Abstract
Recent research revealed decreased access to semantic and associative networks in acute cocaine withdrawal. In autism, such behavioral outcomes are associated with decreased functional connectivity using functional magnetic resonance imaging. Therefore, we wished to determine whether connectivity is also decreased in acute cocaine withdrawal. Eight subjects in acute cocaine withdrawal were compared to controls for connectivity in language areas while performing a task involving categorization of words according to semantic and phonological relatedness. Acute withdrawal subjects had significantly less overall connectivity during semantic relatedness, and a trend towards less connectivity during phonological relatedness. Of potential future interest is whether this might serve as an imaging marker for treatment in patients.
Introduction
Acute cocaine withdrawal (CW) is characterized by high levels of anxiety and stress (Aronson & Craig, 1986), a range of cognitive impairments including cognitive flexibility (Kelley, Yeager, Pepper, & Beversdorf, 2005), and high rates of relapse. Individuals in acute cocaine withdrawal show cognitive impairments, particularly involving flexibility of access to semantic and associative networks (Kelley et al., 2005). As an initial pilot investigation to determine whether an imaging correlate of these cognitive impairments may be present, we examined functional connectivity in patients in acute withdrawal from cocaine during language tasks.
Functional connectivity, as defined by Friston, is the ‘temporal correlation between spatially remote neurophysiological events’ (Friston, 1994). Functional connectivity has been found to be decreased in autism spectrum disorders during a range of cognitive tasks including language tasks and cognitive flexibility tasks (Just, Cherkassky, Keller, & Minshew, 2004; Koshino et al., 2005; Kana, Keller, Cherkassky, Minshew, & Just, 2006; Just, Cherkassky, Keller, Kana, & Minshew, 2007). This is believed to be an imaging correlate of restricted network access in autism spectrum disorder (Belmonte et al., 2004), proposed in network models of autism (Cohen, 1994; McClelland, 2000; Beversdorf, Narayanan, Hillier, & Hughes, 2007), and based on neuropsychological findings in autism (Frith & Happé, 1994; Beversdorf et al., 2000; Hillier, Campbell, Keillor, Phillips, & Beversdorf, 2007), from which our understanding of autism has evolved as a syndrome characterized by underconnectivity between remote cortical regions (Belmonte et al., 2004). Neuropsychological effects in autism have been observed on a range of tasks involving cognitive flexibility and language, related to this underconnectivity (Frith & Happé, 1994; Beversdorf et al., 2000).
A range of cognitive impairments have been reported in cocaine withdrawal, with varying reports of impairment on aspects of cognitive flexibility and language performance (Gillen et al., 1998; Ardila, Rosselli, & Strumwasser, 1991; Beatty et al., 1995; O’Malley et al., 1992; Hoff et al., 1996). However, early in withdrawal, characterized by the highest degrees of anxiety (Aronson & Craig, 1986), there is a significant degree of impairment on language tasks and cognitive flexibility (Kelley et al., 2005), believed to be due to the apparent increased noradrenergic activation in chronic cocaine use and acute cocaine withdrawal (Macey et al., 2003; McDougle et al., 1994). Therefore, with neuropsychological findings potentially consistent with restricted network access in acute cocaine withdrawal (Beversdorf, 2010), as has been observed in autism (Frith & Happé, 1994; Beversdorf et al., 2000), we would predict decreased functional connectivity in acute cocaine withdrawal due to the cognitive impairments observed in this setting.
Furthermore, these cognitive impairments in acute cocaine withdrawal are improved with administration of the β-adrenergic antagonist, propranolol (Kelley, Yeager, Pepper, Bornstein, & Beversdorf, 2007). Due to our finding that propranolol appears to increase functional connectivity in autism spectrum disorder (Narayanan et al., 2010), we are interested in the future exploration of the effects of propranolol on connectivity in other populations. Therefore we selected language tasks previously used by us in examining the effects of pharmacological agents on connectivity (Tivarus, Hillier, Schmalbrock, & Beversdorf, 2008; Narayanan et al., 2010; Kim, Goel, Tivarus, Hillier, & Beversdorf, 2010), in order to determine whether acute cocaine withdrawal is characterized by decreased functional connectivity.
Materials and Methods
Research Participants
Eight individuals in the acute stage of cocaine withdrawal (6 male), aged 20–45 (mean 31 ± 8.8), and 10 normal age matched control subjects (8 male) aged 21–37 (mean 25.1 ± 4.6) (p=n.s.) were recruited for this study. The cocaine withdrawal subjects were between 1 and 7 days from last drug use with an average of 3.8 ± 1.8 days. All subjects were right handed native-English speakers. Subjects were without a history of learning disabilities such as dyslexia. None of the subjects were pregnant. Subjects with a history of schizophrenia, major depression, bipolar disorder, major head trauma, or neuroleptic use were excluded, in order to avoid a confound on effects on imaging from these commonly co-occurring conditions in withdrawal. All addicts met the criteria for cocaine withdrawal according to DSM-IV (Association, 1995), using the Structured Clinical Interview for DSM-IV (First, Gibbon, Spitzer, & Williams, 1996), and had a history of daily use of cocaine. The cocaine withdrawal subjects were given a drug screen upon entering the program and were interviewed, assessed, and diagnosed by a board certified Psychiatrist and Addictionologist (THP) and placed in a treatment program in accordance with the criteria of the American Society of Addiction Medicine. Severity of addiction was assessed with the Addiction Severity Index (Carise, McLellan, Cacciola, Love, Cook et al., 2001). All participants either reported normal vision or were provided corrective lenses for viewing the fMRI stimuli. Participants were also screened to comply with the MRI safety requirements (no metallic implants or prostheses, no metal objects in their bodies, no claustrophobia).
