Abstract
Older patients with AML have a worse outcome compared to young patients. To study for potential contributors to their poor prognosis, we compared two NK-AML cohorts, young (< 60 years old) and old (> 60 years old), via high density SNP array analysis. Older patients had more genomic changes (1.83±0.23 vs. 1.16±0.2, p=0.037) and a trend for a higher number of copy number neutral loss of heterozygosity (0.5±0.2 vs. 0.24±0.08, p=0.088) compared to young patients. We speculate that complex genomic changes in NK-AML may be a sign of an increase in genomic instability and an indicator of a worse prognosis.
Keywords: AML, Normal karyotype, SNP array, Old age
Introduction
Increasing age is an adverse prognostic factor for survival in AML patients [1]. Older patients, with a cutoff age of ≥ 60 years old in most reports, have a higher incidence of high risk cytogenetic changes and antecedent hematological disorders, and often have a poor performance status. Nevertheless, when accounting for these risk factors, age independently affects outcome [1-3]. Normal karyotype AML (NK-AML) comprises approximately 45% of adult AML cases and is a highly heterogeneous group with regard to treatment outcomes [4, 5]. While NK-AML is less common in older patients, the paucity of known changes makes it a good candidate to study further the pathogenesis of older age AML. While NK-AML in young patients has been previously studied for acquired genomic changes [6], this cohort was not studied in relation to a cohort of older patients.
In the current study, we have used high density single nucleotide polymorphism (SNP) array analysis to detect genomic changes in older NK-AML patients as well as a cohort of young patients; we report our findings and compare them between the two cohorts.
Design and Methods
Patient samples
Diagnostic samples were obtained from 49 NK-AML (mean number of analyzed metaphases-20, range 15-35); 24 old (age ≥ 60 years old) and 25 young (age < 60 years old). All but 2 patients (1 young, 1 old) had de novo AML, and they were treated with standard AML chemotherapy regimens. Clinical data including age, gender, baseline blood count, molecular abnormalities and outcome were extracted from the patients' data files. The study was approved by the local ethics committees.
High density single nucleotide polymorphism (SNP) array analysis
Genomic DNA was isolated from bone marrow cells and was subjected to 250 K GeneChip Human mapping microarray (SNP-chip, Affymetrix, Santa Clara, CA, USA) according to the manufacturer's protocol. Microarray data were analyzed for determination of both total and allelic-specific copy numbers using the CNAG program and the AsCNAR algorithm using non-matched controls as previously described [7, 8]. Exclusion of known copy number variations (CNV), as well as the determination of the size, position and location of specific genes was done with the UCSC Genome Browser (http://genome.ucsc.edu/). All copy number abnormalities and CNN-LOH were verified by visual inspection.
Validation of acquired copy number neutral loss of heterozygosity (CNN-LOH) and copy number abnormalities (CNA)
Presence of CNN-LOH was supported by sequencing random SNPs selected from within the area of CNN-LOH in chromosome 1p (case #43) and demonstrating LOH compared to random SNP sequences outside of the CNN-LOH area demonstrating heterozygous SNP calls. Gene dosage of chromosome 11q (case #14) was determined using real time quantitative (RQ) PCR in order to support allelic loss, as previously described [9, 10]. Validation results are shown in Supplement Figures 1 and 2.
Statistical analysis
Comparison of rates of genomic changes between young and older patients was determined using unpaired student T test, chi square and Fisher's exact test analysis. Event free survival (EFS) and overall survival (OS) curves were calculated and compared using the Kaplan-Meier and log rank tests.
Results
Baseline characteristics of patients are given in Table 1. Median age was 43 years (range 23-59) for the young patients and 69 years (range 61-77) for older patients. The rate of molecular markers including CEBPA, NPM1, FLT3-ITD, MLL-PTD and FLT3-TKD mutations was similar in the study cohorts.
Table 1. Characteristics of patients.
