Abstract
Digital peripheral arterial tonometry (PAT) is an emerging, non-invasive method to assess vascular function. The physiology underlying this phenotype, however, remains unclear. Therefore, we evaluated the relationship between digital PAT and established brachial artery ultrasound measures of vascular function under basal conditions and following reactive hyperemia. Using a cross-sectional study design, digital PAT and brachial artery ultrasound with pulsed wave Doppler were simultaneously completed at baseline and following reactive hyperemia in both individuals with established coronary artery disease (n=99) and healthy volunteers at low cardiovascular disease risk (n=40). Under basal conditions, the digital pulse volume amplitude demonstrated a significant positive correlation with the brachial artery velocity-time integral, that was independent of arterial diameter, in both the healthy volunteer (rs=0.64, P<0.001) and coronary artery disease (rs=0.63, P<0.001) cohorts. Similar positive relationships were observed with baseline brachial artery blood flow velocity and blood flow. In contrast, no relationship between the reactive hyperemia-evoked digital PAT ratio and either brachial artery flow-mediated dilation or shear stress was observed in either cohort (P=NS). In conclusion, these findings demonstrate that digital PAT measures of vascular function more closely reflect basal blood flow in the brachial artery than reactive hyperemia-induced changes in arterial diameter or flow velocity, and the presence of vascular disease does not modify the physiology underlying the digital PAT phenotype.
Keywords: endothelial function, peripheral arterial tonometry, flow-mediated dilation, coronary artery disease
Endothelial dysfunction is integral to the pathogenesis and progression of coronary artery disease (CAD).1 Brachial artery flow-mediated dilation (FMD) is the most widely used non-invasive method to assess endothelial function; however, technical complexity and lack of standardization limit its clinical application.2 Digital peripheral arterial tonometry (PAT) quantifies reactive hyperemia-induced changes in pulse volume amplitude (PVA) in the finger tip, and is an emerging, automated method to non-invasively assess endothelial function.3 Digital PAT is predictive of the presence of cardiovascular disease risk factors,4–7 impaired vasodilator responses to acetylcholine in coronary arteries,8,9 and adverse cardiovascular events in a single study.10 Although prior studies have demonstrated a direct contribution of nitric oxide to brachial artery FMD11 and the digital PAT ratio,12 conflicting results regarding their correlation have been reported.5,6,13–15 Despite growing interest in the utility of digital PAT, the physiology underlying the digital PVA signal and its relationship with established vascular function phenotypes remains unclear. In particular, the relationship between digital PVA and brachial artery blood flow velocity, and the impact of vascular disease on these phenotypes, has not been rigorously evaluated. Therefore, we characterized the relationship between digital PAT and brachial artery ultrasound vascular function phenotypes, under both basal and reactive hyperemia-evoked conditions, in healthy volunteers and individuals with established CAD.
METHODS
Individuals with established CAD (defined as ≥50% stenosis in at least one major epicardial coronary artery by coronary angiography) were identified in the University of North Carolina Cardiac Catheterization Laboratory. Exclusion criteria included pregnancy, atrial fibrillation, Raynaud’s disease, left-ventricular ejection fraction ≤35%, current use of insulin or long-acting nitrates, active autoimmune disease, history of severe aortic stenosis, history of solid organ transplant or dialysis, or history of cancer within the previous 5 years.
A parallel cohort of healthy volunteers (HV) was identified by advertisement from the local Chapel Hill, NC community. Following a detailed history and a fasting serum chemistry and cholesterol panel, potential participants were excluded if they had a history of cardiovascular disease, risk factors for CAD [including physician-diagnosed hypertension or diabetes, cigarette smoking within the previous 6 months, high cholesterol (defined as total cholesterol >240 mg/dL, triglycerides >200 mg/dL or low density lipoprotein cholesterol >160 mg/dL), or a body mass index >30 kg/m2], or were currently taking any medication for a chronic medical condition.