Of the eight subjects, four reported concurrent heroin abuse, three each reported concurrent alcohol abuse and sedative use, two reported use of cannabis, and one reported using hallucinogens concurrently as well. While in treatment, two patients were being given buprenorphine, and one each were being given escitalopram, valproic acid, and a benzodiazepine. Cocaine withdrawal subjects reported an average anxiety of 5.5 ± 2.4 on a 10 point scale on the day of the MRI scan, and reported an overall anxiety of 6.4 ± 3.2 since cessation of drug abuse. Scores among controls were at floor, as no anxiety was reported by any of the control subject. Written consent was obtained from all participants after explaining the nature of the study and the nature of the procedure to each participant in accordance with the regulations of the Institutional Review Board of The Ohio State University, subsequent to approval.
Materials
Separate sets of word-lists were created for phonological and semantic processing by modifying previously used stimuli utilized in an fMRI experiment to demonstrate the overlapping and distinct areas involved in semantic and phonological processing (McDermott, Petersen, Watson, & Ojemann, 2003). For the phonological word lists, all phonologically-related words rhymed with the cue word, and for the semantic word lists, all semantically-related words were related in meaning to the cue word (McDermott et al., 2003). Median word length was 5 letters for both the semantic and phonological lists, and the Kucera-Francis written word fluency was 30–32 per million for both lists (Kucera & Francis, 1967; Tivarus et al., 2008). These particular stimuli were chosen because they have demonstrated robust activation for both the phonological and semantic tasks, and revealed a high degree of functional connectivity between language areas in normal individuals (Tivarus et al., 2008), and reactivity to pharmacological agents (Tivarus et al, 2008; Narayanan et al., 2010; Kim et al., 2010). A total of eight lists, four each for phonological and semantic processing, were presented to each participant during the study (Tivarus et al., 2008).
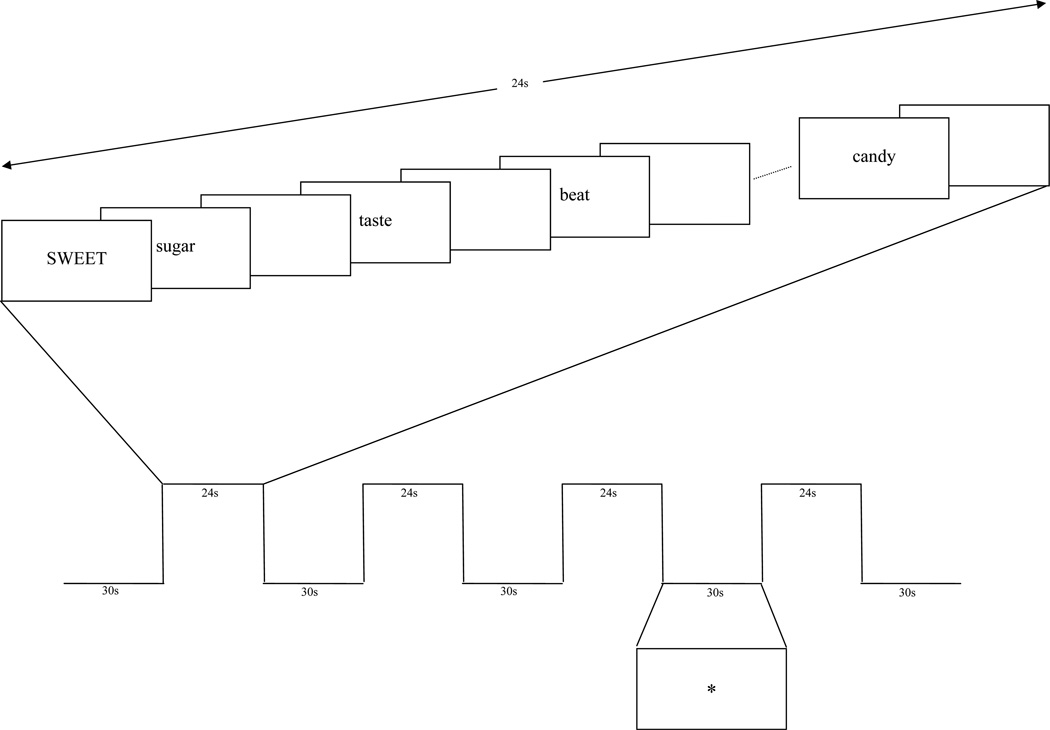
Every participant was instructed in performing the task prior to imaging during the fMRI visit. Tasks were presented in a block design (Figure 1) with 4 task blocks (24s) interspersed between 5 rest blocks (30s) for a total of 4 minutes and 6 seconds. During each task block, the cue word was presented in capitalized letters (3s), followed by a stream of corresponding target words from the word-list (1.1s for each word followed by 300ms inter-stimulus interval, consisting of a blank screen). A ‘*’ sign was shown during the rest blocks. Presentation and recording was done using E-Prime version 1.1 and a radiofrequency interference-free LCD monitor placed inside the scanner room. Each subject was instructed to attend to each word in the word list. For the phonological task, the subject was asked to respond regarding whether each target word from the word-list rhymed with the cue word presented at the beginning of the block. For the semantic task, the subject was instructed to attend to the meaning of the word and respond regarding whether each target word related semantically with the cue word. The response was made using an fMRI-compatible response system (Lumina LP 400, Cedrus Corp., San Pedro, CA). Of the 15 words in each list, 10 rhymed with or were related to the cue word, while 5 did not.