| Young (n=25) | Old (n=24) | |
|---|---|---|
| Age median (range) | 43 (23-59) | 68 (61-76.6) |
| Gender M (%) | 13 (54) | 12 (48) |
| de novo AML (%) | 23 (96)a | 24 (96)b |
| Diagnostic CBC | ||
| mean ±sem (range) | ||
| Hbg/dL | 9.6±0.35 (6.4-11.9) | 10.2±0.54 (3.2-14.2) |
| WBC × 109/L | 67.7±15.4 (4.0-190) | 74.5±21.7 (2.4-386) |
| PLT × 109/L | 67.9±13 (6.0-194) | 65.3±10 (10-197) |
| Molecular changes N/N | ||
| studied (%) | ||
| FLT3-ITD | 12/25 (50) | 7/19 (35) |
| FLT-TKD mutation | 2/18(12) | 2/13 (15) |
| NPM1 mutation | 14/25 (58) | 11/19(55) |
| CEBPA mutation | 3/16(19) | 1/10(10) |
| MLL-PTD | 2/25 (8) | 2/19(10) |
| NPM1 mutation/ FLT3- | 4/25 (16) | 4/19(21) |
| ITD negative |
patient had therapy-related AML
patient had AML secondary to MDS
CBC complete blood count; Hb hemoglobin; WBC white blood cells; PLT platelets; FLT3-ITD FLT3 internal tandem duplication; FLT-TKD FLT3 tyrosine kinase domain; NPM1 nucleophosmin; CEBPA CCAAT/enhancer-binding protein-alpha; MLL- PTD MLL partial tandem duplication
Older patients had a higher frequency of genomic changes in SNP array analysis compared to young patients (1.83±0.23 vs.16±0.2 per patient, p=0.037) (Figure 1). Changes were detected in 96% of older (23/24) and 72% (18/25) of young patients, respectively (p=ns).
Figure 1.
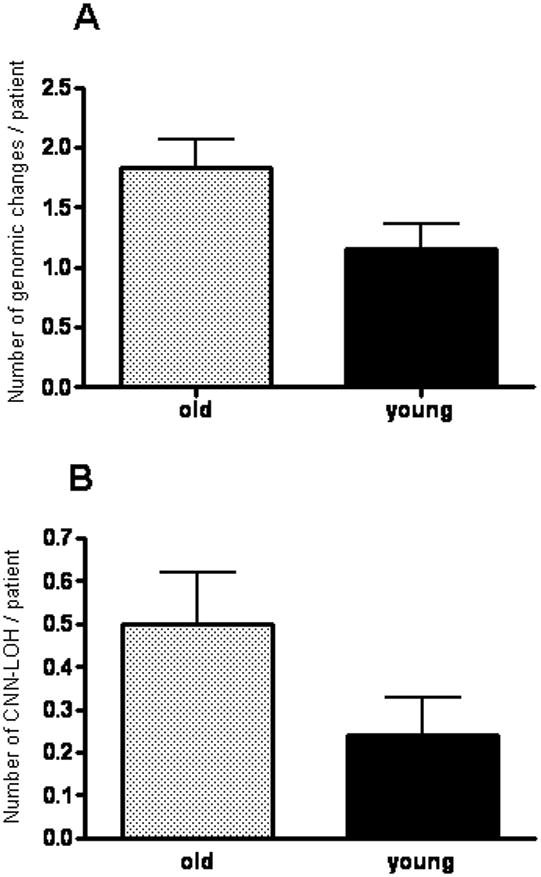
Rates of genomic changes (A. p=0.037) and CNN-LOH (B. p=0.088) per patient as detected in SNP array analysis in old compared to young patients.
Copy number neutral-loss of heterozygosity (CNN-LOH)
Copy number neutral-loss of heterozygosity (CNN-LOH) strongly suggests that one affected allele becomes mutated and is duplicated while loss of the normal allele occurs. The retained allele can result in either loss of tumor suppressor activity or gain of oncogenic activity. Sites of CNN-LOH occurred in 11/24 (46%) of the older individuals and 6/25 (24%) of the young patients, p=ns and a trend for higher number of CNN-LOH per patient in older patients was noted (p=0.088) (Figure 1 and Table 2). Recurring CNN-LOH occurred at chromosome 13q (n=5, 10%), 1p (n=3, 7.5%), 6p (n=2, 4%) and 11q (n=2, 4%) (Figure 2). Notably, 4/4 patients with 13q CNN-LOH examined for FLT3-ITD had the FLT3-ITD mutation. Thus, FLT3-ITD was duplicated. A candidate gene in the common 11q LOH region was CBL; therefore, we screened CBL exons 8 and 9 for mutations in the 2 cases with CNN-LOH. We found a C416S CBL mutation in one case. The second case did not have a detectable CBL mutation but did have a MLL-PTD mutation on chromosome 11q that may have promoted its duplication with loss of the normal allele. We identified the common deleted region (CDR) on chromosome 1p for 3 samples and interrogated the DNA for mutations of FGR, RUNX3, CDC42 and PINK1, which all reside in this CDR. No mutations were detected.
