin/100 μg/ml streptomycin, and 2 mM L-glutamine at 37°C with 5% CO2. All the R2 transfected cells [PC43-10, RT16, R2/hPCFT4, R2(VC)] were routinely cultured in α-MEM plus 1.5 mg/ml G418. Prior to the cytotoxicity assays (see below), RT16 cells were cultured in complete folate-free RPMI 1640 (without added folate) for three days. R2/hPCFT4 and R2(VC) cells were cultured in complete folate-free RPMI 1640 including dialyzed fetal bovine serum (Invitrogen) and 25 nM LCV plus 1.5 mg/ml G418. KB human nasopharyngeal carcinoma cells were purchased from the American Type Culture Collection (Manassas, VA) whereas IGROV1 ovarian carcinoma cells were a gift of Dr. Manohar Ratnam (University of Toledo). KB and IGROV1 cells were routinely cultured in folate-free RPMI 1640 medium, supplemented with 10% fetal bovine serum, penicillin-streptomycin solution, and 2 mM L-glutamine at 37°C with 5% CO2.
For growth inhibition assays, cells (CHO, KB, or IGROV1) were plated in 96 well dishes (~2500–5000 cells/well, total volume of 200 μl medium) with a range of antifolate concentrations. The medium was RPMI 1640 (contains 2.3 μM folic acid) with 10% dialyzed fetal bovine serum and antibiotics for experiments with R2 and PC43-10 cells. For RT16, KB, and IGROV1 cells, cells were cultured in folate-free RPMI media with 10% dialyzed fetal bovine serum and antibiotics supplemented with 2 nM LCV and 2 mM L-glutamine. The requirement for FR-mediated drug uptake in these assays was established in parallel incubations including 200 nM folic acid. For R2/hPCFT4 cells, the medium was folate-free RPMI 1640 (pH 7.2) containing 25 nM LCV, supplemented with 10% dialyzed fetal bovine serum, antibiotics, and L-glutamine. Cells were routinely incubated for up to 96 h and metabolically active cells (a measure of cell viability) were assayed with CellTiter-blue Cell Viability Assay (Promega, Madison, WI), with fluorescence measured (590 nm emission, 560 nm excitation) using a fluorescence plate reader. Raw data were exported from Softmax Pro software to an Excel spreadsheet for analysis and determinations of IC50s, corresponding to the drug concentrations that result in 50% loss of cell growth.
For some of the in vitro growth inhibition studies, the inhibitory effects of the antifolate inhibitors on de novo thymidylate biosynthesis (i.e., thymidylate synthase) and de novo purine biosynthesis (GARFTase and AICARFTase) were tested by co-incubations with thymidine (10 μM) and adenosine (60 μM), respectively. For de novo purine biosynthesis, additional protection experiments used AICA (320 μM) to distinguish inhibitory effects at GARFTase from those at AICARFTase.10–12
For assays of clonogenicity (colony formation), KB cells (500 cells) were plated in folate-free RPMI1640 medium supplemented with 2 nM LCV, 10% dialyzed fetal bovine serum, penicillin-streptomycin solution, and 2 mM L-glutamine, in the presence of antifolate drugs. The dishes were incubated at 37°C with 5% CO2 for 10–14 days. To stain the colonies for counting, the dishes were rinsed with Dulbecco's phosphate-buffered saline (DPBS), 5% trichloroacetic acid, then borate buffer (10 mM, pH 8.8), followed by 30 min incubation in 1% methylene blue in the borate buffer. The dishes were rinsed with the borate buffer and colonies were counted for calculating percent colony-forming efficiency normalized to control.
