Abstract
Lapatinib, a dual EGFR/HER2 kinase inhibitor, is approved for use in patients with trastuzumab-refractory HER2-overexpressing breast cancer. Increased PI3K signaling has been associated with resistance to trastuzumab, although its role in lapatinib resistance remains unclear. The purpose of the current study was to determine if PI3K/mTOR activity affects lapatinib sensitivity. Reduced sensitivity to lapatinib was associated with an inability of lapatinib to inhibit Akt and p70S6K phosphorylation. Transfection of constitutively active Akt reduced lapatinib sensitivity, while kinase-dead Akt increased sensitivity. Knockdown of 4EBP1 also increased lapatinib sensitivity, in contrast to p70S6K knockdown, which did not affect response to lapatinib. Pharmacologic inhibition of mTOR using rapamycin or ridaforolimus increased lapatinib sensitivity and reduced phospho-Akt levels in cells that showed poor response to single-agent lapatinib, including those transfected with hyperactive Akt. Finally, combination mTOR inhibition plus lapatinib resulted in synergistic inhibition of proliferation, reduced anchorage-independent growth, and reduced in vivo tumor growth of HER2-overexpressing breast cancer cells that have primary trastuzumab resistance. Our data suggest that PI3K/mTOR inhibition is critical for achieving optimal response to lapatinib. Collectively, these experiments support evaluation of lapatinib in combination with pharmacologic mTOR inhibition as a potential strategy for inhibiting growth of HER2-overexpressing breast cancers that show resistance to trastuzumab and poor response to lapatinib.
Keywords: Akt, breast cancer, drug resistance, erbB2, HER2, Herceptin, lapatinib, MK-8669, mTOR, PI3K, p70S6K, rapamycin, ridaforolimus, Tykerb, trastuzumab
INTRODUCTION
The HER2 gene is amplified and overexpressed in approximately 25%–30% of metastatic breast cancers, and is associated with an aggressive clinical course resulting in reduced disease-free and overall survival compared with other breast cancer subtypes [1,2]. Trastuzumab (Herceptin) is a recombinant humanized monoclonal antibody directed against the HER2 extracellular domain. Initial clinical trials of single-agent trastuzumab demonstrated overall response rates ranging from 11% to 21% in patients with HER2-overexpressing metastatic breast cancer [3,4]. Thus, almost two-thirds of patients demonstrated primary resistance to trastuzumab, although response rates were improved when combined with chemotherapy [5,6]. The dual EGFR/HER2 tyrosine kinase inhibitor lapatinib (Tykerb) (Figure 1) is approved in combination with capecitabine for use against HER2-overexpressing breast cancers with prior disease progression on trastuzumab and as first-line therapy in combination with letrozole for hormone receptor-positive, HER2-positive metastatic breast cancer. Combination lapatinib plus chemotherapy achieved an overall response rate of 22% and clinical benefit rate of 27%, with median time to progression of 8.4 months [7]. As a single agent, lapatinib showed clinical benefit rates ranging from 12.4% to 25% in trastuzumab-pretreated populations [8,9]. Thus, lapatinib shows benefit in a subset of trastuzumab-refractory breast cancers, although the majority of trastuzumab-resistant disease shows poor response to lapatinib.
Figure 1. Chemical structures of kinase inhibitors.
Structures for lapatinib, rapamycin, and the rapamycin analogue ridaforolimus (MK-8669) were downloaded from the ChemACX database (CambridgeSoft, Cambridge, MA) and drawn in ChemDraw (CambridgeSoft).
Resistance to trastuzumab has been closely associated with increased PI3K signaling due to either loss of the PTEN phosphatase gene [10] or hyper-activating mutations in the PIK3CA catalytic subunit of PI3K [11]. Esteva et al [12] recently showed that phosphorylation of Akt or the mTOR substrate p70S6K were not independently associated with trastuzumab resistance, but when considered together, p-Akt, p-p70S6K, and loss of PTEN were strongly associated with poor response to trastuzumab. A genome-wide loss-of-function short hairpin RNA screen performed to identify mediators of lapatinib resistance showed that loss of PTEN or PIK3CA mutations also contributed to lapatinib resistance [13]. Further, treatment with a dual inhibitor of PI3K/mTOR inhibited colony formation and proliferation of lapatinib-resistant cells harboring genetic defects in PI3K signaling [13]. In contrast, O’Brien et al. [14] suggested that lapatinib resistance was not associated with loss of PTEN or PIK3CA mutations, and that lapatinib could block the hyperactive PI3K signaling associated with trastuzumab resistance. Wang et al. [15] examined 57 primary tumor samples from lapatinib-treated patients with HER2-overexpressing breast cancer heavily pretreated with chemotherapy and trastuzumab. Patients with loss of PTEN or hyper-activating mutations in PIK3CA had a significantly lower clinical benefit rate (36.4% versus 68.6%) and significantly lower overall response rate (9.1% versus 31.4%) in contrast to those patients whose tumors did not show PI3K pathway activation.
Blocking the PI3K pathway with mTOR inhibition has been demonstrated to be beneficial in trastuzumab-resistant cancers. Response rates of more than 40% and disease control rates of more than 70% were achieved in metastatic HER2-positive breast cancers resistant to trastuzumab and taxanes, when treated with trastuzumab, paclitaxel and everolimus [16]. The combination of trastuzumab, chemotherapy and everolimus has been demonstrated to be beneficial in patients with HER2-positive metastatic breast cancer, resistant to both trastuzumab and lapatinib. The use of mTOR inhibition, with everolimus, is currently being evaluated in a phase 3 trial of patients with metastatic HER2-positive breast cancers resistant to trastuzumab. Patients entering this trial can have received lapatinib. Therefore, there is a clear need to understand the role of PI3K signaling in HER2-positive breast cancers that are resistant to currently approved HER2-directed agents, including lapatinib.
Thus, although some studies suggest that PI3K pathway activation correlates with reduced response to lapatinib, controversy exists in the literature regarding the role of PI3K/mTOR in lapatinib sensitivity. We examined the ability of lapatinib to inhibit proliferation in multiple HER2-overexpressing breast cancer cell lines that have primary trastuzumab resistance. Lower response to lapatinib was associated with an inability of lapatinib to reduce p-Akt or p-p70S6K levels. Transfection of constitutively active Akt into lapatinib-sensitive cells abrogated response to lapatinib, while kinase-dead Akt improved lapatinib sensitivity, further suggesting that inhibition of Akt phosphorylation is critical to achieving response to lapatinib. In contrast, knockdown of p70S6K alone did not improve response to lapatinib. However, knockdown of 4EBP1 or pharmacologic mTOR inhibition increased lapatinib sensitivity. Inhibition of mTOR reduced p-Akt levels and increased response to lapatinib in cells that showed poor response to single-agent lapatinib, even those transfected with hyperactive Akt. Single agent mTOR inhibition was associated with feedback signaling activating Akt and Erk1/2, which was overcome by co-treatment with lapatinib. Combination mTOR inhibition plus lapatinib resulted in synergistic growth inhibition of HER2-overexpressing trastuzumab-resistant breast cancer cells and xenografts. Our data indicate that p-Akt is a critical downstream target of lapatinib, whose inhibition must be intact in order to achieve optimal response to lapatinib. In cases where lapatinib alone does not effectively block Akt or p70S6K phosphorylation, our data support strategies that combine lapatinib with mTOR inhibition in the context of primary trastuzumab-resistant HER2-overexpressing breast cancer.
MATERIALS AND METHODS
Materials
Lapatinib for in vitro studies was purchased from Santa Cruz, Biotech (Santa Cruz, CA), and dissolved in DMSO at a stock concentration of 10 mM. Lapatinib for in vivo studies was purchased from the Winship Cancer Institute pharmacy and dissolved in 1% Tween 80 at a stock concentration of 7.5 mg/mL. Rapamycin mTOR inhibitor (Sigma-Aldrich; St. Louis, MO) (Figure 1) was supplied as a 2.74 mM solution in DMSO. MK-8669 (ridaforolimus, supplied by Merck through an MTA) (Figure 1) was dissolved in DMSO at stock concentration of 10 mM for in vitro studies. For in vivo studies, MK-8669 was dissolved fresh daily in 10% Tween 80 and 40% PEG-400 in sterile water at stock 1 mg/mL as recommended by Merck. Trastuzumab was purchased from the Winship Cancer Institute pharmacy and dissolved in sterile water at a stock concentration of 20 mg/mL.
