Abstract
Previously, we reported that stimulation of selective serotonin (5-HT) receptor subtypes in the nucleus accumbens shell differentially affected consumption of freely available food. Specifically, activation of 5-HT6 receptors caused a dose-dependent increase in food intake, while the stimulation of 5-HT1/7 receptor subtypes decreased feeding [34]. The current experiments tested whether similar pharmacological activation of nucleus accumbens serotonin receptors would also affect appetitive motivation, as measured by the amount of effort non-deprived rats exerted to earn sugar reinforcement. Rats were trained to lever press for sugar pellets on a progressive ratio 2 schedule of reinforcement. Across multiple treatment days, three separate groups (N = 8–10) received bilateral infusions of the 5-HT6 agonist EMD 386088 (at 0.0, 1.0 and 4.0 μg/0.5 μl/side), the 5-HT1/7 agonist 5-CT (at 0, 0.5, 1.0, or 4.0 μg/0.5 μl/side), or the 5-HT2C agonist RO 60-0175 fumarate (at 0, 2.0, or 5.0 μg/0.5 μl/side) into the anterior medial nucleus accumbens prior to a 1-hr progressive ratio session. Stimulation of 5-HT6 receptors caused a dose-dependent increase in motivation as assessed by break point, reinforcers earned, and total active lever presses. Stimulation of 5-HT1/7 receptors increased lever pressing at the 0.5 μg dose of 5-CT, but inhibited lever presses and break point at 4.0 μg/side. Injection of the 5- HT2C agonist had no effect on motivation within the task. Collectively, these experiments suggest that, in addition to their role in modulating food consumption, nucleus accumbens 5-HT6 and 5-HT1/7 receptors also differentially regulate the appetitive components of food-directed motivation.
Keywords: Nucleus accumbens, serotonin, appetitive motivation, progressive ratio
The prevalence of obesity within the US has risen to approximately 33% [6], costing an estimated 147 billion dollars a year in health management and over 11 billion dollars in lost productivity in the workplace [12, 36]. Treatment of obesity continues to be difficult, with the weight loss following traditional diet and exercise plans commonly being regained within a 5-year period [7, 24]. Although pharmacotherapies targeting weight loss have had limited efficacy, the most successful to date have been drugs that increase serotonin output in the central nervous system. However, those drugs that globally enhance central serotonergic tone and promote weight loss, such as fenfluramine-phentermine combination therapy or sibutramine hydrochloride, have also been associated with increased cardiovascular risk and therefore have not remained viable treatment options. Nevertheless, the pursuit of serotonin-based treatment for obesity continues to be of interest, particularly as drugs are developed that specifically target selective serotonin receptors (of which there are 14 known types) that may have the potential for promoting healthier body weight without deleterious side-effects [19, 33, 39].
Serotonin signaling is known to affect feeding and satiety mechanisms due to its actions within the hypothalamus [8, 29], which regulates food intake and satiety in response to peripheral signals indicating the presence or absence of sufficient energy stores. However, serotonin projections from the raphe nuclei also innervate brain pathways involved in the sensory and motivational processing of food stimuli. Recent evidence has demonstrated that serotonin receptor stimulation of hindbrain feeding circuitry or the nucleus accumbens affects food intake -patterns [20, 23, 28, 34, 40]. Given that the current obesity epidemic has been caused, at least in part, by the relative over-abundance of palatable, highly caloric foods that activate neural reward circuitry [4, 27], it is of interest to understand how serotonergic signaling within sensory and motivational circuits may impact not only consummatory behavior, but also the appetitive motivation to seek out food.
