Abstract
2,3,7,8- Te trachlorodibenzo- p-dioxin (TCDD) adversely affects many mammalian organs and tissues. These effects are mediated by the aryl hydrocarbon receptor (AHR). CYP1A1, CYP1A2 and CYP1B1 are upregulated by the liganded AHR. These (and other) cytochromes P450 can metabolize arachidonic acid into a variety of bioactive eicosanoids. Towards investigating a potential role of eicosanoids in TCDD toxicity, arachidonic acid, two other unsaturated long-chain fatty acids, and up to twenty-three eicosanoids were measured in five organs/tissues of male and female wild-type and Ahr null mice treated or untreated with TCDD. TCDD generally increased the levels of the four dihydroxyeicosatrienoic acids (DHETs) and (where measured) 5,6-epoxyeicosatrienoic acid and 18-, 19- and 20-hydroxyeicosatrienoic acids (HETEs) in the serum, liver, spleen and lungs, but not the heart, of both sexes, and increased the levels in the serum, liver and spleen of several metabolites that are usually considered products of lipoxygenase activity, but which may also be generated by cytochromes P450. TCDD also increased the levels of the esterified forms of these eicosanoids in the liver in parallel with the corresponding free forms. The levels of prostanoids were generally not affected by TCDD. The above changes did not occur in Ahr null mice, and are therefore mediated by the AHR. TCDD increased the mRNA levels of Cyp1a1, Cyp1a2, Cyp1b1 and the Pla2g12a form of phospholipase A2 to varying degrees in the different organs, and these increases correlated with some but not all the changes in eicosanoids levels in the organs, suggesting that other enzymes may also be involved.
Keywords: 2,3,7,8-tetrachlorodibenzo-p-dioxin; aryl hydrocarbon receptor; eicosanoids; arachidonic acid; linoleic acid; docosahexaenoic acid; cytochrome P450
Introduction
Eicosanoids are derivatives of twenty carbon polyunsaturated fatty acids, particularly arachidonic acid (AA). Equivalent derivatives of eighteen and twenty-two carbon unsaturated fatty acids, such as linoleic (LA) acid and docosahexaenoic acid (DHA), may have similar biological activities, although less is known about the biological effects of these latter compounds. Eicosanoids are generally short-lived in vivo. They have effects on many organs and tissues (Nebert and Karp, 2008). Drugs targeting the arachidonate cascade represent over 25% of the world’s pharmaceutical sales (Li et al, 2011).
Arachidonic acid (and the other aforementioned polyunsaturated fatty acids) are metabolized via three pathways: the cyclooxygenase, lipoxygenase and cytochrome P450 epoxidation/hydroxylation pathways (although cytochromes P450 are or can be involved in all three pathways). The least is known about the last pathway. Mammalian cytochromes P450 from many different subfamilies, including the CYP1A, CYP1B, CYP2B, CYP2C, CYP2D, CYP2E, CYP2F, CYP2G, CYP2J, CYP2P, CYP2U, CYP3A, CYP4A, CYP4B and CYP4F subfamilies exhibit arachidonic acid epoxidation and/or hydroxylation activities. The immediate products of the epoxidation/hydroxylation pathway of arachidonic acid metabolism include four cis-epoxyeicosatrienoic acids (EETs), 5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET, the “terminal” hydroxides, 16-HETE, 17-HETE, 18-HETE, 19-HETE and 20-HETE, and certain “midchain” hydroxides, including 5-HETE, 8-HETE, 12-HETE and 15-HETE. The midchain hydroxides are also products of the lipoxygenase catalyzed metabolism of arachidonic acid (Zeldin, 2001; Rifkind, 2006). Several of the cis-epoxyeicosantrienoic acids and hydroxides, including some of the terminal hydroxides (particularly 20-HETE), exhibit potent biological activities. For example, EETs can affect a number of functions, including angiogenesis, apoptosis, fibrinolysis, mitogenesis, hormone action, vasodilation, bronchodilation, inflammation, and neuropathic pain (Norwood et al., 2010). The epoxides can be further metabolized, particularly by soluble epoxide hydrolase, which converts them to the dihydroxyeicosatrienoic acids (DHETs). DHETs are generally less biologically active, although some activities have been ascribed to them (Buczynski et at, 2009; Nebert and Karp, 2008).
2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD) elicits a wide variety of toxic, teratogenic and carcinogenic responses in animals and in humans. Most if not all the effects of dioxin are mediated by the aryl hydrocarbon receptor (AHR), which after binding TCDD, transolocates to the nucleus, dimerizes with the aryl hydrocarbon nuclear translocator protein (ARNT) and activates transcription of a large number of genes, including those for CYP1A1, CYP1A2 and CYP1B1, and represses others (White and Birnbaum, 2009; Ma et al, 2009; Denison et al, 2011). A wide variety of other compounds, including halogenated aromatic hydrocarbons and polycyclic aromatic hydrocarbons (PAH) can bind to, and activate the AHR. Many if not most of the biological effects of the AHR are probably mediated by its effect on these transcriptional responses (Bunger et al, 2008; Fujii-Kuriyama and Kawajiri, 2010). Nebert and Karp pointed out that “the myriad AHR-mediated processes mirror the vast universe of action of the eicosanoids” and they proposed that many of the biological effects of AHR are mediated by synthesis of eicosanoids by the CYP1 subfamily (Nebert and Karp, 2008). Compatible with this notion, several investigators have shown that microsomes from organs of TCDD- or PAH-treated mammals catalyze the conversion of arachidonic acid to certain eicosanoids at different rates compared with microsomes from non-treated mammals (Capdevilla et al, 1990; Lee et al, 1998; Aboutabl et al, 2009). Furthermore, Dalton and coworkers showed that TCDD exposure increased the levels of three cyclooxygenase-derived arachidonic metabolism in the urine of mice (Dalton et al, 2001). However, to directly address the potential role of eicosanoids in TCDD toxicity, it would be highly advantageous to measure these compounds in the relevant organs and tissues. Recent advances in liquid chromatography-tandem mass spectrometry (LC-MS/MS) have allowed for the identification and quantitation of a large number of eicosanoids in tissue extracts (Blaho et al, 2009; Yang et al, 2009). We report here such a “lipidomics” approach to quantitate up to twenty-three eicosanoids and three polyunsaturated fatty acids (Figure 1) in five different organs/tissues from TCDD-treated and untreated mice. Our results demonstrate that the levels of eicosanoids derived from the cytochrome P450-dependent epoxidation/hydroxylation pathway were the most widely and markedly elevated, and that the levels of some of mid-chain hydroxides and other metabolites generally considered to be products of the lipoxygenase pathways were also increased, although there were differences in these regards between organs/tissues. Products of the cyclooxygenase pathway were generally not affected by TCDD treatment. By utilizing an Ahr−/− null mouse, we also demonstrate that the changes in eicosanoids levels elicited by TCDD are dependent upon the AHR. Altogether, these studies lay the foundation for future experiments addressing the potential role of eicosanoids in mediating the toxic effects of TCDD and other ligands of the AHR.
