Abstract
Intestinal epithelial restitution is the first part in the process of mucosal repair after injury in the intestine. Integrity of the intestinal mucosal barrier is important as a first line of defense against bacteria and endotoxin. Necrotizing enterocolitis (NEC) is a major cause of morbidity and mortality in extremely low birth weight infants, but its mechanisms are not well defined. Abnormal bacterial colonization, immature barrier function, innate immunity activation and inflammation likely play a role. Lipopolysaccharide (LPS) binding protein (LBP) is secreted by enterocytes in response to inflammatory stimuli and has concentration-dependent effects. At basal concentrations, LBP stimulates the inflammatory response by presenting LPS to its receptor. However, at high concentrations, LBP is able to neutralize LPS and prevent an exaggerated inflammatory response. We sought to determine how LBP would affect wound healing in an in vitro model of intestinal cell restitution and protect against intestinal injury in a rodent model of NEC. Immature intestinal epithelial cells (IEC-6) were seeded in poly-l-lysine coated 8 chamber slides and grown to confluence. A 500μm wound was created using a cell scraper mounted on the microscope to achieve uniform wounding. Media was replaced with media containing LPS +/− LBP. Slide wells were imaged after 0, 8, and 24 hours and then fixed. Cellular restitution was evaluated via digital images captured on an inverted microscope and wound closure was determined by automated analysis. TLR4 was determined by rtPCR after RNA isolation from wounded cells 24 hours after treatment. LPS alone attenuated wound healing in immature intestinal epithelium. This attenuation is reversed by 24 hours with increasing concentrations of LBP so that wound healing is equivalent to control (p< 0.001). TLR4 was increased with LPS alone but levels returned to that of control after addition of LBP in the higher concentrations. LBP had no effect on the development of intestinal injury when given during our rodent model of NEC. Abnormal bacterial colonization and activation of innate immunity by LPS are likely involved in the pathogenesis of NEC. The attentuation of wound healing was reversed when LBP was added to LPS but only in the higher concentrations. At these same concentrations of LBP, TLR4 was decreased to that of control. These results indicate that LBP may be a novel therapeutic strategy to facilitate wound healing after the acute phase of NEC and other forms of intestinal injury.
Introduction
The mucosal lining of the gastrointestinal tract is composed of a rapidly proliferating and continually renewing sheet of epithelial cells that when damaged in the course of daily events of digestion and motility rapidly reseal to prevent penetration and absorption of toxic and immunogenic factors [1] [2]. These toxic and immunogenic factors, if absorbed, may lead to a generalized systemic inflammatory and uncontrolled immune response, as occurs in necrotizing enterocolitis (NEC) [1] [2]. NEC is a disease of mainly premature infants and is characterized by abnormal bacterial colonization, altered barrier function, exaggerated inflammatory response and impaired intestinal epithelial wound healing [3–6]. Intestinal mucosal surface repair occurs in several steps, the first of which involves adjacent epithelial cells migrating into the wound. This is a process known as epithelial restitution which begins almost immediately following injury and does not require cell proliferation [1] [2].
Multiple factors have been shown to alter intestinal epithelial cell migration, including lipopolysaccharide (LPS). LPS, or bacterial endotoxin, is the main component of gram negative bacterial cell wall and is a potent activator of the immune system. Studies have shown that human infants with NEC have an increased amount of pathogenic gram negative bacteria and corresponding endotoxin in their stool [7] [3] and that in animal models experimental NEC is increased with exposure to LPS or live bacteria [8]. Others have demonstrated that LPS impairs intestinal epithelial restitution in vitro and in vivo [6] [9].
LPS binding protein (LBP) is an acute-phase protein synthesized in the liver and other organs and serves as a key modulator of cellular and systemic responses to LPS [13]. LBP binds to the lipid A moiety of LPS and transfers LPS to membrane-bound CD14 (mCD14) which is one part of the LPS-receptor [14] [15]. LBP is present in serum of healthy humans at concentrations of 5 to 15 mcg/ml but increases to ≥ 50 mcg/ml during the acute-phase response [16]. Recent studies have shown a duality in the impact of LBP binding to LPS; at lower basal concentrations LBP enhances cell responses to LPS by accelerating the binding of LPS to mCD14, thus facilitating receptor agonism and ensuing host defense responses[17]. In contrast, at high LBP concentrations (as in settings of an acute-phase response), LPS cellular activation can be inhibited [18].