The study protocol was approved by the University of North Carolina at Chapel Hill Biomedical Institutional Review Board. Eligible participants (108 CAD, 41 HV) provided written informed consent and returned to the clinical research unit for a single morning study visit after fasting overnight and withholding their morning medications. Participants were instructed to refrain from tobacco products, caffeine, and vigorous exercise the morning of the study visit, and from use of vitamin C, vitamin E, fish oil, niacin or arginine supplements, oral decongestants, non-steroidal anti-inflammatory drugs (other than low-dose aspirin), or erectile dysfunction medications for at least 7 days prior to the study visit. Individuals who experienced a respiratory tract infection within 4 weeks prior to the study visit were not eligible to participate.
Endothelial function measurements were completed in a quiet, dimly lit, temperature-controlled room and obtained while the participant lay in a supine position. Following 10 minutes of rest, brachial artery reactivity was assessed by quantifying arterial diameter and blood flow velocity at baseline and following reactive hyperemia using a 12.5-MHz linear-array transducer (Philips HDI 5000 system) with pulsed wave Doppler, as described.16,17 Reactive hyperemia was induced by inflating a blood pressure cuff around the right forearm for 5 minutes to a pressure of at least 70 mmHg greater than the systolic blood pressure. All images were electrocardiogram gated and obtained by the same sonographer throughout the study. Data were analyzed by the Brachial Tools software (Medical Imaging Applications, Coralville, IA).
Brachial artery diameter was measured at end diastole from the lumen-intimal interface of the proximal and distal walls. Baseline diameter was averaged from 10 consecutive frames. Arterial diameter was assessed for 90 seconds following cuff deflation, and the diameter during peak dilation was averaged from 3 consecutive frames. The peak change in arterial dilation was observed 51 ± 12 (mean ± standard deviation) seconds after cuff deflation. Brachial artery FMD (endothelium-dependent) was calculated as the percent change in brachial artery diameter from baseline [= 100*(diameter Peak − diameter Baseline)/(diameter Baseline)]. The reproducibility of this method in our laboratory has been previously reported.16 Following 10 minutes of rest, a second baseline image was acquired, which was highly correlated with the initial baseline measurement (r=0.99, P<0.001). Endothelial-independent vasodilation was assessed 5 minutes following sublingual administration of nitroglycerin (0.4 mg) as the percent change in arterial diameter [= 100*(diameter Nitroglycerin − diameter Baseline)/(diameter Baseline)].
Brachial artery blood flow velocity was measured by pulsed wave Doppler at baseline and for 15 seconds following cuff deflation. The velocity-time integral (VTI) was measured by automated planimetry of the velocity profile and averaged over 3 consecutive cardiac cycles at baseline and during peak reactive hyperemia. The reactive hyperemia-induced increase in VTI relative to baseline was calculated as a ratio [= VTI RH/VTI Baseline]. The mean flow velocity (V) was calculated by dividing the VTI (cm) by the duration of the cycle (s). Mean flow velocity was converted to local shear stress [= 8*μ*Vx/diameter Baseline] and blood flow [= Vx*π*(diameter Baseline/2)2], as described,18,19 which each normalize flow velocity to arterial diameter. The subscript x indicates either baseline or peak reactive hyperemia, and μ indicates blood viscosity at an assumed value of 0.035 dyne*s/cm2. Since reactive hyperemia-induced flow velocity changes in conduit arteries are regulated by dilation of distal resistance vessels, brachial shear stress following reactive hyperemia (SSRH) is as an index of microvascular function.18,19