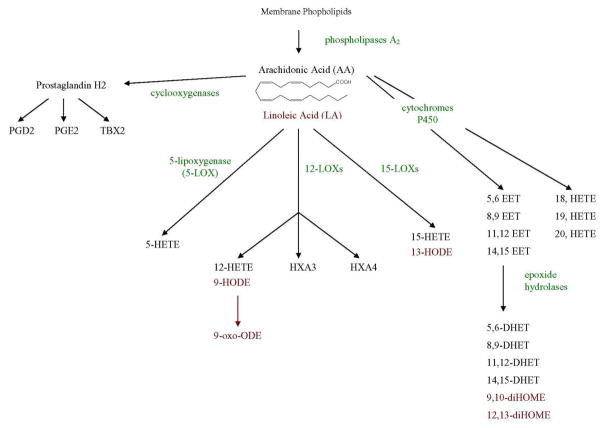
Figure 1.
Metabolism of arachadonic acid (and linoleic acid) by the cyclooxygenase, lipoxygenase and cytochrome P450 pathways, showing the matabolites that were measured. Linoleic acid metabolites are shown in red. Enzymes are shown in green. Abbreviations here and in Table 1 are as follows:- TBX, thromboxane; HETE, hydroxyeicosatetraenoic acid; HODE, hydroxyoctadecadienoic acid; 9-oxo-ODE, 9-oxo-octadecadienoic acid; HX, hepoxilin; EET, epoxyeicosatrienoic acid; DHET, dihydroxyeicosatrienoic acid; diHOME, dihydroxyoctadecenoic acid.
Materials and Methods
Chemical and Reagents
HPLC solvents (HPLC grade) were obtained from Sigma Aldrich (St. Louis, MO). The C18 reversed-phase column (Discovery R C18, Supelco, 2.2 mm x 150 mm, 5 μm) was purchased from Supelco Sigma Alrich (St. Louis, MO). 9α,11α-epidioxy-15S-hydroxy-prosta-5Z,13E-dien-1-oic acid (PGH2), 9-oxo-11α,15S-dihydroxy-prosta-5Z,13E-dien-1-oic acid (pGE2), 9α,15S-dihydroxy-11-oxo-prosta-5Z,13E-dien-1-oic acid (PGD2), 9α,11,15S-trihydroxythromba-5Z,13E-dien-1-oic acid (TXB2), 6-oxo-9α,11α,15S-trihydroxy-prost-13E-en-1-oic acid (6-k-PGF1α), 9α,15S-dihydroxy-11-oxo-prosta-5Z,13E-dien-1-oic-3,34,4-d4 acid (PGD2-d4), 9,15-dioxo-11α-hydroxy-prosta-5Z,13E-dien-1-oic acid (15-keto-PGE2), 5S,12R-dihydroxy-6Z,8E,10E,14Z-eicosatetraenoic acid (LTB4), 7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid (Resolvin D1), 10(S),17(S)-dihydroxy-4Z,7Z,11E,13Z,15E,19Z-docosahexaenoic acid (Protectin D1), 12,13-dihydroxy-9Z-octadecenoic acid (12,13-DiHOME), (±)5,6-dihydroxy-8Z,11Z,14Z-eicosatrienoic acid (5,6-DHET), (±)8,9-dihydroxy-5Z,11Z,14Z-eicosatrienoic acid (8,9-DHET), (±)11,12-dihydroxy-5Z,8Z,14Z-eicosatrienoic acid (11,12-DHET), (±)14,15-dihydroxy-5Z,8Z,11Z-eicosatrienoic acid (14,15-DHET), (±)5(6)-epoxy-8Z,11Z,14Z-eicosatrienoic acid (5(6)-EET), (±)8(9)-epoxy-5Z,11Z,14Z-eicosatrienoic acid (8(9)-EET), (±)11(12)-epoxy-5Z,8Z,14Z-eicosatrienoic acid (11(12)-EET), (±)14(15)-epoxy-5Z,8Z,11Z-eicosatrienoic acid (14(15)EET), 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid (5-oxo-ETE), 12-oxo-5Z,8Z,10E,14Z-eicosatetraenoic acid (12-oxoETE), 15-oxo-5Z,8Z,10E,14Z-eicosatetraenoic acid (15-oxoETE), 13-oxo-9Z,11E-octadecadienoic acid (13-oxoODE), 5S-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid (5-HETE), 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12-HETE), 15S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (15-HETE), 13S-hydroxy-9Z,11E-octadecadienoic acid (13-HODE), (±)18-hydroxy-5Z,8Z,11Z,14Z-eicosatetraenoic acid (18-HETE), (±)19-hydroxy-5Z,8Z,11Z,14Z-eicosatetraenoic acid (19-HETE), (±)20-hydroxy-5Z,8Z,11Z,14Z-eicosatetraenoic acid (20-HETE), (±)17-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid (17-HDOHE), Linoleic acid (LA), Docosahexaenoic Acid (DHA), Arachidonic acid (AA), 5S-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic-5,6,8,9,11,12,14,15-d8 acid (5-HETE-d8), 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic-5,6,8,9,11,12,14,15-d8 acid (12-HETE-d8), 15(S)-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic-5,6,8,9,11,12,14,15-d8 acid (15(S)-HETE-d8), and 13-HODE-d4 were purchased from Cayman Chemical (Ann Arbor, MI). Oasis HLB (1cc/10mg, 30μm) was purchased from Waters Corporation (Milford, MA, USA). Protease inhibitors cocktail was purchased from Roche. TCDD was purchased from Wellington Laboratories, Guelph, ON, Canadaand was handled with extreme caution.
Animals
Ahr −/− null mice were a kind gift of Christopher Bradfield (Schmidt et al, 1996). They were backcrossed at least seventeen times to C57BL/6 mice and therefore were of the C57BL/6 genetic background. Male and female Ahr −/− null mice and their sibling Ahr +/+ wild type mice were obtained from crossing heterozygous Ahr +/− mice. Genotyping of mice was performed by PCR as described by the Jackson Laboratories (http://jaxmice.jax.org/protocolsdb/f?p=116:2:4212526675950722::NO:2:P2_MASTER_PROTOCOL_ID,P2_JRS_CODE:195,002831). All mice were housed and bred at UCLA in a specific-pathogen-free facility. Mice were allowed free access to food (chow diet) and water before being used for the experiments. Mice were kept under a 12-h light/dark cycle and house at 25°C. Two to three-month-old mice were used for all experiments.