The intestinal lumen contains high amounts of endotoxin and the intestinal mucosa forms the interface between this potentially harmful material and the interior of the host [19]. There is evidence for the synthesis of LBP in the intestinal mucosa[20], suggesting that its presence regulates or contributes to intestinal response to LPS. Given these previous reports we sought to determine if recombinant LBP could serve as a potential approach to blunt enterocyte response to LPS, and thus may have value as a strategy for NEC treatment. Here we tested the hypothesis that exogenously administered LBP attenuates the effects of LPS and improves intestinal epithelial cell wound healing. We also tested the hypothesis that orally administered LBP decreases the incidence and severity of intestinal injury in a newborn rat model of NEC.
Materials and Methods
Materials
Neonatal rat enterocytes (IEC-6 cells, passage < 25) were obtained from ATTC (Manassas, VA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, Manassas, VA) containing 10% fetal bovine serum (Hyclone), 2mM L-Glutamine (Mediatech, Manassas, VA), penicillin and streptomycin (Mediatech, Manassas, VA) and insulin (10μg/ml; Gibco). Purified LPS from Escherichia coli 0155:B4 was obtained from Sigma (St. Louis, MO). Recombinant human LBP (25 mcg) was purchased from R&D Systems (Minneapolis, MN). Bromodeoxyuridine (BrDU) for the assessment of epithelial cell proliferation was obtained fromAldrich (St. Louis, MO).
Animals
Pregnant Sprague-Dawley rats (Harlan Laboratories Indianapolis, IN.) were obtained at gestation E16 and were allowed access to standard chow and water ad libitum until delivery of the pups via C-section at E21 (term). All animal studies were approved by The Research Institute at Nationwide Children’s Hospital’s Institutional Animal Care and Use Committee (IACUC).
Wounding assays
Wounding assays were performed as follows: Briefly, enterocytes were seeded (400,000 cells/ml) in poly-l-lysine coated 8-chamber slides (Labtek II; Nunc, Rochester, NY) and grown to confluence. After the cells achieved confluence, a wound was created using a modified cell scraper attached to a microscope. The microscope stage controls allows the creation of a straight consistent wound (width ≈ 500μm). After wounding, media was replaced with serum-free media with or without LPS (50–100 mcg/ml) in the presence or absence of LBP (0.1 – 50 mcg/ml). LPS was mixed with LBP for 1 hr prior to treatment of the wounded cells. Digital images of wounds were captured using a 4x objective on an inverted microscope (Olympus, Melville, NY) with an attached digital camera (Qimaging, Surrey, BC, Canada) at 0, 8 and 24 hours after wounding. Image analysis was performed using Image Pro Plus software (Media Cybernetics, Silver Springs, MD) and a custom-written macro. Migration was assessed by measuring the amount of area that is occupied by cells in the wound area compared to the 0hr wound. Results are expressed as the microns of wound closure as compared to the 0 hr wound area. Experiments were performed in quadruplicate and three images from each well were used to quantitate the effects of LPS and LBP on migration.
TLR4 mRNA by rt-PCR analysis
Cells treated with LPS in the presence or absence of LBP were harvested and total RNA was extracted with guanidine using a previously published protocol (Current Protocols in Molecular Biology).
Isolation of RNA
Enterocyte cells were lysed with 4M Guanidine thiocyanate, mixed sequentially with 2 M sodium acetate pH 4, extracted with phenol, Phenol/chloroform/isoamy alcohol, and then precipitated with isopropanol. The RNA pellet was washed with 75% ethanol and then dissolved in DEPC water. RNA quality was assessed examining 28s and 18s bands after agarose/formaldehyde gel electrophoresis..
Reverse Transcriptase Real-time PCR
RNA was reverse transcripted by MuLV Reverse Transcriptase (Applied Biosystems) with Oligo dT primer. Real-time PCRs were performed on an iCycler (Bio-Rad laboratories, Hercules, CA.) with one-step PCR SYBR green, 3min 95°C denaturation setp, 40-cycle thermal cycling program composed of a 30-s denaturation step at 95°C, 10-s annealing step at 60°C and 30-s extension step at 72°C. Real-time PCR results were normalized to the ribosomal protein L30 (RPL30) housekeeping gene. Primer sequence: TLR4 sense, 5′-GGA TTT ATC CAG GTG TGA AA-3′, TLR4 antisense, 5′-TTT GTC TCC ACA GCC ACC A-3′. Products are 160bp.
Determination of cell proliferation
Enterocytes were seeded in 8-chamber slides, grown to confluence in DMEM with 10% FBS and wounded as described in the wounding assays section. Media was replaced with serum-free media with or without LPS. After 8 hrs of incubation at 37°C, BrDU (10 M) was added and incubated for 30 min. Following BrDU incubation, cells were fixed in ice cold methanol and stained using an anti-BrDU primary antibody (Millipore, Billerica, MA), according to manufacturer’s instructions, and Alexafluor 488 goat anti-mouse secondary antibody (Molecular Probes). Cells were counterstained with DAPI (Pierce, Rockford, IL). Images were taken of each wound using DAPI and FITC filters.
