Abstract
Analogues of the δ opioid antagonist peptide TIPP (H-Tyr-Tic-Phe-Phe-OH; Tic = 1,2,3,4-tetrahydroisoquinoline3-carboxylic acid) containing various 4′-[N-(alkyl or aralkyl)carboxamido]phenylalanine analogues in place of Tyr1 were synthesized. The compounds showed subnanomolar or low nanomolar δ opioid receptor binding affinity and various efficacy at the δ receptor (antagonism, partial agonism, full agonism) in the [35S]GTPγS binding assay. Two analogues, [1-Ncp1]TIPP (1-Ncp = 4′-[N-(2-(naphthalene-1-yl)ethyl)carboxamido] phenylalanine) and [2-Ncp1]TIPP (2-Ncp = 4′-[N-(2-(naphthalene-2-yl)ethyl)carboxamido]phenylalanine), were identified as potent and selective δ opioid agonists.
Keywords: amino acid synthesis, peptide synthesis, opioid peptides, δ opioid agonists, δ partial opioid agonists, δ opioid antagonist
Selective δ opioid receptor agonists are of interest both as pharmacological tools and as potential therapeutic agents (for a review, see ref.1). While they have low analgesic efficacy in acute pain models, their potential for the treatment of inflammatory and neuropathic pain has been clearly demonstrated.2,3 Furthermore, there is evidence to indicate that δ opioid agonists produce anxiolytic and antidepressant effects4 and that they may be useful as neuroprotective agents as well as for the treatment of addictive disorders.1 Both peptide and non-peptide δ opioid agonists have been reviewed.1,5 Examples of highly selective peptide δ opioid agonists are the cyclic opioid peptide analogues H-Tyr-c[D-Pen-Gly-Phe(pF)-Pen]Phe-OH6 and H-Tyr-c[D-Cys-Phe-D-Pen]OH (JOM-13)7, and the linear tripeptide UFP-512.8
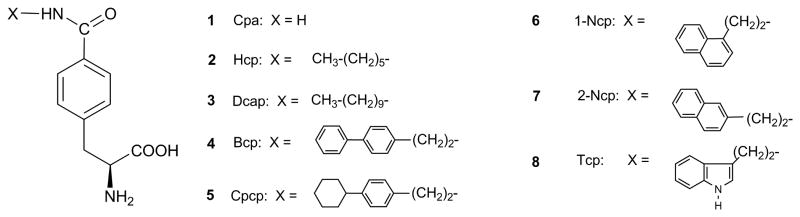
The tetrapeptide TIPP (H-Tyr-Tic-Phe-Phe-OH) is a highly selective δ opioid antagonist.9 Recently, we prepared a TIPP analogue containing 4′-[N-((4′-phenyl)phenethyl)carboxamido]phenylalanine (Bcp, Fig. 1) in place of Tyr1, [Bcp1]TIPP, which displayed high δ receptor binding affinity and δ agonist activity in the MVD assay.10 Here we describe analogues of TIPP in which the Tyr1 residue was replaced by p-carboxamidophenylalanine (Cpa, 1) and its derivatives containing various substituents at the p-carboxamido group, including 4′-[N-(hexyl)carboxamido]phenylalanine (Hcp, 2), 4′-[N-(decyl)carboxamido]phenylalanine (Dcap, 3), 4′-[N-((4′-cyclohexyl)phene-thyl)carboxamido]phenylalanine (Cpcp, 5), 4′-[N-(2-(naphthalene-1-yl)ethyl)carboxa-mido]phenylalanine (1-Ncp, 6), 4′-[N-(2-naphthalene-2-yl)ethyl)carboxamido]phenylalanine (2-Ncp, 7), and 4′-N-(2-(indole-3-yl)ethyl)carboxamido]phenylalanine (Tcp, 8) (Fig. 1). The effects of these various substitutions on binding affinity and efficacy at the δ and μ opioid receptors were determined in binding- and functional assays.
