Abstract
Alcohol activates orosensory circuits that project to motivationally relevant limbic forebrain areas that control appetite, feeding and drinking. To date, limited data exists regarding the contribution of chemosensory-derived ethanol reinforcement to ethanol preference and consumption. Measures of taste reactivity to intra-orally infused ethanol have not found differences in initial orofacial responses to alcohol between alcohol-preferring (P) and – nonpreferring (NP) genetically selected rat lines. Yet, in voluntary intake tests P rats prefer highly-concentrated ethanol upon initial exposure, suggesting an early sensory-mediated attraction. Here, we directly compared self-initiated chemosensory responding for alcohol and prototypic sweet, bitter, and oral trigeminal stimuli among selectively bred P, NP, and non-selected Wistar (WI) outbred lines to determine whether differential sensory responsiveness to ethanol and its putative sensory components are phenotypically associated with genetically-influenced alcohol preference. Rats were tested for immediate short-term lick responses to alcohol (3–40%), sucrose (0.01–1 M), quinine (0.01–3 mM) and capsaicin (0.003–1 mM) in a brief-access assay designed to index orosensory-guided behavior. P rats exhibited elevated short-term lick responses to both alcohol and sucrose relative to NP and WI lines across a broad range of concentrations of each stimulus and in the absence of blood alcohol levels that would produce significant postabsorptive effects. There was no consistent relationship between genetically-mediated alcohol preference and orosensory avoidance of quinine or capsaicin. These data indicate that enhanced initial chemosensory attraction to ethanol and sweet stimuli are phenotypes associated with genetic alcohol preference and are considered within the framework of downstream activation of oral appetitive reward circuits.
Keywords: Chemosensory, ethanol, genetics, reinforcement, taste, trigeminal
Introduction
Investigation of genetically-mediated differences in responsiveness to ethanol that may contribute to vulnerability or resistance to high levels of ethanol ingestion has focused largely on the postabsorptive effects of the drug following entry of pharmacologically relevant levels of ethanol into brain (Bell et al. 2006, for review). Acting alongside its distinctive postabsorptive effects, ethanol also possesses highly salient sensory stimulus properties, including activation of sensory receptor and brain pathways that process appetitive sweet taste information (Blednov et al. 2008; Brasser, Norman & Lemon 2010; Hellekant et al. 1997; Kiefer & Mahadevan 1993; Kiefer et al. 1990; Lemon, Brasser & Smith 2004; Scinska et al. 2000) as well as oral trigeminal circuits sensitive to noxious or irritant stimulus input (Carstens, Kuenzler & Handwerker 1998; Danilova & Hellekant 2002). These sensory pathways are linked to limbic forebrain and cortical areas involved in controlling ingestive motivation and feeding (Hajnal, Smith & Norgren 2004; Norgren, Hajnal & Mungarndee 2006; Yamamoto 2006). A limited number of studies have addressed the contribution of orosensory-derived ethanol reinforcement to alcohol intake, despite the fact that ethanol sensory signals gain immediate access to the brain (within ms) temporally prior to the drug’s delayed postabsorptive effects under natural conditions of oral self-administration.
Although sensory-derived reinforcement from ethanol has often been assumed to play a minor role in ethanol intake relative to more well-characterized postingestive effects of the drug, recent data demonstrate that direct manipulation of taste substrates substantially alters oral alcohol preference and intake in alcohol-preferring rodents. Independent genetic deletion of several proteins critical for sweet taste reception and transduction in mammals [taste receptor type 1 member 3 (T1r3), alpha-gustducin, and transient receptor potential channel melastatin-5 (TRPM5)] eliminates alcohol preference in C57BL/6J mice (Blednov et al. 2008; Brasser et al. 2010). The reduction in oral ethanol preference in T1r3 receptor-deficient mice is accompanied by a suppression of central neural gustatory responses to ethanol in the nucleus of the solitary tract (NTS; Brasser et al. 2010), the first brain area to receive and process taste information. In humans, genetic risk for alcoholism as indexed by a positive family history of the disorder has repeatedly been associated with heightened preference for concentrated sweet solutions (Kampov-Polevoy, Garbutt & Khalitov 2003; Pepino & Mennella 2007; Wronski et al. 2007). This relationship has recently been identified in children with a positive family history of alcoholism but no prior experience with alcohol (Mennella et al. 2010). These findings indicate that genetic variation in sensory receptor or neural substrates involved in peripheral detection or central hedonic processing in the taste system may importantly regulate avidity for ethanol, consistent with a functional evolution of this sensory system to mediate ingestion.
To date, examination of variation in chemosensory responses to alcohol as a potential contributing factor to differential alcohol preference in alcohol-preferring and -avoiding rodents has involved measures of taste reactivity to intra-orally infused ethanol. In this paradigm, no inherent differences in initial ingestive or aversive orofacial responses (e.g., tongue protrusions, gapes, passive drip) to alcohol have been observed between alcohol-preferring (P) and –nonpreferring (NP) or high alcohol-drinking (HAD1) and low alcohol-drinking (LAD1) selectively bred rat lines (Bice & Kiefer 1990; Kiefer, Badia-Elder & Bice 1995). Nevertheless, in voluntary consumption tests P rats display high levels of initial preference for concentrated ethanol (72% preference for a 20% ethanol solution) within their first 22 hr of exposure (Olney et al. 2010), suggesting an early attraction to and minimal sensory avoidance of orally self-administered ethanol in this genetic line. P rats will also operantly self-administer oral ethanol over water at concentrations up to 30%, whereas NP rats avoid ethanol concentrations of 10% and higher (Murphy et al. 1989). Prior work in outbred rats has demonstrated that measurement of initial orosensory avidity for alcohol via short-term self-generated lick rates predicts alcohol consumption more readily than taste reactivity responses (Bice, Kiefer & Elder 1992; Kiefer & Dopp 1989), indicating that these indices of alcohol palatability may reflect different processes (Bice et al. 1992). These data are consistent with other studies that have shown differences in taste-elicited behavior when a tastant is administered via a self-generated response vs. experimenter-administered infusion (Fouquet, Oberling & Sandner 2001; Yamamoto, Fresquet & Sandner 2002).
In the present study, we directly measured self-initiated chemosensory responses to ethanol among selectively bred P, NP, and nonselected WI outbred lines utilizing a brief access orosensory assay to determine whether enhanced sensory responsiveness to ethanol is a phenotype associated with genetically-mediated alcohol preference. Psychophysical functions were additionally generated for stimuli representing ethanol’s putative individual sensory components (sweet, bitter, and trigeminal) to dissect the relationship between genetic alcohol preference and chemosensory sensitivity to these stimuli.