Newborn Rodent Model of NEC
Following delivery by caesarian section, rat pups were placed on the rat NEC model protocol. Each pup was given enteral LPS (1 mg/Kg E. coli lipopolysaccharide) via gavage once per day and enteral orogastric artificial feedings 6 times a day. The feedings consisted of 15 grams of Similac 60/40 powder (Ross Pediatrics, Columbus, OH) added to 75 mL Esbilac Liquid Milk Replacer (Pet-Ag, New Hampshire, IL), to provide 200 Kcal/Kg/day. Starting within 2–3 hours of delivery, pups were exposed to hypoxia (100% nitrogen) for 2 minutes followed immediately by exposure to hypothermia (10 minutes at 4° C) twice a day. Pups were also treated daily with either LBP via gavage (10ug/ml) (n=13) or equal amounts of sterile water (n=20 control) four hours prior to the LPS dose. Pups were sacrificed at 4 days of age or sooner if they developed evidence of distress, including increased work of breathing or lethargy. Following sacrifice, the intestines were removed and placed in 10% formalin for 24 hours followed by saline. Following fixation of the intestines with formalin, the intestine was placed in paraffin block, and histologic sections were prepared and stained with hematoxylin and eosin for further analysis.
Statistical analysis
All results are expressed as mean ± standard deviation (SD). Statistical comparisons were made using one-way analysis of variance. Statistical associations between variables were made by nonparametric correlation analyses (Spearmans test). All statistical analyses were performed on raw data using GraphPad Prism4.0 software.
Results
LPS Impairs Intestinal Epithelial Cell Restitution, in vitro
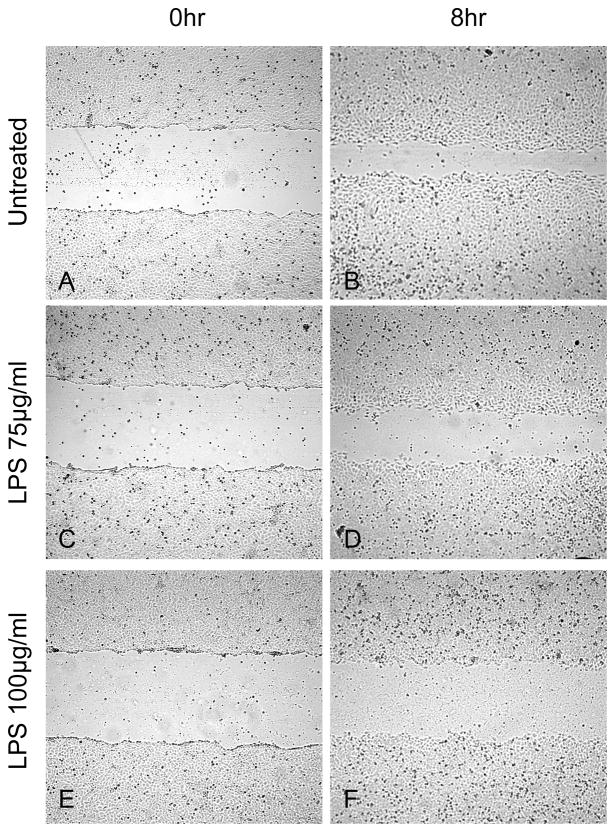
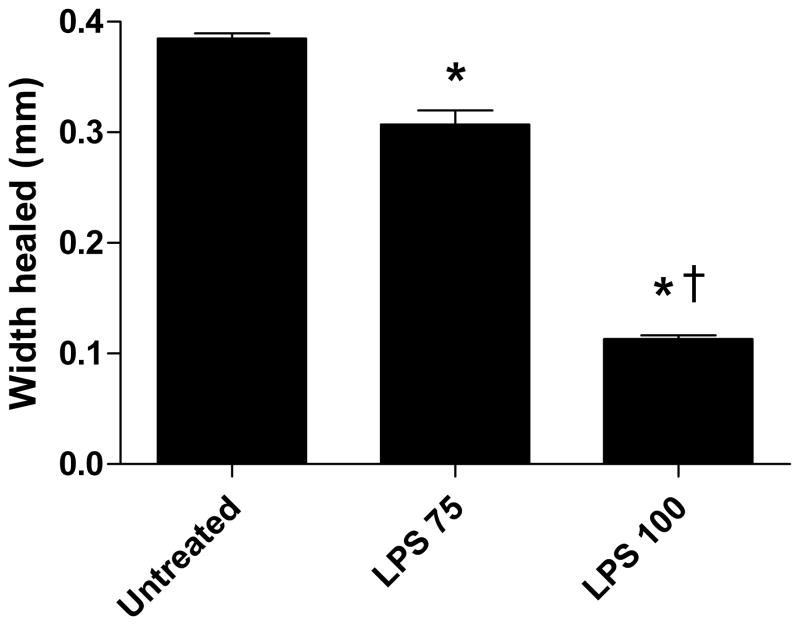
Our results are consistent with other previously published results demonstrating that LPS impairs the ability of enterocytes to migrate across a wounded monolayer [6] [9]. We examined the effect of differing concentrations of LPS (75 and 100 mcg/ml) on IEC-6 cell restitution, in vitro. Enterocyte restitution was impaired in a dose dependent fashion where the highest concentration of LPS led to the smallest amount of healing (Figure 1G.). Representative digital images of control and LPS 75 and 100 mcg/ml treated wounds at 0 (Fig. 1; A, C, E) and 8 hr (Fig. 1; B, D, F) are shown. These data demonstrate that LPS impairs enterocyte restitution, in vitro.
