Abstract
3,3′,4,4′,5-Pentachlorobiphenyl (PCB 126), an aryl hydrocarbon receptor (AhR) agonist and most potent dioxin-like PCB congener, significantly alters gene expression, lipid metabolism, and oxidative stress in the liver. PON1, an antioxidant and anti-atherogenic enzyme, is produced in the liver and secreted into the blood where it is incorporated into high density lipoprotein (HDL) and protects LDL and cellular membranes against lipid peroxidation. To explore the regulation of PON1, male Sprague-Dawley rats were treated with ip injections of 0, 1 or 5 μmol/kg PCB 126 and euthanized up to two weeks afterwards. Serum total and HDL-cholesterol were increased by low dose and decreased by high dose exposure, while LDL-cholesterol was unchanged. PCB 126 significantly increased hepatic PON1 gene expression and liver and serum PON1 activities. Liver and serum thiobarbituric acid reactive substances levels were not elevated except for high dose and long exposure times. Serum antioxidant capacity was unchanged across all exposure doses and time points. This study, the first describing the regulation of gene expression of PON1 by a PCB congener, raises interesting questions whether elevated PON1 is able to ameliorate PCB 126-induced lipid peroxidation and whether serum PON1 levels may serve as a new biomarker of exposure to dioxin-like compounds.
Keywords: Paraoxonase 1, PCB 126, TBARS, rat, liver, AhR, plasma lipids
1. Introduction
Polychlorinated biphenyls (PCBs) are persistent organic pollutants and ubiquitous in the environment even though their commercial production has been banned for decades. Many of the 209 individual PCB congeners are highly resistant to biotransformation and bioaccumulate. Certain congeners with non-ortho or mono-ortho chlorine substitution are called “dioxin-like PCBs” because their binding to the arylhydrocarbon receptor (AhR) and biochemical and toxic activities are similar to those of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Bandiera et al., 1982; Hestermann et al., 2000). PCB 126 is the most potent AhR agonist among all PCB congeners tested (Bandiera et al., 1982) and has a toxic equivalency factor (TEF) of 0.1 compared to TCDD, which qualifies it as the most toxic PCB in this family (Van den Berg et al., 2006). Like TCDD, PCB 126 significantly alters the expression of numerous genes and increases enzyme activities, particularly CYP 1A1/2 (Kopec et al., 2010). PCBs also increase intracellular oxidative stress which has been associated with a variety of disease processes, including carcinogenesis and cardiovascular damage (Hennig et al., 2002; Hennig et al., 2005; Ludewig et al., 2008). Several studies have shown that the dioxin-like PCBs (e.g., PCB77 and PCB 126) induce ROS and cause lipid oxidation in endothelial cells which is associated with atherosclerosis (Hassoun et al., 2002; Choi et al., 2009).
Paraoxonase 1 (PON1), a member of a three gene family which includes PON2 and PON3, is a calcium-dependent hydrolase with substrate specificity toward esters, phosphotriester lactones, carbonates and other compounds (Costa et al., 2005a; Costa et al., 2005b). PON1 is primarily synthesized in the liver and secreted into the blood as a major high density lipoprotein (HDL)-associated enzyme (La Du et al., 1999). Besides chemoprotection through detoxification of organophosphorus (OP) pesticides, it displays preventive properties against cardiovascular disease, mainly through removal of oxidized lipids in low density lipoproteins and cell membranes (Furlong et al., 2005; Furlong et al., 2010).
Recently, XRE- (xenobiotic response element)-like sequences were described in the promoter region of PON1 and these sequences were identified as possible binding sites for the activated AhR (Gouedard et al., 2004). In this publication dietary polyphenols and 3-methylcholanthrene (3-MC) were shown to induce PON1 through the AhR-dependent pathway. We therefore hypothesized that PCB 126, as an AhR agonist, may influence PON1 expression and thereby hepatic and serum PON1 activities, a possible protection mechanism against PCB-induced ROS. Here we show that PCB 126 increased hepatic PON1 expression and liver and serum PON1 activity as well as serum HDL levels and hepatic CYP1A1 and AhR transcription in male Sprague Dawley rats in vivo, while no consistent change in lipid peroxides (TBARS) and serum total antioxidant activity was observed.
2. Materials and methods
2.1. Chemicals and reagents
PCB 126 was synthesized and purified using a modified Suzuki-coupling method of 3,4,5-trichlorobromobenzene with 3,4-dichlorophenyl boronic acid utilizing a palladium-catalyzed cross coupling reaction as previously reported (Luthe et al., 2009). All reagents were obtained from Fischer Scientific (Pittsburgh, PA) and were the highest purity available, if not stated otherwise.
2.2. Animals and treatment protocol
Male Sprague-Dawley rats (75–100 g) from Harlan Laboratories, Inc (Indianapolis, IN) were housed in a controlled environment maintained at 22 °C with a 12 hour dark-light cycle with food and water available ad libitum. In the dose-response study, animals were divided into 3 groups of 3 rats each, received a single ip injection of either corn oil (control), or 1 or 5 μmol/kg PCB 126, and were euthanized two weeks after the injection. In the time-course study, rats were divided into 2 groups of 6 animals each and received 2 ip injections (days 1 & 3) of either corn oil (control) or 1 μmol/kg of PCB 126, resulting in a total dose of 2 μmol/kg in the PCB group. Half of the animals in each group were sacrificed one week, the remaining animals two weeks after the first injection. The doses of PCB 126 and exposure time periods were chosen based on a previous study in which a 1 μmol/kg dose of PCB 126 was shown to elicit mild fatty liver (Lai et al., 2010). All animals were euthanized by carbon dioxide asphyxiation and cervical dislocation. The animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Whole blood was stored at 4°C immediately after collecting and coagulation and serum separation were performed at 4°C to minimize any artificial increase in oxidized lipids during processing. Sera were stored at −80 °C until serum endpoints measurement were performed. Livers were removed immediately and a part of them was quickly frozen in liquid nitrogen for later RNA isolation, while the rest was homogenized in Tris-KCl buffer (20mM Tris and 1.15% KCl, ph 7.4) to produce an approximate 10% (w/v) liver homogenate. All liver samples were stored at −80 °C until further use.
2.3. Analysis of serum lipid profile
Rat serum samples were used to measure total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C) using a lipid assay kit from BioVision Inc (Mountain View, CA). Briefly, rat serum was separated into HDL and LDL fractions according the manufacture’s protocol for analysis of HDL-C and LDL-C, respectively. TC was determined in serum directly.
