Abstract
Pericellular proteolysis by ADAM family metalloproteinases has been widely implicated in cell signaling and development. We recently found that Xenopus ADAM13, an ADAM metalloproteinase, is required for activation of canonical Wnt signaling during cranial neural crest (CNC) induction by regulating a novel crosstalk between Wnt and ephrin B (EfnB) signaling pathways (Wei et al., 2010b). In the present study we show that the metalloproteinase activity of ADAM13 also plays important roles in eye development in X. tropicalis. Knockdown of ADAM13 results in reduced expression of eye field markers pax6 and rx1, as well as that of the pan-neural marker sox2. Activation of canonical Wnt signaling or inhibition of forward EfnB signaling rescues the eye defects caused by loss of ADAM13, suggesting that ADAM13 functions through regulation of the EfnB-Wnt pathway interaction. Downstream of Wnt, the head inducer Cerberus was identified as an effector that mediates ADAM13 function in early eye field formation. Furthermore, ectopic expression of the Wnt target gene snail2 restores cerberus expression and rescues the eye defects caused by ADAM13 knockdown. Together these data suggest an important role of ADAM13-regulated Wnt activity in eye development in Xenopus.
Keywords: ADAM13, Xenopus, eye, Wnt, ephrin, Snail2, Cerberus
Introduction
Members of the ADAM (A Disintegrin And Metalloproteinase Domain) family are type I transmembrane proteins with an extracellular metalloproteinase domain, a disintegrin domain and a cysteine-rich domain. More than half of the known ADAMs contain a conserved zinc-binding motif in the metalloproteinase domain, which is required for protease activity (Edwards et al., 2008; White et al., 2005). These proteolytically active ADAMs, together with other related proteases such as members of the ADAMTS (disintegrin metalloproteinase with thrombospondin type I motifs) and MMP (matrix metalloproteinase) families, form a superfamily of secreted and cell-surface metalloproteinases that regulates turnover of the extracellular matrix, cell-cell and cell-matrix interactions, and cell signaling. Dysregulated metalloproteinase activities often lead to developmental defects and other diseases (Gomis-Ruth, 2009).
Several proteolytically active ADAMs are known to have important roles in cell signaling, mainly through ectodomain cleavage (“shedding”) of cell-surface protein substrates. A growing list of substrates has been identified for ADAMs, among which are some key signaling molecules, including receptors and/or ligands of the Notch, EGFR, TNF and Efn signaling pathways (Edwards et al., 2008; White et al., 2005). Ectodomain shedding by ADAMs may have different outcomes for signaling, depending on the cellular and developmental context. For example, cleavage of receptors or ligands has been proposed to be an efficient way to eliminate a functional signaling molecule and, thereby, terminate a signal (Paland et al., 2008; Sapir et al., 2005; Sun et al., 2008). On the other hand, cleavage may also be required to generate a functional fragment of a signaling molecule and activate a pathway. Such a fragment could be the soluble form of a membrane-bound ligand that can signal over a long distance (Black et al., 1997; Blobel, 2005), or the cytoplasmic end of a transmembrane receptor that is further cleaved by γ-secretase and subsequently translocated to the nucleus to regulate target gene expression (Pan and Rubin, 1997; Sardi et al., 2006). For these reasons, several ADAM metalloproteinases have been shown to be indispensable for embryogenesis in mouse and other model animals (Hartmann et al., 2002; Peschon et al., 1998; Rooke et al., 1996).
The diploid amphibian species Xenopus tropicalis has emerged as a new and powerful model system to study early vertebrate development (Hellsten et al., 2010). With the aid of bioinformatics tools, we have identified more than a dozen ADAM genes in the X. tropicalis genome (Wei et al., 2010a). Loss-of-function studies suggest that ADAM13, one of the proteolytically active ADAMs, is essential for early CNC induction in X. tropicalis. We further found that ADAM13 cleaves Efns B1 and B2, two class B Efns, and that such cleavage alleviates the inhibition of canonical Wnt signaling by these Efns and allows proper induction of CNC (Wei et al., 2010b). Given the co-existence of class B Efns and Wnt components in multiple tissues and the involvement of canonical Wnt signaling in many developmental events, it would be interesting to examine if this ADAM13-EfnB-Wnt cascade also functions in the morphogenesis of other tissues.
Here we show that ADAM13 metalloproteinase activity is required for normal eye development and early eye field formation in X. tropicalis. Rescue experiments suggest that ADAM13 functions in this developmental event by regulating EfnB and Wnt signaling. We further identified snail2 and cerberus, two genes that are known to be controlled by the canonical Wnt pathway, as potential downstream effectors that contribute to eye field formation. These data provide new insight into the mechanisms of action for ADAMs and Wnt signaling in vertebrate eye development.
Materials and Methods
Embryo manipulation and injection
Wild-type X. tropicalis adults were purchased from NASCO, and the γ1-crys/GFP3 transgenic line was generated in a previous study (Offield et al., 2000). Embryos were obtained by in vitro fertilization or natural mating, and injected as described (Ogino et al., 2006). Red dextran was co-injected as a lineage tracer. Morpholino (MO) oligos were designed and synthesized by Gene Tools, as described previously (Wei et al., 2010b). The sequences for MOs 13-1 and 13-3 are 5′-TGTGCAGCCAACCCTCCGTCCCCAT-3′and 5′-CCCCGGCTCAGTCCGCTCTCAGCC-3′, respectively. Embryos were cultured in 0.1× MBS to desired stages, and in situ hybridization and phenotype scoring were carried out as described below.