Administration of TCDD to mice and harvesting of tissues/organs
TCDD was received in a pre-weighed vial
50 μg/kg of TCDD in corn oil was administered by intraperitoneal injection. Corn oil was used as vehicle control. Five to 8 animals were used per treatment group. Mice were euthanized with carbon dioxide on day 3 after injection. Immediately, blood was collected via cardiac puncture using heparin-coated needles; 0.2% of BHT (butylated hydroxytoluene) and TPP (triphenylphosphine) were added directly to the blood to prevent auto-oxidation of fatty acids. Serum was prepared using serum separator tubes (BD). Heart, lung, liver and spleen were also collected and stored in −80 o C for later analysis. Hearts from only the wild type mice were collected and analyzed. All TCDD procedures with mice, including exposure, euthanasia and dissections were performed in two dedicated laboratories, constituting a “Carcinogen Suite” in the containment area of the UCLA vivarium. The suite is under negative pressure. Isolation of mouse tissues from the TCDD-treated mice was done in a vertical laminar flow hood (Class II B1) certified for use with chemical carcinogens. Cages which had been used with TCDD-treated mice were wiped down with methanol and then water. Bedding was treated as hazardous waste. Mouse carcasses were placed in a designated refrigerator for disposal by UCLA’s Environmental Health and Safety Office (E.H. & S.). After use, solutions containing TCDD solutions were extracted with chloroform for disposal. The chloroform fraction was sent to the UCLA Division of Environmental (E.H. & S.) for disposal by incineration. These procedures have been approved by the UCLA Division of Laboratory Animal Medicine, the UCLA Division of E.H. &S, and the UCLA Office for the Protection of Research Subjects
Extraction of fatty acids from different tissues
i) Serum
A 150 μL volume of serum was transferred to a 2 mL polypropylene tube. The sample was spiked with 100 μL of internal standards mixture (PGD2-d4, 5-HETE-d8, 12-HETE-d8, 15-HETE-d8, 13-HODE-d4, 10 ng/ml each) in methanol. Subsequently, 1.75 ml of water (0.1% acetic acid, pH 3.0) was added. The samples were left for 15 minutes on ice for complete acidification and equilibration. The resulting sample was then extracted using the solid-phase extraction method described below.
ii) Liver, Lung, Spleen and Heart
Each tissue was cut into pieces and transferred into 2 ml polypropylene tubes. 450 μl of 50 mM Tris-HCl, pH 7.5 plus protease inhibitors (complete mini protease inhibitor cocktail, Roche, Nutley, New Jersey) was added into each tube. Samples were homogenized for 60 seconds with the medium setting, followed by sonication with 7–8 pulses at 50 % output and a 5 pulser setting using a VibraCell sonicator (Sonics and Materials, Inc., Newtown, CT). 250 μl of lysate was then mixed with 100 μl of MeOH (0.2% BHT and TPP) followed by centrifugation at 10,000xg for 15 minutes. The supernatant was then transferred to 2 ml tube containing 1700 μl 0.1%acetic acid, pH3.0 and 100 μl of internal standards mixture ( see above) were added. The supernatant was then treated as for the serum samples above. For female mice, the liver tissue was treated the same as for the male mice except that the liver was suspended in 250 μl H2O plus 250 μl of MeOH (0.2% BHT and TPP).
Total fatty acid extraction
Only wild type male mice liver samples were used for total lipid extraction. 250 μl of liver lysate samples were transferred to a 2 ml tube containing 250 μl water. 500 μl of 1M KOH was added to hydrolyze fatty acids from their conjugates, and the tubes incubated at 37oC for 30 minutes. The samples were then acidified with 500 μl of 1M HCl followed by centrifugation at 10,000xg for 15 minutes. The supernatant was transferred to a 15 ml tube containing 1500 μl H2O and 100 μl of internal standard mixture (10 ng/ml in methanol). The samples were left for 15 minutes on ice for complete acidification and equilibration. The sample was then extracted using the solid-phase extraction method.
Solid phase extraction
Processed samples from different tissues were loaded onto a preconditioned 1cc Oasis HLB solid-phase extraction (SPE) cartridge on a vacuum manifold (Waters). The SPE cartridge was equilibrated with 2 ml methanol followed by 2ml water (0.1% acetic acid, pH 3.0) before sample loading. After slowly loading the cartridge, it was washed with 2ml 5% methanol in water (0.1% acetic acid). Fatty acid analytes were subsequently eluted with 2 ml methanol. The eluate was then evaporated to dryness under a stream of argon. 100 μl of methanol was added to the dried extract, vortexed for 30s, and the reconstituted extract was centrifuged at 13,200 rpm for 20 min at 4°C.
LC/MS/MS analysis
LC/MS/MS was performed using a quadrupole mass spectrometer (4000 QTRAP; Applied Biosystems, Foster City, CA) equipped with electrospray ionization (ESI). The HPLC system utilized an Agilent 1200 series LC pump equipped with a thermostated autosampler (Agilent Technologies, Santa Clara, CA). Chromatography was performed using C18 reversed-phase column, (Discovery R C18, Supelco, 2.2 mm x 150 mm, 5 μm), plus a C18 guard column held at 45oC. Mobile phase A consisted of 0.1% formic acid in water, and mobile phase B consisted of 0.1% formic acid in acetonitrile. The autosampler was set at 4oC. The injection volume was 20 μl and the flow rate was controlled at 0.4 mL/min. Chromatography was optimized to separate all analytes in 30 minutes. The gradient is given in Table S-1 in the supplemental material. Data acquisition and instrument control were accomplished using Analyst 1.4.2 software (Applied Biosystems). Detection was accomplished by using the multiple reaction monitoring (MRM) mode with negative ion detection. The parameter settings are described in table S-2. Mass spectrometry measurement was divided into 4 periods to increase dwell time and lower the limits of detection. Dwell time was found to play a very important role in increasing the signal-to-noise ratio with a shorter dwell time resulting in a much higher noise (Yang et al., 2009). In our method, the dwell times were set above 75 mili-seconds which is longer than the dwell time used by Yang and coworkers (Yang et al., 2009) (Table S-2). In addition, MRM transitions, collision energy, declustering potential, and collision cell exit potential were optimized for each compound by direct infusion into the mass spectrometer to obtain optimum sensitivity (see Table S-3). For several analytes for which we did not possess a standard, MRM transition parameters were adopted from previous publications (Blaho et al., 2009; Yang et al., 2009). For most analytes, four concentrations (5, 10, 20, and 40 ng/ml) were used to create standard curves. In the case of docosahexaenoic acid (DHA), 10, 20, 40, and 80 ng/ml were used, whereas 50, 100, 200, and 400 ng/ml were used for arachidonic acid (AA) and linoleic acid (LA). All the standard curves had R2 values (for linear regression analysis) of 0.990 or greater for each analyte. The limit of detection for each standard was below 0.5 ng/ml. The inter- and intra-day accuracy and precision were determined based on the % CV (coefficient of variation) using data for 13-HODE and 12-HETE (see supplemental material table S-4-5). In addition, to ensure the consistency of the instrument during every experimental run, the standards were always submitted first and resubmitted again at the end of each run. The % CV between the two submissions of the same sample were routinely less than 15%.
RNA extraction, cDNA synthesis, and real-time PCR
RNA was isolated using RNEasy Mini columns (Qiagen, Valencia, CA), according to the manufacturer’s instructions for frozen animal tissues, and quantified on a NanoPhotometer (Implen, Westlake Village, CA). One microgram of total RNA was used for cDNA synthesis in a total reaction volume of 20 μl. Reverse transcription reactions were performed using Superscript III reverse transcriptase (Invitrogen) and primed with random hexamers (Invitrogen) according to the manufacturer’s instructions. Following cDNA synthesis, all reactions were diluted into a total of 200 μl of RNase-free water, and were then used as templates for real-time PCR.