Cell culture
HCC1419 and HCC1954 cells were maintained in RPMI with 10% fetal bovine serum (FBS); MDA-MB-361 was maintained in RPMI with 20% FBS; JIMT-1, BT474, and MDA-MB-453 were maintained in DMEM with 10% FBS; all cells were maintained in 1% penicillin/streptomycin and cultured in humidified incubators at 37°C with 5% CO2. JIMT-1 cells were purchased from DSMZ, Germany; all other cell lines were purchased from American Type Culture Collection, Manassas, VA.
Cell proliferation assay
Cells were plated at 3000 per well in 96-well format, and treated with lapatinib and/or rapamycin or MK-8669 versus DMSO control corresponding to the volume found in the highest dose combination. For another experiment, cells were treated with 20 μg/mL trastuzumab versus untreated control. Six replicates were run per group. After 6 days of treatment, proliferation was measured by MTS assay as directed by the manufacturer (Promega; Madison, WI). Combination index (C.I.) values were determined using the commercial software package Calcusyn (Biosoft, Cambridge, United Kingdom) by the method of Chou and Talalay [17]. Experiments were repeated on at least two independent occasions to ensure reproducibility.
Anchorage-independent growth
Cells were plated at 15 × 105 in 6-well plate format in 1mL matrigel (BD Biosciences; Franklin Lakes, NJ) diluted 3:1 (media:matrigel). The matrigel-cell suspension was allowed to solidify for 2 hours at 37Co. Then 2 mL of media containing lapatinib, mTOR inhibitor (rapamycin or MK-8669), combination lapatinib plus mTOR inhibitor, or DMSO control at the same volume found in the drug combination group, was added to the matrigel-cell culture. Media plus drug was changed twice a week for approximately 3–4 weeks. Photographs were taken with an Olympus IX50 inverted microscope at 4X magnification. Matrigel was then digested using dispase (BD Biosciences), and viable cells were counted by trypan blue exclusion. Experiments were repeated on at least two independent occasions to ensure reproducibility.
Transfection
Cells were plated in antibiotic-free media. The next day, cells were transfected using Lipofectamine 2000 (Invitrogen; Carlsbad, CA) with 1μg of one of the following plasmids: dominant negative kinase dead Akt1 mutant (pcDNA3-Akt1-K179A), constitutively active Akt1 mutant (pcDNA3-Akt1-T308D/S473D), or pcDNA3 empty vector control (plasmids generously provided by Dr. Keqiang Ye, Emory). After 24h, protein lysates were Western blotted for p-Akt to confirm transfection, or cells were treated with vehicle control (DMSO), lapatinib, rapamycin, or combination rapamycin plus lapatinib for an additional 72h, at which point cells were counted by trypan blue exclusion. In an independent set of experiments, 100 nM p70/85 S6 Kinase siRNA II (Cell Signaling), 4EBP1 siRNA (Cell Signaling) or control siRNA (Santa Cruz Biotech) were transfected using Lipofectamine. After 24 h, cells were treated with vehicle control or lapatinib for an additional 72h, at which point cells were counted by trypan blue exclusion. Knockdown was confirmed after 48 hours of transfection by Western blotting. Experiments were repeated three times to confirm reproducibility.
Western blotting
Cells were lysed in RIPA buffer (consists of 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin; Cell Signaling; Danvers, MA) supplemented with protease and phosphatase inhibitors (Sigma-Aldrich). Total protein extracts (30 μg) were run on SDS-PAGE and blotted onto nitrocellulose. Blots were probed overnight using the following antibodies: from Cell Signaling, p-S473 Akt-XP used at 1:1000, phospho-Thr389 p70S6K clone 1A5 at 1:750; and polyclonal antibodies against total Akt (1:1000), p-Thr202/Tyr204 p42/p44 Erk1/2 (1:1000), total p42/p44 Erk1/2 (1:1000), total p70S6K (1:1000); β-actin monoclonal AC-15 (Sigma-Aldrich) at 1:10,000. Protein bands were detected using the Odyssey Imaging System (Li-Cor Biosciences; Lincoln, NE). Experiments were repeated on at least two independent occasions to ensure reproducibility.
Xenograft studies
Flank xenografts were established in 8–10 week female athymic nu/nu mice (Harlan) by s.c. injection with 1 × 106 JIMT-1 cells and 50% Matrigel (BD Biosciences). Tumor volumes were calculated as the product of the length, width and height of the tumor measured twice a week with a caliper. Animals were administered lapatinib by oral gavage at a dose of 75 mg/kg, and MK-8669 by i.p. injection at a dose of 1 mg/kg. Control mice received vehicle diluent (10% Tween 80, 40% PEG-400 in sterile water). All treatments were done daily for 5 days, then off for 2 days, for a total of 17 days. Animals were euthanized by CO2 inhalation in accordance with institutional IACUC regulations.
RESULTS
Analysis of lapatinib response in HER2-overexpressing breast cancer cell lines with primary resistance to trastuzumab
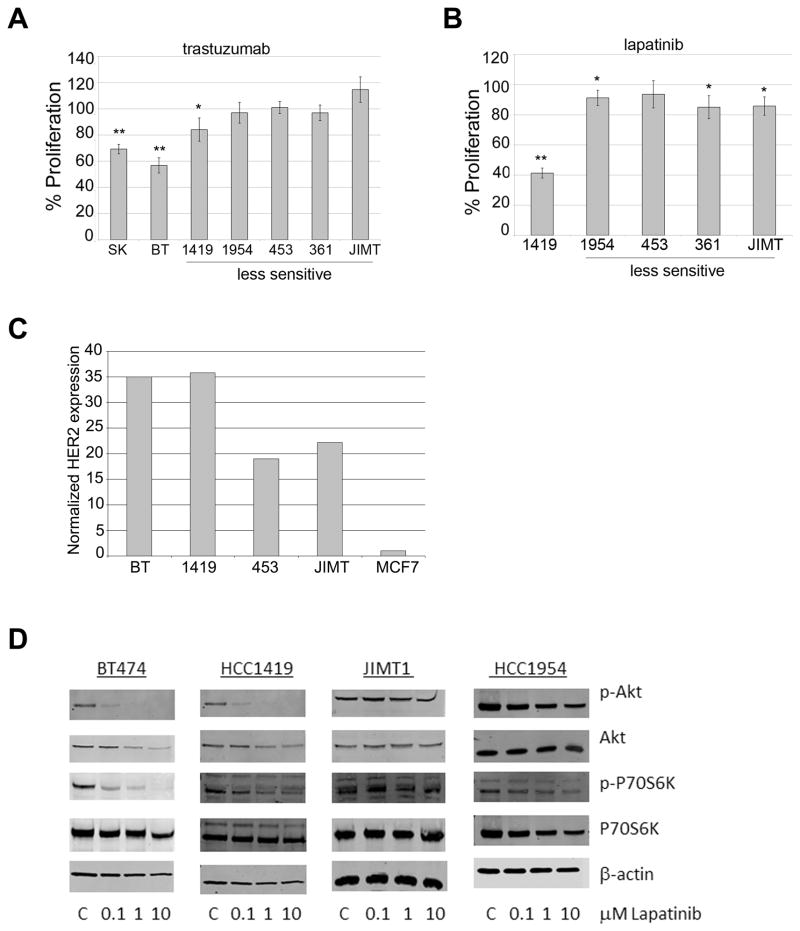
The HER2-overexpressing breast cancer cell lines SKBR3, BT474, HCC1419, HCC1954, MDA453, MDA361, and JIMT-1 were examined for sensitivity to trastuzumab by MTS proliferation assay (Figure 2A). A clinically relevant concentration of trastuzumab (20 μg/mL) inhibited proliferation of SKBR3 and BT474 cells. HCC1419 cells showed slightly lower although statistically significant inhibition of proliferation in response to trastuzumab. HCC1954, MDA453, MDA361, and JIMT-1 cells showed primary resistance to trastuzumab. Lapatinib (0.1 μM) inhibited proliferation of HCC1419 cells by 50–60%, but inhibited the remaining four primary trastuzumab-resistant cell lines by only 10–20% (Figure 2B). Thus, 4 out of 5 lines (approximately 80%) of cells with trastuzumab resistance also showed poor response to lapatinib, which mimics clinical response rates to lapatinib in trastuzumab-pretreated patients [7–9]. To confirm HER2 over-expression in lines used in this study, Western blotting for total HER2 was performed relative to the MCF-7 cell line (Figure 2C). In addition to measuring differential response to lapatinib by proliferation assay, Western blotting for p-Akt and p-p70S6K was performed. Lapatinib blocked phosphorylation of Akt and p70S6K in lapatinib-sensitive BT474 and HCC1419 cells, but not in JIMT-1 or HCC1954 cells (Figure 2D). These results suggest that inhibition of PI3K and mTOR may be important for achieving inhibition of proliferation in response to lapatinib.
