Abstract
The mechanisms that deprive HDL of its cardioprotective properties are poorly understood. One potential pathway involves oxidative damage of HDL proteins by myeloperoxidase (MPO) a heme enzyme secreted by human artery wall macrophages. Mass spectrometric analysis demonstrated that levels of 3-chlorotyrosine and 3-nitrotyrosine—two characteristic products of MPO—are elevated in HDL isolated from patients with established cardiovascular disease. When apolipoprotein A-I (apoA-I), the major HDL protein, is oxidized by MPO, its ability to promote cellular cholesterol efflux by the membrane-associated ATP-binding cassette transporter A1 (ABCA1) pathway is diminished. Biochemical studies revealed that oxidation of specific tyrosine and methionine residues in apoA-I contributes to this loss of ABCA1 activity. Another potential mechanism for generating dysfunctional HDL involves covalent modification of apoA-I by reactive carbonyls, which have been implicated in atherogenesis and diabetic vascular disease. Indeed, modification of apoA-I by malondialdehyde (MDA) or acrolein also markedly impaired the lipoprotein’s ability to promote cellular cholesterol efflux by the ABCA1 pathway. Tandem mass spectrometric analyses revealed that these reactive carbonyls target specific Lys residues in the C-terminus of apoA-I. Importantly, immunochemical analyses showed that levels of MDA-protein adducts are elevated in HDL isolated from human atherosclerotic lesions. Also, apoA-I co-localized with acrolein adducts in such lesions. Thus, lipid peroxidation products might specifically modify HDL in vivo. Our observations support the hypotheses that MPO and reactive carbonyls might generate dysfunctional HDL in humans.
Keywords: Myeloperoxidase, malondialdehyde, acrolein, dysfunctional HDL, 3-chlorotyrosine, coronary artery disease
1. Introduction
Despite enormous progress in preventing and treating cardiovascular disease, atherosclerosis remains the leading cause of death in industrialized societies (1). One important risk factor is an elevated level of low-density lipoprotein (LDL), the major carrier of cholesterol in the blood of humans on a Western diet (2). However, a high LDL level may not by itself be sufficient, because in vitro studies suggest that LDL must be modified to be atherogenic (3–5). For example, macrophages become foam cells, a hallmark of atherosclerosis, by taking up excess lipid, but they fail to accumulate lipid in vitro when incubated with high concentrations of native LDL. In contrast, they rapidly take up oxidized LDL.
Macrophage foam cells are abundant at all stages of the atherosclerotic process. Moreover, oxidative modifications of LDL are known to promote cholesterol uptake by the macrophage scavenger receptors that trigger foam cell formation (3). Significantly, oxidized LDL has also been detected in human atherosclerotic lesions, raising the possibility that oxidative modification of lipoproteins may be clinically important.
Unlike LDL, high-density lipoprotein (HDL) protects the artery wall from atherosclerosis (6). Many potential mechanisms have been proposed for HDL’s anti-atherogenic effects, including the ability of HDL to inhibit inflammation and regulate NO production by endothelial cells (7–9). However, the best established cardioprotective effect of HDL is its role in reverse cholesterol transport (10). In this scenario, HDL removes excess cholesterol from artery wall macrophages and transports it back to the liver for excretion in bile. Apolipoprotein A-I (apoA-I), the major protein of HDL, plays a critical role in the first step of reverse cholesterol transport by enhancing the sterol efflux from macrophages. Thus, a severe deficiency of apoA-I increases CAD in both humans and mice (11). In contrast, overexpression of apoA-I in transgenic mice and rabbits increased HDL levels and consistently reduced CAD (12–14). Administering small apolipoprotein-mimetic peptides to mice also reduced atherogenesis (15, 16). These findings support the possibility that the arterial supply or activity of apoA-I and HDL particles can influence the progression and/or regression of lesions.
Active removal of cholesterol from macrophages to HDL is mediated by ABCA1 and ABCG1, two membrane-bound proteins called ATP-binding cassette (ABC) transporters (17–19). Another membrane-bound protein termed scavenger receptor B1 (SR-B1) can also play a role in removing cholesterol from macrophages (20). However, factors that might prevent apoA-I and HDL from interacting with cholesterol transporters have not yet been identified. One important pathway may involve reactive intermediates that modify artery wall proteins.
In this article, we review evidence that several reactive intermediates, including malondialdehyde (MDA), acrolein, and oxidants generated by myeloperoxidase (MPO), modify HDL in humans. We emphasize our recent findings that these reactive intermediates target specific amino acid residues in apoA-I, and discuss how such damage impairs HDL’s ability to transport cholesterol by the ABCA1 pathway.
2. One key cardioprotective property of HDL involves reverse cholesterol transport
HDL—originally classified by its density on ultracentrifugation—is a noncovalent assembly of proteins and lipids (21). About ~70% of its protein mass is apoA-I (22), a 28-kDa peptide. Although most circulating apoA-I is found in HDL, ~5% of the apoA-I in plasma is thought to be lipid-free or lipid-poor (22, 23). ApoA-I has 243 amino acid residues, and its 10 repeated sequences of 11 or 22 amino acids (often termed helical repeats) play a key role in its secondary and tertiary structure (24).
Clinical, epidemiological, and animal studies have demonstrated a strong inverse relationship between HDL level and risk for coronary artery disease (25–27), suggesting that HDL is cardioprotective. This property has been attributed to several distinct pathways (6, 25). Many lines of evidence, including studies of myeloid cells deficient in ABC transporters, indicate that one key protective mechanism is cholesterol removal from macrophages by HDL and/or apoA-I (17, 28, 29). HDL components can remove cellular cholesterol by multiple mechanisms in vitro (30, 31). For example, plasma membrane cholesterol can be transferred to HDL phospholipids by passive diffusion, a process that is facilitated by interaction of HDL particles with SR-B1 (20).
An energy-dependent pathway for reverse cholesterol transport to apoA-I involves ABCA1 and ABCG1. ABCA1 is highly expressed in the liver and tissue macrophages (32, 33). When mutated, it can cause Tangier disease, which involves abnormally low levels of HDL and premature atherosclerosis (6, 17, 18, 34–37). Moreover, myeloid-specific deletion of ABCA1 markedly increased atherosclerosis in mice without affecting plasma lipoprotein levels (6). The ABCA1 pathway mediates the transport of cholesterol, phospholipids, and other lipophilic molecules across cellular membranes, where they are removed from cells mainly by lipid-free or lipid-poor apoA-I (17, 38).
The other major transporter, ABCG1, is highly expressed in tissue macrophages, and it mediates cholesterol transport from cells to mature, lipid-containing HDL particles (39, 40). The lungs of mice deficient in ABCG1 accumulate massive quantities of sterol- and lipid-laden macrophages (41), indicating that the transporter is also involved in cholesterol efflux from macrophages in vivo (39, 40). However, selective ABCG1 deficiency does not always promote atherosclerosis in hypercholesterolemic mouse models (42–44). In contrast, atherosclerosis is markedly enhanced when mouse macrophages lack both ABCA1 and ABCG1 (45, 46). Thus, the two pathways appear to work together in mice to prevent foam cell formation and atherosclerosis.
3. HDL’s anti-inflammatory properties may contribute to its cardioprotective effects
Many lines of evidence support this proposal (47). For example, systemic inflammation and marked atherosclerosis are evident in hypercholesterolemic mice that are also deficient in apoA-I (48). Recent studies indicate that the animals’ immune system also is abnormal (49). Macrophages harvested from mice that lack ABCA1 alone or both ABCA1 and ABCG1 release markedly higher levels of inflammatory cytokines when stimulated with lipopolysaccharide (LPS), a bacterial ligand for the TLR4 receptor (31, 50, 51).
HDL also suppresses the type 1 interferon response in macrophages (9), which is of central importance in atherogenesis (52). Moreover, proteins carried by HDL play key roles in the acute-phase response, proteolysis, and the complement system, strongly linking the lipoprotein to modulation of inflammation (53). Indeed, inflammation significantly remodels both HDL lipids and proteins during acute inflammation (54, 55). Both mouse and human studies suggest that inflammatory HDL can become dysfunctional and lose its cardioprotective effects (28, 47, 56, 57), though the underlying mechanisms are poorly understood. One important pathway could involve oxidative damage (58–60).
4. Myeloperoxidase (MPO), a heme enzyme that produces reactive chlorinating and nitrating species, modifies artery wall proteins in humans
One potential pathway for generating dysfunctional HDL involves myeloperoxidase (MPO), a heme enzyme in neutrophils, monocytes, and some macrophage populations. Co-localization of macrophages with immunostaining for MPO has been observed in human atherosclerotic lesions, which also yield active enzyme (61). These observations suggest that lipid-laden macrophages, the cellular hallmark of the atherosclerotic lesion, express MPO in response to inflammatory stimuli associated with atherosclerosis.
Macrophages also produce reactive oxygen species, such as superoxide and hydrogen peroxide (H2O2). MPO uses H2O2 to generate reactive intermediates (62). The major end product at plasma concentrations of chloride ion is generally thought to be hypochlorous acid (HOCl), a potent anti-microbial agent (63, 64). HOCl is a powerful chlorinating reagent that reacts with a wide variety of biomolecules (65). For example, HOCl produced by MPO converts free and protein-bound tyrosine (Tyr) residues to 3-chlorotyrosine (66, 67), which is generated by acute inflammation in mice. Importantly, mice deficient in MPO fail to produce 3-chlorotyrosine (7), demonstrating that this abnormal amino acid is a molecular fingerprint that implicates the enzyme in oxidative damage to proteins (67–69).
Another pathway for oxidizing artery wall proteins involves the nitric oxide (NO) to promote vasodilation (70, 71). NO reacts rapidly with superoxide (O2•−) to form peroxynitrite (ONOO−), a reactive nitrofen species (72). Macrophages are a rich source of both O2•− and NO, suggesting that ONOO− may be an important reactive nitrogen species in vivo. Furthermore, oxidation of NO produces nitrite (NO2−), which MPO and H2O2 convert to nitrogen dioxide radical (NO2•), another potent nitrating intermediate (73, 74). Both ONOO− and NO• generate 3-nitrotyrosine when they react with tyrosine residues. Such reactive nitrogen species might promote inflammation by nitrating lipoproteins and other artery wall proteins.
Mass spectrometric analyses have detected high levels of 3-chlorotyrosine and 3-nitrotyrosine in LDL and HDL isolated from human atherosclerotic tissue (69), strongly suggesting that MPO is one pathway for oxidative damage in the human artery wall. Moreover, tyrosine chlorination and nitration are impaired when mice are deficient in MPO (68). Thus, the MPO pathway might both nitrate and chlorinate lipoproteins in vivo.