SYBR Green real-time PCR was performed according to standard protocols using the 7500 Fast (Applied Biosystems, Foster City, CA) and quantities were normalized to those for the GAPDH glycolytic enzyme housekeeping gene. All primers were designed using Primer Express 3.0 (Applied Biosystems, Foster City, CA), and purchased from Fisher Scientific (Pittsburgh, PA). The forward and reverse primers used for quantification are listed in Supplementary Table S-6. Data were analyzed using the ABI software and Microsoft Excel, and significance was evaluated using Student’s t-test.
Results
The effects of TCDD on eicosanoid levels
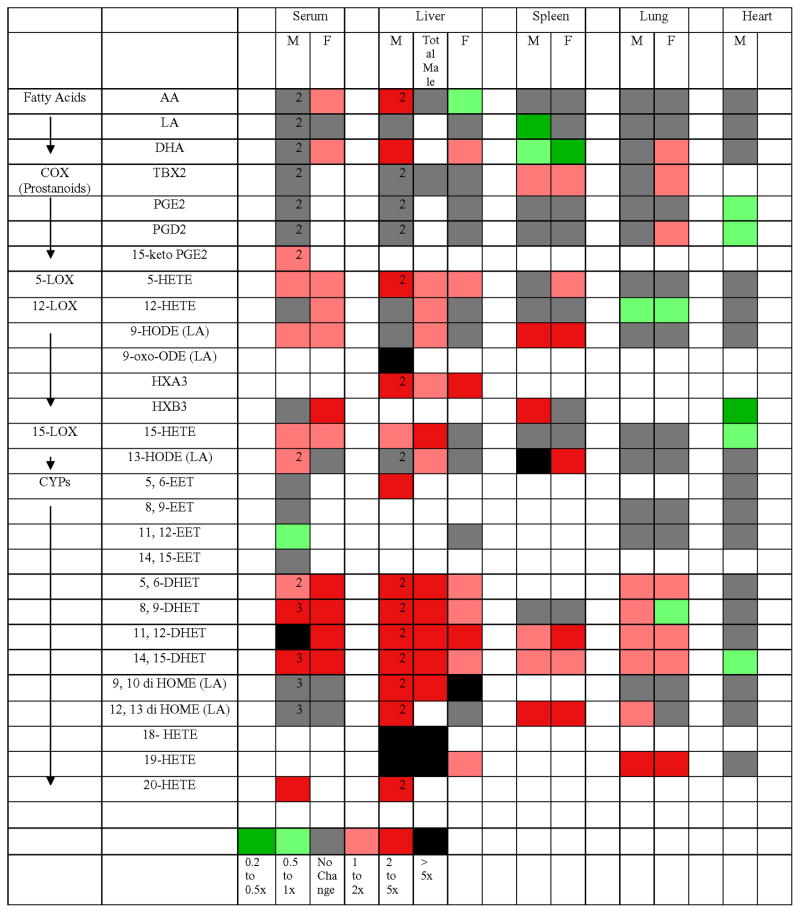
Adult C57BL/6 wild-type or knockout mice were injected intraperitoneally with 50 ug/kg TCDD or with vehicle (corn oil). Three days later their organs/tissues were harvested and subsequently analyzed for the levels of certain free (i.e. non-esterified) eicosanoids by HPLC followed by MS/MS (see Materials and Methods). This dose and duration of exposure were used as they are known to maximally induce most TCDD-responsive genes (Hayes et al, 2007; Forgacs et al, 2011) Three separate experiments were performed. In each independent experiment, 5 to 8 mice were analyzed per gender per genotype. In experiment 1, male and female wild-type and Ahr−/− knockout mice were analyzed for the levels of certain free eicosanoids in serum, liver, lung, spleen, and heart (wild-type males only). The data for experiment 1 are presented in Supplementary Table S-7. In experiment 2 (Supplementary Table S-8), the levels of total eicosanoids as well as free eicosanoids were measured in the livers of wild-type male mice. To obtain total eicosanoids, liver extracts were treated with KOH in order to hydrolyze membrane phospholipids. In the third experiment, free eicosanoids were measured in the serum from wild-type mice (data not shown). The results from all three experiments are summarized as a “heat map” in Figure 2. The major points that can be made from these data are as follows:-
Figure 2.
“Heat map” summarizing the results from the three experiments measuring eicosanoids levels. Colored segments indicate that the values were significantly increased or decreased by TCDD treatment at p<0.05 in wild-type mice. Where the data are averaged from more than one experiment, the number of independent experiments is indicated in the corresponding segment in the figure. Where a particular segment is empty, this indicates that the compound was not measured or was below the level of detection.
TCDD treatment increased the levels of arachidonic acid in the liver.
In wild-type mice, TCDD had little if any effect on the levels of the cyclooxygenase pathway metabolites in any of the five organs/tissues examined.
TCDD increased the levels of many metabolites of the cytochrome P450 epoxidation/hydroxylation pathway in the serum, liver, lung and spleen of wild-type mice, but not in the heart.
In the serum, liver and spleen but not the lungs or heart of wild-type mice, TCDD treatment increased the levels of many metabolites of arachadonic acid that are generally categorized as lipoxygenase products (but which can also be generated by particular cytochromes P450).
The levels of the total metabolites in the liver were in all cases greater than the levels of the corresponding free metabolites, generally exceeding them by 2- to 10-fold (Supplementary Table S-8). The levels of total prostanoids could not be measured as they are degraded by KOH.
For those metabolites in the liver affected by TCDD treatment, total levels generally increased in parallel with the free levels of the same metabolite. This was particularly striking for the cytochrome P450-derived DHETs and terminal hydroxides.
TCDD significantly increased the levels of only four metabolites and only in one tissue/organ examined (male serum) of male and female Ahr −/− knockout mice (Supplementary Table S-7). Thus the observed increases in the eicosanoids induced by TCDD in the wild-type mice are generally dependent on AHR.
There were significant differences in the levels of only a few eicosanoids between wild-type and Ahr−/− knockout mice in the absence of TCDD (Supplementary Table S-7)
There were markedly different levels of the prostanoids and putative lipoxygenase products between different (DMSO-treated) organs; with the levels generally decreasing in the following order: spleen, lung, heart, liver, serum; such that the spleen contained up to 200-fold higher levels of some of these metabolites than the liver. Such differences were not observed for the metabolites derived from the cytochrome P450 epoxidation/hydroxylation pathway (Supplementary Table S-7).
Although not a major focus of this study, we observed differences in the levels of certain eicosanoids between (DMSO-treated) male and female mice. However, the differences that were observed generally did not exceed three-fold.
