Abstract
To date, effective nickel-catalyzed enantioselective cross-couplings of alkyl electrophiles that bear oxygen leaving groups have been limited to reactions of allylic alcohol derivatives with Grignard reagents. In this report, we establish that, in the presence of a nickel/pybox catalyst, a variety of racemic propargylic carbonates are suitable partners for asymmetric couplings with organozinc reagents. The method is compatible with an array of functional groups and uti-lizes commercially available catalyst components. The development of a versatile nickel-catalyzed enantioselective cross-coupling process for electrophiles that bear a leaving group other than a halide adds a significant new dimension to the scope of these reactions.
During the past several years, we have pursued the development of nickel-catalyzed asymmetric crosscoupling reactions of racemic secondary alkyl electrophiles.1,2,3 Although both activated and unactivated electrophiles can serve as suitable coupling partners, our progress to date has been limited to substrates in which the leaving group is a halide. Of course, oxygen-based leaving groups are widely used in organic chemistry, and the conditions for their synthesis from alcohols can complement those employed for the genera-tion of halides (e.g., Brønsted-basic vs. Brønsted-acidic). We therefore sought to add a new dimension to our stereoconvergent cross-coupling reactions of alkyl electrophiles by developing a method that can utilize oxygen leaving groups. In this report, we de-scribe the achievement of this objective, specifically, nickel-catalyzed asymmetric Negishi reactions of racemic propargylic carbonates (eq 1).
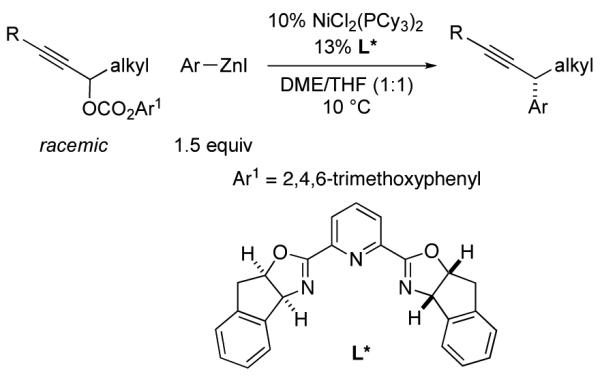 |
(1) |
Oxygen leaving groups are widely employed in crosscoupling reactions of aryl electrophiles (Csp2-O bond cleavage), including nickel-catalyzed carbon-carbon bond-forming processes.4 In contrast, there are far fewer examples of corresponding nickel-catalyzed couplings of alkyl electrophiles (Csp3-O bond cleavage), es-pecially secondary or tertiary electrophiles. Further-more, to the best of our knowledge, enantioselective reactions are limited to a rather narrow set of allylic electrophiles, and good ee’s and yields are observed only with highly reactive Grignard reagents.5
We sought to expand the scope of such processes beyond allylic alcohol derivatives and beyond Grignard reagents as coupling partners. For some of the nickelcatalyzed cross-coupling methods that we have developed, we have hypothesized that oxidative addition proceeds through a two-step inner-sphere electron-transfer pathway, beginning with abstraction of the leaving group (X) by nickel (eq 2).6,7 Because SH2 reactions at oxygen are uncommon, we decided to pursue a Barton-McCombie-like approach8 to achieving C-O bond cleavage, specifically, the use of acylated alcohols as substrates, which provides nickel with the oppor-tunity to initially interact with a carbon-heteroatom double bond and to cleave the target C-O bond in a subsequent step.
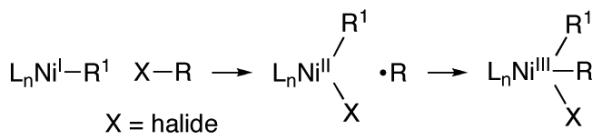 |
(2) |
In view of the synthetic utility of alkynes,9 we at-tempted to apply a method that we have developed for stereoconvergent Negishi reactions of propargylic halides10 to the cross-coupling of an acylated propargylic alcohol (eq 3). Unfortunately, we obtained virtually none of the desired coupling product.