Table 2. Chromosomal regions containing CNN-LOH.
| Patient # | Age (years) | Chromosomal Region | Start (MB) | End (MB) | Size (MB) |
|---|---|---|---|---|---|
| 35 | 61 | lpter-p31.2 | 1.59 | 68.5 | 66.91 |
| 43 | 76 | lpter-p21.3 | 0.8 | 98.3 | 97.5 |
| 8 | 52 | lpter-p35.3 | 0.82 | 27.9 | 27.08 |
| 2 | 61 | 2pter-p22.2 | 0.02 | 37.8 | 37.78 |
| 32 | 68 | 6pter-p21.1 | 0.19 | 47.7 | 47.51 |
| 53 | 74 | 6pter-p21.2 | 0.22 | 39.3 | 39.08 |
| 70 | 73 | 7qll.23-7qter | 76.1 | 158.5 | 82.4 |
| 64 | 66 | llql3.5-qter | 74.4 | 134.3 | 59.9 |
| 65 | 25 | llpter-pl2 | 0.2 | 40 | 39.8 |
| 85 | 59 | llql3.2-qter | 65.5 | 134.4 | 68.9 |
| 30 | 36 | 13ql2.11-qter | 21.31 | 114 | 92.69 |
| 44 | 68 | whole 13q | 18.5 | 113.6 | 95.1 |
| 56 | 45 | whole 13q | 18.8 | 114 | 96 |
| 83 | 73 | whole 13q | 19.6 | 114 | 94.4 |
| 132 | 39 | whole 13q | 18.5 | 113.9 | 95.4 |
| 4 | 65 | whole 14q | 19.4 | 106.2 | 86.8 |
| 2 | 61 | whole 17q | 22.6 | 78.5 | 55.9 |
| 6 | 61 | 19ql3.11-qter | 38.3 | 63.7 | 25.4 |
Figure 2.
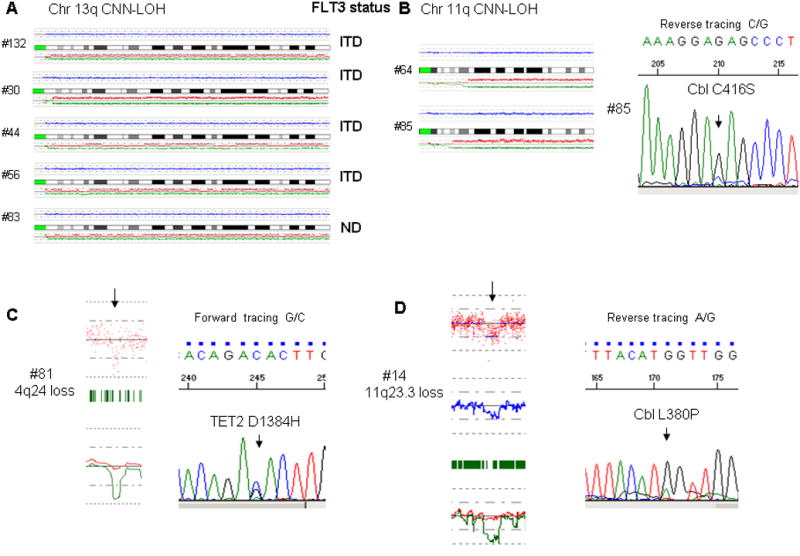
Selected single nucleotide polymorphism (SNP) array findings with correlating molecular features: (A) Alignment of chromosome 13q CNN-LOH cases and corresponding FLT3 aberrations. (B) Alignment of chromosome 11q CNN-LOH cases and a sequence trace showing an acquired CBL mutation in one of the chromosome 11q cases. (C) SNP array tracing showing a deletion in chromosome 4q24 with a corresponding sequence tracing showing an acquired TET2 mutation in this case (background normal allele can be seen probably due to contamination with normal cells) (D) SNP array tracing demonstrating a deletion in chromosome 11q with a corresponding sequence tracing showing an acquired Cbl mutation in this case.
Copy number abnormalities (CNA)
CNA were found in 22 older patients (88%) (11 deletions, 11 gains) and in 19 young patients (79%) (12 deletions, 7 gains), p=ns (Table 3).
Table 3. Acquired copy number changes.