Recently, we reported the effects of selective stimulation of 5-HT6, 5-HT1/7, and 5-HT2C receptors of the anterior nucleus accumbens shell on the intake of rat chow in food-restricted rats and on the intake of a palatable, sweetened fat diet in animals that were not food-deprived [34]. In both hungry and sated animals, stimulation of nucleus accumbens 5-HT1/7 receptors dose-dependently decreased food intake and locomotion, while stimulation of 5-HT6 receptors had the opposite effect, enhancing intake of both rat chow and the sweetened fat diet in the respective animal groups. 5-HT2C receptor stimulation had only modest effects on food intake; antagonism of 5-HT6, 5-HT7, and 5-HT2C receptors were generally without effect. Those findings suggested that individual serotonin receptors of the nucleus accumbens shell have separable functional roles on the consummatory phases of food-directed motivation. The purpose of the present set of experiments was to expand the examination of these serotonin receptor classes of the nucleus accumbens into a behavioral task that assesses the appetitive phase of food motivation. To do this, three groups of rats were trained in a progressive ratio 2 task, and subsequently tested following selective stimulation of 5-HT6, 5-HT1/7, or 5-HT2C receptors of the anterior medial nucleus accumbens.
All experiments were conducted in accordance to NIH animal care guidelines and were approved by the Wake Forest University Animal Care and Use Committee. Thirty adult male Sprague-Dawley rats (Harlan, Madison, WI) were acclimated to dual housing in a colony room maintained at ~21 °C with a 12-hr light–dark cycle. Following approximately one week of habituation to the laboratory environment and handling procedures, rats were slowly reduced to 90% of their ad libitum body weight.
Upon reaching their target weights, rats were habituated to standard operant chambers (Med Associates, St. Albans, VT) with three daily 30 min sessions of a random-time 30 sec (RT-30) reinforcement schedule, in which a sugar pellet was delivered to the food magazine approximately once every 30 sec. On the day following the final RT-30 session, two levers were extended into the chambers (one on each side of the food magazine). Presses on one lever were reinforced on a fixed ratio 1 (FR-1) schedule; presses on the opposite lever were never reinforced. Operant training proceeded for three sessions each on FR-1, FR-3, and FR-5 schedules of reinforcement, at which point all rats had achieved reliable responding on the active lever. On the day after the final FR-5 training session, rats were switched to a progressive ratio 2 (PR-2) schedule of reinforcement. In this schedule, the rat was reinforced for the first lever press and was then required to increase the number of responses by two lever presses for each subsequent pellet delivery. Thus, progressively more effort was required to earn each reinforcer. The number of responses required in the final completed ratio is referred to as the break point, a well-validated measure reflecting the strength of the reinforcer and the motivational state of the animal [1, 22]. At the end of 7 days with the PR-2 schedule, all rats had achieved high levels of lever responding. Rats were then given free access to food in their home cages for 5–7 days prior to the placement of intracranial guide cannulas. Rats remained on ad libitum feeding for the remainder of the experiment.
Standard aseptic surgical procedures were used to implant indwelling stainless steel guide cannulas (23 gauge) bilaterally above the anterior medial nucleus accumbens (with the nose bar set at +5.0 mm above the interaural plane; 3.1 mm anterior and 1.0 mm lateral to bregma, 5.0 mm ventral to skull surface). This region of the nucleus accumbens was targeted for the following three reasons: 1) we have previously shown that serotonin receptor stimulation of this region affects food consumption [34]; 2) both 5-HT2C and 5-HT6 receptors are expressed heavily in the anterior aspects of the nucleus accumbens shell [44]; and 3) it has been shown to be functionally connected with hypothalamic feeding and motivational circuitry [42].
