JAK2V617F, an activation mutant form of tyrosine kinase JAK2, is found in the majority of patients with myeloproliferative neoplasms (MPNs) including polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis. Overwhelming studies have demonstrated the pathogenicity of JAK2V617F.1 However, some still doubt that JAK2V617F is a primary molecular defect in causing the malignant disease. This uncertainty partly comes from the finding of biallelic JAK2V617F mutations.2, 3, 4 By using allele-specific PCR amplification of genomic DNAs and subcloning, Olcaydu et al.2 demonstrated an infrequent occurrence of biallelic JAK2V617F mutations in <5% of MPN patients. However, a subsequent study by Lambert et al.3 employed cDNA and identified biallelic JAK2V617F mutations in the majority of patients with ET. Based on this finding, it was concluded that independent JAK2V617F mutation events may occur in ET patients on a polyclonal background, and therefore, the presence of a JAK2 mutation in ET patients should not be equated with a malignant disease. A recent study by Beer et al.4 essentially confirmed the results using each individual method but cautioned that PCR artifacts may affect the outcome, and they concluded that the true prevalence of biallelic JAK2 mutations in ET is approximately 5–10%.
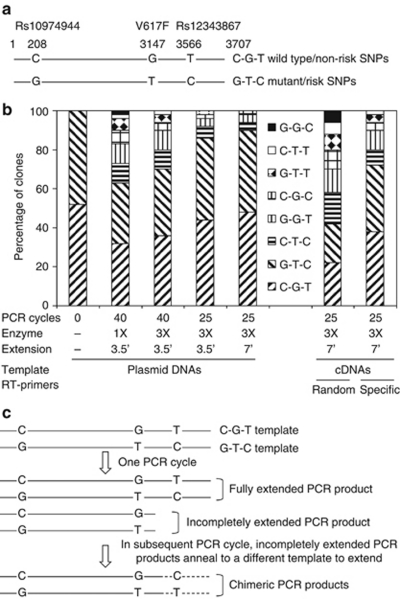
Although the previous studies were performed with patient DNA samples of unknown compositions, we believe it is important to clarify them by using DNAs with absolute allelic sequence information. For this purpose, we amplified a 3707-bp DNA fragment from blood genomic DNAs of a heterozygous JAK2V617F-positive PV patient with primers P1 5′-CAGGCTGGAATGCAGAAATACTAT-3′ and P2 5′-ATCTAAGAAGCACAATAAAGCAAGGT-3′ by using the high-fidelity Phusion polymerase. The fragment spans intron 12–14 of the JAK2 gene and contains single-nucleotide polymorphism (SNP) rs10974944, the V617F mutation site, and SNP rs12343867. Upon cloning into the pBluescript KS vector, two clones, representing two distinct JAK2 alleles, were verified by complete DNA sequencing and were designated C–G–T for wild-type JAK2 with two non-risk SNPs and G–T–C for mutant JAK2V617F DNA with two risk SNPs as illustrated in Figure 1a. The DNAs were purified and mixed in equal proportions for further PCR amplification. PCR was conducted with primers derived from the T3 and T7 promoter regions of the vector, giving rise to a 3883-bp product. We first ran the PCR under the standard conditions recommended by the manufacturer of the Phusion polymerase, except that the extension time was 3.5 min instead of the suggested 15 s/kb. A typical reaction with a total volume of 20 μl contained 10 ng mixed DNA templates, 0.4 U of Phusion DNA polymerase, 0.2 m dNTP (each) and 0.2 μ primers (each). The PCR was run for 25 or 40 cycles. Reactions were also conducted with three times the amount of Phusion polymerase of the standard procedure and a longer extension time of 7 min. The PCR products were digested with EcoRI and XhoI, and then ligated into the pBluescript KS vector opened with the same enzymes. The ligation products were used to transform Escherichia coli cells. At least 50 plasmid DNA clones were sequenced for each set of PCR conditions. The percentages of different clones are summarized in Figure 1b. In control experiments without PCR amplification, the sequencing results revealed approximately equal portions of C–G–T and G–T–C DNA clones without any chimeric products, indicating that the restriction enzyme digestion, DNA ligation and E. coli cell transformation process are highly reliable. However, after PCR amplification under the standard conditions for 40 cycles, eight different DNA clones were found, six of which were new chimeric products, representing all possible chimeric recombinations. The frequency of each chimeric product apparently correlated with the relative position of each site with C–T–C/G–G–T>C–G–C/G–T–T>C–T–T/G–G–C. Interestingly, using more Phusion polymerase, increasing the extension time and shortening PCR cycles significantly reduced the percentage of chimeric products, although they could not be completely eliminated. It should be noted that generation of the chimeric products is likely caused by template-switching during PCR rather than by new point mutations. Out of 500 clones sequenced, not a single point mutation was found, confirming the high fidelity of the Phusion polymerase.
Figure 1.
