Abstract
The TNF superfamily members including Fas ligand and TRAIL have been studied extensively for cancer therapy, including as components of gene therapy. We examined the use of FasL expression to achieve tumor selective replication of an oncolytic poxvirus (vFasL) and explored its biology and therapeutic efficacy for FasR− and FasR+ cancers. Infection of FasR+ normal and MC38 cancer cells by vFasL led to abortive viral replication due to acute apoptosis and subsequently displayed both reduced pathogenicity in non-tumor bearing mice and reduced efficacy in FasR+ tumor-bearing mice. Infection of FasR− B16 cancer cells by vFasL led to efficient viral replication, followed by late induction of FasR and subsequent apoptosis. Treatment with vFasL compared to its parental virus (vJS6) led to increased tumor regression and prolonged survival of mice with FasR− cancer (B16), but not with FasR+ cancer (MC38). The delayed induction of FasR by viral infection in FasR− cells provides for potential increased efficacy beyond the limit of the direct oncolytic effect. FasR induction provides one mechanism for tumor selective replication of oncolytic poxviruses in FasR− cancers with enhanced safety. The overall result is both a safer and more effective oncolytic virus for FasR− cancer.
Keywords: vaccinia virus, oncolytic virus, Fas ligand, Fas receptor, apoptosis
Introduction
Oncolytic virotherapy has been actively investigated as a novel approach for treating cancer.1–3 Poxviruses possess many properties that make them attractive vehicles for tumor directed gene therapy and oncolytic virotherapy.2–4 Investigators have genetically engineered a number of oncolytic vaccinia virus (VACV) that target various features of cancer cells or tumor tissues.5–9 Most importantly, results from a phase I clinical trial suggested that the oncolytic VACV, JX-594, is effective in human patients with hepatocellular carcinomas.10 While VACV is a promising oncolytic agent, enhanced safety and efficacy will improve this effective antitumor agent. Oncolytic viruses armed with genes encoding apoptosis-inducing proteins (such as TRAIL) have also been actively studied for cancer treatment.11–13 Combinatorial strategies have been shown to be highly effective in comparison to monotherapy.14–17 Despite the fact that oncolytic viruses have been tested in cancer patients in a number of clinical trials, improved efficacy and safety are needed for this to be a viable treatment option.
Fas receptor (FasR; Fas; CD95; APO-1, TNFRSF6) and Fas ligand (FasL; CD95L; TNFSF6) belong to the tumor necrosis factor and receptor superfamilies.18 FasR is a 48-kDa, type I transmembrane glycoprotein while FasL is a 40-kDa, type II transmembrane protein. The death factor FasL was identified as the natural trigger of FasR signaling pathway and as an inducer of FasR-dependent activation-induced cell death. Tumor cell expression of FasL has been proposed to aid in immune evasion through a FasR-mediated “tumor counterattack” mechanism,19 but it has also been described as a proinflammatory factor.18 FasR and FasL interactions are important in the control of malignant disease. In several cancer types such as breast cancer, lung cancer and osteosarcoma, FasR loss-of-function tracks with an aggressive disease presentation and decreased patient survival.20–22 A recent study demonstrated that an additional FasR deficiency in ApcMin/+ mice causes a dramatic increase in the number of intestinal tumors.23
FasL exists in both soluble and membrane–bound forms. The two forms of FasL display opposing effects on inflammation and tumor cell survival.24 The soluble form of FasL (26 kDa), a trimer as the bioactive form, is thought to be released from tumor cells after enzymatic cleavage of membrane-bound FasL by matrix metalloproteinases. Its abilityto cross-link the receptor and induce apoptosis of FasR+ cells is reduced relative to membrane-bound FasL. Membrane-bound FasL is constitutively expressed in lungs, testes, anterior chambers of the eyes, activated B cells, T cells and NK cells. When sFasL or activating anti-FasR antibodies are given systemically to mice, it results in a rapid death due to hepatic injury.
FasR-FasL engagement results in apoptosis. As apoptotic cell death is a natural response to cellular stress and a means of shutting down viral replication, VACV encodes numerous anti-apoptotic proteins as a means of self preservation.25 We hypothesized that replication of an oncolytic poxvirus expressing FasL would be aborted by FasR mediated apoptosis in FasR+ cells. This should lead to enhanced attenuation in normal tissues while retaining efficacy in FasR− tumors. The late induction of FasR in FasR− tumors may even lead to an enhanced therapeutic effect. Any apoptosis-induction signaling late in the infection cycle would work synergistically or additively with the oncolytic virus to enhance tumor cell killing. The results presented in this study support our hypothesis and indicate that vFasL may be a novel and highly effective agent to treat FasR negative cancers which are difficult to treat with currently available therapies.
Materials and Methods
Cell lines
CV-1 cells and murine melanoma (B16) and colorectal (MC38) cancer cells have been used in the laboratory.6, 7 Other cell lines, such as human breast cancer MCF7 and melanoma MeWo, and mouse normal hepatocyte AML12 cells were obtained from American Type Culture Collection (Manassas, VA).
Construction of vFasL
In order to generate cDNA encoding murine FasL, total RNA was isolated from splenocytes of a female C57BL/6 mouse using TRIzol reagent according to a protocol provided by the supplier (Invitrogen, Carlsbad, CA). PCR primers were generated from the published murine FasL sequence with flanking SalI or EcoRI sites, respectively. RT-PCR using these primers and total RNA from mouse splenocytes was performed to obtain the cDNA encoding FasL (GenBank accession no. NM_010177). The cDNA was then isolated and digested with SalI and EcoRI and inserted into the transfer plasmid pCB023-II, in which the transgene is driven by vaccinia viral pSE/L promoter. The plasmid pCB023-II-FasL was amplified in E. coli DH5α cells. The inserted DNA was sequenced to confirm its identity.
To construct the new virus (vFasL), a tk-gene deleted WR strain of VACV vJS6 was used as the parental virus.5 vFasL was made in CV-1 cells by homologous recombination of transfected plasmid pCB023-II-FasL and infection of cells with the parental virus vJS6. The caspase inhibitor z-VAD-fmk (20 μM) was included in the media of the transfection mixture and subsequent clone isolation was performed. PCR was then used to confirm the correct identity of viral construct. The virus vFasL was expanded in HeLa cells without the presence of z-VAD-fmk in the growth medium. All viral tiers were determined by plaque assays on CV1 cells.