DNA constructs and in vitro transcription
The expression construct for dominant-negative X. laevis EphB1 receptor (pCS107-ephB1ΔC) was kindly provided by Dr. Ira Daar, and constructs encoding full-length and mutant forms (ΔDix and ΔDep) of Xdsh were provided by Dr. Mungo Marsden. X. tropicalis cDNA clones for snail2 (in pCS107; clone ID: Ttba075D05; from Geneservice) and cerberus (in pCS108; clone ID: 7579004; from OpenBiosystems) were identified by bioinformatics and used directly for expression (sequences of all clones were confirmed by DNA sequencing). Cloning of X. tropicalis adam13 has been described previously (Wei et al., 2010a). The coding sequence for ADAM13 was then subcloned into a pCS2+ expression vector modified to append a C-terminal myc6-tag. To generate rescue constructs, site-directed mutagenesis was carried out using a QuickChange mutagenesis kit (Stratagene). Sense mutations were introduced by using the primers 5′-TATGCGGCCACATGGGCACCGAAGGCTGGTTACATACATGGCTGG-3′ (forward) and 5′-CCAGCCATGTATGTAACCAGCCTTCGGTGCCCATGTGGCCGCATA-3′ (reverse). The protease-inactive E340A mutation was introduced by using the primers 5′-GCTGCTGCAACAATGGCCCATGCAATTGGACACAAT-3′ (forward) and 5′-ATTGTGTCCAATTGCATGGGCCATTGTTGCAGCAGC-3′ (reverse). Capped mRNA with poly(A) tail was generated by in vitro transcription as described in Sive et al. (Sive et al., 2000).
In situ hybridization
Clones of X. tropicalis pax6 and rx1, as well as X. laevis sox2, chordin and xbra, were used to produce digoxigenin-UTP labeled antisense RNA probes. To generate cerberus probe, the coding sequence of X. tropicalis cerberus gene was amplified by PCR using the Sp6 promoter primer and a reverse primer that introduced a NotI site (5′-ATATGCGGCCGCTTTAATTGTGCAGGGTGG-3′). The PCR product was digested with SalI and NotI, and subcloned into pCS108. Antisense RNA probes were generated by in vitro transcription using T3 RNA polymerase (Promega). Whole-mount in situ hybridization was carried out as described (Sive et al., 2000).
Cell fate analyses
Embryos were injected with different MOs and red dextran as a lineage tracer and cultured to stage 37/38. Whole and sectioned embryos were analyzed for the presence of red fluorescence in the eye field as described (Lee et al., 2006; Moody, 1987).
Phenotype scoring and statistics
Embryos were cultured to stage ~35 and scored for defects in eye morphology (see Fig. 1A for examples of phenotypes). Cartilage phenotypes of the same embryos as those shown in Fig. 1A, 2B, 3B and 4B have been reported elsewhere (Wei et al., 2010b). The percentage of normal, moderate and severe phenotypes was calculated for each experiment, and averaged for multiple independent experiments. For statistics, the percentage of normal embryos in each experiment was used for comparison, and Student’s t tests were performed for a series of independent experiments.
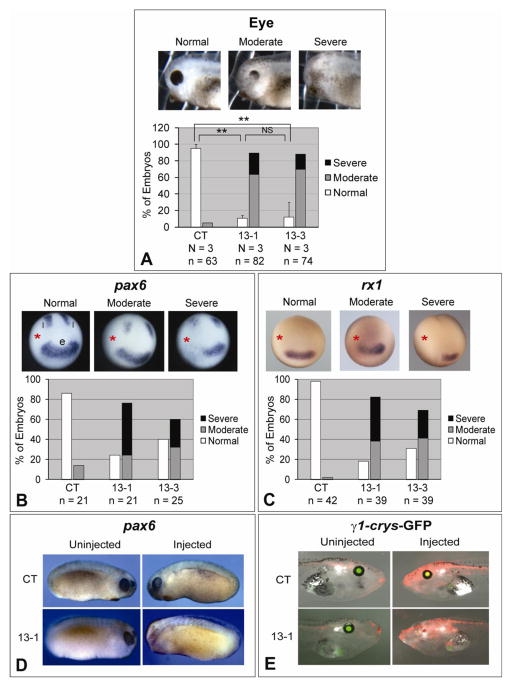
Fig. 1.
Knockdown of ADAM13 affects eye development in Xenopus. Eight-cell stage embryos were injected in one dorsal-animal blastomere with the indicated MO (1.5 ng), and cultured to desired stages. (A) Embryos were scored at stage ~35 for eye defects. One example of each phenotype is shown in the upper panels, and results of multiple experiments are graphed in the lower panels. **, p < 0.001 for comparison between control (CT) and 13-1 morphants, and p = 0.002 for comparison between CT and 13-3 morphants; NS, not significant (p = 0.91). (B and C) Embryos were cultured to stage ~12.5, and in situ hybridization was carried out for pax6 (B) or rx1 (C). The injected side is denoted with a red asterisk. e, eye field; l, lateral stripes. (D and E) Wild-type (D) or γ1-crys/GFP3 transgenic (E) embryos were injected with MO CT (upper panels) or 13-1 (lower panels). Embryos were cultured to stage 21–24 and processed for in situ hybridization for pax6 (D), or to stage ~45 (E). Representative embryos were photographed on both the uninjected (left panels) and injected (right panels) sides. Images taken in red (for co-injected red dextran) and green (for GFP) channels and bright field were merged in E. Note the lack of eye structures and GFP expression on the injected side of the 13-1 morphant. N = number of independent experiments; n = number of embryos scored (same below).
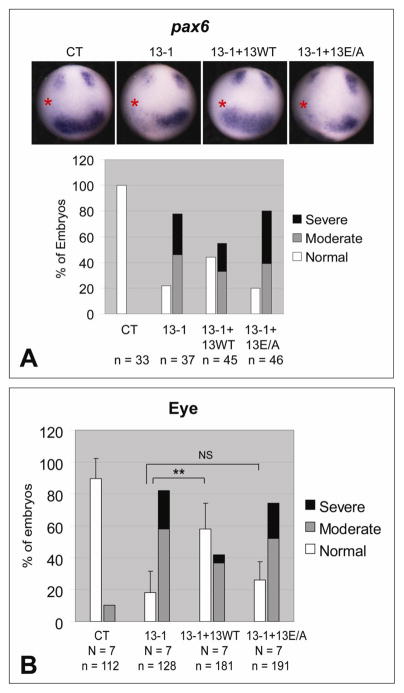
Fig. 2.