Figure 1.
Representative images of dose-dependent inhibition of intestinal epithelial wound healing with LPS 75 and 100 mcg/ml. Wounding assay. Images A, C, and E are 0 hr wounds and images B, D, and F are 8 hrs after wounding incubated with serum-free media and LPS treatment. G) LPS impairs wound healing in a dose-dependent fashion. Width healed (μm) was calculated by subtracting the area of the remaining wound at 8 hrs from the initial wound image at 0 hr. Values are means ± SD of two separate experiments. *, p < 0.001 vs untreated by ANOVA; †, p < 0.001 vs LPS 75 by ANOVA.
Intestinal Epithelial Cell Restitution is due to Migration not Proliferation
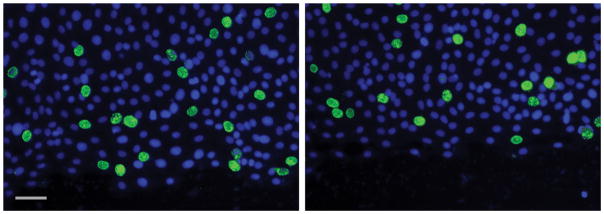
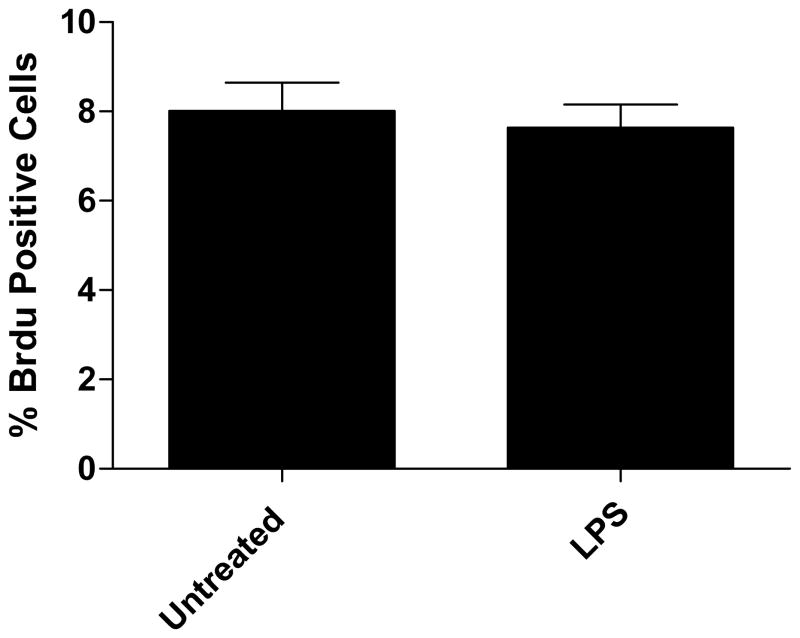
In order to assess whether intestinal epithelial cellular proliferation was contributing to restitution of the wounded monolayer as compared to migration, control and treated wounded monolayers were incubated with bromodeoxyuridine which gets incorporated into proliferating cells. There was no statistical difference in the number of proliferating cells between control and LPS treated wounds at 8 hours after wounding (Fig. 2A). A representative composite image of proliferating FITC stained BrDU positive cells overlayed with the DAPI stained image is shown in Figure 2B.
Figure 2.
A) Representative image of BrDU staining for cellular proliferation. The % FITC-positive cells were not significantly different in LPS treated as compared to untreated. B) Cellular proliferation not contributing to wound healing. Cells were counterstained with DAPI and images were taken of each wound using DAPI and FITC filters. Values are means of % FITC positive cells as compared to DAPI positive ± SD, with 4 wounds examined per treatment. Untreated 8.01% ± 2.2% vs LPS 7.64% ± 1.6%, NS.
