Abstract
Polybrominated diphenyl ethers (PBDEs) are persistent organic chemicals used as flame retardants in textiles, plastics, and consumer products. Although PBDE accumulation in humans has been noted since the 1970s, few studies have investigated PBDEs within the gestational compartment, and none to date has identified levels in amniotic fluid. The present study reports congener-specific brominated diphenyl ether (BDE) concentrations in second-trimester clinical amniotic fluid samples collected in 2009 from fifteen women in southeast Michigan, USA. Twenty-one BDE congeners were measured by GC/MS/NCI. The average total PBDE concentration was 3795 pg/ml amniotic fluid (range: 337 – 21842 pg/ml). BDE-47 and BDE-99 were identified in all samples. Based on median concentrations, the dominant congeners were BDE-208, 209, 203, 206, 207, and 47 representing 23, 16, 12, 10, 9 and 6%, respectively, of the total detected PBDEs. PBDE concentrations were identified in all amniotic fluid samples from southeast Michigan, supporting a need for further investigations of fetal exposure pathways and potential impacts on perinatal health.
Keywords: Polybrominated diphenyl ethers (PBDEs), amniotic fluid, human exposure, pregnancy
Introduction
Polybrominated diphenyl ethers (PBDEs) are a class of widely used brominated flame retardants that have been incorporated into many consumer electronics, textiles and furniture (Birnbaum and Staskal, 2004). These compounds typically are not chemically bound within the products and thus may migrate into the environment. Because of their environmental persistence and toxicity, PBDEs have been identified as a priority human health concern (U.S. Environmental Protection Agency, 2006).
Commercial formulations of PBDEs contain mixtures of the individual brominated diphenyl ether (BDE) congeners: the penta-BDE formulation has BDE congeners with 3 to 6 bromine atoms; the octa-BDE formulation has 6 to 9 bromine atoms; and the deca-BDE formulation is composed primarily of the fully brominated deca-congener with variable levels of nona-BDE congeners. The penta-BDE and octa-BDE formulations were voluntarily withdrawn from the United States marketplace in 2004. In 2009, the 4th meeting of the Convention of Parties of the Stockholm Convention on Persistent Organic Pollutants listed tetra-, penta-, hexa- and hepta-BDEs as persistent organic compounds, and banned their use in over 160 member countries (Stockholm Convention on Persistent Organic Polutants, 2010). In the United States, the use of deca-BDE is scheduled to be phased out by 2013. Despite recent restrictions, PBDEs remain a human health concern due to continued production in some regions, import/export of goods containing PBDEs, disposal of PBDE-containing materials, remaining stock of PBDE-containing materials, and PBDE's environmental persistence (Birnbaum and Staskal, 2004).
Currently, human reproductive and developmental health risks associated with PBDE exposure have not been thoroughly addressed and many uncertainties remain. Animal studies show that PBDEs exhibit neurodevelopmental (Branchi et al., 2003; Viberg et al., 2006), hepatic (Zhou et al., 2001; Zhou et al., 2002), immunological (Fowles et al., 1994; Thuvander and Darnerud, 1999) and thyroid toxicity (Zhou et al., 2002). Rabbits orally exposed to PBDEs show decreased gestation length (Breslin et al., 1989). PBDEs are lipophilic and accumulate in human tissues, with many reports of PBDE concentrations in various human matrices (Frederiksen et al., 2009). Although human biomonitoring has primarily focused on breast milk and serum (Schecter et al., 2003; Schecter et al., 2006; Schecter et al., 2010), several studies address the partitioning among gestational compartments through paired sampling of maternal blood, fetal blood and placenta (Bi et al., 2006; Gomara et al., 2007; Herbstman et al., 2007; Main et al., 2007; Mazdai et al., 2003). Recently, we identified PBDE accumulation in human extraplacental gestational membranes (Miller et al., 2009). Although amniotic fluid plays an important role in gestation and provides a possible route ofexposure for the fetus, the potential for PBDEs to accumulate in human amniotic fluid has not been addressed to date. The aim of the present study was to characterize PBDE profiles, including congener-specific and total PBDE concentrations, in amniotic fluid samples from women in southeast Michigan, USA.