ADAM13 metalloproteinase activity is required for eye field formation and eye morphology. One dorsal-animal blastomere of 8-cell stage embryos was injected with the indicated MO (1.5 ng) and, as indicated, rescue mRNA encoding wild-type or the E/A mutant of ADAM13 (25 pg). (A) Embryos were allowed to develop to stage ~12.5 and then processed by in situ hybridization for pax6. A representative embryo of each injection is shown in the upper panels (the injected side is denoted with a red asterisk), and combined results summarized in graphs. (B) Embryos were scored for eye phenotype at stage ~35. **, p < 0.001; NS, not significant (p = 0.28). See Fig. 1A and Materials and Methods for phenotype scoring.
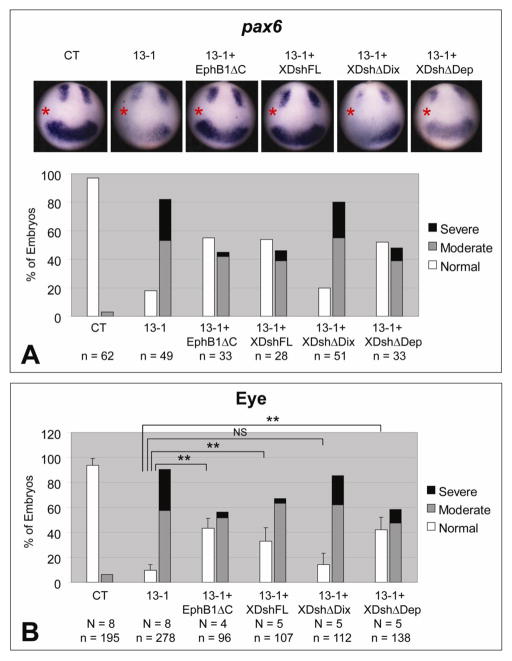
Fig. 3.
Effects of ADAM13 MO on eye development can be rescued by blocking EfnB signaling or by restoring canonical Wnt signaling. One dorsal-animal blastomere of 8-cell stage embryos was injected with the indicated MO (1.5 ng) and, as indicated, with 100 pg mRNA encoding EphB1ΔC, or full-length (FL) or deleted forms of Xdsh. (A) Embryos were cultured to stage ~12.5 and then processed by in situ hybridization for pax6. The injected side is denoted with a red asterisk. (B) Embryos were scored for eye phenotype at stage ~35. **, p < 0.001 in each case; NS, not significant (p = 0.17). See Fig. 1A and Materials and Methods for phenotype scoring.
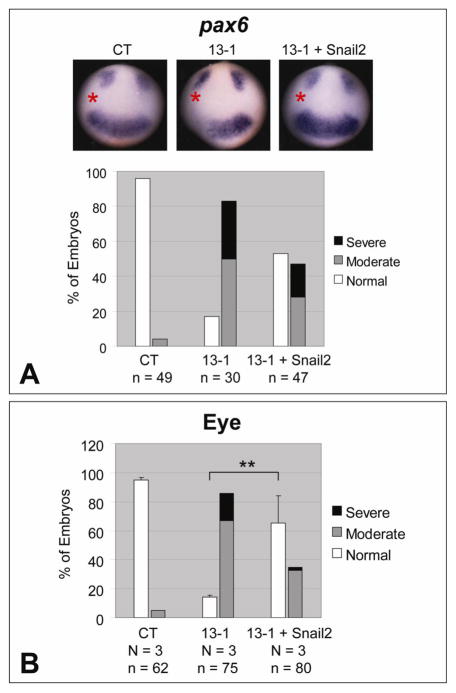
Fig. 4.
Snail2 rescues the eye phenotypes caused by ADAM13 MO. One dorsal-animal blastomere of 8-cell stage embryos was injected with the indicated MO (1.5 ng) together with or without Snail2 transcript (200 pg). Embryos were processed for in situ hybridization for pax6 at stage ~12.5 (A), or scored for eye defects at stage ~35 (B). The injected side is denoted with a red asterisk. See Fig. 1A and Materials and Methods for phenotype scoring. **, p = 0.008.
Results
Knockdown of ADAM13 in X. tropicalis leads to defects in eye morphology
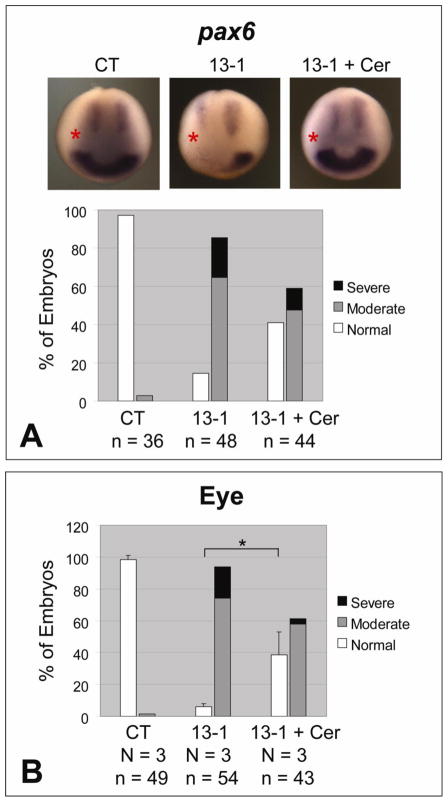
Two antisense MOs (MOs 13-1 and 13-3) with no overlap between their target sequences, as designed in earlier studies (Wei et al., 2010b), were used to block ADAM13 translation. Western blot analyses demonstrated that both MOs effectively knock down endogenous ADAM13 protein, as well as translation of exogenous ADAM13 expressed by a co-injected transcript that contains the MO binding sites (Wei et al., 2010b). When either MO 13-1 or 13-3 was injected into a dorsal-animal blastomere of 8-cell stage embryos, we observed defects in eye morphology with high penetrance at tadpole stages (Fig. 1A), in addition to the aberrant head cartilage structures reported previously (Wei et al., 2010b). More than 90% of such morphants developed abnormal eye phenotypes on the injected side, ranging from reduced eye pigment (moderate) to little or no visible eye structure (severe). Both 13-1 and 13-3 morphants displayed the same eye phenotypes with no significant difference in penetrance (Fig. 1A). The uninjected side (not shown), as well as embryos injected with the same dose of the standard control MO, appeared to have normal eye morphology (Fig. 1A), indicating that the eye phenotypes were caused specifically by loss of ADAM13 function.
