Abstract
Objective
A common gain-of-function LPL variant, LPLS447X, has favorable clinical features and involves a C→G base change at nucleotide 1595 of the LPL cDNA, along with a haplotype, which includes other non-coding SNPs. The mechanism for the LPL gain-in-function is not clear. LPL translation is regulated by epinephrine by an RNA-protein complex, consisting of PKA subunits and an A kinase anchoring protein (AKAP), which targets the 3’UTR.
Methods
To examine LPL translation of the LPLS447X variant, in vitro translation of LPL mRNA constructs was studied in the presence of cytoplasmic extracts from 3T3-F442A adipocytes treated with/without epinephrine.
Results
When the C→G base change at nucleotide 1595 was introduced, LPL mRNA was less susceptible to inhibition by the adipocyte extract. Similarly, a lessened susceptibility to translation inhibition occurred when the complete haplotype was constructed in the full-length 3.6 kb LPL mRNA, when an irrelevant coding sequence was introduced into the LPL mRNA construct, and in response to the use of components of the RNA binding complex (PKA C and R subunits, and KH region of AKAP149).
Conclusion
These studies suggest that the LPLS447X gain of function may be due to the base change in the LPL mRNA resulting in a decreased susceptibility to translational inhibition.
Supplementary key words: cardiovascular disease, triglycerides, epinephrine, A kinase anchoring protein, protein kinase A
Introduction
Lipoprotein lipase (LPL) is a central enzyme in lipoprotein metabolism and hydrolyses the triglyceride core of plasma chylomicrons and VLDL, releasing free fatty acids [1]. A number of common conditions, such as diabetes and renal insufficiency, are associated with low levels of LPL, resulting in elevated levels of triglycerides and potentially leading to increased atherosclerosis [2]. Many different point mutations in LPL have been identified in patients with LPL deficiency, and heterozygotes for these mutations demonstrate modest levels of hypertriglyceridemia [3].
In addition to many causes of decreased LPL, a common human LPL mutation, LPL S447X, is a gain-of-function variant. This variant involves a C→G change at nucleotide 1595 of the LPL cDNA, which leads to a change in amino acid 447 from a serine to a stop codon, truncating the LPL protein of the two C-terminal amino acids [4]. The resulting increase in LPL activity leads to a modest decrease in triglycerides, an increase in HDL cholesterol, and has been associated with a modest protection from cardiovascular disease [5, 6]. A more detailed genetic mapping of LPL revealed that the S447X LPL variant occurs as part of a haplotype and tightly linked to the base change causing the premature stop codon were 6 additional exonic SNPs located further 3’ on the LPL cDNA [7].
Because of the change in protein sequence, some studies have hypothesized that the LPL S447X variant involves a more active LPL protein. When the LPLS447X variant was expressed, one study found increased LPL specific activity [8], but others found no difference when compared to wild type LPL [9–13].
Another possible mechanism for the LPL S447X gain of function is altered LPL gene expression, especially at the level of translation. LPL translation is altered by catecholamines, thyroid hormone, and by poor glycemic control in diabetic subjects [14–17]. In response to catecholamines in vitro, LPL translation is inhibited by the formation of an RNA binding complex containing the catalytic and regulatory subunits of PKA and A kinase anchoring protein (AKAP) which interacts with the 3’UTR of LPL mRNA [15, 18].
Because the translational regulation of LPL is inhibitory, and involves the 3’UTR and the region very close to the LPL S447X point mutation, we hypothesized that the LPL S447X mutation resulted in an altered LPL mRNA which would demonstrate reduced binding to the RNA binding complex, and would therefore be better translated. An increased efficiency of LPL translation would therefore result in increased LPL activity, and the clinical phenotype described by others.
Methods
LPL S447X is rs328 in the National Center for Biotechnology Information SNP database, dbSNP (http://www.ncbi.nlm.nih.gov/SNP/).