1. Materials and Methods
1.1. Sample collection
In February and March 2009, clinical amniotic fluid samples were collected at the University of Michigan Women’s Hospital in Ann Arbor, Michigan, USA. De-identified samples containing amniotic fluid from 15 women undergoing clinically-indicated amniocentesis for fetal chromosomal screening during their second trimester were analyzed. An average volume of 15 ml of sterile amniotic fluid per sample was received in the cytogenetics laboratory and centrifuged at 280 g for 10 min to remove the cellular component. The supernatant was transferred into tubes without patient identification and stored at −80 °C for no longer than 90 days. The resulting supernatant was used for all analyses. The amniotic fluid samples were collected in an anonymous manner without personal identification or demographic information, and without interaction between the investigators and human subjects. The use of these de-identified clinical samples, which would have been otherwise discarded, was in compliance with the University of Michigan Institutional Review Board.
1.2. Lipid analysis
Amniotic fluid supernatants were evaluated for lipid content. A 1-ml aliquot of each amniotic fluid sample was denatured with 1 ml HCl (35–38%) followed by 6 ml isopropyl alcohol, and then extracted using 5 ml of a mixture of methyl tert-butyl ether and hexane (1:1). Sample fractions were separated by centrifugation and the organic fraction collected. Extractions were repeated three times and organic fractions were combined in a previously weighed beaker. The organic fraction was volatilized under a nitrogen stream until dry, and baked at 100°C for 12 h to remove all moisture. Beakers were reweighed and lipid weight per ml amniotic fluid was calculated.
1.3. PBDE analysis
Amniotic fluid supernatants were evaluated for 21 BDE congeners using methods previously described (Batterman et al., 2009). For each amniotic fluid sample, 7 ml of amniotic fluid was denatured with 2 ml concentrated HCl (35–38%), followed by 12 ml isopropyl alcohol. Samples were extracted using a mixture of 10 ml methyl tert-butyl ether and hexane (1:1). Sample fractions were separated by centrifugation and the organic fraction collected. The extraction process was repeated three times and organic fractions were combined and volatilized under a flowing nitrogen stream until nearly dry. Samples were diluted in 5 ml of hexane and cleaned with 3 ml of sulfuric acid. The organic fraction was removed and neutralized using dissolved sodium carbonate.
Instrumental analyses used GC/MS (Agilent 6890, Palo Alto, CA, USA), negative chemical ionization mode, a DB-5 column (30 m, 0.25 mm i.d., 0.25 µm film thickness, J&W Sci, Folsom, CA, USA) and a 2 µl splitless injection. The carrier gas was helium (0.7 ml/min, inlet pressure 5.43 psi, average velocity 37 cm/s), and methane was the reagent gas. The injector was set at 280°C. The oven temperature was started at 80°C, held for 2 min, ramped at 10°C/min to 300°C, and held for 40 min. Calibration standards included BDEs 17, 28, 75, 49, 71, 47, 66, 100, 99, 85, 154, 153, 138, 166, 183, 190, 203, 208, 207, 206 and 209 (Cambridge Isotope Laboratories, Inc., Andover, MA, USA). Standards were run for a wide range of concentrations (100 to 5000 ng/ml). The MS was operated in selected ion monitoring mode, and quality matching routines included at least two ions. Structural verification used additional ions, and confirmed by requiring proper ratios of ions for each analyte. Complete details of the instrumental analysis have been published previously (Batterman et al., 2009).
1.4. Quality assurance
In parallel with the analyses, field, lab and method blanks were processed and shown to be clear of contamination. A standard reference material (SRM), linearity and drift checks were analyzed with each sample batch. Repeat analysis of a standard mixture injected every fifth sample varied by less than 10%, and linearity plots produced r2 values greater than 0.999. Surrogate spike recoveries ranged from 82–106%. An inter-laboratory comparison of PBDE analyses in different media confirmed the performance quality of our analyses.
1.5. Data analysis
The lower limits of detection (LOD) for the 21 congeners ranged from 0.01–0.65 pg/ml amniotic fluid and are detailed in Table 1. Measurements falling below the LOD were assigned a value of one-half the LOD. The addition of one-half the LOD did not exceed 0.5% the total PBDE (∑PBDE) concentration for any of the samples. Descriptive statistics, including the mean, the standard deviation, geometric mean, median, 25th – 75th quartiles, and the percentage of observations above the LOD, were calculated for each congener. For each sample, ∑PBDE concentrations were calculated as the sum of the 21 congeners. The grand mean and grand median were calculated as the average and median, respectively, of the sample ∑PBDEs. Concentrations were expressed as volumetric and lipid-based fractions. To construct the homologue profile, congener-specific concentrations for each sample were divided by the sample ∑PBDE, multiplied by 100, then summed across samples within each homologue group and normalized to provide the percent homologue contribution to ∑PBDE. Sample statistics utilized the mean, median and sum of these concentrations. The relationship of lipid content to BDE levels was evaluated using the Spearman rank order correlation. Similarly, the Spearman rank order correlation was used to assess associations among individual BDE congeners,
Table 1.