Western blot analysis
CV1 cells were infected with vJS6 or vFasL at MOI of 5.0 in serum-free growth media. Infected CV1 cells and supernatants were harvested at 24 h post infection and lysed in NuPAGE sample buffer (Invitrogen, Carlsbad, CA). Cell lysates and supernatants were run on NuPAGE gel according to manufacturer’s instructions (45 min at 200 V), and then blotted onto nitrocellulose membrane for 1 h at 30 V. The presence of murine FasL protein in cells after infection with vFasL was confirmed by the Western Breeze immune-detection system performed as per manufacturer’s instructions using an anti-FasL mAb (H11) (Alexis Biochemicals; San Diego, CA).
Flow cytometry
Mouse and human cells were harvested and probed using a PE-conjugated anti-mouse FasR mAb with isotype control (mouse IgG1) or anti-human FasR mAb and isotype control (eBioscience, San Diego, CA). To test for FasR upregulation in virus-infected cells, mouse and human cancer cells were infected with vJS6 at MOI of 0.1 and harvested at various time points and probed with PE-conjugated anti-FasR mAb.
In vitro viral growth
MC38, B16 and AML12 cells were infected with vJS6 or vFasL at MOI of 1.0, and the cells were harvested at 12 h intervals. The cells were homogenized and the quantity of infectious virus was determined by plaque assays on CV1 cells.
The Apo-BrdU TUNEL assay
Cancer cells were infected with vJS6 or vFasL at MOI of 1.0 or 0.1 and cells were then harvested, fixed with 4% paraformaldehyde, and stored at −20°C in 70% ethanol at various time intervals. The Apo-BrdU assay was performed using a kit per manufacturer’s instructions (eBioscience, Inc.), then run on FACS. Results presented are a composite of three repeated experiments.
Mice
Female athymic nude mice of 5–6 weeks old, were obtained from either the NCI animal Facility (Frederick, MD) or Taconic Corporation (Germantown, NY). All animal studies conducted at the two institutions were approvedby the Institutional Animal Care and Use Committees at the two institutions.
Viral pathogenecity and biodistribution of the viruses
For assays of viral pathogenecity, ten nude mice per group were injected i.p. with 1.0 × 108 PFU/mouse of either vFasL or vJS6. The time of death was recorded and plotted on a Kaplan-Meier curve.
The biodistribution of the viruses were determined in tumor-bearing nude mice. Bilateral flank tumors were obtained by injection of 2.0 × 105 MC38 and B16 cancer cells into left and right flanks, respectively. Tumors were allowed to grow to approximately 60–70 mm3 in size (7–14 days). Mice were then injected i.p. with 1.0 × 108 PFU/mouse of vFasL or vJS6. The tumors and normal tissues were harvested under sterile conditions, homogenized, and the infectious virus was quantified by plaque assays on CV1 cells. Titer results were then normalized to tissue protein concentration and recorded as PFU/mg of protein, as described previously.6, 7
Tumor models and treatments
MC38 and B16 tumors were established with 2.0 × 105 cells in the right flank of each nude mouse. When the tumors reached a volume of ~100 mm3, the mice were given i.p. injection of either 1× PBS, vJS6, or vFasL in equal volumes with 1.0 × 107 PFU being administered to each/mouse. The tumor growth was monitored, and tumor sizes measured at regular intervals.
Results
Construction and characterization of new virus vFasL
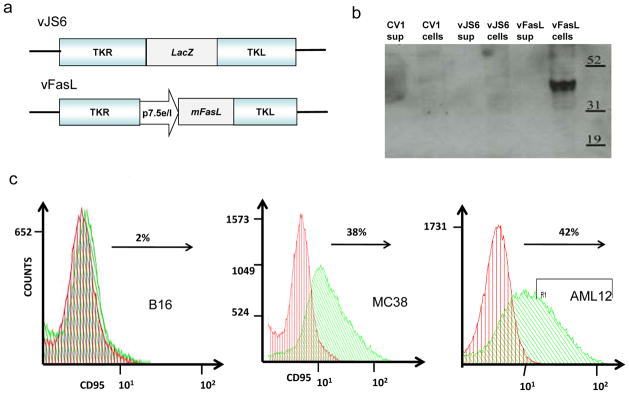
A new recombinant VACV with the murine membranous FasL gene inserted into the TK locus (vFasL) was created, isolated and expanded. The parental tk-deleted virus vJS6 was used as the control (Figure 1A). CV1 cells were infected with vJS6 or vFasL, then supernatants and cells were collected separately and analyzed by Western blot analysis (Figure 1B). A band of approximately 40 kDa, identified with a monoclonal antibody against murine FasL, is associated only with the cellular component of vFasL, but not vJS6-infected CV1 cells, consistent with membrane bound FasL in vFasL- infected cells. Of note, there is no band from the supernatant of the vFasL-infected cells, indicating that the protein sFasL is not secreted to a level detectable by Western blot analysis.
Figure 1. Construction and characterization of new virus vFasL.
(a) Schematic representation of the new vaccinia virus vFasL versus parental virus vJS6. Both are derivatives of WR strain virus with tk gene mutated. (b) Western blots analysis showing a ~40 kDa band in the vFasL-infected CV1 cells and not in the supernatants. (c) FasR (CD95) expression on B16 and MC38 cancer cells, and AML12 normal hepatocytes. Cells were stained with a PE-conjugated anti-mouse Fas antibody and then analyzed by flow cytometry. Green shading corresponds to cells stained the antibody for FasR and red shading corresponds to the isotype control.