Figure 1.
Structures of Phe(4′-CONHX) amino acids.
For the preparation of the various amino acids, Boc-Phe(4′-COOH)-OMe was synthesized by activating the hydroxyl group of Boc-Tyr-OMe as the triflate, followed by carbonylation with carbon monoxide, as described.11 Boc-Phe-(4′-CONH2)-OMe (Boc-Cpa, 1)12 was prepared by reacting Boc-Phe(4′-COOH)-OMe with ammonium chloride using HBTU as coupling agent, followed by hydrolysis with 2N NaOH. The other Boc-protected amino acids (2, 3, 5–9)12 were synthesized by reacting Boc-Phe(4′-COOH)-OMe with the appropriate alkyl- or aralkylamine using HBTU as coupling agent, followed by hydrolysis with 2N NaOH. All amines were commercially available, except for 2-((4′-cyclohexyl)phenyl)ethylamine (5a)12 required for the synthesis of Cpcp-OH. The latter amine was prepared by catalytic hydrogenation of 2-(4′-biphenyl)ethylamine in EtOH using palladium on carbon as catalyst with hydrogen pressure of 140 psig during 45 hours at 95°C. 2-((4′-cyclohexyl)phenyl)ethylamine was obtained as the predominant product (45%) with unreacted 2-(4′-biphenyl)ethylamine (35%) and 2-((4′-phenyl)cyclohexyl)ethylamine (20%) also present. This mixture was used in the synthesis of Boc-Cpcp-OH which was isolated from the reaction product by reversed-phase HPLC.
Peptides were synthesized by the solid-phase method using a Merrifield resin with Boc-protection and DIC/Cl-HOBt or HBTU/DIPEA as coupling agents, and were cleaved from the resin by HF/anisole treatment. The crude products were purified by preparative reversed-phase HPLC and their purity (> 98%) and structural identity were established by TLC, analytical HPLC and ES-MS.13
Binding affinities (Ki-values) for μ and δ opioid receptors were determined by displacing, respectively, [3H]DAMGO and [3H]DSLET from rat brain membrane binding sites and κ receptor binding affinities were measured by displacement of [3H]U69,593 from guinea pig brain membrane binding sites, as described.14 None of the compounds showed significant κ receptor binding affinity (Kiκ > 10 μM). Opioid agonist potencies (IC50s) or antagonist activities (Ke-values) were determined in the mouse vas deferens (MVD) assay (δ receptor-representative) and in the guinea pig ileum (GPI) assay using previously described protocols.14 Furthermore, δ receptor agonist potencies and efficacies were determined in the [35S]GTPγS binding assay using HEK293 cells stably expressing the human δ opioid receptor15 (efficacies were indicated relative to the efficacy of DPDPE [e = 1.0]).
In comparison with the TIPP parent peptide, [Cpa1]TIPP (10) showed comparable δ and μ receptor binding affinities and δ vs. μ selectivity (Table 1), and about 3-fold lower δ antagonist activity in the MVD assay (Table 2). In the [35S]GTPγS binding assay both TIPP and [Cpa1]TIPP behaved as δ neutral antagonists (e = 0). These data indicate that replacement of Tyr1 in TIPP with Cpa had little effect on the binding affinity and efficacy at the δ receptor and are in agreement with earlier results indicating that Cpa was also a good surrogate for Tyr1 in opioid agonist peptides.16,17 The Hcp1-analogue (11) displayed about 2.5-fold higher δ receptor binding affinity than 10 and retained high δ vs. μ receptor selectivity. Peptide 11 was a δ full agonist in the MVD assay with subnanomolar potency and a weak partial agonist in the GPI assay. It showed δ partial agonist activity in the [35S]GTPγS binding assay (e = 0.486) with an ED50 of 0.103 nM. In comparison with 11, the [Dcap1]analogue (12) displayed somewhat diminished δ receptor binding affinity and lower δ vs. μ selectivity in the binding assays, 4-fold lower δ agonist potency in the MVD assay, and slightly decreased δ partial agonist activity (ED50 = 0.239 nM) with comparable efficacy (e = 0.527) in the [35S]GTPγS binding assay.