Preparation of constructs
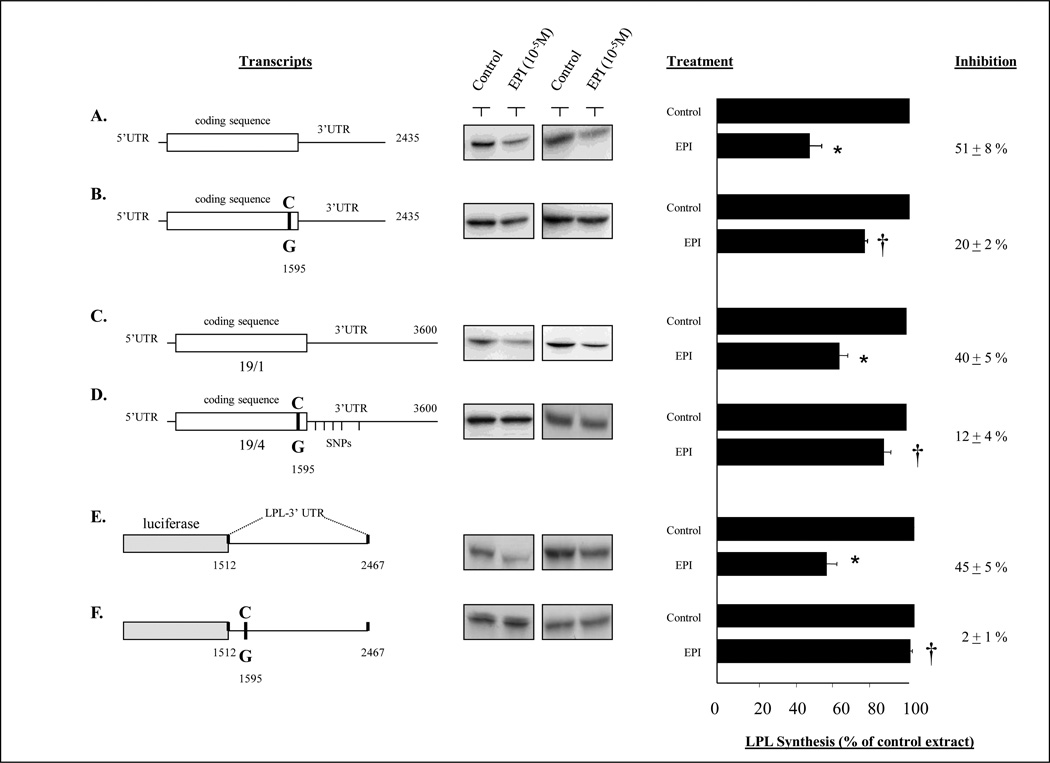
Six different LPL mRNA constructs were used in in vitro translation reactions to assess their response to inhibition. These constructs are illustrated in Figure 1 and labeled as A, B, C, D, E, and F.
Figure 1.
Effects of an epinephrine-treated adipocyte extract on LPL constructs. A cytoplasmic extract was prepared from 3T3-F442A adipocytes with or without treatment with epinephrine 10−5M for 2 hr. In previous studies, this epinephrine-treated cytoplasmic extract inhibited LPL translation in an in vitro translation system [20]. The control and epinephrine-treated extracts were added to an in vitro translation system containing different LPL mRNA constructs which contained different components of the LPL S447X variant sequence. Constructs A and B differed only in the absence or presence of the C→G variant at nucleotide 1595. Constructs C and D represented the entire 3.6 LPL mRNA sequence without or with the full haplotype, which included the variant at nucleotide 1595. Constructs E and F contained an irrelevant coding sequence (luciferase) followed by LPL sequence from nucleotide 1512 to 2467, without and with the C→G variant at 1595. Each experiment was repeated at least thrice. The middle panel illustrates representative autoradiograms from the in vitro translation reactions. The mean (±SEM) percent inhibition of each reaction is shown on the far right. *p<0.05 vs control cell extract; †p<0.05 vs the wildtype construct (without the C→G variant).
Construct A is LPL35, described by Wion et al [19] and contains 174 nucleotides of 5’ UTR, the complete coding sequence and 822 nucleotides of the 3’UTR. Construct B is Construct A with a C→G point mutation at nt 1595. This point mutation was made using the QuickChange 11 Site-Directed mutagenesis Kit (Stratagene. CA 92037). Complementary primers were designed spanning nt 1573–1609 of human LPL cDNA [19] and containing the desired mutation C→G at nt: 1595. The primer sequences were: F-5’CAGAAGTCTCTGAATAAGAAGTGAGGCTGAAACTGGG-3’; R- 5’-CCCCAGTTTCAGCCTCACTTCTTATTCAGAGACTTCTG-3’. The mutagenesis and cloning were performed according to manufacturer’s instructions using Pfu-Ultra high-fidelity DNA polymerase. In brief, the plasmid LPL35 was amplified using the two complementary primers containing the desired mutation. Each primer amplifies the complementary strand on the plasmid DNA during temperature cycling without primer displacement. Extension of the oligo-nucleotide primer generates a mutated plasmid with nicks. The product is then digested with Dpn 1endonuclease which digests methylated starter plasmid DNA template. The nicked newly synthesized DNA containing the desired mutation is transformed into competent cells. Discrete colonies are selected and the presence of the single point mutation C→G at nt 1595 is confirmed by sequencing and is referred to as LPL2435 C→G.