After one week of recovery from surgery, rats were returned to the operant chambers, and the session length for the PR-2 schedule was increased to 1 hr. Once stable break points were achieved, rats were habituated to the microinjection procedure across two days. During the first mock infusion, injectors were lowered to the end of the guide cannula. On the second day, injectors were lowered 2.5 mm below the end of the guides into the medial nucleus accumbens. No solutions were delivered on mock injection days. Experimental treatments for each group began 72 hrs after the last mock injection. Three groups of rats were tested, with each group receiving 3–4 doses of a selective 5-HT receptor agonist across multiple treatment days, the order of which was randomly determined for each rat. In Experiment 1, rats received nucleus accumbens infusions of the 5-HT6 agonist EMD 386088 (at 0.0, 1.0 and 4.0 μg/0.5 μl/side; Tocris Biosciences). Rats in Experiment 2 received intracranial infusions of the 5-HT1/7 receptor agonist 5-CT (at 0.0, 0.5, 1.0, or 4.0 μg/0.5 μl/side; Tocris Biosciences). Experiment 3 tested the effects of medial nucleus accumbens infusions of the 5-HT2C receptor agonist RO 60-0175 fumarate (at 0.0, 2.0 or 5.0 μg/0.5 μl/side; Tocris Biosciences). 5-CT and RO 60-0175 were dissolved in sterile saline; EMD 386088 was dissolved in sterile saline containing 10% 2-hydroxypropyl-β-cyclodextrin (Sigma). To maintain solubility, 5-CT drug solutions were pH-balanced to the saline vehicle and Ph levels of RO 60-0175 solutions were raised to ~7.0. The chosen concentrations for each serotonergic agent were based upon solubility and effective doses in other behavioral paradigms [11, 14, 34]. Each drug treatment was separated by at least 2 days of additional PR-2 training to stabilize baseline performance. When the experiments were completed, the rats were sacrificed and the location of the injection site was verified utilizing standard histological procedures. Four rats were excluded from final analysis due to misplacement of cannula tips or necrosis at the injection site.
Dependent measures included the number of bar presses on the active and inactive levers, the total number of reinforcers earned, and the last completed reinforcement ratio (break point). Data from each experiment was analyzed using one-way repeated measures ANOVAs comparing the dependent measures across drug doses. Post-hoc analyses utilized Tukey’s HSD, as appropriate.
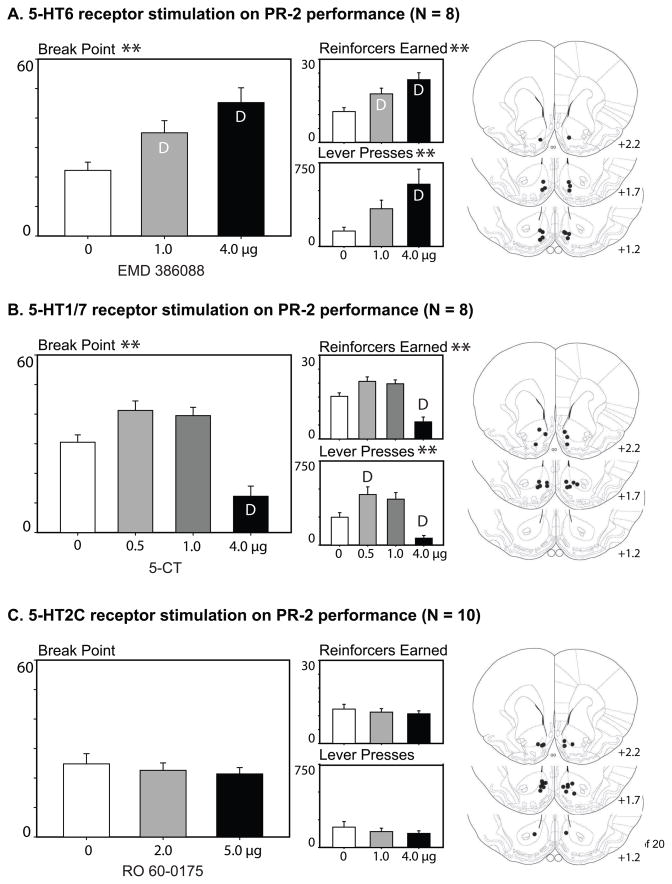
As shown in panel A of Figure 1, stimulation of nucleus accumbens 5-HT6 receptors with EMD 386088 significantly and dose-dependently increased the break point and number of reinforcers earned within the progressive ratio task (F2,14 = 16.88, p < .001). The total number of active lever presses were also increased (F2,14 = 9.73, p = .002). Tukey’s post-hoc analysis indicated a significant increase in break point and reinforcers earned at the 1.0 and 4.0 microgram dose of EMD 386088 as compared to the vehicle injection. Active lever presses significantly differed at the 4.0 microgram/side dose. Presses on the inactive lever did not differ between drug treatments, suggesting that a general locomotor increase was not responsible for the increases observed on the active lever (F2,14 = 2.52, p > 0.1; data not shown).