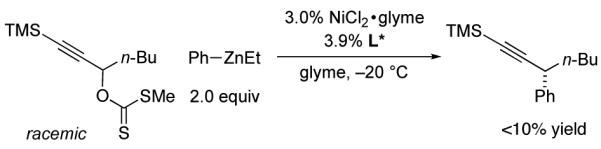 |
(3) |
After considerable effort, we have developed a method that achieves enantioselective cross-couplings of propargylic electrophiles that bear an oxygen leaving group. As illustrated in Table 1, whereas a Negishi reaction of a propargylic xanthate proceeds with very modest ee and yield (entry 1), the corresponding carbonate couples with promising enantioselectivity (but still unsatisfactory yield; entry 2). Substitution of the methyl group of the carbonate with a phenyl group leads to a significant improvement in ee and product formation (entry 3), and, finally, the addition of electron-donating substituents to the 2, 4, and 6 positions of the aromatic ring results in a small enhancement in enantioselectivity and a substantial increase in yield (entries 4-6).
Table 1.
The Impact of the Structure of the Oxygen Leaving Group on the Catalytic Asymmetric Cross-Coupling of a Propargylic Electrophilea
 | |||
|---|---|---|---|
| entry | LG | ee (%) | yield (%) b |
| 1 |
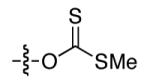
|
8 | 24 |
| 2 |
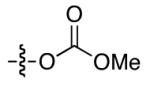
|
60 | 9 |
| 3 |
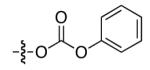
|
85 | 33 |
| 4 |
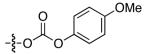
|
88 | 53 |
| 5 |
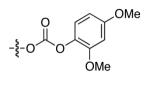
|
89 | 56 |
| 6 |
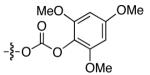
|
90 | 83 |
All data are the average of two experiments.
The yield was determined by GC analysis with the aid of a calibrated internal standard.
Table 2 provides a variety of examples of this new stereoconvergent cross-coupling of organozinc reagents with propargylic electrophiles via C-O bond cleavage.11,12 Thus, an array of TMS-substituted propargylic carbonates couple with a range of arylzinc reagents, including ortho-,13 meta-, and para-substituted nucleo-philes (entries 3-5). The method is compatible with a diverse set of functional groups, such as aryl methyl ethers (entries 3-5 and 9),14 acetals (entries 8 and 12), silyl ethers (entry 9), esters (entry 10), aryl chlorides and fluorides (entry 10),15 olefins (entry 11), alkyl chlorides (entry 12),16 and a Boc-protected nitrogen heterocycle (entry 13). Both of the catalyst components (NiCl2(PCy3)2 and L*) are commercially available and air-stable.
Table 2.
Catalytic Asymmetric Cross-Couplings of TMS-Protected Racemic Propargylic Carbonates (for the reaction conditions, see eq 1, R = TMS)a
| entry | alkyl | Ar | ee (%) | yield (%)b |
|---|---|---|---|---|
| 1 | Me |
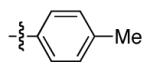
|
93 | 69 |
| 2 | n-Bu | Ph | 90 | 81 |
| 3 | n-Bu |
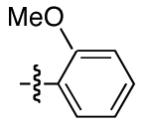
|
93 | 66 |
| 4 | n-Bu |
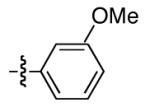
|
92 | 73 |
| 5 | n-Bu |
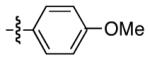
|
89 | 76 |
| 6 | i-Bu |
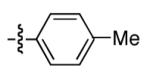
|
93 | 57 |
| 7 |
|
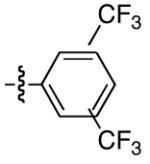
|
85 | 85 |
| 8 |
|
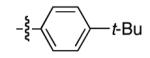
|
92 | 87 |
| 9 |
|
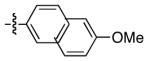
|
91 | 94 |
| 10 |
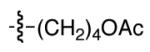
|
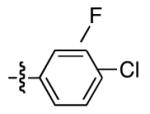
|
86 | 81 |
| 11 |
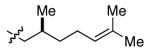
|
Ph | 89c | 79 |
| 12 |
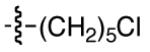
|
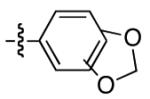
|
84 | 65 |
| 13 |
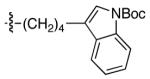
|
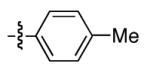
|
90 | 72 |
All data are the average of two experiments.