Figure 2. Analysis of anti-proliferative activity of lapatinib in primary trastuzumab-resistant HER2-overexpressing breast cancer cell lines.
Proliferation was examined by MTS assay in cell lines treated with (A) 20 μg/mL trastuzumab, or (B) 0.1 μM lapatinib for 6 days. Values represent the average of 6 replicates per group as a percentage of untreated control cells (for trastuzumab) or DMSO-treated cells (for lapatinib). Error bars represent standard deviation between replicates. P-values were determined by t-test; *p<0.05, **p<0.005. Experiments were repeated three times with reproducible results. A representative immunoblot of total HER2 is shown for all cell lines. (C) Total protein lysates of cell lines were examined by Western blotting for total HER2. Bands were quanitated and values were normalized to actin levels. Total HER2 level is shown relative to MCF-7 cell line. (D) BT474, HCC1419, JIMT-1, and HCC1954 cells were treated with 0.1, 1 or 10 μM lapatinib, or with DMSO at the volume found in the highest dose of lapatinib (C, control) for 48 h. Whole cell protein lysates were immunoblotted for p-S473 Akt, total Akt, p-T389 p70S6K, total p70S6K, or actin loading control. Blots were repeated on at least two separate occasions with reproducible results.
Constitutively active Akt reduces lapatinib sensitivity, while kinase dead Akt improves lapatinib sensitivity
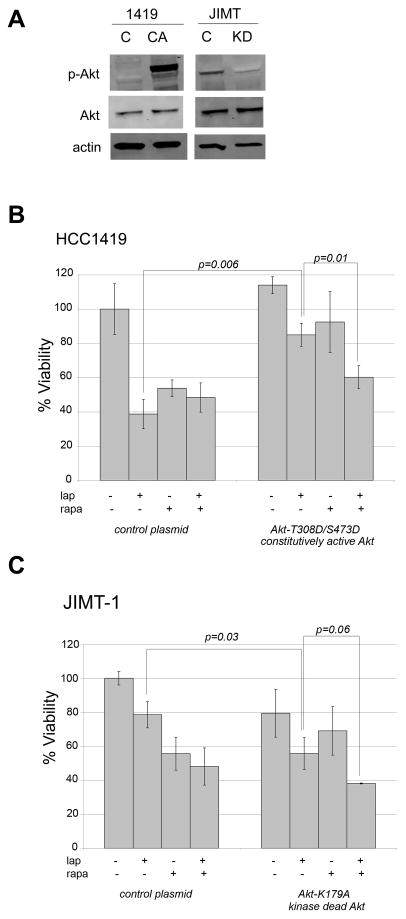
Based on this initial data (Figure 2) showing that lapatinib sensitivity correlates with reduced p-Akt in BT474 and HCC1419 cells in contrast to JIMT-1 and HCC1954 cells, we hypothesized that Akt inhibition is important for achieving response to lapatinib. To test this hypothesis, HCC1419 cells, which are relatively sensitive to lapatinib, were transfected with constitutively active Akt double mutant T308D/S473D. Alternatively, JIMT-1 cells, which showed relatively low response to single-agent lapatinib, were transfected with dominant-negative kinase-dead Akt mutant K179A. Transfection was confirmed by blotting for p-Akt and total Akt (Figure 3A). In comparison to control transfectants, HCC1419 cells transfected with constitutively active Akt showed significantly reduced sensitivity to lapatinib (p=0.006) (Figure 3B). Sensitivity to the mTOR inhibitor rapamycin was also reduced in the presence of constitutively active Akt, although this effect did not reach statistical significance (p=0.08). In the presence of hyperactive Akt, combination mTOR inhibition plus lapatinib resulted in a significant reduction in cell viability versus either drug alone (p=0.01). These results suggest that combination lapatinib plus rapamycin may be an effective therapeutic strategy in tumors that show elevated PI3K signaling and low response to single-agent lapatinib. In a background of kinase dead Akt, JIMT-1 cells showed a statistically significant increase in lapatinib sensitivity (p=0.03) (Figure 3C). In contrast, sensitivity to rapamycin was not significantly affected. The combination of lapatinib plus rapamycin showed a trend towards reduced cell viability versus single-agent lapatinib, although this did not reach statistical significance (p=0.06). However, JIMT-1 cells did retain sensitivity to this drug combination in the presence of kinase dead Akt. Collectively, these results suggest that Akt activation status affects lapatinib sensitivity. Hyperactive Akt signaling significantly reduced response to lapatinib in HCC1419 cells, whereas kinase dead Akt significantly improved response to lapatinib in JIMT-1 cells. In addition, pharmacologic inhibition of mTOR significantly increased response to lapatinib in HCC1419 cells transfected with constitutively active Akt. Thus, this combination may be effective in HER2-overexpressing breast cancers that show poor response to lapatinib and high baseline Akt activity.
Figure 3. Akt activation status affects lapatinib sensitivity.
(A) HCC1419 cells were transiently transfected with 1 μg pcDNA3 vector control (C) or pcDNA3-Akt-T308D/S473D plasmid (CA), which expresses constitutively active Akt. JIMT-1 cells were transiently transfected with 1 μg pcDNA3 vector control (C) or pcDNA3-Akt-K179A plasmid (KD), which expresses kinase dead Akt. Total protein lysates were collected after 48 h and immunoblotted for phosphorylated S473 Akt and total Akt to confirm p-Akt level after transfection. (B) HCC1419 cells were transiently transfected with 1 μg pcDNA3 vector control (C) or pcDNA3-Akt-T308D/S473D constitutively active Akt plasmid. (C) JIMT-1 cells were transiently transfected with 1 μg pcDNA3 vector control (C) or pcDNA3-Akt-K179A kinase dead Akt plasmid. After 24 h transfection, cells were treated for an additional 72 h with DMSO, 10 μM lapatinib, 100 nM rapamycin, or a combination of 100 nM rapamycin plus 10 μM lapatinib. Viable cells were then counted by trypan blue exclusion. Viability is presented as a percentage of DMSO-treated control vector group, and reflects the average of 3 replicates per treatment group. Error bars represent standard deviation between replicates. P-values were determined by t-test for each treatment group in Akt-transfected cells versus corresponding treatment group in control-transfected cells; p=0.006 for lapatinib-treated constitutively active Akt-transfected HCC1419 cells versus lapatinib-treated vector control; p=0.01 for constitutively active Akt-transfected HCC1419 cells treated with combination lapatinib plus rapamycin versus treated with lapatinib alone; p=0.03 for lapatinib-treated, kinase-dead Akt-transfected JIMT-1 versus JIMT-1 lapatinib-treated vector control; no other statistically significant differences were found between treatment groups. Experiments were repeated at least twice with reproducible results.