In order to explore the utility of vFasL in cancer therapy, we have also examined the status of FasR expression on the cell surface by flow cytometry (Figure 1C). B16 cancer cells express little, if any, FasR on the cell surface, with 2% positive; while MC38 (cancer cells) and AML12 (normal cells) express high levels of FasR, with over 38% and 42% of cells positive respectively. We have also examined the expression of FasR in human breast cancer cells with MCF7 showing 18.3% FasR+ and melanoma MeWo cells which showing only 0.3% FasR+ (data not shown).
vFasL and vJS6 displayed contrasting kinetics of viral replication and apoptosis induction in Fas− versus Fas+ cells
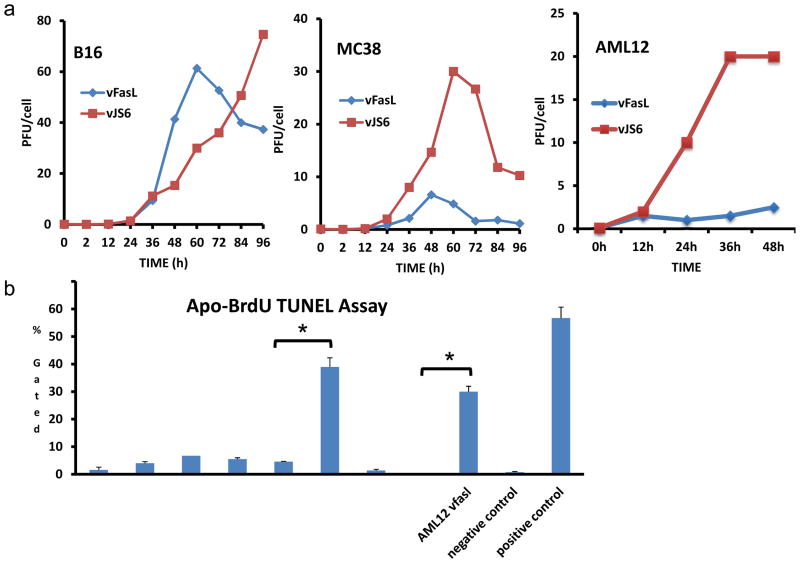
The replication of vJS6 and vFasL in FasR− or FasR+ cells was then examined. B16 (FasR−) melanoma cells, MC38 (FasR+) colon cancer cells and AML12 (FasR+) normal hepatocytes were infected with vJS6 or vFasL at an MOI of 1.0, and harvested at various time points. The titers were determined by plaque assays. The viral growth curves in those cells were then plotted (Figure 2A). In FasR− B16 cells, after a latent period of time (~12 h), vJS6 replicated exponentially up to the endpoint of the assay, while vFasL replicated exponentially at the initial period of time following latency, the titer peaked at 60 h (~42 PFU/cell) and then decreased. In FasR+ MC38 cells, vJS6 replicated exponentially, plateaued at 60 h (~15 PFU/cell) and then declined over time (because cells were mostly dead). In contrast, the replication peak of vFasL was much lower at 6 PFU/cell, and occurred at an earlier time, compared to vJS6. In FasR+ AML12 normal cells, the replication of vFasL was similarly inhibited compared to vJS6 (Figure 2A). We have also examined the viral growth characteristics in cancer cells infected at an MOI of 1, and similar patterns were observed (data not shown). We examined the apoptosis of cells at 24 h after infection by TUNEL assays to confirm the functional exression of mFasL from vFasL(Figure 2B). The results showed that vJS6 virus caused minimal apoptosis in each of the three cell lines at this time point. In contrast, vFasL infection caused 39.6% and 29.8% of apoptosis in FasR+ MC38 and FasR+ AML12 cells respectively, while only 4% apoptosis in FasR− B16 cells.
Figure 2. Viral replication and induction of apoptosis in infected cells as determined by TUNEL assay.
(a) Viral replication curves for vJS6 and vFasL in (FasR−) B16 cancer and (FasR+) MC38 cancer and normal AML12 (FasR+) cells infected at MOI of 0.1. Infected cells were harvested at indicated times and infectious viruses were determined by plaque assays on CV1 cells. Data are presented as mean +/− s.d. for PFU/cell. Time points where p<0.05 is indicated as *. (b) Apoptosis of infected cells was determined by Apo-BrdU TUNEL assays, confirming the functional ability of induction of apoptosis by vFasL at 24 h at MOI of 1.0 (mean+/− s.d., * p<0.01).
Delayed and gradual induction of apoptosis in Fas− cells by vFasL
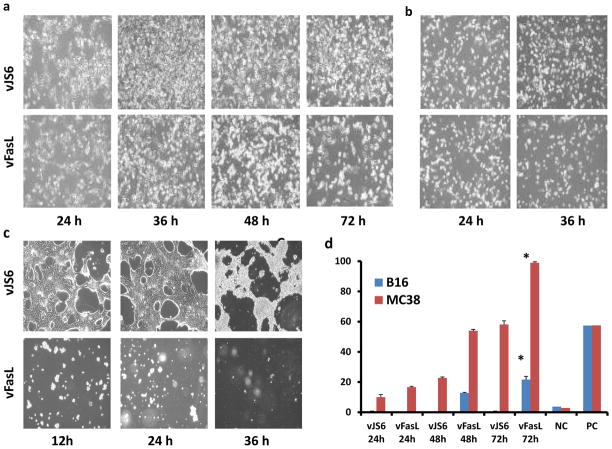
To seek a correlation of apoptosis, viral replication and the status of FasR expression of host cells, we examined the cell morphology and apoptosis of cells infected with viruses at a lower MOI (MOI = 0.1) at multiple times post infection. Cells were first examined by light microscopy for cell death and apoptosis were confirmed by Apo-Brdu TUNEL assays by flow cytometry, and tried to correlate with the yield of viruses in the cells. In FasR− B16 cells, we found little apoptosis in vJS6-infected cells, but significant and gradual increase in apoptosis of vFasL-infected cells at 48 and 72 h post infection (Figure 3A,D). In FasR+ MC38 cancer cells, more cells underwent apoptosis at early time points, with more death in vFasL-infected cells (Figure 3B,D). In FasR+ AML12 normal cells, cell death in vFasL-infected cells was evident as early as 12 h, while vJS6-infected cells showed signs of cell death at 48 h (Figure 3C).
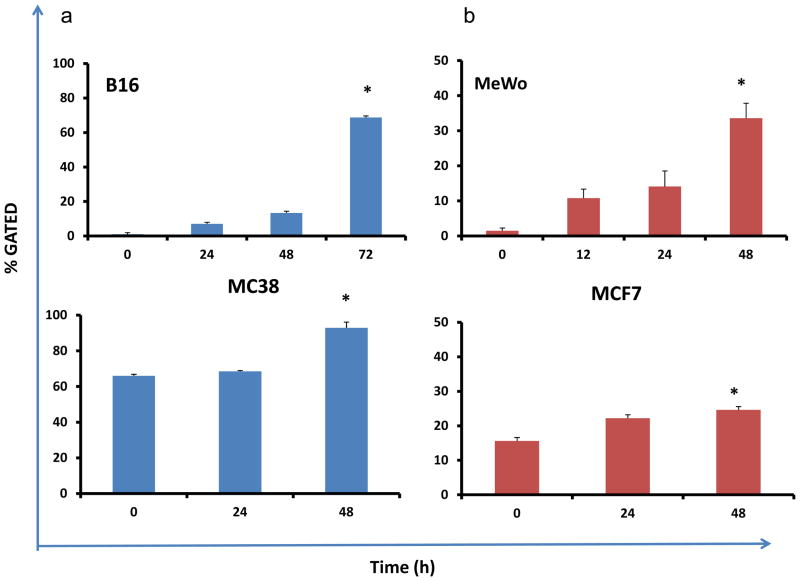
Figure 3. FasR upregulation in both murine and human cancer cells infected with vJS6.