Figure 1.
Effects of selective serotonin receptor stimulation of the medial nucleus accumbens upon performance of the PR-2 task. Stimulation of 5-HT6 receptors with EMD 38606 dose- dependently increased break point, the number of reinforcers earned, and the number of lever presses on the active lever (A). In contrast, stimulation of 5-HT1/7 receptors with 5-CT caused an inhibition in break point and lever presses at the 4.0 micrograms/side, although the lowest dose (0.5 micrograms/side) increased the number of presses on the active lever (compared to vehicle injections) without significantly increasing breakpoint or reinforcers earned (B). 5-HT2C receptor stimulation with RO 60-0175 had no effect on performance in the progressive ratio task (C). The locations of the injector tips for animals included in the behavioral analyses are shown to the right for each experimental group. Brain drawings were adapted from The Rat Brain in Stereotaxic Coordinates, 4th ed., G. Paxinos and C. Watson, Figures 10, 11, and 13, copyright 1998.*p < 0.05, **p < 0.01 for drug effects; D indicates a significant difference from vehicle infusion according to post-hoc Tukey HSD analyses.
Stimulation of the 5-HT1/7 receptor subtypes of the nucleus accumbens shell significantly altered the total number of active lever presses across the drug doses of 5-CT (F3, 21 = 13.93, p < .001; see Figure 1, panel b). This pattern was biphasic, with the number of active lever presses significantly increased at the low dose of 5-CT, and decreased at the highest dose (according to Tukey’s HSD). Breakpoint and the number of reinforcers earned also were significantly affected by 5-HT1/7 receptor stimulation (F3, 21 = 20.08, p < .001); follow-up analyses with Tukey’s HSD verified significant decreases in both measures between the vehicle injection and the highest dose of 5-CT injected. However, the apparent increase in break point observed at the lower drug doses missed significance according to post-hoc analysis. There was no effect of 5-HT1/7 receptor stimulation on presses of the inactive lever (F3, 21 = 0.19, p = .90).
In contrast to the behavioral effects seen following 5-HT6 or 5-HT1/7 receptor agonism, the stimulation of 5-HT2C receptors of the medial nucleus accumbens with RO 60-0175 had no effect on the total number of active lever presses in this progressive ratio paradigm (F2,18 = 1.59, p = .23; See Figure 1, panel c), nor did it affect break point or the number of reinforcers earned (F2,18 = 1.15, p = .34). Lever presses on the inactive lever were also unaffected by drug dose (F2,18 = 0.75, p = .49).
The goal of these experiments was to determine if selective serotonin receptor stimulation of the anterior medial nucleus accumbens, with treatments that had been previously shown to alter the consumption of freely available food, would also affect the effort that rats exert to earn sugar reinforcement on a PR-2 paradigm. Stimulation of both the 5-HT6 and 5-HT1/7 receptors altered progressive ratio performance, while activation of 5-HT2C receptors had no effect on the effort rats exerted to earn sugar pellets. To our knowledge, these are the first data to report that serotonin receptors within the nucleus accumbens differentially regulate appetitive motivation for food reward.
The observed increase in break point following nucleus accumbens 5-HT6 receptor stimulation is consistent with our prior findings that similar treatments augment food consumption in hungry rats offered rat chow and also in non-restricted rats given 2-hr access to a palatable diet [34]. 5-HT6 receptors are distributed throughout the striatum [18, 37, 44], and have recently received attention as a potential target for pharmacotherapies targeting weight maintenance and cognitive deficits [17, 31, 43]. Genetically engineered mice that lack a functional 5-HT6 receptor gain less weight on a high-fat diet than wild-type mice [16], suggesting an important role for the 5-HT6 receptor for promoting food intake in the response to a palatable diet. However, the mechanisms underlying changes in food intake following 5-HT6 receptor manipulations remain unclear. There is evidence that both 5-HT6 receptor agonists and antagonists, when applied systemically, reduce food intake and body weight [13, 21, 46, 47]. Further research will be needed to understand the locus and mechanisms of action of the systemic treatments. The current data suggest that 5-HT6 receptors within the nucleus accumbens serve an important role in appetitive motivation for food reward.