PBDE concentrations in human amniotic fluid (N=15).
| PBDE Concentration (pg/ml amniotic fluid) | ||||||
|---|---|---|---|---|---|---|
| PBDE | LODa | % Samples > LOD |
Mean (SD) | Geometric Mean |
Median | 25th–75th Quartile |
| 17 | 0.01 | 80 | 108 (351) | 3.29 | 11 | 2.88 – 21.36 |
| 28 | 0.02 | 27 | 206 (796) | 0.07 | <LOD | LOD – 1.39 |
| 47 | 0.05 | 100 | 380 (1141) | 66.60 | 49 | 23.75 – 146.58 |
| 49 | 0.02 | 40 | 50 (168) | 0.22 | <LOD | LOD – 7.85 |
| 66 | 0.02 | 53 | 11 (23) | 0.39 | 4.92 | LOD – 10.10 |
| 71 | 0.01 | 33 | 31 (112) | 0.07 | <LOD | LOD – 3.40 |
| 75 | 0.02 | 40 | 12 (31) | 0.19 | <LOD | LOD – 5..64 |
| 85 | 0.01 | 73 | 75 (98) | 3.74 | 24 | LOD – 140.58 |
| 99 | 0.06 | 100 | 188 (368) | 40.22 | 31 | 13.18 – 114.33 |
| 100 | 0.05 | 93 | 187 (492) | 24.23 | 37 | 7.98 – 57.14 |
| 138 | 0.01 | 67 | 565 (1210) | 5.19 | 37 | LOD – 492.58 |
| 153 | 0.03 | 67 | 143 (298) | 4.42 | 18 | LOD – 108.78 |
| 154 | 0.02 | 53 | 192 (544) | 2.22 | 36 | LOD – 71.96 |
| 166 | 0.01 | 20 | 20 (70) | 0.03 | <LOD | LOD – LOD |
| 183 | 0.05 | 40 | 28 (67) | 0.48 | <LOD | LOD – 35.78 |
| 190 | 0.05 | 27 | 36 (98) | 0.19 | <LOD | LOD – 7.39 |
| 203 | 0.05 | 60 | 177 (238) | 5.75 | 97 | LOD – 285.86 |
| 206 | 0.22 | 60 | 149 (266) | 8.05 | 79 | LOD – 122.18 |
| 207 | 0.22 | 67 | 559 (346) | 17.83 | 71 | LOD – 427.23 |
| 208 | 0.22 | 67 | 362 (700) | 19.58 | 189 | LOD – 249.13 |
| 209 | 0.65 | 80 | 319 (396) | 62.26 | 133 | 41.02 – 598.24 |
| ∑PBDE | 3795 (5921) | 1732.72 | 1253 | 809.20 – 3187.28 | ||
| ∑PBDE (ng/g lipid) | 404 (630) | 184.39 | 133 | 86.11 – 339.07 | ||
| ∑Tri-octaBDEs | 2407 (4582) | 843.23 | 788 | 294.17 – 2635.5 | ||
| ∑Tri-octaBDEs (ng/g lipid) | 256 (487) | 89.73 | 84 | 31.31 – 280.39 | ||
LOD, limit of detection (pg/ml amniotic fluid).
2. Results and Discussion
2.1. Lipid content
The lipid content of the amniotic fluid samples ranged from 0.74 to 1.25% and averaged 0.94 ± 0.03%, substantially lower than the average lipid content of 22.5 ± 1.2% reported previously for second trimester amniotic fluid (Das et al., 1975). The lower lipid content in the present study likely results from our use of amniotic fluid supernatants from which lipid-rich cellular debris was removed by centrifugation prior to lipid and PBDE measurements. However, because amniotic fluid sample centrifugation was part of the clinical laboratory procedure performed prior to our acquisition of the samples, we were unable to compare lipid values in centrifuged versus noncentrifuged samples.
2.2. PBDE concentrations
A wide range of BDE congeners were identified, and all 21 target congeners were measured above LODs in at least three of the fifteen samples (20%) (Table 1). Because lipid content is extremely low in our samples, BDE concentrations are presented as pg/ml amniotic fluid. Because of the low lipid content and low variability in lipid content among the samples, adjustment for lipid weight has a nominal (≤0.31%) impact on BDE concentrations. Moreover, lipid concentration was not significantly associated with ∑PBDE concentration (p=0.86) nor with any of the individual BDE congeners (p=0.15–0.99). The ∑PBDE concentration in amniotic fluid averaged 3795 ± 1529 pg/ml (median of ∑PBDE = 1253 pg/ml; n=15; Table 1), which is equivalent to a mean concentration of 404 ± 163 ng/g lipid (median = 133 ng/g). Because PBDEs are lipophilic and the lipid-rich cellular debris was stripped by centrifugation from the amniotic fluid used in this study, the total PBDE loading of amniotic fluid prior to centrifugation was likely higher than the values reported here.