Table 1.
Receptor binding affinities of TIPP analogues
| Compound | Kiδ [nM]a | Ki μ [nM]a | Kiμ/Kiδ |
|---|---|---|---|
| 10 H-Cpa-Tic-Phe-Phe-OH | 0.978 ± 0.195 | 2190 ± 170 | 2240 |
| 11 H-Hcp-Tic-Phe-Phe-OH | 0.369 ± 0.028 | 507 ± 17 | 1370 |
| 12 H-Dcap-Tic-Phe-Phe-OH | 1.54 ± 0.25 | 174 ± 40 | 113 |
| 13 H-Bcp-Tic-Phe-Phe-OHb | 0.605 ± 0.058 | 87.7 ± 8.0 | 145 |
| 14 H-Cpcp-Tic-Phe-Phe-OH | 2.72 ± 0.40 | 47.6 ± 17.3 | 17.5 |
| 15 H-1-Ncp-Tic-Phe-Phe-OH | 1.65 ± 0.31 | 850 ± 190 | 515 |
| 16 H-2-Ncp-Tic-Phe-Phe-OH | 1.01 ± 0.10 | 249 ± 32 | 247 |
| 17 H-Tcp-Tic-Phe-Phe-OH | 1.84 ± 0.28 | 3600 ± 1250 | 1960 |
| H-Tyr-Tic-Phe-Phe-OH | 1.22 −± 0.07 | 1720 ± 50 | 1410 |
| DPDPE | 8.39 ± 0.70 | 2820 ± 0.7 | 336 |
Mean of 3 determinations ± SEM. Data taken from ref.10
Table 2.
Opioid activities of TIPP analogues in the MVD and GPI assaysa
| Compound | MVD | GPI | |||
|---|---|---|---|---|---|
| IC50 [nM] | Keδ [nM]b | IC50 [nM] | Keμ [nM]c | ||
|
|
|
||||
| 10 H-Cpa-Tic-Phe-Phe-OH | 18.3 ± 2.1 | 6030 ± 1600 | |||
| 11 H-Hcp-Tic-Phe-Phe-OH | 0.392 ± 0.051 | 875 ± 171 (IC30)d | |||
| 12 H-Dcap-Tic-Phe-Phe-OH | 1.54 ± 0.25 | 730 ± 109 (IC30)d | |||
| 13 H-Bcp-Tic-Phe-Phe-OHe | 3.42 ± 0.36 | 223 ± 37 (IC40)d | |||
| 14 H-Cpcp-Tic-Phe-Phe-OH | 6.20 ± 0.40 | 23.4 ± 5.1 (IC35)d | |||
| 15 H-1-Ncp-Tic-Phe-Phe-OH | 0.533 ± 0.047 | inactive at 10 μM | |||
| 16 H-2-Ncp-Tic-Phe-Phe-OH | 0.950 ± 0.142 | P.A.d | 734 ± 17 | ||
| 17 H-Tcp-Tic-Phe-Phe-OH | 10.1 ± 1.4 | P.A.d | |||
| H-Tyr-Tic-Phe-Phe-OH | 4.80 ± 0.20 | > 10000 (inactive) | |||
| DPDPE | 2.02 ± 1.6 | 6340 ± 410 | |||
Mean of 3 determinations ± SEM.
Determined against DPDPE.
Determined against TAPP.
Partial agonist.
Data taken from ref.10
As previously reported,10 [Bcp1]TIPP (13) was a potent δ full agonist in the MVD assay; however, it was now characterized as a δ partial agonist (e = 0.678) in the [35S]GTPγS binding assay with an overall in vitro activity profile similar to that of 12. The results obtained with peptides 11–13 in the [35S]GTPγS binding assay indicate that they all behave as δ partial agonists with regard to G protein activation. The fact that these compounds show δ full agonist behavior in the MVD assay could be due to the presence of spare δ receptors in the vas preparation or to a different signal transduction mechanism.