Constructs C and D were LPL cDNA constructs corresponding to haplotypes 19-1 and 19-4 were constructed. LPL 2435 [19] or LPL 2435 C→G were cut with MSC1 (nt 1812) and BamH1 (vector). The fragment containing nt 1–1812 of hLPL cDNA and the vector was gel purified and ligated with PCR products corresponding to the region nt 1812–3600 of the hLPL isolated from the lyphoblastoid cells from subjects with 19-1 or 19-4 haplotypes, as described [7]. The primers used were FR. 5’-GTATAGTGGCCAAATAGCA. 3’RV. 5’-GGTAATAAAATGTTGTCA. 3’, and the resulting PCR products were cloned into PCR 2.1 topo vector and the region containing nt 1812–3600 was cut out using MSC1 and BamH1. The 19-4 cDNA contained the early serine stop at nucleotide 1595 and 6 additional exonic SNPs in the 3’UTR of hLPL at nt 2454, 2825, 3272, 3342, 3406, 3446. The 19-1 cDNA clone did not contain these SNPs.
Constructs E and F involved the reporter construct Luciferase-LPL 3’UTR (shown in Figure 1 E) has been described by us previously and contains the region between nucleotides 1512 and 2451 of LPL cDNA cloned 3’ to luciferace coding sequence [20]. Construct E was mutated at nucleotide 1595 C→G using QuickChange 11 Site-Directed mutagenesis Kit (Stratagene. CA 92037) as described above in the preparation of construct B from construct A.
All constructs were sequenced and the presence of the appropriate SNP was confirmed. Transcripts were obtained from each construct after digestion at the unique poly linker restriction site, and in vitro transcription using T7 RNA polymerase.
Cell culture and differentiation
3T3-F442A cells were obtained from Dr. Howard Green (Harvard Medical School, Boston, MA). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco BRL), supplemented to 10% with calf serum. For experiments, cells were grown to confluence and stimulated to differentiate in DMEM containing 10% fetal bovine serum and 100nM insulin for 14 days.
Preparation of cytoplasmic extracts
Approximately 100 dishes (100 mm) containing 3T3-F442A adipocytes, representing ~109 cells differentiated with insulin for 14 days were used for the experiment. Cells were treated with 10−5M epinephrine for 2 h. A cytoplasmic extract was prepared as described previously [21]. In brief, adipocytes were homogenized in lysis buffer (50mM Tris-HCl, pH 7.4, 250mM sucrose, 35mM KCl, 10mM MgCl2, 0.5mM EDTA, 7mM β-mercaptoethanol), 2mM phenylmethylsulfonyl fluoride and protease inhibitor cocktail (Sigma). The post-nuclear extract was used to prepare a high-speed supernatant fraction (S-100); this cytosolic fraction was precipitated with ammonium sulfate to 60% saturation for 30 minutes on ice. Precipitated proteins were collected, dissolved and dialyzed against Buffer A (20mM Tris-HCl, pH 7.4, 20mM KCl, 10% glycerol, 7mM β-mercaptoethanol, 0.1mM EDTA, and 2mM phenylmethylsulfonyl fluoride.
In vitro translation
RNA transcripts were made from LPL constructs described previously [21] using T7 polymerase. In vitro translation of RNA transcripts was performed using rabbit reticulocyte in vitro translation system as described [21]. Equal quantities of RNA transcripts (0.1µg) were translated in a rabbit reticulocyte lysate system (Promega, WI) in the presence of [35S] methionine. Reactions were incubated at 30°C for 1 h and terminated by transferring to 4°C. The products of reactions were analyzed on 10%-SDS PAGE followed by autoradiography. Images are quantitated using Image Quant software (Amersham Biosciences, NJ). The control or epinephrine treated S-100 cell extract or expressed GST fusion proteins [18] as well as PKA subunits were added to the in vitro translation reactions as specified.
Statistics
All data were expressed as mean ± SEM, and Student’s two-sample t-tests were used to compare groups.
Results
To examine the impact of the LPL S447X variant on LPL translation, we prepared constructs with different point mutations. In previous studies, we determined that LPL translation was inhibited by transacting factors that bound to a region near the first 40 nt of the LPL 3’ UTR [20, 21]. The 1595 C→G point mutation is very near the 3’UTR, so we wished to examine the effects of this variant on LPL translation. On the other hand, the 1595 C→G variant occurs as part of a haplotype, involving mutations further 3’ [7], and we wished to examine the impact of these mutations on LPL translation. Therefore, we created LPL constructs containing the 1595 C→G variant both with and without the additional six SNPs present on the 3’UTR, as is found in the 19-4 haplotype.
LPL translation inhibition can be demonstrated in vitro by the addition of a cytoplasmic extract from cells treated with epinephrine [20, 21]. To determine the functional significance of each construct, we prepared a cytoplasmic extract from control and epinephrine-treated 3T3-F442A adipocytes, and added these cytoplasmic extracts to the in vitro translation reaction containing different LPL constructs.