Consistent with previous reports (discussed recently by She et al. (2007)), the distribution of ∑PBDE concentrations is right-skewed (to high concentrations), with sample ∑PBDE levels ranging from 337 to 21842 pg/ml. Significantly, the highest amniotic fluid ∑PBDE level (equivalent to 2324 ng/g lipid) is comparable to the highest PBDE level reported in U.S. breast milk samples (1900 ng/g lipid), although it is below that reported in adipose tissue (9600 ng/g lipid) (Johnson-Restrepo et al., 2005). Tri- to octa-BDE congeners accounted for 82% of the ∑PBDEs in the sample with the highest ∑PBDE concentration, well above the 63% average for all samples.
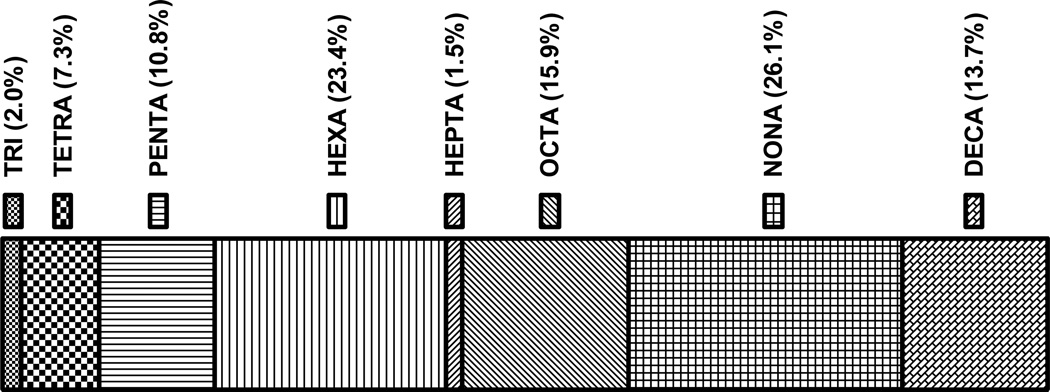
BDE-47 and BDE-99 were found in all samples tested, consistent with most previous studies of breast milk and serum (Frederiksen et al., 2009). However, BDE-100, BDE-209, and BDE-17 were identified in 93%, 80% and 80% of amniotic fluid samples, respectively. Based on median concentrations, the dominant congeners in amniotic fluid were, in descending order, BDE-208, 209, 203, 206, 207, and 47 representing 23, 16, 12, 10, 9 and 6%, respectively, of the ∑PBDEs. Grouped by PBDE homologues based on bromine number, the PBDE profile of amniotic fluid shows that the higher brominated nona and deca congeners significantly contributed to the ∑PBDEs (26.1% and 13.7%, respectively; Fig. 1). Differences in relative congener concentrations depend, in part, on which congeners are included in the total PBDE concentrations. As noted in recent reports (Frederiksen et al., 2009; Gomara et al., 2011), few studies of human fluids and tissues have included the higher brominated BDE-209 congener in their analysis, making comparisons of congener profiles problematic. Consistent with the high levels observed in our amniotic fluid samples, recent reports found that BDE-209 was the predominant congener in human breast milk samples from women in Spain (Gomara et al., 2011) and China (Sudaryanto et al., 2008), and a significant contributor to total PBDEs in Taiwanese breast milk samples (Chao et al., 2007). Moreover, BDE-209 was detected in all analyzed breast milk samples of women from Norway (Polder et al., 2008b), Russia (Polder et al., 2008a), and the US/Canadian Pacific Northwest (She et al., 2007), though a study of Texan breast milk detected BDE-209 in only 6 of 23 samples (Schecter et al., 2003). A study of human blood found that BDE-209 contributed approximately 15% of total PBDEs in children (Fangstrom et al., 2005a). However, none of these studies included analysis for BDE-203, 206, and 208, yet these congeners had similar concentrations as BDE-209 in our amniotic fluid samples. Of particular relevance to the present study, BDE-209 concentrations were higher in umbilical cord blood and placenta than in maternal blood of Spanish subjects (Gomara 2007), suggesting that higher brominated congeners may preferentially distribute to the gestational compartment. However, congener differences between umbilical cord and maternal blood was not observed in a study of US Midwestern women (Mazdai et al., 2003). In addition, exposure to PBDEs and commercial formulations have varied geographically and historically (Frederiksen et al., 2009). Thus, the variation in congener profiles among the studies could be related to differences in sampling procedures, specific congeners analyzed, differences in PBDE exposure, small sample sizes, differences in tissue distribution, and other factors.