4.1 MPO oxidizes HDL in humans with cardiovascular disease
Despite considerable interest in the atherogenic potential of oxidized lipoproteins, the pathways that promote HDL oxidation in vivo remain unclear One potential mechanism for the formation of dysfunctional HDL involves reactive intermediates generated by MPO. Indeed, recent studies from two different groups strongly suggest that MPO oxidizes HDL in humans (58–60).
Our group isolated HDL from plasma of subjects with established CAD and from age- and sex-matched healthy subjects (58, 59). Using isotope dilution mass spectrometry (MS), we detected much higher levels of protein-bound 3-chlorotyrosine and 3-nitrotyrosine in circulating HDL from the CAD patients than in HDL from the controls (58, 59). Moreover, levels of both abnormal amino acids were significantly higher in HDL isolated from atherosclerotic lesions than in plasma HDL (58, 59). Other investigators have reported similar results (60). As MPO is the only known source of chlorinating intermediates in humans and is a potent source of reactive nitrogen species in a mouse model of acute inflammation (68, 74), these observations strongly support the hypothesis that MPO oxidizes HDL in humans.
Because MPO oxidation products were undetectable in plasma LDL or total plasma proteins in these studies, it is unlikely that HDL was oxidized in the circulation. However, it might have been damaged before entering the circulation, perhaps in a microenvironment rich in MPO and depleted of antioxidants. One likely environment is the inflamed atherosclerotic lesion. It is important to note that HDL is thought to be cardioprotective by entering the artery wall, interacting with macrophages to remove cholesterol, and then leaving the artery wall and returning the cholesterol to the liver for excretion. In this scenario, chlorinated HDL detected in the plasma is derived from atherosclerotic lesions. Indeed, using antibodies to 3-nitrotyrosine and to proteins modified by HOCl, we demonstrated that chlorinated and nitrated adducts co-localize with macrophages in human atherosclerotic lesions (58, 59). Thus, plasma HDL in subjects with established CAD, but not in control subjects, may be enriched with chlorotyrosine, a marker of damage by MPO.
4.2 Macrophage-specific expression of MPO promotes atherosclerosis in mice
Mouse models have provided major insights into atherogenesis. However, macrophages in mouse atherosclerotic tissue do not express MPO (75). Thus, it has not been possible to use knockout mice to study the role of MPO in atherosclerosis because this only affects levels of the enzyme in circulating neutrophils and monocytes.
To address this important limitation of mouse models, we generated a transgenic mouse that expresses human MPO selectively in macrophages (76). This MPO transgenic (MPO-Tg) mouse line allowed us to directly examine the impact of MPO expression in macrophages on atherosclerosis. After LDL receptor knock-out (LDLR−/−) mice received bone marrow from these MPO-Tg mice and ate a high-fat, high-cholesterol diet, they had significantly more atherosclerosis than mice transplanted with bone marrow from either wild type or LDLR−/− mice. These observations strongly suggest that expression of MPO in macrophages promotes atherosclerosis in this mouse model of hypercholesterolemia.
4.3 MPO-mediated chlorination, but not nitration, of apoA-I impairs cholesterol transport by ABCA1
For oxidized HDL to promote atherosclerosis, oxidation would need to compromise HDL’s cardioprotective functions, such as cholesterol removal. In the artery wall, membrane-associated ABCA1 interacts with lipid-free or poorly-lipidated apoA-I to remove excess cholesterol from resident macrophages (17, 18, 77, 78). ApoA-I contains amphipathic α-helices, a structural motif that promotes lipid association (22, 24, 79, 80). Synthetic peptides that have α-helices but differ in primary sequence from apoA-I can also interact with ABCA1 to promote reverse cholesterol transport from cells. Therefore, interaction with ABCA1 appears to require α-helices, and agents that disrupt helical structure might inhibit cholesterol removal.
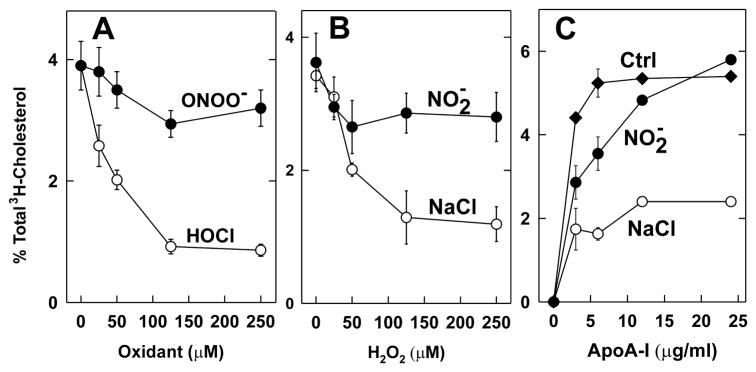
Because HDL isolated from humans with CAD contains elevated levels of 3-chlorotyrosine and 3-nitrotyrosine (58–60), we determined how oxidation affected apoA-I’s ability to promote cholesterol efflux from cells (58, 81). Oxidizing the protein with H2O2 had no effect. In striking contrast, exposing it to increasing concentrations of H2O2 in the presence of MPO and NaCl (the MPO-H2O2-NaCl system, which generates HOCl) progressively and severely impaired its ability to remove cellular cholesterol from BHK cells transfected with ABCA1 (Fig. 1B; ref. (81)). Similar results were observed with increasing concentrations of reagent HOCl (Fig. 1A, ref. (81)). This impairment correlated closely with the extent to which one particular tyrosine residue—Tyr192—was chlorinated (Fig. 2A; ref. (81, 82)). Although Zheng et al. reported similar results (60), they also found that nitrating apoA-I with MPO impaired its activity with ABCA1. In contrast, we found that nitrating apoA-I with ONOO- or H2O2 in the presence of MPO and NaNO2 (the MPO-H2O2-NaNO2 system) barely affected cholesterol efflux activity (Fig. 1; ref. (81)), despite extensive nitration of Tyr192 (Fig. 2B; ref. (81, 83)).
Figure 1. Cholesterol efflux activities of lipid-free apoA-I oxidized with HOCl, ONOO−, MPO-H2O2-chloride, or MPO-H2O2-nitrite.
ApoA-I (5 μM) was incubated with the indicated concentrations of HOCl, ONOO−, or H2O2 for 60 min at 37 °C in phosphate buffer. The reaction was terminated by adding methionine. Where indicated, the system was supplemented with 50 nM myeloperoxidase (MPO) and 100 μM nitrite (NO2−) or 100 mM NaCl (NaCl). (A, B) [3H]Cholesterol-labeled ABCA1-transfected BHK cells were incubated for 2 h with native (0 μM oxidant), HOCl-oxidized, ONOO−-oxidized, or MPO-H2O2-oxidized apoA-I (5 μg/mL). (C) [3H]Cholesterol-labeled ABCA1-transfected BHK cells were incubated with the indicated concentration of apoA-I for 2 h. ApoA-I was incubated with MPO and 0 μM oxidant (Ctrl), 125 μM H2O2 plus 100 μM nitrite (NO2), or 100 mM NaCl (NaCl). At the end of the incubation, [3H]cholesterol efflux to the acceptor apolipoprotein was measured (81).
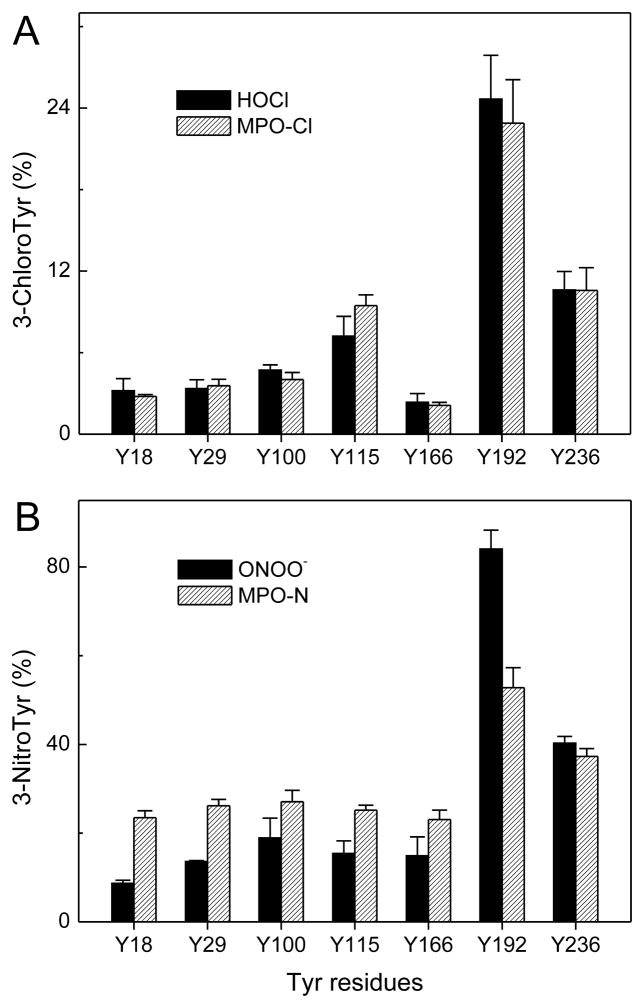
Figure 2. Site-specific chlorination of tyrosine residues in apoA-I exposed to HOCl or the MPO-H2O2-NaCl system (A) and nitration of tyrosine residues in apoA-I exposed to ONOO− or the MPO-H2O2-NaNO2 system (B).
ApoA-I (10 μM) was exposed to HOCl (A, solid bars), ONOO− (B, solid bars), or H2O2 in the MPO-chloride system (A, single-line, shaded bars) or MPO-nitrite system (B, single-line, shaded bars) at molar ratio of 25:1 (oxidant/apoA-I) for 60 min at 37 °C in phosphate buffer (100 μM DTPA, 20 mM sodium phosphate, pH 7.4). After the reaction was terminated with L-methionine, a tryptic digest of oxidized apoA-I was analyzed by MS and tandem MS, and the oxidized peptides were detected and quantified, using reconstructed ion chromatograms of precursor and product peptides. Product yield (%) = peak area of product ion/sum (peak area of precursor ion + peak areas of product ions) × 100. Peptide sequences were confirmed using tandem MS. Results are from 3 independent experiments (mean ± SD) (83).
Our observations suggest that oxidation of apoA-I by HOCl or the MPO chlorinating system, but not by the MPO nitrating system, selectively impairs the protein’s ability to remove cholesterol from cells by a pathway requiring ABCA1. Thus, HOCl generated by MPO might be a mechanism for generating dysfunctional HDL, for impairing ABCA1-dependent cholesterol efflux from macrophage foam cells in the human artery wall, and therefore for promoting the development of atherosclerotic plaque.