2.4. Measurement of liver and serum PON1 activity
Paraoxon and phenylacetate were used as two individual substrates in PON1 activity measurements. Briefly, the enzyme activities were determined spectrophotometrically by following the initial rate of substrate hydrolysis to p-nitrophenyl (412nm) or phenol (270nm), respectively, in 1000 μl assay mixture (100mM Tris-HCl, 2.0mM CaCl2, 2.0 mM paraoxon or 4.0 mM phenylacetate, pH 8.0) at 25°C. A blank sample containing the assay buffer without sample was run simultaneously to correct for spontaneous substrate breakdown. The units (U) of enzyme activity were calculated from the molar extinction coefficients, E412 (18,290 M−1cm−1) and E270 (1310 M−1cm−1), respectively, and expressed as U/ml serum or U/mg protein in liver homogenate. Each unit of enzyme is defined as that hydrolyzing 1 nmol of paraoxon or 1 μmol of phenylacetate per minute (Beltowski et al., 2005). The protein concentration in liver homogenates was determined using the Bradford protein assay regent (Bio-Rad Laboratories, Inc CA).
2.5. Gene expression analysis
Total RNA was isolated from rat liver samples using the RNeasy Mini Kit from Qiagen, Inc (Valencia, CA) according to the manufacture’s protocol. RNA concentration and purity was determined by spectrophotometric measurement of A260/A280. cDNA templates were generated using 1 μg of total RNA per 20-μL reaction and a High-Capacity cDNA Reverse Transcription Kit from Applied Biosystems, Inc (Foster City, CA) according to the manufacture’s protocol.
Real-time PCR was performed in a 20-μL reaction with 4 ng of cDNA template and 900 nM primer using a SYBR Green Master Mix kit from Applied Biosystems, Inc (Foster City, CA) according to the manufacture’s protocol. The primers used here were taken from previous publications as indicated below and synthesized by Integrated DNA Technologies, Inc (Coralville, IA). The specificity of these primers was further verified by the Primer-BLAST online program provided by National Library of Medicine. The primer sequences are: PON1: forward 5′TGCTGGCTCACAAGA TTCA C3′, reverse 5′TTCCTTTGTACACAGCAGCG3′ (Varatharajalu et al., 2009); RPL13a: forward 5′CCC TCCACCCTATGACAAGA3′, reverse 5′ CCTTTT CCTTCCGTTTCTCC3′ (Gaub et al., 2010); ApoA1: forward 5′CCTGGATGAATTCCAGGAGA′3, reverse 5′ TCGCTGTAGAGCCCAAACTT′3 (Bettzieche et al., 2009); AhR: forward 5′ GGGCCAAGAG CTTCTTTGATG′3, reverse 5′GCAAGTCCTGCCAGTC TCTGA′3 (Shipley and Waxman, 2006); CYP1A1: forward 5′ ATGTCCAGCTCTCAGATGATAAGGTC′3, reverse 5′ATCCCTGCCAATCACTGTGTCTAAC′3 (Vondracek et al., 2006).
The PCR program used started with 95° for 10 min followed by 40 cycles of 95° for 30s, 55° for 30s and 72° for 1 min performed on an Eppendorf RealPlex2 Mastercycler® (Hamburg, Germany). A melting curve analysis was carried out after the reaction to check for false amplification caused by primer dimmers, non-specific binding or other contamination. The relative gene expression levels were calculated using the relative standard curve method. The target gene expression levels were adjusted to the house keeping gene, ribosomal protein L13a (RPL13a). Results are presented as fold change which was calculated by dividing the adjusted expression level of each target gene in the treatment group with the expression level of the gene in the corn oil control group.
2.6. Measurement of liver and serum TBARS levels
The level of thiobarbituric acid reactive substances (TBARS) expressed in terms of malondialdehyde (MDA) was used as index of liver and serum lipid peroxidation and performed according to the methods described previously (Ohkawa et al., 1979; Yagi, 1998). Briefly, liver homogenate or the serum lipid fraction were incubated with thiobarbituric acid at 95 °C for 60 minutes and the red pigment produced in the reaction was extracted using n-butanol or a n-butanol-pyridine mixture. The pigment concentration was determined spectrophotometrically (at 535nm, liver samples) or spectrofluorometrically (at excitation 515nm and emission 553nm, serum samples).
2.7. Determination of total serum antioxidant capacity
The total serum antioxidant capacity was evaluated by measuring the ferric reducing ability of serum as described (Benzie and Strain, 1996). This method determines the capacity of antioxidants contained in the sample to reduce ferric-tripyridyltriazine (Fe3+-TPTZ) to a ferrous form (Fe2+) which is then used as putative index of antioxidant or otherwise reducing potential in the sample. Results are expressed as μmol/L.
2.9. Statistics
All data are expressed as means ± SEM. Statistical analysis of the time-course study was performed by two-way ANOVA to evaluate the factors of time and PCB 126 as well as the interaction effect. One-way ANOVA was used for each individual factor (time or PCB 126) in the time-course and dose-response study. All analyses were carried out by General Linear Models (GLM) in SAS 9.2, P<0.05) followed by Dunnett’s T comparison test. A P-value of <0.05 was considered to be statistically significant. Pearson correlation analysis was used to establish the relationship between various endpoints measured in this study.
3. Results
3.1. Biometrical parameters
In the dose-response study (Table 1, upper half) the growth of rats treated with the high dose (5 μmol/kg) of PCB 126 was significantly decreased (P<0.05) and the final body weights were decreased by 31% (P<0.05). Liver weights were significantly increased by the lower dose of 1μmol/kg PCB 126 (46%, P<0.05), and both doses significantly increased the relative liver weight (51% and 54%, respectively). In the time-response study (Table 1, lower half) weight gain of PCB 126-treated rats was significantly decreased from the early time point onwards (P<0.05) with significant decreases in final body weights visible at the late time point (2 weeks). PCB 126 increased both the absolute liver weights and relative liver weights at both time points compared to the corresponding corn oil controls, an effect that was more pronounced at the later time point (34% vs 23% for liver weight and 49% vs 31% for relative liver weight after 2 and 1 week exposure, respectively).
Table 1.
Growth, body and organ weights In rats treated with PCB 126 compared to the corn oil control group two weeks after injection (upper) or at different time points after receiving a total of 2 umol/kg PCB126
|
Dose-Response PCB126 (μmol/kg) |
Body weight gain (%) | Final body weight (g) | Final liver weight (g) | Liver/body weight (%) |
|---|---|---|---|---|
| Corn oil | 26.1 ±1.0 | 325 ±11 | 13.2 ±0.2 | 4.1 ±0.1 |
| 1 | 21.5 ±5.9 | 312 ±8a | 19.3 ±1.1a | 6.2 ±0.4a |
| 5 | −8.1 ±0.4a,b | 225 ±10a,b | 14.0 ±0.6 | 6.3 ±0.1a |
|
| ||||
|
Time-Course Time after injection |
Body weight gain (%) | Final body weight (g) | Final liver weight (g) | Liver/body weight (%) |
|
| ||||
| Corn oil – 1 week | 21.3 ±2.0 | 209 ±7 | 9.3 ±0.5 | 4.5 ±0.2 |
| PCB126 – 1 week | 8.3 ±0.2a | 195 ±4 | 11.4 ±0.1a | 5.9 ±0.1a |
|
| ||||
| Corn oil – 2 weeks | 55.1 ±1.6 | 268 ±6 | 11.1 ±0.7b | 4.1 ±0.2 |
| PCB126 – 2 weeks | 37.6 ±2.6a | 243 ±2a | 14.9 ±0.4a | 6.1 ±0.1a |
Results are expressed as mean ± SEM (n=3).