| Patient # | Age (years) | Type of change | Chromosomal Region | Start (MB) | End (MB) | Size (MB) | Involved genes | |
|---|---|---|---|---|---|---|---|---|
| 6 | 61 | loss | lq41 | 212.248859 | 212.250274 | 0.0001 | USH2A | |
| 30 | 36 | loss | lq21.3 | 150.129082 | 150.182082 | 0.053 | PGLYRP4 | |
| 165 | 54 | loss | 2p23.3 | 25.324960 | 27.191067 | 1.866 | DNMT3A, DTNB, ASXL2, RAB10, KIF3C, HADHA, HADHB, GPR113, OTOF, VIGC16372, KCNK3, CENPA, DPYSL5, MAPRE3 | |
| 25 | 23 | loss | 2pl4 | 68.290689 | 68.459811 | 0.169 | PPP3R1 | |
| 25 | 23 | loss | 2pl3.3 | 68.508120 | 68.525361 | 0.017 | PLEK | |
| 15 | 43 | loss | 2p25.1-p24.3 | 12.094633 | 12.117474 | 0.022 | None | |
| 168 | 43 | loss | 2ql3 | 111.142569 | 113.071551 | 1.92 | BUB1, BCL2L11, ACOXL, ANPC1, MERTK, ZC3HDC8, IANBP2L1, BC036819, POLR1B | |
| 47 | 36 | loss | 3pl4.1-pl2.3 | 67.545370 | 76.755140 | 9.2 | SUCLG2, FAM19A1, FAM19A4, AER61, TMF1, UBE1C, ARL6IP5, LMOD3, MITF, FOXP1, GPR27, EIF4E3, PROK2, SHQ1, PPP4R2, PDZRN3 | |
| 3 | 24 | loss | 3p21.1-pl4.3 | 54.224729 | 54.537572 | 0.312 | CACNA2D3 | |
| 45 | 66 | loss | 3p24.3 | 20.532192 | 20.716157 | 0.183 | None | |
| 2 | 61 | loss | 4pl3 | 44.473770 | 44.601164 | 0.127 | GNPDA2 | |
| 81 | 58 | loss | 4q24 | 106.137398 | 106.497922 | 0.036 | TET2 | |
| 14 | 73 | loss | 5q21.3 | 106.725384 | 106.764256 | 0.038 | EFNA5 | |
| 73 | 55 | loss | 4q22.3 | 93.677775 | 93.996483 | 0.318 | GRID2 | |
| 45 | 66 | loss | 5pl4.1 | 28.755438 | 28.822601 | 0.067 | None | |
| 44 | 68 | loss | 6ql6.1 | 94.544,526 | 94.631590 | 0.087 | TSG1 | |
| 60 | 72 | loss | 6q21 | 107.064167 | 107.087442 | 0.023 | AIM1 | |
| 124 | 41 | loss | 6q26 | 162.459944 | 162.512495 | 0.052 | PARK2 | |
| 2 | 61 | loss | 7pl2.2-12.1 | 50.078130 | 51.141658 | 1.06 | IKZF1, FINGL1, DDC, GRB10, COBL | |
| 5 | 69 | loss | 7p21.1 | 17.493502 | 17.560451 | 0.67 | None | |
| 6 | 61 | loss | 7p36.2 | 153.684383 | 153.957254 | 0.272 | DPP6 | |
| 165 | 54 | loss | 8p22 | 15.995420 | 16.079209 | 0.083 | MSR1 | |
| 165 | 54 | loss | 9p24.3 | 2.138966 | 2.150105 | 0.011 | SMARCA2 | |
| 3 | 24 | loss | 9p21.1 | 28.187129 | 28.285916 | 0.098 | FLJ31810 | |
| 45 | 66 | loss | 9p21.3 | 22.120389 | 22.151212 | 0.03 | None | |
| 60 | 72 | loss | 9q21.13 | 72.137151 | 72.219197 | 0.082 | ZA20D2 | |
| 68 | 76 | loss | 9q33.2 | 120.038756 | 120.151232 | 0.11 | None | |
| 6 | 61 | loss | llpl4.3 | 23.169551 | 23.857193 | 0.687 | None | |
| 14 | 73 | loss | llq23.3 | 117.886279 | 119.789979 | 1.9 | MLL, TMEM2, ARCN1, PHLDB1, DDX6, BLR1, BCL9L, UPK2, FOXR1, TRAPPC4, RPS25, HYOU1, HMBS, DPAGT1, TMEM24, MIZF, ABCG4, NOD9, PDZK2, CBL, MCAM, RNF26, C1QTNF5, MFRP,USP2, THY1, PVRL1, TRIM29, POU2F3, ARHGEF12 | |
| 4 | 65 | loss | 12pl2.3 | 19.685480 | 19.763724 | 0.078 | None | |
| 60 | 72 | loss | 13q32.3 | 98.668215 | 98.735083 | 0.066 | UBAC2, GPR18 | |
| 20 | 40 | loss | 15q22.33 | 65.179831 | 65.195295 | 0.015 | SMAD3 | |
| 20 | 40 | loss | 15q23 | 65.845701 | 65.866142 | 0.02 | MAP2K5 | |
| 10 | 72 | gain | lp33 | 49.140323 | 49.477458 | 0.334 | AGBL4 | |
| 85 | 59 | gain | lq25.3 | 180.298570 | 180.324251 | 0.025 | None | |
| 56 | 45 | gain | lq41 | 217.469324 | 217.509794 | 0.04 | None | |
| 82 | 69 | gain | lq42.2 | 228.783719 | 228.910865 | 0.127 | SIPA1L2 | |
| 143 | 67 | gain | lq24.3 | 169.064905 | 169.086789 | 0.021 | DNM3 | |
| 70 | 73 | gain | 2q37.1 | 231.005389 | 231.135031 | 0.129 | SP100 | |
| 5 | 69 | gain | 3ql2.2 | 101.836860 | 101.937829 | 0.1 | GPR128 | |
| TFG | ||||||||