All cancer cells were infected with vJS6 at MOI of 0.1, and cells were harvested at indicated time points and stained with anti-murine Fas or anti-human Fas antibodies and subject them to flow cytometry. (a) Fas expression on B16 (Fas−) and MC38 (Fas+) mouse cancer cells. (b) Fas expression on MeWo (Fas−), and MCF7 (Fas+) human cancer cells. Data presented are mean +/− s.d., *- p<0.001 compared to its baseline (before viral infection).
In summary, a significant degree of apoptosis occurred at later time points in FasR− B16 cells infected with vFasL, but not vJS6. In addition, a progressive degree of apoptosis occurred in FasR+ MC38 cells infected with vFasL over time (Figure 3D). Thus, it was reasonable to postulate that FasR was induced in both B16 and MC38 cells infected by viruses and this signal triggered cellular apoptosis at later time points.
The induction of FasR in cancer cells infected by oncolytic vaccinia virus
In order to test our hypothesis that VACV may induce the expression of FasR in infected cancer cells, we used flow cytometry to examine the amounts of FasR in mock or vaccinia virus-infected cancer cells (Figure 3). Four lines of cancer cells, with low levels (B16 and MeWo) and high levels (MC38 and MCF7) of FasR expression (both human and murine origin), were infected with vJS6 at an MOI of 0.1, and cells were collected and probed with monoclonal antibodies against FasR and subjected to flow cytometry. In FasR− B16 cancer cells, FasR expression increased from undetectable to ~5% positive at 24 h, 11% at 48 h and ~70% at 72 h. In FasR+ MC38 cells, the basal level was ~65%, and we observed the value increased to 95% at 48 h (Figure 3B). We also observed the time-dependent enhanced FasR expression in two human cancer cell lines, MeWo and MCF7. Even though FasR induction was seen in all four cell lines, FasR− cell lines showed a more considerable upregulation from their baseline when compared to the FasR+ cell lines which have higher baselines of expression.
vFasL displayed reduced pathogenicity in nude mice and reduced viral titers in normal tissues
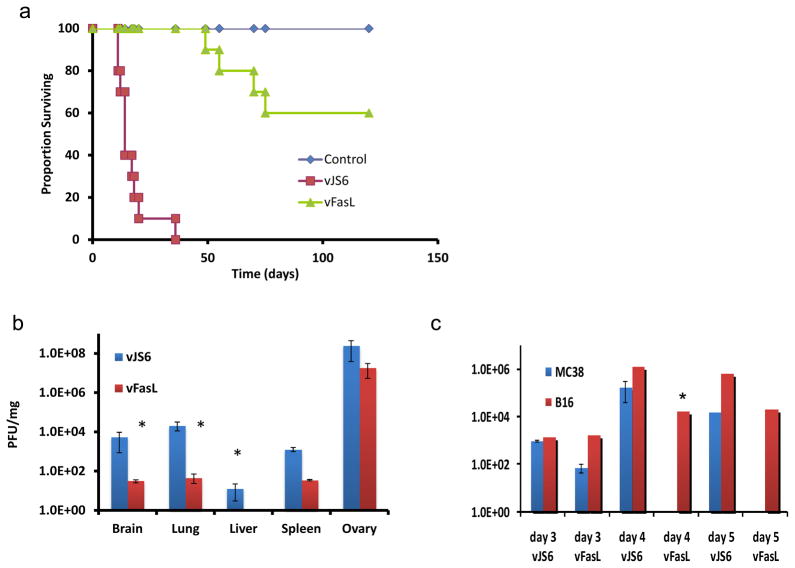
We then examined the pathogenicity of the two viruses in nude mice. Athymic nude mice were injected with either vJS6 or vFasL and followed for survival. As expected, vJS6, the control parental virus, was quite virulent, with mice surviving a median of 17 days. The virus of interest, vFasL, displayed much milder virulence, with median survival not yet reached after 120 days (p < 0.0001) (Figure 5A). The biodistribution of normal tissues in non-tumor bearing nude mice showed reduced viral titers in vFasL treated mice compared to vJS6 (Figure 5B). Brain and lung showed a two log decrease, while liver and spleen showed one log decrease (independent sample t test, p < 0.02, mean+/− SEM). This reduced pathogenicity correlated with abortive viral replication in normal tissues.
Figure 5. Viral pathogenecity in athymic nude mice.
(a) Athymic mice were injected with vJS6 or vFasL intraperitoneally (i.p.) at a dose of 1.0 × 107 pfu/mouse, or PBS (n = 10 each). Median survival of the mice treated with vJS6 was 15 days while that of the mice with vFasL was 120 days (P < 0.00001 by log rank test). (b) Recovery of viruses on day 7 from normal tissues in non-tumor-bearing nude mice. Compared to vJS6, vFasL showed a significant decrease in viral titers from normal tissues at day 7 (p<0.02). (c) Viral titers of vJS6 and vFasL on days 3, 4, 5 in bilateral MC38 and B16 flank tumors. The virus vFasL showed a faster clearance in MC38 cancers even at day 3 compared to vJS6. vFasL persisted in FasR− B16 cancer like vJS6 virus, even though the titers are decreased on day 5.