Statistically significant differences
between PCB 126 and corresponding control, and
between low and high dose of PCB 126; P<0.05
3.2. Serum lipid profile changes during PCB 126 treatments
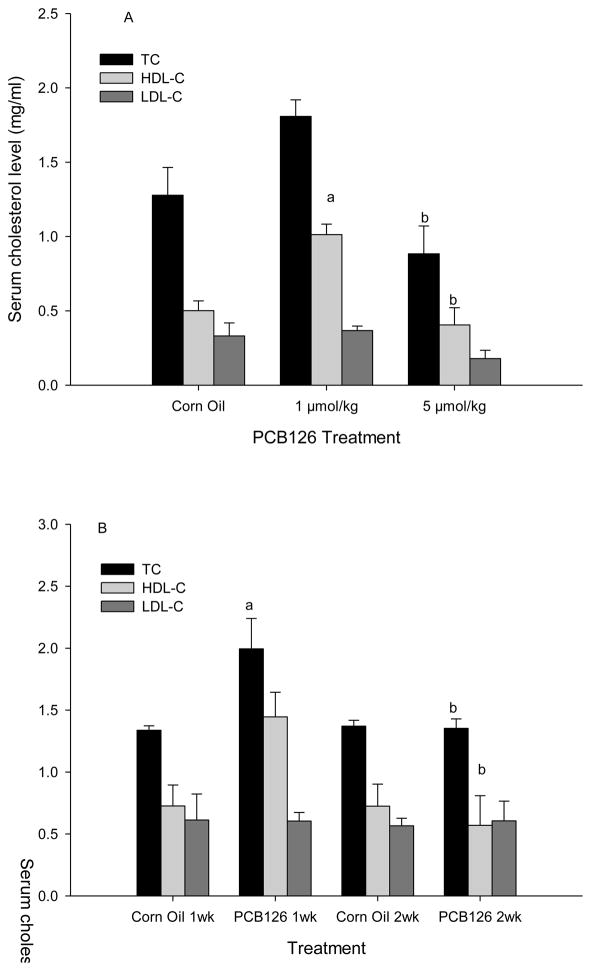
In the dose-response study a significant increase in HDL-C was seen with the 1 μmol/kg dose (Fig 1A). Total cholesterol was slightly increased with the low dose (1 μmol/kg), but decreased with the high dose (5 μmol/kg) compared to the corn oil control; these changes were non-significant, but high enough to produce a statistical difference between the two doses (Fig 1A). Similarly, in the time-course study (two 1 μmol/kg injections within 3 days) a non significant increase in HDL-C after 1 week and decrease after 2 weeks compared to the control were observed, resulting in a significant difference between 1 and 2 week exposure to PCB 126. In addition a transient, significant increase in total cholesterol after 1 week exposure compared to the control and week 2 was visible (Fig 1B). No effect of PCB 126 on LDL-C was seen in either assay. These changes resulted in higher HDL-C/TC and HDL-C/LDL-C ratios with the low (1 μmol/kg) dose of PCB 126 and the 1 week time point with two 1 μmol/kg injections, but these increases did not reach significance (Supplemental Table 1). The two-way ANOVA analysis (Supplemental Table 2) suggests that both, time and PCB, have no significant overall effects on HDL-C and LDL-C, but that both had a significant overall and interactive effect on total cholesterol (TC) levels.
Fig. 1.
Serum lipid profile 2 weeks after a single ip injection of PCB 126 (A) and 1 or 2 weeks after the 1st of 2 injections of 1 umol/kg PCB 126 (B). Results are expressed as mean ± SEM (n=3). Statistically significant differences a between PCB 126 and corresponding control, and b between low and high dose of PCB 126 or 1st and 2nd week; P<0.05
3.3. Serum and liver PON1 activities in control and PCB 126-treated animals
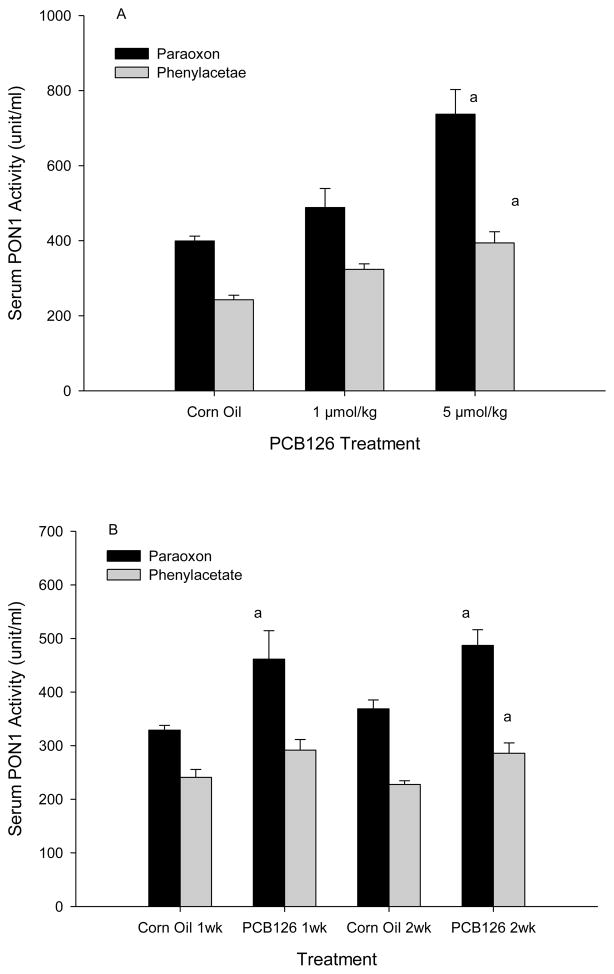
PON1 activities in serum were measured with 2 different substrates, paraoxon (paraoxonase activity) and phenylacetate (arylesterase activity of PON1). Serum PON1 paraoxonase activity in corn oil treated control animals was 399 and 349 and the arylesterase activity was 242 U/ml and 234 U/ml in the dose-response and time-response study, respectively (Fig 2). PCB 126 increased PON1 activity in serum which was significant with 5 μmol/kg where PON1 activity was nearly doubled compared to the control. A clear linear dose-dependent pattern was visible with a R2 of 0.99 with phenylacetate as substrate (Fig 2A). The twice 1 μmol/kg-treatment in the time-response study (Fig 2B) produced a significant increase with paraoxon at both and phenylacetate at the later time point. No significant change in PON1 activity was seen between week 1 and week 2 in controls or in PCB 126 animals. Accordingly, as shown in the two-way ANOVA analysis (Supplemental Table 2), time did not significantly affect serum PON1 activity overall.