Fig. 1.
PBDE homologue profile in human amniotic fluid. The relative contributions of PBDE homologues (based on bromine number) to ΣPBDE are shown; the percent contribution is specified above the segment representing each homologue group.
Because BDE-209 has a short half-life (estimated at 15 days; Fangstrom et al. (2005b)), the BDE-209 concentrations observed in our Michigan samples of amniotic fluid suggest that exposure to this congener is common and frequent. Although the lower brominated congeners (with 3–8 bromine atoms) are no longer produced in the United States, the homologue profile shows that they still constitute the bulk (60%) of the ∑PBDE loading. Mobilization of body burdens, exposure from the import of goods containing PBDEs, disposal of PBDE-containing materials, the wide stock of PBDE-containing materials still in use, and environmental persistence may explain the legacy of those congeners detected in nascent amniotic fluid. Future studies that include matched maternal blood samples collected at the time of amniotic fluid sampling may provide further insight into BDE congener patterns in amniotic fluid.
Because midgestation amniotic fluid sample collection is invasive, we used samples collected during the second trimester for diagnostic purposes related to the donor’s medical condition, and we had no access to individual donor information. However, many factors can affect an individual’s PBDE body burden, including maternal age, gestational age, and differences in PBDE exposure before and during pregnancy. Moreover, concentrations of PBDEs in amniotic fluid may be affected by the total volume of amniotic fluid, which increases in volume from approximately 200 ml at 16 weeks gestation to a peak of 1000 ml at 28 weeks gestation (Brace, 1997). These factors were not controlled in this study, and they represent limitations in our study design.
2.3. Correlations among BDE Congeners
Spearman rank order correlations for the PBDE measurements from human amniotic fluid are detailed in Table 2. Deca- and nona-BDE congeners were highly correlated, which is unsurprising since nona-BDEs are often found as contaminants of the commercial deca-BDE mixture. Interestingly, BDE-71 was also strongly correlated with the deca- and nona-congeners. Although previously unreported, this correlation raises the question of whether the higher order congeners can break down into lower order congeners, as shown by Stapleton et al. (2006) in fish which can debrominate deca-BDEs into nona-congeners.
Table 2.
Spearman rank order correlations matrix for PBDE congeners in human amniotic fluid.a
| BDE- 28 |
BDE- 75 |
BDE- 49 |
BDE- 71 |
BDE- 47 |
BDE- 66 |
BDE- 100 |
BDE- 99 |
BDE- 85 |
BDE- 154 |
BDE- 153 |
BDE- 138 |
BDE- 166 |
BDE- 183 |
BDE- 190 |
BDE- 203 |
BDE- 208 |
BDE- 207 |
BDE- 206 |
BDE- 209 |
∑ P B D E | ∑ P B D E -deca |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDE-17 | 0.09 | 0.04 | 0.04 | 0.12 | 0.01 | 0.35 | 0.00 | 0.00 | 0.47 | 0.01 | 0.01 | 0.31 | 0.45 | 0.58 | 0.05 | 0.80 | 0.08 | 0.06 | 0.11 | 0.21 | 0.00 | 0.00 |
| BDE-28 | 0.02 | 0.06 | 0.03 | 0.38 | 0.73 | 0.17 | 0.29 | 0.50 | 0.19 | 0.91 | 0.81 | 0.03 | 0.04 | 0.21 | 0.57 | 0.18 | 0.18 | 0.22 | 0.16 | 0.37 | 0.70 | |
| BDE-75 | 0.00 | 0.00 | 0.31 | 0.36 | 0.04 | 0.16 | 0.61 | 0.37 | 0.32 | 0.40 | 0.13 | 0.22 | 0.43 | 0.29 | 0.11 | 0.06 | 0.07 | 0.09 | 0.04 | 0.30 | ||