Figure 1.
Task design for one semantic task presented in this experiment. In the example, sugar, taste, and candy are related to the cue word, SWEET, while the word beat is not related.
Imaging Data Acquisition and Analysis
Images were acquired with a 3 Tesla (T) Philips scanner with an 8-channel SENSE head coil. Structural T1-weighted images were acquired using the T1 weighted 3D FFE pulse sequence (TR=25ms; TE=3.6ms; 512×512 matrix; 240mm FOV; 64 axial slices; 2.2mm slice thickness). BOLD contrast functional scans were acquired using a gradient echo EPI pulse sequence (TR=3s; TE=35ms; 80×80 matrix; 230mm FOV; 35 axial slices; 4mm slice thickness; α=90°). During functional scans, the first two imaging volumes were acquired to allow stabilization of longitudinal magnetization, and were discarded prior to analysis. The imaging data were analyzed using SPM8. Each bold series was corrected for slice acquisition timing, motion corrected for respiratory and other motion artifacts, normalized to the Montreal Neurological Institute (MNI) template after segmentation of the structural image, and resampled and smoothed using a 5mm Gaussian kernel to decrease spatial noise.
Statistical analysis was performed on individual data using the general linear model (GLM) as implemented in SPM8 using the six motion parameters as regressors. Functional connectivity was measured as the correlation between the average time series of different ROI-pairs. Four language-related ROI’s known to be activated by the phonological and semantic tasks (McDermott et al., 2003), including the left inferior frontal cortex (BA 44/45/46)-LIFG, left fusiform gyrus (BA37)-LFUS, left parietal cortex (BA7)-LPAR and left middle temporal gyrus (BA 21/22)-LMTG, were selected a priori. Spherical (10mm diameter) ROIs centered within the abovementioned regions were drawn and confirmed by a fellowship trained Behavioral and Cognitive Neurologist (D. Q. B.), on the MNI standard template.
Average time series for all voxels included in the ROI were extracted for each participant for each drug condition using MarsBar (Brett, Anton, Valabregue, & Poline, 2002), a SPM toolbox in MATLAB. To assess functional connectivity, correlations between the time series for these a priori pairs of ROIs were computed by calculating the correlation coefficient between the time series for each ROI pair for each subject. Fisher’s Z-transformation was applied to the computed correlations for each a priori ROI-pair to enable statistical comparison between groups and ROI-pairs.
Results
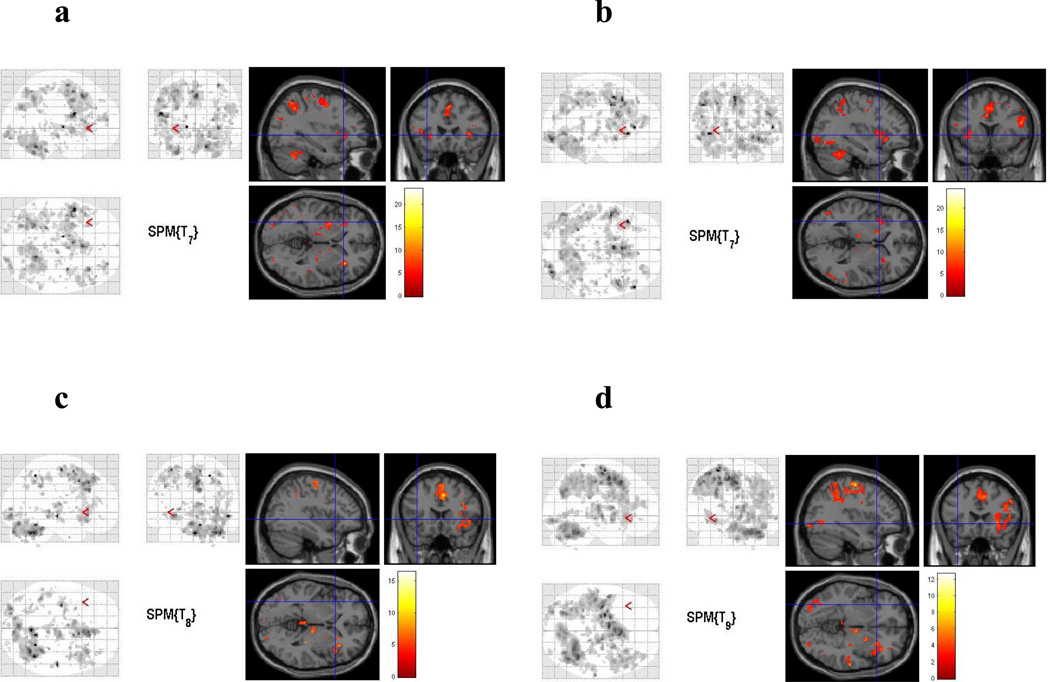
Average group activations maps generated through SPM8 revealed a pattern of activity similar to previous studies (McDermott et al., 2003; Tivarus et al., 2008) for all groups (Figure 2), including a set of brain regions comprised of the LIFG extending to the premotor and motor areas, bilateral middle frontal gyrus, left posterior middle temporal gyrus, left fusiform gyrus, bilateral occipital cortex and bilateral premotor cortex. There were no significant differences detected between the cocaine withdrawal and control groups in any of the a priori language-related ROIs for either the semantic or phonological task. Errors were rare on the task, with no subject responding with less than 90% correct under any condition for either task and either group.