Yield of purified product.
de.
Although this method was optimized for asymmetric cross-couplings of readily deprotected TMS-substituted alkynes, we have determined that it can be applied without modification to other families of alkynes. Thus, regardless of whether the distal carbon of the propargylic carbonate bears a small or a large alkyl group, or an aromatic substituent, the stereoconvergent Negishi reaction proceeds with promising enantioselectivity and yield (eq 4-6).
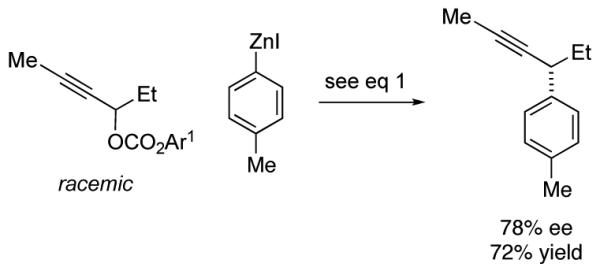 |
(4) |
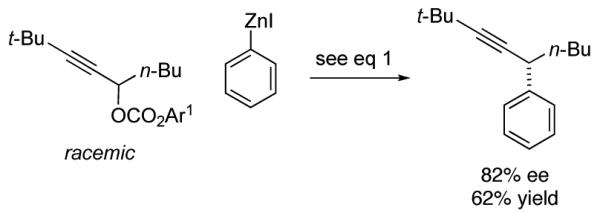 |
(5) |
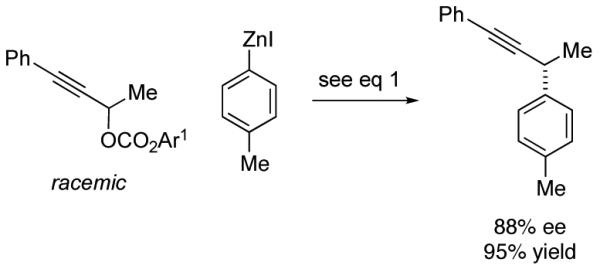 |
(6) |
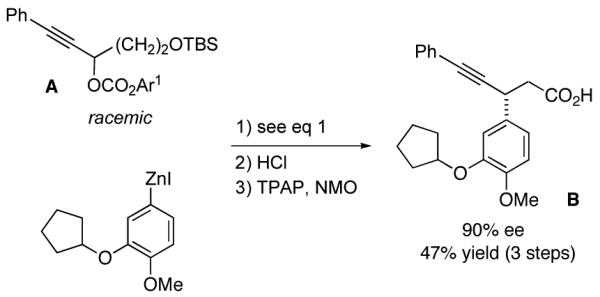
We have applied our method to the synthesis of alkyne B, which has potential utility for the treatment of allergic and inflammatory diseases.17 Thus, on a gram-scale, a catalytic asymmetric Negishi cross-coupling of propargylic carbonate A with a functionalized arylzinc reagent, followed by desilylation and oxidation, fur-nishes the target alkyne in 90% ee and 47% overall yield (three steps; eq 7).
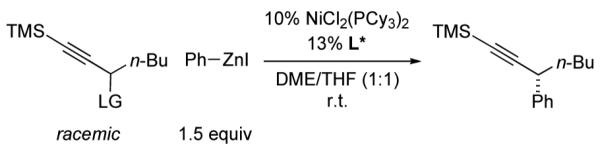
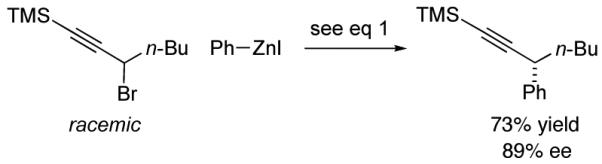
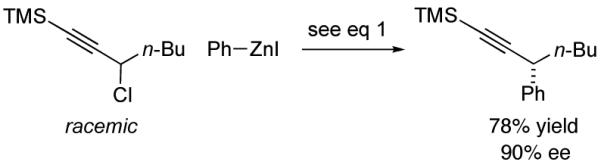
Although propargylic carbonates are not suitable cross-coupling partners (<2% ee and 5% yield) using our earlier procedure for Negishi reactions of propargylic bromides,10 our new method is fairly versatile, effective not only for propargylic carbonates (Table 2), but, without modification, also for propargylic bromides and chlorides (eq 8 and eq 9).