| BDE-49 | 0.00 | 0.36 | 0.29 | 0.04 | 0.19 | 0.63 | 0.41 | 0.26 | 0.37 | 0.23 | 0.26 | 0.29 | 0.37 | 0.15 | 0.11 | 0.10 | 0.18 | 0.04 | 0.28 | |||
| BDE-71 | 0.51 | 0.58 | 0.10 | 0.22 | 1.00 | 0.71 | 0.76 | 0.94 | 0.17 | 0.58 | 0.83 | 0.66 | 0.02 | 0.02 | 0.01 | 0.02 | 0.12 | 0.84 | ||||
| BDE-47 | 0.04 | 0.00 | 0.02 | 0.08 | 0.00 | 0.00 | 0.03 | 0.30 | 0.29 | 0.21 | 0.43 | 0.77 | 0.51 | 0.40 | 0.39 | 0.00 | 0.00 | |||||
| BDE-66 | 0.20 | 0.31 | 0.00 | 0.02 | 0.05 | 0.00 | 0.10 | 0.03 | 0.02 | 0.25 | 0.29 | 0.17 | 0.42 | 0.87 | 0.09 | 0.04 | ||||||
| BDE-100 | 0.00 | 0.14 | 0.00 | 0.00 | 0.11 | 0.27 | 0.51 | 0.07 | 0.37 | 0.23 | 0.10 | 0.11 | 0.15 | 0.00 | 0.00 | |||||||
| BDE-99 | 0.17 | 0.00 | 0.02 | 0.17 | 0.09 | 0.57 | 0.31 | 0.36 | 0.13 | 0.07 | 0.13 | 0.08 | 0.00 | 0.00 | ||||||||
| BDE-85 | 0.03 | 0.08 | 0.00 | 0.05 | 0.01 | 0.08 | 0.45 | 0.03 | 0.12 | 0.15 | 0.57 | 0.28 | 0.03 | |||||||||
| BDE-154 | 0.00 | 0.04 | 0.09 | 0.06 | 0.11 | 0.21 | 0.71 | 0.65 | 0.62 | 0.33 | 0.00 | 0.00 | ||||||||||
| BDE-153 | 0.02 | 0.33 | 0.31 | 0.08 | 0.90 | 1.00 | 0.55 | 0.49 | 0.55 | 0.00 | 0.00 | |||||||||||
| DBE-138 | 0.16 | 0.10 | 0.13 | 0.99 | 0.04 | 0.17 | 0.15 | 0.38 | 0.07 | 0.00 | ||||||||||||
| BDE-166 | 0.00 | 0.45 | 0.70 | 0.79 | 0.73 | 0.65 | 0.05 | 0.18 | 0.13 | |||||||||||||
| BDE-183 | 0.17 | 0.79 | 0.41 | 0.48 | 0.64 | 0.43 | 0.58 | 0.21 | ||||||||||||||
| BDE-190 | 0.82 | 0.58 | 0.47 | 0.73 | 0.42 | 0.16 | 0.05 | |||||||||||||||
| BDE-203 | 0.95 | 0.59 | 0.96 | 0.80 | 0.78 | 0.77 | ||||||||||||||||
| BDE-208 | 0.00 | 0.00 | 0.00 | 0.15 | 0.95 | |||||||||||||||||
| BDE-207 | 0.00 | 0.00 | 0.07 | 0.70 | ||||||||||||||||||
| BDE-206 | 0.00 | 0.07 | 0.86 | |||||||||||||||||||
| BDE-209 | 0.08 | 0.72 | ||||||||||||||||||||
| ∑PBDE | 0.00 |
P-values are shown for each congener pair. Statistically significant P-values < 0.05 are shown in bold. All correlations are positive.
Strong correlations were seen between congeners within specific homologue groups. Hexa-BDEs 138, 153 and 154 were all found to have significant correlations with each other. In addition, tetra-BDE congeners 49, 71 and 75 also showed significant correlations. The latter findings are expected because these BDE homologues are often found together in the commercial mixtures of PBDE used prior to 2004.
3. Conclusions
This study is the first to report congener-specific concentrations of PBDEs in human amniotic fluid. Although the sample size was modest, and personal and demographic information from donors was unavailable, PBDE concentrations in amniotic fluid were significant. Fetal intake of amniotic fluid into the gastrointestinal and respiratory tracks along with dermal exposure provide routes of transport into the fetus for toxicants that enter the amniotic fluid. Consequently, PBDEs in amniotic fluid are likely highly correlated with the exposure of the fetus, and thus our results suggest a potential fetal exposure pathway not considered previously: the amniotic fluid-to-fetus route. Our results also suggest the need for further investigation examining the partitioning and uptake of PBDEs between amniotic fluid, the fetus, and other gestational tissues.
Highlights.
21 BDE congeners were measured in amniotic fluid samples from fifteen women.
The average total PBDE concentration was 3795 pg/ml amniotic fluid.