Digital PVA was measured on the index finger of each hand using plethysmographically based probes and the Endo-PAT 2000 device (Itamar Medical Ltd, Caesarea, Israel) simultaneously with the brachial artery ultrasound measurements. Measurements were obtained continuously during the baseline (5 minutes), cuff occlusion (5 minutes), and post-cuff deflation (5 minutes) periods, and the data were automatically derived using the Endo-PAT v3.0.4 software. The digital PAT ratio was calculated as the ratio of post-cuff deflation PVA (in 30 second intervals) to baseline PVA in the arm undergoing cuff occlusion (occluded), and then normalized to the contralateral (control) arm. The digital PAT ratio in the 90–120 second post-cuff deflation interval [= (PVA occluded, 90–120sec/PVA occluded, Baseline)/(PVA control, 90–120sec/PVA control, Baseline)] is presented, as described,4 unless otherwise noted. The digital PAT test was unsuccessful in 10 study participants (9 CAD, 1 HV), consistent with prior reports.4,6 The reproducibility of this automated method has been previously established.20
Data presented as mean ± standard deviation or median (interquartile range). Study population characteristics were compared using a one-way analysis of variance or Wilcoxon rank-sum test, where appropriate. Relationships between each digital PAT and brachial artery ultrasound phenotype were evaluated by Spearman rank correlation (rs). Partial correlations were also evaluated to account for the potential impact of demographic (age, sex, race) and vascular (baseline brachial artery diameter, baseline brachial artery VTI, baseline digital PVA, SSRH) variables on these relationships. Parameters that were not normally distributed, including digital PVA and PAT ratio, were log10-transformed prior to graphical presentation. Since the units of each digital PAT and brachial artery ultrasound measure were not the same, Bland–Altman plots could not be constructed to evaluate the agreement between each phenotype.21 Analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). Statistical significance was set at P<0.05.
RESULTS
The cohort characteristics are provided in Table 1. Participants in the CAD cohort returned for their study visit 61 ± 33 days following cardiac catheterization. High rates of statin, renin-angiotensin system inhibitor, beta blocker, aspirin and clopidogrel use were reported, consistent with management of the CAD cohort according to current clinical practice guidelines.
Table 1.
Study participant characteristics.
| Characteristic | Coronary Artery Disease (n=99) | Healthy Volunteer (n=40) |
|---|---|---|
| Age (years) | 59 ± 9 | 51 ± 8 * |
| Women | 33 (33%) | 23 (58%) * |
| African-American | 18 (18%) | 7 (18%) |
| Body mass index (kg/m2) | 30 ± 6 | 26 ± 3 * |
| ≥ 30 kg/m2 | 53 (54%) | 0 † |
| Current smoker | 23 (23%) | 0 † |
| Diabetes | 24 (24%) | 0 † |
| Hypertension | 82 (83%) | 0 † |
| Disease Severity | ||
| 1-vessel disease | 35 (35%) | - |
| 2-vessel disease | 36 (36%) | - |
| 3-vessel disease | 28 (28%) | - |
| Revascularization Procedure ‡ | 65 (66%) | - |
| Systolic blood pressure (mmHg) | 136 ± 17 | 122 ± 14 * |
| Diastolic blood pressure (mmHg) | 80 ± 10 | 74 ± 8 * |
| Total cholesterol (mg/dL) | 159 (49) | 192 (30) * |
| Low density lipoprotein cholesterol (mg/dL) | 85 (41) | 117 (30) * |
| High density lipoprotein cholesterol (mg/dL) | 48 (17) | 65 (23) * |
| Triglycerides (mg/dL) | 97 (79) | 69 (32) * |
| Total: high density lipoprotein cholesterol | 3.4 (1.6) | 3.1 (1.4) |
| High sensitivity C-reactive protein (mg/L) | 1.6 (3.3) | 0.7 (1.7) * |
| Statin use | 92 (93%) | 0 † |
| Renin-angiotensin system inhibitor use § | 61 (62%) | 0 † |
| Beta-blocker use | 82 (83%) | 0 † |
| Aspirin use | 96 (97%) | 0 † |
| Clopidogrel use | 79 (80%) | 0 † |
Data presented as mean ± standard deviation, median (interquartile range) or count (proportion).