Fig 2.
Serum PON1 activity from does-response study (A) and time-Course study (B)
Results are expressed as mean ± SEM (n=3). a significant difference between PCB 126 and corresponding control, P<0.01
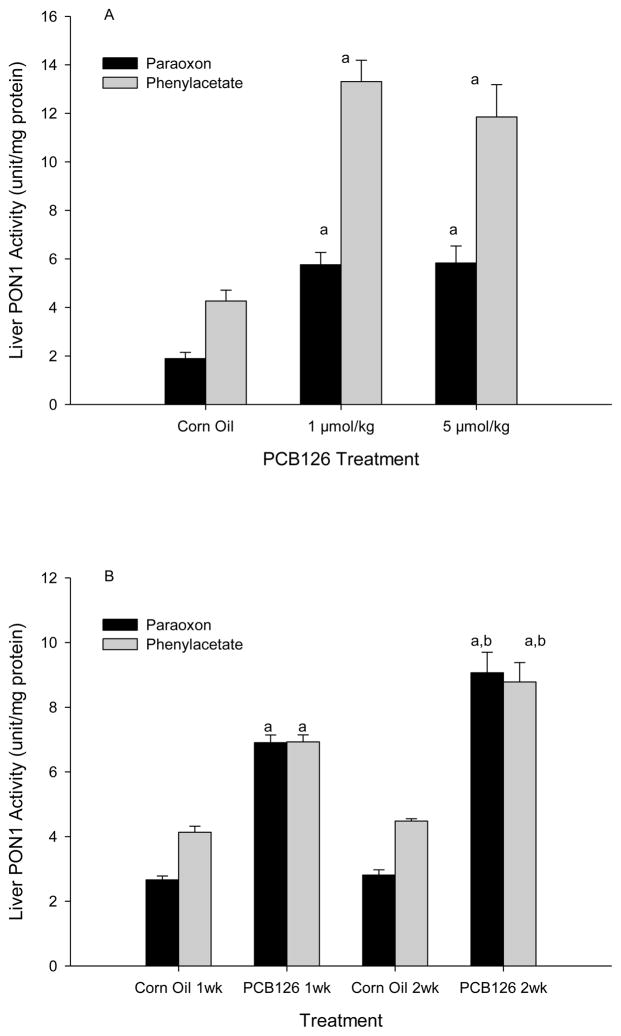
The hepatic PON1 levels in corn oil controls were about 2.4 and 4.3 U/mg with paraoxon and phenylacetate, respectively. Liver PON1 activities tripled due to PCB 126-exposure. No dose-response effect was seen (Fig 3A), but a time-dependent increase was visible (Fig 3B), which is reflected in the significant overall effect of both factors, time and PCB 126, in the time-course study (Supplemental Table 2).
Fig 3.
Liver PON1 Activity from does-response study (A) and time-course study (B) Results are expressed as mean ± SEM (n=3)
a significant difference between PCB 126 and control, P<0.01 and b between 1 week and 2 weeks of exposure, P<0.05
3.4. Direct effects of PCB 126 on serum PON1 paraoxon activity
To analyze whether PCB 126 can directly affect PON1 activity in the serum, we incubated serum samples from a healthy, unexposed rat with 1 μM PCB 126 (stock solution in DMSO, 1% final concentration in serum) in vitro for various lengths of time (0, 5, 10, 20 min) at 37 °C followed by spectrophotometrical PON1 activity determination with paraoxon as substrate. No significant change in PON1 activity was observed at any time points compared to the DMSO vehicle control and untreated serum (Supplemental Fig 1).
3.5. Effect of PCB 126 on hepatic gene expression
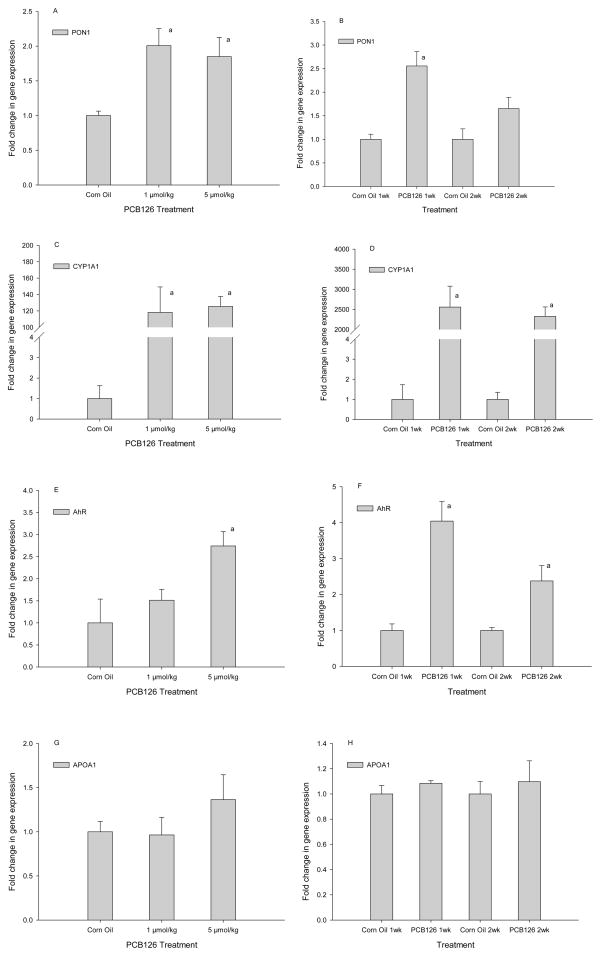
Hepatic gene expression analysis by RT-PCR showed a doubling of PON1 mRNA with both doses, 1 and 5 μmol/kg PCB 126 (Fig 4A). This effect was even stronger in the time-response study after 1 week of exposure, but lower and no longer significant in the second week (Fig 4B). As expected, CYPIAI mRNA was increased by PCB 126 more than 100-fold (dose-response study) and even more than 2000-fold (time-course study) (Fig 4C, D); this effect was also not dose-dependent and only a small, non-significant reduction was seen with time. PCB 126 also increased the amount of AhR mRNA 2 to-4-fold; a statistically significant dose effect and reduction with time was visible (Fig 4E, 4F). The mRNA level of ApoA1, a PON1 stabilizing protein, was higher with 5 μmol/kg PCB 126 than in controls, but this change was not significant (Fig 4G, 4H). No changes were observed with lower concentrations of PCB 126.