Figure 2.
Average group activation maps during phonological and semantic processing for both groups, a) semantic, cocaine withdrawal, b) phonological, cocaine withdrawal, c) semantic, control, d) phonological, control. The left portion of each image demonstrates the Maximum Intensity Projection (MIP) generated trough SPM8 (presented in neurological convention).
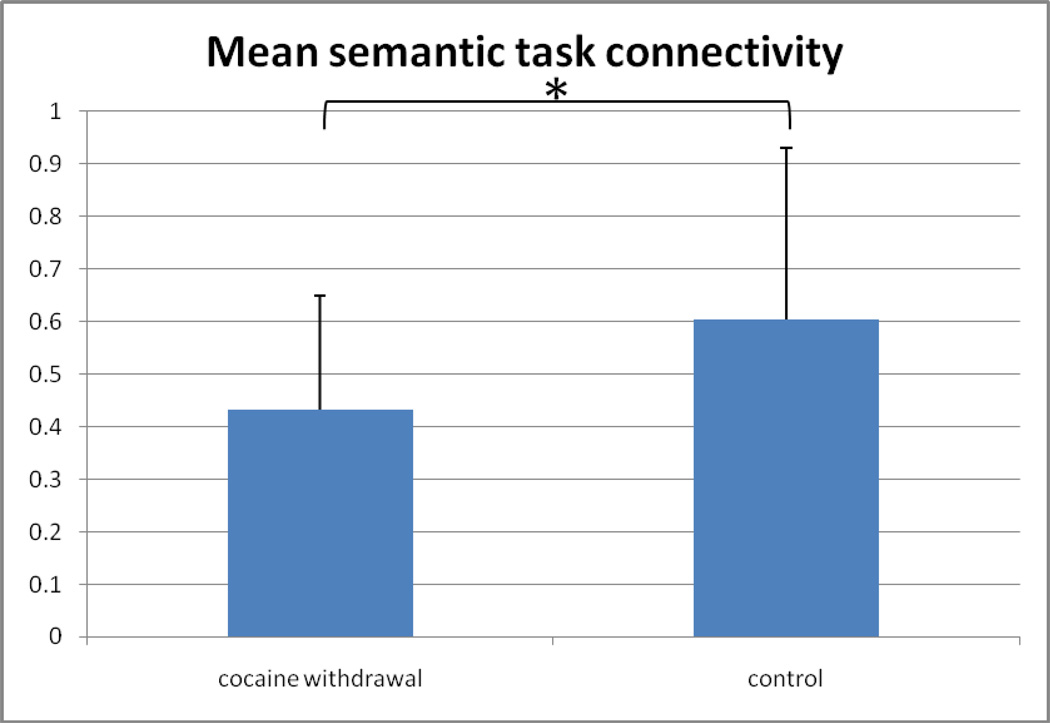
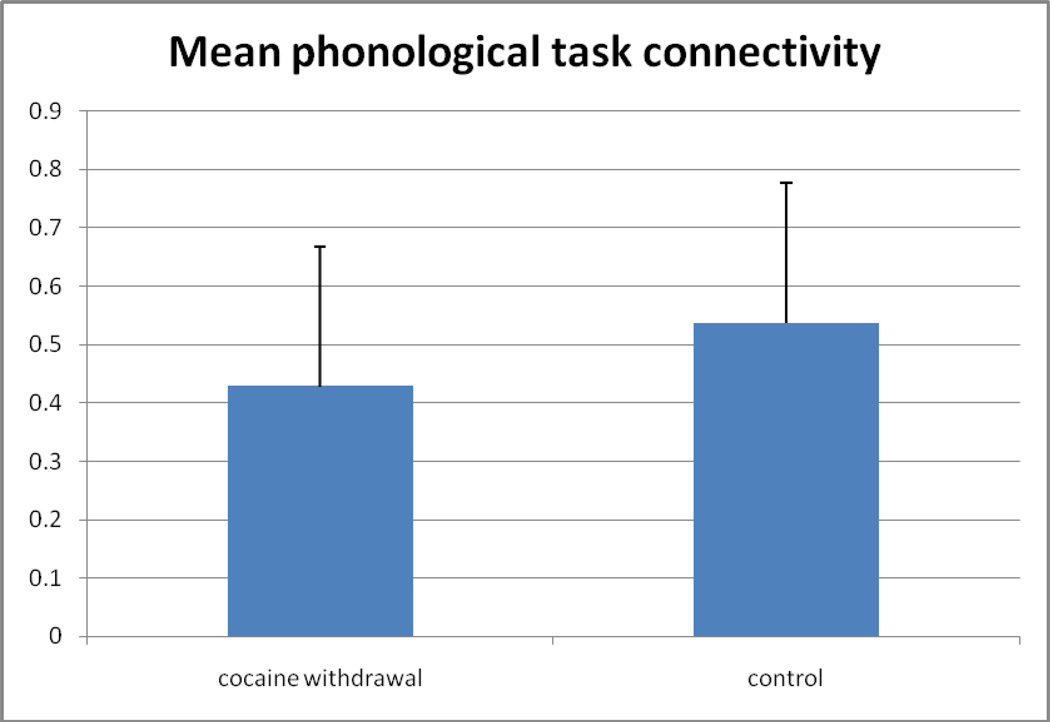
A 2 * 6 ANOVA (group*ROI-pair) revealed a main effect of greater connectivity for the control group (mean r=0.499; SDev=0.23, range 0.28–0.79, with one outlier below 0.15) as compared to the cocaine withdrawal group (mean r=0.381; SDev=0.17, range 0.22–0.64, with one outlier below 0.15) for the semantic task [F(1,11)=7.026; P=0.009] (Figure 3a), and a trend was observed in the same direction for greater connectivity for the control group (mean r=0.461; SDev=0.20, range 0.39–0.71, with one outlier below 0.15) as compared to the cocaine withdrawal group (mean r=0.375; SDev=0.20, range 0.23–0.59, with one outlier below 0.15) for the phonological task [F(1,11)=3.195; P=0.077] (Figure 3b). There was no significant drug*ROI-pair interaction revealed for either the phonological or semantic task. There was no main effect of ROI for the semantic task, but an isolated main effect for ROI-pair was observed for the phonological task [F(1,11)=2.504; P=0.035]. However, there was no significant difference between groups detected in connectivity between any of the individual ROI pairs for the phonological task. With the one outlier removed from each group, the main effect of connectivity became significant for both the semantic (P=0.0012) and phonological (P=0.036) tasks.
Figure 3.
Comparison of functional connectivity between groups. a) Mean z-transformed correlation coefficient (average over all ROI-pairs with standard deviation error bars) for the semantic task for both groups. b) Mean z-transformed correlation coefficient (average over all ROI-pairs with standard deviation error bars) for the ponological task for both groups. (* = p<0.01)
Since half of the cocaine withdrawal group was also using heroin, we compared functional connectivity between those with heroin exposure and those without heroin exposure. No difference was found with this comparison for either the semantic or phonological task (p>0.6 for both comparisons). Similarly, no differences were detected between those with and without recent alcohol, sedative, or cannabis abuse for either the semantic or phonological task (p>0.75 for all comparisons)
To determine whether anxiety might relate to functional connectivity among the cocaine withdrawal group, a Pearson correlation coefficient was performed between functional connectivity and anxiety scores. The correlation in the expected direction (decreasing connectivity with greater anxiety) did not reach significance for either the phonological (r=−0.21, p=n.s.) or semantic (r=−0.47, p=n.s.) task with this small sample size.