The decrease in progressive ratio performance following the stimulation of 5-HT1/7 receptors at the high (4.0 μg) dose of 5-CT was consistent with previously reported decreases in food intake and locomotor activity following similar injections. 5-CT was initially chosen for our experiments due to its known action for stimulating neuronal activity of striatal acetylcholine-containing interneurons at the 5-HT7 receptor [5; also see discussion in 34]. However, in addition to its actions at the 5-HT7 receptor, 5-CT also has strong affinity at the 5-HT1-type receptors. That the lowest dose of 5-CT (0.5 μg/side) increased lever pressing was unexpected, and suggests the possibility that the different receptor classes that are stimulated by 5-CT may themselves serve differential roles in food-directed motivation. Systemically, 5-HT1A and 5-HT1B agonists alter food intake and shift feeding [9, 39]. Within the brain, activation of 5- HT1B receptors in the parabrachial nucleus of the pons inhibit food intake [28, 40]. Furthermore, stimulation of 5-HT1A receptors within the hypothalamus advance satiety processes [30], although activation of 5-HT1A autoreceptors in the raphe nuclei causes hyperphagia [2, 10]. Ongoing experiments, utilizing more selective ligands, are required to determine if the motivation, feeding and locomotor effects observed following nucleus accumbens 5-CT treatment are separable by individual receptor type.
The stimulation of nucleus accumbens 5-HT2C receptors had no effects on appetitive motivation for food as measured in this progressive ratio paradigm. This is interesting, given that intra-accumbens infusions of RO 60-0175 (at doses from 0.5 to 5.0 μg/side) are known to increase cocaine-induced locomotion, and enhance rats’ discrimination of low doses of cocaine [11]. Dopamine release within the nucleus accumbens has been argued to be under the regulation of local 5-HT2C receptors [32, 45, 48]. Dopamine has been heavily implicated in the signaling of incentive motivational processes, and dopamine receptor manipulations of nucleus accumbens circuitry regulate incentive motivation and effortful responding to achieve food reward [3, 27, 38]. It could be argued that 5-HT2C activation of the nucleus accumbens might increase appetitive motivation due to actions on promoting dopamine output, but that was not observed here. The current data (in combination with a similar lack of effect in food consumption as previously reported) suggest that intra-accumbens 5-HT2C receptor treatments that may be effective in modulating the behavioral effects of drugs of abuse may not have a significant impact on food-directed motivation, an important consideration in terms of deriving effective and specific pharmacotherapies targeted toward addiction.
The current experiments add to a growing literature that suggests that serotonergic signaling within the nucleus accumbens serves important and diverse roles in food-directed motivation [15, 23, 34, 35, 41]. Previous research has suggested that the hypophagic actions of systemic serotonergic agonists are primarily mediated by actions on hypothalamic circuitry involved in the homeostatic regulation of energy balance, with secondary actions on hindbrain circuitry involved in the sensory evaluation of foods [8, 20, 28, 29]. The nucleus accumbens, however, is a critical node in the evaluation of and direction of behavior towards rewards, whether they be natural (as in the case of food) or drug mediated (as in the case of drugs of abuse). Glutamate, dopamine, opioid, and acetylcholine signaling within the nucleus accumbens have been shown to be critical for regulating incentive motivational processes and the learning of operant responses to obtain food reward [3, 25–27, 38]. Little is yet known about the specific cellular distribution of individual serotonin receptors within striatum, or how they interface with the other neurotransmitters known to be involved in motivational processes [although see 44]. As it becomes apparent that serotonergic mechanisms within brain reward circuits modulate both natural and drug-reinforced behaviors, it will become critical to characterize the interactions of serotonin receptors with other neurotransmitter receptor classes, from the level of neuroanatomical examination of receptor location to the modulation of cellular- and circuit-level signaling to the behavior of the whole animal.
Research Highlights.
Selective 5-HT receptor agonism of the nucleus accumbens affects appetitive motivation.