Biodistribution of the viruses in tumor-bearing mice
We then examined the replication and persistence of the viruses in tumor tissues in mice bearing (FasR−) B16 and (FasR+) MC38 tumors in the right and left flanks. When tumors reached the size of about 5 × 5 mm in diameters, the viruses were injected into mice i.p at the dose of 1.0 × 108 pfu. On days 3, 4 and 5, groups of mice were sacrificed and tumor tissues were collected. Infectious virions were determined by plaque assays on CV1 cells (Figure 5 C). On day 3, we observed similar titers of vJS6 from both tumors, but lower titers of vFasL from (FasR+) MC38 than (FasR−) B16 cancer. This trend was maximized on days 4 and 5, when we did not recover any vFasL virus from MC36 tumors, yet high levels of vFasL from (FasR−) B16 tumors (independent sample t test, mean +/− SEM, p ≤ 0.05). In contrast, we observed high and comparable titers of vJS6 from both B16 and MC38 tumors.
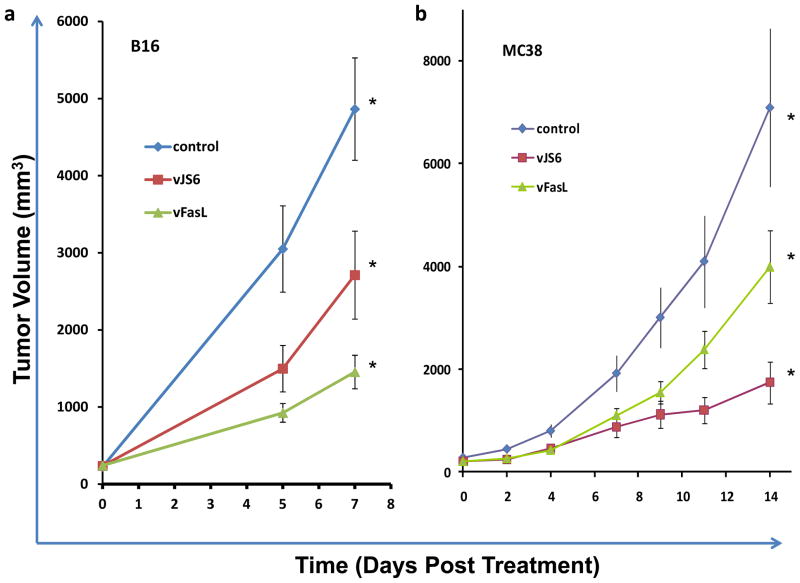
The anti-tumoral effect of vFasL in FasR− and FasR+ tumor models
The efficacy of vFasL versus vJS6 in treating Fas negative and positive tumors were examined in FasR− B16 and FasR+ MC38 tumor models (Figure 6). In the FasR− B16 tumor model, vFasL is more effective than vJS6, (on day 7: 2711 mm3 vs 1453 mm3, ANOVA=0.001, one-tailed, independent sample t test, p=0.04). On the other hand, vFasL is less efficacious than vJS6 in the FasR+ MC38 tumors (on Day 14: 1746 mm3 vs 3999 mm3, ANOVA= 0.011, one-tailed independent sample t test, p=0.046). Paradoxically, vFasL displayed a superior antitumor effect in the FasR negative cancer model, while vJS6 was superior to vFasL in the FasR positive tumor model. This is consistent with our original hypothesis and it correlates with the viral recovery data reported above.
Figure 6. Inhibition of MC38 and B16 tumor growth in nude mice treated with the two viruses.
Subcutaneous MC38 or B16 cancers were established in nude mice. When cancers reached about 5 × 5 mm (~ day 14), mice were treated with PBS saline, vJS6 or vFasL at 1.0 × 107 pfu/mouse (n = 8 to 10). (a) The growth curves of FasR− B16 melanoma. Significant difference in B16 tumor volumes in groups of mice was observed [p = 0.003 by ANOVA; vJS6 versus vFasL: p < 0.05 by one tailed, two samples, equal variance T-test]. (b) The growth curves of FasR+ MC38 colon cancer. Significant difference in tumor volumes in groups of mice was observed [p = 0.011 by ANOVA; vJS6 versus vFasL: p < 0.05 by one tailed, two samples, equal variance T-test].
Discussion
Cancer therapy using genetically engineered and multi-mechanistic VACV is efficacious and safe in both preclinical models and early clinical trials.3, 10 We have previously demonstrated the tumor-selectivity and oncolytic potency of an oncolytic VACV with a mutated viral tk gene 5, 26. In the current study, we set out to further enhance the safety and selectivity of this oncolytic VACV by arming it with the gene encoding the membrane-bound FasL. Our goal was to inhibit replication in normal cells through the induction of apoptosis, while the virus would replicate normally in tumor cells which lack FasR.
Our data demonstrated enhanced safety of the vFasL virus compared to the parent VJS6 virus. The median survival of nude mice treated with 1 × 107 pfu of VACV, improved from 15 days to 120 days with the addition of FasL expression. While all cells infected with the virus ultimately die, the inhibition of viral replication leads to a safer virus. While other factors may contribute to this safety in vivo, including the immune response (even in nude mice), the enhanced safety correlates with decreased viral recovery from normal tissue. This also correlated with the induction of apoptosis and decreased replication in FasR+ cells in vitro.
The induction of apoptosis is a cell’s natural defense to viral infection, and VACV produces many proteins which inhibit different cellular apoptotic pathways for its own protection 25. The intentional induction of apoptosis for the purpose of inhibiting replication and improving safety is a novel strategy. Investigators have utilized gene therapy to produce pro-apoptotic proteins for the purpose of an anti-tumor response, and have tried to selectively avoid the induction of apoptosis in normal tissues. In general, the efficiency of gene transduction has limited the effectiveness of this approach. For efficient, replicating viruses like VACV, the anti-tumor effect of the replicating viral-mediated cellular destruction outweighs the effect of the therapeutic transgene expression, especially if that transgene inhibits viral replication.26 The toxicity to organs and tissues such as the brain, depends not on the initial infection of the virus at the time of injection, but on ongoing viral replication in the tissue. By inhibiting viral replication, normal tissues are spared destruction and safety is enhanced.
In FasR+ tumor cells, the viral delivery of FasL will also lead to apoptosis and tumor cell death. However, our hypothesis suggests that this will paradoxically lead to decreased viral replication and a decreased anti-tumor response. In fact, our data demonstrated this paradox. The FasL expressing virus was statistically inferior to the parent virus in the FasR+ MC-38 tumor model. This effect has not been previously reported on by other investigators examining the expression of FasL. Based on our hypothesis, our expectation for FasR− tumors was that the FasL expressing virus would be equally effective to the parent virus. Our data, however, demonstrated a statistical advantage for FasL expression over the parent virus in the FasR− B16 tumor model. This suggests that FasL is having an anti-tumor effect unrelated to FasR expression and the induction of apoptosis, or that FasR is being induced in the tumor cell over time. If FasR is being induced, the timing of induction must be such that the virus is reaching the end of its effect as an oncolytic virus (due to immune clearance) and the apoptotic effect extends the response. This seems to be the case in our models. The FasR is being induced in FasR− tumors, and this is leading to late apoptosis and an enhanced anti-tumor effect in vivo.