The Cpcp1-analogue (14) showed diminished δ vs. μ receptor binding selectivity and somewhat reduced δ full agonist potency in the MVD assay. It was essentially a δ full agonist (e = 0.933) with an ED50 of 1.69 nM in the [35S]GTPγS binding assay. [1-Ncp1]TIPP (15) (1-NIPP) was a potent and selective δ opioid agonist with an IC50 of 0.533 nM in the MVD assay and with δ full agonist properties (e = 0.894) in the [35S]GTPγS binding assay. Similarly, [2-Ncp1]TIPP (16) (2-NIPP) displayed high δ receptor binding affinity (Kiδ = 1.01 nM), high δ agonist potency in the MVD assay (IC50 = 0.950 nM) and δ full agonist potency (ED50 = 0.475 nM, e = 0.989) in the [35S]GTPγS binding assay. In a direct comparison with the standard δ opioid agonist DPDPE, 1-NIPP and 2-NIPP show 5–8 fold higher δ receptor binding affinity, comparable δ vs. μ receptor selectivity (Kiμ/Kiδ = 250–520 vs. Kiμ/Kiδ for DPDPE), 2–4-fold higher δ agonist potency in the MVD assay and 7–14-fold higher δ agonist potency in the [35S]GTPγS binding assay. Finally, replacement of the naphthyl moiety in 15 with an indole moiety resulted in a compound, [Tcp1]TIPP (17) with somewhat diminished δ agonist potency in the MVD assay and reduced δ partial agonist activity in the [35S]GTPγS binding assay.
In conclusion, all compounds described here displayed high δ opioid receptor binding affinity, δ antagonist or δ full agonist activity in the MVD assay and various efficacy, ranging from δ antagonism to δ partial agonism to δ full agonism, in the [35S]GTPγS binding assay. Two of the compounds, 1-NIPP and 2-NIPP, turned out to be potent δ full agonists in both functional assays. The δ partial agonist vs. δ full agonist behavior of the compounds in G protein activation can be explained with two different receptor activation models. Assuming that the receptor exists in only two functional conformational states, inactive and active, the partial agonists may simply induce a smaller fraction of receptors to undergo the transition to the active state than do the full agonists. The more likely explanation is that the partial agonists induce a receptor conformation distinct from that induced by the full agonists. It has been convincingly demonstrated that β2-adrenergic receptor partial agonists and full agonists induce distinct receptor conformations.18,19 In the case of the δ opioid partial agonists and full agonists described here, distinct δ receptor conformations could be induced through diverse interactions of the various large lipophilic substituents at the 1-position residue of these peptides with hydrophobic residues of an accessory receptor binding site. Because these structurally flexible peptides contain 4–6 aromatic rings they may have unique ability to selectively induce or stabilize a number of distinct receptor conformations through diverse hydrophobic interactions with the numerous aromatic and aliphatic residues present in the receptor binding site.10 For this reason, they need to be further examined in various assay systems for possible functional selectivity.
Table 3.
[35S]GTPS binding assay of TIPP analogues
| Compound | ED50 [nM]a | ea |
|---|---|---|
| 10 H-Cpa-Tic-Phe-Phe-OH | antagonist | 0 |
| 11 H-Hcp-Tic-Phe-Phe-OH | 0.103 ± 0.012 | 0.486 ± 0.060 |
| 12 H-Dcap-Tic-Phe-Phe-OH | 0.239 ± 0.023 | 0.527 ± 0.080 |
| 13 H-Bcp-Tic-Phe-Phe-OH | 0.413 ± 0.015 | 0.678 ± 0.076 |
| 14 H-Cpcp-Tic-Phe-Phe-OH | 1.69 ± 0.25 | 0.933 ± 0.026 |
| 15 H-1-Ncp-Tic-Phe-Phe-OH | 1.03 ± 0.05 | 0.894 ± 0.009 |
| 16 H-2-Ncp-Tic-Phe-Phe-OH | 0.475 ± 0.064 | 0.989 ± 0.44 |
| 17 H-Tcp-Tic-Phe-Phe-OH | 7.08 ± 2.19 | 0.643 ± 0.051 |
| H-Tyr-Tic-Phe-Phe-OH | antagonist | 0 |
| DPDPE | 6.81 ± 0.68 | 1 |
Mean of 3–6 determinations ± SEM.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (MOP-89716) and the U.S. National Institutes of Health (DA004443).