Materials and methods
Animals
Naive adult male selectively bred alcohol-preferring (P) and alcohol-nonpreferring (NP) rats (62nd-64th generations, n = 38/line; Indiana University School of Medicine Alcohol Research Center, Indianapolis, IN) and nonselected WI rats (n = 38; Harlan, Indianapolis, IN) ere used. The selectively bred lines were originally derived from a WI foundation stock as described by Lumeng and colleagues (1977) and thus the WI strain served as the appropriate control line. Rats were 17–19 weeks of age at the start of the experiment with mean body weights of 496.82 g (±5.63 SEM), 494.58 g (±8.85 SEM) and 508.18 g (±7.10 SEM) for P, NP, and WI lines, respectively. Animals were housed individually in standard tub cages (47 × 25.5 × 20.5 cm) in a vivarium maintained on a 12-h light/dark cycle and at an ambient temperature of approximately 23°C. All training and testing occurred during the light phase of the cycle. Food and water were available ad libitum in the home cage except for water restriction conditions noted below. Rats did not have access to food during chemosensory test sessions (20–40 min/day). All procedures were approved by the Institutional Animal Care and Use Committee at San Diego State University and were in accordance with National Institutes of Health guidelines.
Lick measurement
Training and testing were conducted in a Davis MS-160 lickometer (DiLog Instruments, Tallahassee, FL). This device allows for automated within-session presentation of multiple stimulus solutions to an animal in the form of individual sampling trials of short duration (e.g., 5–10 s) during which immediate lick responses are monitored. Rats gained access to a stainless steel drinking spout on each trial through a small aperture in the front wall of a 30 × 14.5 × 15 cm testing chamber, with availability of the spout determined by the opening and closing of a motorized shutter. Delivery of a given stimulus solution was determined by the positioning of a motorized table/block apparatus just outside of the chamber that could accommodate up to 16 different stimulus tubes. Lick activity was detected via a high-frequency AC contact circuit and all data collection (i.e., lick counts and latencies), as well as presentation and timing of all stimuli, were controlled precisely via computer and associated software.
Training
Rats initially were given four days of training with water as the only available stimulus in order to familiarize them with the lickometer apparatus and to train them to lick the spout to receive fluid. During the training phase, an overnight water restriction schedule was in effect in order to motivate performance on the task, with rats receiving their sole daily fluid intake in the apparatus. On the first two training days, subjects were given a 30-min period of continuous access to water through a single sipper tube that began when the animal took its first lick. On the last two days of training, rats were allowed access to water during 40 5-s trials separated by 10-s inter-presentation intervals to familiarize them with the brief access trial procedure. All subjects successfully completed Days 1–2 sipper tube training with an average of 2407.05 (±51.58 SEM) licks/session and Days 3–4 brief access training with an average of 92.85% (±1.20 SEM) trials sampled/session. Water was returned to the home cage immediately following completion of the training phase and testing commenced 72 h later.
Stimulus testing
Rats (n = 22/line) were tested for short-term lick responses to the following four stimuli: ethanol (3, 5, 10, 15, 25 and 40%), sucrose (0.01, 0.03, 0.06, 0.1, 0.3 and 1 M), quinine HCl (0.01, 0.03, 0.1, 0.3, 1 and 3 mM), and capsaicin (0.003, 0.01, 0.03, 0.1, 0.3 and 1 mM). Each stimulus was tested over five consecutive daily sessions, and stimuli were presented in serial order (ethanol, sucrose, quinine, capsaicin) during separate weeks with a 1-week break interposed between testing of different stimuli. Within each test session, rats were presented with all six concentrations of a given stimulus and a vehicle control (see below) during discrete, brief-access exposure trials (10-s trials: ethanol, sucrose and capsaicin; 5-s trials: quinine). Solutions were presented randomly within blocks of 7 trials (4 blocks total), such that animals were allowed to sample each stimulus concentration and the vehicle control once/block and four times during a given test session (20 trial replications total at each concentration over the five test days for each stimulus). Each stimulus trial was preceded by a 2-s deionized water rinse trial that served to rinse the oral cavity and minimize sensory adaptation effects. Upon opening of the shutter on each presentation, 30 s was allowed for trial initiation and the trial duration began with the animal’s first lick on the sipper tube. If a rat failed to initiate sampling during the 30-s period, the shutter closed and the table was automatically repositioned for the next trial. All presentations were separated by 10-s inter-presentation intervals during which the shutter remained closed. Test sessions were approximately 20–40 min in length. Water was available ad libitum in the home cage during ethanol and sucrose testing and during the weeks intervening stimulus testing. Overnight water restriction was in effect during testing of solely non-preferred stimuli (quinine, capsaicin) in order to promote sampling of these stimuli. Brief-access testing has been used extensively to measure orosensory responsiveness in rodents (Brasser, Mozhui & Smith 2005; Dotson & Spector 2004; Ellingson, Silbaugh & Brasser 2009). The specific trial durations for each stimulus were based on those previously shown to produce reliable concentration-response functions for these stimuli in brief-access tests (Brasser et al. 2005; Ellingson et al. 2009). Longer (10-s) trial durations for ethanol and sucrose prevent truncating the expression of any appetitive responses to these stimuli, and 10-s trial durations for capsaicin are employed to allow for the delayed response latency to this stimulus on the tongue (Okuni 1977).
Blood ethanol determination
An additional group of rats from each line (n = 16/line) were tested for short-term lick responses to ethanol alone using an identical protocol to that described above, and tail blood samples were collected 1 h after the onset of their last session of ethanol testing for assessment of blood alcohol level (BAL). Blood alcohol concentrations in P rats have previously been shown to peak 1 hr following the onset of oral self-administration in a limited access paradigm (Murphy et al. 1986). Forty µl of blood was collected from the tip of the tail of each subject into a heparinized capillary tube. Samples were centrifuged and frozen (−80° C) immediately after collection and ethanol concentrations of 5.0 µl plasma aliquots were later assessed with an Analox GL5 alcohol analyzer (Analox Instruments, Lunenburg, MA). This instrument determines alcohol concentration as a direct proportion to the rate of oxygen consumption in a controlled reaction between sample alcohol and alcohol oxidase. Values < 3.0 mg/dl are functionally equivalent to zero (due to measurement floor). Before analyzing test samples, the instrument was calibrated with a 50 mg/dl ethanol standard and the accuracy verified with a quality control serum of known concentration. To ensure measurement stability, calibration was checked with a standard sample after every 10 test samples and the instrument recalibrated if the error was greater than ±5 mg/dl.