As illustrated in Figure 1, LPL Construct A contained the 175 nucleotides of 5’UTR, the complete coding sequence and 836 nucleotides of 3’UTR. Construct B was identical to construct A but contained the 1595 C→G point mutation. Translation of transcript A was inhibited by 51±8% by the epinephrine-treated cell extract, compared to the control extract, and Transcript B was only inhibited 20± 2% by the epinephrine treated extract (p<0.05 vs inhibition of translation in transcript A). Construct C utilized the full length 3.6 kb LPL cDNA, containing the entire 3’UTR, and was constructed using DNA amplified from exon 10 of the wild type 19-1 haplotype, whereas Construct D was used DNA amplified from exon 10 of the 19-4 haplotype and contained the 1595 C→G mutation. Translation of transcript C was inhibited by 40± 5% by the epinephrine treated extract as compared to control extract, whereas transcript D demonstrated no significant translation inhibition by the addition of the epinephrine treated extracts. Construct E contained an irrelevant coding sequence from luciferase, along with LPL sequence 3’ to luciferase. The LPL sequence included nt 1512 –2467, which involves 87 bases of LPL coding sequence followed by 868 nt of the proximal 3’ UTR. Construct F was made by introducing a single point mutation at nucleotide 1595 C→G in construct E using site directed mutagenesis. Translation of transcript E was inhibited by 45± 5% in the presence of epinephrine treated extract, but the insertion of a single point mutation at nucleotide 1595 C→G (Transcript F) resulted in complete loss of the inhibitory response to epinephrine treated extract.
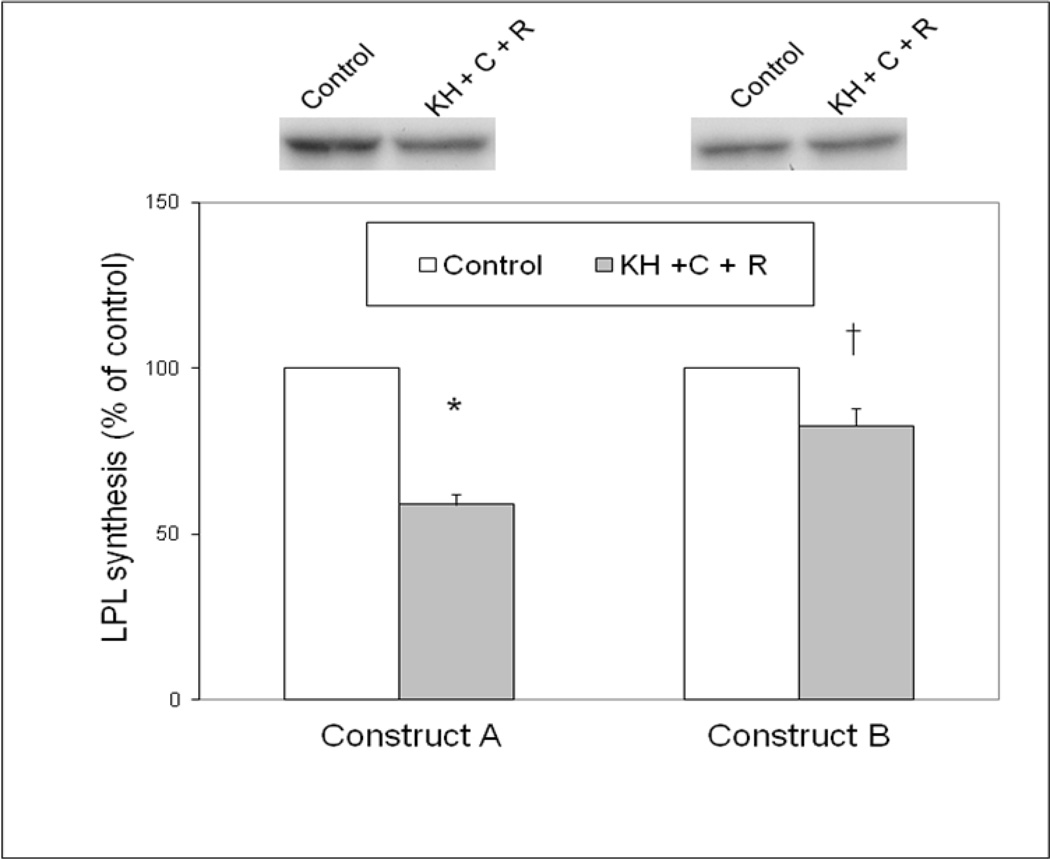
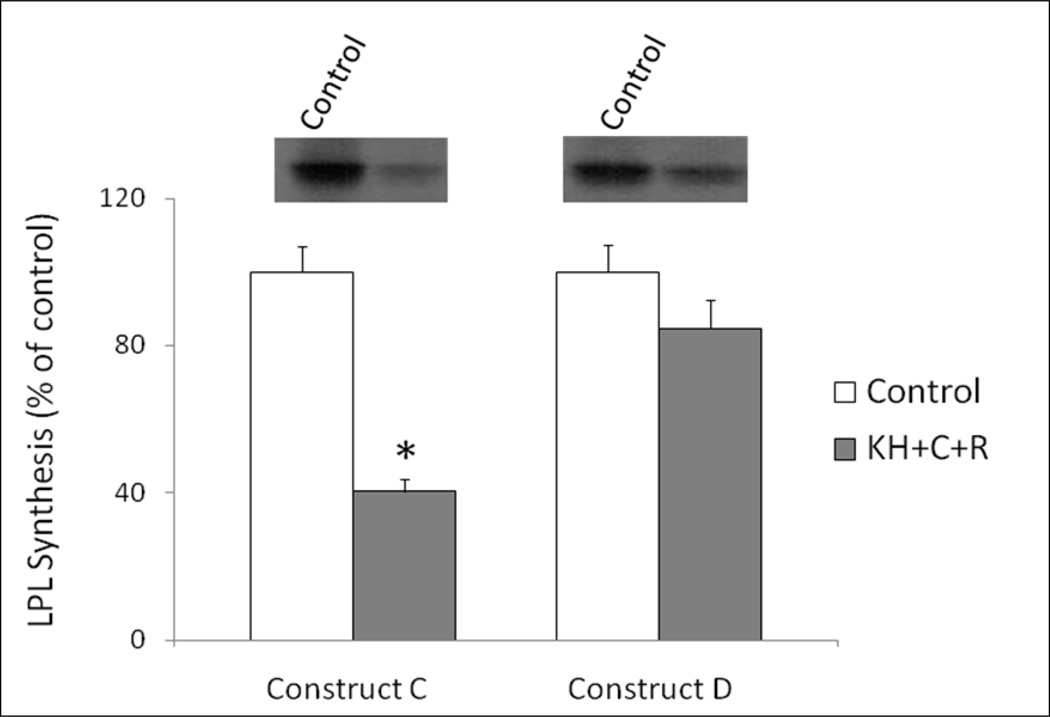
The components of the RNA binding complex that impact LPL translation in response to epinephrine include the C and R subunits of PKA along with the KH region of AKAP149 [15, 18]. To further clarify the role of the 1595 C→G point mutation, transcript A and transcript B were translated in the presence of PKA C subunit, R subunit, and a GST fusion protein containing the KH region of AKAP149. Figure 2 shows that transcript A was inhibited by 55± 5% by the addition of the purified RNA binding proteins and transcript B was not significantly inhibited by the addition of the same proteins. This same experiment was performed using Construct C and Construct D, corresponding to the 19-1 and 19-4 haplotypes, respectively (Figure 3). Whereas the addition of the purified RNA binding proteins inhibited translation of the Construct C by 60%, there was no significant inhibition of translation of Construct D, confirming the results of Figure 1 using an adipocyte cytoplasmic extract.