Abbreviations
- Boc
tert-butyloxycarbonyl
- Cl-HOBt
6-chloro-1-hydroxybenzotriazole
- DAMGO
H-Tyr-D-Ala-Gly-Phe(NMe)-Gly-ol
- DIC
1,3-diisopropylcarbodiimide
- DIPEA
N,N-diisopropylethylamine
- DPDPE
H-Tyr-c[D-Pen-Gly-Phe-D-Pen]OH
- DSLET
H-Tyr-D-Ser-Gly-Phe-Leu-Thr-OH
- ES-MS
electrospray mass spectrometry
- GPI
guinea pig ileum
- HBTU
2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
- HPLC
high performance liquid chromatography
- MVD
mouse vas deferens, Pen, penicillamine
- Tic
1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
- TIPP
H-Tyr-Tic-Phe-Phe-OH
- U69
593, (5α,7α,8β-(—)-N-methyl-N-[7-(1pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]benzeneacetamide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Pradhan AA, Befort K, Nozaki C, Gavériaux-Ruff C, Kieffer BL. Trends Pharmacol Sci. 2011;32:581. doi: 10.1016/j.tips.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cahill CM, Morinville A, Hoffert C, O’Donnell D, Beaudet A. Pain. 2003;101:199. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- 3.Holdridge SV, Cahill CM. Eur J Pain. 2007;11:685. doi: 10.1016/j.ejpain.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J. J Pharmacol Sci. 2004;95:374. doi: 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- 5.Hruby VJ, Agnes RS. Biopolymers (Peptide Sci) 1999;51:391. doi: 10.1002/(SICI)1097-0282(1999)51:6<391::AID-BIP3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 6.Hruby VJ, Bartosz-Bechowski H, Davis P, Slaninova J, Zalewska T, Stropova D, Porreca F, Yamamura HI. J Med Chem. 1997;40:3957. doi: 10.1021/jm9704762. [DOI] [PubMed] [Google Scholar]
- 7.Mosberg HI, Omnaas JR, Medzihradsky F, Smith GB. Life Sci. 1988;43:1013. doi: 10.1016/0024-3205(88)90547-4. [DOI] [PubMed] [Google Scholar]
- 8.Vergura R, Balboni G, Spagnolo G, Gavioli E, Lambert DG, McDonald J, Trapella C, Lazarus LH, Regoli D, Guerrini R, Salvadori S, Caló G. Peptides. 2008;29:93. doi: 10.1016/j.peptides.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Schilller PW, Nguyen TM-D, Weltrowska G, Wilkes BC, Marsden BJ, Lemieux C, Chung NN. Proc Natl Acad Sci USA. 1992;89:11871. doi: 10.1073/pnas.89.24.11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berezowska I, Chung NN, Lemieux C, Wilkes BC, Schiller PW. J Med Chem. 2009;52:6941. doi: 10.1021/jm9004913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Obeyesekere NU, McMurray JS. Tetrahedron Lett. 1996;37:6661. [Google Scholar]