Stimuli
Solutions were prepared fresh prior to testing using reagent grade chemicals (Sigma-Aldrich, St. Louis, MO) and were presented at room temperature. Ethanol, sucrose and quinine HCl were dissolved in deionized water and capsaicin in a vehicle of 1.5% ethanol/1.5% Tween 80 in deionized water. Stimulus concentrations were selected to encompass a full behavioral orosensory response range based upon previous work (Brasser et al. 2005; Ellingson et al. 2009).
Statistical analysis
For each individual rat, the mean number of licks to each stimulus concentration and to the vehicle control was calculated across all trials sampled over the five test days for each stimulus. Nonsampled trials (i.e., those with 0 licks) were excluded from the data analysis such that only valid trials for which rats were attending to the tube and had initiated sampling were evaluated. In order to standardize responses to each stimulus solution with responses to vehicle (which serves to control for individual and/or line differences in licking behavior that are not stimulus driven), lick difference scores for each rat were determined by subtracting the mean number of licks to vehicle from the mean number of licks to each stimulus concentration. A lick difference score of zero indicates equal responding to a given stimulus concentration relative to vehicle, with positive and negative difference scores representing, respectively, increased or decreased licking behavior to a stimulus relative to vehicle. To additionally assess potential line differences in olfactory and motivational responses to each stimulus, mean latency to initiate licking across all sampled trials at each concentration and total number of trials sampled at each concentration across the five test days were also calculated. Concentration-dependent differences in latency to initiate licking in brief-access tests have previously been suggested to reflect the influence of olfactory input (Rhinehart-Doty et al. 1994).
Mean lick count, latency to first lick and trials sampled data for each stimulus were subsequently analyzed using 3 (line) × 7 (concentration) mixed analyses of variance (ANOVAs), with line as a between-subject factor and concentration as a within-subject factor.Lick difference score data were analyzed using 3 (line) × 6 (concentration) mixed ANOVAs. Blood ethanol concentrations were assessed via one-way ANOVA with line as a factor. Only rats that had a complete set of lick data for a given stimulus (i.e., sampled at least one trial at every concentration) were included in the analyses for that stimulus (ethanol: 28 P, 11 NP, 26 WI; sucrose: 21 P, 17 NP, 17 WI; quinine: 22 P, 22 NP, 19 WI; capsaicin: 22 P, 22 NP, 13 WI). Significant interactions from the overall ANOVAs were further analyzed using one-way ANOVAs to test for simple effects followed by Fisher’s least significant difference (LSD) test (α = 0.015) where appropriate. Alpha level for all other statistical tests was 0.05.
Results
Chemosensory responses to ethanol
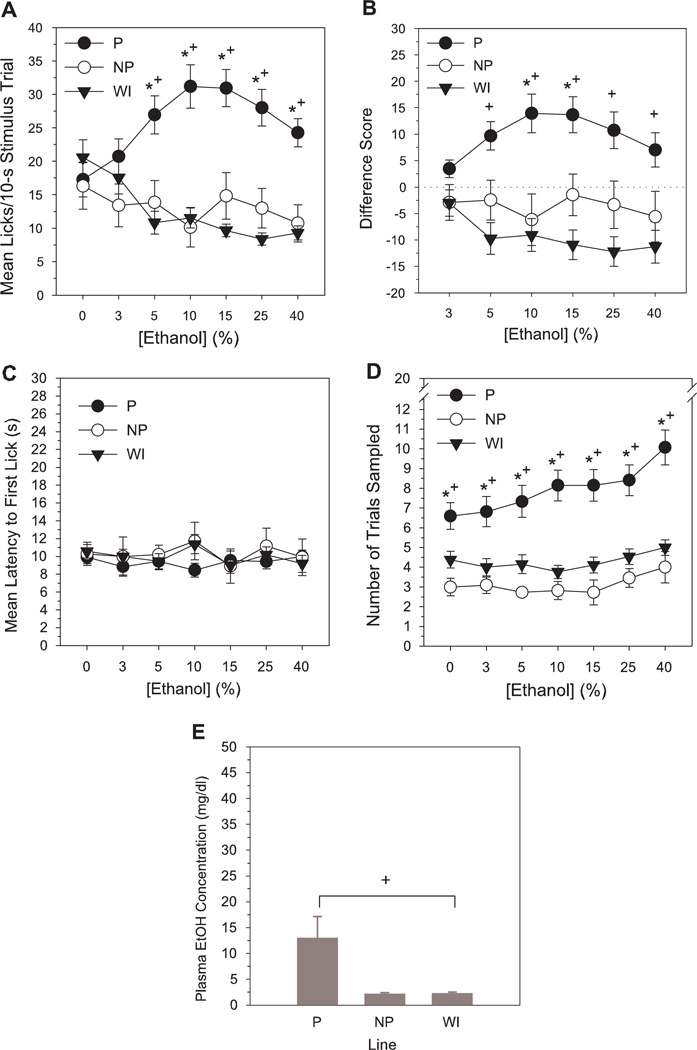
P rats displayed elevated short-term lick responses to ethanol relative to NP and WI rats across a broad range of concentrations (Table 1, Fig. 1A). One-way analyses to test for simple effects of line at each concentration indicated higher mean lick counts/10-s trial in P rats compared to NP or WI rats at 5–40% ethanol, but no line differences in responses to vehicle or 3% ethanol (LSD test, P’s < 0.015, Table 2). Across-concentration analyses for each line indicated that mean lick responses of P rats to 5–40% ethanol were greater than to water vehicle; 5–25% > 3%; and 10–15% > 40% (LSD test, P’s < 0.015). By contrast, WI rats exhibited suppressed responding to 5–40% ethanol compared to vehicle or 3% (LSD test, P’s < 0.015). Lick responses of NP rats did not significantly differ across concentration (Table 2). Analyses of the standardized lick difference scores indicated equivalent responses across line to 3% ethanol but significantly higher lick difference scores in P rats than in NP and WI rats at 10–15% and 5–40%, respectively (LSD test, P’s < 0.015, Tables 1 & 2, Fig. 1B).Across-concentration differences for the standardized lick scores were similar to that of the absolute mean lick responses [P—5–25% > 3%; and 10–15% > 40%; WI—5–40% < 3%; NP— no across-concentration differences (LSD test, P’s < 0.015, Table 2)].
Table 1.