Knockdown of 4EBP1 but not p70S6K improves lapatinib sensitivity
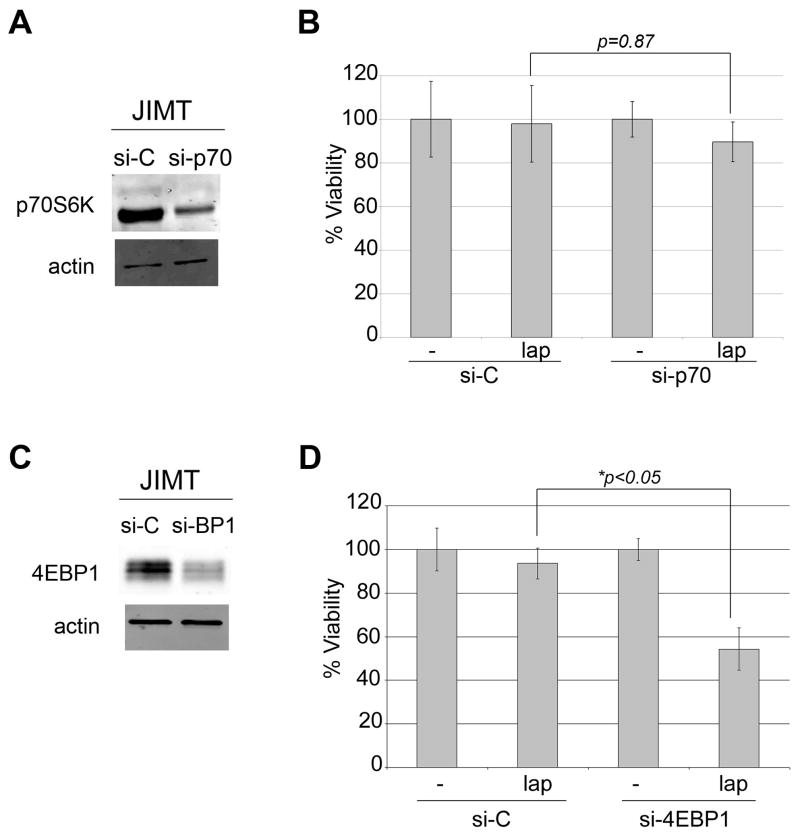
Inhibition of p70S6K phosphorylation correlated with the anti-proliferative activity of lapatinib (Figure 2). In addition, mTOR inhibition by rapamycin increased lapatinib activity in JIMT-1 cells (Figure 3). Thus, we hypothesized that p70S6K inhibition is critical for achieving response to lapatinib. We transfected lapatinib-resistant JIMT-1 cells with p70S6K siRNA, and confirmed knockdown by Western blot (Figure 4A). Surprisingly, knockdown of p70S6K alone was not sufficient to increase the growth inhibitory activity of lapatinib (Figure 4B), suggesting that additional signaling molecules regulated by mTOR must be inhibited in order to achieve optimal response to lapatinib. Knockdown of 4EBP1 confirmed by Western blotting (Figure 4C) significantly increased the sensitivity of JIMT1 cells to lapatinib (Figure 4D). Thus, mTOR inhibition, particularly 4EBP1 inhibition, increases lapatinib-mediated cytotoxicity.
Figure 4. Knockdown of 4EBP1 but not p70S6K improves lapatinib sensitivity.
(A) JIMT-1 cells were transfected with 100 nM control siRNA (si-C) or p70S6K siRNA (si-p70) for 48 h. Total protein lysates were immunoblotted for total p70S6K and actin loading control to confirm knockdown. (B) JIMT-1 cells were transfected with 100 nM control siRNA (si-C) or p70S6K siRNA (si-p70) for 24 h, and then treated with DMSO control or 1 μM lapatinib (lap). After 72 h, viable cells were counted by trypan blue exclusion. Viability is presented as a percentage of DMSO-treated si-C group, and reflects the average of 3 replicates per treatment group. Error bars represent standard deviation between replicates. P-values were determined by t-test for p70 knockdown plus lapatinib versus si-C plus lapatinib. Experiments were repeated three times with reproducible results. (C) JIMT-1 cells were transfected with 100 nM control siRNA (si-C) or 4EBP1 siRNA (si-BP1) for 48 h. Total protein lysates were immunoblotted for total 4EBP1 and actin loading control to confirm knockdown. (D) JIMT-1 cells were transfected with 100 nM control siRNA (si-C) or 4EBP1 siRNA (si-4EBP1) for 24 h, and then treated with DMSO control or 1 μM lapatinib (lap). After 72 h, viable cells were counted by trypan blue exclusion. Viability is presented as a percentage of DMSO-treated si-C group, and reflects the average of 3 replicates per treatment group. Error bars represent standard deviation between replicates. P-values were determined by t-test for 4EBP1 knockdown plus lapatinib versus si-C plus lapatinib. Experiments were repeated three times with reproducible results.
Rapamycin increases lapatinib sensitivity in HER2-overexpressing breast cancer cells that have primary trastuzumab resistance
Pharmacologic mTOR inhibitors appear to improve response to trastuzumab [16,18]. Our data indicates that pharmacologic inhibition of mTOR may also improve response to lapatinib in cells with primary trastuzumab resistance. We performed drug combination analysis to determine whether true synergy is achieved by addition of rapamycin to lapatinib. Treatment of JIMT-1 and MDA361 cells with rapamycin plus lapatinib resulted in significantly reduced proliferation versus either drug alone (Figure 5A). Using the method of Chou and Talalay [17] (CalcuSyn; Biosoft), we analyzed data to determine if these drugs act synergistically (Table 1). Drug combination index (C.I.) values less than 1.0 were achieved in JIMT-1 cells, indicating strong pharmacologic synergy. Higher C.I. values were measured in MDA361 cells, suggesting lower drug synergy versus what was observed in JIMT-1 cells, although increased benefit was still observed with the combination versus single agent treatments.
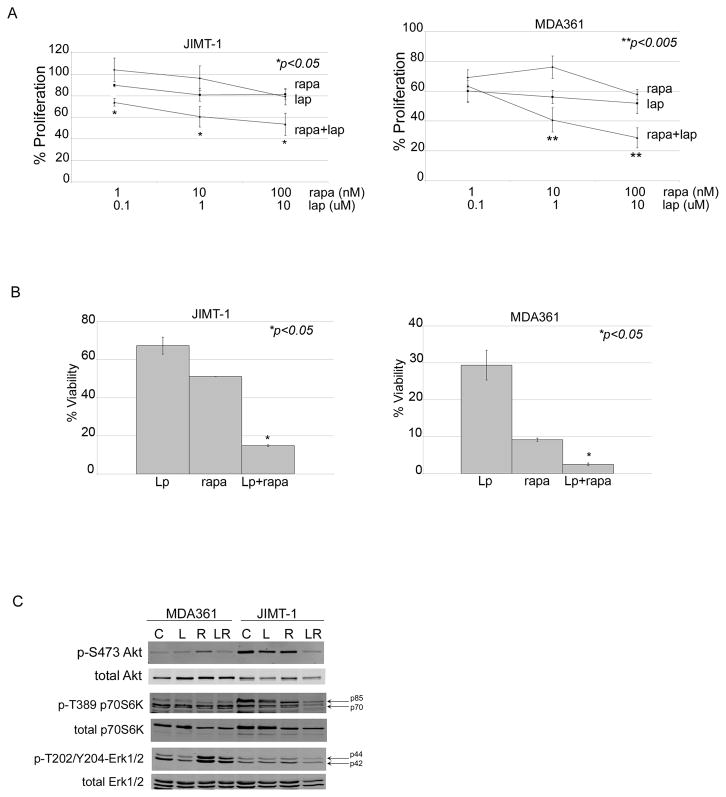
Figure 5. Rapamycin increases lapatinib sensitivity of HER2-overexpressing breast cancer cells with primary trastuzumab resistance.