Figure 2.
Effects of RNA binding complex on translation of LPL constructs. Previously identified components of the LPL inhibitory RNA binding complex include the C and R subunits of PKA, as well as the KH region of AKAP149 [18]. The C and R subunits were added to the in vitro translation reaction along with a GST fusion protein containing the KH region of AKAP149 in the presence of LPL Construct A and Construct B, corresponding to the LPL transcript without or with the C→G variant at nucleotide 1595, as described in methods.. *p<0.05 vs control cell extract; †p<0.05 vs the wildtype construct (without the C→G variant).
Figure 3.
Effects of RNA binding complex on translation of LPL haplotype constructs. This experiment was performed as described for Figure 2, except the C and R subunits of PKA, as well as the KH region of AKAP149, were added to Construct C and Construct D, as described in Methods. These constructs correspond to the 19-1 and 19-4 haplotypes, respectively. *p<0.05 vs control cell extract;
Discussion
The LPL gene contains many polymorphisms and mutations, many of which result in decreased lipolytic activity and clinical features which include hypertriglyceridemia and cardiovascular disease [3]. In contrast, the LPL S447X variant results in a gain of function and has been extensively studied [4]. The increase in LPL activity that results from the variant is subtle, and results in protection against cardiovascular disease, presumably due to the lower triglycerides and higher HDL cholesterol [22, 23], although hypertensive patients with the S447X variant have a higher incidence of left ventricular hypertrophy, perhaps due to increased FFA delivery to the heart [24].
Notwithstanding the increased LPL activity and the mostly favorable clinical data, the mechanisms underlying the increased LPL activity of the S447X variant are not clear. A number of studies have examined LPL catalytic activity, and the findings have not consistently supported the hypothesis that the S447X variant is a more active enzyme than wild type LPL [8–13]. It is possible that there is an increase in LPL activity in the variant, but it is clouded by the variability of the non-standardized assays for LPL activity and mass. On the other hand, the increased LPL activity of the S447X variant could be due to other mechanisms, and this study was performed to consider an alternative explanation involving changes in LPL protein expression.