| 15 | 43 | gain | 3p26.1 | 6.291534 | 6.303872 | 0.012 | None | |
| 25 | 23 | gain | 5q23.1 | 118.729125 | 119.000084 | 0.27 | TNFAIP8, HSD17B4 | |
| 25 | 23 | gain | 5q23.2 | 124.358421 | 124.429690 | 0.071 | None | |
| 83 | 73 | gain | 6p24.2 | 11,289,695 | 11,311,011 | 0.021 | NEDD9 | |
| 2 | 61 | gain | 6ql4.3 | 85.721165 | 86.156062 | 0.43 | None | |
| 10 | 72 | gain | 10q24.32 | 103.187709 | 103.391506 | 0.2 | BTRC, POLL, FBXW4 | |
| 28 | 67 | gain | llpl3 | 34.381903 | 34.447223 | 0.065 | CAT | |
| 1 | 77 | gain | 13q31.3 | 90.716800 | 90.843580 | 0.126 | hsa-mir- 17, 18a, 19a, 20a, 19bl, 92-1 | |
| 39 | 61 | gain | 13ql4.11 | 42.426411 | 42.632690 | 0.206 | EPSTI1, DNAJD1 | |
| 47 | 36 | gain | 14q21.3 | 49.162652 | 49.386407 | 0.223 | POLE2, KLHDC1, KLHDC2, SDCCAG1 | |
| 82 | 69 | gain | 16q24.3 | 87.750726 | 87.798162 | 0.047 | ACSF3, | |
| CDH15 | ||||||||
| 148 | 74 | gain 17ql2 | 28.969011 | 30.024199 | 1.055 | ACCN1, CCL2, CCL7, CCL11, CCL8, CCL13, CCL1, | ||
| 1 | 77 | gain 17q25.1 | 69.345596 | 70.223957 | 0.878 | RPL38, TTYH2, DNAI2, GPC142, GPRC5C, CD300A, CD300LB, CD300C, CD300LE CD300LF, RAB37 | ||
| 5 | 69 | gain 17qll.2 | 26.238513 | 26.516549 | 0.278 | CENTA2, RNF135, NF1 | ||
| 27 | 49 | gain 17q25.1 | 70.667982 | 71.448248 | 0.78 | SUM02, PCNT1, GGA3, MRPS7, SLC25A19, GRB2, CASKIN2, TSEN54, LLGL2, RECQL5, HCNGP, ITGB4, GALK1, H3F3B, ZC3HDC5,UNCI 3D, WBP2,TRIM47, MRPL38, FBF1 | ||
| 135 | 44 | 19ql3.43 | 61.023747 | 61.067752 | 0.044 | NALP11, NALP4 | ||
Although not statistically significant, genomic losses tended to be larger than gains with an average size of 0.6 MB compared to 0.22 MB. Losses were larger in the young (average size-0.87 MB) compared to older patients (average size- 0.34 MB), while the size of gains was similar in both groups with an average of 2.21 and 2.23 MB for young and older patients, respectively. Genomic areas containing genes of interest including 4q24 (#81), 11q23.3 (#14) and 7p12.2-12.1 (#2) were screened for mutations in TET2, CBL and IKZF1, respectively (Figure 2), revealing TET2 D1384H and CBL L380P mutations but none involving the IKZF1 gene. No areas of recurrent copy number changes were detected in either of the study cohorts.
Correlations between clinical data and SNP array results
No correlations between baseline blood counts and the presence of genomic changes were found. The frequency of SNP array changes in the different molecular subgroups is given in Table 4. Of notable significance, the presence FLT3-ITD was associated with genomic changes in SNP array analysis (Figure 3). For example, 19/20 (95%) of patients with the FLT3-ITD mutation had genomic changes (CNN-LOH, deletions or gains) compared to 16/24 (67%), p=0.027. When analyzed according to the nature of the genomic changes, patients with FLT3-ITD mutation were more likely to harbor CNN-LOH compared to patients without FLT3-ITD, p=0.025. In contrast, no difference occurred in the rates of CNA in patients either with or without FLT3-ITD. Young patients with FLT3-ITD mutations had a higher rate of genomic changes compared to young patients with no FLT3-ITB mutations (p=0.002) and a higher rate of CNN-LOH (p=0.015). The association of FLT3-ITD mutation with genomic changes was not seen in the cohort of older patient. Of note, although the FLT3-ITD mutation was associated with chromosome 13q CNN-LOH in 4 patients, 6 additional patients with FLT3-ITD had CNN-LOH in other chromosomes, suggesting that FLT3-ITD may be associated with a propensity for CNN-LOH in all chromosomes.