- 12.All new Boc-protected amino acids were fully characterized by optical rotation measurements, melting point determinations, 1H and 13C NMR spectra and HRMS:1. [α]D20 +15.7° (c 1, MeOH); mp 240–242° C; 1H NMR (500 MHz, CD3OD) δ 7.88–7.79 (d, 2H, J = 8.0 Hz), 7.40-7.32 (d, 2H, J = 7.8 Hz), 4.46-4.36 (m, 1H), 3.27-3.21 (m, 1H), 3.02-2.95 (m, 1H), 1.41-1.37 (s, 8H), 1.36-1.34 (s, 1H); 13C NMR (125 MHz, CD3OD) δ 173.9, 171.1, 156.6, 142.0, 132.1, 129.4, 127.6, 79.4, 54.8, 37.3, 27.5; HRMS (EI) m/e calcd for C15H20N2NaO5 [M+Na]+ 331.1270, found 331.1023.2. [α]D20 −5.8° (c 1, DMSO); mp 152–153° C; 1H NMR (500 MHz, DMSO-d6) δ 12.64-12.50 (br s, 1H), 8.38-8.32 (t, 1H, J = 5.5 Hz), 7.77-7.71 (d, 2H, J = 7.8 Hz), 7.34-7.29 (d, 2H, J = 8.0 Hz), 7.14-7.10 (d, 1H, J = 8.3 Hz), 4.15-4.08 (m, 1H), 3.24-3.20 (m, 2H), 3.09-3.03 (m, 1H), 2.90-2.83 (m,1H), 1.54-1.46 (m, 2H), 1.33-1.30 (s, 8H), 1.30-1.23 (m 7H), 0.89-0.84 (m, 3H); 13C NMR (125 MHz, DMSO-d6) δ 174.1, 166.5, 156.1, 141.9, 133.5, 129.6, 127.7, 78.8, 55.6, 39.8, 36.9, 31.7, 29.8, 28.8, 26.8, 22.8, 14.6; HRMS (EI) m/e calcd for C21H32O5N2Na [M+Na]+ 415.2209, found 415.2201.3. [α]D20 −3.2° (c 1, DMSO); mp 136–137° C; 1H NMR (500 MHz, DMSO-d6) δ 12.74-12.34 (br s, 1H), 8.36-8.32 (t, 1H, J 5.6 Hz), 7.77-7.72 (d, 2H, J = 8.1 Hz), 7.34-7.28 (d, 2H, J = 8.1 Hz), 7.14-7.08 (d, 1H, J = 8.3 Hz), 4.15-4.08 (m, 1H), 3.26-3.19 (m, 2H), 3.09-3.03 (m, 1H), 2.90-2.83 (m, 1H), 1.54-1.46 (m, 2H), 1.34-1.30 (s, 8H), 1.29-1.21 (m, 15H), 0.88-0.83 (m, 3H); 13C NMR (125 MHz, DMSO-d6) δ 174.2, 166.5, 156.1, 141.9, 133.5, 129.6, 127.7, 78.8, 55.6, 39.8, 36.9, 32.0, 29.8, 29.7, 29.6, 29.5, 29.4, 28.8, 27.2, 22.8, 14.7; HRMS (EI) m/e calcd for C25H40O5N2Na [M+Na]+ 471.2835, obsd 471.2823.5. [α]D20 +12.2° (c 1, MeOH); mp 206–208° C; 1H NMR (500 MHz, DMSO-d6) δ 12.75-12.50 (br s, 1H), 8.53-8.47 (t, 1H, J = 5.5 Hz), 7.77-7.72 (d, 2H, J = 8.0 Hz), 7.36-7.29 (d,2H, J = 8.0 Hz),7.17-7.11 (m,5H), 4.18-4.09 (m,1H), 3.48-3.41 (m, 2H), 3.10-3.04 (m, 1H), 2.91-2.85 (m, 1H), 2.81-2.73 (m, 2H), 2.49-2.40 (m, 1H), 1.80-1.66 (m, 6H), 1.40-1.34 (m, 4H), 1.33-1.30 (s, 8H), 1.27-1.24 (s, 1H); 13C NMR 125 MHz, DMSO-d6) δ 175.4, 166.6, 146.0, 142.2, 137.6, 133.0, 129.6, 129.2, 127.7, 127.3, 78.8, 55.6, 45.0, 44.1, 36.9, 35.4, 34.7, 26.3, 25.5; HRMS (EI) m/e calcd for C29H38N2O5Na [M+Na]+ 517.2678, obsd 517.3116. 