(A) JIMT-1 and MDA361 cells were treated with rapamycin alone, lapatinib alone, or combination rapamycin plus lapatinib at indicated doses for 6 days. Proliferation was then measured by MTS assay. Values represent the average of 6 replicates per group as a percentage of DMSO-treated cells per treatment group. Error bars represent standard deviation between replicates. P-values were determined by t-test for each combination versus corresponding dose of lapatinib; *p<0.05, **p<0.005. Experiments were repeated twice with reproducible results. (B) JIMT-1 and MDA361 cells were plated in matrigel and treated with DMSO, 10 nM rapamycin (rapa), 1 μM lapatinib (Lp), or a combination of 10 nM rapamycin plus 1 μM lapatinib. Media plus drugs were changed every 3 days for approximately 3–4 weeks. Matrigel was dissolved with dispase, and viable cells were counted by trypan blue. Viability is shown as a percentage of the DMSO control group, and reflects an average of 3 replicates per treatment group. Error bars represent standard deviation between replicates. P-value was determined by t-test for combination treatment versus lapatinib alone; *p<0.05, **p<0.005. (C) MDA361 and JIMT-1 cells were treated with DMSO (C), 10 μM lapatinib (L), 100 nM rapamycin (R), or a combination of 100 nM rapamycin plus 10 μM lapatinib (LR) for 48 h, lysed for total protein, and immunoblotted for p-S473 Akt, total Akt, p-T389 p70S6K, total p70S6K, p-T202/Y204 Erk1/2, or total Erk1/2.
Table 1.
Combination Index (C.I.) values for lapatinib + rapamycin (1:10)
| Cell line | ED50 | ED75 | ED90 | Dm | m | r |
|---|---|---|---|---|---|---|
| MDA361 | 0.03638 | 1849.48693 | 9.6203e+007 | 0.63550 | −0.36817 | 0.97157 |
| JIMT | 0.20320 | 0.00555 | 0.00569 | 14.51422 | −0.20104 | 0.98597 |
Cells were treated with lapatinib (0.1, 1, or 10 μM), rapamycin (1, 10, or 100 nM), or combination lapatinib plus rapamycin. After 6 days, proliferation was measured by MTS assay. The fraction of cells proliferating relative to DMSO control-treated cells was determined, and C.I. values were determined for the combination using CalcuSyn software. C.I. values are listed for effective doses at which 50%, 75%, or 90% (ED50, ED75, and ED90, respectively) of cells were killed. Statistically, drug synergy is defined by C.I. values less than 1.0, and very strong synergy is defined by C.I. values less than 0.1. Dm, the median-effect (ED50) drug concentration; m < 1 indicates a negative sigmoidal shape to the dose-effect curve; r states the linear correlation coefficient.
Next, anchorage-independent (AI) colony growth of JIMT-1 and MDA361 cells was examined. Drug combination inhibited AI growth more significantly than either drug alone (Figure 5B). In addition, a stronger response to single-agent lapatinib was observed under anchorage-independent conditions, which may be due to differences in 3-dimensional cultures versus adherent cultures or may be due to longer-term treatment (3–4 weeks) versus short-term treatment (6 days) in MTS assays. Western blot analysis showed that combination drug treatment inhibited Akt S473 phosphorylation in JIMT-1, whereas neither drug was able to do so when given as a single agent (Figure 5C). In MDA361 cells, the combination inhibited Akt phosphorylation to a similar degree as individual lapatinib. Treatment with single-agent rapamycin resulted in feedback signaling, as shown by increased phosphorylation of Akt and Erk1/2 in MDA361 cells. Activation of PI3K and MAPK signaling in response to rapamycin has been previously reported [19–21], and is thought to be a mechanism driving resistance to pharmacologic mTOR inhibition. Importantly, co-treatment with lapatinib was able to overcome rapamycin-induced feedback signaling, as we previously observed [21]. Phosphorylation of the mTOR substrate p70S6K remained largely unaffected by the combination of rapamycin and lapatinib in MDA361 cells. However the combination achieved increased inhibition of p-p70S6K in JIMT-1 cells versus single agents. Thus, our data indicate that mTOR inhibition increases lapatinib sensitivity in association with reduced Akt and possibly reduced p70S6K phosphorylation.
Pharmacologic mTOR inhibitor MK-8669 increases lapatinib sensitivity of HER2-overexpressing breast cancer cells
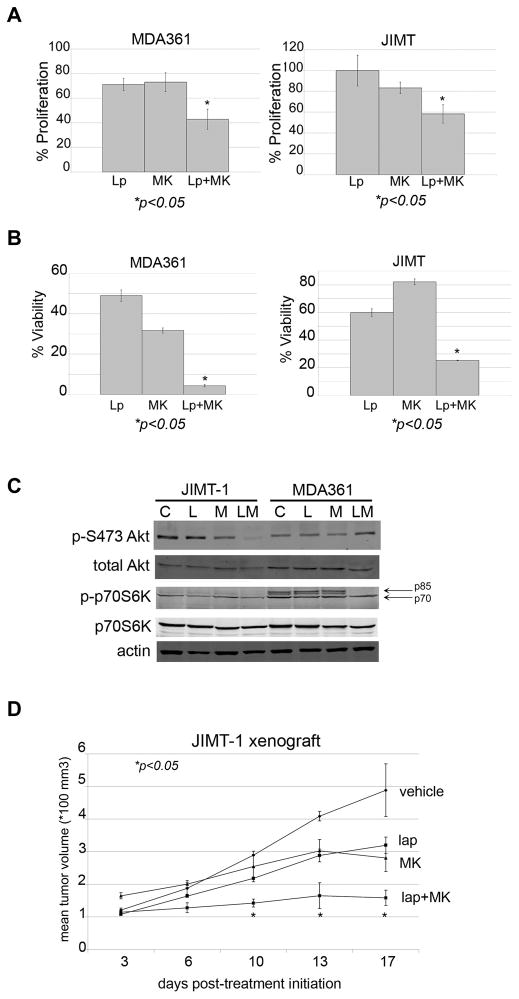
The novel rapamycin analogue, MK-8669 (AP23573, ridaforolimus; provided by Merck), has shown promising tumor inhibitory effects in early phase clinical trials [22,23]. We combined MK-8669 with lapatinib in MDA361 and JIMT-1 HER2-overexpressing breast cancer cells. The combination achieved statistically significant improvements in inhibition of proliferation versus either drug alone (Figure 6A). Drug combination index values (Table 2) showed synergy between lapatinib and MK-8669 in both MDA361 and JIMT-1 cells. Furthermore, combined lapatinib plus MK-8669 dramatically reduced anchorage-independent growth of MDA361 and JIMT-1 cells (Figure 6B). Similar to what was observed with combination lapatinib plus rapamycin, the combination of MK-8669 and lapatinib inhibited phosphorylation of Akt in JIMT-1 cells without effect on p70S6K phosphorylation (Figure 6C). In contrast, although no change in p-Akt level was detected in MDA361 cells, treatment with the drug combination reduced phosphorylation of the p70 isoform p85 S6K with slight inhibition of p70 phosphorylation. Thus, synergistic growth inhibitory effects of combination MK-8669 plus lapatinib were associated with inhibition of PI3K-Akt-mTOR signaling. Finally, we determined if the combination of MK-8669 and lapatinib was synergistic in vivo by treating xenografts of primary trastuzumab-resistant JIMT-1 cells with each drug alone or with the drug combination (Figure 6D). Treatment with single-agent lapatinib or MK-8669 resulted in reduced tumor growth versus control. When administered in combination, however, lapatinib plus MK-8669 achieved statistically significant inhibition of tumor growth versus either drug alone. Thus, our data support the clinical evaluation of lapatinib in combination with pharmacologic mTOR inhibitors as a potential strategy for inhibiting growth of HER2-overexpressing breast cancers that show resistance to trastuzumab and poor response to single agent lapatinib.