Previous studies have shown that FasR and/or FasL are induced in certain types of host cells following viral infection. This occurs following infection with influenza virus in cultured cells,27 herpes simplex virus in neonatal neutrophils,28 respiratory syncytial virus in respiratory epithelial cells,29 reovirus in the brain,30 simian immunodeficiency virus, human T cell lymphotropic virus type I and human immunodeficiency virus in immune cells,31–33 dengue virus in human endothelial cells 34, and ectromelia virus (a poxvirus) in mouse brain.35 An oncolytic vesicular stomatitis virus induce apoptosis via signaling through PKR, Daxx and FasR, by inducing the movement of Fas death receptor to cell surface.36 Based on these observations, we hypothesized that VACV induces FasR in infected cancer cells, and we found evidence of this in multiple experiments. We found that FasR was upregulated in the B16 cancer cells infected by vJS6 virus as analyzed by flow cytometry. Further experiments showed that FasR was strongly induced in FasR-cancer cells, and further induced in Fas+ cells (such as MC38 cells). One possible mechanism is the stress response of host cells to the viral infection. Activated HSF-1, an early response to stress (including infection by a virus), positively regulates the transcription of the FasR gene with delayed kinetics.37 VACV encodes a number of early genes (such as M2L) to inhibit NF-κB and inflammation.38, 39 However, NF-κB was eventually induced in the late phase of the viral life cycle, which leads to the activation of FasR.40
It is well known that FasL can cause inflammation. FasL exerts its pro-inflammatory effects via neutrophil recruitment because sFasL is a potent neutrophil chemoattractant 41. Expressing FasL in CT26 colon cancer leads to rejection of the implanted tumor due to the inflammatory response.42 FasL can also have a negative impact on the immune response, inducing apoptosis in infiltrating lymphocytes. While beyond the scope of this manuscript, the immune system may have an effect on the in vivo results that are presented. We have been careful to support our hypothesis through in vitro work where the immune system does not play a role, and much of the immunologic effect of FasL is expected to be independent of the FasR status of the tumor cells.
Our results show that vFasL can selectively replicate in FasR− cancer cells, and induce the expression of cellular FasR in the later stage of infection. The potential utility for this FasL expressing VACV will be in FasR− tumors. In breast, lung and certain other types of cancer, low expression of FasR by tumor cells often correlates with poor prognosis.20, 21 In osteosarcoma, FasR also plays a role in metastasis. FasR negative cancer cells are selected during metastasis to the lung, and increased Fas expression reduces the metastatic potential of human osteosarcoma cells.43, 44 Therefore, the targeting of FasR-tumors and the induction of FasR in cancer cells may sensitize these cells to other therapeutic strategies targeting this pathway.
The FasL-FasR signaling may pose severe toxicity. Severe toxicity by FasL in normal tissues hampers its application to cancer therapy. FasL is a type 2 transmembrane protein; upon cleavage by specific proteases, it can form soluble homotrimeric molecules. Soluble FasL is a very weak agonist and can antagonize the function of membrane-associated FasL, which has potent apoptosis-inducing activity.45 FasR is constitutively expressed in cells of the immune system and in normal tissues by a broad panel of epithelial cells.46 As suggested previously by other investigators, FasL expressed from tumor might pose toxicity to lymphonoid organs and in the liver.47 Even though our oncolytic poxvirus expresses a membrane-bound FasL which should pose minimal toxicity, it could be shed from dying tumor cells and diffuse to the circulation and into normal tissue. We observed that mice died rapidly due to acute toxicity when a high titer of vvFasL was administered (data not shown). Minimal toxicity was observed when virus at the dose used in our experiments was used to treat tumor-bearing mice. Our results were consistent with another published report showing that when FasL is expressed from an oncolytic adenovirus selectively in the tumor, it displays minimal toxicity.48
In summary, we have established that the selective induction of apoptosis is a mechanism for tumor selective viral replication and enhanced safety of oncolytic viruses. The Fas pathway is only one of many mechanisms by which this can be accomplished, and the application of this strategy to other oncolytic viruses is certainly possible. The delayed induction of FasR provides the added benefit of a bystander killing beyond the limit of the oncolytic effect. The result is both a safer and more effective oncolytic virus.
Figure 4. Phase contrast pictures and TUNEL assays of cancer and normal cells infected with vFasL undergoing apoptosis.
(a) Phase contrast pictures of FasR− B16 cancer cells infected at MOI of 0.1 with vJS6 or vFasL at 24, 36, 48, 72 h post infection, showing increased apoptosis at 72 h by vFasL. (b) FasR+ MC38 cancer cells infected with vJS6 and vFasL. The apoptosis was more severe in vFasL-infected cells at 36 h. (c) Normal hepatocyte AML12 cells (FasR+) were infected with either vJS6 or vFasL. The pictures show rapid induction of cell death even at 12 h post infection in cells infected by vFasL, but not with vJS6. (d) TUNEL assay by apo-Brdu staining by flow cytometry from the cells in the corresponding phase contrast pictures. In FasR− B16 cancer cells, apoptosis is evident only at later time in cells infected with vFasL. In FasR+ MC38 cancer cells, apoptosis started early and increased over time. Data are presented as mean +/− s.d, *-p<0.01 when compared to 24hr post infection. The controls are, NC: negative control; and PC, positive control.
Acknowledgments
This work was supported in part by the NIH grant R01CA100415, the Intramural Research Program of the NIH, and by the David C. Koch Regional Therapy Cancer Center.
We thank Dr. Ravikumar Muthusamy at the University of Pittsburgh for his technical assistance in flow cytometry and real time PCR, Dr. Shyam Sukumar at the University of Michagan for statistical analyses of data presented in Figure 5B.
Footnotes
Competing Interests
DLB serves as a consult of Jennerex Biotherapeutics, Inc., a company developing oncolytic viruses. The other authors have declared that no competing interests exist.