Summary of major ANOVA effects
| Line | Concentration | Line × Concentration | |||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | P | df | F | P | df | F | P | |
| Ethanol | |||||||||
| Mean licks | 2,62 | 17.99 | 0.00* | 6,372 | 1.06 | 0.39 | 12,372 | 8.18 | 0.00* |
| Difference score | 2,62 | 13.77 | 0.00* | 5,310 | 1.42 | 0.22 | 10,310 | 5.28 | 0.00* |
| Latency | 2,62 | 0.84 | 0.44 | 6,372 | 0.58 | 0.75 | 12,372 | 0.61 | 0.83 |
| Trials sampled | 2,61 | 19.15 | 0.00* | 6,366 | 6.91 | 0.00* | 12,366 | 2.43 | 0.00* |
| Sucrose | |||||||||
| Mean licks | 2,52 | 14.87 | 0.00* | 6,312 | 190.82 | 0.00* | 12,312 | 5.73 | 0.00* |
| Difference score | 2,52 | 12.46 | 0.00* | 5,260 | 162.95 | 0.00* | 10,260 | 3.84 | 0.00* |
| Latency | 2,52 | 15.79 | 0.00* | 6,312 | 4.55 | 0.00* | 12,312 | 1.39 | 0.17 |
| Trials sampled | 2,51 | 11.60 | 0.00* | 6,306 | 48.78 | 0.00* | 12,306 | 2.05 | 0.02* |
| Quinine | |||||||||
| Mean licks | 2,60 | 24.58 | 0.00* | 6,360 | 551.84 | 0.00* | 12,360 | 10.32 | 0.00* |
| Difference score | 2,60 | 0.47 | 0.63 | 5,300 | 520.78 | 0.00* | 10,300 | 12.44 | 0.00* |
| Latency | 2,60 | 15.93 | 0.00* | 6,360 | 67.90 | 0.00* | 12,360 | 1.07 | 0.38 |
| Trials sampled | 2,57 | 12.45 | 0.00* | 6,342 | 75.97 | 0.00* | 12,342 | 3.74 | 0.00* |
| Capsaicin | |||||||||
| Mean licks | 2,54 | 29.93 | 0.00* | 6,324 | 180.90 | 0.00* | 12,324 | 7.11 | 0.00* |
| Difference score | 2,54 | 1.48 | 0.24 | 5,270 | 181.93 | 0.00* | 10,270 | 8.62 | 0.00* |
| Latency | 2,54 | 25.83 | 0.00* | 6,324 | 15.48 | 0.00* | 12,324 | 0.63 | 0.82 |
| Trials sampled | 2,54 | 19.05 | 0.00* | 6,324 | 26.14 | 0.00* | 12,324 | 0.81 | 0.64 |
Significant effect, P < 0.05
Figure 1.
Mean number of licks/stimulus trial (A), lick difference scores (B), latency to initiate licking (s) (C) and total number of trials sampled (D) for ethanol as a function of concentration in selectively bred P, NP and non-selected WI rats tested in a brief-access exposure assay assessing orosensory responsiveness. Lower panel (E) represents mean blood ethanol levels (mg/dl) in each line 1 h after the onset of their final brief-access test session. Lick difference score = mean licks to stimulus - mean licks to deionized water. Mean (±SEM) data are shown. Significant difference between *P vs NP, +P vs WI, #NP vs WI (P< 0.015).
Table 2.
Simple effects of line and concentration for significant interaction effects from omnibus ANOVAs
| Mean licks | Difference score | Trials sampled | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Line | df | F | P | df | F | P | df | F | P |
| Ethanol (%) | |||||||||
| 0 | 2,62 | 0.58 | 0.56 | -- | -- | -- | 2,61 | 8.18 | 0.00* |
| 3 | 2,62 | 1.34 | 0.27 | 2,62 | 2.76 | 0.07 | 2,61 | 8.49 | 0.00* |
| 5 | 2,62 | 12.56 | 0.00* | 2,62 | 12.52 | 0.00* | 2,61 | 11.04 | 0.00* |
| 10 | 2,62 | 19.31 | 0.00* | 2,62 | 12.74 | 0.00* | 2,61 | 20.16 | 0.00* |
| 15 | 2,62 | 25.76 | 0.00* | 2,62 | 16.53 | 0.00* | 2,61 | 16.71 | 0.00* |
| 25 | 2,62 | 23.48 | 0.00* | 2,62 | 13.72 | 0.00* | 2,61 | 15.60 | 0.00* |
| 40 | 2,62 | 21.44 | 0.00* | 2,62 | 8.66 | 0.00* | 2,61 | 19.57 | 0.00* |
| Sucrose (M) | |||||||||
| 0 | 2,52 | 1.09 | 0.34 | -- | -- | -- | 2,51 | 12.21 | 0.00* |
| 0.01 | 2,52 | 2.17 | 0.12 | 2,52 | 1.11 | 0.34 | 2,51 | 12.75 | 0.00* |
| 0.03 | 2,52 | 12.44 | 0.00* | 2,52 | 16.34 | 0.00* | 2,51 | 12.37 | 0.00* |
| 0.06 | 2,52 | 14.17 | 0.00* | 2,52 | 10.76 | 0.00* | 2,51 | 11.43 | 0.00* |
| 0.1 | 2,52 | 17.04 | 0.00* | 2,52 | 13.16 | 0.00* | 2,51 | 7.94 | 0.00* |
| 0.3 | 2,52 | 16.87 | 0.00* | 2,52 | 8.06 | 0.00* | 2,51 | 6.31 | 0.00* |
| 1 | 2,52 | 18.98 | 0.00* | 2,52 | 5.75 | 0.01* | 2,51 | 5.88 | 0.01* |