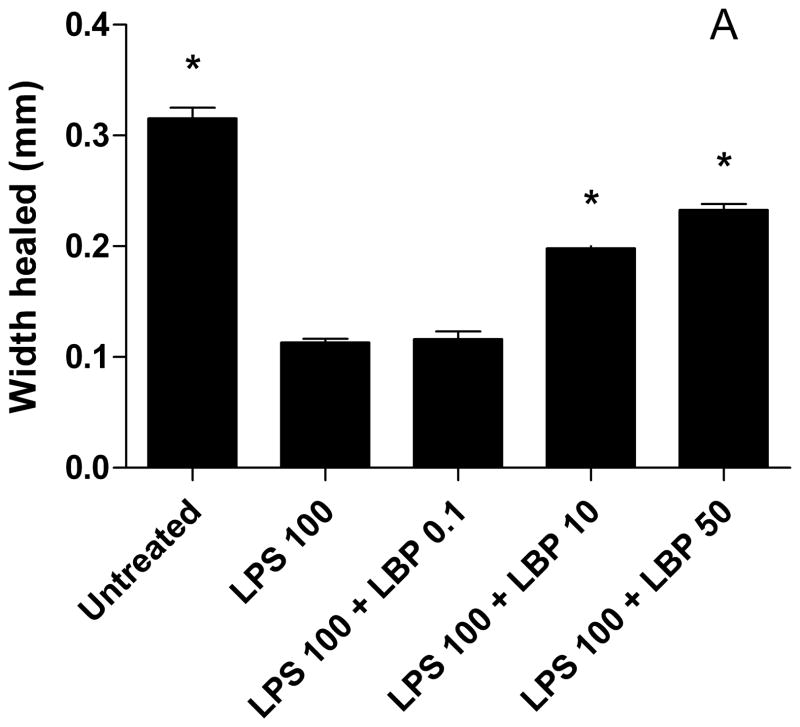
LBP Improves Enterocyte Restitution Despite LPS Exposure In vitro
Next, we investigated whether enterocyte migration could be restored with LBP despite exposure to LPS. We found that the higher concentrations of LBP (10 and 50 mcg/ml) improved enterocyte migration with LPS exposure (100 mcg/ml) so that at 24 hours the wounds were healed to that of control (Fig. 3). Enterocyte migration was not significantly different at the lowest concentration of LBP and LPS as compared to LPS alone.
Figure 3.
LBP improves wound healing despite LPS exposure. IEC-6 cells were grown to confluence in 8-well chamber slides. Monolayers were wounded with cell scraper and media was replaced with serum-free media containing LPS 75 or 100 mcg/ml ± LBP 0.1, 10, or 50 mcg/ml. Digital images of the wounds were captured on a camera mounted to an inverted microscope. Width healed (μm) was calculated by subtracting the area of the remaining wound at 8 hrs from the initial wound image at 0 hr. Values are means ± SD of two separate experiments. * p < 0.001 compared to LPS 100 or LPS 100 + LBP 0.1 by ANOVA.
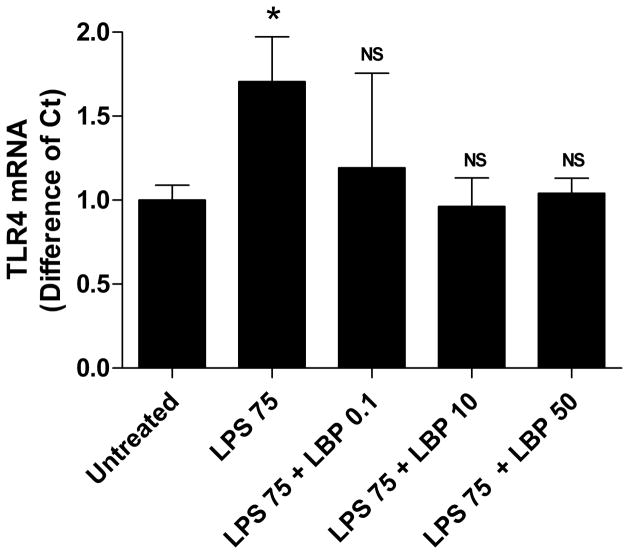
Expression of TLR4 in Enterocytes
We sought to examine a possible mechanism by which enterocyte migration was improved with LBP in the face of LPS exposure. Therefore, we examined the expression of the TLR4 by RT-PCR. TLR4 expression was increased when cells were exposed to LPS (p<0.01) and expression returned to control levels when LBP was present (Fig. 4).