Discussion
These results did, as predicted, reveal decreased functional connectivity during language categorization among the cocaine withdrawal subjects as compared to controls. This effect was greatest for the semantic task, perhaps due to a greater effect on the more distributed semantic network in contrast to the phonological network, as is suggested by our previous work comparing functional connectivity between semantic phonological and semantic tasks (Tivarus et al., 2008). Thus, functional connectivity may have potential as an imaging marker for the cognitive impairments in acute cocaine withdrawal.
Cocaine withdrawal has been associated with a range of impairments on aspects of cognitive flexibility and language, with prominent effects observed in acute cocaine withdrawal (Kelley et al., 2005). Related findings are also observed in autism (Frith & Happé, 1994; Beversdorf et al., 2000), and are associated with decreased functional connectivity (Just et al., 2004; Koshino et al., 2005; Kana et al., 2006; Just et al., 2007). This also appears to be the case in acute cocaine withdrawal. In autism this is believed to be, to a significant degree, due to a structural decrease in connectivity between remote brain regions (Belmonte et al., 2004). It is uncertain as to whether this is the case in cocaine withdrawal, as well as whether it contributes to or results from cocaine use. Studies examining repeat imaging over the course of cocaine withdrawal would help to address this latter question.
Stress and anxiety are well known contributors to relapse in cocaine withdrawal, in both human and animal models (Erb, Shaham, & Stewart, 1996; Erb, Shaham, & Stewart, 1998; Sinha, Catapano, & O'Malley, 1999; Shaham, Erb, & Stewart, 2000; Stewart, 2003). While a large amount of literature in cocaine withdrawal is focused on the dopaminergic system (Volkow, Folwer, Wang, & Swanson, 2004), the noradrenergic system is also significantly impacted by cocaine use (Zhu, Shamburger, Li, & Ordway, 2000). Alterations in the noradrenergic system during chronic cocaine use in primates may be equivalent to or greater than the changes in the dopaminergic system (Macey, Smith, Nader, & Porrino, 2003; Stewart, 2003). Furthermore, the noradrenergic system is associated with aspects of the symptomatology of cocaine withdrawal, including anxiety (Charney, Woods, Krystal, Nagy, & Heninger, 1992). Further work will be necessary to determine whether administration of propranolol in acute cocaine withdrawal, known to improve the cognitive impairments in cocaine withdrawal (Kelley et al., 2007), increases connectivity as is observed in autism spectrum disorder (Narayanan et al., 2010). This is also of potential interest since propranolol is known to reverse the cognitive impairments resulting from stress in healthy controls (Alexander, Hillier, Smith, Tivarus, & Beversdorf, 2007).