| Quinine (mM) | |||||||||
| 0 | 2,60 | 11.92 | 0.00* | -- | -- | -- | 2,57 | 8.49 | 0.00* |
| 0.01 | 2,60 | 19.22 | 0.00* | 2,60 | 3.69 | 0.03* | 2,57 | 6.13 | 0.00* |
| 0.03 | 2,60 | 19.22 | 0.00* | 2,60 | 6.27 | 0.00* | 2,57 | 5.64 | 0.01* |
| 0.1 | 2,60 | 27.64 | 0.00* | 2,60 | 7.61 | 0.00* | 2,57 | 9.94 | 0.00* |
| 0.3 | 2,60 | 22.94 | 0.00* | 2,60 | 1.46 | 0.24 | 2,57 | 11.00 | 0.00* |
| 1 | 2,60 | 14.00 | 0.00* | 2,60 | 2.17 | 0.12 | 2,57 | 14.94 | 0.00* |
| ws3 | 2,60 | 0.43 | 0.65 | 2,60 | 9.98 | 0.00* | 2,57 | 14.51 | 0.00* |
| Capsaicin (mM) | |||||||||
| 0 | 2,54 | 16.23 | 0.00* | -- | -- | -- | -- | -- | -- |
| 0.003 | 2,54 | 23.98 | 0.00* | 2,54 | 1.51 | 0.23 | -- | -- | -- |
| 0.01 | 2,54 | 26.83 | 0.00* | 2,54 | 1.19 | 0.31 | -- | -- | -- |
| 0.03 | 2,54 | 28.56 | 0.00* | 2,54 | 0.55 | 0.58 | -- | -- | -- |
| 0.1 | 2,54 | 23.42 | 0.00* | 2,54 | 0.61 | 0.55 | -- | -- | -- |
| 0.3 | 2,54 | 9.16 | 0.00* | 2,54 | 2.91 | 0.06 | -- | -- | -- |
| 1 | 2,54 | 10.34 | 0.00* | 2,54 | 10.75 | 0.00* | -- | -- | -- |
| Concentration | |||||||||
| Ethanol | |||||||||
| P | 6,162 | 8.69 | 0.00* | 5,135 | 6.59 | 0.00* | 6,156 | 9.89 | 0.00* |
| NP | 6,60 | 0.64 | 0.70 | 5,50 | 0.50 | 0.78 | 6,60 | 1.22 | 0.31 |
| WI | 6,150 | 8.66 | 0.00* | 5,125 | 5.97 | 0.00* | 6,150 | 1.62 | 0.14 |
| Sucrose | |||||||||
| P | 6,120 | 155.13 | 0.00* | 5,100 | 116.97 | 0.00* | 6,120 | 19.59 | 0.00* |
| NP | 6,96 | 35.22 | 0.00* | 5,80 | 35.49 | 0.00* | 6,90 | 18.59 | 0.00* |
| WI | 6,96 | 46.11 | 0.00* | 5,80 | 39.80 | 0.00* | 6,96 | 11.83 | 0.00* |
| Quinine | |||||||||
| P | 6,126 | 387.23 | 0.00* | 5,105 | 356.66 | 0.00* | 6,120 | 26.02 | 0.00* |
| NP | 6,126 | 157.49 | 0.00* | 5,105 | 144.80 | 0.00* | 6,120 | 40.62 | 0.00* |
| WI | 6,108 | 108.42 | 0.00* | 5,90 | 101.51 | 0.00* | 6,102 | 16.37 | 0.00* |
| Capsaicin | |||||||||
| P | 6,126 | 121.77 | 0.00* | 5,105 | 120.55 | 0.00* | -- | -- | -- |
| NP | 6,126 | 32.64 | 0.00* | 5,105 | 30.09 | 0.00* | -- | -- | -- |
| WI | 6,72 | 48.34 | 0.00* | 5,60 | 48.27 | 0.00* | -- | -- | -- |
Significant effect, P < 0.05
Latency to initiate licking on sampled trials did not differ as a function of ethanol concentration or line (Table 1, Fig. 1C). P rats, however, sampled more trials during ethanol testing than either NP or WI lines and increased their trial sampling frequency with increasing ethanol concentration (Table 1, Fig. 1D). Tests for simple effects of line indicated a greater number of trials sampled by P rats relative to NP and WI rats at every concentration, including vehicle (LSD test, P’s < 0.015, Table 2). Across concentration, neither NP or WI rats displayed significant differences in trial sampling frequency. In P rats, number of trials sampled at 10–40% was greater than for vehicle and 3%; and 40% > all other concentrations (LSD test, P’s < 0.015, Table 2). The latter concentration-dependent increase in trial sampling frequency indicates that P rats were able to distinguish among ethanol solutions based upon sensory input and displayed an avidity for higher concentrations.
Blood ethanol levels
In the subset of ethanol-tested subjects from which blood samples were collected following brief-access testing, ethanol in plasma of NP and WI rats was undetectable [2.17 (±0.24 SEM) and 2.30 (±0.22 SEM) mg/dl, respectively] and in P rats was present at very low levels [12.98 (±4.17 SEM) mg/dl]. Mean blood ethanol values of P rats were greater than those of WI rats (LSD test, P < 0.015) and differed at a borderline level (P = 0.03) from those of NP rats (effect of line: F2, 24 = 4.69, P < 0.05, Fig. 1E). These results indicate that detectable levels of ethanol in blood are present in P rats following brief access testing, but at a magnitude (<13 mg/dl) below that likely to produce significant postabsorptive effects.Mean total duration of ethanol exposure (across all concentrations) per brief access test session was 97.85 (±8.78 SEM), 37.64 (±4.15 SEM) and 51.15 (±3.49 SEM) s for P, NP, and WI rats, respectively.