P<0.05 versus coronary artery disease.
Exclusion criteria in the healthy volunteer cohort.
In the coronary artery disease cohort, 55/99 underwent a percutaneous coronary intervention and 10/99 underwent a coronary artery bypass grafting procedure between screening and the study visit.
Use of either an angiotensin converting enzyme inhibitor or angiotensin receptor blocker.
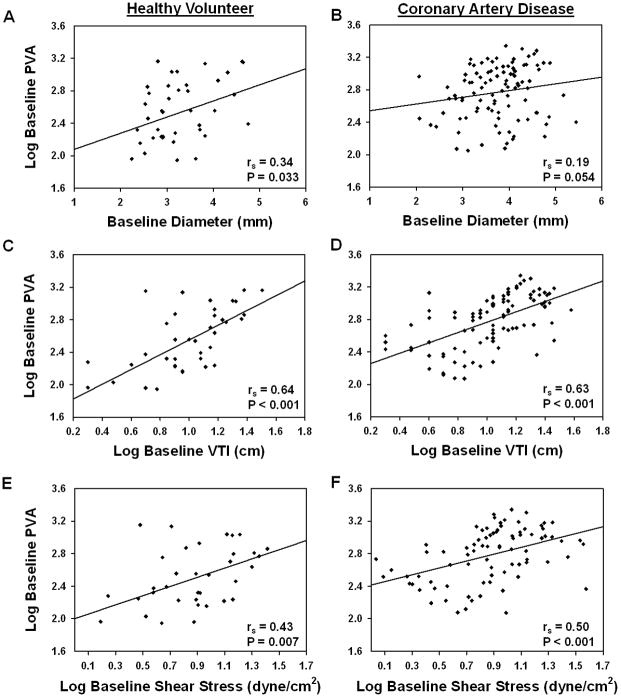
Under basal conditions, the digital PVA and brachial artery diameter were modestly correlated in both cohorts (Figure 1A, 1B); however, these correlations were not statistically significant when adjusting for age, sex and race (HV: rs=0.21, P=0.208; CAD: rs=0.12, P=0.260). The baseline digital PVA demonstrated a significant positive correlation with basal brachial artery VTI that was similar in both cohorts (Figure 1C, 1D). After stratifying by sex, significant relationships were observed in women (HV: rs=0.80, P<0.001; CAD: rs=0.65, P<0.001) and men (HV: rs=0.55, P=0.023; CAD: rs=0.66, P<0.001). Similar relationships were also observed after adjusting for age, sex, race and brachial artery diameter (HV: rs=0.71, P<0.001; CAD: rs=0.72, P<0.001), and when the data were expressed as mean flow velocity (HV: rs=0.56, P<0.001; CAD: rs=0.62, P<0.001), blood flow (HV: rs=0.78, P<0.001; CAD: rs=0.72, P<0.001) or shear stress (Figure 1E, 1F), further demonstrating a positive relationship between basal digital PVA and brachial artery blood flow velocity that is independent of arterial size.
Figure 1. Correlations between digital pulse volume amplitude and brachial artery diameter and velocity under basal conditions.
Correlations between the log10-transformed digital pulse volume amplitude (PVA) at baseline and the (A, B) brachial artery diameter at baseline, (C, D) log10-transformed brachial artery velocity-time integral (VTI) at baseline, and (E, F) log10-transformed brachial artery shear stress at baseline in the (A, C, E) healthy volunteer and (B, D, F) and coronary artery disease cohorts are presented. The Spearman rank correlation coefficient (rs) and P-value for each comparison is provided.