Effects of TCDD on the levels of the mRNAs for Cyp1a1, Cyp1a2, Cyp1b1, cytosolic phospholipase A2 (Pla2g4a and Pla2g12a), soluble epoxide hydrolase (Ephx2) and prostaglandin endoperoxide synthase 2 (cyclooxygenase 2 {Ptgs2}) in male mice
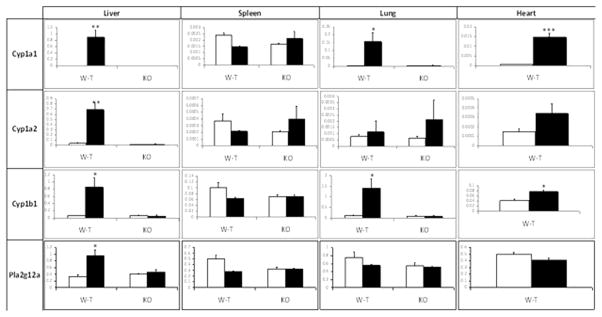
The levels of the Cyp1a1 and Cyp1b1 mRNAs were increased by TCDD treatment in the wild-type male mouse in the liver, lung and heart (although the mRNA levels were 10-fold or more lower in the heart). Cyp1a2 increased only in the liver. None of the mRNA levels were increased by TCDD in the spleen. Interestingly, the levels of Cyp1a1 mRNAs in the different organs correlate with the known distribution of TCDD to these organs (De Jongh et al, 1995). No induction of any of these enzymes occurred in the Ahr−/− mouse (Figure 3). It should be noted that TCDD characteristically affects the levels of these enzymes exclusively at the transcriptional level, and increases in their mRNAs are reflected in increases in the corresponding proteins (Hankinson, 1995). The mRNA for the phosopholipase A2 form Plag2g12a has been reported to be inducible by TCDD in the liver of both male and female C57BL/6 mice in whole genome microarray studies (Tijet et al, 2005: Boutros et al 2008; Kopec et al, 2010). We found that Plag2g12a mRNA was inducible in the male liver about 2.5-fold in our studies (Figure 3). Pla2g4a was reported to be increased by TCDD treatment in the Hepa-1 mouse hepatoma cell line (Kinehara et al., 2009). This enzyme has been reported to represent a major form of phosopholipase A2 with regard to the metabolism of arachidonic acid to eicosanoids (Kita et al., 2006). However, neither this enzyme, nor Ephx2 were induced by TCDD in our studies in mice (Supplementary Figure 1). The levels of the prostaglandin endoperoxide synthase 2 mRNA was not increased in any of the organs after a three day treatment with (50 ug/kg) TCDD (Supplementary Figure 1). This is in agreement with the results of Vogel and coworkers (Vogel et at., 1998). However, those workers did find that 10 μg/kg TCDD transiently increased cyclooxygenase 2 mRNA levels: inducing the mRNA maximal 4.5-fold and 3.5-fold in the lung and spleen, respectively, of female C57BL/6 mice after 24 hours of treatment. (No increase occurred in the liver.) Nevertheless, any increase in cyclooxygenase that might have occurred at earlier time points had minimal effects on the levels of prostanoids in our experiments. In conclusion, TCDD induction of Cyp1a1, Cyp1a2, Cyp1b1 and Plag2g12a correlated with TCDD-induced increases in eicosanoid levels in some organs (lung and liver), but not others (spleen and heart).
Figure 3.
Effect of TCDD on Cyp1a1, Cyp1a2, Cyp1b1, and Pla2g12a mRNA levels in liver, spleen, lung and heart of male wild-type mice. The levels of each mRNA are represented relative to the levels of the constitutively expressed GAPDH glycolytic enzyme in each organ. The means and standard errors were derived from four mice treated with TCDD (filled bars) or vehicle (open bars). *, ** and *** represent significantly different from the DMSO control at p<0.05, p<0.01 and 0.001, respectively.
Discussion
In this study, we show that TCDD increases the levels of a number of eicosanoids in several organs/tissues of the mouse. DHETs were elevated in many of the organs/tissues by TCDD. The levels of the DHETs we measured fell in the 4 to 40 nM range in the absence of TCDD in serum. These values are similar to those previously reported for mouse serum (Kubala et al., 2010). Although these compounds are generally considered to be inactive metabolites of EETs, they do exhibit some of the same properties as EETs, including vasodilatory activity (Oltman et al, 1998), and they are ligands for perioxisome proliferator activated receptors (PPAR) α and γ (Buczynski et al, 2009; Konkel and Schunck, 2011). Importantly, they are rapidly generated from the corresponding EETs by epoxide hydrolase, and so their levels probably reflect the levels of the more biologically active EETs. We did not report the levels of the EETs themselves in most organs/tissues either because they were below the level of detection, or because we had not developed the means for their analysis. Nevertheless, we found that 5,6-EET was increased by TCDD in the liver and spleen. Schlezinger and coworkers previously reported that TCDD treatment increased the levels of three EETs in the liver of fish (Schlezinger et al, 1998). The equivalent derivatives of linoleic acid, 9,10-diHOME and 12,13-diHOME, which are proinflammatory (Slim et al., 2001), were increased by TCDD in some organs.
We did not possess the means for measuring the terminal hydroxides in most experiments. However, where measured, these metabolites were consistently elevated by TCDD in all organs/tissues studied (except the heart). 20-HETE is known to be biologically active, for example exhibiting potent vasoconstrictive activity (Ishizuka et al, 2008). Little is known about the potential functions of 18-HETE and 19-HETE, however, they can induce vasodilation by inhibiting the effects of 20-HETE (Carroll et al, 1996). Interestingly, many metabolites that are traditionally considered products of lipoxygenase metabolism, including 5-HETE, 12-HETE, 9-HODE, HXA3, HXB3, 15-HETE and 13-HODE were increased by TCDD treatment in serum, liver and spleen. These compounds all exhibit biologically activity, of varying potency (Buczynski et al, 2009).
TCDD increased the level of free arachidonic acid in the liver of wild-type mice, as has been described previously (Lin et al., 2011), and this may explain to some degree the increases in eicosanoids that occur after TCDD treatment in this organ. However the increases in these metabolites are not likely to be completely due to the increase in arachidonic acid, because (i) increases did not occur in the levels of the prostanoid products of arachidonic acid metabolism, ii) increases in the many arachidonic acid metabolites occurred in female liver (although to a lesser extent), despite the fact that no increase free in arachidonic acid occurred in this organ, and (iii) an increase did not occur in the amount of total arachidonic acid in male liver. The total levels of several eicosanoids were increased by TCDD in male liver. Since the metabolites are likely generated from free arachidonic or linoleic acid rather than the esterefied forms, the free metabolites are probably incorporated rapidly into phospholipids, and the phospholipids so formed therefore represent a reservoir for these metabolites.
The TCDD-induced increases in the levels of the eicosanoids in the spleen and other solid organs cannot be ascribed to the (relatively small amount of) blood co-harvested with them, as the levels in the organs were generally of the same magnitude or greater than in serum. Nevertheless, it is very possible that, since several of the eicosanoids were elevated by TCDD in serum, increases in one organ may be transmitted to another via transport of the compound in the blood.
Since there was little effect of TCDD in eicosanoids levels in Ahr−/− mice, the Ahr mediates most if not all the effects of TCDD on the eicosanoids. Cyp1a1, Cyp1a2 and Cyp1b1 are known to be among the most highly TCDD-induced genes in the liver in vertebrates. Purified (human) CYP1A1, CYP1A2, and CYP1B1 can metabolize arachidonic acid to midchain HETEs, terminal HETEs and EETs (Choudhary et al, 2004; Schwarz et al, 2004; Fer et al, 2008; Rifkind et al, 2006), and so these cytochromes P450 may be responsible for much of the increases in these metabolites in the liver after TCDD treatment. However, in some (but not all) microarray experiments, TCDD has been shown to elevate the levels of the arachidonic acid-metabolizing Cyp2c29, Cyp2c47 and Cyp2c50 enzymes (Hayes et al, 2007; Forgacs et al, 2011), and benzo(a)pyrene, a ligand for the AHR has been shown to increase the levels of the mRNAs for the arachidonic acid-metabolizing Cyp4f4 and Cyp4f5 enzymes in the rat (Aboutabl et al, 2011). Therefore other cytochromes P450 may contribute to the increase in eicosanoids levels occurring upon TCDD treatment. The heart did not exhibit an increase in any eicosanoids after TCDD treatment, despite the fact that Cyp1a1 and Cyp1b1 mRNAs were inducible in this organ. However, this may be explained by the fact that the induced levels of these cytochromes P450 were considerably lower than they were in the liver and lung.