4.4 The YXXK motif directs site-specific tyrosine chlorination in apoA-I
Most studies of protein oxidation have focused on the vulnerability of individual amino acid side chains. Remarkably little is known about the influence of nearby residues or specific motifs. To explain the selective oxidation of Tyr192 (58, 81, 82), we hypothesized that Lys residues located in a YXXK motif (Y = tyrosine, K = lysine, X = unreactive amino acid) promote Tyr chlorination in proteins. Thus, Tyr would be oxidized permanently but Lys only transiently.
When we tested our model by using synthetic peptides, we found that lysine residues located in a YXXK motif can direct the regiospecific chlorination of nearby tyrosine residues by a pathway involving chloramine formation (82). Long-lived chloramines (63), which have been proposed to chlorinate Tyr in synthetic peptides (66, 82), form when HOCl (or other chlorinating agents) reacts rapidly with the ε-amino group of Lys. As Tyr192 is two residues away from Lys195 in apoA-I’s primary sequence, we proposed that site-specific chlorination of Tyr192 in apoA-I by MPO requires the participation of Lys195, which is located in a YXXK motif.
Using hydrogen-deuterium exchange, Zheng et al. showed that MPO interacts with the region of apoA-I that contains Tyr192 (60). Based on that observation, the group proposed an alternative model in which MPO must bind directly to that region to promote site-specific chlorination of Tyr192, perhaps via interactions with positively charged amino acids such as Lys. To distinguish between their model and ours, we engineered a series of mutations in the cDNA of human apoA-I, using site-directed mutagenesis (84). Studies with those mutations provided strong evidence that YXXK can direct the regiospecific chlorination of tyrosine residues in apoA-I. For example, chlorination of Tyr192 was blocked when we mutated Lys195 to arginine. Also, tyrosine residues that normally resist chlorination were chlorinated in high yield when we introduced the KXXY motif into that region of the protein.
Importantly, we observed virtually identical results when we used reagent HOCl or the complete MPO chlorinating system (84). The reaction with reagent HOCl clearly cannot involve direct interaction or binding of MPO with apoA-I. These observations strongly support our proposal that the YXXK motif orchestrates the regiospecific chlorination of tyrosine residues in proteins. They argue against the hypothesis that MPO must interact directly with apoA-I to selectively chlorinate Tyr192.
4.5 MPO impairs ABCA1-dependent cholesterol export from cells by oxidizing methionine residues and promoting site-specific chlorination of tyrosine 192 in apoA-I
Amino acid analysis identified tyrosine, methionine, and phenylalanine residues as the major targets when HOCl oxidizes apoA-I in vitro (85–87). However, those studies did not determine whether residues at specific locations are especially vulnerable to chlorination. Using tandem MS, we identified a single tyrosine residue, Tyr192, as the major chlorination site when HOCl oxidizes apoA-I (Fig. 2A) (81–83). Moreover, we noted a strong linear association between the extent of Tyr192 chlorination and loss of ABCA1 transport activity (81).
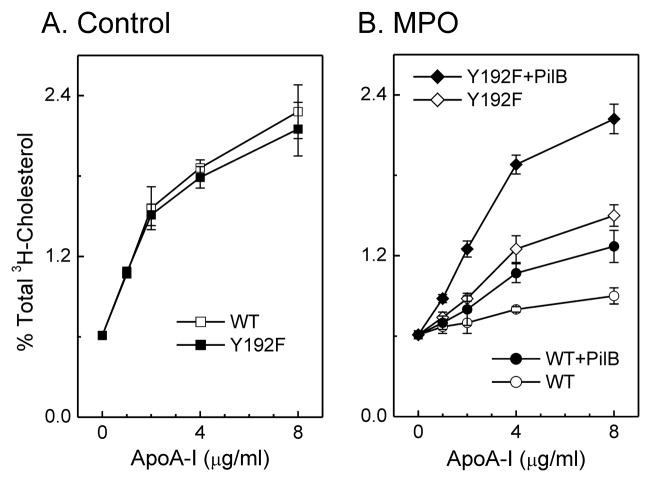
To determine whether tyrosine chlorination in apoA-I reduces ABCA1 activity, we converted the Tyr192 in apoA-I to phenylalanine (Tyr192Phe). The relationship between protein concentration and ABCA1-dependent cholesterol efflux was virtually identical for the mutant protein and the wild type protein (Fig. 3A; ref. (84)), strongly suggesting that the Tyr192Phe mutation had no major impact on the structure of lipid-free apoA-I. When the mutant protein was exposed to either HOCl or the MPO chlorinating system, the substitution offered a small but significant protection against inactivation (Fig. 3B) (84). Other investigators replaced all seven tyrosine residues in apoA-I with phenylalanine, which is resistant to chlorination. As with wild type apoA-I, this mutant was unable to promote ABCA1-dependent cholesterol efflux after being oxidized by MPO (88). However, our Tyr192Phe mutant appeared somewhat resistant to inactivation when the concentration of oxidant was high (Fig. 3B) (84). The differences between the two mutant proteins may reflect our use of transfected cells that express high levels of ABCA1, which may be more sensitive to subtle changes in apoA-I’s activity.
Figure 3. Cholesterol efflux activities of apoA-I (WT) and Tyr192Phe (Y192F) apoA-I exposed to the MPO system and reduced by PilB.
(A) [3H]Cholesterol-labeled ABCA1-transfected BHK cells were incubated with the indicated concentration of apoA-I (WT) or Tyr192Phe apoA-I (Y192F) for 2 h. At the end of the incubation, [3H]cholesterol efflux to the acceptor apolipoprotein was measured. (B) ApoA-I or Tyr192Phe apoA-I (5 μM) was oxidized by the MPO-H2O2-NaCl system (25:1, mol/mol, H2O2/apoA-I) for 60 min at 37 °C in phosphate buffer (100 μM DTPA, 20 mM sodium phosphate, pH 7.4). The reaction was terminated by adding methionine. Where indicated, apoA-I was incubated with the methionine sulfoxide reductase PilB. [3H]Cholesterol-labeled ABCA1-transfected BHK cells were incubated with the indicated concentration of lipoproteins, and cholesterol efflux was measured at the end of the incubation. Results are means ± SD of 3 determinations and are representative of 3 independent experiments (84).
HOCl reacts more strongly with the alkylated thiol of methionine than with Tyr residues (89). Moreover, methionine sulfoxide [Met(O)] has been detected in circulating HDL (90). However, the role of methionine oxidation in apoA-I’s cholesterol efflux activity has been unclear. Using tandem MS, we confirmed that HOCl or the complete MPO system quantitatively oxidized all three methionine residues in apoA-I to Met(O) (84). To determine whether Met(O) contributed to the loss of ABCA1 activity, we used a bacterial methionine sulfoxide reductase that converts both the R- and S-forms of Met(O) residues back to methionine (91). Methionine sulfoxide reductase completely reversed methionine oxidation in apoA-I that had been exposed to MPO (84). However, the protein’s cholesterol efflux activity was only partly restored (Fig. 3B; ref (84)), implying that oxidation of Met residues alone is insufficient to eliminate that apoA-I function. Thus, neither inhibition of Tyr192 chlorination nor reduction of Met(O) alone markedly protected apoA-I from oxidative inactivation.
We next determined if a combination of Tyr chlorination and Met oxidation contributes to the loss of ABCA1 activity when apoA-I is exposed to MPO. When Tyr192Phe apoA-I was exposed to HOCl or the MPO system, subsequent treatment with methionine sulfoxide reductase almost completely restored its ability to promote cholesterol efflux by ABCA1 (Fig. 3B, ref. (84)). It is noteworthy that Tyr192 is one of eight amino acid residues that are completely conserved in every species of apoA-I sequenced (24). Moreover, the conserved pair of amino acids Glu191-Tyr192 has been proposed to play a key role in a solvent-exposed loop-helix-loop that regulates the structure and function of helical repeats 9 and 10 (24). These observations indicate that a combination of Tyr192 chlorination (84) and methionine oxidation (90)—perhaps together with other structural changes—impairs the ability of apoA-I to activate sterol efflux by the ABCA1 pathway (84).
HOCl can also oxidize tryptophan residues in apoA-I (87, 92). However, oxidation failed to prevent a mutant apoA-I in which Phe had replaced all four Trp residues from activating ABCA1 (93). Analysis by circular dichroism suggested that the α-helical content of lipid-free control protein was 56% while that of the mutant protein was 71% (93), which matches that (~70%) of lipid-associated native apoA-I (85). These observations indicate that the tryptophan substitutions significantly alter the tertiary structure of lipid-free apoA-I. Thus, the mutant protein’s oxidation resistance might result from structural alterations.
4.6 Chlorination of apoA-I reduces cholesterol efflux by impairing the protein’s ability to interact directly with ABCA1
Many lines of evidence support the hypothesis that lipid removal by apolipoproteins involves three steps. First, apolipoproteins bind directly to ABCA1, stabilizing the protein and trigger sterol enrichment of the exofacial leaf of the plasma membrane. Next, apoA-I binds to exovesiculated lipid domains formed by ABCA1. Finally, apoA-I promotes the excretion of exovesiculated lipids (17, 94–96). Any of these steps could be impaired if apoA-I were oxidized by the MPO pathway.
4.6.1 Oxidation impairs apoA-I binding to ABCA1
To investigate potential mechanisms, we used a competitive assay to test whether chlorination reduces the direct binding of apoA-I to ABCA1 (83). BHK cells transfected with ABCA1 were incubated with 125I-apoA-I in the absence or presence of unlabeled and unmodified apoA-I or with oxidized apoA-I. The cells were then treated with the cross-linking agent DSP. Following immunoprecipitation of ABCA1 and then SDS-PAGE, we detected any 125I-apoA-I that had cross-linked to ABCA1, using autoradiography and phosphorimaging. Unlabeled control apoA-I or nitrated apoA-I reduced the amount of complex detected, indicating that these forms of apoA-I competed equally well with 125I-apoA-I for binding to ABCA1. In contrast, apoA-I exposed to either HOCl or the MPO-H2O2-Cl- system was much less effective, suggesting impaired binding (83). These observations indicate that chlorinated apoA-I is less able to bind to ABCA1 than is native or nitrated apoA-I. Thus, MPO-mediated chlorination of apoA-I impairs the first step in cholesterol transport.
Most of the apoA-I binding sites on the surfaces of cells that express ABCA1 are lipid domains formed by ABCA1 (95–98). To determine how oxidation by the MPO pathway affected apoA-I’s ability to bind to such cells, we exposed apoA-I to HOCl or H2O2 plus MPO and NaCl. Chlorination of apoA-I by either HOCl or the MPO-H2O2-NaCl system impaired its ability to compete with 125I-apoA-I for binding to BHK cells expressing ABCA1 (83). In contrast, apoA-I nitrated by ONOO− or the MPO-nitrite system was able to bind normally. These results indicate that chlorination—but not nitration—severely impairs the high-affinity binding of apoA-I to cells that express ABCA1.