Chemosensory responses to sucrose
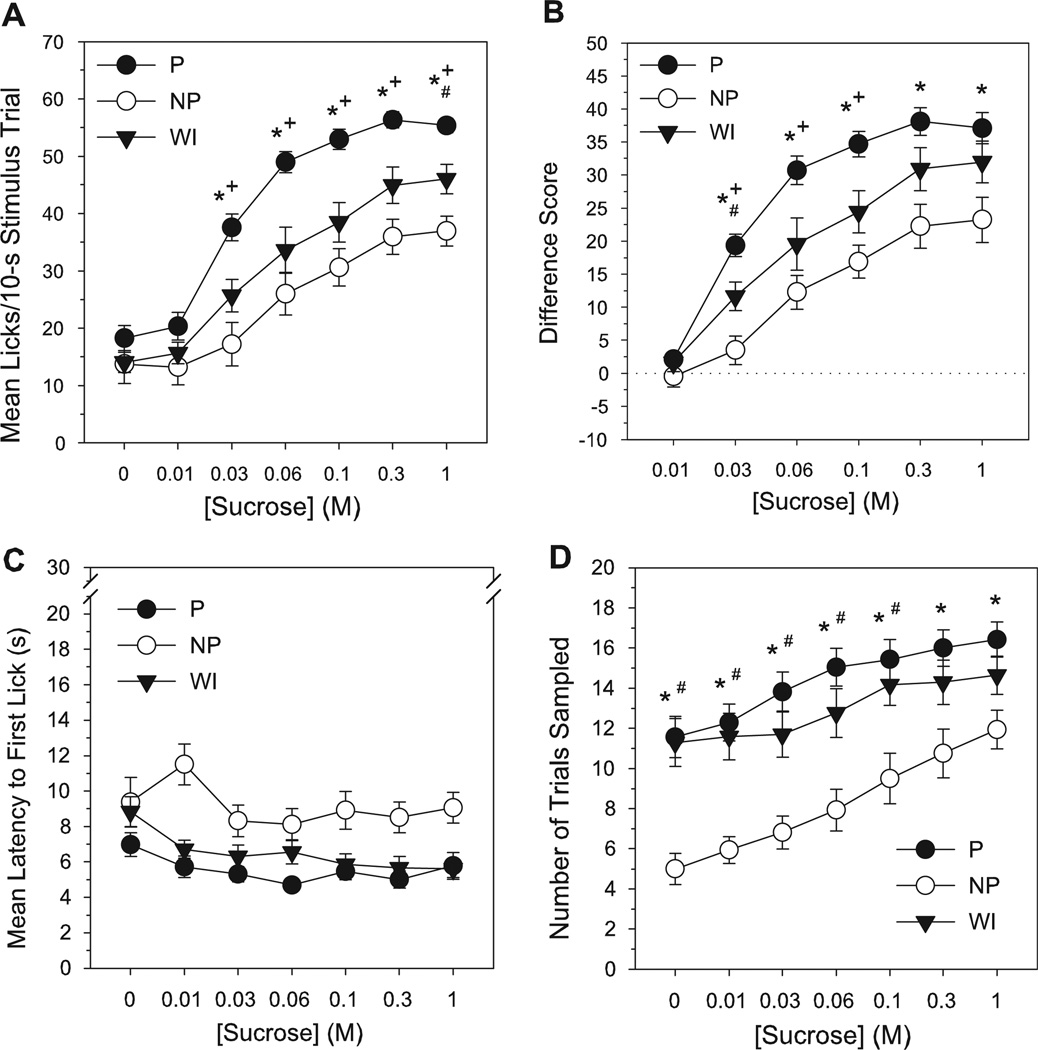
P rats exhibited higher mean lick counts to sucrose compared to NP and WI lines at multiple concentrations (Table 1, Fig. 2A). Lick responses to sucrose were elevated in P rats relative to NP and WI rats at concentrations of 0.03–1 M, but no line differences existed in responses to vehicle or 0.01 M (LSD test, P’s < 0.015, Table 2). Additionally, WI rats displayed a greater number of raw lick counts to sucrose than NP rats at the 1 M concentration (LSD test, P < 0.015). All lines displayed an increase in responding to sucrose with increasing concentration (P—0–0.01 M < 0.03–1 M; 0.03 M < 0.06–1 M; and 0.06 M < 0.3–1 M; NP—0–0.03 M < 0.06–1 M; 0.06 M < 0.3–1 M; and 0.1 M < 1 M; WI—0–0.01 M < 0.03–1 M; 0.03 M < 0.06–1 M; 0.06 M < 0.3–1 M; and 0.1 M < 1 M, LSD test, P’s < 0.015, Table 2). When individual responses to sucrose were standardized relative to vehicle, lick difference scores in P rats were higher than those in NP at 0.03–1 M and above those of WI rats at 0.03–0.1 M (LSD test, P’s < 0.015, Tables 1 & 2, Fig. 2B). Lick difference scores for sucrose in WI rats were also greater than those in NP at the 0.03 M concentration (LSD test, P < 0.015). Responses to 0.01 M sucrose did not differ across line (Table 2). All lines showed a monotonic increase in sucrose difference scores with rising concentration (all concentration differences similar to those reported above for raw lick counts, LSD test, P’s < 0.015, Table 2). Thus, while all lines displayed an avidity for sucrose, P rats exhibited the most vigorous sensory-guided licking behavior for sucrose, NP the least, and responses of nonselected WI controls were intermediate to the two selected lines.
Figure 2.
Mean number of licks/stimulus trial (A), lick difference scores (B), latency to initiate licking (s) (C) and total number of trials sampled (D) for sucrose as a function of concentration in selectively bred P, NP and non-selected WI rats during brief-access testing. Lick difference score = mean licks to stimulus - mean licks to deionized water. Mean (±SEM) data are shown. Significant difference between *P vs NP, +P vs WI, #NP vs WI (P < 0.015).
Regardless of concentration, NP rats were slower to initiate licking during sucrose testing than either P or WI rats, with the latter two lines not differing in latency to sample (LSD test, P’s < 0.015, Table 1, Fig. 2C). The overall ANOVA on the latency data also revealed a main effect of concentration, with latencies to initiate lick responses to 0.03–1 M sucrose significantly shorter than to vehicle, and latencies to sample 0.06 and 0.3 M < 0.01 M (LSD test, P’s < 0.015, Table 1). NP rats also sampled significantly fewer trials than P rats at every concentration during sucrose testing and fewer trials than WI rats at 0–0.1 M (LSD test, P’s < 0.015, Tables 1 & 2, Fig. 2D). All lines, however, increased their trial sampling frequency as sucrose concentration increased, indicating greater attraction to higher sucrose concentrations relative to lower concentrations (P—0–0.01 M < 0.03–1 M; and 0.03 M < 0.1–1 M; NP—0 M < 0.06–1 M; 0.01–0.03 M < 0.1–1 M; 0.06 M < 0.3–1 M; and 0.1 M <1 M; WI— 0 M < 0.06–1 M; 0.01–0.03 M < 0.1–1 M; and 0.06 M < 0.3–1 M, LSD test, P’s < 0.015, Table 2).