It should be noted that although analysis of arachidonic acid metabolism by purified cytochromes P450 is useful, the metabolic profile of arachidonic acid in a particular organ is difficult to predict from its content of cytochromes P450, for several reasons, including the effects of regulatory interactions between metabolic products of some cytochromes P450 (Rifkind, 2006). Interestingly, Schlezinger and coworkers found that TCDD had a stronger effect on the in vivo metabolism of arachidonic acid than its in vitro metabolism by liver microsomes (Schlezinger et al, 1998). Lipidomic analysis as we report here is therefore essential for advancing understanding of the roles of the eicosanoids in biological processes in the whole organism.
In the absence of TCDD, Ahr−/− mice exhibited different levels of only a few eicosanoids compared with wild-type mice. (Supplementary Table S-7). Furthermore, the number of compounds involved was much less than the number increased by TCDD treatment in wild-type mice; more compounds showed elevated levels in knockout mice than showed reduced levels; and the five metabolites that were significantly elevated in wild-type mice did not exhibit an obvious expression pattern (i.e. they were not focused in any particular metabolic group or organ). Thus, it seems unlikely that the eicosanoids that were measured contribute to the known differences in the physiological phenotypes of Ahr−/− and wild-type mice in the absence of exogenous ligand (Fujii-Kuriyama and Kawajiri, 2010), although this needs further study.
TCDD causes toxicity in all the organs we analyzed. TCDD induces many adverse effects in the liver, including hepatocellular hypertrophy and hyperplasia, fatty change, necrosis, inflammation, portal fibrosis, and liver tumor promotion and progression (Yoshizawa et al., 2007; Bock and Kohle, 2009). TCDD causes several forms of pulmonary disease, such as keratinizing epithelioma, bronchiolar metaplasia, and squamous metaplasia of the alveolar epithelium (Yoshizawa et al., 2007). TCDD causes cardiomyopathy, defects in several heart functions, and elevated arterial blood pressure (Korashy and El-Kadi, 2006; Yoshizawa et al., 2007). AHR agonists cause modest splenic lymphoid atrophy in rodents in some but not all studies (Sulentic and Kaminski, 2011; Yoshizawa et al., 2007). (We observed a decrease in spleen weight of 44% and 27% in male and female mice, respectively, in the current study that was dependent on the AHR {data not shown}). It is important to consider whether increasese in eicosanoids levels cause or contribute to any of the TCDD-induced toxicities of these and other organs. Insights into this question can be provided by studies addressing the potential roles of Cyp1a1, Cyp1a2 and Cyp1b1 in TCDD toxicity, since these cytochromes P450 are probably responsible for a large portion of the increased levels of the eicosanoids after TCDD treatment. Many toxic responses to a high TCDD dose, including lethality and wasting, were found to be abrogated in Cyp1a1−/− or Cyp1a2−/− null mice demonstrating that these cytochromes P450 are essential for these toxic responses to TCDD (Smith et al., 2001; Uno et al., 2004). Certain rat strains that were resistant to about half of the multi-organ toxicities of TCDD that were analyzed exhibit normal induction of Cyp1a1, Cyp1a2 and Cyp1b1, indicating that although they may be necessary, these cytochromes P450 are not sufficient for the development of these toxic manifestations (Pohjanvirta et al., 2011). With regard to the hepatic toxicity, induction of Cyp1a1and Cyp1a2 appear to protect against some toxic responses but to enhance others (Nukaya et al., 2009; Nukaya et al., 2010 (a); Nukaya et al., 2010 (b)). TCDD induction of splenic lymphoid atrophy appears not to depend upon induction of Cyp1a1 (Uno et al, 2004). Interestingly, studies with Cyp1a1 knockout mice demonstrated that this enzyme is required for vascular dysfunction and hypertension induced by TCDD (Kopf et al, 2010). .
These studies lay the foundation for future experiments addressing the potential role of eicosanoids in mediating the toxic effects of TCDD and other ligands of the AHR. Comparing the kinetic and dose-response parameters for the TCDD-mediated increases in eicosanoids levels with those for the induction of potentially relevant enzymes could provide insight into the identities of the enzymes involved. Such studies would be complemented by studies using knockout mice for the relevant genes. It will be also of interest to ascertain whether other ligands for the AHR, particularly nutrient-derived ligands such as indole-3-carbinol have the same effect as TCDD. Our studies on whole organs may mask much greater changes in eicosanoids levels in individual cell types in these organs, and this warrants examination. In addition, analysis of additional eicosanoids may identify metabolites that are even more elevated by TCDD than those studied here. It will also be of interest to investigate the effect of TCDD on the levels of eicosanoids in other mouse organs that are targets of TCDD toxicity. Finally, it will be most important to determine whether elevated eicosanoids levels contribute to the deleterious effects of TCDD and other toxic agonists of the AHR.
Supplementary Material
Highlights.
TCDD treatment increases the levels of many eicosanoids in several mouse organs.
Products of both the cytochrome P450 and classical lipoxygenase pathways are increased.
These increases are dependent on the aryl hydrocarbon receptor.
Cyp1a1, Cyp1a2 and Cyp1b1 appear to be responsible for much but not all of the increases.
Acknowledgments
Funding
This work was supported by grants from National Institute of Health [R01CA28868 and R01ES015384 (to O.H.]). PB and PS were partially supported by fellowships from training grant T32ES015457 from the National Institute of Environmental Health Sciences. PB was also partially supported by a fellowship from Clinical Medical Genetics training grant from the NIH [T32-GM0843].