References
- 1.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 2.Guo ZS, Thorne SH, Bartlett DL. Oncolytic virotherapy: molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. Biochim Biophys Acta. 2008;1785:217–231. doi: 10.1016/j.bbcan.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breitbach CJ, Reid T, Burke J, Bell JC, Kirn DH. Navigating the clinical development landscape for oncolytic viruses and other cancer therapeutics: no shortcuts on the road to approval. Cytokine Growth Factor Rev. 2010;21:85–89. doi: 10.1016/j.cytogfr.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Guo ZS, Bartlett DL. Vaccinia as a vector for gene delivery. Expert Opin Biol Ther. 2004;4:901–917. doi: 10.1517/14712598.4.6.901. [DOI] [PubMed] [Google Scholar]
- 5.Puhlmann M, Brown CK, Gnant M, Huang J, Libutti SK, Alexander HR, et al. Vaccinia as a vector for tumor-directed gene therapy: biodistribution of a thymidine kinase-deleted mutant. Cancer Gene Ther. 2000;7:66–73. doi: 10.1038/sj.cgt.7700075. [DOI] [PubMed] [Google Scholar]
- 6.McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61:8751–8757. [PubMed] [Google Scholar]
- 7.Guo ZS, Naik A, O’Malley ME, Popovic P, Demarco R, Hu Y, et al. The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2. Cancer Res. 2005;65:9991–9998. doi: 10.1158/0008-5472.CAN-05-1630. [DOI] [PubMed] [Google Scholar]
- 8.Kirn DH, Wang Y, Liang W, Contag CH, Thorne SH. Enhancing poxvirus oncolytic effects through increased spread and immune evasion. Cancer Res. 2008;68:2071–2075. doi: 10.1158/0008-5472.CAN-07-6515. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q, Liang C, Yu YA, Chen N, Dandekar T, Szalay AA. The highly attenuated oncolytic recombinant vaccinia virus GLV-1h68: comparative genomic features and the contribution of F14.5L inactivation. Mol Genet Genomics. 2009;282:417–435. doi: 10.1007/s00438-009-0475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu TC, Hwang T, Park BH, Bell J, Kirn DH. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol Ther. 2008;16:1637–1642. doi: 10.1038/mt.2008.143. [DOI] [PubMed] [Google Scholar]
- 11.Chalikonda S, Kivlen MH, O’Malley ME, Eric Dong XD, McCart JA, Gorry MC, et al. Oncolytic virotherapy for ovarian carcinomatosis using a replication-selective vaccinia virus armed with a yeast cytosine deaminase gene. Cancer Gene Ther. 2008;15:115–125. doi: 10.1038/sj.cgt.7701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kottke T, Hall G, Pulido J, Diaz RM, Thompson J, Chong H, et al. Antiangiogenic cancer therapy combined with oncolytic virotherapy leads to regression of established tumors in mice. J Clin Invest. 2010;120:1551–1560. doi: 10.1172/JCI41431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziauddin MF, Guo ZS, O’Malley ME, Austin F, Popovic PJ, Kavanagh MA, et al. TRAIL gene-armed oncolytic poxvirus and oxaliplatin can work synergistically against colorectal cancer. Gene Ther. 2010;17:550–559. doi: 10.1038/gt.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong F, Wang L, Davis JJ, Hu W, Zhang L, Guo W, et al. Eliminating established tumor in nu/nu nude mice by a tumor necrosis factor-alpha-related apoptosis-inducing ligand-armed oncolytic adenovirus. Clin Cancer Res. 2006;12:5224–5230. doi: 10.1158/1078-0432.CCR-06-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez-Breckenridge C, Kaur B, Chiocca EA. Pharmacologic and chemical adjuvants in tumor virotherapy. Chem Rev. 2009;109:3125–3140. doi: 10.1021/cr900048k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridle BW, Stephenson KB, Boudreau JE, Koshy S, Kazdhan N, Pullenayegum E, et al. Potentiating cancer immunotherapy using an oncolytic virus. Mol Ther. 2010;18:1430–1439. doi: 10.1038/mt.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC, McCart JA. Intelligent design: combination therapy with oncolytic viruses. Mol Ther. 2010;18:251–263. doi: 10.1038/mt.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF Receptor Superfamilies: Integrating Mammalian Biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 19.Bennett MW, O’Connell J, O’Sullivan GC, Brady C, Roche D, Collins JK, et al. The Fas counterattack in vivo: apoptotic depletion of tumor-infiltrating lymphocytes associated with Fas ligand expression by human esophageal carcinoma. J Immunol. 1998;160:5669–5675. [PubMed] [Google Scholar]
- 20.Koomagi R, Volm M. Expression of Fas (CD95/APO-1) and Fas ligand in lung cancer, its prognostic and predictive relevance. Int J Cancer. 1999;84:239–243. doi: 10.1002/(sici)1097-0215(19990621)84:3<239::aid-ijc7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.Mottolese M, Buglioni S, Bracalenti C, Cardarelli MA, Ciabocco L, Giannarelli D, et al. Prognostic relevance of altered Fas (CD95)-system in human breast cancer. Int J Cancer. 2000;89:127–132. doi: 10.1002/(sici)1097-0215(20000320)89:2<127::aid-ijc5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Takeda K, Stagg J, Yagita H, Okumura K, Smyth MJ. Targeting death-inducing receptors in cancer therapy. Oncogene. 2007;26:3745–3757. doi: 10.1038/sj.onc.1210374. [DOI] [PubMed] [Google Scholar]
- 23.Guillen-Ahlers H, Suckow MA, Castellino FJ, Ploplis VA. Fas/CD95 deficiency in ApcMin/+ mice increases intestinal tumor burden. PLoS One. 2010;5:e9070. doi: 10.1371/journal.pone.0009070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hohlbaum AM, Moe S, Marshak-Rothstein A. Opposing effects of transmembrane and soluble Fas ligand expression on inflammation and tumor cell survival. J Exp Med. 2000;191:1209–1220. doi: 10.1084/jem.191.7.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor JM, Barry M. Near death experiences: poxvirus regulation of apoptotic death. Virology. 2006;344:139–150. doi: 10.1016/j.virol.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 26.McCart JA, Puhlmann M, Lee J, Hu Y, Libutti SK, Alexander HR, et al. Complex interactions between the replicating oncolytic effect and the enzyme/prodrug effect of vaccinia-mediated tumor regression. Gene Ther. 2000;7:1217–1223. doi: 10.1038/sj.gt.3301237. [DOI] [PubMed] [Google Scholar]