Altered networks have previously been observed for sensorimotor control in chronic cocaine use (Hanlon, Wesley, Roth, Miller, and Porrino, 2010), and decreased functional connectivity has also been reported in the human visual motor cortices in association with cocaine administration (Li et al., 2000). During withdrawal, there is significant alteration in activation in response to working memory as well as to visuospatial attention during fMRI in cocaine withdrawal (Tomasi et al., 2007a; Tomasi et al., 2007b). Recently, mesencephalic activation has been reported with fMRI in cocaine addicts exposed to drug-related words, suggesting activation of the dopaminergic system in this setting (Goldstein et al., 2009). Furthermore, relative thamamic deactivation during working memory, believed to be related to mesocortical and mesolimbic dopaminergic projections, was found to predict poorer treatment response in cocaine withdrawal (Moeller et al., 2010). It will be of interest to determine whether our finding is related to outcomes as well, either as a predictor of outcomes or as a marker of response. The degree of variability in individual connectivity results will be important for this question, as well as what factors contribute to this variability. Aside from one low connectivity outlier in each of our groups, our variability was modest. Heroin and other drug use did not appear to contribute to variability, but future studies will need to further explore the role of anxiety, as well as the roles of any other potential factors, treatments, or other drugs of abuse.
Our finding of decreased connectivity during cocaine withdrawal, particularly during semantic tasks, may be at least in part a reflection of altered white matter integrity in cocaine withdrawal. This is supported by diffusion tensor imaging studies revealing greater radial diffusivity in the genu of the corpus callosum in cocaine withdrawal (Moeller et al., 2007), and decreased fractional anisotropy in the inferior frontal white matter in cocaine dependence (Lim et al., 2008). Subsequent studies have revealed that findings of increased diffusivity in the isthmus and rostral body of the corpus callosum in cocaine dependence persisted after correction for alcohol use, whereas the findings in fractional anisotropy became no longer significant after correction for history of alcohol use (Ma et al., 2009). However, reduced fractional anisotropy in the anterior corpus callosum was shown to relate to impulsivity and ability to discriminate target from catch stimuli in cocaine withdrawal patients (Moeller et al., 2005). White matter integrity as assessed by diffusion tensor imaging has been shown to predict treatment outcomes in cocaine withdrawal, with longer abstinence associated with greater white matter integrity (Xu et al., 2010). However, white matter integrity would not be expected to be affected by pharmacological agents. Functional connectivity, though, has in our previous work been shown to be affected by pharmacological agents (Narayanan et al., 2010), which are associated with cognitive effects as well (Beversdorf, Carpenter, Miller, Cios, & Hillier, 2008; Kelley et al., 2007). Future research will be necessary to examine how findings in white matter integrity, as assessed by diffusion tensor imaging, might interact with our findings.
Given the effects of cocaine on the dopaminergic system (Volkow et al., 2004), the effects of dopamine on neurovascular coupling (Choi et al., 2006) lead to some caution in interpretation of fMRI studies of cocaine withdrawal. These complex hemodynamic and metabolic changes have been directly observed in fMRI studies of cocaine administration (Schmidt et al., 2006; Lou et al., 2009). Furthermore, in addition to the dopaminergic effects, the potential upregulation of noradrenergic activity in acute withdrawal may have effects on fMRI that will need to be taken into account in future studies (Narayanan et al.,2010).
Many patients seeking treatment for help with cocaine withdrawal are also using other substances. This was an issue with our sample, resulting in a limitation to this study as the other substances could be contributing to our findings. This appears not to have been the case with heroin, alcohol, sedatives, or cannabis in our data, as above, but this will have to be further explored in subsequent studies examining patients only using cocaine. Furthermore, as suggested above, in future studies, reassessment after withdrawal is completed would be important to help confirm that the findings are due to acute withdrawal rather than due to baseline issues with the patient population. One further limitation of this study is the limited psychometric data available on these subjects during this work. Previous studies have demonstrated relationships between development of intelligence and functional connectivity during peri-adolescent development (Schmithorst & Holland, 2006). Therefore, future studies will need to closely monitor neuropsychological outcomes and how these relate to the imaging findings. Finally, the subjects performed at ceiling on our task, which was selected for its potential use in future pharmacological studies. Future work should examine tasks where performance can be simultaneously monitored, and performance can be compared to connectivity. This future work would also need to explore the potential contributions from attention and processing speed, which could also be affected by cocaine withdrawal and could impact functional connectivity, and larger studies would be needed to explore whether this effect is global or whether effects specific to certain ROI pairs can also be detected. Also, as our task required a target word to be retained for comparison to stimulus words, future studies would need to disentangle effects of working memory and its interactions with cocaine withdrawal and anxiety.
However, with the tasks utilized in this experiment, decreased connectivity does appear to be observed, particularly during semantic tasks, in acute cocaine withdrawal, as we had predicted based on the neuropsychological impairments observed in acute cocaine withdrawal. The greater effect on the semantic task may be due to semantic tasks utilizing the neocortex in a more distributed manner than tasks involving phonological processing (Tivarus et al., 2008), thus being more sensitive to conditions involving less flexible access to networks. Future studies will be needed to explore the potential of functional connectivity in this setting as a marker for treatment response or as a predictor of treatment response in cocaine withdrawal.