Figure 6. Efficacy of combination MK-8669 plus lapatinib in primary trastuzumab-resistant HER2-overexpressing breast cancer cells.
(A) MDA361 and JIMT-1 cells were treated with 1 μM lapatinib (Lp), 10 nM MK-8669 (MK), or a combination of 1 μM lapatinib plus 10 nM MK-8669 for 6 days. Proliferation was then measured by MTS assay. Values represent the average of 6 replicates per group as a percentage of DMSO-treated cells. Error bars represent standard deviation between replicates. P-values were determined by t-test for combination treatments versus lapatinib alone for each cell line; *p<0.05. Experiments were repeated twice with reproducible results. (B) MDA361 and JIMT-1 cells were plated in matrigel and treated with 10 nM MK-8669, 1 μM lapatinib, or a combination of 10 nM MK-8669 plus 1 μM lapatinib. Media plus drugs were changed every 3 days for approximately 3–4 weeks. Matrigel was dissolved with dispase, and viable cells were counted by trypan blue. Viability is presented as a percentage of DMSO control group, and reflects an average of 3 replicates per treatment group. Error bars represent the standard deviation between replicates. P-value was determined by t-test for combination treatment versus lapatinib alone; *p<0.05. (C) JIMT-1 and MDA361 cells were treated with DMSO (C), 10 μM lapatinib (L), 100 nM MK-8669 (M), or a combination of 10 μM lapatinib plus 100 nM MK-8669 (LM) for 48 h, lysed for total protein, and immunoblotted for p-S473 Akt, total Akt, p-T389 p70S6K, total p70S6K, or actin loading control. (D) JIMT-1 cells were injected s.c. in the flank of athymic mice. After palpable tumors formed, tumors were treated daily (5 days on, 2 days off) with vehicle control (n=2), 75 mg/kg oral lapatinib (n=2), 1 mg/kg i.p. MK-8669 (n=2), or combination lapatinib plus MK-8669 (n=3). Mean tumor volume (x 100 mm3) is shown per treatment group, with error bars representing the standard deviation between replicates. P-value was determined by t-test for combination treatment versus lapatinib alone for each day that measurements were taken; *p<0.05.
Table 2.
Combination Index (C.I.) values for lapatinib + MK-8669 (1:10)
| Cell line | ED50 | ED75 | ED90 | Dm | m | r |
|---|---|---|---|---|---|---|
| MDA361 | 1581.46933 | 3.8312e-012 | 0.04294 | 0.01883 | −0.043822 | 0.99312 |
| JIMT | 0.01445 | 0.00286 | 0.00128 | 7.70049 | −0.29234 | 0.95796 |
Cells were treated with lapatinib (0.1, 1, or 10 μM), MK-8669 (1, 10, or 100 nM), or combination lapatinib plus MK-8669. After 6 days, proliferation was measured by MTS assay. The fraction of cells proliferating relative to solvent control-treated cells was determined, and C.I. values were determined for the combination using CalcuSyn software. C.I. values are listed for effective doses at which 50%, 75%, or 90% (ED50, ED75, and ED90, respectively) of cells were killed. Statistically, drug synergy is defined by C.I. values less than 1.0, and very strong synergy is defined by C.I. values less than 0.1. Dm, the median-effect (ED50) drug concentration; m < 1 indicates a negative sigmoidal shape to the dose-effect curve; r states the linear correlation coefficient.
DISCUSSION
Our data indicated that inhibition of Akt is essential in order to achieve optimal response to lapatinib, with hyperactivation of Akt abrogating lapatinib sensitivity. Co-treatment with an mTOR inhibitor significantly improved response to lapatinib, as measured by reduced proliferation, anchorage-independent growth, and in vivo tumor growth. Although many HER2-overexpressing breast cancers are initially sensitive to trastuzumab, many recur and all metastatic cancers eventually develop resistance. Lapatinib has been approved for use in trastuzumab-refractory breast cancers, although response rates to single agent lapatinib are low. Our findings suggest that pharmacologic inhibition of mTOR should be tested in combination with lapatinib in HER2-overexpressing breast cancers exhibiting resistance to trastuzumab. Further, our results suggest that p-Akt levels may be measured as a marker of response in patients whose cancers are treated with lapatinib.
Resistance to trastuzumab has been strongly associated with increased PI3K signaling [10–12]; however, conflicting data exists regarding the relationship between resistance to lapatinib and Akt activity. Eichhorn et al. [13] showed that PTEN loss or dominant activating PIK3CA mutations (E545K and H1047R) reduced lapatinib sensitivity in vitro and in vivo. In contrast, data presented by O’Brien et al. [14] suggested that there is not any association between increased PI3K signaling and response to lapatinib. Our data indicated that HCC1954, JIMT-1, MDA361, and MDA453 cells, all of which possess activating PIK3CA mutations and primary resistance to trastuzumab, also exhibit reduced sensitivity to lapatinib. In contrast, HCC1419 cells, which express wild-type PIK3CA, showed higher response to lapatinib. Thus, hyperactive PI3K signaling due to activating mutations in PIK3CA appeared to reduce lapatinib sensitivity (although cells were not fully resistant). These data are consistent with that of O’Brien et al. [14], in that cells with activating PIK3CA mutations are not completely resistant to lapatinib, although our results indicate that these cells do show reduced sensitivity to lapatinib and resistance to trastuzumab. Reduced sensitivity to lapatinib was associated with an inability to block phosphorylation of Akt and p70S6K. In addition, overexpression of constitutively active Akt reduced response to lapatinib, while kinase-dead Akt improved sensitivity to lapatinib. Thus, our data support a direct association between Akt activity, the ability to inhibit p-Akt levels, and sensitivity to lapatinib. In contrast, although inhibition of p70S6K phosphorylation correlated with lapatinib sensitivity, knockdown of p70S6K alone was not sufficient to improve response to lapatinib. P70S6K is one downstream target of mTOR. Since inhibition of mTOR was sufficient to improve lapatinib sensitivity, our data suggest that other downstream effectors of mTOR must be inhibited in order to achieve optimal response to lapatinib. Indeed, knockdown of the mTOR substrate 4EBP1 resulted in a statistically significant improvement in the response to lapatinib. Thus, mTOR inhibition, and in particular, reduced expression and function of 4EBP1, is likely to achieve optimal response to lapatinib.
PI3K/Akt signaling controls expression of multiple cell cycle and apoptotic regulators. Sensitivity to lapatinib has been associated with modified expression of many of these proteins, consistent with the concept that PI3K inhibition is required for optimal response to lapatinib. Reduced expression of FOX03a transcription factor downstream of PI3K inhibition has been reported in lapatinib-sensitive cells, leading to increased p27 and estrogen receptor transcription [24–26]. Inhibition of PI3K-survivin and MEK-Erk-Bim signaling has also been associated with lapatinib-mediated apoptosis [27,28]. In addition to inhibiting kinase activities of EGFR and HER2, lapatinib has been shown to block nuclear translocation of these receptors [29]. Nuclear HER2 acts as a transcription factor, inducing expression of cell cycle regulators such as thymidylate synthase, which is required for DNA synthesis. Lapatinib suppresses DNA replication in part by blocking TS transcription due to inhibition of HER2 translocation to the nucleus [29]. Thus, inhibition of multiple PI3K-dependent cell cycle and apoptotic pathways appears to be required for lapatinib sensitivity. In addition, PI3K-independent mechanisms such as inhibition of HER2 nuclear localization may contribute to achieving complete response to lapatinib.