LPL regulation is mechanistically complex, and may involve changes in LPL mRNA levels [25], or posttranscriptional changes at different levels [26]. Translational regulation of LPL occurs in response to catecholamines, thyroid hormone, and diabetes [16, 17, 27]. In response to epinephrine, an RNA binding complex is formed that is composed of the C and R subunits of PKA, along with a member of the A-kinase-anchor-protein family [15, 18]. In studies using different RNA constructs in vitro, the region of the LPL mRNA that interacted with the RNA binding complex included the first 40 nucleotides of the LPL 3’UTR [20].
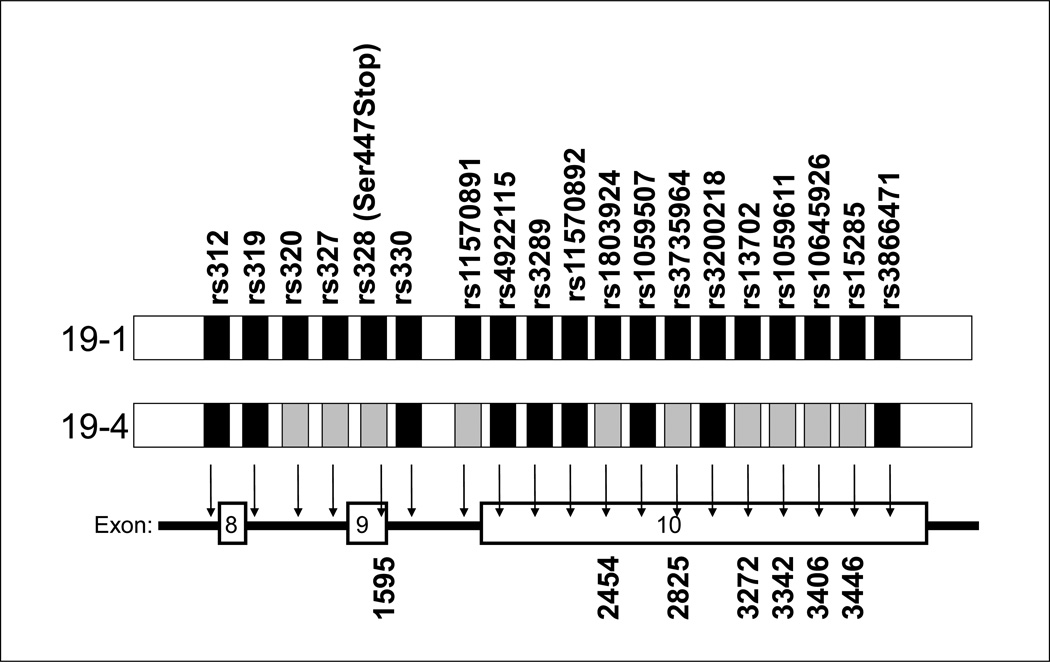
This study was intended to determine whether the changes in the LPL mRNA associated with the S447X variant alter LPL translation. As shown in Figure 4, the S447X variant occurs in the 19-4 haplotype, which includes 6 base changes in the LPL 3’UTR, along with the 1595 C→G change that results in the serine→stop, and the change in amino acid sequence, along with other intronic base changes. Although none of the variants in the haplotype is within the 40 nucleotides of the 3’ UTR, the base change that results in the serine→stop is only 4 nucleotides from the 3’UTR, and could be involved in a complex binding site of the RNA binding complex.
Figure 4.
Schematic illustration of the 19-1 and 19-4 haplotypes. The grey boxes represent the variant alleles that are specific to 19-4, and their position on the LPL gene is shown with arrows. Distances are not to scale. The numbers at the bottom are the corresponding positions on the LPL cDNA.
To examine LPL translation, we constructed different mRNAs that contained either a point mutation of C→G at position 1595, or which contained this mutation along with the complete haplotype. In vitro translation was used, whereby the mRNA of interest was translated in a reticulocyte lysate system in the presence of a cytoplasmic extract from adipocytes that had been treated with epinephrine, as has been described previously [20]. The inclusion of the SNP at position 1595 resulted in a blunted response to the epinephrine treated adipocyte extract. This occurred when the SNP was introduced into a construct containing 836 nucleotides of 3’UTR, and was also present when an irrelevant coding sequence (luciferase) was used. The blunting of LPL translation inhibition was similar when the entire haplotype was studied in the full-length 3.6 kb LPL mRNA construct. Additional studies were performed using purified components of the RNA binding complex. When the PKA C and R subunits, along with the KH region of AKAP 149 was added to the in vitro translation reaction, LPL translation was inhibited. These data suggest that the 1595 C→G mutation is important in the epinephrine-mediated inhibition of LPL translation. It is possible that more distal point mutations in the haplotype play a role in the translation inhibition, but this was not evident from these studies.
LPL is subject to positive hormonal regulation by insulin and other elements of the fed state, and under negative regulation by catecholamines and other factors in the post-absorptive state. To the extent that the S447X variant diminishes the inhibitory influences of PKA activation, LPL activity is higher, and this increase in lipoprotein metabolism could lead to a reduction in triglycerides, an increase in HDL, and a subsequent resistance to heart disease, as has been observed in epidemiologic studies. The studies describe in this report indicate a potential new mechanism for this well-described clinical observation.