We thank Dr. Srinivasa Reddy for advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aboutabl M, Zordoky B, El-Kadi A. 3-Methylcholanthrene and benzo(a)pyrene modulate cardiac cytochrome P450 gene expression and arachidonic acid metabolism in male Sprague Dawley rats. Br J Pharmacol. 2009;158:1808–1819. doi: 10.1111/j.1476-5381.2009.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaho V, Buczynski M, Brown C, Dennis E. Lipidomic Analysis of Dynamic Eicosanoid Responses during the Induction and Resolution of Lyme Arthritis. J Biol Chem. 2009;283(32):21599–21612. doi: 10.1074/jbc.M109.003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bock K, Kohle C. The mammalian aryl hydrocarbon (Ah) receptor: from mediator of dioxin toxicity toward physiological functions in skin and liver. J Biol Chem. 2009;390:1225–1235. doi: 10.1515/BC.2009.138. [DOI] [PubMed] [Google Scholar]
- 4.Boutros P, Yan R, Moffat I, Pohjanvirta R, Okey A. Transcriptomic responses to 2,3,7,8-tetrachlorodibenzi-p-dioxin (TCDD) in liver: comparison of rat and mouse. BMC Genomics. 2008;9(419):1471–2164. doi: 10.1186/1471-2164-9-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buczynski M, Dumlao D, Dennis E. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50:1015–1038. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunger M, Glover E, Moran S, Walisser J, Lahvis G, Hsu E, Bradfield C. Abnormal Liver Development and Resistance to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Toxicity in Mice Carrying a Mutation in the DNA-Binding Domain of the Aryl Hydrocarbon Receptor. Toxicol Sci. 2008;106(1):83–92. doi: 10.1093/toxsci/kfn149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capdevilla J, Karara A, Waxman D, Martin M, Falck J, Guenguerich P. Cytochrome P-450 enzyme-specific control of the regio- and enantiofacial selectivity of the microsomal arachidonic acid epoxygenase. J Biol Chem. 1990;265(19):10865–10871. [PubMed] [Google Scholar]
- 8.Carroll M, Balazy M, Margiotta P, Huang D, Falck J, McGiff J. Cytochrome P-450-dependent HETEs: profile of biological activity and stimulation by vasoactive peptides. Am J Physiol. 1996;271(4 Pt 2):R863–869. doi: 10.1152/ajpregu.1996.271.4.R863. [DOI] [PubMed] [Google Scholar]
- 9.Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman J. Metabolism of Retinoids and Arachidonic Acid by Human and Mouse Cytochrome P450 1B1. Drug Metab Dispos. 2004;32(8):840–847. doi: 10.1124/dmd.32.8.840. [DOI] [PubMed] [Google Scholar]
- 10.Dalton T, Kerzee K, Wang B, Miller M, Dieter M, Lorenz J, Shertzer H, Nebert D, Puga A. Dioxin Exposure Is an Environmental Risk Factor for Ischemic Heart Disease. Cardiovasc Toxicol. 2009;1(4):285–298. doi: 10.1385/ct:1:4:285. [DOI] [PubMed] [Google Scholar]
- 11.De Jongh J, DeVito M, Diliberto J, Van den Berg M, Birnbaum L. The effects of 2,2’ 4,4’ ,5,5’-hexachlorobiphenyl cotreatment on the disposition of 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice. Toxicol Lett. 1995;80:131–137. doi: 10.1016/0378-4274(95)03387-z. [DOI] [PubMed] [Google Scholar]
- 12.Denison M, Soshilov A, He G, DeGroot D, Zhao B. Exactly the Same but Different: Promiscuity and Diversity in the Molecular Mechanisms of Action of the Aryl Hydrocarbon (Dioxin) Receptor. Toxicol Sci. 2011;124(1):1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fer M, Dreano Y, Lucas D, Corcos L, Salaun J, Berthou F, Amet Y. Metabolismo f eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Arch Biochem Biophys. 2008;471:116–125. doi: 10.1016/j.abb.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Forgacs A, Kent M, Makley M, Mets B, DelRaso N, Jahns G, Burgoon L, Reo N, Zacharewski T. Comparative Metabolomic and Genomic Analyses of TCDD-Elicited Metabolic Disruption in Mouse and Rat Liver. Toxicol Sci. 2011 doi: 10.1093/toxsci/kfr262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujii-Kuriyama Y, Kawajiri K. Molecular mechanisms of the physiological functions of the aryl hydrocarbon (dioxin) receptor, a multifunctional regulator that senses and responds to environmental stimuli. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(1):40–53. doi: 10.2183/pjab.86.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 17.Hayes K, Zastrow G, Nukaya M, Pande K, Glover E, Maufort J, Liss A, Liu Y, Moran S, Vollrath A, Bradfield C. Hepatic Transcriptional Networks Induced by Exposure to 2,3,7,8-Tetrachlorodibenzo-p-dioxin. Chem Res Toxicol. 2007;20:1573–1581. doi: 10.1021/tx7003294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishizuka T, Cheng J, Singh H, Vitto M, Manthati V, Falk J, Laniado-Schwartzman M. 20-Hydroxyeicosatetraenoic Acid Stimulates Nuclear Factor-κB Activation and the Production of Inflammatory Cytokines in Human Endothelial Cells. J Pharmacol Exp Ther. 2007;324:103–110. doi: 10.1124/jpet.107.130336. [DOI] [PubMed] [Google Scholar]
- 19.Kinehara M, Fukada I, Yoshida K, Ashida H. High-throughput evaluation of aryl hydrocarbon receptor-binding sites selected via chromatin immunoprecipitation-based screening in Hepa-1c1c7 cells stimulated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biosci Bioeng. 2009;108(4):277–281. doi: 10.1266/ggs.83.455. [DOI] [PubMed] [Google Scholar]
- 20.Kita Y, Ohto T, Uozumi N, Shimizu T. Biochemical properties and pathophysiological roles of cytosolic phospholipase A2s. Biochim Biophys Acta. 2006;1761:1317–1322. doi: 10.1016/j.bbalip.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Konkel A, Schunck W. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim Biophys Acta. 2011;1814:210–222. doi: 10.1016/j.bbapap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Kopec A, Burgoon L, Ibrahim-Aibo D, Burg A, Lee A, Tashiro C, Potter D, Sharratt B, Harkema J, Rowlands J, Budinsky R, Zacharewski T. Automated Dose-Response Analysis and Comparative Toxicogenomic Evaluation of the Hepatic Effects Elicited by TCDD, TCDF, and PCB126 in C57BL/6 Mice. Toxicol Sci. 2010;118(1):286–297. doi: 10.1093/toxsci/kfq236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopf P, Scott J, Agbor L, Boberg J, Elased K, Huwe J, Walker M. Cytochrome P4501A1 is Required for Vasuclar Dysfunction and Hypertension Induced by 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. Toxicol Sci. 2010;117(2):537–546. doi: 10.1093/toxsci/kfq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korashy H, El-Kadi A. The Role of Aryl Hydrocarbon Receptor in the Pathogenesis of Cardiovascular Diseases. Drug Metab Rev. 2006;38:411–450. doi: 10.1080/03602530600632063. [DOI] [PubMed] [Google Scholar]
- 25.Kubala L, Schmelzer K, Klinke A, Kolarova H, Baldus S, Hammock B, Eiserich J. Modulation of arachidonic and linoleic acid metabolites in myeloperoxidase-deficient mice during acute inflammation. Free Radical Biol Med. 2010;48:1311–1320. doi: 10.1016/j.freeradbiomed.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee C, Lawrence B, Kerkvliet N, Rifkind A. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induction of cytochrome P450-dependent arachidonic acid metabolism in mouse liver microsomes: evidence for species-specific differences in responses. Toxicol Appl Pharmacol. 1998;153(1):1–11. doi: 10.1006/taap.1998.8468. [DOI] [PubMed] [Google Scholar]