Fig 4.
Hepatic gene expression of PON1 (A, B), CYP1A1(C, D), AhR (E,F), ApoA1(G,H). Results are mean ± SEM (n=3); a indicates significant differences to control, P<0.05
3.6. Correlation of the various parameters
A Pearson analysis of all data revealed that indeed the hepatic PON1 activity and mRNA levels as well as PON1 and CYP1A1mRNA levels were highly significantly correlated (Table 3). In the time-response study AhR mRNA levels were also significantly correlated to PON1 and CYP1A1 mRNA levels (Table 2, Figure 4). ApoA1 expression was not correlated with any other factor.
Table 3.
TBARS and Antioxidant Capacity: Dose- Response and Time-Course
|
Dose-Response PCB126 (μmol/kg) |
Liver TBARS (nmol/100mg protein) | Serum TBARS (nmol/ml) | Serum Antioxidant Capacity(μmol/l) |
|---|---|---|---|
| Corn oil | 297 ±17 | 3.0 ±0.3 | 723 ±107 |
| 1 | 389 ±14a | 3.7 ±0.2 | 765 ±201 |
| 5 | 305 ±23 | 4.1 ±0.1a | 783 ±94 |
|
| |||
|
Time-Course Time after injection |
Liver TBARS (nmol/100mg protein) | Serum TBARS (nmol/ml) | Serum Antioxidant Capacity(μmol/l) |
|
| |||
| Corn oil – 1 week | 397 ±39 | 6.1 ±0.6 | 881 ±42.9 |
| PCB126 – 1 week | 339 ±58 | 6.9 ±0.2 | 923 ±97.7 |
| Corn oil – 2 weeks | 237 ±32 | 5.9 ±0.2 | 804 ±50.4 |
| PCB126 – 2 weeks | 560 ±93a,b | 5.0 ±0.3b | 763 ±94.3 |
Results are expressed as mean ± SEM (n=3).
Statistically significant differences
between PCB 126 and corresponding control, and
between 1 and 2 weeks of exposure to PCB 126; P<0.05
Table 2.
Pearson correlation analysis
| AhR | CYP1A1 | ApoA1 | PON1 | PON1LPX | PON1LPH | PON1SPX | PON1SPH | |
|---|---|---|---|---|---|---|---|---|
| Dose-response-- | ||||||||
|
| ||||||||
| AhR | -- | 0.63 | 0.62 | 0.43 | 0.63 | 0.49 | 0.85 a | 0.81 a |
| CYP1A1 | -- | -- | 0.35 | 0.81 a | 0.88 a | 0.89 a | 0.68 a | 0.85 a |
| ApoA1 | -- | -- | -- | −0.07 | 0.13 | 0.01 | 0.71 | 0.58 |
| PON1 | -- | -- | -- | -- | 0.90 a | 0.87 a | 0.51 a | 0.69 a |
| PON1LPX | -- | -- | -- | -- | -- | 0.96 a | 0.62 | 0.72 a |
| PON1LPH | -- | -- | -- | -- | -- | -- | 0.48 | 0.64 |
| PON1SPX | -- | -- | -- | -- | -- | -- | -- | 0.94 a |
| PON1SPH | -- | -- | -- | -- | -- | -- | -- | -- |
|
| ||||||||
| Time-course | ||||||||
|
| ||||||||
| AhR | -- | 0.91 a | 0.27 | 0.86 a | 0.78 a | 0.75 a | 0.65 a | 0.65 a |
| CYP1A1 | -- | -- | 0.34 | 0.91 a | 0.80 a | 0.74 a | 0.82 a | 0.81 a |
| ApoA1 | -- | -- | -- | 0.34 | −0.04 | −0.11 | 0.17 | 0.50 |
| PON1 | -- | -- | -- | -- | 0.63 a | 0.59 a | 0.77 a | 0.72 a |
| PON1LPX | -- | -- | -- | -- | -- | 0.99 a | 0.76 a | 0.67 a |
| PON1LPH | -- | -- | -- | -- | -- | -- | 0.72 a | 0.62 a |
| PON1SPX | -- | -- | -- | -- | -- | -- | -- | 0.89 a |
| PON1SPH | -- | -- | -- | -- | -- | -- | -- | -- |
Value denotes the Pearson correlation coefficient r,
indicates a statistically significant correlation (p<0.05)
Abbreviation: PON1LPX (liver PON1 activity towards paraoxon), PON1LPH (liver PON1 activity towards phenylacetate), PON1SPX (serum PON1 activity towards paraoxon), PON1SPH (serum PON1 activity towards phenylacetate)
3.7. Liver and serum lipid peroxidation and serum antioxidant capacity
Hepatic and serum TBARS levels, expressed in terms for MDA, and serum antioxidant capacity, measured as the ferric reducing ability, are shown in Table 3. In the dose-response study a significant increases (P<0.05) in TBARS level was seen in 1μmol/kg exposed animals in the liver and in 5μmol/kg PCB 126-treated rats in the serum. A marginal dose-dependent but not significant increase of serum antioxidant capacity was seen. In the time-course study, a significant increase in hepatic TBARS after 2 weeks exposure to PCB 126 compared to the corn oil control and the PCB 126-1 week group was observed. The only change in serum TBARS was a decrease in the 2-week vs 1-week PCB-exposed group. These results agree with the two-way ANOVA analysis (Supplemental Table 2) which shows no significant overall effect of time or PCB 126 on liver TBARS but a significant effect of time of exposure on serum TBARS and a significant interaction effect on liver and serum TBARS. No significant changes were seen in the serum antioxidant capacity in either study (Table 3). However, the sensitivity of these endpoints may be very low due to the small number of animals (n=3) in each group, as the difference between the two corn oil groups in TBARS and serum antioxidant capacity levels and the large SEM indicate.
4. Discussion
Recently, researchers reported that PCB 126, the most potent dioxin-like PCB congener and a conventional food chain persistent organic pollutant, was found in Chicago air (Zhao et al., 2010). This suggests a new exposure source besides the food chain. PCB 126 was shown to induce oxidative stress and cause lipid peroxidation (Hassoun et al., 2002). PON1 is believed to protect against lipid peroxidation and to lower the risk of developing coronary artery disease and atherosclerosis (Shih et al., 1998; Furlong et al., 2000; Costa et al., 2005a). The goal of this study was to investigate the effects of PCB 126 on PON1 and the associated risk of lipid peroxidation.