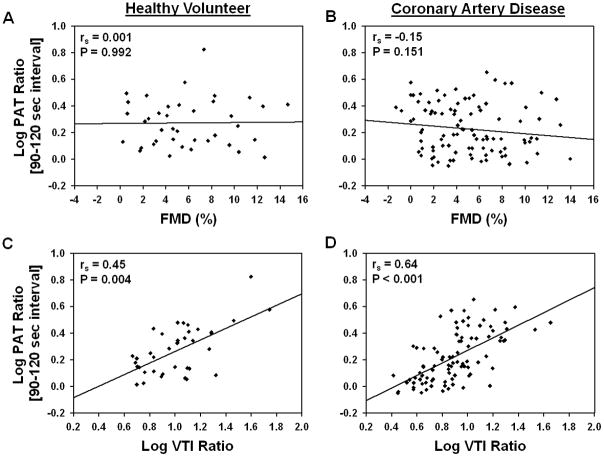
A significant positive relationship between brachial artery FMD and SSRH (HV: rs=0.64, P<0.001; CAD: rs=0.54, P<0.001), which serves as the stimulus for arterial dilation, and a significant inverse relationship between digital PAT ratio and baseline PVA (HV: rs=−0.63, P<0.001; CAD: rs=−0.79, P<0.001) was observed in both cohorts. A significant inverse relationship between age and brachial artery FMD (HV: rs=−0.33, P=0.037; CAD: rs=−0.33, P=0.001), but not digital PAT ratio (HV: rs=0.05, P=0.737; CAD: rs=0.08, P=0.403), was observed in both cohorts. No significant relationship between digital PAT ratio and FMD was observed in either cohort (Figure 2A, 2B). Similar results were observed after adjusting for age, sex, race and SSRH (HV: rs=−0.02, P=0.925; CAD: rs=−0.01, P=0.930), as well as in each 30-second post-cuff deflation interval utilized for the digital PAT ratio calculation (data not shown). No relationship between digital PAT ratio and nitroglycerin-mediated dilation was observed in either cohort (HV: rs=0.13, P=0.454; CAD: rs=0.17, P=0.092).
Figure 2. Correlations between digital PAT and brachial artery ultrasound phenotypes following reactive hyperemia.
Correlations between the log10-transformed digital peripheral arterial tonometry (PAT) ratio and the (A, B) brachial artery flow-mediated dilation (FMD) and (C, D) log10-transformed brachial artery velocity-time integral (VTI) ratio in the (A, C) healthy volunteer and (B, D) coronary artery disease cohorts are presented. The Spearman rank correlation coefficient (rs) and P-value for each comparison is provided.
In contrast, a significant positive relationship between digital PAT ratio and the brachial artery VTI ratio was observed in both cohorts (Figure 2C, 2D). A similar correlation was also observed with the reactive hyperemia-induced increase in blood flow relative to baseline (HV: rs=0.41, P=0.012; CAD: rs=0.65, P<0.001). Although the relationship between the digital PAT and brachial artery VTI ratio persisted after adjusting for age, sex, and race (HV: rs=0.48, P=0.004; CAD: rs=0.66, P<0.001), the correlations were substantially weakened after also adjusting for baseline digital PVA (HV: rs=0.19, P=0.272; CAD: rs=0.34, P=0.001) or baseline brachial artery VTI (HV: rs=0.25, P=0.145; CAD: rs=0.11, P=0.295). Moreover, no significant relationship between SSRH and the digital PVA following reactive hyperemia was observed in either the healthy volunteer (rs=0.05, P=0.748) or CAD (rs=0.13, P=0.244) cohort. Similar results were observed in each 30-second post-cuff deflation interval (data not shown), further demonstrating the lack of a relationship between digital PAT and brachial artery ultrasound phenotypes following reactive hyperemia.
After stratifying the CAD cohort by sex, disease severity, a recent revascularization procedure, and use of clopidogrel, beta blockers or renin-angiotensin system inhibitors, similar relationships between each basal and reactive hyperemia-evoked digital PAT and brachial artery ultrasound phenotype were observed within all subsets of the population (data not shown).