Highlights.
Lipoprotein lipase clears triglyceride-rich lipoproteins from plasma, and there is a common gain-of-function variant with favorable clinical features.
We determined that the base change in this variant that alters the amino acid sequence also changes the manner by which the LPL mRNA is translated.
LPL RNA constructs with the variant base change were less susceptible to inhibition by an RNA binding complex composed of protein kinase A subunits.
Acknowledgments
Grant support: Merit Review Grants (GR) from the Veterans Administration, and NIH grants R37-DK039176 (PAK), R01-DK080327 (PAK), P01-HL060030 (W. Hsueh), R01-HL088457 (JIR), R01-DK079888 (MOG), M01-RR00425, and UL1-RR033173.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Merkel M, Eckel RH, Goldberg IJ. Lipoprotein lipase: genetics, lipid uptake, and regulation. J Lipid Res. 2002;43:1997–2006. doi: 10.1194/jlr.r200015-jlr200. [DOI] [PubMed] [Google Scholar]
- 2.Zilversmit DB. Atherogenesis - a post-prandial phenomenon. Circulation. 1979;60:473–485. doi: 10.1161/01.cir.60.3.473. [DOI] [PubMed] [Google Scholar]
- 3.Murthy V, Julien P, Gagne C. Molecular pathobiology of the human lipoprotein lipase gene. Pharmacol Ther. 1996;70:101–135. doi: 10.1016/0163-7258(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 4.Rip J, Nierman MC, Ross CJ, Jukema JW, Hayden MR, Kastelein JJ, Stroes ES, Kuivenhoven JA. Lipoprotein lipase S447X: a naturally occurring gain-of-function mutation. Arterioscler Thromb Vasc Biol. 2006;26:1236–1245. doi: 10.1161/01.ATV.0000219283.10832.43. [DOI] [PubMed] [Google Scholar]
- 5.Wittrup HH, Tybjaerg-Hansen A, Nordestgaard BG. Lipoprotein lipase mutations, plasma lipids and lipoproteins, and risk of ischemic heart disease. A meta-analysis. Circulation. 1999;99:2901–2907. doi: 10.1161/01.cir.99.22.2901. [DOI] [PubMed] [Google Scholar]
- 6.Sagoo GS, Tatt I, Salanti G, Butterworth AS, Sarwar N, van MM, Jukema JW, Wiman B, Kastelein JJ, Bennet AM, de FU, Danesh J, Higgins JP. Seven Lipoprotein Lipase Gene Polymorphisms, Lipid Fractions, and Coronary Disease: A HuGE Association Review and Meta-Analysis. Am J Epidemiol. 2008 doi: 10.1093/aje/kwn235. [DOI] [PubMed] [Google Scholar]
- 7.Goodarzi MO, Wong H, Quinones MJ, Taylor KD, Guo X, Castellani LW, Antoine HJ, Yang H, Hsueh WA, Rotter JI. The 3' untranslated region of the lipoprotein lipase gene: haplotype structure and association with post-heparin plasma lipase activity. J Clin Endocrinol Metab. 2005;90:4816–4823. doi: 10.1210/jc.2005-0389. [DOI] [PubMed] [Google Scholar]
- 8.Kozaki K, Gotoda T, Kawamura M, Shimano H, Yazaki Y, Ouchi Y, Orimo H, Yamada N. Mutational analysis of human lipoprotein lipase by carboxy- terminal truncation. J Lipid Res. 1993;34:1765–1772. [PubMed] [Google Scholar]
- 9.Reymer PWA, Gagne E, Groenemeyer BE, Zhang H, Forsyth I, Jansen H, Seidell JC, Kromhout D, Lie KE, Kastelein J, Hayden MR. A lipoprotein lipase mutation (Asn291Ser) is associated with reduced HDL cholesterol levels in premature atherosclerosis. Nature Genetics. 1995;10:28–34. doi: 10.1038/ng0595-28. [DOI] [PubMed] [Google Scholar]
- 10.Previato L, Guardamagna O, Dugi KA, Ronan R, Talley GD, Santamarina-Fojo S, Brewer HB., Jr A novel missense mutation in the C-terminal domain of lipoprotein lipase (Glu410-->Val) leads to enzyme inactivation and familial chylomicronemia. J Lipid Res. 1994;35:1552–1560. [PubMed] [Google Scholar]
- 11.Zhang H, Henderson H, Gagne SE, Clee SM, Miao L, Liu G, Hayden MR. Common sequence variants of lipoprotein lipase: standardized studies of in vitro expression and catalytic function. Biochim Biophys Acta. 1996;1302:159–166. doi: 10.1016/0005-2760(96)00059-8. [DOI] [PubMed] [Google Scholar]