When apoA-I stabilizes ABCA1, it does not need to bind directly to the transporter (99). We therefore compared the abilities of control and oxidized apoA-I to stabilize ABCA1 protein. For these studies, we used J774 macrophages that express ABCA1, because those cells rapidly degrade ABCA1 protein in the absence of apolipoproteins and ABCA1 inducers (99–101). Indeed, when macrophages were treated with 8-Br-cAMP (to induce ABCA1) and subsequently incubated without 8-Br-cAMP, most of the induced ABCA1 protein disappeared within 4 h (83). When we included control, chlorinated, or nitrated apoA-I in the media, this loss was minimized (83). Thus, although chlorinated apoA-I was markedly less able to interact with ABCA1 and to remove lipid, it stabilized ABCA1 protein just as effectively as untreated or nitrated apoA-I.
4.6.2 Oxidation of apoA-I has variable effects on phospholipid binding
Different investigators have reached different conclusions regarding the impact of apoA-I oxidation on lipid binding, a key step in sterol export by ABCA1 (83, 102, 103). For example, Stocker and colleagues demonstrated that oxygenation of methionine residues in apoA-I resulted in a greater affinity for lipid, as determined by the rate of clearance of multilamellar phospholipid vesicles (102). However, Kinter, Smith and colleagues found that chlorination or nitration of apoA-I impaired its ability to bind lipid, using an assay that monitored the ability of the apoA-I to inhibit LDL aggregation (103). Using two different assays, we found that chlorination or nitration of apoA-I could either improve or impair lipid binding (83).
The conflicting results of the different studies suggest that oxidation of apoA-I has complex effects on phospholipid binding, perhaps because different method were used to assess protein-lipid interaction. In future studies it will be important to clarify the impact of apoA-I oxidation on its ability to interact with phospholipids.
5. Reactive carbonyls: potential intermediates for generating dysfunctional HDL in humans
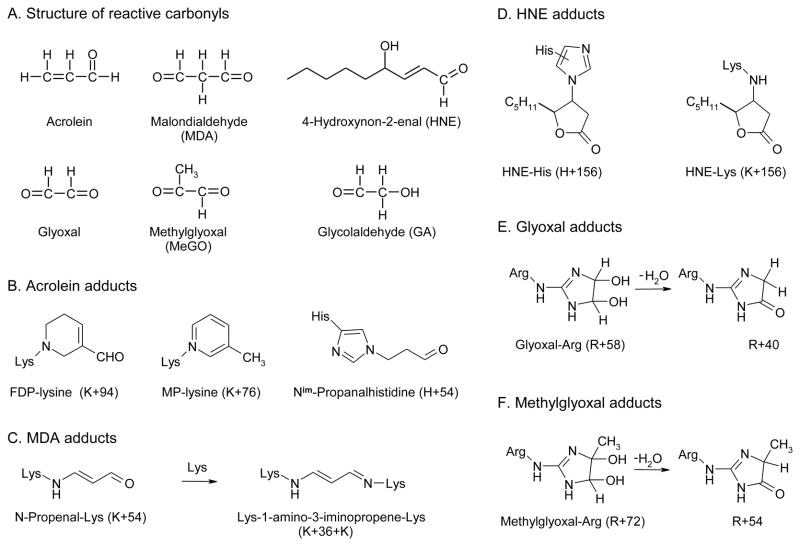
Reactive carbonyls, which can also modify proteins (104, 105), have been implicated in atherogenesis and diabetic vascular disease (3, 106, 107). They can result from lipid peroxidation or oxidation of carbohydrates or amino acids (104, 105, 108). Major carbonyl products of carbohydrate oxidation in vivo are thought to be glyoxal, methylglyoxal, and glycolaldehdye, which are precursors of advanced glycation end products (AGEs). Lipid peroxidation yields a different spectrum of reactive carbonyl compounds, including malondialdehyde (MDA), 4-hydroxynonenal (HNE), and acrolein (106, 108), which have been termed advanced lipoxidation end products (ALEs; ref. (104)). Figure 4A shows the structure of major physiologically relevant reactive carbonyls. Importantly, ALE and AGE levels are elevated in diabetes, a disease that greatly increases the risk for CAD (104, 109).
Figure 4.
Structures of physiologically relevant reactive carbonyls and carbonyl adducts detected by mass spectrometry.
We therefore determined whether reactive carbonyls can interrupt cholesterol efflux by the ABCA1 pathway. Treating cultured macrophages with glycolaldehyde or glyoxal—two AGEs—strongly inhibited ABCA1-dependent transport of cholesterol from cells to apoA-I (110). Glycolaldehyde and glyoxal destabilized ABCA1 and nearly abolished its binding to apoA-I, indicating that these carbonyls inhibit cholesterol export by directly modifying ABCA1. Reactive carbonyls may also impair cholesterol transport by modifying HDL lipoproteins, because HDL is the major carrier of lipid hydroperoxides in plasma and may be constantly exposed to reactive carbonyls (111). Moreover, when plasma is oxidized ex vivo, apoA-I is a major target for lipid oxidation products (112). Reactive carbonyls are also known to impair HDL’s function in vitro. For example, high concentrations of carbonyls modified apoA-I and inhibited LCAT activation by HDL, but the underlying mechanisms were not identified (113). When apoA-I incorporated into discoidal reconstituted HDL was exposed to methylglyoxal, it lost its ability to activate lecithin:cholesterol acyltransferase (LCAT) (114). This loss associated with modification of arginine, lysine, and tryptophan residues. These observations suggest that reactive carbonyls can potentially generate dysfunctional HDL.
5.1 Acrolein impairs ABCA1-dependent cholesterol export from cells by modifying C-terminal lysine residues of apoA-I
Acrolein is the most reactive α,β-unsaturated aldehyde, and it rapidly modifies biological nucleophiles (115). This potent electrophile may be produced in the body through lipid peroxidation or peroxidation of threonine by MPO, and high levels are found in cigarette smoke (116–118). Biomarkers of lipid peroxidation products, renal disease, and cigarette smoking all strongly associate with an increased risk of vascular disease (5, 104, 119). Moreover, epitopes recognized by a monoclonal antibody specific for lysine adducts of acrolein have been detected in macrophages of atherosclerotic lesions (118). Thus, acrolein might be another reactive species that damages artery wall proteins.
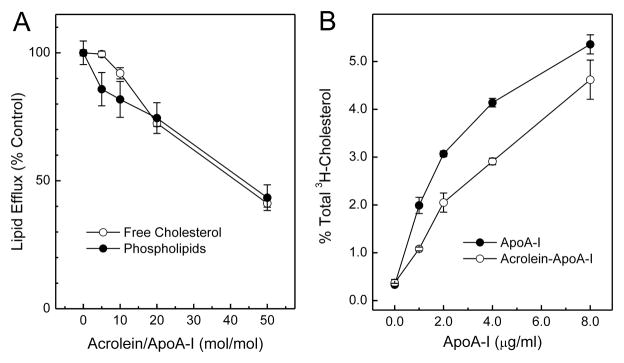
We investigated the possibility that acrolein might promote the formation of macrophage foam cells in the artery wall by impairing apoA-I’s ability to remove cholesterol from cells by the ABCA1 pathway. When BHK cells transfected with ABCA1were incubated with apoA-I and exposed to increasing concentrations of acrolein, the apolipoprotein progressively lost its ability to promote cholesterol and phospholipid efflux (Fig. 5A) (120). When increasing amounts of apoA-I were incubated with those cells, acrolein-modified apoA-I was significantly less able than untreated apoA-I to remove cholesterol (Fig. 5B) (120). Thus, modification by acrolein, like chlorination by MPO, reduces apoA-I’s ability to promote cholesterol and phospholipid efflux by the ABCA1 pathway.
Figure 5. Cholesterol and phospholipid efflux activities of acrolein-modified apoA-I.
ApoA- I (25 μM) was incubated with the indicated concentrations of acrolein (A) or with 500 μM acrolein (B) for 24 h at 37°C in 50 mM sodium phosphate buffer (pH 7.4) containing 100 μM DTPA. Reactions were initiated by adding acrolein, and terminated by adding a 20-fold molar excess (relative to acrolein) of aminoguanidine. (A) [3H]Cholesterol- or phospholipid-labeled ABCA1-transfected BHK cells were incubated for 2 h with 5 μg/ml of native (0 μM acrolein) or acrolein-modified apoA-I. (B) [3H]Cholesterol-labeled ABCA1-transfected BHK cells were incubated with the indicated concentrations of untreated or acrolein-treated apoA-I (20:1, mol/mol, acrolein/protein) for 2 h. At the end of the incubation, [3H]cholesterol efflux to the acceptor apolipoprotein was measured. Results represent those from 2 independent experiments (120).
Like other α,β-unsaturated aldehydes, acrolein reacts with the sulfhydryl group of cysteine, the imidazole group of histidine, and the ε-amino group of lysine (106, 121, 122). Indirect evidence suggests that it might also modify arginine (123). To investigate the reactions of amino acid side chains with acrolein, we synthesized model peptides containing Lys, His, and Arg (apoA-I contains no cysteine). We found that acrolein reacted with Lys and His but not with Arg residues in the peptides (120).
In vitro studies have identified a number of acrolein adducts with His and Lys in proteins (Fig. 4B). ApoA-I contains five His, 21 Lys, and 19 Arg. We determined that all 21 Lys residues are modified by acrolein, suggesting that Lys residues are acrolein’s major targets in apoA-I protein (120). Importantly, our approach demonstrated that Lys226 was the major residue modified by acrolein (120), strongly implying that the local amino acid environment and perhaps the α-helical structure of apoA-I significantly influence acrolein’s reactions with specific residues in this protein.
To determine whether modification of Lys226 might account for the loss of apoA-I function, we examined the relationship between the disappearance of the peptide containing this residue and loss of ABCA1 activity. Loss of the peptide associated strongly with a decrease in the ability of acrolein-modified apoA-I to promote cholesterol efflux (120). Moreover, there was a strong linear correlation between the appearance of MP-Lys226 and decreased lipid efflux from cells (120).
We cannot exclude the possibility that other modifications to apoA-I helped impair cholesterol efflux, but our observations suggest that modification of Lys226, which lies in helix 10, is particularly important. Previous studies demonstrated that deleting helix 10 and/or helices 9 plus 10 of apoA-I greatly diminishes ABCA1-dependent cholesterol efflux (124, 125). Moreover, a synthetic peptide that mimics the 9/10 helix of apoA-I mediated high-affinity cholesterol efflux via ABCA1 (126). Those investigators suggested that a specific structural element containing a linear array of acidic residues spanning two apoA-I amphipathic α-helices mediates cholesterol efflux and stabilizes ABCA1. It is noteworthy that modification of Lys226 could disrupt this linear array, which might account for acrolein’s ability to impair cholesterol transport.