 |
(7) |
 |
(8) |
 |
(9) |
In summary, with respect to nickel-catalyzed enantioselective cross-couplings of alkyl electrophiles that bear oxygen leaving groups, only reactions of a small set of allylic alcohol derivatives with Grignard reagents had previously been reported to proceed in good ee and yield. We have established that a diverse array of racemic propargylic carbonates are suitable coupling partners in nickel/pybox-catalyzed asymmetric Negishi reactions. The method is compatible with a range of functional groups and employs commercially available catalyst components. The development of a versatile nickel-catalyzed enantioselective cross-coupling process for electrophiles that bear a leaving group other than a halide adds a significant new dimension to the scope of these reactions. Further investigations into the use of non-halide leaving groups, as well as studies to eluci-date the mechanism of this transformation, are under-way.
Supplementary Material
ACKNOWLEDGMENT
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences, grant R01-GM62871) and the Alexander von Humboldt Foundation (research fellowship for A.J.O.). We thank Dr. Wataru Muramatsu for a preliminary investigation.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Experimental procedures and compound characterization data (PDF). This material is available free of charge via the Internet at http://pubs.acs.org
REFERENCES
- (1).(a)For leading references, see: Zultanski SL, Fu GC. J. Am. Chem. Soc. 2011;133:15362–15364. doi: 10.1021/ja2079515. Owston NA, Fu GC. J. Am. Chem. Soc. 2010;132:11908–11909. doi: 10.1021/ja105924f. (b) For an initial study, see: Fischer C, Fu GC. J. Am. Chem. Soc. 2005;127:4594–4595. doi: 10.1021/ja0506509. (c) For multi-gram reactions: Lou S, Fu GC. Org. Synth. 2010;87:317–329. Lou S, Fu GC. Org. Synth. 2010;87:330–338.
- (2).For work by others, see: Caeiro J, Sestelo JP, Sarandeses LA. Chem. Eur. J. 2008;14:741–746. doi: 10.1002/chem.200701035.
- (3).For reviews and leading references, see: Rudolph A, Lautens M. Angew. Chem., Int. Ed. 2009;48:2656–2670. doi: 10.1002/anie.200803611. Glorius F. Angew. Chem., Int. Ed. 2008;47:8347–8349. doi: 10.1002/anie.200803509.
- (4).For a recent review with leading references, see: Rosen BM, Quasdorf KW, Wilson DA, Zhang N, Resmerita A-M, Garg NK. Percec, V. Chem. Rev. 2011;111:1346–1416. doi: 10.1021/cr100259t.
- (5).Enantioselective coupling reactions of secondary allylic electrophiles: Indolese AF, Consiglio G. Organometallics. 1994;13:2230–2234. and references therein. Nomura N, RajanBabu TV. Tetrahedron Lett. 1997;38:1713–1716. Gomez-Bengoa E, Heron NM, Didiuk MT, Luchaco CA, Hoveyda AH. J. Am. Chem. Soc. 1998;120:7649–7650. Nagel U, Nedden HG. Inorg. Chim. Acta. 1998;269:34–42. Chen H, Deng M-Z. J. Organomet. Chem. 2000;603:189–193. Chung K-G, Miyake Y, Uemura S. J. Chem. Soc., Perkin Trans. 2000;1:15–18. Chung K-G, Miyake Y, Uemu-ra S. J. Chem. Soc., Perkin Trans. 2000;1:2725–2729. No-vak A, Fryatt R, Woodward S. Comptes Rendus Chimie. 2007;10:206–212.