- 27.Takizawa T, Matsukawa S, Higuchi Y, Nakamura S, Nakanishi Y, Fukuda R. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J Gen Virol. 1993;74:2347–2355. doi: 10.1099/0022-1317-74-11-2347. [DOI] [PubMed] [Google Scholar]
- 28.Ennaciri J, Menezes J, Proulx F, Toledano BJ. Induction of apoptosis by herpes simplex virus-1 in neonatal, but not adult, neutrophils. Pediatr Res. 2006;59:7–12. doi: 10.1203/01.pdr.0000191816.57544.b4. [DOI] [PubMed] [Google Scholar]
- 29.O’Donnell DR, Milligan L, Stark JM. Induction of CD95 (Fas) and apoptosis in respiratory epithelial cell cultures following respiratory syncytial virus infection. Virology. 1999;257:198–207. doi: 10.1006/viro.1999.9650. [DOI] [PubMed] [Google Scholar]
- 30.Clarke P, Beckham JD, Leser JS, Hoyt CC, Tyler KL. Fas-mediated apoptotic signaling in the mouse brain following reovirus infection. J Virol. 2009;83:6161–6170. doi: 10.1128/JVI.02488-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu XN, Screaton GR, Gotch FM, Dong T, Tan R, Almond N, et al. Evasion of cytotoxic T lymphocyte (CTL) responses by nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J Exp Med. 1997;186:7–16. doi: 10.1084/jem.186.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Zachar V, Zdravkovic M, Guo M, Ebbesen P, Liu X. Role of the Fas/Fas ligand pathway in apoptotic cell death induced by the human T cell lymphotropic virus type I Tax transactivator. J Gen Virol. 1997;78 (Pt 12):3277–3285. doi: 10.1099/0022-1317-78-12-3277. [DOI] [PubMed] [Google Scholar]
- 33.Badley AD, McElhinny JA, Leibson PJ, Lynch DH, Alderson MR, Paya CV. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J Virol. 1996;70:199–206. doi: 10.1128/jvi.70.1.199-206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao H, Xu J, Huang J. FasL/Fas pathway is involved in dengue virus induced apoptosis of the vascular endothelial cells. J Med Virol. 2010;82:1392–1399. doi: 10.1002/jmv.21815. [DOI] [PubMed] [Google Scholar]
- 35.Krzyzowska M, Cymerys J, Winnicka A, Niemialtowski M. Involvement of Fas and FasL in Ectromelia virus-induced apoptosis in mouse brain. Virus Res. 2006;115:141–149. doi: 10.1016/j.virusres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Gaddy DF, Lyles DS. Oncolytic vesicular stomatitis virus induces apoptosis via signaling through PKR, Fas, and Daxx. J Virol. 2007;81:2792–2804. doi: 10.1128/JVI.01760-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shunmei E, Zhao Y, Huang Y, Lai K, Chen C, Zeng J, et al. Heat shock factor 1 is a transcription factor of Fas gene. Mol Cells. 2010;29:527–531. doi: 10.1007/s10059-010-0065-4. [DOI] [PubMed] [Google Scholar]
- 38.Seet BT, Johnston JB, Brunetti CR, Barrett JW, Everett H, Cameron C, et al. Poxviruses and immune evasion. Annu Rev Immunol. 2003;21:377–423. doi: 10.1146/annurev.immunol.21.120601.141049. [DOI] [PubMed] [Google Scholar]
- 39.Gedey R, Jin XL, Hinthong O, Shisler JL. Poxviral regulation of the host NF-kappaB response: the vaccinia virus M2L protein inhibits induction of NF-kappaB activation via an ERK2 pathway in virus-infected human embryonic kidney cells. J Virol. 2006;80:8676–8685. doi: 10.1128/JVI.00935-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Starace D, Riccioli A, D’Alessio A, Giampietri C, Petrungaro S, Galli R, et al. Characterization of signaling pathways leading to Fas expression induced by TNF-alpha: pivotal role of NF-kappaB. FASEB J. 2005;19:473–475. doi: 10.1096/fj.04-2726fje. [DOI] [PubMed] [Google Scholar]
- 41.Dupont PJ, Warrens AN. Fas ligand exerts its pro-inflammatory effects via neutrophil recruitment but not activation. Immunology. 2007;120:133–139. doi: 10.1111/j.1365-2567.2006.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen JJ, Sun Y, Nabel GJ. Regulation of the proinflammatory effects of Fas ligand (CD95L) Science. 1998;282:1714–1717. doi: 10.1126/science.282.5394.1714. [DOI] [PubMed] [Google Scholar]
- 43.Koshkina NV, Khanna C, Mendoza A, Guan H, DeLauter L, Kleinerman ES. Fas-negative osteosarcoma tumor cells are selected during metastasis to the lungs: the role of the Fas pathway in the metastatic process of osteosarcoma. Mol Cancer Res. 2007;5:991–999. doi: 10.1158/1541-7786.MCR-07-0007. [DOI] [PubMed] [Google Scholar]
- 44.Lafleur EA, Koshkina NV, Stewart J, Jia SF, Worth LL, Duan X, et al. Increased Fas expression reduces the metastatic potential of human osteosarcoma cells. Clin Cancer Res. 2004;10:8114–8119. doi: 10.1158/1078-0432.CCR-04-0353. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 46.Leithauser F, Dhein J, Mechtersheimer G, Koretz K, Bruderlein S, Henne C, et al. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993;69:415–429. [PubMed] [Google Scholar]
- 47.Lombard C, McKallip RJ, Hylemon PB, Nagarkatti PS, Nagarkatti M. Fas Ligand-dependent and -independent mechanisms of toxicity induced by T cell lymphomas in lymphoid organs and in the liver. Clin Immunol. 2003;109:144–153. doi: 10.1016/s1521-6616(03)00179-7. [DOI] [PubMed] [Google Scholar]
- 48.Li X, Liu YH, Zhang YP, Zhang S, Pu X, Gardner TA, et al. Fas ligand delivery by a prostate-restricted replicative adenovirus enhances safety and antitumor efficacy. Clin Cancer Res. 2007;13:5463–5473. doi: 10.1158/1078-0432.CCR-07-0342. [DOI] [PubMed] [Google Scholar]