Table 4. Frequency of SNP array changes in molecular subgroups.
| Molecular change | Any SNP array change N/N studied (%) | CNN-LOH N/N studied (%) | Median EFS (days) |
|---|---|---|---|
| FLT3-ITD | 19/20 (95) | 10/20 (50) | 223 |
| NPM1 mutation | 21/26(81) | 9/26 (35) | 281 |
| CEBPA mutation | 3/4 (75%) | 2/4 (40) | NR |
| NPM1 mutation/FLT3-ITD negative | 4/8 (50) | 0/8 (0) | 325 |
FLT3-ITD FLT3 internal tandem duplication; NPM1 nucleophosmin; NR not reached
Figure 3.
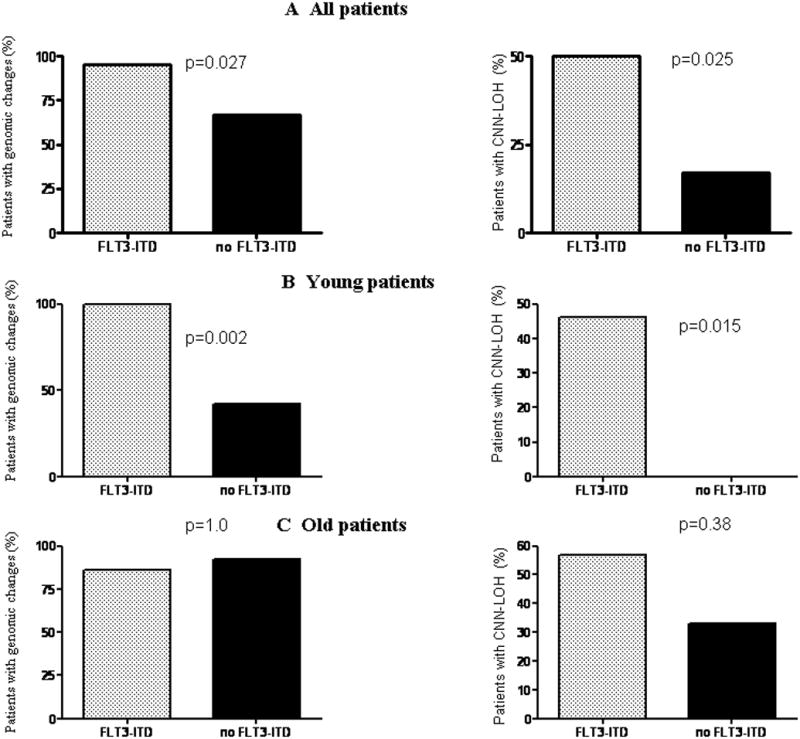
Correlation of FLT3-ITD mutational data with SNP array findings. (A) Frequency of genomic changes (left panel) and CNN-LOH (right panel) in patients either with or without FLT3-ITD in the entire cohort. (B) and (C) show frequency of genomic changes (left panel) and of CNN-LOH (right panel) in young and older patients, respectively, either with or without FLT3-ITD. p = level of significance (Fisher exact test).
No correlation occurred between genomic changes and the presence of either a CEBPA, NPM1, MLL-PTD or FLT3-TKD mutation. However, a lower rate of any genomic change and CNN-LOH was found in the subgroup of patient with who had NPM1 mutation and no FLT3-ITD mutation, compared to any other mutation combination (50% compared to 86%, p=0.042 and 0 compared to 39%, p=0.041, respectively).
Correlation between clinical outcome and genomic changes
Median event free survival (EFS) was 807 and 324 days in the young and older patients cohorts, respectively, p=0.2; while the median overall survival was not reached in the young patients cohort, it was 324 days in older patients, p=0.05 (Figure 4).
Figure 4.
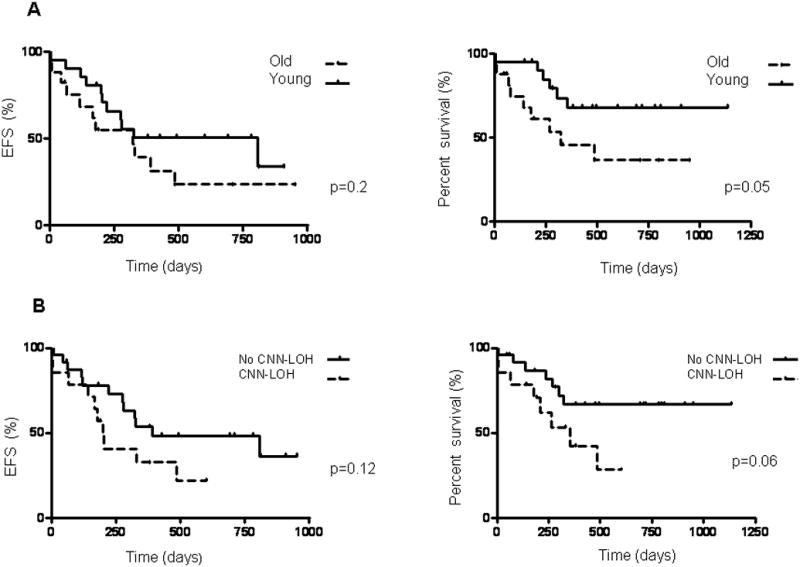
Kaplan-Meier curves for event free survival (EFS) and overall survival (OS). (A) EFS (left panel) and OS (right panel) in young compared to older patients. (B) EFS (left panel) and OS (right panel) in patients either with or without CNN-LOH.