- 12.Salinelli S, Lo JY, Mims MP, Zsigmond E, Smith LC, Chan L. Structure-function relationship of lipoprotein lipase-mediated enhancement of very low density lipoprotein binding and catabolism by the low density lipoprotein receptor. Functional importance of a properly folded surface loop covering the catalytic center. J Biol Chem. 1996;271:21906–21913. doi: 10.1074/jbc.271.36.21906. [DOI] [PubMed] [Google Scholar]
- 13.Faustinella F, Chang A, Van Biervliet JP, Rosseneu M, Vinaimont N, Smith LC, Chen S-H, Chan L. Catalytic triad residue mutation (Asp156 -->Gly) causing familial lipoprotein lipase deficiency. Co-inheritance with a nonsense mutation (Ser447 -->Ter) in a Turkish family. J Biol Chem. 1991;266:14418–14424. [PubMed] [Google Scholar]
- 14.Ranganathan G, Li C, Kern PA. The translational regulation of lipoprotein lipase in diabetic rats involves the 3'-untranslated region of the lipoprotein lipase mRNA. J Biol Chem. 2000;275:40986–40991. doi: 10.1074/jbc.M008775200. [DOI] [PubMed] [Google Scholar]
- 15.Ranganathan G, Phan D, Pokrovskaya ID, McEwen JE, Li C, Kern PA. The translational regulation of lipoprotein lipase by epinephrine involves an RNA binding complex including the catalytic subunit of protein kinase A. J Biol Chem. 2002;277:43281–43287. doi: 10.1074/jbc.M202560200. [DOI] [PubMed] [Google Scholar]
- 16.Saffari B, Ong JM, Kern PA. Regulation of adipose tissue lipoprotein lipase gene expression by thyroid hormone in rats. J Lipid Res. 1992;33:241–249. [PubMed] [Google Scholar]
- 17.Simsolo RB, Ong JM, Saffari B, Kern PA. Effect of improved diabetes control on the expression of lipoprotein lipase in human adipose tissue. J Lipid Res. 1992;33:89–95. [PubMed] [Google Scholar]
- 18.Ranganathan G, Pokrovskaya I, Ranganathan S, Kern PA. Role of A kinase anchor proteins in the tissue specific regulation of lipoprotein lipase. Mol Endocrinol. 2005;19:2527–2534. doi: 10.1210/me.2005-0144. [DOI] [PubMed] [Google Scholar]
- 19.Wion KL, Kirchgessner TG, Lusis AJ, Schotz MC, Lawn RM. Human lipoprotein lipase complementary DNA sequence. Science. 1987;235:1638–1641. doi: 10.1126/science.3823907. [DOI] [PubMed] [Google Scholar]
- 20.Ranganathan G, Vu D, Kern PA. Translational regulation of lipoprotein lipase by epinephrine involves a transacting binding protein interacting with the 3' untranslated region. J Biol Chem. 1997;272:2515–2519. doi: 10.1074/jbc.272.4.2515. [DOI] [PubMed] [Google Scholar]
- 21.Yukht A, Davis RC, Ong JM, Ranganathan G, Kern PA. Regulation of lipoprotein lipase translation by epinephrine in 3T3-L1 cells - Importance of the 3' untranslated region. J Clin Invest. 1995;96:2438–2444. doi: 10.1172/JCI118301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–219. [PubMed] [Google Scholar]
- 23.Wittrup HH, Nordestgaard BG, Steffensen R, Jensen G, Tybjaerg-Hansen A. Effect of gender on phenotypic expression of the S447X mutation in LPL: the Copenhagen City Heart Study. Atherosclerosis. 2002;165:119–126. doi: 10.1016/s0021-9150(02)00183-1. [DOI] [PubMed] [Google Scholar]
- 24.Talmud PJ, Flavell DM, Alfakih K, Cooper JA, Balmforth AJ, Sivananthan M, Montgomery HE, Hall AS, Humphries SE. The lipoprotein lipase gene serine 447 stop variant influences hypertension-induced left ventricular hypertrophy and risk of coronary heart disease. Clin Sci (Lond) 2007;112:617–624. doi: 10.1042/CS20060344. [DOI] [PubMed] [Google Scholar]
- 25.Ong JM, Kirchgessner TG, Schotz MC, Kern PA. Insulin increases the synthetic rate and messenger RNA level of lipoprotein lipase in isolated rat adipocytes. J Biol Chem. 1988;263:12933–12938. [PubMed] [Google Scholar]
- 26.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab. 2009;297:E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 27.Ong JM, Saffari B, Simsolo RB, Kern PA. Epinephrine inhibits lipoprotein lipase gene expression in rat adipocytes through multiple steps in posttranscriptional processing. Mol Endocrinol. 1992;6:61–69. doi: 10.1210/mend.6.1.1738372. [DOI] [PubMed] [Google Scholar]