Function of ADAM13 in early eye field formation
In Xenopus, the presumptive eye tissue (eye field) is specified at the anterior neural plate as early as stage 12.5, when the embryos are transitioning from gastrula to early neural plate stages (Li et al., 1997). A number of eye field transcription factors (EFTFs) are expressed before stage 15, with the homeobox genes pax6 and rx1 among the earliest to appear in the forming eye field (Zuber et al., 2003). The expression domain of pax6 is slightly larger than that of rx1 in the eye field, and also includes two lateral stripes (Casarosa et al., 1997; Hirsch and Harris, 1997; Zuber et al., 2003; Figs. 1B and 1C). Injection of either MO 13-1 or 13-3 resulted in a significant decrease in pax6 and rx1 mRNA in the anterior neural plate at stage 12.5, as shown by in situ hybridization (Figs. 1B and 1C), suggesting that early formation of the eye field was perturbed. We also observed a dose-dependent reduction in the transcript of the pan-neural marker sox2 caused by ADAM13 MO (Supplementary Fig. 1). Downregulation of pax6 and sox2 persisted in ADAM13 morphants at later stages (Fig. 1D and data not shown). By contrast, the control MO had virtually no effect on pax6, rx1 or sox2 expression (Figs. 1B–1D and Supplementary Figs. 1A and 1B). To investigate if the entire forebrain area is reduced, in situ hybridization was carried out for the forebrain/midbrain marker otx2. In contrast to the highly penetrant reduction in EFTF expression (Figs. 1B––1D), none of the ADAM13 morphants we examined displayed any apparent reduction in otx2 expression; instead knockdown of ADAM13 caused a moderate posterior-lateral expansion of the forebrain and a disruption of the forebrain/midbrain boundary, as suggested by the altered expression pattern of otx2 (Supplementary Fig. 2). No apparent change was observed for the expression pattern of either xbra or chordin upon ADAM13 knockdown (Supplementary Figs. 3 and 4), arguing against the possibility of the eye phenotypes being a secondary effect resulting from aberrant mesoderm induction or patterning.
To address whether lens induction was affected, we employed a transgenic line that drives expression of the green fluorescent protein (GFP) under the control of the γ1-crystallin promoter (Offield et al., 2000). MO 13-1 strongly suppressed GFP expression on the injected side of the embryos, indicating that lens induction was also severely impaired (Fig. 1E). Together these results suggest an essential role for ADAM13 in early eye field formation and eye/lens morphogenesis.
Roles of the metalloproteinase activity of ADAM13 in Xenopus eye development
To test whether the eye phenotypes caused by ADAM13 MOs can be rescued by exogenously expressed ADAM13, we generated expression constructs with sense mutations in the coding sequences, so that they cannot be targeted by MO 13-1 (Wei et al., 2010b). Two such rescue constructs were used in this study, one encoding wild-type ADAM13 and the other carrying an E to A mutation in the consensus zinc-binding motif in the metalloproteinase domain. In a series of in vitro shedding assays described previously, we found that both Efns B1 and B2 are cleaved by wild-type ADAM13 but not the E/A mutant in HEK293T cells (Wei et al., 2010b), indicating that the latter is proteolytically inactive. When co-injected with MO 13-1, the transcript encoding wild-type ADAM13 partially reversed the decrease in pax6 expression at stage ~12.5, as well as the defects in eye morphology at later stages. In contrast, the E/A mutant transcript failed to rescue (Figs. 2A and 2B). Thus we conclude that ADAM13 metalloproteinase activity is required for eye development in X. tropicalis.
ADAM13 functions in eye development by regulating EfnB and Wnt signaling
We showed previously that ADAM13 activates canonical Wnt signaling during CNC induction by cleaving class B Efns. Upon ADAM13 knockdown, intact Efns B1 and B2 accumulate in X. tropicalis embryos and antagonize Wnt activity (Wei et al., 2010b). Transcripts of adam13, efnB1 and efnB2 were detected in dorsal mesoderm during gastrulation (Supplementary Figs. 5A–5C; Wei et al., 2010b), and in an area of anterior neural plate corresponding to the eye field during early neurulation (Supplementary Figs. 5F–5H). Similar patterns of adam13 and efnB1 have been reported previously for X. laevis (Alfandari et al., 1997; Lee et al., 2006). We therefore ask whether accumulation of EfnBs upon ADAM13 knockdown can also lead to the deficiencies in eye development as seen in Fig. 1. If this were the case, downregulating EfnB signaling in developing Xenopus embryos should rescue the eye phenotypes caused by ADAM13 MOs. To test this hypothesis, we used a dominant-negative EphB1 receptor construct that blocks forward and receptor-independent EfnB signaling (EphB1ΔC) (Durbin et al., 1998; Lee et al., 2009). As shown in Figs. 3A and 3B, co-injection of EphB1ΔC transcript in X. tropicalis embryos rescued the defects in both early pax6 expression and later eye morphology caused by MO 13-1, suggesting that EfnB signaling mediates ADAM13 function in eye development.
The Xenopus dishevelled protein Xdsh plays important roles in two different Wnt signaling pathways, with the Dix and the Dep domains of Xdsh mediating canonical β-catenin dependent Wnt signaling and non-canonical PCP signaling, respectively (Boutros and Mlodzik, 1999; Park et al., 2005; Sokol et al., 1995). To investigate the possible involvement of Wnt signaling pathways in ADAM13-mediated eye development, we used transcripts encoding wild-type as well as two mutant forms of Xdsh, one with the Dix domain deleted (ΔDix) and the other with the Dep domain deleted (ΔDep; Wei et al., 2010b). As shown in Figs. 3A and 3B, ectopic expression of full-length Xdsh partially rescued pax6 expression and eye phenotypes caused by MO 13-1. Between the two Xdsh mutants, XdshΔDep rescued the effects caused by ADAM13 knockdown with an activity comparable to that of the full-length Xdsh, whereas XdshΔDix was unable to exert a significant rescue (Figs. 3A and 3B). The requirement of Dix domain for the rescue points to canonical Wnt signaling as the downstream target in this developmental event. This is further supported by a partial rescue of the eye defects in ADAM13 morphants by exogenous β-catenin (Supplementary Figs. 6A and 6B). Because it has been shown that EfnB1-mediated PCP signaling is required for the migration of retinal progenitor cells into the eye field (Lee et al., 2006), we also tested if the reduced eye field in ADAM13 morphants was due to failure of retinal progenitor cell migration. To do this we injected MO 13-3 with a fluorescent lineage tracer into the D1.1 blastomere of 16-cell stage embryos, which is a major contributor (>60%) to the retinal progeny (Huang and Moody, 1993; Moody, 1987). As shown in Supplementary Figs. 7A–7D, knockdown of ADAM13 in the D1.1 lineage resulted in loss of eye structures in most embryos injected (similar to what was seen when targeting a dorsal-animal blastomere of 8-cell stage embryos, Fig. 1A), but did not prevent the population of the retina by fluorescent cells. Therefore, the migration of the retinal progenitor cells and thus PCP signaling are likely not compromised. Taken together, these data indicate that ADAM13 functions in Xenopus eye development by regulating the EfnB-canonical Wnt signaling cascade.