The presence of genomic changes was not significantly associated with EFS and OS rates. The median EFS in the entire study group was 200 days in patients with CNN-LOH compared to 391 days in patients without CNN-LOH, p=0.12, and the respective median OS were 356 and not reached, p=0.06 (Figure 4). CNN-LOH was not significantly associated with either EFS or OS when analyzed separately for the young and older patients' cohort (Figure 4).
Discussion
This is the first study to look specifically at genomic changes in older (≥ 60 years old) patients with NK-AML as a group and compare these changes to those in a young (<60 years old) patient's cohort. Our main findings are a higher frequency of genomic changes in older compared to young patients, and in particular a higher prevalence of CNN-LOH in older patients. The 46% frequency of CNN-LOH in older patients in our study is 2-3 times higher than the 24% and 15%) prevalence found in younger patients in this study and in a recent report [6], respectively. In addition, a trend towards a worse outcome was noted in patients with CNN-LOH, although this must be cautiously interpreted due to the relatively small patients numbers. Two questions arise from these results: 1. why do more genomic changes / CNN-LOH occur in older patients? 2. Are these changes directly related to the worse outcome of older patients?
Genomic instability in AML has been linked to increased ROS production as a mediator of endogenous DNA damage. FLT3-ITD, in particular, was shown to increase ROS production via STAT5 signaling and activation of RAC1 [11]. Indeed, the higher frequency of genomic changes including CNN-LOH in patients with FLT3-ITD in our study supports this concept. Interestingly, the increased risk was maintained in subgroup analysis in the young and not the older patients' cohort, suggesting another unidentified mechanism for genomic instability in older patients. In support of our finding of an increased rate of genomic changes in older patients, a recent analysis of genomic changes in myeloproliferative neoplasms (MPN) found a higher rate of genomic changes in older compared to younger patients [12]. We found a higher rate of 1p CNN-LOH than was previously described in young patients with NK-AML [6, 13, 14]. Combined with our previous report [15] the frequency of 1p CNN-LOH was 9% in older patients compared to 2.8% in young patients, and in other reports on young patients the frequencies were either 1.3% [6], or 0 [13, 14]. Although the numbers are small, the CDR in chromosome lp detected in our study may be an area to study further for gene mutations and epimutations in older patients AML.
Complex karyotype is a well-known adverse prognostic factor in AML [5], not only because of the simple summation of specific abnormalities, but most probably because it is a sign of increased genomic instability. In parallel, we speculate that in NK-AML, abundance of genomic changes, which can be detected in SNP array analysis, are a sign of increased genomic instability which may be associated with a worse outcome. A recent SNP array analysis in unselected AML patients showed, in a similar manner, that an increase in the number of genomic changes was associated with worse overall survival rates [16]. This can partially explain the poor outcome in older patients.
In conclusion, we found a higher rate of genomic changes, and in particular of CNN-LOH in older compared to younger patients. The number or type of genomic changes may become additional prognostic indicators in NK-AML.
Supplementary Material
Acknowledgments
The authors would like to thank Dror Berel, MS, Sr. Biostatistician, Samuel Oschin Comprehensive Cancer Institute at Cedars-Sinai Medical Center, for statistical analysis support.
Funding Source: This work was supported in part by National Institutes of Health grant 5R01CA026038-31, Inger Fund, Parker Hughes Trust, NSFC30470980, and A*STAR grant from Singapore. HPK is a member of the Molecular Biology Institute and Jonsson Comprehensive Cancer Center at UCLA, holds the endowed Mark Goodson Chair of Oncology Research at Cedars-Sinai Medical Center/UCLA School of Medicine and is Deputy Director of Research of the National Cancer Institute of Singapore.
Footnotes
Authors' Contributions: S.O. and H.P.K. contributed equally to the work; M.K.M performed the research, analyzed the data, and wrote the paper; A.S.O performed; S.N.P. array analysis; A.N. and T.H. assisted with the research and edited the paper; S.O and H.P.K directed the overall study.