Survival outcomes for patients with HER2-overexpressing breast cancer have dramatically improved with the introduction of trastuzumab. Despite its efficacy, however, a subset of tumors show primary resistance and many may develop acquired resistance. Additional HER2-targeted agents, such as the dual EGFR/HER2 kinase inhibitor lapatinib, have shown promise clinically. However, many trastuzumab-pretreated cancers fail to respond to lapatinib therapy, and all eventually develop resistance. While these cancers are initially addicted to HER2 signaling, it remains possible that cancers that fail trastuzumab and/or lapatinib therapy have become addicted to additional signaling pathways, such as the PI3K/Akt-mTOR pathway, consistent with the concept of “oncogenic switch” [30,31]. Our current data and recent clinical trials [16,32] support inhibition of mTOR as a potentially effective strategy for treating breast cancers that are resistant to trastuzumab, suggesting some level of dependence of these cancers on this molecular pathway. Single-agent rapamycin has been shown to induce feedback signaling via increased PI3K and/or MAPK signaling [19–21]. However, this compensatory feedback signaling activated by rapamycin appears to be overcome by co-treatment with lapatinib. Collectively, the experiments support further study of combination lapatinib plus mTOR inhibition as a treatment approach in HER2-overexpressing breast cancers that show poor response to trastuzumab or lapatinib.
Akt phosphorylation can be activated by multiple upstream molecular alterations, including HER2 overexpression; thus, PI3K-Akt inhibition has been explored as a strategy for improving outcome of HER2-amplified breast cancers. Preclinical work has shown that PI3K inhibitors improve response to trastuzumab in trastuzumab-resistant breast cancer cells [33,34]. Clinically, PI3K inhibitors were slower to evolve as targeted therapies in breast cancer due to selectivity issues; however, inhibitors of downstream mTOR have shown great promise in the context of refractory HER2-overexpressing breast cancer. Phase II trial of the mTOR inhibitor ridaforolimus plus trastuzumab in patients with HER2-positive trastuzumab-refractory metastatic breast cancer showed early evidence of anti-cancer activity with 2 partial responses reported out of 22 patients enrolled [23]. Our results support additional trial of ridaforolimus plus lapatinib in trastuzumab-refractory disease. Phase 1 and 2 trials combining the mTOR inhibitor, everolimus, with trastuzumab with or without chemotherapy in patients with trastuzumab-resistant HER2-positive metastatic breast cancer showed encouraging results [16,32,36]. A retrospective analysis of two phase 1 trials was performed to determine the safety and efficacy of everolimus in combination with trastuzumab-based chemotherapy in patients who had received prior treatment with both trastuzumab and lapatinib [36,37]. The overall response rate (ORR) was higher in patients who had not received lapatinib (31%), compared to those who had received lapatinib (18%). Time to progression (TTP) was shorter in patients who had received lapatinib (29 weeks) compared to those who did not receive lapatinib (41 weeks). These findings may be a reflection of heavier pretreatment in the lapatinib group. Despite the reduced ORR and TTP, the clinical benefit was approximately equal in both groups: 89% in the lapatinib pre-treated group and 84% in the lapatinib-free group. These results are important, as they suggest that patients who have progressed on both trastuzumab and lapatinib in the metastatic setting may derive clinical benefit from combination trastuzumab plus mTOR inhibitor. These results are being confirmed in ongoing phase 3 trials in first-line and trastuzumab-resistant settings. In summary, our results strongly support future clinical trials of combination lapatinib plus mTOR inhibitor in the context of metastatic breast cancers that show resistance to trastuzumab and poor sensitivity to single-agent lapatinib. Given the results presented here and the encouraging results of recent trials [37,38], future studies should also examine combination treatments of trastuzumab, lapatinib, and mTOR inhibitor as first-line therapy in HER2-overexpressing breast cancers that are resistant to available HER2-targeted agents.
CONCLUSION
In conclusion, we show that inhibition of Akt and p70S6K phosphorylation correlates with anti-proliferative activity of lapatinib. Further, kinase dead Akt, 4EBP1 knockdown, and mTOR inhibition increased lapatinib sensitivity, although p70S6K knockdown alone did not improve response to lapatinib (Figure 7). Our data suggest that PI3K/mTOR inhibition is critical for achieving optimal response to lapatinib. Collectively, these experiments support further study of combination lapatinib plus mTOR inhibition as a treatment approach in HER2-overexpressing breast cancers that show poor response to trastuzumab and lapatinib.
Figure 7. Proposed model and summary.
Lapatinib, a dual EGFR/HER2 kinase inhibitor, inhibits cell proliferation and survival in part by blocking PI3K/Akt/mTOR signaling. Based on our data, we propose that lapatinib is unable to block proliferation when PI3K/mTOR is not inhibited. Genetic or pharmacologic strategies that improved sensitivity to lapatinib (marked with an asterisk) included expression of dominant negative kinase-dead Akt, knockdown of 4EBP1, and mTOR inhibition by rapamycin or MK-8669. In contrast, knockdown of p70S6K alone did not increase the anti-proliferative activity of lapatinib.
Acknowledgments
We are grateful to Merck and Co., Inc. for providing MK-8669 for research use. We thank Tuba Ozbay, Halima Garba, and Rami Yacoub for technical assistance. S.S.G. thankfully acknowledges funding from The NIEHS T32 Toxicology Training Grant through the Emory University MSP program. R.N. gratefully acknowledges funding from the National Cancer Institute (K01CA118174 and 3K01CA118174-5S1) and the Georgia Cancer Coalition (Distinguished Cancer Scholar Award).
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 4.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, Rosen PP, Twaddell T, Henderson IC, Norton L. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14(3):737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 5.Esteva FJ, Valero V, Booser D, Guerra LT, Murray JL, Pusztai L, Cristofanilli M, Arun B, Esmaeli B, Fritsche HA, Sneige N, Smith TL, Hortobagyi GN. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(7):1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 7.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 8.Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, Baselga J, O’Shaughnessy J. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 9.Toi M, Iwata H, Fujiwara Y, Ito Y, Nakamura S, Tokuda Y, Taguchi T, Rai Y, Aogi K, Arai T, Watanabe J, Wakamatsu T, Katsura K, Ellis CE, Gagnon RC, Allen KE, Sasaki Y, Takashima S. Lapatinib monotherapy in patients with relapsed, advanced, or metastatic breast cancer: efficacy, safety, and biomarker results from Japanese patients phase II studies. Br J Cancer. 2009;101(10):1676–1682. doi: 10.1038/sj.bjc.6605343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, Hortobagyi GN, Hung MC, Yu D. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6(2):117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, Beijersbergen RL, Mills GB, van de Vijver MJ, Bernards R. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12(4):395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Esteva FJ, Guo H, Zhang S, Santa-Maria C, Stone S, Lanchbury JS, Sahin AA, Hortobagyi GN, Yu D. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177(4):1647–1656. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eichhorn PJ, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, Beijersbergen RL, Valero V, Seoane J, Bernards R, Baselga J. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68(22):9221–9230. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien NA, Browne BC, Chow L, Wang Y, Ginther C, Arboleda J, Duffy MJ, Crown J, O’Donovan N, Slamon DJ. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010;9(6):1489–1502. doi: 10.1158/1535-7163.MCT-09-1171. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Zhang Q, Zhang J, Sun S, Guo H, Jia Z, Wang B, Shao Z, Wang Z, Hu X. PI3K pathway activation results in low efficacy of both trastuzumab lapatinib. BMC Cancer. 2011;11:248–258. doi: 10.1186/1471-2407-11-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andre F, Campone M, O’Regan R, Manlius C, Massacesi C, Sahmoud T, Mukhopadhyay P, Soria JC, Naughton M, Hurvitz SA. Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol. 2010;28(34):5110–5115. doi: 10.1200/JCO.2009.27.8549. [DOI] [PubMed] [Google Scholar]