Stimulation of 5-HT6 receptors increased break point in a progressive ratio task.
Activation of 5-HT1/7 receptors inhibited break point at a 4.0 μg/side dose of 5-CT.
Stimulation of medial nucleus accumbens 5-HT2C receptors was without effect.
Acknowledgments
We would like to thank Kara Clissold, Clifton Lewis, and Emma Edgar for their technical assistance during various phases of these projects. These studies were supported by R15 DA030618 and the Wake Forest University Department of Psychology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arnold JM, Roberts DCS. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–7. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- 2.Bendotti C, Samanin R. 8-Hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) elicits eating in free-feeding rats by acting on central serotonin neurons. Eur J Pharmacol. 1986;121:147–50. doi: 10.1016/0014-2999(86)90405-x. [DOI] [PubMed] [Google Scholar]
- 3.Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 4.Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1266–77. doi: 10.1152/ajpregu.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonsi P, Cuomo D, Ding J, Sciamanna G, Ulrich S, Tscherter A, Bernardi G, Surmeier DJ, Pisani A. Endogenous serotonin excites striatal cholinergic interneurons via the activation of 5-HT 2C, 5-HT6, and 5-HT7 serotonin receptors: implications for extrapyramidal side effects of serotonin reuptake inhibitors. Neuropsychopharmacology. 2007;32:1840–54. doi: 10.1038/sj.npp.1301294. [DOI] [PubMed] [Google Scholar]
- 6.CDC Website. [Accessed 12/20/2011.];Overweight and Obesity: US Obesity Trends. http://www.cdc.gov/obesity/data/trends.html.
- 7.Christiansen T, Bruun JM, Madsen EL, Richelsen B. Weight loss maintenance in severely obese adults after an intensive lifestyle intervention: 2- to 4-year follow-up. Obesity (Silver Spring) 2007;15:413–20. doi: 10.1038/oby.2007.530. [DOI] [PubMed] [Google Scholar]
- 8.Currie PJ, Coscina DV. Metergoline potentiates natural feeding and antagonizes the anorectic action of medical hypothalamic 5-hydroxytryptamine. Pharmacol Biochem Behav. 1996;53:1023–8. doi: 10.1016/0091-3057(95)02097-7. [DOI] [PubMed] [Google Scholar]
- 9.De Vry J, Schreiber R. Effects of selected serotonin 5-HT(1) and 5-HT(2) receptor agonists on feeding behavior: possible mechanisms of action. Neurosci Biobehav Rev. 2000;24:341–53. doi: 10.1016/s0149-7634(99)00083-4. [DOI] [PubMed] [Google Scholar]
- 10.Dourish CT, Hutson PH, Kennett GA, Curzon G. 8-OH-DPAT-induced hyperphagia: its neural basis and possible therapeutic relevance. Appetite. 1986;7(Suppl):127–40. doi: 10.1016/s0195-6663(86)80058-7. [DOI] [PubMed] [Google Scholar]
- 11.Filip M, Cunningham KA. Serotonin 5-HT(2C) receptors in nucleus accumbens regulate expression of the hyperlocomotive and discriminative stimulus effects of cocaine. Pharmacol Biochem Behav. 2002;71:745–56. doi: 10.1016/s0091-3057(01)00741-9. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–31. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 13.Fisas A, Codony X, Romero G, Dordal A, Giraldo J, Merce R, Holenz J, Vrang N, Sorensen RV, Heal D, Buschmann H, Pauwels PJ. Chronic 5-HT6 receptor modulation by E-6837 induces hypophagia and sustained weight loss in diet-induced obese rats. Br J Pharmacol. 2006;148:973–83. doi: 10.1038/sj.bjp.0706807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher PJ, Chintoh AF, Sinyard J, Higgins GA. Injection of the 5-HT2C receptor agonist Ro60–0175 into the ventral tegmental area reduces cocaine-induced locomotor activity and cocaine self-administration. Neuropsychopharmacology. 2004;29:308–18. doi: 10.1038/sj.npp.1300319. [DOI] [PubMed] [Google Scholar]