Snail2 and Cerberus are downstream effectors of ADAM13 in eye development
The neural crest marker snail2 is a direct target of canonical Wnt signaling (Vallin et al., 2001), and we found previously that snail2 expression is downregulated by ADAM13 MO as early as gastrula stages (Wei et al., 2010b). Moreover, exogenous Snail2 rescues the deficiencies in CNC induction and head cartilage morphology caused by ADAM13 knockdown (Wei et al., 2010b). Surprisingly, we also observed a significant rescue of the eye phenotypes by Snail2 in ADAM13 morphants (Fig. 4B). In situ hybridization confirmed that pax6 expression at early stages was also rescued (Fig. 4A), suggesting a possible involvement of Snail2 in ADAM13-regulated eye development.
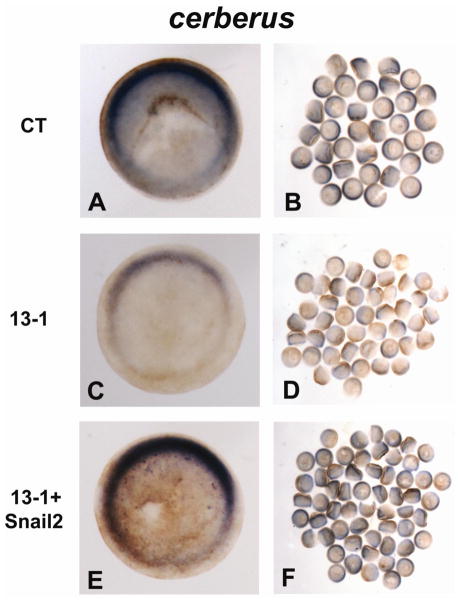
It has been reported that both snail2 and the head inducer cerberus are expressed in dorsal mesoendoderm of gastrula stage X. laevis embryos, and that ectopic expression of a dominant-negative form of Snail2 inhibits cerberus expression and head induction (Mayor et al., 2000). In X. tropicalis gastrulae we observed similar expression patterns for snail2 and cerberus, which overlap with those of adam13, efnB1 and efnB2 (Supplementary Figs. 5A–5E). We therefore asked whether Snail2 functions by regulating cerberus transcription during eye development. As seen in Figs. 5A–5D, embryos injected with MO 13-1 had less cerberus expression than siblings injected with the control MO. Downregulation of cerberus mRNA was also apparent in 13-3 morphants (data not shown). Co-injection of a transcript encoding Snail2 was able to rescue cerberus expression in ADAM13 morphants (Figs. 5E and 5F). These results are consistent with our hypothesis that Snail2 acts upstream of Cerberus in a signaling cascade that is controlled by ADAM13.
Fig. 5.
ADAM13 controls cerberus expression through Snail2. One-cell stage embryos were injected with 12 ng of MO CT (A and B) or 13-1 (C and D), or MO 13-1 with 1 ng mRNA encoding Snail2 (E and F), and cultured to stage ~11. In situ hybridization was carried out for cerberus, and embryos were cleared with 2:1 benzyl benzoate/benzyl alcohol before photographed. One representative embryo of each injected group is shown in animal pole view (with dorsal at the top) in the left panels, and all embryos of each injected group are shown in the right panels.
Cerberus was originally identified as a secreted protein that can induce a secondary head with eyes (Bouwmeester et al., 1996). Previous studies showed a robust activity of Cerberus in inducing the EFTF gene rx1 in animal cap assays (Lupo et al., 2002), prompting us to investigate if the early defects in eye field induction in ADAM13 morphants could be reversed by exogenous Cerberus. Indeed, co-injection of cerberus mRNA rescued pax6 expression as well as the eye morphology defects caused by MO 13-1 (Figs. 6A and 6B), supporting a role of Cerberus as a downstream effector in mediating ADAM13-regulated eye development.
Fig. 6.
The effects of ADAM13 knockdown on pax6 expression and eye morphology can be rescued by Cerberus. One dorsal-animal blastomere of 8-cell stage embryos was injected with the indicated MO (1.5 ng) with or without an mRNA encoding Cerberus (50 pg). Embryos were allowed to develop and then processed by in situ hybridization for pax6 (stage ~12.5; A), or scored for eye phenotype (stage ~35; B). See Fig. 1A and Materials and Methods for phenotype scoring. *, p = 0.02.
Discussion
Our data suggest a previously unknown function of ADAM13 in Xenopus eye development, and a mechanism of action for ADAM13 in early eye field formation. We have proposed that ADAM13 activates canonical Wnt signaling by cleaving the Wnt antagonists Efns B1 and B2 (Wei et al., 2010b). In this study we found that ADAM13-regulated Wnt activity upregulates the level of cerberus mRNA, likely by inducing the expression of snail2. The activity of Cerberus in turn induces EFTF genes such as rx1 and pax6, leading to formation of the early eye field.