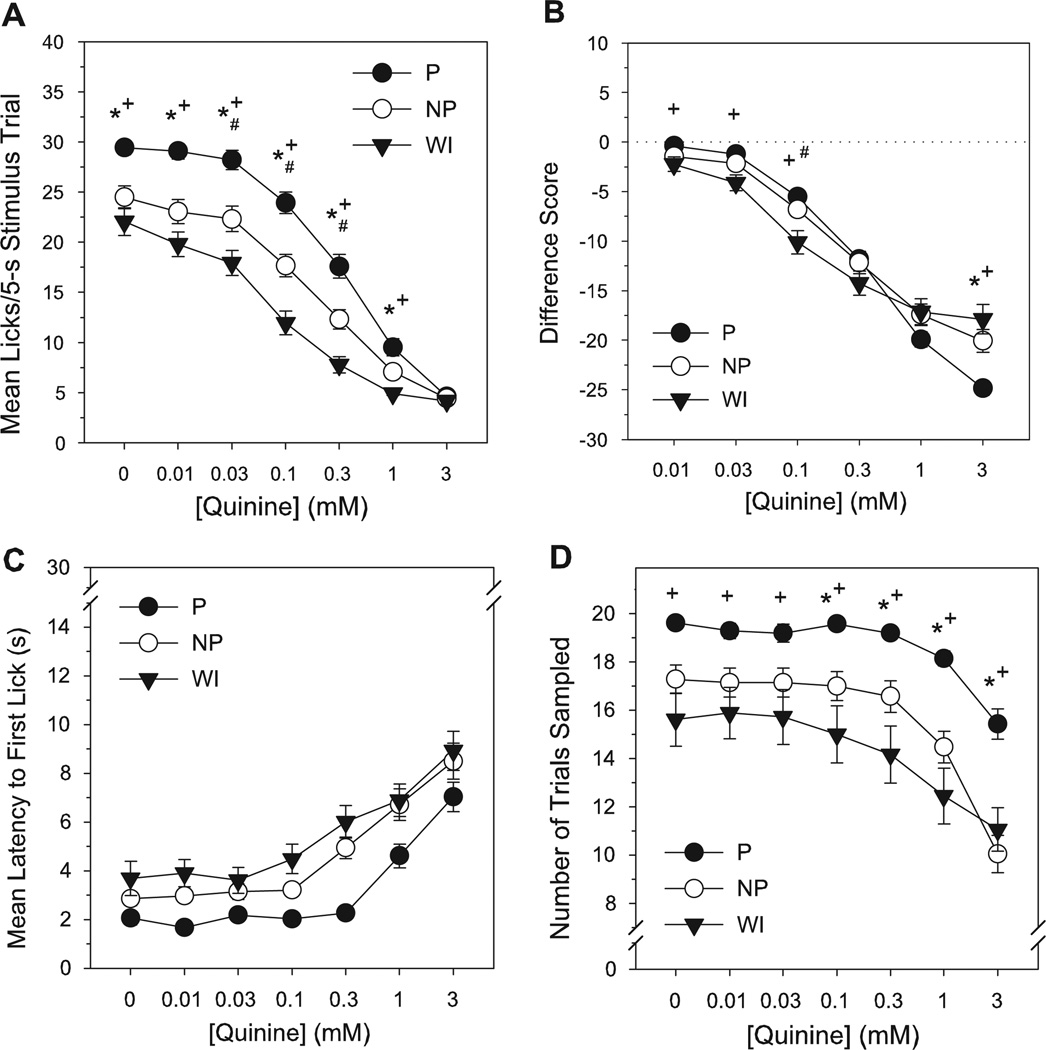
Chemosensory responses to quinine
NP and WI rats displayed lower mean lick responses than P rats for 0.01–1 mM quinine, but also exhibited fewer responses than Ps to the water vehicle during quinine testing (LSD test, P’s < 0.015, Tables 1 & 2, Fig. 3A). WI rats also licked significantly less than NP at 0.03–0.3 mM concentrations (LSD test, P’s < 0.015), but did not differ from NP rats in responding to vehicle, 0.01 or 1 mM (P’s ≥ 0.02). No line differences existed at the highest quinine concentration (3 mM), which produced a uniformly low number of lick counts (Table 2). All lines displayed a concentration-dependent avoidance of quinine, with lick responses declining as quinine concentration increased (P, NP—0–0.03 mM > 0.1–3 mM; 0.1 mM > 0.3–3 mM; 0.3 mM > 1–3 mM; and 1 mM > 3 mM; WI—0 mM > 0.03–3 mM; 0.01–0.03 mM > 0.1–3 mM; 0.1 mM > 0.3–3 mM; and 0.3 mM > 1–3 mM, LSD test, P’s < 0.015, Table 2). When responses to quinine were standardized to account for individual/line differences in responses to vehicle, WI rats showed slightly suppressed difference scores for quinine relative to P and NP rats at 0.01–0.1 mM and 0.1 mM concentrations, respectively (LSD test, P’s < 0.015, Tables 1 & 2, Fig. 3B). Additionally, lick difference scores in P rats at the highest quinine concentration were lower than in NP and WI lines (P’s < 0.01). The latter difference, however, appeared to result from a floor effect in raw lick counts to quinine among all lines at the 3 mM concentration, and thus a reciprocal reflection of line differences in responses to vehicle vs. a true difference in quinine sensitivity. Across concentration, lick difference scores in all lines decreased in a monotonic fashion (all concentration differences similar to those reported above for raw lick counts, LSD test, P’s < 0.015, Table 2). Taken together, the data indicate a somewhat greater aversion to quinine among WI rats compared to P and NP, and no difference in quinine avoidance between the selected lines.
Figure 3.
Mean number of licks/stimulus trial (A), lick difference scores (B), latency to initiate licking (s) (C) and total number of trials sampled (D) for quinine as a function of concentration in selectively bred P, NP and non-selected WI rats during brief-access testing. Lick difference score = mean licks to stimulus - mean licks to deionized water. Mean (±SEM) data are shown. Significant difference between *P vs NP, +P vs WI, #NP vs WI (P < 0.015).
NP and WI rats were slower to initiate sampling than P rats during quinine testing, regardless of concentration (LSD test, P’s < 0.015, Table 1, Fig. 3C). Latency to sample also varied as a function of concentration, with no difference in trial response latencies up to 0.1 mM, but progressively longer latencies to initiate licking with each successive increase in quinine concentration from 0.1 to 3 mM (LSD test, P’s < 0.015, Table 1). WI rats sampled significantly less trials than P rats at every concentration during quinine testing, but did not differ from NP rats in quinine trials sampled (LSD test, P’s < 0.015, Tables 1 & 2, Fig. 3D). Trial sampling frequency was also lower in NP rats relative to P at 0.1–3 mM concentrations (LSD test, P’s < 0.015). For all lines, the number of trials sampled for quinine declined at concentrations above 0.3 mM, but did not differ at lower concentrations (P, NP—0–0.3 mM >1–3 mM; and 1 mM > 3 mM; WI—0–0.3 mM > 1–3 mM, LSD test, P’s < 0.015, Table 2). Thus, olfactory input appeared to similarly guide the sampling behavior of all lines to quinine, with a decreased propensity and increased latency to sample intermediate to high quinine concentrations.