- 27.Li N, Liu JY, Qiu H, Harris TR, Sirish P, Hammock BD, Chiamvimonvat N. Use of metabolomic profiling in the study of arachidonic acid metabolism in cardiovascular disease. Congest Heart Fail. 2011;17(1):42–6. doi: 10.1111/j.1751-7133.2010.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin S, Yang Z, Liu H, Cai Z. Metabolomic analysis of liver and skeletal muscle tissues in C57BL/6J and DBA/2J mice exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol BioSyst. 2011;7:1956–1965. doi: 10.1039/c1mb05057e. [DOI] [PubMed] [Google Scholar]
- 29.Ma C, Marlowe J, Puga A. The aryl hydrocarbon receptor at the crossroads of multiple signaling pathways. EXS. 2009;99:231–257. doi: 10.1007/978-3-7643-8336-7_9. [DOI] [PubMed] [Google Scholar]
- 30.Nebert D, Karp L. Endogenous Functions of the Aryl Hydrocarbon Receptor (AHR): Intersection of Cytochrome P450 1 (CYP1)-metabolized Eicosanoids and AHR Biology. J Biol Chem. 2008;283(52):36061–36065. doi: 10.1074/jbc.R800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norwood S, Liao J, Hammock B, Yang G. Epoxyeicosatrienoic acids and soluble epoxide hydrolase: potential therapeutic targets for inflammation and its induced carcinogenesis. Am J Transl Res. 2010;2(4):447–457. [PMC free article] [PubMed] [Google Scholar]
- 32.Nukaya M, Moran S, Bradfield C. The role of the dioxin-responsive element cluster between the Cyp1a1 and Cyp1a2 loci in aryl hydrocarbon receptor biology. Proc Natl Acad Sci USA. 2009;106(12):4923–4928. doi: 10.1073/pnas.0809613106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nukaya M, Lin B, Glover E, Moran S, Kennedy G, Bradfield C. The Aryl Hydrocarbon Receptor-interacting Protein (AIP) Is Required for Dioxin-induced Hepatotoxicity but Not for the Induction of the Cyp1a1 and Cyp1a2 Genes. J Biol Chem. 2010a;285(46):35599–35605. doi: 10.1074/jbc.M110.132043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nukaya M, Walisser J, Moran S, Kennedy G, Bradfield C. Aryl Hydrocarbon Receptor Nuclear Translocator in Hepatocytes Is Required for Aryl Hydrocarbon Receptor-Mediated Adaptive and Toxic Responses in Liver. Toxicol Sci. 2010b;118(2):554–563. doi: 10.1093/toxsci/kfq305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oltman C, Weintraub N, VanRollins M, Dellsperger K. Epoxyeicosatrienoic Acids and Dihydroxyeicosatrienoic Acids are Potent Vasodilators in the Canine Coronary Microcirculation. Circ Res. 1998;83:932–939. doi: 10.1161/01.res.83.9.932. [DOI] [PubMed] [Google Scholar]
- 36.Pohjanvirta R, Korkalainen M, Moffat I, Boutros P, Okey A. The AH Receptor in Biology and Toxicology. John Wiley & Sons; New Jersey: 2011. Role of the AHR and its structure in TCDD toxicity; pp. 93–97. [Google Scholar]
- 37.Rifkind A. CYP1A in TCDD Toxicity and in Physiology-with Particular Reference to CYP Dependent Arachidonic Acid Metabolism and Other Endogenous Substrates. Drug Metab Rev. 2006;38:291–335. doi: 10.1080/03602530600570107. [DOI] [PubMed] [Google Scholar]
- 38.Schlezinger J, Parker C, Zeldin D, Stegeman J. Arachidonic Acid Metabolism in the Marine Fish Stenotomus chrysops (Scup) and the Effects of Cytochrome P450 1A Inducers. Arch Biochem Biophys. 1998;353(2):265–275. doi: 10.1006/abbi.1998.0651. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci USA. 1996;93(13):6731–6. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarz D, Kisselev P, Ericksen S, Szklarz G, Chernogolov A, Horneck H, Schnuck W, Roots I. Arachidonic and eicosapentaenoic acid metabolism by human CYP1A1: highly stereoselective formation of 17(R),18(S)-epoxiyeicosatetraenoic acid. Biochem Pharmacol. 2004;67:1445–1457. doi: 10.1016/j.bcp.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 41.Slim R, Hammock B, Toborek M, Robertson L, Newman J, Morisseau C, Watkins B, Saraswathi V, Hennig B. The role of methyl-linoleic acid epoxide and diol metabolites in the amplified toxicity of linoleic acid and polychlorinated biphenyls to vascular endothelial cells. Toxicol Appl Pharmacol. 2001;171(3):184–193. doi: 10.1006/taap.2001.9131. [DOI] [PubMed] [Google Scholar]
- 42.Smith A, Clothier B, Carthew P, Childs N, Sinclair P, Nebert D, Dalton T. Protection of the Cyp1a2 (−/−) Null Mouse against Uroporphyria and Hepatic Injury Following Exposure to 2,3,7,8-Tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 2001;173:89–98. doi: 10.1006/taap.2001.9167. [DOI] [PubMed] [Google Scholar]
- 43.Smith K, Pinkerton K, Watanabe T, Pedersen T, Ma S, Hammock B. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc Natl Acad Sci USA. 2005;102(6):2186–2191. doi: 10.1073/pnas.0409591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sulentic C, Kaminski N. The long Winding Road toward Understanding the Molecular Mechanisms for B-Cell Suppression by 2,3,7,8-Tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2010;120(S1):S171–S191. doi: 10.1093/toxsci/kfq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tijet N, Boutros P, Moffat I, Okey A, Tuomisto J, Pohjanvirta R. Aryl Hydrocarbon Receptor Regulates Distinct Dioxin-Dependent and Dioxin-Independent Gene Batteries. Mol Pharmacol. 2005;69:140–153. doi: 10.1124/mol.105.018705. [DOI] [PubMed] [Google Scholar]
- 46.Uno S, Dalton T, Sinclair P, Gorman N, Wang B, Smith A, Miller M, Shertzer H, Nebert D. Cyp1a1 (−/−) male mice: protection against high-dose TCDD-induced lethality and wasting syndrome, and resistance to intrahepatocyte lipid accumulation and uroporphyria. Toxicol Appl Pharmacol. 2004;196:410–421. doi: 10.1016/j.taap.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 47.Vogel C, Schuhmacher U, Degen G, Bolt H, Pineau T, Abel J. Modulation of Prostaglandin H Synthase-2 mRNA Expression by 2,3,7,8-Tetrachlorodibenzo-p-dioxin in Mice. Arch Biochem Biophys. 1998;352(2):265–271. doi: 10.1006/abbi.1997.0555. [DOI] [PubMed] [Google Scholar]
- 48.White S, Birnbaum L. An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. J Environ Sci Health, Part C. 2009;27(4):197–211. doi: 10.1080/10590500903310047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, Schmelzer K, Georgi K, Hammock B. Quantitative Profiling Method for Oxylipin Metabolome by Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry. Anal Chem. 2009;81(19):8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshizawa K, Heatherly A, Malarkey D, Walker N, Nyska A. A Critical Comparison of Murine Pathology and Epidemiological Data of TCDD, PCB126, and PeCDF. Toxicol Pathol. 2007;35:865–879. doi: 10.1080/01926230701618516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeldin D. Epoxygenase Pathways of Arachidonic Acid Metabolism. J Biol Chem. 2001;276(39):36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.