Regulation of Efn and Wnt signaling by ADAM13 in Xenopus eye development
The eye phenotypes we observed here with ADAM13 knockdown are similar to those reported by Lee et al. with EfnB1 or Xdsh knockdown (Lee et al., 2006). However, instead of a loss of EfnB1 in the experiments shown by Lee et al., which causes impaired PCP signaling and retinal progenitor cells migration, we found that the endogenous and exogenous EfnB1 and/or B2 accumulate in ADAM13 morphants (Wei et al., 2010b). Furthermore, results of our lineage tracing experiments also indicate a normal migration of retinal progenitor cells into the eye field and hence, a likely intact PCP signaling pathway upon ADAM13 knockdown (Supplementary Fig. 7). Finally, we were able to rescue the eye phenotypes in ADAM13 morphants by restoring the canonical Wnt pathway (or the expression of known canonical Wnt targets Snail2 or Cerberus) but not the PCP pathway (Figs. 3, 4 and 6; Supplementary Fig. 6). These data suggest that similar to the CNC induction defects that we described earlier (Wei et al., 2010b), the eye phenotypes displayed by ADAM13 morphants are also caused by reduced canonical Wnt activity.
We have reported previously that knockdown of ADAM13 “protects” endogenous EfnB1/B2, and that higher levels of class B Efns can antagonize canonical Wnt signaling, leading to an inhibition of CNC induction (Wei et al., 2010b). Here we show that downregulation of the EfnB signaling by overexpressing a dominant-negative EphB1 receptor partially rescues the eye phenotypes caused by ADAM13 MO (Figs. 3A and 3B). Therefore, cleavage of EfnBs by ADAM13 is important for Wnt activation during both CNC induction and eye field formation. However, our data do not rule out a potential contribution of other ADAM13-mediated events to the activation of canonical Wnt signaling. The mammalian ADAM10 is known to process N- and E-cadherins, resulting in the redistribution of β-catenin (which can be “tethered” to the plasma membrane through interaction with these cadherins) and enhanced expression of Wnt target genes (Maretzky et al., 2005; Reiss et al., 2005). In our in vitro cleavages assays, we did not detect any processing of N- or E-cadherin by ADAM13 (data not shown). Studies with X. laevis have shown that another cadherin, cadherin-11, can be cleaved by ADAM13 in vivo, but such cleavage seems to have no effect on canonical Wnt signaling (McCusker et al., 2009).
The canonical Wnt pathway, acting through maternally encoded β-catenin, is required for induction of dorsal structures very early during embryogenesis (Heasman et al., 1994). However, most of the dorsal structures, including the somites, appeared normal in our ADAM13 morphants (Figs. 1D and 1E), arguing against an early effect on dorsal-ventral patterning by ADAM13. Zygotic expression of β-catenin begins at the midblastula stage (Wylie et al., 1996), and is necessary and sufficient for posteriorizing neural tissues in the developing neural plate (Kiecker and Niehrs, 2001). Although it is generally thought that eye and forebrain development requires inhibition of the Wnt/β-catenin pathway, it has also been proposed that a low level of canonical Wnt signaling is necessary for this process (Esteve and Bovolenta, 2006; Fuhrmann, 2008). Many components of the canonical Wnt pathway are expressed in the Xenopus forebrain, and knockdown of Pygopus, a nuclear co-activator of Wnt/β-catenin signaling, has been shown to inhibit rx1 and pax6 expression (Lake and Kao, 2003). Moreover, Heasman et al. demonstrated an essential role for β-catenin in head induction in X. laevis, which is independent of its function in dorsal-ventral patterning (Heasman et al., 2000). We have observed a similar effect in X. tropicalis by using an MO targeting β-catenin (data not shown). Together these results suggest that canonical Wnt signaling may have an important role in eye development.
Cerberus and Snail2 are Wnt effectors in eye field formation
The organizer gene cerberus is known to be regulated by canonical Wnt signaling in both X. laevis and X. tropicalis (Heasman et al., 2000; Wills et al., 2008). Whether this regulation is direct or indirect remains a question. Because nuclear localization of β-catenin on the dorsal side of early cleavage stage embryos is critical for establishing the Spemann organizer, the decrease in cerberus expression in ADAM13 morphants (Fig. 5) could be caused by early deficiencies in organizer formation. However, the expression level of chordin, another organizer gene, remains largely intact in ADAM13 morphants (Supplementary Fig. 4), suggesting that the effect of ADAM13 MO on cerberus expression is gene-specific instead of part of a general defect in organizer induction. In fact our data support a positive regulation of cerberus by the Wnt target gene snail2 (Fig. 5). In gastrula stage Xenopus embryos, snail2 is expressed in dorsal mesoendoderm overlapping with cerberus (Supplementary Figs. 5D and 5E; Mayor et al., 2000). This spatiotemporal expression pattern of snail2 was proposed to be important for its function in inducing head structures, probably through inhibition of BMP signaling (Mayor et al., 2000). Previous reports on the interaction between snail2 and cerberus genes in X. laevis are, nevertheless, controversial. While Snail2 has been shown to induce cerberus expression (Mayor et al., 2000), a recent study suggests that it may have a negative effect on cerberus mRNA levels (Zhang and Klymkowsky, 2009). Although our results (Fig. 5) are more consistent with the former study, we cannot rule out the possibility that Snail2 rescues cerberus expression and/or eye development by mimicking the activity of another protein, such as the closely related transcription factor Snail, or Bcl-xL or Twist (Zhang et al., 2006; Zhang and Klymkowsky, 2009). However, our data do suggest that a Snail2-like activity is involved in upregulating cerberus expression during Xenopus eye field formation.
Effects of ADAM13 on forebrain and neural development
In Xenopus, the eye field is established by the EFTF genes and the forebrain/hindbrain marker otx2 (Esteve and Bovolenta, 2006; Zuber et al., 2003). Because the eye is a forebrain structure, and otx2 has been reported to induce EFTF expression (Zuber et al., 2003), the reduced eye field caused by ADAM13 MO could be associated with a diminished forebrain territory. Instead we found a moderate posterior-lateral expansion of the forebrain expression domain of otx2 upon ADAM13 knockdown (Supplementary Fig. 2), which resembles the phenotype caused by inactivation of canonical Wnt signaling (Wu et al., 2005). Therefore the effect of ADAM13 MO on eye development appears to be independent of its effect on the overall size of the forebrain. We also observed a decrease in the transcript of sox2, a pan-neural marker, in neural plate stage embryos (Supplementary Fig. 1). Because sox2 does not seem to affect early EFTF expression and that sox2 transcription can be induced by canonical Wnt (Van Raay et al., 2005), it is likely that the downregulated sox2 expression is caused separately by Wnt inactivation. However, the reduced sox2 expression suggests a more general effect of ADAM13 knockdown on neural development, and we cannot rule out the possibility that all or part of the eye phenotypes displayed by ADAM13 morphants are secondary to this general defect in early neural development.