Our results supported the hallmark toxic changes in response to TCDD and dioxin-like compounds, increase of liver weight and decreases of body weight gain (Denison and Heath-Pagliuso, 1998). Another hallmark is oxidative stress, which should elevate TBARS levels. Total antioxidant capacity, an index of the overall antioxidant environment in serum, should theoretically be inversely correlated to the level of serum lipid peroxides. On the other hand, PON1 is known to remove and prevent lipid peroxidation, which should lower TBARS and preserve the total serum antioxidant capacity. We were interested to see whether and how PCB 126 would influence these interrelated parameters. However, our TBARS results are difficult to interprete since it is not known to which extent the PCB treatment increased oxidative stress in these animals and/or to which extend is was counteracted by PON1.
PCBs have been shown to cause an elevation of serum total cholesterol (TC) and high density lipoprotein cholesterol (HDL-C) (Quazi et al., 1983). We observed a significant elevations of serum TC and HDL-C levels by the low dose PCB 126 exposure while the low density lipoprotein cholesterol (LDL-C) level was not changed. With the high dose or long time exposure both total and HDL-C levels were significantly decreased compared to the low dose or 1 week exposure, probably because the hepatic synthesis of cholesterol was impaired due to liver damage. The increase in total cholesterol, mediated by elevation of HDL-C, commonly known as ‘good cholesterol’, could be beneficial with respect to cardiovascular disease and atherosclerosis due to its association with PON1 (Aviram, 2006; Kaur and Bansal, 2009; Ferretti et al., 2010).
PON1 has been shown to protect both HDL and LDL against oxidation, which is considered a trigger of atherosclerosis (Aviram et al., 1998; Aviram and Rosenblat, 2005; Mackness and Mackness, 2010). Low levels of PON1 activity is a risk factor for cardiovascular disease (Aviram, 2006). Numerous factors can affect PON1 activity including oxidative stress (Franco-Pons et al., 2008). Since PCB 126 is known to increase oxidative stress, we expected to find a decrease in PON1 activity, but the opposite emerged: PCB 126 significantly increased hepatic and serum PON1 activities towards both substrates. Our in vitro experiment showed that this effect was not due to a direct effect of PCB 126 on PON1 activity. In vivo liver PON1 activity tripled and serum PON1 activity increased dose-dependently up to 100% with PCB 126 exposure. The dose-related ceiling of PON1 activity in the liver may be due to the onset of liver toxicity with the high dose of PCB 126 as indicated by the decreased cholesterol secretion and the biometric findings, or by the limits of gene induction. PON1 is synthesized in the liver and partly secreted into the blood where it is associated to HDL. No difference in serum PON1 between 1st and 2nd week of exposure was observed, suggesting that PON1 secretion was an early response to PCB exposure. Interestingly, no linear relationship between serum HDL-C and PON1 activity was found, indicating that binding of these two components is not a limiting factor.
To elucidate the mechanism of the increase in PON1 activity, we determined the hepatic levels of PON1 mRNA as well as those of CYP1A1, AhR and ApoA1. We observed a more than doubling in PON1 mRNA levels and, similar to the induction of CYP1A1 transcription, the highest levels was already reached with the low dose of PCB 126. Indeed, according to the Pearson calculations, CYP1A1 transcription correlated with PON1 transcription and PON1 activities, and PON1 mRNA correlated with PON1 activities. This suggests that the increase of PON1 activity was due to increases PON1 gene transcription which was up-regulated by the PCB 126 exposure. It was report that rat hepatic PON1 mRNA was increased by TCDD treatment (Boverhof et al., 2006) and hepatic PON1 protein levels were increased in rats treated with 3-MC (Rodrigo et al., 2001). PCB 126, 3-MC and TCDD bind to the AhR; the ligand-AhR complex is then translocated to the nucleus where it binds to ARNT and the ligand/AHR/ARNT complex binds to the XRE (xenobiotic response element) sequence in the promoter region of specific genes, resulting in their up regulation (Abel and Haarmann-Stemmann, 2010). The PON1 gene promoter does not have an XRE core sequence (GCGTG), but it has several so called XRE-like sequences (GCGGG). It was shown that the induction of PON1 transcription was AhR dependent and the localization of the AhR\ARNT complex was in the XRE-like sequence (Gouedard et al., 2004). Thus it is most likely that the up regulation of PON1 gene transcription by PCB 126 is also mediated by the AhR through binding to XRE-like sequences, resulting in increased PON1 activities in the liver and serum.
Interestingly, the mRNA of AhR was also significantly increased by PCB 126 treatments and the induction pattern is qualitatively and quantitatively similar to that of PON1. Similarly PCB77, another AhR agonist, was reported to increase the AhR mRNA (Mortensen and Arukwe, 2008), and aspirin caused an AHR-dependent upregulation of both, PON1 and AhR gene expression, in HepG2, primary rat hepatocytes, and mice in vivo (Jaichander et al., 2008). On the other hand, in our time-course study CYP1A1 gene remained upregulated whereas the AhR and PON1 transcription was only transiently increased. One possible explanation may be that the XRE consensus sequence and XRE-like sequence have different binding intensity to the PCB 126-AhR complex as described previously for other ligands (Gouedard et al., 2004). In addition, the overall efficiency and time course of gene transcription may also involve different recruitment of co-factors to the complex. The underlying mechanism needs to be further investigated. Independently of this, the induction of AhR gene expression may increase the chance for the ligand-receptor complex to be formed and therefore an increase in target gene transcription.
Another component of HLD-C is apolipoprotein A1 (ApoA1). ApoA1 was reported to stabilize the PON1-HDL complex (Gaidukov and Tawfik, 2005). Also, a combined down-regulation of PON1 and ApoA1 was observed in murine hepatocytes (Han et al., 2006) and Aroclor 1248, but not 3-MC and phenobarbitol caused an increase in ApoA1 hepatic mRNA and serum levels in rats (Oda et al., 2000). In contrast, no significant change of ApoA1 mRNA was seen in our studies, although a non-significant elevation in mRNA levels was observed with the high dose of PCB 126. This discrepancy deserves further evaluation, but shows that ApoA1 may not be a limiting factor in PCB 126-increased PON1 regulation and activity.
In summary, this is the first report of the regulation of PON1 status by PCB 126 exposure. Our results show that PCB 126-treatment increases liver and serum PON1 activities through the up-regulation of PON1 gene expression. This increase in PON1 activity, a protective antioxidant enzyme, may ameliorate oxidative stress induced by dioxin-like PCB congeners or other compounds. Also, serum PON1 activity may be useful in future studies as biomarker of exposure to dioxin-like compounds.
Supplementary Material
Highlights.