Importantly, immunohistochemical studies with a monoclonal antibody that reacts specifically with MP-Lys (118, 121) found that acrolein adducts co-localized with apoA-I in human atherosclerotic lesions (120), suggesting that apoA-I is targeted and modified by acrolein in atherosclerotic intima. Thus, our observations suggest the following model. Lipid peroxidation and amino acid oxidation by MPO in the artery wall generate acrolein (116, 127). The aldehyde then reacts with apoA-I. Modification of Lys residues in helix 10 impairs apoA-I’s ability to remove cholesterol from lipid-laden macrophages, contributing to the formation of atherosclerotic lesions. Our findings suggest that acrolein production by phagocytes is a physiological mechanism for modifying apoA-I to produce dysfunctional HDL and promote atherogenesis.
5.2 Malondialdehyde impairs ABCA1-dependent cholesterol efflux by pathways associated with cross-linking C-terminal lysine residues in apoA-I
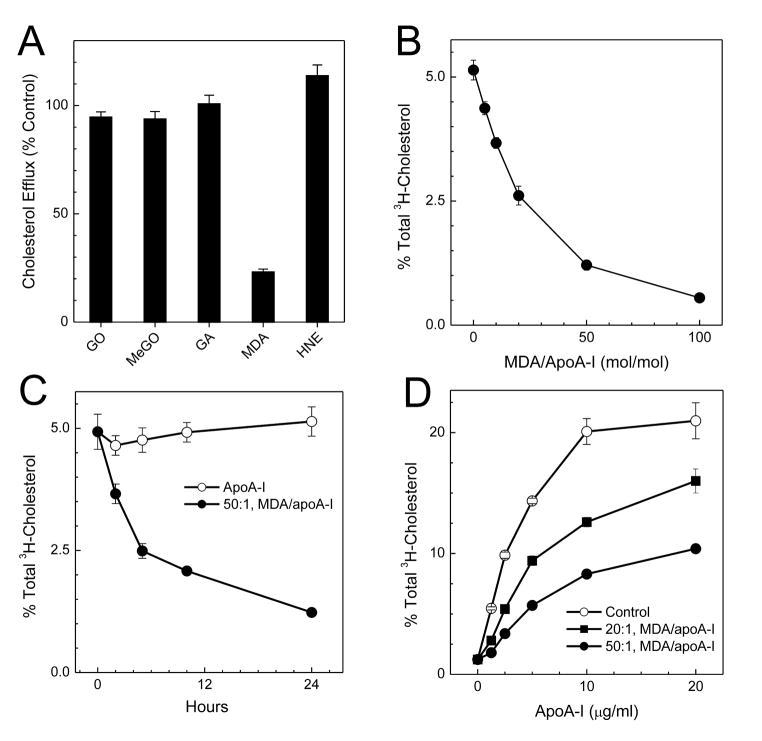
We next investigated whether other physiologically relevant reactive carbonyls resulting from lipid peroxidation (MDA and HNE) or carbohydrate oxidation (glycolaldehyde, glyoxal, and methylglyoxal) also impair apoA-I’s ability to promote cholesterol efflux. When we transfected BHK cells with ABCA1 and incubated them with apoA-I that had been exposed to carbonyls, the only carbonyl that affected cholesterol efflux was MDA (Fig. 6A) (128).
Figure 6. Impact of carbonyl modification on apoA-I’s ability to promote cholesterol efflux by ABCA1.
ApoA-I (5 μM) was incubated with 250 μM glyoxal, methylglyoxal, glycolaldehdye, MDA, or HNE (A), or with the indicated concentrations of MDA for 24 h (B and D), or with 250 μM MDA for the indicated times (C) at 37°C in 50 mM sodium phosphate buffer (pH 7.4) containing 100 μM DTPA. Reactions were initiated by adding carbonyl, and terminated by adding a 20-fold molar excess (relative to carbonyl) of aminoguanidine. (A, B, and C) [3H]Cholesterol-labeled ABCA1-transfected BHK cells were incubated for 2 h with 3 μg/mL of control (0 μM carbonyl) or carbonyl-modified apoA-I. (D) [3H]Cholesterol-labeled ABCA1- transfected BHK cells were incubated with the indicated concentrations of untreated or MDA- treated apoA-I (20:1 or 50:1, mol/mol, MDA/protein) for 2 h. At the end of the incubation, [3H]cholesterol efflux to the acceptor apolipoprotein was measured. Results represent 2 independent experiments (128).
Exposure to increasing concentrations of MDA progressively and dramatically deprived apoA-I of its ability to promote cholesterol efflux (Fig. 6B) (128). When increasing amounts of apoA-I were incubated with BHK cells transfected with ABCA1, cholesterol removal was dramatically lower with MDA-modified apoA-I than with native apoA-I (Fig. 6D) (128). Thus, MDA significantly reduces apoA-I’s ability to promote cholesterol efflux by the ABCA1 pathway. In contrast, modification by HNE, glyoxal, methylglyoxal, or glycolaldehdye has little effect.
We used tandem MS to elucidate the molecular basis for apoA-I’s selective vulnerability to MDA. Previous studies identified Lys residues as major targets, and the adducts included N-propenal-lysine (K+54, Fig. 4C) and dihydropyridine (DHP)-lysine (K+134) (106, 129). To determine which adducts form in apoA-I, we exposed the protein to MDA, digested it, and analyzed the resulting peptides. Tandem MS detected K+54 (128). In contrast, we failed to detect DHP-Lys when we exposed apoA-I to a wide range of MDA concentrations.
The N-propenal-Lys adduct contains a carbonyl group that could cross-link with a second Lys residue to form Lys-MDA-Lys (Fig. 4C). Indeed, when apoA-I was exposed to MDA, tandem MS identified K+36+K (128), the Lys-1-amino-3-iminopropene-Lys (Lys-MDA-Lys) cross-link in which two Lys residues have gained 36 amu (130). To determine whether MDA targets specific Lys residues in apoA-I, we used isotope dilution MS to quantify the product yields of modified Lys residues (128). Certain Lys residues were converted in high yield to MDA-Lys (K+54) or Lys-MDA-Lys (K+36+K). Moreover, six of the 10 most reactive Lys residues resided in helical repeats 7 to 10 of apoA-I’s C-terminus (128), suggesting that ABCA1 activity might be seriously impaired when this region is modified.
Consistent with this proposal, we identified the C-terminus of apoA-I as the major site for cross-linking by MDA (128). A molecular dynamic model based on energy minimization suggested that cross-linking drastically condenses the C-terminus of apoA-I, affecting its conformation and conformational adaptability. Importantly, studies of deletion mutants of apoA-I suggest that repeat 10 of the C-terminus helps activate ABCA1 (131, 132). Taken together, these observations support the proposal that the protein loses its ability to promote cholesterol efflux by the ABCA1 pathway when MDA modifies its C-terminus.
MDA is one of the most abundant products of lipid peroxidation (106). Moreover, MDA levels appear to be elevated in diseases such as diabetes mellitus that increase the risk of cardiovascular disease (133). Importantly, immunochemical analyses of HDL with MDA2, a monoclonal antibody that binds with high affinity to a variety of MDA-modified proteins (134), demonstrated that HDL isolated from atherosclerotic tissue contained higher levels of MDA-modified proteins than HDL from plasma of apparently healthy humans (128). This suggests that MDA modifies HDL in human atherosclerotic tissue. It also suggests that cross-linking of Lys residues in apoA-I’s C-terminus by MDA in the artery wall could promote the formation of macrophage foam cells, because cholesterol efflux by the ABCA1 pathway would be impaired.
5.3 HNE modifes His residues in apoA-I without affecting cholesterol efflux by ABCA1
HNE (a reactive carbonyl generated by lipid peroxidation) (106) can react with imidazoles (His), thiols (Cys), or free amino (Lys) groups of proteins to form stable Michael adducts with hemiacetal structures (Michael adducts form when a nucleophile is added to an α,β-unsaturated carbonyl compound, Fig. 4D) (130, 135). After we exposed apoA-I to a 50:1 molar ratio of HNE, all 5 peptides containing His were lost in near-quantitative yield (~90%) (128). Tandem MS revealed that the His residue in each peptide had gained 158 amu or 156 amu without NaBH4 reduction (128), suggesting that His had been converted to the hemiacetal adduct (136). Remarkably, this concentration of HNE had no effect on apoA-I’s ability to promote cholesterol efflux by the ABCA1 pathway, likely because all five His residues lie in the protein’s central region, which does not appear to play a major role in activating the transporter.
6. Mechanisms for generating dysfunctional HDL in humans
Our in vitro studies have shown that chlorination of apoA-I by MPO hampers the lipoprotein’s ability to transport cholesterol by the ABCA1 pathway. Modification of one or more Met residues and chlorination of Tyr192 are responsible for this impairment. Moreover, chlorination of apoA-I by MPO hinders the direct interaction between apoA-I and ABCA1, while apoA-I’s ability to solubilize phospholipids is minimally affected. Our studies further demonstrated that plasma HDL isolated from humans with CAD contains higher levels of 3-chlorotyrosine and 3- nitrotyrosine than HDL from healthy subjects, suggesting that chlorinated and/or nitrated HDL might serve as a marker of active cardiovascular disease in humans. Thus, MPO could potentially generate dysfunctional HDL in humans. In future studies, it will be important to identify the specific sites of chlorination and nitration of apoA-I in vivo. It will also be critical to establish whether chlorinated and nitrated Tyr192 and oxidized Met residues in plasma apoA-I can indeed serve as CAD markers. Studies using MPO inhibitors will be necessary to determine whether HDL oxidation is causally linked to atherosclerosis in humans.
We also demonstrated that MDA and acrolein impaired apoA-I’s ability to transport cholesterol by the ABCA1 pathway. The mechanisms likely involve modification and cross-linking of Lys residues in repeats 9 and 10 in the protein’s C-terminus. Our observations raise the possibility that modification of apoA-I by MDA and acrolein generates dysfunctional HDL that can no longer remove cholesterol from macrophages. In future studies, it will be of interest to determine whether these carbonyls impair cholesterol transport by impairing the direct interaction between apoA-I and ABCA1, as chlorination does. We would also like to determine whether reactive carbonyls inhibit other key steps in reverse cholesterol transport—such as LCAT activation—that depend on apoA-I. It will be crucial to obtain direct evidence that HDL is modified by MDA and acrolein in vivo by detecting MDA and acrolein modification and/or cross-linking products in HDL isolated from humans. It will also be important to determine whether HDL modified by MDA and acrolein can serve as markers for CAD.