Figure 4.
Treatment with LBP decreased TLR4 expression to control levels by RT-PCR. IEC-6 cells were grown, wounded and treated as described in Figure 1. RNA was obtained from cells 24 hours after wounding and treatment. TLR4 copy numbers were determined and expressed as difference from control (untreated). Bars represent means ± SD.
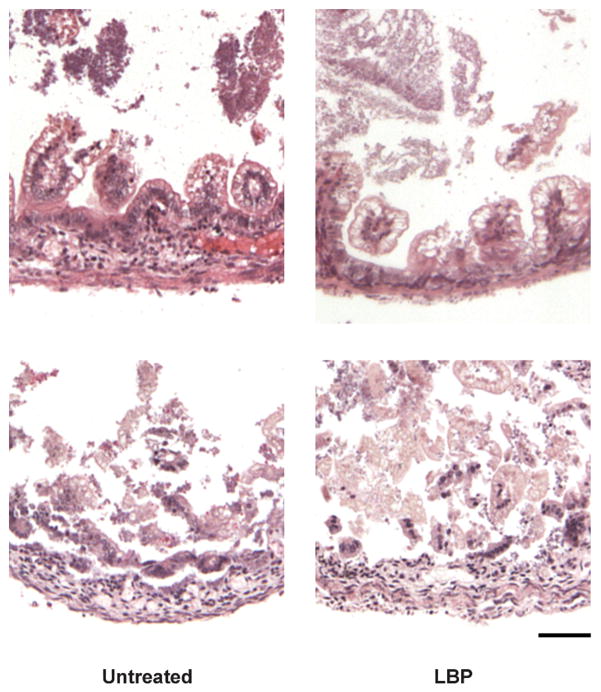
LBP Treatment Did Not Affect the Degree of Intestinal Injury Incurred in the In Vivo Newborn Rat Model of NEC
Given the clearly favorable effects of LBP in our in vitro enterocyte experiments we hypothesized that this approach would have protective effects in the neonatal rat model of NEC we and others have previously established. We investigated whether oral treatment with LBP had any protective or adverse effects on newborn intestinal injury in our established newborn rat model of NEC. In these studies we found that the LBP dosing did not provide any untoward effects, and that the general appearance and health status of both treatment groups were undistinguishable throughout the repeated handling requirements of the NEC model protocol. At sacrifice of each animal intestinal specimens were excised for histological assessments and grading of NEC severity; there were no differences observed in the development of any degree of NEC between the two treatment groups (55% NEC in controls vs. 53% in treatment) or grades > 2 (35% in controls vs. 30% in LBP treatment). Results and representative images are shown in are shown in Table 1 and Figure 5 respectively.
Table 1.
Treatment with LBP does not decrease the degree of intestinal injury incurred in a newborn rodent model of NEC. Statistics performed by Student t-test.
| - | LPS only | LPS + LPB |
|---|---|---|
| Percent of pups with NEC | 55% | 54% |
| Percent of pups with NEC grade > 2 | 35% | 30% |
Figure 5.
Representative images of intestinal injury incurred in both the control pups and LBP treated pups when placed in the newborn rat model of NEC.
Discussion
In this study, we demonstrated that LBP improves neonatal enterocyte wound healing despite exposure to LPS in a concentration-dependent manner, in vitro. This improvement in wound healing by LBP correlated with a down-regulation of the LPS cellular activation receptor, toll-like receptor 4 (TLR4), which corroborates findings by others that LPS through TLR4 mediates intestinal epithelial cell repair [6] [10]. LPS acts through the (TLR4) which is found on enterocytes [6, 8, 10]. In addition, the impairment in the intestinal barrier may allow translocation of endotoxin and activation of the systemic inflammatory system [11]. LPS binding to TLR4 results in the release of proinflammatory cytokines and a significant systemic inflammatory response that contributes to the multiorgan system failure and death that can be seen with severe sepsis and NEC [12]. TLR4 is up-regulated in animals and humans with NEC and in mice with a mutation in the TLR4 gene there was reduced NEC severity [6, 8, 10].
A study by Cetin et al demonstrated that LPS activation of TLR4 inhibits enterocyte migration leading to the activation of focal adhesion kinase (FAK) and an increase in focal adhesions [6]. In addition, a study by Leaphart et al found that TLR4 immunoprecipitated with FAK and that transfection of IEC-6 cells with siRNA against FAK significantly reversed the inhibitory effect of LPS on migration [10]. Yet, another study demonstrated that LPS leads to increased expression and function of integrins in enterocytes which results in increased cell-matrix adhesion and decreased migration [9]. Together these data indicate that LPS has a direct effect on intestinal epithelial cell migration and, in turn, repair after injury, likely through TLR4 activation.