- 17.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 18.Miller TW, Forbes JT, Shah C, Wyatt SK, Manning HC, Olivares MG, Sanchez V, Dugger TC, de Matos Granja N, Narasanna A, Cook RS, Kennedy JP, Lindsley CW, Arteaga CL. Inhibition of mammalian target of rapamycin is required for optimal antitumor effect of HER2 inhibitors against HER2-overexpressing cancer cells. Clin Cancer Res. 2009;15(23):7266–7276. doi: 10.1158/1078-0432.CCR-09-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65(16):7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Hawk N, Yue P, Kauh J, Ramalingam SS, Fu H, Khuri FR, Sun SY. Overcoming mTOR inhibition-induced paradoxical activation of survival signaling pathways enhances mTOR inhibitors’ anticancer efficacy. Cancer Biol Ther. 2008;7(12):1952–1958. doi: 10.4161/cbt.7.12.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu T, Yacoub R, Taliaferro-Smith LD, Sun SY, Graham TR, Dolan R, Lobo C, Tighiouart M, Yang L, Adams A, O’Regan RM. Combinatorial effects of lapatinib and rapamycin in triple-negative breast cancer cells. Mol Cancer Ther. 2011;10(8):1460–1469. doi: 10.1158/1535-7163.MCT-10-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perotti A, Locatelli A, Sessa C, Hess D, Vigano L, Capri G, Maur M, Cerny T, Cresta S, Rojo F, Albanell J, Marsoni S, Corradino I, Berk L, Rivera VM, Haluska F, Gianni L. Phase IB study of the mTOR inhibitor ridaforolimus with capecitabine. J Clin Oncol. 2010;28(30):4554–4561. doi: 10.1200/JCO.2009.27.5867. [DOI] [PubMed] [Google Scholar]
- 23.Yardley D, Seiler M, Ray-Coquard I, Melichar B, Hart L, Dieras V, Barve M, Melnyk A, Dorer D, Turner C, Dodion P. Ridaforolimus (AP23573; MK-8669) in Combination with Trastuzumab for Patients with HER2-Positive Trastuzumab-Refractory Metastatic Breast Cancer: A Multicenter Phase 2 Clinical Trial. Cancer Res; Proceedings of the 32nd Annual CTRC-AACR San Antonio Breast Cancer Symposium; San Antonio, TX. December 10–13 2009; p. 3091. [Google Scholar]
- 24.Hegde PS, Rusnak D, Bertiaux M, Alligood K, Strum J, Gagnon R, Gilmer TM. Delineation of molecular mechanisms of sensitivity to lapatinib in breast cancer cell lines using global gene expression profiles. Mol Cancer Ther. 2007;6(5):1629–1640. doi: 10.1158/1535-7163.MCT-05-0399. [DOI] [PubMed] [Google Scholar]
- 25.D’Alessio A, De Luca A, Maiello MR, Lamura L, Rachiglio AM, Napolitano M, Gallo M, Normanno N. Effects of the combined blockade of EGFR and ErbB-2 on signal transduction and regulation of cell cycle regulatory proteins in breast cancer cells. Breast Cancer Res Treat. 2010;123(2):387–396. doi: 10.1007/s10549-009-0649-x. [DOI] [PubMed] [Google Scholar]
- 26.Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, Paulazzo G, Lyass L, Trusk P, Hill J, Harris J, Spector NL. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A. 2006;103(20):7795–7800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia W, Bisi J, Strum J, Liu L, Carrick K, Graham KM, Treece AL, Hardwicke MA, Dush M, Liao Q, Westlund RE, Zhao S, Bacus S, Spector NL. Regulation of survivin by ErbB2 signaling: therapeutic implications for ErbB2-overexpressing breast cancers. Cancer Res. 2006;66(3):1640–1647. doi: 10.1158/0008-5472.CAN-05-2000. [DOI] [PubMed] [Google Scholar]
- 28.Tanizaki J, Okamoto I, Fumita S, Okamoto W, Nishio K, Nakagawa K. Roles of BIM induction and survivin downregulation in lapatinib-induced apoptosis in breast cancer cells with HER2 amplification. Oncogene. 2011;30(39):4097–4106. doi: 10.1038/onc.2011.111. [DOI] [PubMed] [Google Scholar]
- 29.Kim HP, Yoon YK, Kim JW, Han SW, Hur HS, Park J, Lee JH, Oh DY, Im SA, Bang YJ, Kim TY. Lapatinib, a dual EGFR and HER2 tyrosine kinase inhibitor, downregulates thymidylate synthase by inhibiting the nuclear translocation of EGFR and HER2. PLoS One. 2009;4(6):e5933. doi: 10.1371/journal.pone.0005933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, Stegh AH, Bradner JE, Ligon KL, Brennan C, Chin L, DePinho RA. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318(5848):287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 31.Valabrega G, Capellero S, Cavalloni G, Zaccarello G, Petrelli A, Migliardi G, Milani A, Peraldo-Neia C, Gammaitoni L, Sapino A, Pecchioni C, Moggio A, Giordano S, Aglietta M, Montemurro F. HER2-positive breast cancer cells resistant to trastuzumab and lapatinib lose reliance upon HER2 and are sensitive to the multitargeted kinase inhibitor sorafenib. Breast Cancer Res Treat. 2011;130(1):29–40. doi: 10.1007/s10549-010-1281-5. [DOI] [PubMed] [Google Scholar]
- 32.Morrow PK, Wulf GM, Ensor J, Booser DJ, Moore JA, Flores PR, Xiong Y, Zhang S, Krop IE, Winer EP, Kindelberger DW, Coviello J, Sahin AA, Nunez R, Hortobagyi GN, Yu D, Esteva FJ. Phase I/II Study of Trastuzumab in Combination With Everolimus (RAD001) in Patients With HER2-Overexpressing Metastatic Breast Cancer Who Progressed on Trastuzumab-Based Therapy. J Clin Oncol. 2011;29(23):3126–3132. doi: 10.1200/JCO.2010.32.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu CH, Wyszomierski SL, Tseng LM, Sun MH, Lan KH, Neal CL, Mills GB, Hortobagyi GN, Esteva FJ, Yu D. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13(19):5883–5888. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- 34.Ozbay T, Durden DL, Liu T, O’Regan RM, Nahta R. In vitro evaluation of pan-PI3-kinase inhibitor SF1126 in trastuzumab-sensitive and trastuzumab-resistant HER2-over-expressing breast cancer cells. Cancer Chemother Pharmacol. 2010;65(4):697–706. doi: 10.1007/s00280-009-1075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nahta R, O’Regan RM. Evolving strategies for overcoming resistance to HER2-directed therapy: targeting the PI3K/Akt/mTOR pathway. Clin Breast Cancer. 2010;10(Suppl 3):S72–S78. doi: 10.3816/CBC.2010.s.015. [DOI] [PubMed] [Google Scholar]
- 36.Jerusalem G, Fasolo A, Dieras V, Cardoso F, Bergh J, Vittori L, Zhang Y, Massacesi C, Sahmoud T, Gianni L. Phase I trial of oral mTOR inhibitor everolimus in combination with trastuzumab and vinorelbine in pre-treated patients with HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat. 2011;125(2):447–455. doi: 10.1007/s10549-010-1260-x. [DOI] [PubMed] [Google Scholar]
- 37.Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, Aura C, De Azambuja E, Gomez H, Dinh P, Fauria K, Van Dooren V, Paoletti P, Goldhirsch A, Chang TW, Lang I, Untch M, Gelber RD, Piccart-Gebhart M on Behalf of the NeoALTTO Study Team. First Results of the NeoALTTO Trial (BIG 01–06/EGF 106903): A Phase III, Randomized, Open Label, Neoadjuvant Study of Lapatinib, Trastuzumab, and and Their Combination Plus Paclitaxel in Women with HER2-Positive Primary Breast Cancer. Cancer Res; Proceedings of the Thirty-Third Annual CTRC-AACR San Antonio Breast Cancer Symposium; San Antonio, TX. December 8–12; 2010. pp. S3–3. [Google Scholar]
- 38.Wu Y, Amonkar MM, Sherrill BH, O’Shaughnessy J, Ellis C, Baselga J, Blackwell KL, Burstein HJ. Impact of lapatinib plus trastuzumab versus single-agent lapatinib on quality of life of patients with trastuzumab-refractory HER2+ metastatic breast cancer. Ann Oncol. 2011 doi: 10.1093/annonc/mdr014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]