7. Conclusions
Our studies suggest that MPO and reactive carbonyls are potential mechanisms for generating dysfunctional HDL in humans. Oxidation of lipid-free apoA-I by MPO or reactive carbonyls impairs cholesterol efflux by the ABCA1 pathway. This deficit in cholesterol removal might promote foam cell formation and therefore atherogenesis.
Research highlights.
MPO impairs ABCA1 cholesterol efflux by modifying Met residues and Tyr192 in apoA-I.
Chlorination of apoA-I by MPO impairs the initial interactions with ABCA1.
Acrolein impairs cholesterol efflux by modifying Lys226 in apoA-I.
MDA impairs cholesterol export by cross-linking of C-terminal Lys residues in apoA-I.
MPO and reactive carbonyls might generate dysfunctional HDL in humans.
Acknowledgments
This work is supported by an NIH K99/R00 award (R00HL091055) from the National Heart, Lung, and Blood Institute and by a Pilot and Feasibility Award from the Diabetes Endocrinology Research Center (NIH P30DK017047). Mass spectrometry experiments were supported by the Mass Spectrometry Core of the Diabetes Endocrinology Research Center, University of Washington.
The abbreviations used are
- ABCA1
ATP-binding cassette transporter A1
- ABCG1
ATP-binding cassette transporter G1
- AGE
advanced glycation end products
- ALE
advanced lipoxidation end products
- apoA-I
apolipoprotein A-I
- BHK
baby hamster kidney
- CAD
coronary artery disease
- DHP-lysine
dihydropyridine-lysine
- DTPA
diethylenetriaminepentaacetic acid
- HDL
high-density lipoproteins
- HNE
4-hydroxynonenal
- HOCl
hypochlorous acid
- H2O2
hydrogen peroxide
- LCAT
lecithin:cholesterol acyltransferase
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LDL
low-density lipoprotein
- Lys-MDA-Lys
Lys-1-amino-3-iminopropene-Lys
- Met(O)
methionine sulfoxide
- MDA
malondialdehyde
- MPO
myeloperoxidase
- MP-lysine
Nε-(3-methylpyridinium)lysine
- MS
mass spectrometry
- NO
nitric oxide
- NO2•
nitrogen dioxide radical
- ONOO−
peroxynitrite
- PilB
methionine sulfoxide reductase
- SR-B1
scavenger receptor B1
- SUV
single unilamellar vesicles
- WT
wild type
- Y192F
Tyr192Phe apoA-I mutant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 2.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 3.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 4.Heinecke JW. Oxidants and antioxidants in the pathogenesis of atherosclerosis: implications for the oxidized low density lipoprotein hypothesis. Atherosclerosis. 1998;141:1–15. doi: 10.1016/s0021-9150(98)00173-7. [DOI] [PubMed] [Google Scholar]
- 5.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 7.Haas MJ, Mooradian AD. Inflammation, high-density lipoprotein and cardiovascular dysfunction. Curr Opin Infect Dis. 2011;24:265–272. doi: 10.1097/QCO.0b013e328344b724. [DOI] [PubMed] [Google Scholar]
- 8.Lowenstein CJ, Cameron SJ. High-density lipoprotein metabolism and endothelial function. Curr Opin Endocrinol Diabetes Obes. 2010;17:166–170. doi: 10.1097/MED.0b013e32833727ee. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki M, Pritchard DK, Becker L, Hoofnagle AN, Tanimura N, Bammler TK, Beyer RP, Bumgarner R, Vaisar T, de Beer MC, de Beer FC, Miyake K, Oram JF, Heinecke JW. High-density lipoprotein suppresses the type I interferon response, a family of potent antiviral immunoregulators, in macrophages challenged with lipopolysaccharide. Circulation. 2010;122:1919–1927. doi: 10.1161/CIRCULATIONAHA.110.961193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis GA. The complexity of HDL. Biochim Biophys Acta. 2010;1801:1286–1293. doi: 10.1016/j.bbalip.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Assman G, von Eckardstein A, Brewer HB. The Metabolic and Molecular Bases of Inherited Disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Familial high density lipoprotein deficiency: Tangier disease. New York, N.Y: McGraw-Hill; 1995. pp. 2053–2072. [Google Scholar]
- 12.Duverger N, Viglietta C, Berthou L, Emmanuel F, Tailleux A, Parmentier-Nihoul L, Laine B, Fievet C, Castro G, Fruchart JC, Houbebine LM, Denefie P. Transgenic rabbits expressing human apolipoprotein A-I in the liver. Arterioscler Thromb Vasc Biol. 1996;16:1424–1429. doi: 10.1161/01.atv.16.12.1424. [DOI] [PubMed] [Google Scholar]
- 13.Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 14.Tangirala RK, Tsukamoto K, Chun SH, Usher D, Pure E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein AI in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 15.Getz GS, Wool GD, Reardon CA. Biological properties of apolipoprotein a-I mimetic peptides. Curr Atheroscler Rep. 2010;12:96–104. doi: 10.1007/s11883-010-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Lenten BJ, Wagner AC, Anantharamaiah GM, Navab M, Reddy ST, Buga GM, Fogelman AM. Apolipoprotein A-I mimetic peptides. Curr Atheroscler Rep. 2009;11:52–57. doi: 10.1007/s11883-009-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oram JF, Heinecke JW. ATP-binding cassette transporter A1: A cell cholesterol exporter that protects against cardiovascular disease. Physiological Reviews. 2005;85:1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 18.Tall AR, Costet P, Wang N. Regulation and mechanisms of macrophage cholesterol efflux. J Clin Invest. 2002;110:899–904. doi: 10.1172/JCI16391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 20.Krieger M. Charting the fate of the “good cholesterol”: identification and characterization of the high-density lipoprotein receptor SR-BI. Annu Rev Biochem. 1999;68:523–558. doi: 10.1146/annurev.biochem.68.1.523. [DOI] [PubMed] [Google Scholar]
- 21.Gotto AM, Jr, Pownall HJ, Havel RJ. Introduction to the plasma lipoproteins. Methods Enzymol. 1986;128:3–41. doi: 10.1016/0076-6879(86)28061-1. [DOI] [PubMed] [Google Scholar]
- 22.Davidson WS, Thompson TB. The structure of apolipoprotein A-I in high density lipoproteins. J Biol Chem. 2007;282:22249–22253. doi: 10.1074/jbc.R700014200. [DOI] [PubMed] [Google Scholar]
- 23.Thomas MJ, Bhat S, Sorci-Thomas MG. Three-dimensional models of HDL apoA-I: implications for its assembly and function. J Lipid Res. 2008;49:1875–1883. doi: 10.1194/jlr.R800010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bashtovyy D, Jones MK, Anantharamaiah GM, Segrest JP. Sequence conservation of apolipoprotein A-I affords novel insights into HDL structure-function. J Lipid Res. 2011;52:435–450. doi: 10.1194/jlr.R012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon DJ, Rifkind BM. High-density lipoprotein--the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 26.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 27.Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, Patsch W. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104:1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 28.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Rader DJ. Molecular regulation of macrophage reverse cholesterol transport. Curr Opin Cardiol. 2007;22:368–372. doi: 10.1097/HCO.0b013e3281ec5113. [DOI] [PubMed] [Google Scholar]
- 31.Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30:139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawn RM, Wade DP, Couse TL, Wilcox JN. Localization of human ATP-binding cassette transporter 1 (ABC1) in normal and atherosclerotic tissues. Arterioscler Thromb Vasc Biol. 2001;21:378–385. doi: 10.1161/01.atv.21.3.378. [DOI] [PubMed] [Google Scholar]
- 33.Wellington CL, Walker EK, Suarez A, Kwok A, Bissada N, Singaraja R, Yang YZ, Zhang LH, James E, Wilson JE, Francone O, McManus BM, Hayden MR. ABCA1 mRNA and protein distribution patterns predict multiple different roles and levels of regulation. Lab Invest. 2002;82:273–283. doi: 10.1038/labinvest.3780421. [DOI] [PubMed] [Google Scholar]
- 34.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 35.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Jr, Hayden MR. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 36.Lawn RM, Wade DP, Garvin MR, Wang X, Schwartz K, Porter JG, Seilhamer JJ, Vaughan AM, Oram JF. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J Clin Invest. 1999;104:R25–31. doi: 10.1172/JCI8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, Assmann G. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 38.Oram JF. HDL apolipoproteins and ABCA1: partners in the removal of excess cellular cholesterol. Arterioscler Thromb Vasc Biol. 2003;23:720–727. doi: 10.1161/01.ATV.0000054662.44688.9A. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura K, Kennedy MA, Baldan A, Bojanic DD, Lyons K, Edwards PA. Expression and regulation of multiple murine ATP-binding cassette transporter G1 mRNAs/isoforms that stimulate cellular cholesterol efflux to high density lipoprotein. J Biol Chem. 2004;279:45980–45989. doi: 10.1074/jbc.M408652200. [DOI] [PubMed] [Google Scholar]
- 40.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wojcik AJ, Skaflen MD, Srinivasan S, Hedrick CC. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J Immunol. 2008;180:4273–4282. doi: 10.4049/jimmunol.180.6.4273. [DOI] [PubMed] [Google Scholar]
- 42.Baldan A, Pei L, Lee R, Tarr P, Tangirala RK, Weinstein MM, Frank J, Li AC, Tontonoz P, Edwards PA. Impaired development of atherosclerosis in hyperlipidemic Ldlr−/− and ApoE−/− mice transplanted with Abcg1−/− bone marrow. Arterioscler Thromb Vasc Biol. 2006;26:2301–2307. doi: 10.1161/01.ATV.0000240051.22944.dc. [DOI] [PubMed] [Google Scholar]
- 43.Out R, Hoekstra M, Hildebrand RB, Kruit JK, Meurs I, Li Z, Kuipers F, Van Berkel TJ, Van Eck M. Macrophage ABCG1 deletion disrupts lipid homeostasis in alveolar macrophages and moderately influences atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2295–2300. doi: 10.1161/01.ATV.0000237629.29842.4c. [DOI] [PubMed] [Google Scholar]
- 44.Ranalletta M, Wang N, Han S, Yvan-Charvet L, Welch C, Tall AR. Decreased atherosclerosis in low-density lipoprotein receptor knockout mice transplanted with Abcg1−/− bone marrow. Arterioscler Thromb Vasc Biol. 2006;26:2308–2315. doi: 10.1161/01.ATV.0000242275.92915.43. [DOI] [PubMed] [Google Scholar]