PCB126 is a strong AhR agonist and reported to increase oxidative stress
PON1 is an important serum antioxidant, produced in the liver and attached to HDL
In rats PCB126 increased hepatic PON1 mRNA and hepatic and serum PON1 activity
No changes in lipid peroxides (TBARS) or total serum antioxidant capacity were seen
Increased PON1 activity may ameliorate oxidative stress produced by PCB126
Acknowledgments
Funding
This study is supported by NIEHS P42 ES013661 and DOD DAMD17-02-1-0241.
The authors would like to thank the members of the Robertson and Ludewig laboratories for help with animal treatments and necropsy.
Footnotes
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel J, Haarmann-Stemmann T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol Chem. 2010;391:1235–1248. doi: 10.1515/BC.2010.128. [DOI] [PubMed] [Google Scholar]
- Aviram M. HDL--associated paraoxonase 1 (PON1) and dietary antioxidants attenuate lipoprotein oxidation, macrophage foam cells formation and atherosclerosis development. Pathophysiol Haemost Thromb. 2006;35:146–151. doi: 10.1159/000093558. [DOI] [PubMed] [Google Scholar]
- Aviram M, Rosenblat M. Paraoxonases and cardiovascular diseases: pharmacological and nutritional influences. Curr Opin Lipidol. 2005;16:393–399. doi: 10.1097/01.mol.0000174398.84185.0f. [DOI] [PubMed] [Google Scholar]
- Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest. 1998;101:1581–1590. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandiera S, Safe S, Okey AB. Binding of polychlorinated biphenyls classified as either phenobarbitone-, 3-methylcholanthrene- or mixed-type inducers to cytosolic Ah receptor. Chem Biol Interact. 1982;39:259–277. doi: 10.1016/0009-2797(82)90045-x. [DOI] [PubMed] [Google Scholar]
- Beltowski J, Jamroz-Wisniewska A, Borkowska E, Wojcicka G. Differential effect of antioxidant treatment on plasma and tissue paraoxonase activity in hyperleptinemic rats. Pharmacol Res. 2005;51:523–532. doi: 10.1016/j.phrs.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bettzieche A, Brandsch C, Eder K, Stangl GI. Lupin protein acts hypocholesterolemic and increases milk fat content in lactating rats by influencing the expression of genes involved in cholesterol homeostasis and triglyceride synthesis. Mol Nutr Food Res. 2009;53:1134–1142. doi: 10.1002/mnfr.200800393. [DOI] [PubMed] [Google Scholar]
- Bolayirli IM, Aslan M, Balci H, Altug T, Hacibekiroglu M, Seven A. Effects of atorvastatin therapy on hypercholesterolemic rabbits with respect to oxidative stress, nitric oxide pathway and homocysteine. Life Sci. 2007;81:121–127. doi: 10.1016/j.lfs.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Boverhof DR, Burgoon LD, Tashiro C, Sharratt B, Chittim B, Harkema JR, Mendrick DL, Zacharewski TR. Comparative toxicogenomic analysis of the hepatotoxic effects of TCDD in Sprague Dawley rats and C57BL/6 mice. Toxicol Sci. 2006;94:398–416. doi: 10.1093/toxsci/kfl100. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Arzuaga X, Kluemper CT, Caraballo A, Toborek M, Hennig B. Quercetin blocks caveolae-dependent pro-inflammatory responses induced by co-planar PCBs. Environ Int. 2009 doi: 10.1016/j.envint.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Furlong CE. Paraoxonase (PON1): from toxicology to cardiovascular medicine. Acta Biomed. 2005a;76(Suppl 2):50–57. [PubMed] [Google Scholar]
- Costa LG, Vitalone A, Cole TB, Furlong CE. Modulation of paraoxonase (PON1) activity. Biochem Pharmacol. 2005b;69:541–550. doi: 10.1016/j.bcp.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Denison MS, Heath-Pagliuso S. The Ah receptor: a regulator of the biochemical and toxicological actions of structurally diverse chemicals. Bull Environ Contam Toxicol. 1998;61:557–568. doi: 10.1007/pl00002973. [DOI] [PubMed] [Google Scholar]
- Ferretti G, Bacchetti T, Masciangelo S, Bicchiega V. HDL-paraoxonase and membrane lipid peroxidation: a comparison between healthy and obese subjects. Obesity (Silver Spring) 2010;18:1079–1084. doi: 10.1038/oby.2009.338. [DOI] [PubMed] [Google Scholar]
- Franco-Pons N, Marsillach J, Joven J, Camps J, Closa D. Serum paraoxonase undergoes inhibition and proteolysis during experimental acute pancreatitis. J Gastrointest Surg. 2008;12:891–899. doi: 10.1007/s11605-008-0502-2. [DOI] [PubMed] [Google Scholar]
- Furlong CE, Cole TB, Jarvik GP, Pettan-Brewer C, Geiss GK, Richter RJ, Shih DM, Tward AD, Lusis AJ, Costa LG. Role of paraoxonase (PON1) status in pesticide sensitivity: genetic and temporal determinants. Neurotoxicology. 2005;26:651–659. doi: 10.1016/j.neuro.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Furlong CE, Li WF, Brophy VH, Jarvik GP, Richter RJ, Shih DM, Lusis AJ, Costa LG. The PON1 gene and detoxication. Neurotoxicology. 2000;21:581–587. [PubMed] [Google Scholar]
- Furlong CE, Suzuki SM, Stevens RC, Marsillach J, Richter RJ, Jarvik GP, Checkoway H, Samii A, Costa LG, Griffith A, Roberts JW, Yearout D, Zabetian CP. Human PON1, a biomarker of risk of disease and exposure. Chem Biol Interact. 2010;187:355–361. doi: 10.1016/j.cbi.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidukov L, Tawfik DS. High affinity, stability, and lactonase activity of serum paraoxonase PON1 anchored on HDL with ApoA-I. Biochemistry. 2005;44:11843–11854. doi: 10.1021/bi050862i. [DOI] [PubMed] [Google Scholar]
- Gaub P, Tedeschi A, Puttagunta R, Nguyen T, Schmandke A, Di Giovanni S. HDAC inhibition promotes neuronal outgrowth and counteracts growth cone collapse through CBP/p300 and P/CAF-dependent p53 acetylation. Cell Death Differ. 2010;17:1392–1408. doi: 10.1038/cdd.2009.216. [DOI] [PubMed] [Google Scholar]
- Gouedard C, Barouki R, Morel Y. Dietary polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor-dependent mechanism. Mol Cell Biol. 2004;24:5209–5222. doi: 10.1128/MCB.24.12.5209-5222.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CY, Chiba T, Campbell JS, Fausto N, Chaisson M, Orasanu G, Plutzky J, Chait A. Reciprocal and coordinate regulation of serum amyloid A versus apolipoprotein A-I and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler Thromb Vasc Biol. 2006;26:1806–1813. doi: 10.1161/01.ATV.0000227472.70734.ad. [DOI] [PubMed] [Google Scholar]