The average total PBDE concentration ranged from 337 to 21842 pg/ml.
BDE-47 and BDE-99 were identified in each sample of human amniotic fluid.
BDE-208, 209, 203, 206, 207, and 47 were the most abundant BDEs in amniotic fluid.
Supplementary Material
Acknowledgements
The authors thank Dr. Diane Roulston in the Department of Pathology at the University of Michigan for assistance collecting amniotic fluid samples.
Role of funding sources
This work was supported by grant ES014860 to R.L.-C. from the United States National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH) and a grant from the Michigan Institute for Clinical and Health Research at The University of Michigan. Additional support for M.F.M. was provided by the Department of Environmental Health Sciences of the University of Michigan School of Public Health, and NRSA Institutional Training Grants from the United States NIEHS (T32 ES07062) and the National Institute of Child Health and Human Development (T32 HD007048). The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or The University of Michigan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Batterman S, Chen TC, Chernyak S, Godwin C. Design and performance evaluation of a medium flow sampler for airborne brominated flame retardants (BFRs) J Environ Monit. 2009;11:858–866. doi: 10.1039/b817298f. [DOI] [PubMed] [Google Scholar]
- Bi X, Qu W, Sheng G, Zhang W, Mai B, Chen D, et al. Polybrominated diphenyl ethers in South China maternal and fetal blood and breast milk. Environ Pollut. 2006;144:1024–1030. doi: 10.1016/j.envpol.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brace RA. Physiology of amniotic fluid volume regulation. Clin Obstet Gynecol. 1997;40:280–289. doi: 10.1097/00003081-199706000-00005. [DOI] [PubMed] [Google Scholar]
- Branchi I, Capone F, Alleva E, Costa LG. Polybrominated diphenyl ethers: neurobehavioral effects following developmental exposure. Neurotoxicology. 2003;24:449–462. doi: 10.1016/S0161-813X(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Breslin WJ, Kirk HD, Streeter CM, Quast JF, Szabo JR. 1,3-dichloropropene: two-generation inhalation reproduction study in Fischer 344 rats. Fundam Appl Toxicol. 1989;12:129–143. doi: 10.1016/0272-0590(89)90068-7. [DOI] [PubMed] [Google Scholar]
- Chao HR, Wang SL, Lee WJ, Wang YF, Papke O. Levels of polybrominated diphenyl ethers (PBDEs) in breast milk from central Taiwan and their relation to infant birth outcome and maternal menstruation effects. Environ Int. 2007;33:239–245. doi: 10.1016/j.envint.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Das SK, Foster HW, Adhikary PK, Mody BB, Bhattacharyya DK. Gestational variation of fatty acid composition of human amniotic fluid lipids. Obstet Gynecol. 1975;45:425–432. [PubMed] [Google Scholar]
- Fangstrom B, Hovander L, Bignert A, Athanassiadis I, Linderholm L, Grandjean P, et al. Concentrations of polybrominated diphenyl ethers, polychlonnated biphenyls, and polychlorobiphenylols in serum from pregnant Faroese women and their children 7 years later. Environmental Science & Technology. 2005a;39:9457–9463. doi: 10.1021/es0513032. [DOI] [PubMed] [Google Scholar]
- Fangstrom B, Strid A, Grandjean P, Weihe P, Bergman A. A retrospective study of PBDEs and PCBs in human milk from the Faroe Islands. Environmental Health: A Global Access Science Source. 2005b;4:12. doi: 10.1186/1476-069X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles JR, Fairbrother A, Baecher-Steppan L, Kerkvliet NI. Immunologic and endocrine effects of the flame-retardant pentabromodiphenyl ether (DE-71) in C57BL/6J mice. Toxicology. 1994;86:49–61. doi: 10.1016/0300-483x(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. Human internal and external exposure to PBDEs--a review of levels and sources. Int J Hyg Environ Health. 2009;212:109–134. doi: 10.1016/j.ijheh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Gomara B, Herrero L, Pacepavicius G, Ohta S, Alaee M, Gonzalez MJ. Occurrence of co-planar polybrominated/chlorinated biphenyls (PXBs), polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in breast milk of women from Spain. Chemosphere. 2011;83:799–805. doi: 10.1016/j.chemosphere.2011.02.080. [DOI] [PubMed] [Google Scholar]