Acknowledgments
This research was funded by a Biomedical Research Grant from the National Alliance for Autism Research (1033/DB//01/201/-005-00-00), by grants from NIDA (R21 DA015734) and NINDS (K23 NS43222), the OSU Research Investment Fund and the Wright Center for Innovation, and the University of Missouri Department of Radiology Research Investment Fund.
References
- Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. J Cogn Neruosci. 2007;19:468–478. doi: 10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th edition) (DSM-IV) Washington: American Psychiatric Association; 1995. [Google Scholar]
- Ardila A, Rosselli M, Strumwasser S. Neuropsychological deficits in chronic cocaine abusers. Int J Neurosci. 1991;57:73–79. doi: 10.3109/00207459109150348. [DOI] [PubMed] [Google Scholar]
- Aronson TA, Craig TJ. Cocaine precipitation of panic disorder. Am J Psychiatry. 1986;143:643–645. doi: 10.1176/ajp.143.5.643. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Katzung VM, Moreland VJ, et al. Neuropsychologicalperformance of recently abstinent alcoholics and cocaine abusers. Drug Alcohol Depend. 1995;37:247–253. doi: 10.1016/0376-8716(94)01072-s. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beversdorf DQ. The role of the noradrenergic system in autism spectrum dosorders. In: Blatt GJ, editor. The Neurochemical Basis of Autism. New York: Springer; 2010. pp. 175–184. [Google Scholar]
- Beversdorf DQ, Carpenter AL, Miller RF, Cios JS, Hillier A. Effect of propranolol on verbal problem solving in autism spectrum disorder. Neurocase. 2008;14:378–383. doi: 10.1080/13554790802368661. [DOI] [PubMed] [Google Scholar]
- Beversdorf DQ, Hughes JD, Steinberg BA, Lewis LD, Heilman KM. Noradrenergic modulation of cognitive flexibility in problem solving. Neuroreport. 1999;10(13):2763–2767. doi: 10.1097/00001756-199909090-00012. [DOI] [PubMed] [Google Scholar]
- Beversdorf DQ, Narayanan A, Hillier A, Hughes JD. Neural network model of decreased context utilization in autism spectrum disorders. J Autism Dev Disord. 2007;37:1040–1048. doi: 10.1007/s10803-006-0242-7. [DOI] [PubMed] [Google Scholar]
- Beversdorf DQ, Smith BW, Crucian JP, et al. Increased discrimination of "false memories" in autism spectrum disorder. Proc Natl Acad Sci. 2000;97:8734–8737. doi: 10.1073/pnas.97.15.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Campbell HL, Tivarus ME, Hillier A, Beversdorf DQ. Increased task difficulty results in greater benefit of noradenergic modulation of cognitive flexibility. Pharmacol Biochem Behav. 2008;88:222–229. doi: 10.1016/j.pbb.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carise D, McLellan AT, Cacciola J, Love M, Cook T, Bovasso G, et al. Suggested specifications for a standardized Addiction Severity Index database. J Subst Abuse Treat. 2001;20(3):239–244. doi: 10.1016/s0740-5472(01)00161-1. [DOI] [PubMed] [Google Scholar]
- Charney DS, Woods SW, Krystal JH, Nagy LM, Heninger GR. Noradrenergic neruonal dysregulation in panic disorder: the effects of intravenous yohimbine and clonidine in panic disorder patients. Acta Psychiatr Scand. 1992;86:273–282. doi: 10.1111/j.1600-0447.1992.tb03266.x. [DOI] [PubMed] [Google Scholar]
- Choi J-K, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. NeuroImage. 2006;30:700–712. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Cohen IL. An artificial neural network analogue of learning in autism. Biol Psychiatry. 1994;36:5–20. doi: 10.1016/0006-3223(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 1996;128(4):408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18(14):5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. User's guide for the SCID-I Structured Clinical Interview for DSM-IV (User's guide for the SCID-I Structured Clinical Interview for DSM-IV) Biometrics Research Department, New York State Psychiatric Institute. 1996 [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- Frith U, Happé FGE. Autism: beyond theory of mind. Cognition. 1994;50:115–132. doi: 10.1016/0010-0277(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Gillen RW, Kranzler HR, Bauer LO, et al. Neuropsychologic findings in cocaine-dependent outpatients. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:1061–1076. doi: 10.1016/s0278-5846(98)00057-8. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, et al. Dopaminergic response to drug words in cocaine addiction. J Neurosci. 2009;29:6001–6006. doi: 10.1523/JNEUROSCI.4247-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Roth AJ, Miller MD, Porrino LJ. Loss of laterality in chronic cocaine users: an fMRI investigation of sensorimotor control. Psychiatry Res: Neuroimaging. 2010;181:15–23. doi: 10.1016/j.pscychresns.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier A, Campbell H, Keillor J, Phillips N, Beversdorf DQ. Decreased false memory for visually presented shapes and symbols among adults on the autism spectrum. J Clin Exp Neuropsychol. 2007;29:610–616. doi: 10.1080/13803390600878760. [DOI] [PubMed] [Google Scholar]
- Hoff AL, Riordan H, Morris L, et al. Effects of crack cocaine on neurocognitive function. Psychiatry Res. 1996;60:167–176. doi: 10.1016/0165-1781(96)02758-8. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an fMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of uncerconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley BJ, Yeager KR, Pepper TH, Beversdorf DQ. Cognitive impairment in acute cocaine withdrawal. Cogn Behav Neurol. 2005;18(2):108–112. doi: 10.1097/01.wnn.0000160823.61201.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley BJ, Yeager KR, Pepper TH, Bornstein RA, Beversdorf DQ. The effect of propranolol on cognitive flexibility and memory in acute cocaine withdrawal. Neurocase. 2007;13:320–327. doi: 10.1080/13554790701846148. [DOI] [PubMed] [Google Scholar]