- Hassoun EA, Wang H, Abushaban A, Stohs SJ. Induction of oxidative stress in the tissues of rats after chronic exposure to TCDD, 2,3,4,7,8-pentachlorodibenzofuran, and 3,3′,4,4′,5-pentachlorobiphenyl. J Toxicol Environ Health A. 2002;65:825–842. doi: 10.1080/00984100290071054. [DOI] [PubMed] [Google Scholar]
- Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, Robertson LW. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicol Appl Pharmacol. 2002;181:174–183. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- Hennig B, Reiterer G, Majkova Z, Oesterling E, Meerarani P, Toborek M. Modification of environmental toxicity by nutrients: implications in atherosclerosis. Cardiovasc Toxicol. 2005;5:153–160. doi: 10.1385/ct:5:2:153. [DOI] [PubMed] [Google Scholar]
- Hestermann EV, Stegeman JJ, Hahn ME. Relative contributions of affinity and intrinsic efficacy to aryl hydrocarbon receptor ligand potency. Toxicol Appl Pharmacol. 2000;168:160–172. doi: 10.1006/taap.2000.9026. [DOI] [PubMed] [Google Scholar]
- Jaichander P, Selvarajan K, Garelnabi M, Parthasarathy S. Induction of paraoxonase 1 and apolipoprotein A-I gene expression by aspirin. J Lipid Res. 2008;49:2142–2148. doi: 10.1194/jlr.M800082-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur HD, Bansal MP. Studies on HDL associated enzymes under experimental hypercholesterolemia: possible modulation on selenium supplementation. Lipids Health Dis. 2009;8:55. doi: 10.1186/1476-511X-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec AK, Burgoon LD, Ibrahim-Aibo D, Burg AR, Lee AW, Tashiro C, Potter D, Sharratt B, Harkema JR, Rowlands JC, Budinsky RA, Zacharewski TR. Automated dose-response analysis and comparative toxicogenomic evaluation of the hepatic effects elicited by TCDD, TCDF, and PCB 126 in C57BL/6 mice. Toxicol Sci. 2010;118:286–297. doi: 10.1093/toxsci/kfq236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Du BN, Aviram M, Billecke S, Navab M, Primo-Parmo S, Sorenson RC, Standiford TJ. On the physiological role(s) of the paraoxonases. Chem Biol Interact. 1999;119–120:379–388. doi: 10.1016/s0009-2797(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Lai I, Chai Y, Simmons D, Luthe G, Coleman MC, Spitz D, Haschek WM, Ludewig G, Robertson LW. Acute toxicity of 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) in male Sprague-Dawley rats: effects on hepatic oxidative stress, glutathione and metals status. Environ Int. 2010;36:918–923. doi: 10.1016/j.envint.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig G, Lehmann L, Esch H, Robertson LW. Metabolic Activation of PCBs to Carcinogens in Vivo - A Review. Environ Toxicol Pharmacol. 2008;25:241–246. doi: 10.1016/j.etap.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthe GM, Schut BG, Aaseng JE. Monofluorinated analogues of polychlorinated biphenyls (F-PCBs): synthesis using the Suzuki-coupling, characterization, specific properties and intended use. Chemosphere. 2009;77:1242–1248. doi: 10.1016/j.chemosphere.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Mackness B, Mackness M. Anti-inflammatory properties of paraoxonase-1 in atherosclerosis. Adv Exp Med Biol. 2010;660:143–151. doi: 10.1007/978-1-60761-350-3_13. [DOI] [PubMed] [Google Scholar]
- Mortensen AS, Arukwe A. Estrogenic effect of dioxin-like aryl hydrocarbon receptor (AhR) agonist (PCB congener 126) in salmon hepatocytes. Mar Environ Res. 2008;66:119–120. doi: 10.1016/j.marenvres.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Oda H, Suzuki Y, Wakayama M, Yoshida A. Apolipoprotein A-I gene expression is upregulated by polychlorinated biphenyls in rat liver. J Nutr Biochem. 2000;11:568–573. doi: 10.1016/s0955-2863(00)00121-2. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Quazi S, Yokogoshi H, Yoshida A. Effect of dietary fiber on hypercholesterolemia induced by dietary PCB or cholesterol in rats. J Nutr. 1983;113:1109–1118. doi: 10.1093/jn/113.6.1109. [DOI] [PubMed] [Google Scholar]
- Rodrigo L, Hernandez AF, Lopez-Caballero JJ, Gil F, Pla A. Immunohistochemical evidence for the expression and induction of paraoxonase in rat liver, kidney, lung and brain tissue. Implications for its physiological role. Chem Biol Interact. 2001;137:123–137. doi: 10.1016/s0009-2797(01)00225-3. [DOI] [PubMed] [Google Scholar]
- Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM, Lusis AJ. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- Shipley JM, Waxman DJ. Aryl hydrocarbon receptor-independent activation of estrogen receptor-dependent transcription by 3-methylcholanthrene. Toxicol Appl Pharmacol. 2006;213:87–97. doi: 10.1016/j.taap.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varatharajalu R, Garige M, Leckey LC, Gong M, Lakshman MR. Betaine protects chronic alcohol and omega-3 PUFA-mediated down-regulations of PON1 gene, serum PON1 and homocysteine thiolactonase activities with restoration of liver GSH. Alcohol Clin Exp Res. 2009;34:424–431. doi: 10.1111/j.1530-0277.2009.01107.x. [DOI] [PubMed] [Google Scholar]
- Vondracek J, Svihalkova-Sindlerova L, Pencikova K, Krcmar P, Andrysik Z, Chramostova K, Marvanova S, Valovicova Z, Kozubik A, Gabelova A, Machala M. 7H-Dibenzo[c,g]carbazole and 5,9-dimethyldibenzo[c,g]carbazole exert multiple toxic events contributing to tumor promotion in rat liver epithelial ‘stem-like’ cells. Mutat Res. 2006;596:43–56. doi: 10.1016/j.mrfmmm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol. 1998;108:101–106. doi: 10.1385/0-89603-472-0:101. [DOI] [PubMed] [Google Scholar]
- Zhao HX, Adamcakova-Dodd A, Hu D, Hornbuckle KC, Just CL, Robertson LW, Thorne PS, Lehmler HJ. Development of a synthetic PCB mixture resembling the average polychlorinated biphenyl profile in Chicago air. Environ Int. 2010;36:819–827. doi: 10.1016/j.envint.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.