- 45.Out R, Hoekstra M, Habets K, Meurs I, de Waard V, Hildebrand RB, Wang Y, Chimini G, Kuiper J, Van Berkel TJ, Van Eck M. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler Thromb Vasc Biol. 2008;28:258–264. doi: 10.1161/ATVBAHA.107.156935. [DOI] [PubMed] [Google Scholar]
- 46.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 48.Zabalawi M, Bhat S, Loughlin T, Thomas MJ, Alexander E, Cline M, Bullock B, Willingham M, Sorci-Thomas MG. Induction of fatal inflammation in LDL receptor and ApoA-I double-knockout mice fed dietary fat and cholesterol. Am J Pathol. 2003;163:1201–1213. doi: 10.1016/S0002-9440(10)63480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilhelm AJ, Zabalawi M, Owen JS, Shah D, Grayson JM, Major AS, Bhat S, Gibbs DP, Jr, Thomas MJ, Sorci-Thomas MG. Apolipoprotein A-I modulates regulatory T cells in autoimmune LDLr−/−, ApoA-I−/− mice. J Biol Chem. 2010;285:36158–36169. doi: 10.1074/jbc.M110.134130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goossens P, Gijbels MJ, Zernecke A, Eijgelaar W, Vergouwe MN, van der Made I, Vanderlocht J, Beckers L, Buurman WA, Daemen MJ, Kalinke U, Weber C, Lutgens E, de Winther MP. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metab. 2010;12:142–153. doi: 10.1016/j.cmet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chait A, Han CY, Oram JF, Heinecke JW. Thematic review series: The immune system and atherogenesis. Lipoprotein-associated inflammatory proteins: markers or mediators of cardiovascular disease? J Lipid Res. 2005;46:389–403. doi: 10.1194/jlr.R400017-JLR200. [DOI] [PubMed] [Google Scholar]
- 55.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev. 2006;58:342–374. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 56.Shao B, Oda MN, Oram JF, Heinecke JW. Myeloperoxidase: an oxidative pathway for generating dysfunctional high-density lipoprotein. Chem Res Toxicol. 2010;23:447–454. doi: 10.1021/tx9003775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heinecke J. HDL and cardiovascular-disease risk--time for a new approach? N Engl J Med. 2011;364:170–171. doi: 10.1056/NEJMe1012520. [DOI] [PubMed] [Google Scholar]
- 58.Bergt C, Pennathur S, Fu X, Byun J, O’Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pennathur S, Bergt C, Shao B, Byun J, Kassim SY, Singh P, Green PS, McDonald TO, Brunzell J, Chait A, Oram JF, O’Brien K, Geary RL, Heinecke JW. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem. 2004;279:42977–42983. doi: 10.1074/jbc.M406762200. [DOI] [PubMed] [Google Scholar]
- 60.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henderson JP. Myeloperoxidase and Eosinophil Peroxidase: Phagocytosis and Microbial Killing. In: Dunford HB, editor. Heme Peroxidases. New York: John Wiley & Sons, Inc; 1999. pp. 349–385. [Google Scholar]
- 63.Hurst JK, Barrette WC., Jr Leukocytic oxygen activation and microbicidal oxidative toxins. Crit Rev Biochem Mol Biol. 1989;24:271–328. doi: 10.3109/10409238909082555. [DOI] [PubMed] [Google Scholar]
- 64.Klebanoff SJ. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980;93:480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- 65.Heinecke JW. Pathways for oxidation of low density lipoprotein by myeloperoxidase: tyrosyl radical, reactive aldehydes, hypochlorous acid and molecular chlorine. Biofactors. 1997;6:145–155. doi: 10.1002/biof.5520060208. [DOI] [PubMed] [Google Scholar]
- 66.Domigan NM, Charlton TS, Duncan MW, Winterbourn CC, Kettle AJ. Chlorination of tyrosyl residues in peptides by myeloperoxidase and human neutrophils. J Biol Chem. 1995;270:16542–16548. doi: 10.1074/jbc.270.28.16542. [DOI] [PubMed] [Google Scholar]
- 67.Hazen SL, Hsu FF, Mueller DM, Crowley JR, Heinecke JW. Human neutrophils employ chlorine gas as an oxidant during phagocytosis. J Clin Invest. 1996;98:1283–1289. doi: 10.1172/JCI118914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gaut JP, Yeh GC, Tran HD, Byun J, Henderson JP, Richter GM, Brennan ML, Lusis AJ, Belaaouaj A, Hotchkiss RS, Heinecke JW. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc Natl Acad Sci U S A. 2001;98:11961–11966. doi: 10.1073/pnas.211190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cooper CE, Patel RP, Brookes PS, Darley-Usmar VM. Nanotransducers in cellular redox signaling: modification of thiols by reactive oxygen and nitrogen species. Trends Biochem Sci. 2002;27:489–492. doi: 10.1016/s0968-0004(02)02191-6. [DOI] [PubMed] [Google Scholar]
- 71.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 72.Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–240. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- 73.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 74.Gaut JP, Byun J, Tran HD, Lauber WM, Carroll JA, Hotchkiss RS, Belaaouaj A, Heinecke JW. Myeloperoxidase produces nitrating oxidants in vivo. J Clin Invest. 2002;109:1311–1319. doi: 10.1172/JCI15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brennan ML, Anderson MM, Shih DM, Qu XD, Wang X, Mehta AC, Lim LL, Shi W, Hazen SL, Jacob JS, Crowley JR, Heinecke JW, Lusis AJ. Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest. 2001;107:419–430. doi: 10.1172/JCI8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McMillen TS, Heinecke JW, LeBoeuf RC. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation. 2005;111:2798–2804. doi: 10.1161/CIRCULATIONAHA.104.516278. [DOI] [PubMed] [Google Scholar]
- 77.Francis GA, Knopp RH, Oram JF. Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier Disease. J Clin Invest. 1995;96:78–87. doi: 10.1172/JCI118082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mendez AJ, Anantharamaiah GM, Segrest JP, Oram JF. Synthetic amphipathic helical peptides that mimic apolipoprotein A-I in clearing cellular cholesterol. J Clin Invest. 1994;94:1698–1705. doi: 10.1172/JCI117515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brouillette CG, Anantharamaiah GM, Engler JA, Borhani DW. Structural models of human apolipoprotein A-I: a critical analysis and review. Biochim Biophys Acta. 2001;1531:4–46. doi: 10.1016/s1388-1981(01)00081-6. [DOI] [PubMed] [Google Scholar]
- 80.Davidson WS, Silva RA. Apolipoprotein structural organization in high density lipoproteins: belts, bundles, hinges and hairpins. Curr Opin Lipidol. 2005;16:295–300. doi: 10.1097/01.mol.0000169349.38321.ad. [DOI] [PubMed] [Google Scholar]
- 81.Shao B, Bergt C, Fu X, Green P, Voss JC, Oda MN, Oram JF, Heinecke JW. Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport. J Biol Chem. 2005;280:5983–5993. doi: 10.1074/jbc.M411484200. [DOI] [PubMed] [Google Scholar]
- 82.Bergt C, Fu X, Huq NP, Kao J, Heinecke JW. Lysine residues direct the chlorination of tyrosines in YXXK motifs of apolipoprotein A-I when hypochlorous acid oxidizes high density lipoprotein. J Biol Chem. 2004;279:7856–7866. doi: 10.1074/jbc.M309046200. [DOI] [PubMed] [Google Scholar]
- 83.Shao B, Tang C, Heinecke JW, Oram JF. Oxidation of apolipoprotein A-I by myeloperoxidase impairs the initial interactions with ABCA1 required for signaling and cholesterol export. J Lipid Res. 2010;51:1849–1858. doi: 10.1194/jlr.M004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shao B, Oda MN, Bergt C, Fu X, Green PS, Brot N, Oram JF, Heinecke JW. Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-I. J Biol Chem. 2006;281:9001–9004. doi: 10.1074/jbc.C600011200. [DOI] [PubMed] [Google Scholar]
- 85.Bergt C, Oettl K, Keller W, Andreae F, Leis HJ, Malle E, Sattler W. Reagent or myeloperoxidase-generated hypochlorite affects discrete regions in lipid-free and lipid-associated human apolipoprotein A-I. Biochem J. 2000;346(Pt 2):345–354. [PMC free article] [PubMed] [Google Scholar]
- 86.Francis GA, Mendez AJ, Bierman EL, Heinecke JW. Oxidative tyrosylation of high density lipoprotein by peroxidase enhances cholesterol removal from cultured fibroblasts and macrophage foam cells. Proc Natl Acad Sci U S A. 1993;90:6631–6635. doi: 10.1073/pnas.90.14.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Panzenboeck U, Raitmayer S, Reicher H, Lindner H, Glatter O, Malle E, Sattler W. Effects of reagent and enzymatically generated hypochlorite on physicochemical and metabolic properties of high density lipoproteins. J Biol Chem. 1997;272:29711–29720. doi: 10.1074/jbc.272.47.29711. [DOI] [PubMed] [Google Scholar]
- 88.Peng DQ, Wu Z, Brubaker G, Zheng L, Settle M, Gross E, Kinter M, Hazen SL, Smith JD. Tyrosine modification is not required for myeloperoxidase-induced loss of apolipoprotein A-I functional activities. J Biol Chem. 2005;280:33775–33784. doi: 10.1074/jbc.M504092200. [DOI] [PubMed] [Google Scholar]
- 89.Hawkins CL, Pattison DI, Davies MJ. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids. 2003;25:259–274. doi: 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- 90.Panzenbock U, Stocker R. Formation of methionine sulfoxide-containing specific forms of oxidized high-density lipoproteins. Biochim Biophys Acta. 2005;1703:171–181. doi: 10.1016/j.bbapap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 91.Weissbach H, Resnick L, Brot N. Methionine sulfoxide reductases: history and cellular role in protecting against oxidative damage. Biochim Biophys Acta. 2005;1703:203–212. doi: 10.1016/j.bbapap.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 92.Shao B, Heinecke JW. Using tandem mass spectrometry to quantify site-specific chlorination and nitration of proteins: model system studies with high-density lipoprotein oxidized by myeloperoxidase. Methods Enzymol. 2008;440:33–63. doi: 10.1016/S0076-6879(07)00803-8. [DOI] [PubMed] [Google Scholar]
- 93.Peng DQ, Brubaker G, Wu Z, Zheng L, Willard B, Kinter M, Hazen SL, Smith JD. Apolipoprotein A-I tryptophan substitution leads to resistance to myeloperoxidase-mediated loss of function. Arterioscler Thromb Vasc Biol. 2008;28:2063–2070. doi: 10.1161/ATVBAHA.108.173815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chroni A, Liu T, Fitzgerald ML, Freeman MW, Zannis VI. Cross-linking and lipid efflux properties of apoA-I mutants suggest direct association between apoA-I helices and ABCA1. Biochemistry. 2004;43:2126–2139. doi: 10.1021/bi035813p. [DOI] [PubMed] [Google Scholar]