FR binding assay
Competitive inhibition of [3H]folic acid binding to FRα with RT16 CHO cells was used to assess relative binding affinities for assorted (anti)folate ligands.10–12 Briefly, RT16 cells (~1.6×106) were rinsed twice with DPBS, followed by two washes with an acidic buffer (10 mM sodium acetate, 150 mM NaCl, pH 3.5) to remove FR bound folates. Cells were washed twice with ice-cold HEPES-buffered saline (20 mM HEPES, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, 5 mM glucose, pH7.4) (HBS), then incubated in HBS with [3H]folic acid (50 nM, specific activity 0.5 Ci/mmol) in the presence and absence of unlabeled folic acid or antifolate (over a range of concentrations) for 15 min at 0°C. The dishes were rinsed three times with ice-cold HBS, after which the cells were solubilized (0.5 N NaOH) and aliquots measured for radioactivity and protein contents. Protein concentrations were measured with Folin phenol reagent.20 [3H]Folic acid bound to FRα was calculated as pmol/mg protein and relative binding affinities were calculated as the inverse molar ratios of unlabeled ligands required to inhibit [3H]folic acid binding by 50%. By definition, the relative affinity of folic acid is 1.
Transport assays
For transport assays, R2/hPCFT4 CHO cells were grown as monolayers and used to seed spinner flasks. For experiments to determine the inhibitions of transport by antifolate substrates, cells were collected from spinners, washed with DPBS and resuspended in 2 ml of HBS, adjusted to pH 6.8, or 4-morpholinepropanesulfonic acid-buffered saline (20 mM MES, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, and 5 mM glucose), adjusted to pH 5.5. For determinations of Kis, cells were incubated with 1 μM [3H]MTX over 2 min at 37°C, in the presence and absence of a range of concentrations of unlabeled antifolates. Controls included a comparable amount of vehicle (DMSO to 0.5%). Uptakes of [3H]MTX were quenched with ice-cold DPBS. Cells were washed with ice-cold DPBS (3×) and solubilized with 0.5 N NaOH. Levels of intracellular radioactivity were expressed as pmol/mg protein, calculated from direct measurements of radioactivity and protein contents of cell homogenates. Protein concentrations were measured with Folin phenol reagent. Kis were calculated from Dixon plots and kinetic constants (Kt, Vmax) for [3H]MTX uptake, calculated from Lineweaver Burk plots, as previously described.14
In situ GARFT enzyme inhibition assay
Incorporation of [14C]glycine into [14C]FGAR, as an in situ measure of endogenous GARFTase activity, was measured as described.10–12 For these experiments, IGROV1 cells were seeded in 4 mL of complete folate free RPMI 1640 plus 2 nM LCV in 60 mm dishes at a density of 2×106 cells per dish. On the next day, the medium was replaced with 2 mL of fresh complete folate free RPMI 1640 plus 2 nM LCV (without supplementing glutamine). Azaserine (4 μM final concentration) was added in the presence and absence of the antifolate inhibitors. After 30 min, L-glutamine (final concentration, 2 mM) and [14C]glycine (tracer amounts; final specific activity 0.1 mCi/L) were added. Incubations were at 37°C for 15 h, at which time cells were washed (one-time) with ice-cold folate-free RPMI 1640 plus serum. Cell pellets were dissolved in 2 ml 5% trichloroacetic acid at 0°C. Cell debris was removed by centrifugation (the cell protein contents in the pellets were measured), and the supernatants were extracted twice with 2 mL of ice-cold ether. The aqueous layer was passed through a 1 cm column of AG1×8 (chloride form), 100–200 mesh (Bio-Rad), washed with 10 mL of 0.5N formic acid and then 10 mL of 4N formic acid, and finally eluted with 8 mL 1N HCI. The eluates were collected and determined for radioactivity. The accumulation of radioactive formyl GAR was calculated as pmol per mg protein over a range of inhibitor concentrations. IC50s were calculated as the concentrations of inhibitors that resulted in a 50% decrease in [14C]formyl GAR synthesis.
Supplementary Material
Acknowledgement
This work was supported in partx by grants from the National Institutes of Health, National Cancer Institute, CA125153 (AG), CA152316 (LHM and AG), and CA53535 (LHM); a grant from the Mesothelioma Applied Research Foundation, a pilot grant from the Barbara Ann Karmanos Cancer Institute; and the Duquesne University Adrian Van Kaam Chair in Scholarly Excellence (AG). Ms. Kugel Desmoulin was supported by a Doctoral Research Award from the Canadian Institutes of Health Research (CIHR). Ms. Mitchell-Ryan was supported by T32 CA009531 (LHM).