LBP is a 50 kDa acute-phase protein synthesized primarily by hepatocytes in response to cytokines and other stimuli, including LPS and gram-negative bacteria [13]. Other cell lines have also been shown to synthesize LBP, including enterocytes [20] [19]. Classically, the role of LBP is to aid in LPS recognition by transferring it to mCD14 which then shuttles LPS to MD2, a co-receptor for TLR4 [13] [21]. This leads to activation of the cell and inflammatory response which is important for host defense against gram-negative bacteria [17]. LBP deficient mice have been shown to have decreased cytokine and inflammatory response to LPS but have increased susceptibility for lethal infections with gram-negative bacteria [22].
However, at high concentrations, LBP has been shown to be protective against LPS or gram-negative bacterial infections [18] [16]. In mice, in vitro and in vivo, high concentrations of LBP, such as those equivalent to acute-phase response, injected intraperitoneally led to decreased cytokine release and improved survival after E. coli LPS and live bacteria [18]. Additionally, serum from septic patients, with acute-phase levels of LBP, incubated with monocytes decreased the binding of LPS and subsequent activation [16]. LBP can inhibit cell responses to LPS by at least three different mechanisms [17]. First, LBP transfers LPS to lipoproteins [17] [23], including apoB containing lipoprotein particles, LDL and VLDL [24], and chylomicrons [23]. Second, LBP forms complexes with LPS which is then internalized by the cell without leading to cellular activation [17] [25]. Lastly, LBP was found to remove LPS already bound to mCD14 attenuating cell responses [12].
In our enterocyte wound healing model cells were incubated without serum after treatment with LPS with or without LBP. Therefore, the action of LBP in neutralizing the impairment of wound healing by LPS was not due to transference of LPS to lipoproteins and since the LPS and LBP were combined for one hour prior to treatment it is not likely that LBP removed already bound LPS. The most likely mechanism of our results is LBP forms complexes with LPS aggregates which may lead to internalization without cellular activation through TLR4. Another possibility is that LPS binds to mCD14 but the LPS-LBP complex shields it from direct interaction with TLR4 [17]. Regardless, these data suggest that LPS impairment in enterocyte migration is mediated by TLR4 and that strategies to block this interaction may improve restitution.
In contrast to the consistently favorable effects of LBP in our in vitro experiments, there were no discernable effects of LPB in the neonatal rat model of NEC in vivo. Since the NEC induction protocol includes daily gavage dosing of LPS we hypothesized that LBP may provide some protection in this setting. Our observation that LBP did not conform any protection with respect neonatal rat intestinal injury in this model may be explained by issues regarding the dose selections employed or the timing of the administrations (e.g. single daily bolus vs other strategies). It is also possible that in this animal preparation the LPS alone (and thus its binding by LBP) is only a component of the intestinal injury observed. This concept is consistent with clinical NEC, wherein several factors other than bacterial presence are known contributors to pathogenesis. An additional possibility is that in the single-cell-type conditions we used in vitro the LBP could clearly elicit favorable effects, whereas the multi-cellular environment of intact intestinal tissue changes the observed outcomes. Although these in vivo studies were not intended to be exhaustive in evaluating the therapeutic potential for LBP in NEC, our observations at least suggest that LBP did not further enhance the LPS intestinal insult. Further studies to determine the potential value of this therapeutic approach for NEC would clearly be required.
In conclusion, we demonstrated that LBP improves intestinal epithelial cell wound healing despite LPS exposure in enterocytes in vitro. This is improvement in epithelial restitution by LBP has not been shown previously and suggests that such an approach strategy may provide a safe and novel therapy in disorders of intestinal inflammation, injury and repair, such as necrotizing enterocolitis.
Acknowledgments
This study was supported in part by NIH grant 1K23DK078909.
Abbreviations
- NEC
necrotizing enterocolitis
- LBP
Lipopolysaccharide Binding Proten
- LPS
Lipopolysaccharide
- TLR4
Toll-like receptor 4
Footnotes
No conflicts of interest to declare.