- Kim N, Goel PK, Tivarus M, Hillier A, Beversdorf DQ. Independent component analysis of the effect of L-dopa on fMRI of language processing. Plos-ONE. 2010;5(8):e11933. doi: 10.1371/journal.pone.0011933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational analysis of present day American English. Providence: Brown University Press; 1967. [Google Scholar]
- Li S-L, Biswal B, Li Z, et al. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med. 2000;43:45–51. doi: 10.1002/(sici)1522-2594(200001)43:1<45::aid-mrm6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, et al. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcolol Depend. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou F, Schmidt KF, Fox GB, Ferris CF. Differential responses in CBF and CBV to cocaine as measured by fMRI: implications for pharmacological MRI signals derived oxygen metabolism assessment. J Psychiatr Res. 2009;43:1018–1024. doi: 10.1016/j.jpsychires.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Ma L, Hasan KM, Steinberg JL, et al. Diffusion tensor imaging in cocaine dependence: regional effects of cocaine on corpus callusom and effect of cocaine administration route. Drug Alcohol Depend. 2009;104:262–267. doi: 10.1016/j.drugalcdep.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Smith HR, Nader MA, Porrino LJ. Chronic cocaine self-administration upregulates the norepinephrine transporter and alters functional activity in the bed nucleus of the stria terminalis of the rhesus monkey. J Neurosci. 2003;23(1):12–16. doi: 10.1523/JNEUROSCI.23-01-00012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL. The basis of hyperspecificity in autism: a preliminary analysis based on properties of neural neta. J Autism Dev Disord. 2000;30:497–502. doi: 10.1023/a:1005576229109. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Petersen SE, Watson JM, Ojemann JG. A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia. 2003;41(3):293–303. doi: 10.1016/s0028-3932(02)00162-8. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Black JE, Malison RT, et al. Noradrenergic dysregulation during discontinuation of cocaine use in addicts. Acrh Gen Psychiatry. 1994;51:713–719. doi: 10.1001/archpsyc.1994.03950090045007. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, et al. Reduced anterior corups callosum white matter integrity is related to increased impulsivity and reduced discriminibility in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, et al. Diffusor tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Psychiatry Res: Neuroimaging. 2007;154:251–258. doi: 10.1016/j.pscychresns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Schmitz JM, et al. Working memory fMRI activation in cocaine-dependent subjects: association with treatment response. Psychiatry Res: Neuroimaging. 2010;181:174–182. doi: 10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan A, White CA, Saklayen S, Scaduto MJ, Carpenter AL, Abduljalil A, Schmalbrock P, Beversdorf DQ. Effect of propranolol on functional connectivity in autism spectrum disorder- a pilot study. Brain Imag Behav. 2010;4:189–197. doi: 10.1007/s11682-010-9098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley S, Adamse E, Heaton RK, et al. Neuropsychological impairment in chronic cocaine abusers. Am J Drug Alcohol Abuse. 1992;18:131–144. doi: 10.3109/00952999208992826. [DOI] [PubMed] [Google Scholar]
- Schmidt KF, Febo M, Shen Q, et al. Hemodynamic and metabolic changes induced by cocaine in anesthetized rat observed with multimodal functional MRI. Psychopharmacology (Berl) 2006;185:479–486. doi: 10.1007/s00213-006-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Functional MRI evidence for disparate developmental processes underlying intelligence in boys and girls. NeuroImage. 2006;31:1366–1379. doi: 10.1016/j.neuroimage.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O'Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999;142(4):343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Stewart J. Stress and relapse to drug seeking: studies in laboratory animals shed light on mechanisms and sources of long-term vulnerability. Am J Addict. 2003;12(1):1–17. [PubMed] [Google Scholar]
- Tivarus ME, Hillier A, Schmalbrock P, Beversdorf DQ. Functional connectivity in an fMRI study of semantic and phonological processes and the effect of L-Dopa. Brain Lang. 2008;104(1):42–50. doi: 10.1016/j.bandl.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, et al. Thalamo-cortical dysfucntion in cocaine abusers: implications in attention and perception. Psychiatry Res: Neuroimaging. 2007b;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, et al. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Res. 2007a;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abus and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Xu J, DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacology. 2010;35:1541–1549. doi: 10.1038/npp.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MY, Shamburger S, Li J, Ordway GA. Regulation of the human norepinephrine transporter by cocaine and amphetamine. J Pharmacol Exp Ther. 2000;295(3):951–959. [PubMed] [Google Scholar]