Abbreviations
- AICA
5-amino-4-imidazolecarboxamide
- AICAR
5-amino-4-imidazolecarboxamide ribonucleotide
- AICARFTase
5-amino-4-imidazolecarboxamide ribonucleotide formyltransferase
- CHO
Chinese hamster ovary
- DPBS
Dulbecco's phosphate-buffered saline
- FR
folate receptor
- GAR
glycinamide ribonucleotide
- GARFTase
glycinamide ribonucleotide formyltransferase
- HBSS
Hank's balanced salts solution
- HBS
HEPES-buffered saline
- IC50
50% inhibitory concentration
- LCV
leucovorin
- LMTX
Lometrexol
- MEM
minimal essential media
- MTX
methotrexate
- PMX
Pemetrexed
- PCFT
proton-coupled folate transporter
- RTX
Raltitrexed
- RFC
reduced folate carrier
- SCID
severe combined immunodeficient
Footnotes
Supporting Information Available: Colony formation assay data and elemental analysis are available free of charge via the internet at http://pubs.acs.org.
REFERENCES
- 1.Matherly LH, Hou Z, Deng Y. Human Reduced Folate Carrier: Translation of Basic Biology to Cancer Etiology and Therapy. Cancer Metastasis Rev. 2007;26:111–128. doi: 10.1007/s10555-007-9046-2. [DOI] [PubMed] [Google Scholar]
- 2.Elnakat H, Ratnam M. Distribution, Functionality and Gene Regulation of Folate Receptor Isoforms: Implications in Targeted Therapy. Adv. Drug Dev. 2004;56:1067–1084. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Zhao R.; Matherly LH, Goldman ID. Membrane Transporters and Folate Homeostasis; Intestinal Absorption, Transport into Systemic Compartments and Tissues. Expert Reviews in Molecular Medicine. 2009;11:e4. doi: 10.1017/S1462399409000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao R, Goldman ID. The Molecular Identity and Characterization of a Proton-Coupled Folate Transporter-PCFT; Biological Ramifications and Impact on the Activity of Pemetrexed. Cancer Metastasis Rev. 2007;26:129–139. doi: 10.1007/s10555-007-9047-1. [DOI] [PubMed] [Google Scholar]
- 5.Kugel Desmoulin S, Wang Y, Tait L, Hou Z, Cherian C, Gangjee A, Matherly LH. Expression Profiling of the Major Folate Facilitative Transporters in Human Tumors and Normal Tissues. Abstracts, American Association for Cancer Research. 2010;51:1103. [Google Scholar]
- 6.Qiu A, Min SH, Jansen M, Malhotra U, Tsai E, Cabelof DC, Matherly LH, Zhao R, Akabas MH, Goldman ID. Rodent Intestinal Folate Transporters (SLC46A1): Secondary Structure, Functional Properties, and Response to Dietary Folate Restriction. Am. J. Physiol. Cell. Physiol. 2007;293:C1669–1678. doi: 10.1152/ajpcell.00202.2007. [DOI] [PubMed] [Google Scholar]
- 7.Kugel Desmoulin S, Wang L, Hales E, Polin L, White K, Kushner J, Stout M, Hou Z, Cherian C, Gangjee A, Matherly LH. Therapeutic Targeting of a Novel 6-substituted Pyrrolo[2,3-d]pyrimidine Thienoyl Antifolate to Human Solid Tumors Based on Selective Uptake by the Proton coupled Folate Transporter. Mol. Pharmacol. 2011;80:1096–1107. doi: 10.1124/mol.111.073833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbs DD, Theti DS, Wood N, Green M, Raynaud F, Valenti M, Forster MD, Mitchell F, Bavetsias V, Henderson E, Jackman AL. BGC 945, A Novel Tumor-selective Thymidylate Synthase Inhibitor Targeted to Alpha-Folate Receptor-Overexpressing Tumors. Cancer Res. 2005;65:11721–11728. doi: 10.1158/0008-5472.CAN-05-2034. [DOI] [PubMed] [Google Scholar]
- 9.Xia W, Low PS. Folate-Targeted Therapies for Cancer. J. Med. Chem. 2010;53:6811–6824. doi: 10.1021/jm100509v. [DOI] [PubMed] [Google Scholar]