DISCUSSION
Digital PAT is an emerging, automated method to non-invasively assess endothelial function. The physiology underlying this phenotype, however, has remained unclear. In the current study, we observed a significant positive correlation between baseline digital PVA and brachial artery blood flow velocity that was independent of arterial diameter. In contrast, no relationship was observed between the reactive hyperemia-evoked digital PAT ratio and brachial artery FMD. Furthermore, no correlation was observed between digital PVA and brachial artery SSRH, which is the stimulus for brachial artery vasodilation and an index of microvascular function.18,19,22,23 Although a significant positive relationship was observed between digital PAT ratio and the reactive hyperemia-induced increase in brachial artery VTI and blood flow, this relationship was driven by the positive correlation between baseline brachial artery blood flow velocity and baseline digital PVA. Collectively, these data demonstrate that digital PAT measures of vascular function more closely reflect brachial artery blood flow velocity under basal conditions than reactive hyperemia-induced changes in arterial diameter or flow velocity.
It is well established that brachial artery FMD correlates with coronary artery vasodilator responses, predicts risk of atherosclerosis development and progression, and predicts risk for adverse cardiovascular events.1 A series of recent studies have demonstrated that the reactive hyperemia-evoked digital PAT ratio also is predictive of the presence of cardiovascular disease risk factors4–7 and impaired endothelial-dependent vasodilator responses in coronary arteries.8,9 Although prior studies have demonstrated a direct contribution of nitric oxide to brachial artery FMD11 and the digital PAT ratio,12 conflicting results regarding correlations between these peripheral endothelial function phenotypes have been reported.5,6,13–15 A significant positive correlation was reported in a small cohort of healthy volunteers (n=40),13 as well as two cohorts of patients presenting for evaluation of chest pain (n=89, n=115).14,15 In contrast, no correlation was observed in a subset of the Framingham Third Generation cohort (n=1843),5 the Gutenberg Heart Study (n=5000),6 or the current investigation. These findings, coupled with the clear lack of an inverse correlation between digital PAT ratio and advancing age in multiple studies,3,4,8 suggest that digital PAT ratio and brachial artery FMD assess distinct vascular phenotypes.
Several important differences across these studies should be considered. First, although different post-cuff deflation intervals were used to calculate the digital PAT ratio in each study (90–150 second;13 60–120 second;14,15 90–120 second5), we observed similar relationships between digital PAT ratio and brachial artery FMD in all post-cuff deflation intervals. Second, the degree of cardiovascular disease risk varied considerably across the populations. In the current study, no relationship was observed in either patients with established CAD, irrespective of disease severity or treatment, or healthy volunteers without significant CAD risk factors. These data demonstrate that the presence of clinically significant vascular disease does not impact the relationship between digital PAT ratio and brachial artery FMD.
In the current investigation, and consistent with two prior reports,5,13 no relationship was observed between the digital PVA following reactive hyperemia and brachial artery SSRH. In contrast, our investigation is the first to report presence of a significant positive relationship between baseline digital PVA and baseline brachial artery blood flow velocity. Importantly, this relationship was independent of sex and arterial diameter, two known predictors of digital PVA and blood flow velocity,4,14,23,24 and was similar in patients with CAD as well as healthy volunteers. Although increases in peripheral arterial blood flow following reactive hyperemia are dependent on nitric oxide availability and more widely recognized as an index of microvascular function, studies have also demonstrated that higher basal brachial artery blood flow is associated with the presence of cardiovascular disease risk factors and may be reflective of a hyperperfusion state and underlying vascular dysfunction.18,23–25 Indeed, baseline digital PVA also has been associated with the presence of cardiovascular disease risk factors in prior studies, and these relationships appear stronger than those reported with the PAT ratio.4,6 Although we acknowledge that our study was not powered to identify clinical predictors of each vascular function phenotype, or designed to assess their predictive value, our findings clearly demonstrate that the digital PVA signal reflects resting peripheral arterial blood flow and suggest that baseline digital PVA is a vascular phenotype that should be considered in future studies as a potential predictor of CAD prevalence and risk of adverse cardiovascular events.