5a. A small quantity of 5a was purified by HPLC for analytical characterization: mp 118–120° C; 1H NMR (500 MHz, CD3OD) δ 7.24-7.17 (m, 4), 3.19-3.13 (t, 2H, J = 7.5 Hz), 2.96-2.89 (t, 2H, J = 7.5 Hz), 2.56-2.46 (m, 1H), 1.91-1.74 (m, 5H), 1.51-1.39 (m, 5H), 1.38-1.26 (m, 2H); 13C NMR (125 MHz, CD3OD) δ 147.2, 133.9, 128.6, 128.5, 127.3, 127.2, 44.4, 40.8, 34.5, 33.0, 26.8, 26.1; HRMS (EI) m/e calcd for C14H22N [M+H]+ 204.1752, found 204.1747.6. [α]D20 −15.5° (c 1, DMSO); mp138–140° C; 1H NMR (500 MHz, DMSO-d6) δ 12.84-2.38 (br s, 1H), 8.68-8.61 (t, 1H, J = 5.6 Hz), 8.31-8.25 (d, 1H, J = 8.3 Hz), 7.96-7.90 (d, 1H, J = 8.0 Hz), 7.82-7.79 (d, 1H, J = 8.3 Hz), 7.79-7.73 (d, 2H, J = 8.3 Hz), 7.62-7.56 (m, 1H), 7.56-7.50 (m, 1H), 7.46-7.38 (m, 1H), 7.38-7.30 (d, 2H, J = 8.3 Hz), 7.17-7.10 (d, 1H, J = 8.5 Hz), 4.18-4.10 (m, 1H), 3.63-3.55 (m, 2H), 3.35-3.28 (m, 2H), 3.10-3.04 (m, 1H), 2.91-2.85 (m,1H), 1.38-1.33 (s, 8H),1.28-1.25 (s 1H); 13C NMR (125 MHz, DMSO-d6) δ 174.2, 166.8, 156.4, 142.1, 136.2, 134.2, 132.4, 129.6, 127.7, 127.6, 126.4, 124.5, 78.8, 55.8, 41.0, 36.5, 33.3, 28.8; HRMS (EI) m/e calcd for C27H30O5N2Na [M+Na]+ 485.2052, found 485.2038.7. [α]D20 −5.5° (c 1, DMSO); mp 226–227° C; 1H NMR (500 MHz, DMSO-d6) δ 12.80-12.40 (br s, 1H), 8.56-8.50 (t, 1H, J = 5.6 Hz), 7.89-7.82 (m, 3H), 7.76-7.70 (m, 3H), 7.50-7.40 (m, 3H), 7.34-7.28 (d, 2H, J = 8.0 Hz), 7.16-7.11 (d, 1H, J = 8.3 Hz), 4.15-4.08 (m, 1H), 3.62-3.56 (m, 2H), 3.09-3.04 (m, 1H), 3.03-2.98 (m, 2H), 2.89-2.82 (m, 1H), 1.33-1.28 (s, 8H), 1.27-1.22 (s, 1H); 13C NMR (125 MHz, DMSO-d6) δ 174.1, 166.7, 156.1, 142.0, 137.9, 133.8, 133.4, 132.4, 129.6, 128.4, 128.2, 128.1, 128.0, 127.7, 127.4, 126.7, 126.0, 78.8, 55.5, 41.4, 36.9, 35.9, 28.8; HRMS (E/I) m/e calcd for C27H30N2O5Na [M+Na]+ 485.2052, found 485.2038.8. [α]D20 −5.8° (c 1, DMSO); mp 118–120° C; 1H NMR (500 MHz, DMSO-d6) δ 12.77-12.58 (br s, 1H), 10.83-10.78 (s, 1H), 8.57-8.52 (t, 1H, J = 5.6 Hz), 7.79-7.74 (d, 2H, J = 8.1 