- 95.Hassan HH, Denis M, Lee DY, Iatan I, Nyholt D, Ruel I, Krimbou L, Genest J. Identification of an ABCA1-dependent phospholipid-rich plasma membrane apolipoprotein A-I binding site for nascent HDL formation: implications for current models of HDL biogenesis. J Lipid Res. 2007;48:2428–2442. doi: 10.1194/jlr.M700206-JLR200. [DOI] [PubMed] [Google Scholar]
- 96.Vedhachalam C, Duong PT, Nickel M, Nguyen D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S, Phillips MC. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J Biol Chem. 2007;282:25123–25130. doi: 10.1074/jbc.M704590200. [DOI] [PubMed] [Google Scholar]
- 97.Lin G, Oram JF. Apolipoprotein binding to protruding membrane domains during removal of excess cellular cholesterol. Atherosclerosis. 2000;149:359–370. doi: 10.1016/s0021-9150(99)00503-1. [DOI] [PubMed] [Google Scholar]
- 98.Vedhachalam C, Ghering AB, Davidson WS, Lund-Katz S, Rothblat GH, Phillips MC. ABCA1-induced cell surface binding sites for ApoA-I. Arterioscler Thromb Vasc Biol. 2007;27:1603–1609. doi: 10.1161/ATVBAHA.107.145789. [DOI] [PubMed] [Google Scholar]
- 99.Tang C, Vaughan AM, Anantharamaiah GM, Oram JF. Janus kinase 2 modulates the lipid-removing but not protein-stabilizing interactions of amphipathic helices with ABCA1. J Lipid Res. 2006;47:107–114. doi: 10.1194/jlr.M500240-JLR200. [DOI] [PubMed] [Google Scholar]
- 100.Oram JF, Lawn RM, Garvin MR, Wade DP. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J Biol Chem. 2000;275:34508–34511. doi: 10.1074/jbc.M006738200. [DOI] [PubMed] [Google Scholar]
- 101.Wang Y, Oram JF. Unsaturated fatty acids inhibit cholesterol efflux from macrophages by increasing degradation of ATP-binding cassette transporter A1. J Biol Chem. 2002;277:5692–5697. doi: 10.1074/jbc.M109977200. [DOI] [PubMed] [Google Scholar]
- 102.Panzenbock U, Kritharides L, Raftery M, Rye KA, Stocker R. Oxidation of methionine residues to methionine sulfoxides does not decrease potential antiatherogenic properties of apolipoprotein A-I. J Biol Chem. 2000;275:19536–19544. doi: 10.1074/jbc.M000458200. [DOI] [PubMed] [Google Scholar]
- 103.Zheng L, Settle M, Brubaker G, Schmitt D, Hazen SL, Smith JD, Kinter M. Localization of nitration and chlorination sites on apolipoprotein A-I catalyzed by myeloperoxidase in human atheroma and associated oxidative impairment in ABCA1-dependent cholesterol efflux from macrophages. J Biol Chem. 2005;280:38–47. doi: 10.1074/jbc.M407019200. [DOI] [PubMed] [Google Scholar]
- 104.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 105.Shaklai N, Garlick RL, Bunn HF. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J Biol Chem. 1984;259:3812–3817. [PubMed] [Google Scholar]
- 106.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 107.Rosenfeld ME, Palinski W, Yla-Herttuala S, Butler S, Witztum JL. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990;10:336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- 108.Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 109.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 110.Passarelli M, Tang C, McDonald TO, O’Brien KD, Gerrity RG, Heinecke JW, Oram JF. Advanced glycation end product precursors impair ABCA1-dependent cholesterol removal from cells. Diabetes. 2005;54:2198–2205. doi: 10.2337/diabetes.54.7.2198. [DOI] [PubMed] [Google Scholar]
- 111.Bowry VW, Stanley KK, Stocker R. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc Natl Acad Sci U S A. 1992;89:10316–10320. doi: 10.1073/pnas.89.21.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Szapacs ME, Kim HY, Porter NA, Liebler DC. Identification of proteins adducted by lipid peroxidation products in plasma and modifications of apolipoprotein A1 with a novel biotinylated phospholipid probe. J Proteome Res. 2008;7:4237–4246. doi: 10.1021/pr8001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McCall MR, Tang JY, Bielicki JK, Forte TM. Inhibition of lecithin- cholesterol acyltransferase and modification of HDL apolipoproteins by aldehydes. Arterioscler Thromb Vasc Biol. 1995;15:1599–1606. doi: 10.1161/01.atv.15.10.1599. [DOI] [PubMed] [Google Scholar]
- 114.Nobecourt E, Davies MJ, Brown BE, Curtiss LK, Bonnet DJ, Charlton F, Januszewski AS, Jenkins AJ, Barter PJ, Rye KA. The impact of glycation on apolipoprotein A-I structure and its ability to activate lecithin:cholesterol acyltransferase. Diabetologia. 2007;50:643–653. doi: 10.1007/s00125-006-0574-z. [DOI] [PubMed] [Google Scholar]
- 115.Witz G. Biological interactions of alpha, beta-unsaturated aldehydes. Free Radic Biol Med. 1989;7:333–349. doi: 10.1016/0891-5849(89)90137-8. [DOI] [PubMed] [Google Scholar]
- 116.Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy- amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha, beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest. 1997;99:424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Uchida K. Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc Med. 1999;9:109–113. doi: 10.1016/s1050-1738(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 118.Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, Suzuki D, Miyata T, Noguchi N, Niki E, Osawa T. Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci U S A. 1998;95:4882–4887. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heinecke JW. Mechanisms of oxidative damage by myeloperoxidase in atherosclerosis and other inflammatory disorders. J Lab Clin Med. 1999;133:321–325. doi: 10.1016/s0022-2143(99)90061-6. [DOI] [PubMed] [Google Scholar]
- 120.Shao B, Fu X, McDonald TO, Green PS, Uchida K, O’Brien KD, Oram JF, Heinecke JW. Acrolein impairs ATP binding cassette transporter A1- dependent cholesterol export from cells through site-specific modification of apolipoprotein A-I. J Biol Chem. 2005;280:36386–36396. doi: 10.1074/jbc.M508169200. [DOI] [PubMed] [Google Scholar]
- 121.Furuhata A, Ishii T, Kumazawa S, Yamada T, Nakayama T, Uchida K. N(epsilon)-(3-methylpyridinium)lysine, a major antigenic adduct generated in acrolein- modified protein. J Biol Chem. 2003;278:48658–48665. doi: 10.1074/jbc.M309401200. [DOI] [PubMed] [Google Scholar]
- 122.Uchida K, Kanematsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem. 1998;273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 123.Richter T, Munch G, Luth HJ, Arendt T, Kientsch-Engel R, Stahl P, Fengler D, Kuhla B. Immunochemical crossreactivity of antibodies specific for “advanced glycation end products” with “advanced lipoxidation endproducts”. Neurobiol Aging. 2005;26:465–474. doi: 10.1016/j.neurobiolaging.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 124.Chroni A, Liu T, Gorshkova I, Kan HY, Uehara Y, Von Eckardstein A, Zannis VI. The central helices of ApoA-I can promote ATP-binding cassette transporter A1 (ABCA1)-mediated lipid efflux. Amino acid residues 220–231 of the wild-type ApoA-I are required for lipid efflux in vitro and high density lipoprotein formation in vivo. J Biol Chem. 2003;278:6719–6730. doi: 10.1074/jbc.M205232200. [DOI] [PubMed] [Google Scholar]
- 125.Panagotopulos SE, Witting SR, Horace EM, Hui DY, Maiorano JN, Davidson WS. The role of apolipoprotein A-I helix 10 in apolipoprotein-mediated cholesterol efflux via the ATP-binding cassette transporter ABCA1. J Biol Chem. 2002;277:39477–39484. doi: 10.1074/jbc.M207005200. [DOI] [PubMed] [Google Scholar]
- 126.Natarajan P, Forte TM, Chu B, Phillips MC, Oram JF, Bielicki JK. Identification of an apolipoprotein A-I structural element that mediates cellular cholesterol efflux and stabilizes ATP binding cassette transporter A1. J Biol Chem. 2004;279:24044–24052. doi: 10.1074/jbc.M400561200. [DOI] [PubMed] [Google Scholar]
- 127.Savenkova ML, Mueller DM, Heinecke JW. Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoprotein. J Biol Chem. 1994;269:20394–20400. [PubMed] [Google Scholar]
- 128.Shao B, Pennathur S, Pagani I, Oda MN, Witztum JL, Oram JF, Heinecke JW. Modifying apolipoprotein A-I by malondialdehyde, but not by an array of other reactive carbonyls, blocks cholesterol efflux by the ABCA1 pathway. J Biol Chem. 2010;285:18473–18484. doi: 10.1074/jbc.M110.118182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ishii T, Kumazawa S, Sakurai T, Nakayama T, Uchida K. Mass spectroscopic characterization of protein modification by malondialdehyde. Chem Res Toxicol. 2006;19:122–129. doi: 10.1021/tx050231p. [DOI] [PubMed] [Google Scholar]
- 130.Requena JR, Fu MX, Ahmed MU, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. Quantification of malondialdehyde and 4-hydroxynonenal adducts to lysine residues in native and oxidized human low-density lipoprotein. Biochem J. 1997;322(Pt 1):317–325. doi: 10.1042/bj3220317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Burgess JW, Frank PG, Franklin V, Liang P, McManus DC, Desforges M, Rassart E, Marcel YL. Deletion of the C-terminal domain of apolipoprotein A-I impairs cell surface binding and lipid efflux in macrophage. Biochemistry. 1999;38:14524–14533. doi: 10.1021/bi990930z. [DOI] [PubMed] [Google Scholar]
- 132.Vedhachalam C, Liu L, Nickel M, Dhanasekaran P, Anantharamaiah GM, Lund-Katz S, Rothblat GH, Phillips MC. Influence of ApoA-I structure on the ABCA1-mediated efflux of cellular lipids. J Biol Chem. 2004;279:49931–49939. doi: 10.1074/jbc.M406924200. [DOI] [PubMed] [Google Scholar]
- 133.Slatter DA, Bolton CH, Bailey AJ. The importance of lipid-derived malondialdehyde in diabetes mellitus. Diabetologia. 2000;43:550–557. doi: 10.1007/s001250051342. [DOI] [PubMed] [Google Scholar]
- 134.Palinski W, Yla-Herttuala S, Rosenfeld ME, Butler SW, Socher SA, Parthasarathy S, Curtiss LK, Witztum JL. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 1990;10:325–335. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- 135.Schaur RJ. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol Aspects Med. 2003;24:149–159. doi: 10.1016/s0098-2997(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 136.Uchida K, Stadtman ER. Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proc Natl Acad Sci U S A. 1992;89:4544–4548. doi: 10.1073/pnas.89.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]