Mechanistic studies have demonstrated that inhibition of nitric oxide and prostaglandin biosynthesis reduces resting forearm blood flow in humans.26 Although the impact of cyclooxygenase inhibition on basal digital PVA has not been evaluated to date, inhibition of nitric oxide biosynthesis had no effect in healthy volunteers.12 In contrast, brachial artery administration of phenylephrine, an α1-adrenergic receptor agonist, reduced digital PVA by approximately 50%.12 Similarly, α-adrenergic tone is a key regulator of basal forearm blood flow,27 and represents a potential common mechanism underlying the observed correlation between baseline digital PVA and brachial artery blood flow velocity. The cross-sectional design of our investigation, however, did not enable a cause-and-effect evaluation of either the direction of the observed correlations or the mechanisms underlying these peripheral vascular function phenotypes, and is a limitation. Moreover, we were unable to elucidate the direct impact of pharmacotherapy, including the anti-platelet effects of clopidogrel and anti-adrenergic effects of beta blockers,28,29 on each phenotype in the CAD population. Future studies remain necessary to dissect the mechanisms underlying the digital PVA phenotype at baseline and following reactive hyperemia, and the impact of drug therapy. Despite these limitations, our results clearly demonstrate that digital PAT measures of vascular function more closely reflect brachial artery blood flow under basal conditions than reactive hyperemia-induced changes in arterial diameter or flow velocity. These data offer novel insight into the physiology underlying digital PVA, and provide a foundation for the design and interpretation of future studies that evaluate the relative utility of digital and brachial artery vascular function phenotypes as tools to risk stratify and guide the treatment of patients at risk for adverse cardiovascular events.
Acknowledgments
Sources of funding: This publication was made possible by a Beginning Grant-in-Aid from the American Heart Association and a pilot grant from the North Carolina Translational and Clinical Services Institute to Dr. Lee, and in part by grants M01RR00046 and UL1RR025747 from the NIH/NCRR. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
The authors gratefully acknowledge the UNC Clinical and Translational Research Center staff for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flammer AJ, Luscher TF. Three decades of endothelium research: from the detection of nitric oxide to the everyday implementation of endothelial function measurements in cardiovascular diseases. Swiss Med Wkly. 2010;140:w13122. doi: 10.4414/smw.2010.13122. [DOI] [PubMed] [Google Scholar]
- 2.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 3.Hamburg NM, Benjamin EJ. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med. 2009;19:6–11. doi: 10.1016/j.tcm.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, Levy D, Mitchell GF, Vita JA, Benjamin EJ. Relation of brachial and digital measures of vascular function in the community: the Framingham Heart Study. Hypertension. 2011;57:390–396. doi: 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnabel RB, Schulz A, Wild PS, Sinning CR, Wilde S, Eleftheriadis M, Herkenhoff S, Zeller T, Lubos E, Lackner KJ, Warnholtz A, Gori T, Blankenberg S, Munzel T. Noninvasive vascular function measurement in the community: cross-sectional relations and comparison of methods. Circ Cardiovasc Imaging. 2011;4:371–380. doi: 10.1161/CIRCIMAGING.110.961557. [DOI] [PubMed] [Google Scholar]
- 7.Toggweiler S, Schoenenberger A, Urbanek N, Erne P. The prevalence of endothelial dysfunction in patients with and without coronary artery disease. Clin Cardiol. 2010;33:746–752. doi: 10.1002/clc.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 9.Matsuzawa Y, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Konishi M, Matsubara J, Sumida H, Kaikita K, Kojima S, Nagayoshi Y, Yamamuro M, Izumiya Y, Iwashita S, Matsui K, Jinnouchi H, Kimura K, Umemura S, Ogawa H. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010;55:1688–1696. doi: 10.1016/j.jacc.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 10.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 11.Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am J Physiol. 1996;270:H1435–1440. doi: 10.1152/ajpheart.1996.270.4.H1435. [DOI] [PubMed] [Google Scholar]