- 15.Francis HM, Kraushaar NJ, Hunt LR, Cornish JL. Serotonin 5-HT4 receptors in the nucleus accumbens are specifically involved in the appetite suppressant and not locomotor stimulant effects of MDMA ('ecstasy') Psychopharmacology (Berl) 2011;213:355–63. doi: 10.1007/s00213-010-1982-9. [DOI] [PubMed] [Google Scholar]
- 16.Frassetto A, Zhang J, Lao JZ, White A, Metzger JM, Fong TM, Chen RZ. Reduced sensitivity to diet-induced obesity in mice carrying a mutant 5-HT6 receptor. Brain Res. 2008;1236:140–4. doi: 10.1016/j.brainres.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Garfield AS, Heisler LK. Pharmacological targeting of the serotonergic system for the treatment of obesity. J Physiol. 2009;587:49–60. doi: 10.1113/jphysiol.2008.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerard C, Martres MP, Lefevre K, Miquel MC, Verge D, Lanfumey L, Doucet E, Hamon M, Mestikawy S. Immuno-localization of serotonin 5-HT6 receptor-like material in the rat central nervous system. Brain Res. 1997;746:207–19. doi: 10.1016/s0006-8993(96)01224-3. [DOI] [PubMed] [Google Scholar]
- 19.Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs : effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67:27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- 20.Hayes MR, Covasa M. Dorsal hindbrain 5-HT3 receptors participate in control of meal size and mediate CCK-induced satiation. Brain Res. 2006;1103:99–107. doi: 10.1016/j.brainres.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 21.Heal DJ, Smith SL, Fisas A, Codony X, Buschmann H. Selective 5-HT6 receptor ligands: progress in the development of a novel pharmacological approach to the treatment of obesity and related metabolic disorders. Pharmacol Ther. 2008;117:207–31. doi: 10.1016/j.pharmthera.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–4. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 23.Jean A, Conductier G, Manrique C, Bouras C, Berta P, Hen R, Charnay Y, Bockaert J, Compan V. Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104:16335–40. doi: 10.1073/pnas.0701471104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, Hill DR. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19:5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 25.Kelley AE, Andrzejewski ME, Baldwin AE, Hernandez PJ, Pratt WE. Glutamate-mediated plasticity in corticostriatal networks: role in adaptive motor learning. Ann N Y Acad Sci. 2003;1003:159–68. doi: 10.1196/annals.1300.061. [DOI] [PubMed] [Google Scholar]
- 26.Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- 27.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry, food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–95. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 28.Lee MD, Aloyo VJ, Fluharty SJ, Simansky KJ. Infusion of the serotonin1B (5-HT1B) agonist CP-93,129 into the parabrachial nucleus potently and selectively reduces food intake in rats. Psychopharmacology (Berl) 1998;136:304–7. doi: 10.1007/s002130050570. [DOI] [PubMed] [Google Scholar]
- 29.Leibowitz SF, Weiss GF, Suh JS. Medial hypothalamic nuclei mediate serotonin's inhibitory effect on feeding behavior. Pharmacol Biochem Behav. 1990;37:735–42. doi: 10.1016/0091-3057(90)90556-w. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Alonso VE, Mancilla-Diaz JM, Rito-Domingo M, Gonzalez-Hernandez B, Escartin-Perez RE. The effects of 5-HT1A and 5-HT2C receptor agonists on behavioral satiety sequence in rats. Neurosci Lett. 2007;416:285–8. doi: 10.1016/j.neulet.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell ES, Neumaier JF. 5-HT6 receptors: a novel target for cognitive enhancement. Pharmacol Ther. 2005;108:320–33. doi: 10.1016/j.pharmthera.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Navailles S, De Deurwaerdere P, Porras G, Spampinato U. In vivo evidence that 5-HT2C receptor antagonist but not agonist modulates cocaine-induced dopamine outflow in the rat nucleus accumbens and striatum. Neuropsychopharmacology. 2004;29:319–26. doi: 10.1038/sj.npp.1300329. [DOI] [PubMed] [Google Scholar]