Functions of the mammalian homologues of ADAM13 in embryonic development
We have shown that ADAM13 is required for both CNC and eye development in X. tropicalis (Wei et al., 2010b and this study). A further question is whether these mechanisms are conserved during vertebrate evolution. In mammals, ADAMs 12, 19 and 33 form a subfamily, and our phylogenetic and syntenic analyses suggest that mammalian ADAM33 is the orthologue of frog ADAM13 (Wei et al., 2010a). However, mammalian ADAMs 19 and 33 have nearly equal similarity to Xenopus ADAM13 in protein sequence, raising the possibility that functions of ADAM13 could be shared between ADAMs 19 and 33 in mammals. While knockout of ADAM33 does not appear to affect growth or development in the mouse (Chen et al., 2006), ADAM19-null mice show deficiencies in cardiac neural crest differentiation and heart morphogenesis (Komatsu et al., 2007; Zhou et al., 2004). For the reasons mentioned above, it would be interesting to generate mice lacking both ADAMs 13 and 19 and examine if there is any redundancy in function between these two ADAMs. We also cannot rule out the possibility that the functions of Xenopus ADAM13 may be carried out by additional proteases (such as ADAM12) in mammals. Alternatively, mammals may use a different mechanism to control head (including eye and CNC) development. For example, our results suggest that ADAM13 functions through Snail2, and there seems to be a lack of conservation in Snail2 function between frogs and mammals (Murray and Gridley, 2006).
Conclusions
In summary, the results presented here provide another example of ADAM13-regulated Wnt activity in early vertebrate development. By activating the canonical Wnt signaling pathway, ADAM13 induces the expression of snail2 and cerberus, which leads to formation of the early eye field in Xenopus. It is likely that similar mechanisms, although possibly carried out by different protease(s), may apply to similar developmental events in a wide range of species, including mammals.
Supplementary Material
Highlights.
ADAM13 metalloproteinase activity plays important roles in early eye field formation and normal eye morphogenesis in Xenopus
ADAM13 controls eye development through regulating EfnB and Wnt signaling
snail2 and cerberus are downstream of Wnt to mediate ADAM13 function in eye development
Acknowledgments
We thank Dr. Ira Daar for kindly providing EphB1(ΔC) construct. We also thank Fred Simon, Tania Rozario and Maureen Bjerke for assistance with X. tropicalis husbandry, and the rest of the DeSimone lab for inspiring discussions. This work was supported by the NIH (DE14365 and HD26402 to D.W.D.) and March of Dimes Foundation (F405-140 to J.M.W. and 1-FY10-399 to D.W.D. and S.W.). S.W. was supported by an American Heart Association postdoctoral fellowship, and L.C.B. was supported by a Ruth L. Kirschstein postdoctoral fellowship and an NIH training grant to the University of Virginia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfandari D, et al. ADAM 13: a novel ADAM expressed in somitic mesoderm and neural crest cells during Xenopus laevis development. Dev Biol. 1997;182:314–30. doi: 10.1006/dbio.1996.8458. [DOI] [PubMed] [Google Scholar]
- Black RA, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–33. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, et al. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- Casarosa S, et al. Xrx1, a novel Xenopus homeobox gene expressed during eye and pineal gland development. Mech Dev. 1997;61:187–98. doi: 10.1016/s0925-4773(96)00640-5. [DOI] [PubMed] [Google Scholar]
- Chen C, et al. ADAM33 is not essential for growth and development and does not modulate allergic asthma in mice. Mol Cell Biol. 2006;26:6950–6. doi: 10.1128/MCB.00646-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin L, et al. Eph signaling is required for segmentation and differentiation of the somites. Genes Dev. 1998;12:3096–109. doi: 10.1101/gad.12.19.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DR, et al. The ADAM metalloproteinases. Mol Aspects Med. 2008;29:258–89. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve P, Bovolenta P. Secreted inducers in vertebrate eye development: more functions for old morphogens. Curr Opin Neurobiol. 2006;16:13–9. doi: 10.1016/j.conb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S. Wnt Signaling in Eye Organogenesis. Organogenesis. 2008;4:60–67. doi: 10.4161/org.4.2.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis-Ruth FX. Catalytic domain architecture of metzincin metalloproteases. J Biol Chem. 2009;284:15353–7. doi: 10.1074/jbc.R800069200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann D, et al. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet. 2002;11:2615–24. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- Heasman J, et al. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Heasman J, et al. Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev Biol. 2000;222:124–34. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- Hellsten U, et al. The genome of the Western clawed frog Xenopus tropicalis. Science. 2010;328:633–6. doi: 10.1126/science.1183670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch N, Harris WA. Xenopus Pax-6 and retinal development. J Neurobiol. 1997;32:45–61. [PubMed] [Google Scholar]
- Huang S, Moody SA. The retinal fate of Xenopus cleavage stage progenitors is dependent upon blastomere position and competence: studies of normal and regulated clones. J Neurosci. 1993;13:3193–210. doi: 10.1523/JNEUROSCI.13-08-03193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- Komatsu K, et al. Meltrin beta expressed in cardiac neural crest cells is required for ventricular septum formation of the heart. Dev Biol. 2007;303:82–92. doi: 10.1016/j.ydbio.2006.10.037. [DOI] [PubMed] [Google Scholar]
- Lake BB, Kao KR. Pygopus is required for embryonic brain patterning in Xenopus. Dev Biol. 2003;261:132–48. doi: 10.1016/s0012-1606(03)00305-1. [DOI] [PubMed] [Google Scholar]
- Lee HS, et al. Dishevelled mediates ephrinB1 signalling in the eye field through the planar cell polarity pathway. Nat Cell Biol. 2006;8:55–63. doi: 10.1038/ncb1344. [DOI] [PubMed] [Google Scholar]