- Gomara B, Herrero L, Ramos JJ, Mateo JR, Fernandez MA, Garcia JF, et al. Distribution of polybrominated diphenyl ethers in human umbilical cord serum, paternal serum, maternal serum, placentas, and breast milk from Madrid population, Spain. Environ Sci Technol. 2007;41:6961–6968. doi: 10.1021/es0714484. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Patterson DG, Halden RU, et al. Determinants of prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in an urban population. Environ Health Perspect. 2007;115:1794–1800. doi: 10.1289/ehp.10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Restrepo B, Kannan K, Rapaport DP, Rodan BD. Polybrominated diphenyl ethers and polychlorinated biphenyls in human adipose tissue from New York. Environ Sci Technol. 2005;39:5177–5182. doi: 10.1021/es050399x. [DOI] [PubMed] [Google Scholar]
- Main KM, Kiviranta H, Virtanen HE, Sundqvist E, Tuomisto JT, Tuomisto J, et al. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ Health Perspect. 2007;115:1519–1526. doi: 10.1289/ehp.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect. 2003;111:1249–1252. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MF, Chernyak SM, Batterman S, Loch-Caruso R. Polybrominated Diphenyl Ethers in Human Gestational Membranes from Women in Southeast Michigan. Environmental Science & Technology. 2009;43:3042–3046. doi: 10.1021/es8032764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polder A, Gabrielsen GW, Odland JO, Savinova TN, Tkachev A, Loken KB, et al. Spatial and temporal changes of chlorinated pesticides, PCBs, dioxins (PCDDs/PCDFs) and brominated flame retardants in human breast milk from Northern Russia. Sci Total Environ. 2008a;391:41–54. doi: 10.1016/j.scitotenv.2007.10.045. [DOI] [PubMed] [Google Scholar]
- Polder A, Thomsen C, Lindstrom G, Loken KB, Skaare JU. Levels and temporal trends of chlorinated pesticides, polychlorinated biphenyls and brominated flame retardants in individual human breast milk samples from Northern and Southern Norway. Chemosphere. 2008b;3:14–23. doi: 10.1016/j.chemosphere.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Schecter A, Pavuk M, Papke O, Ryan JJ, Birnbaum L, Rosen R. Polybrominated diphenyl ethers (PBDEs) in U.S. mothers' milk. Environ Health Perspect. 2003;111:1723–1729. doi: 10.1289/ehp.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She J, Holden A, Sharp M, Tanner M, Williams-Derry C, Hooper K. Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in breast milk from the Pacific Northwest. Chemosphere. 2007;67:S307–S317. doi: 10.1016/j.chemosphere.2006.05.154. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Brazil B, Holbrook RD, Mitchelmore CL, Benedict R, Konstantinov A, et al. In vivo and in vitro debromination of decabromodiphenyl ether (BDE 209) by juvenile rainbow trout and common carp. Environ Sci Technol. 2006;40:4653–4658. doi: 10.1021/es060573x. [DOI] [PubMed] [Google Scholar]
- Stockholm Convention on Persistent Organic Polutants. Stockholm Convention on Persistent Organic Polutants. Articles in use. [Date accessed: May 15, 2011];Amendments to Annexes A, B and C. http://chm.pops.int/Implementation/Exemptions/Articlesinuse/tabid/452/language/en-US/Default.aspx.
- Sudaryanto A, Kajiwara N, Tsydenova OV, Isobe T, Yu H, Takahashi S, et al. Levels and congener specific profiles of PBDEs in human breast milk from China: implication on exposure sources and pathways. Chemosphere. 2008;73:1661–1668. doi: 10.1016/j.chemosphere.2008.07.088. [DOI] [PubMed] [Google Scholar]
- Thuvander A, Darnerud PO. Effects of polybrominated diphenyl ether (PBDE) and polychlorinated biphenyl (PCB) on some immunological parameters after oral exposure in rats and mice. Toxicological & Environmental Chemistry. 1999;70:229–242. [Google Scholar]
- U.S. Environmental Protection Agency. Office of Pollution Prevention & Toxics. 2006. Polybrominated Diphenyl Ethers (PBDEs) Project Plan. editor. [Google Scholar]
- Viberg H, Johansson N, Fredriksson A, Eriksson J, Marsh G, Eriksson P. Neonatal exposure to higher brominated diphenyl ethers, hepta-, octa-, or nonabromodiphenyl ether, impairs spontaneous behavior and learning and memory functions of adult mice. Toxicol Sci. 2006;92:211–218. doi: 10.1093/toxsci/kfj196. [DOI] [PubMed] [Google Scholar]
- Zhou T, Ross DG, DeVito MJ, Crofton KM. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci. 2001;61:76–82. doi: 10.1093/toxsci/61.1.76. [DOI] [PubMed] [Google Scholar]
- Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol Sci. 2002;66:105–116. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.