- 10.Deng Y, Wang Y, Cherian C, Hou Z, Buck SA, Matherly LH, Gangjee A. Synthesis and Discovery of High Affinity Folate Receptor-Specific Glycinamide Ribonucleotide Formyltransferase Inhibitors with Antitumor Activity. J. Med. Chem. 2008;51:5052–5063. doi: 10.1021/jm8003366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng , Zhou X, Kugel Desmoulin S, Wu J, Cherian C, Hou Z, Matherly LH, Gangjee A. Synthesis and Biological Activity of a Novel Series of 6-Substituted Thieno[2,3-d]pyrimidine Antifolate Inhibitors of Purine Biosynthesis with Selectivity for High Affinity Folate Receptors Over the Reduced Folate Carrier and Proton-Coupled Folate Transporter for Cellular Entry. J. Med. Chem. 2009;52:2940–2951. doi: 10.1021/jm8011323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Cherian C, Kugel Desmoulin S, Polin L, Deng Y, Wu J, Hou Z, White K, Kushner J, Matherly LH, Gangjee A. Synthesis and Antitumor Activity of a Novel Series of 6-substituted Pyrrolo[2,3-d]pyrimidine Thienoyl Antifolate Inhibitors of Purine Biosynthesis with Selectivity for High Affinity Folate Receptors and the Proton-coupled Folate Transporter Over the Reduced Folate Carrier for Cellular Entry. J. Med. Chem. 2010;53:1306–1318. doi: 10.1021/jm9015729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Kugel Desmoulin S, Cherian C, Polin L, White K, Kushner J, Fulterer A, Chang M-H, Mitchell S, Stout M, Romero MF, Hou Z, Matherly LH, Gangjee A. Synthesis, Biological and Antitumor Activity of a Highly Potent 6-substituted Pyrrolo[2,3-d]pyrimidine Thienoyl Antifolate Inhibitor with Proton-coupled Folate Transporter and Folate Receptor Selectivity Over the Reduced Folate Carrier That Inhibits β-glycinamide Ribonucleotide Formyltransferase. J. Med. Chem. 2011;54:7150–7064. doi: 10.1021/jm200739e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kugel Desmoulin S, Wang Y, Wu J, Stout M, Hou Z, Fulterer A, Chang MH, Romero MF, Cherian C, Gangjee A, Matherly LH. Targeting the Proton-coupled Folate Transporter for Selective Delivery of 6-Substituted Pyrrolo[2,3-d]pyrimidine Antifolate Inhibitors of de novo Purine Biosynthesis in the Chemotherapy of Solid Tumors. Mol.Pharmacol. 2010;78:577–587. doi: 10.1124/mol.110.065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 Gradients in Solid Tumors In Vivo: High-Resolution Measurements Reveal a Lack of Correlation. Nat. Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 16.Raghunand N, Altbach MI, van Sluis R, Baggett B, Taylor CW, Bhujwalla ZM, Gillies RJ. Plasmalemmal pH-gradients in Drug-sensitive and Drug-resistant MCF-7 Human Breast Carcinoma Xenografts Measured by 31P Magnetic Resonance Spectroscopy. Biochem. Pharmacol. 1999;57:309–312. doi: 10.1016/s0006-2952(98)00306-2. [DOI] [PubMed] [Google Scholar]
- 17.Wike-Hooley JL, Haveman J, Reinhold HS. The Relevance of Tumour pH to the Treatment of Malignant Disease. Radiother. Oncol. 1984;2:343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]
- 18.Wong SC, Proefke SA, Bhushan A, Matherly LH. Isolation of Human cDNAs That Restore Methotrexate Sensitivity and Reduced Folate Carrier Activity in Methotrexate Transport-Defective Chinese Hamster Ovary Cells. J. Biol. Chem. 1995;270:17468–17475. doi: 10.1074/jbc.270.29.17468. [DOI] [PubMed] [Google Scholar]
- 19.Beardsley GP, Moroson BA, Taylor EC, Moran RG. A New Folate Antimetabolite, 5,10-dideaza 5,6,7,8-tetrahydrofolate is a Potent Inhibitor of de novo Purine Synthesis. J. Biol. Chem. 1989;264:328–333. [PubMed] [Google Scholar]
- 20.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.