Conflict of Interest: All authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- 1.Juliusson G, Antunovic P, Derolf A, Lehmann S, Mollgard L, Stockelberg D, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–87. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–5. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Büchner T, Berdel WE, Haferlach C, Haferlach T, Schnittger S, Müller-Tidow C, et al. Age-Related Risk Profile and Chemotherapy Dose Response in Acute Myeloid Leukemia: A Study by the German Acute Myeloid Leukemia Cooperative Group. Journal of Clinical Oncology. 2009;27:61–9. doi: 10.1200/JCO.2007.15.4245. [DOI] [PubMed] [Google Scholar]
- 4.Mrozek K, Marcucci G, Paschka P, Whitman SP, Bloomfield CD. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431–48. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimwade D. The clinical significance of cytogenetic abnormalities in acute myeloid leukaemia. Best Practice & Research Clinical Haematology. 2001;14:497–529. doi: 10.1053/beha.2001.0152. [DOI] [PubMed] [Google Scholar]
- 6.Bullinger L, Kronke J, Schon C, Radtke I, Urlbauer K, Botzenhardt U, et al. Identification of acquired copy number alterations and uniparental disomies in cytogenetically normal acute myeloid leukemia using high-resolution single-nucleotide polymorphism analysis. Leukemia. 2009;24:438–49. doi: 10.1038/leu.2009.263. [DOI] [PubMed] [Google Scholar]
- 7.Nannya YSM, Nakazaki K, Hosoya N, Wang L, Hangaishi A, Kurokawa M, Chiba SBD, Kennedy GC, Ogawa S. A robust algorithm for copy number detection using high-density oligonucleotide single nucleotide polymorphism genotyping arrays. Cancer Res. 2005;65:6071–9. doi: 10.1158/0008-5472.CAN-05-0465. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto GNY, Kato M, Sanada M, Levine RL, Kawamata N, Hangaishi A, Kurokawa MCS, Gilliland DG, Koeffler HP, Ogawa S. Highly sensitive method for genomewide detection of allelic composition in nonpaired, primary tumor specimens by use of affymetrix single-nucleotide-polymorphism genotyping microarrays. Am J Hum Genet. 2007;81:114–26. doi: 10.1086/518809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weksberg RHS, Moldovan L, Bassett AS, Chow EW, Squire JA. A method for accurate detection of genomic microdeletions using real-time quantitative PCR. BMC Genomics. 2005;6:180–90. doi: 10.1186/1471-2164-6-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawamata N, Ogawa S, Zimmermann M, Kato M, Sanada M, Hemminki K, et al. Molecular allelokaryotyping of pediatric acute lymphoblastic leukemias by high-resolution single nucleotide polymorphism oligonucleotide genomic microarray. Blood. 2008;111:776–84. doi: 10.1182/blood-2007-05-088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sallmyr A, Fan J, Rassool FV. Genomic instability in myeloid malignancies: Increased reactive oxygen species (ROS), DNA double strand breaks (DSBs) and error-prone repair. Cancer Letters. 2008;270:1–9. doi: 10.1016/j.canlet.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 12.Thorsten Klampfl AH, Berg Tiina, Gisslinger Bettina, Passamonti Francesco MD, Rumi Elisa, Pietra Daniela, Olcaydu Damla, Jäger Roland, Cazzola Mario, Gisslinger Heinz, Kralovics Robert. Chromosomal Aberration Network In Myeloproliferative Neoplasms. ASH Annual Meeting Abstracts. 2010;116:145. [Google Scholar]
- 13.Walter MJ, Payton JE, Ries RE, Shannon WD, Deshmukh H, Zhao Y, et al. Acquired copy number alterations in adult acute myeloid leukemia genomes. Proceedings of the National Academy of Sciences. 2009;106:12950–5. doi: 10.1073/pnas.0903091106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorletta TA, Gasparini P, D'Elios MM, Trubia M, Pelicci PG, Di Fiore PP. Frequent loss of heterozygosity without loss of genetic material in acute myeloid leukemia with a normal karyotype. Genes, Chromosomes and Cancer. 2005;44:334–7. doi: 10.1002/gcc.20234. [DOI] [PubMed] [Google Scholar]
- 15.Akagi T, Ogawa S, Dugas M, Kawamata N, Yamamoto G, Nannya Y, et al. Frequent genomic abnormalities in acute myeloid leukemia/myelodysplastic syndrome with normal karyotype. Haematologica. 2009;94:213–23. doi: 10.3324/haematol.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkin B, Erba H, Ouillette P, Roulston D, Purkayastha A, Karp J, et al. Acquired genomic copy number aberrations and survival in adult acute myelogenous leukemia. Blood. 116:4958–67. doi: 10.1182/blood-2010-01-266999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