- 12.Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101:545–548. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 13.Dhindsa M, Sommerlad SM, DeVan AE, Barnes JN, Sugawara J, Ley O, Tanaka H. Interrelationships among noninvasive measures of postischemic macro- and microvascular reactivity. J Appl Physiol. 2008;105:427–432. doi: 10.1152/japplphysiol.90431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heffernan KS, Karas RH, Mooney PJ, Patel AR, Kuvin JT. Pulse wave amplitude is associated with brachial artery diameter: implications for gender differences in microvascular function. Vasc Med. 2010;15:39–45. doi: 10.1177/1358863X09349523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 16.Sherwood A, Bower JK, McFetridge-Durdle J, Blumenthal JA, Newby LK, Hinderliter AL. Age moderates the short-term effects of transdermal 17beta-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol. 2007;27:1782–1787. doi: 10.1161/ATVBAHA.107.145383. [DOI] [PubMed] [Google Scholar]
- 17.Schneider A, Neas L, Herbst MC, Case M, Williams RW, Cascio W, Hinderliter A, Holguin F, Buse JB, Dungan K, Styner M, Peters A, Devlin RB. Endothelial dysfunction: associations with exposure to ambient fine particles in diabetic individuals. Environ Health Perspect. 2008;116:1666–1674. doi: 10.1289/ehp.11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 19.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonetti PO, Holmes DR, Jr, Lerman A, Barsness GW. Enhanced external counterpulsation for ischemic heart disease: what’s behind the curtain? J Am Coll Cardiol. 2003;41:1918–1925. doi: 10.1016/s0735-1097(03)00428-5. [DOI] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG. Applying the right statistics: analyses of measurement studies. Ultrasound Obstet Gynecol. 2003;22:85–93. doi: 10.1002/uog.122. [DOI] [PubMed] [Google Scholar]
- 22.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123:163–169. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 23.Kullo IJ, Malik AR, Santos S, Ehrsam JE, Turner ST. Association of cardiovascular risk factors with microvascular and conduit artery function in hypertensive subjects. Am J Hypertens. 2007;20:735–742. doi: 10.1016/j.amjhyper.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 25.Malik AR, Kondragunta V, Kullo IJ. Forearm vascular reactivity and arterial stiffness in asymptomatic adults from the community. Hypertension. 2008;51:1512–1518. doi: 10.1161/HYPERTENSIONAHA.107.106088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffy SJ, New G, Tran BT, Harper RW, Meredith IT. Relative contribution of vasodilator prostanoids and NO to metabolic vasodilation in the human forearm. Am J Physiol. 1999;276:H663–670. doi: 10.1152/ajpheart.1999.276.2.H663. [DOI] [PubMed] [Google Scholar]
- 27.Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Post-junctional alpha-adrenoceptors and basal limb vascular tone in healthy men. J Physiol. 2002;540:1103–1110. doi: 10.1113/jphysiol.2001.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller O, Hamilos M, Bartunek J, Ulrichts H, Mangiacapra F, Holz JB, Ntalianis A, Trana C, Dierickx K, Vercruysse K, De Bruyne B, Wijns W, Barbato E. Relation of endothelial function to residual platelet reactivity after clopidogrel in patients with stable angina pectoris undergoing percutaneous coronary intervention. Am J Cardiol. 2010;105:333–338. doi: 10.1016/j.amjcard.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 29.Heffernan KS, Suryadevara R, Patvardhan EA, Mooney P, Karas RH, Kuvin JT. Effect of atenolol vs metoprolol succinate on vascular function in patients with hypertension. Clin Cardiol. 2011;34:39–44. doi: 10.1002/clc.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]