- 33.Nilsson BM. 5-Hydroxytryptamine 2C (5-HT2C) receptor agonists as potential antiobesity agents. J Med Chem. 2006;49:4023–34. doi: 10.1021/jm058240i. [DOI] [PubMed] [Google Scholar]
- 34.Pratt WE, Blackstone K, Connolly ME, Skelly MJ. Selective serotonin receptor stimulation of the medial nucleus accumbens causes differential effects on food intake and locomotion. Behav Neurosci. 2009;123:1046–57. doi: 10.1037/a0016882. [DOI] [PubMed] [Google Scholar]
- 35.Pratt WE, Connolly ME. Contrasting effects of systemic and central sibutramine administration on the intake of a palatable diet in the rat. Neurosci Lett. 2010;484:30–4. doi: 10.1016/j.neulet.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Ricci JA, Chee E. Lost productive time associated with excess weight in the U.S. workforce. J Occup Environ Med. 2005;47:1227–34. doi: 10.1097/01.jom.0000184871.20901.c3. [DOI] [PubMed] [Google Scholar]
- 37.Ruat M, Traiffort E, Arrang JM, Tardivel-Lacombe J, Diaz J, Leurs R, Schwartz JC. A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem Biophys Res Commun. 1993;193:268–76. doi: 10.1006/bbrc.1993.1619. [DOI] [PubMed] [Google Scholar]
- 38.Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- 39.Simansky KJ. Serotonergic control of the organization of feeding and satiety. Behav Brain Res. 1996;73:37–42. doi: 10.1016/0166-4328(96)00066-6. [DOI] [PubMed] [Google Scholar]
- 40.Simansky KJ, Nicklous DM. Parabrachial infusion of D-fenfluramine reduces food intake. Blockade by the 5-HT(1B) antagonist SB-216641. Pharmacol Biochem Behav. 2002;71:681–90. doi: 10.1016/s0091-3057(01)00740-7. [DOI] [PubMed] [Google Scholar]
- 41.Soria-Gomez E, Marquez-Diosdado MI, Montes-Rodriguez CJ, Estrada-Gonzalez V, Prospero-Garcia O. Oleamide administered into the nucleus accumbens shell regulates feeding behaviour via CB1 and 5-HT2C receptors. Int J Neuropsychopharmacol. 2010;13:1247–54. doi: 10.1017/S1461145710000702. [DOI] [PubMed] [Google Scholar]
- 42.Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–8. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Upton N, Chuang TT, Hunter AJ, Virley DJ. 5-HT6 receptor antagonists as novel cognitive enhancing agents for Alzheimer's disease. Neurotherapeutics. 2008;5:458–69. doi: 10.1016/j.nurt.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward RP, Dorsa DM. Colocalization of serotonin receptor subtypes 5-HT2A, 5-HT2C, and 5-HT6 with neuropeptides in rat striatum. J Comp Neurol. 1996;370:405–14. doi: 10.1002/(SICI)1096-9861(19960701)370:3<405::AID-CNE10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 45.Willins DL, Meltzer HY. Serotonin 5-HT2C agonists selectively inhibit morphine-induced dopamine efflux in the nucleus accumbens. Brain Res. 1998;781:291–9. doi: 10.1016/s0006-8993(97)01267-5. [DOI] [PubMed] [Google Scholar]
- 46.Woolley ML, Bentley JC, Sleight AJ, Marsden CA, Fone KC. A role for 5-ht6 receptors in retention of spatial learning in the Morris water maze. Neuropharmacology. 2001;41:210–9. doi: 10.1016/s0028-3908(01)00056-9. [DOI] [PubMed] [Google Scholar]
- 47.Woolley ML, Marsden CA, Fone KC. 5-ht6 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:59–79. doi: 10.2174/1568007043482561. [DOI] [PubMed] [Google Scholar]
- 48.Yan QS. Activation of 5-HT2A/2C receptors within the nucleus accumbens increases local dopaminergic transmission. Brain Res Bull. 2000;51:75–81. doi: 10.1016/s0361-9230(99)00208-7. [DOI] [PubMed] [Google Scholar]