- (6).For suggestions of radical intermediates in nickel-catalyzed cross-coupling reactions of unactivated alkyl electro-philes, see: (a) Suzuki: Zhou J, Fu GC. J. Am. Chem. Soc. 2004;126:1340–1341. doi: 10.1021/ja039889k. (b) Hiyama: Powell DA, Fu GC. J. Am. Chem. Soc. 2004;126:7788–7789. doi: 10.1021/ja047433c. (c) Reference 1a. We expect that the pathway for oxidative addition will depend on a variety of factors, including the ligand, the leaving group, and the nature of the electrophile (e.g., unactivated vs. activat-ed).
- (7).For mechanistic proposals for Ni/terpyridine-catalyzed Negishi reactions of unactivated alkyl electrophiles, see: Jones GD, Martin JL, McFarland C, Allen OR, Hall RE, Haley AD, Brandon RJ, Konovalova T, Desrochers PJ, Pulay P, Vicic DA. J. Am. Chem. Soc. 2006;128:13175–13183. doi: 10.1021/ja063334i. Lin X, Phillips DL. J. Org. Chem. 2008;73:3680–3688. doi: 10.1021/jo702497p.
- (8).For a review and leading references, see: Mancuso J. In: Name Reactions for Homologations. Li JJ, editor. Vol. 1. John Wiley & Sons; Hoboken, NJ: 2009. pp. 614–632.
- (9).For leading references to the chemistry of alkynes, see: (a) Science of Synthesis; Thomas EJ, editor. Stuttgart. Vol. 43 2008. Thieme. Diederich F, Stang PJ, Tykwinski RR, editors. Acetylene Chemistry. Wiley-VCH; New York: 2005.
- (10).Smith SW, Fu GC. J. Am. Chem. Soc. 2008;130:12645–12647. doi: 10.1021/ja805165y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Notes: (a) Under our standard reaction conditions: es-sentially no cross-coupling product (<2%) is formed in the absence of NiCl2(PCy3)2, and very little (~5%) is generated in the absence of L*; a propargylic carbonate that includes a terminal alkyne is not a suitable substrate; an attempt to couple a hindered electrophile (alkyl = i-Pr) led to the formation of an allene; the corresponding propargylic iodide cross-couples in lower yield (<30%); there is no kinetic resolution of the propargylic carbonate during the course of a coupling reaction. (b) The cross-coupling illustrated in entry 2 of Table 2 proceeds in 91% ee and 48% yield (with 20% unreacted electrophile) in the presence of 5% NiCl2(PCy3)2 and 6.5% L*. (c) In a preliminary study under related conditions, a benzylic carbonate couples with an arylzinc reagent in 64% ee and 72% yield.
- (12).Using a nickel catalyst, but with a different leaving group (alkoxy), a different activating substituent (naphthyl and other extended aromatic), a different nucleophile (MeMgI), a different ligand (phosphine), etc., Jarvo has reported cross-couplings that proceed with inversion of stereochemistry: Taylor BLH, Swift EC, Waetzig JD, Jarvo ER. J. Am. Chem. Soc. 2011;133:389–391. doi: 10.1021/ja108547u. and references therein.
- (13).In the case of asymmetric Negishi reactions of propargylic bromides (Reference 10), ortho-substituted arylzinc reagents are not suitable coupling partners (yield <40%).
- (14).For examples of nickel-catalyzed cross-couplings of aryl methyl ethers, see Reference 4.
- (15).For a recent report of nickel-catalyzed Negishi reactions of aryl chlorides, see: Liu N, Wang L, Wang Z-X. Chem. Commun. 2011:1598–1600. doi: 10.1039/c0cc03064c.
- (16).For examples of nickel-catalyzed cross-couplings of unactivated primary alkyl chlorides, see: González-Bobes F, Fu GC. J. Am. Chem. Soc. 2006;128:5360–5361. doi: 10.1021/ja0613761.
- (17).Christensen SB, Karpinski JM, Frazee JS. Substituted-Pent-4-Ynoic Acids. U.S. Patent 6,037,367. 2000 March 14;
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