PCR artifacts cause production of chimeric DNA molecules in reactions with mixed templates. (a) Generation of DNA clones with allele-specific wild-type or mutant forms of JAK2. A 3707-bp DNA fragment was obtained by PCR amplification of genomic DNAs of a JAK2V617F-positive PV patient and cloned into the pBluescript KS vector. The plasmid DNAs were verified by DNA sequencing and were designated C–G–T for wild-type JAK2 with two non-risk SNPs (Rs10974944 and Rs12343867) and G–T–C for mutant JAK2V617F DNA with two risk SNPs as indicated. (b) Production of chimeric DNA molecules by PCR amplification of mixed templates. Purified C–G–T and G–T–C plasmid DNAs mixed in equal proportions were non-amplified or amplified with PCR under the indicated conditions and then digested with EcoRI and XhoI restriction enzymes. The DNA fragments were ligated into the pBluescript KS vector opened with EcoRI and XhoI. Alternately, total RNAs were isolated from recombinant E. coli cells carrying the C–G–T or G–T–C plasmid DNA, treated with DNase I, mixed in equal proportions and reversely transcribed by using either random primers or specific primer P2. The single-stranded cDNAs were amplified using PCR under the indicated conditions and the PCR products were ligated into the pBluescript KS vector opened with EcoRV restriction enzyme. The DNA ligation products were used to transform E. coli cells, and plasmid DNAs from transformed E. coli colonies were sequenced to verify their identities. Data represent percentages of colonies carrying different plasmid DNAs. Anything other than C–G–T and G–T–C represents a chimeric product produced by PCR. A minimum of 50 clones were sequenced for each set of PCR conditions. (c) Template-switch caused by incomplete PCR extension is a possible mechanism to explain the generation of chimeric DNA molecules.
To review the method employed in the study by Lambert et al.,3 we also conducted experiments with cDNA. For this purpose, total RNAs were isolated from recombinant E. coli cells carrying the C–G–T and G–T–C plasmid DNAs by using a hot phenol extraction/ethanol precipitation protocol. This was followed by treatment with DNase I to remove contaminated DNAs. The RNAs were then mixed in equal proportions for synthesis of single-strand cDNAs by reverse transcription with either random primers or specific primer P2. PCR was conducted for 25 cycles with primer P1 and P2 under the conditions described above with 3 × Phusion enzyme and 7 min extension time. PCR products were cloned into the pBluescript KS vector opened with EcoRV. E. coli colonies were picked based on blue/white selection, and over 50 plasmid DNAs were sequenced. The data are presented in Figure 1b (two far right columns). When random primers were used for reverse transcription, nearly 50% of the clones contained chimeric DNA products. With specific primers, the percentage was reduced to <30%. We believe that random primers more likely produce various cDNA fragments, which are combined using PCR to generate chimeric DNA products. Our data suggest that the PCR amplification of reversely transcribed cDNA is a very unreliable way to define allele specificity, especially if random primers were used for cDNA synthesis.
By using purified plasmid DNAs, our study demonstrated that PCR could generate chimeric products when mixed DNA templates were used. This is likely caused by template-switching resulting from incomplete extension of the DNA strand as illustrated in Figure 1c. In principle, an incompletely extended PCR product will serve as a primer and may anneal to a different strand in the subsequent PCR cycle, thereby producing a chimeric DNA product. This is particularly likely to occur when two SNP or mutation sites are distant from each other, which requires extra extension time to synthesize fully extended products, when DNA polymerase activity is reduced due to inhibition by impurities in DNA samples or exhaustion after many PCR cycles and when fragmented DNAs are present in the templates, such as in the case of reversely transcribed cDNA and shared genomic DNAs. Our study demonstrated that the production of chimeric PCR products in reactions with mixed templates appears to be inevitable. We can adjust PCR conditions to reduce the possibility but can never completely prevent this from occurring. Therefore, our study demonstrates that the PCR amplification can give rise to the artifactual appearance of biallelic JAK2V617F mutations. Of course, our data does not necessarily disprove previous conclusions about the presence of biallelic JAK2V617F mutations made by other investigators. After all, in at least one ET patient, the conclusion is also supported by the presence of JAK2V617F-positive erythroid colonies with different X-allele inactivations.3 Whether or not biallelic JAK2V617F mutations occur certainly warrants further investigation using more reliable methodologies.
Acknowledgments
This work was supported by grants HL079441 and HL094591 from the National Institutes of Health and a grant from Oklahoma Center for the Advancement of Science and Technology (to ZJZ).
The authors declare no conflict of interest.
References
- Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7:673–683. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- Olcaydu D, Harutyunyan A, Jäger R, Berg T, Gisslinger B, Pabinger I, et al. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet. 2009;41:450–454. doi: 10.1038/ng.341. [DOI] [PubMed] [Google Scholar]
- Lambert JR, Everington T, Linch DC, Gale RE. In essential thrombocythemia, multiple JAK2-V617F clones are present in most mutant-positive patients: a new disease paradigm. Blood. 2009;114:3018–3023. doi: 10.1182/blood-2009-03-209916. [DOI] [PubMed] [Google Scholar]
- Beer PA, Ortmann CA, Campbell PJ, Green AR. Independently acquired biallelic JAK2 mutations are present in a minority of patients with essential thrombocythemia. Blood. 2010;116:1013–1014. doi: 10.1182/blood-2010-05-284356. [DOI] [PMC free article] [PubMed] [Google Scholar]