- Lee HS, et al. Fibroblast growth factor receptor-induced phosphorylation of ephrinB1 modulates its interaction with Dishevelled. Mol Biol Cell. 2009;20:124–33. doi: 10.1091/mbc.E08-06-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, et al. A single morphogenetic field gives rise to two retina primordia under the influence of the prechordal plate. Development. 1997;124:603–15. doi: 10.1242/dev.124.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo G, et al. Induction and patterning of the telencephalon in Xenopus laevis. Development. 2002;129:5421–36. doi: 10.1242/dev.00095. [DOI] [PubMed] [Google Scholar]
- Maretzky T, et al. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci U S A. 2005;102:9182–7. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor R, et al. A novel function for the Xslug gene: control of dorsal mesendoderm development by repressing BMP-4. Mech Dev. 2000;97:47–56. doi: 10.1016/s0925-4773(00)00412-3. [DOI] [PubMed] [Google Scholar]
- McCusker C, et al. Extracellular cleavage of cadherin-11 by ADAM metalloproteases is essential for Xenopus cranial neural crest cell migration. Mol Biol Cell. 2009;20:78–89. doi: 10.1091/mbc.E08-05-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody SA. Fates of the blastomeres of the 16-cell stage Xenopus embryo. Dev Biol. 1987;119:560–78. doi: 10.1016/0012-1606(87)90059-5. [DOI] [PubMed] [Google Scholar]
- Murray SA, Gridley T. Snail family genes are required for left-right asymmetry determination, but not neural crest formation, in mice. Proc Natl Acad Sci U S A. 2006;103:10300–4. doi: 10.1073/pnas.0602234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offield MF, et al. The development of Xenopus tropicalis transgenic lines and their use in studying lens developmental timing in living embryos. Development. 2000;127:1789–97. doi: 10.1242/dev.127.9.1789. [DOI] [PubMed] [Google Scholar]
- Ogino H, et al. High-throughput transgenesis in Xenopus using I-SceI meganuclease. Nat Protoc. 2006;1:1703–10. doi: 10.1038/nprot.2006.208. [DOI] [PubMed] [Google Scholar]
- Paland N, et al. Reduced display of tumor necrosis factor receptor I at the host cell surface supports infection with Chlamydia trachomatis. J Biol Chem. 2008;283:6438–48. doi: 10.1074/jbc.M708422200. [DOI] [PubMed] [Google Scholar]
- Pan D, Rubin GM. Kuzbanian controls proteolytic processing of Notch and mediates lateral inhibition during Drosophila and vertebrate neurogenesis. Cell. 1997;90:271–80. doi: 10.1016/s0092-8674(00)80335-9. [DOI] [PubMed] [Google Scholar]
- Park TJ, et al. Subcellular localization and signaling properties of dishevelled in developing vertebrate embryos. Curr Biol. 2005;15:1039–44. doi: 10.1016/j.cub.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Peschon JJ, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–4. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- Reiss K, et al. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. Embo J. 2005;24:742–52. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooke J, et al. KUZ, a conserved metalloprotease-disintegrin protein with two roles in Drosophila neurogenesis. Science. 1996;273:1227–31. doi: 10.1126/science.273.5279.1227. [DOI] [PubMed] [Google Scholar]
- Sapir A, et al. Unidirectional Notch signaling depends on continuous cleavage of Delta. Development. 2005;132:123–32. doi: 10.1242/dev.01546. [DOI] [PubMed] [Google Scholar]
- Sardi SP, et al. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127:185–97. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Sive HL, et al. A Laboratory Manual. Cold Spring Harbor Laboratory Press; 2000. Early Development of Xenopus Laevis. [Google Scholar]
- Sokol SY, et al. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development. 1995;121:1637–47. doi: 10.1242/dev.121.6.1637. [DOI] [PubMed] [Google Scholar]
- Sun D, et al. The role of Delta-like 1 shedding in muscle cell self-renewal and differentiation. J Cell Sci. 2008;121:3815–23. doi: 10.1242/jcs.035493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallin J, et al. Cloning and characterization of three Xenopus slug promoters reveal direct regulation by Lef/beta-catenin signaling. J Biol Chem. 2001;276:30350–8. doi: 10.1074/jbc.M103167200. [DOI] [PubMed] [Google Scholar]
- Van Raay TJ, et al. Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron. 2005;46:23–36. doi: 10.1016/j.neuron.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Wei S, et al. Conservation and divergence of ADAM family proteins in the Xenopus genome. BMC Evol Biol. 2010a;10:211. doi: 10.1186/1471-2148-10-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, et al. ADAM13 induces cranial neural crest by cleaving class B Ephrins and regulating Wnt signaling. Dev Cell. 2010b;19:345–52. doi: 10.1016/j.devcel.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM, et al. Introduction to the ADAM family. In: Hooper NM, Lendeckel U, editors. The ADAM Family of Proteases. Springer; 2005. pp. 1–28. [Google Scholar]
- Wills A, et al. Bmp signaling is necessary and sufficient for ventrolateral endoderm specification in Xenopus. Dev Dyn. 2008;237:2177–86. doi: 10.1002/dvdy.21631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, et al. Neural crest induction by the canonical Wnt pathway can be dissociated from anterior-posterior neural patterning in Xenopus. Dev Biol. 2005;279:220–32. doi: 10.1016/j.ydbio.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Wylie C, et al. Maternal beta-catenin establishes a ‘dorsal signal’ in early Xenopus embryos. Development. 1996;122:2987–96. doi: 10.1242/dev.122.10.2987. [DOI] [PubMed] [Google Scholar]
- Zhang C, et al. An NF-kappaB and slug regulatory loop active in early vertebrate mesoderm. PLoS One. 2006;1:e106. doi: 10.1371/journal.pone.0000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Klymkowsky MW. Unexpected functional redundancy between Twist and Slug (Snail2) and their feedback regulation of NF-kappaB via Nodal and Cerberus. Dev Biol. 2009;331:340–9. doi: 10.1016/j.ydbio.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HM, et al. Essential role for ADAM19 in cardiovascular morphogenesis. Mol Cell Biol. 2004;24:96–104. doi: 10.1128/MCB.24.1.96-104.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber ME, et al. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130:5155–67. doi: 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.