Hz), 7.62-7.58 (d, 1H, J = 7.8 Hz), 7.35-7.30 (m, 3H), 7.18-7.16 (s, 1H), 7.16-7.12 (d, 1H, J = 8.5 Hz); 7.09-7.04 (m, 1H), 7.00-6.96 (m, 1H), 4.15-4.08 (m, 1H), 3.56-3.49 (m, 2H), 3.10-3.04 (m, 1H), 2.97-2.90 (m, 2H), 2.90-2.84 (m, 1H), 1.33-1.30 (s, 8H), 1.28-1.25 (s, 1H); 13C NMR (125 MHz, DMSO-d6) δ 174.1, 166.6, 156.1, 142.0, 137.0, 133.5, 129.7, 128.0, 127.7, 123.2, 121.6, 118.9, 112.6, 78.8, 56.6, 45.0, 36.9, 28.8, 25.9; HRMS (E/I) m/e calcd for C25H30O5N3 [M+H]+ 452.2185, found 452.2178.
- 13.Analytical data of peptides. TLC in the following systems (all v/v): (I) n-BuOH/AcOH/H2O (4:1:1), (II) n-BuOH/pyridine/AcOH/H2O (15:10:3:12); analytical reversed-phase HPLC performed on a Vydac column (10×250 mm) with a linear gradient of 45–95% MeOH in 0.1% TFA over 50 min (K′ = (tr-tm)/tm); ES-MS (m/e). 10. Rf 0.571 (I), Rf 0.768 (II); K′ 1.72; ES-MS m/e 662. 11. Rf 0.457 (I), Rf 0.840 (II); K′ 4.65; ES-MS m/e 746. 12. Rf 0.493 (I), Rf 0.852 (II); K′ 6.17, ES-MS m/e 802. 14. Rf 0.593 (I), Rf 0.841 (II); K′ 4.97; ES-MS m/e 848. 15. Rf 0.709, Rf 0.860 (II); K′ 4.86; ES-MS m/e 816. 16. Rf 0.463 (I), Rf 0.846 (II); K′ 4.77; ES-MS m/e 816. 17. Rf 0.743 (I), Rf 0.797 (II), K′ 3.74; ES-MS m/e 805.
- 14.Berezowska I, Lemieux C, Chung NN, Wilkes BC, Schiller PW. Chem Biol Drug Des. 2009;74:329. doi: 10.1111/j.1747-0285.2009.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Audet N, Paquin-Gobeil M, Landry-Paquet O, Schiller PW, Piñeyro G. J Biol Chem. 2005;280:7808. doi: 10.1074/jbc.M411695200. [DOI] [PubMed] [Google Scholar]
- 16.Dolle RE, Machaut M, Martinez-Teipel B, Belanger S, Cassel JA, Stabley GJ, Graczyk TM, DeHaven RN. Bioorg Med Chem Lett. 2004;14:3545. doi: 10.1016/j.bmcl.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 17.Weltrowska G, Lemieux C, Chung NN, Schiller PW. J Peptide Res. 2005;65:36. doi: 10.1111/j.1399-3011.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- 18.Ghanouni P, Gryczynski Z, Steenhuis JJ, Lee TW, Farrens DL, Lakowicz JR, Kobilka BK. J Biol Chem. 2001;276:24433. doi: 10.1074/jbc.C100162200. [DOI] [PubMed] [Google Scholar]
- 19.Swaminath G, Deupi X, Lee TW, Zhu W, Thian FS, Kobilka TS, Kobilka B. J Biol Chem. 2005;280:22165. doi: 10.1074/jbc.M502352200. [DOI] [PubMed] [Google Scholar]