References
- 1.Mammen JM, Matthews JB. Mucosal repair in the gastrointestinal tract. Crit Care Med. 2003;31(8 Suppl):S532–7. doi: 10.1097/01.CCM.0000081429.89277.AF. [DOI] [PubMed] [Google Scholar]
- 2.Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol. 2008;14(3):348–53. doi: 10.3748/wjg.14.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caplan MS, Jilling T. The pathophysiology of necrotizing enterocolitis. NeoReviews. 2001;2(5):e103–9. [Google Scholar]
- 4.Martin CR, Walker WA. Intestinal immune defences and the inflammatory response in necrotising enterocolitis. Semin Fetal Neonatal Med. 2006;11(5):369–77. doi: 10.1016/j.siny.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Lee JS, Polin RA. Treatment and prevention of necrotizing enterocolitis. Semin Neonatol. 2003;8(6):449–59. doi: 10.1016/S1084-2756(03)00123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cetin S, et al. Endotoxin inhibits intestinal epithelial restitution through activation of Rho-GTPase and increased focal adhesions. J Biol Chem. 2004;279(23):24592–600. doi: 10.1074/jbc.M313620200. [DOI] [PubMed] [Google Scholar]
- 7.Duffy LC, et al. Concordance of bacterial cultures with endotoxin and interleukin-6 in necrotizing enterocolitis. Dig Dis Sci. 1997;42(2):359–65. doi: 10.1023/a:1018826204819. [DOI] [PubMed] [Google Scholar]
- 8.Jilling T, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177(5):3273–82. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qureshi FG, et al. Increased expression and function of integrins in enterocytes by endotoxin impairs epithelial restitution. Gastroenterology. 2005;128(4):1012–22. doi: 10.1053/j.gastro.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 10.Leaphart CL, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179(7):4808–20. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 11.Anand RJ, et al. The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock. 2007;27(2):124–33. doi: 10.1097/01.shk.0000239774.02904.65. [DOI] [PubMed] [Google Scholar]
- 12.Thompson PA, et al. Lipopolysaccharide (LPS)-binding protein inhibits responses to cell-bound LPS. J Biol Chem. 2003;278(31):28367–71. doi: 10.1074/jbc.M302921200. [DOI] [PubMed] [Google Scholar]
- 13.Zweigner J, Schumann RR, Weber JR. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infect. 2006;8(3):946–52. doi: 10.1016/j.micinf.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Schumann RR, et al. Structure and function of lipopolysaccharide binding protein. Science. 1990;249(4975):1429–31. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 15.Wright SD, et al. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249(4975):1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 16.Zweigner J, et al. High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood. 2001;98(13):3800–8. doi: 10.1182/blood.v98.13.3800. [DOI] [PubMed] [Google Scholar]
- 17.Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res. 2005;11(4):225–9. doi: 10.1179/096805105X46565. [DOI] [PubMed] [Google Scholar]
- 18.Lamping N, et al. LPS-binding protein protects mice from septic shock caused by LPS or gram-negative bacteria. J Clin Invest. 1998;101(10):2065–71. doi: 10.1172/JCI2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vreugdenhil AC, et al. Lipopolysaccharide binding protein and serum amyloid A secretion by human intestinal epithelial cells during the acute phase response. J Immunol. 1999;163(5):2792–8. [PubMed] [Google Scholar]
- 20.Su GL, et al. Molecular cloning, characterization, and tissue distribution of rat lipopolysaccharide binding protein. Evidence for extrahepatic expression. J Immunol. 1994;153(2):743–52. [PubMed] [Google Scholar]
- 21.Akira S, Sato S. Toll-like receptors and their signaling mechanisms. Scand J Infect Dis. 2003;35(9):555–62. doi: 10.1080/00365540310015683. [DOI] [PubMed] [Google Scholar]
- 22.Jack RS, et al. Lipopolysaccharide-binding protein is required to combat a murine gram-negative bacterial infection. Nature. 1997;389(6652):742–5. doi: 10.1038/39622. [DOI] [PubMed] [Google Scholar]
- 23.Vreugdenhil AC, et al. Lipopolysaccharide (LPS)-binding protein mediates LPS detoxification by chylomicrons. J Immunol. 2003;170(3):1399–405. doi: 10.4049/jimmunol.170.3.1399. [DOI] [PubMed] [Google Scholar]
- 24.Vreugdenhil AC, et al. LPS-binding protein circulates in association with apoB- containing lipoproteins and enhances endotoxin-LDL/VLDL interaction. J Clin Invest. 2001;107(2):225–34. doi: 10.1172/JCI10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gegner JA, Ulevitch RJ, Tobias PS. Lipopolysaccharide (LPS) signal transduction and clearance. Dual roles for LPS binding protein and membrane CD14. J Biol Chem. 1995;270(10):5320–5. doi: 10.1074/jbc.270.10.5320. [DOI] [PubMed] [Google Scholar]
- 26.Giannone PJ, et al. Poly(ADP-Ribose) Polymerase-1: A Novel Therapeutic Target in Necrotizing Enterocolitis. Pediatr Res. 2011 Jul;70(1):67–71. doi: 10.1203/PDR.0b013e31821928ff. [DOI] [PMC free article] [PubMed] [Google Scholar]