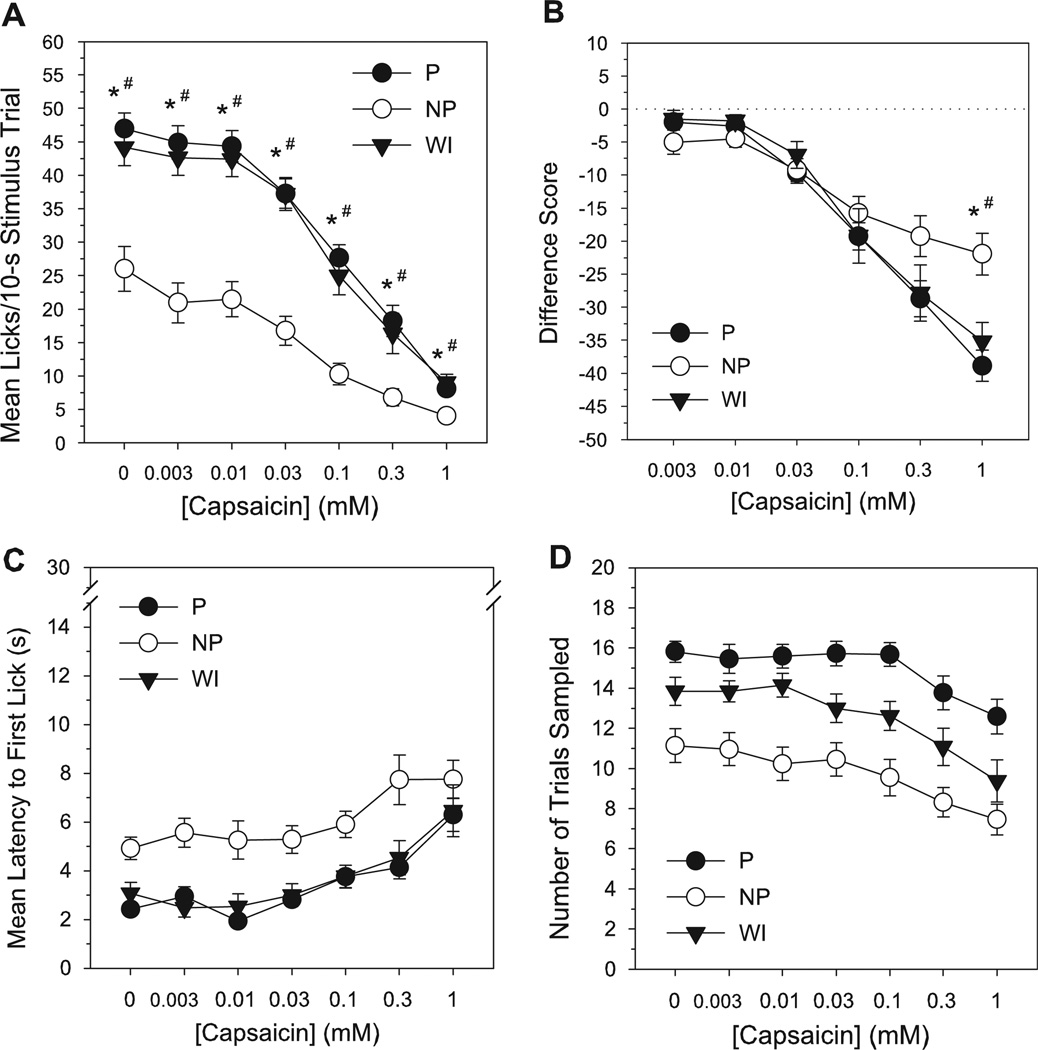
Chemosensory responses to capsaicin
NP rats made fewer licks than P and WI lines to all concentrations during capsaicin testing, including vehicle (LSD test, P’s < 0.015, Tables 1 & 2, Fig. 4A). P and WI rats did not differ in short-term lick responses to capsaicin at any concentration (LSD test, P’s ≥ 0.39). All lines exhibited an orderly suppression of licking behavior as capsaicin concentration increased (P—0–0.01 mM > 0.03–1 mM; 0.03 mM > 0.1–1 mM; 0.1 mM > 0.3–1 mM; and 0.3 mM > 1 mM; NP—0 mM > 0.03–1 mM; 0.003–0.03 mM > 0.1–1 mM; and 0.1 mM > 1 mM; WI—0–0.03 mM > 0.1–1 mM; 0.1 mM > 0.3–1 mM; and 0.3 mM > 1 mM, LSD test, P’s < 0.015, Table 2). Analyses of the lick difference scores indicated a lack of line differences in capsaicin responding for all except the highest (1 mM) concentration, at which lick difference scores in NP rats were higher compared to P or WI (LSD test, P’s < 0.015, Tables 1 & 2, Fig. 4B). However, this difference again appeared to be the product of a near floor on absolute lick counts to 1 mM capsaicin for all lines, and thus an inverse reflection of line differences in vehicle responses vs. a reduced sensitivity to capsaicin in the NP line at the 1 mM concentration. Lick difference scores in all lines decreased as a function of concentration (all concentration differences similar to those reported above for raw lick counts, LSD test, P’s < 0.015, Table 2).
Figure 4.
Mean number of licks/stimulus trial (A), lick difference scores (B), latency to initiate licking (s) (C) and total number of trials sampled (D) for capsaicin as a function of concentration in selectively bred P, NP and non-selected WI rats during brief-access testing. Lick difference score = mean licks to stimulus - mean licks to vehicle (1.5% ethanol/1.5% Tween 80 in deionized water). Mean (±SEM) data are shown. Significant difference between *P vs NP, +P vs WI, #NP vs WI (P < 0.015).
NP rats had longer trial response latencies overall during capsaicin testing than P and WI rats, while the latter two lines displayed equivalent latencies to sample (LSD test, P’s < 0.015, Table 1, Fig. 4C). Across concentration, latency to initiate licking was elevated at the two highest capsaicin concentrations (0.3 and 1 mM), but did not differ for concentrations < 0.1 mM (LSD test: 0–0.003 mM < 0.3–1 mM; 0.01 mM < 0.1–1 mM; 0.03 mM < 0.3–1 mM; and 0.1–0.3 mM < 1 mM, P’s < 0.015, Table 1). NP rats also sampled fewer trials during capsaicin testing than both P and WI lines regardless of concentration, but P and WI lines did not significantly differ in number of capsaicin trials sampled (LSD test, P’s < 0.015, Table 1, Fig. 4D). For all lines, trial sampling frequency at the two highest capsaicin concentrations was less than at lower concentrations (LSD test: 0 > 0.1–1 mM; 0.003–0.1 mM > 0.3–1 mM; and 0.3 mM > 1 mM, P’s < 0.015, Table 1).
Discussion
The present data indicate that elevated initial chemosensory attraction to ethanol is a behavioral phenotype associated with genetically-mediated ethanol preference in the alcohol-preferring (P) line of rats. In a brief access orosensory assay measuring self-initiated immediate lick responses to a range of ethanol concentrations, P rats exhibited increased short-term lick rates to 5–40% ethanol relative to vehicle, while NP and WI rats respectively displayed uniformly low lick rates to ethanol or a concentration-dependent sensory avoidance. P rats further displayed an orderly increase in the number of trials sampled as a function of ethanol concentration, indicating a chemosensory avidity for higher concentrations, whereas NP and WI lines showed no variation in trial sampling frequency across ethanol concentration. These findings are consistent with previous data that the P line of rats prefers highly concentrated (20%) ethanol in voluntary intake tests upon initial exposure (Olney et al. 2010) and will operantly self-administer oral ethanol over water at concentrations up to 30% (Murphy et al. 1989). Furthermore, recent neurophysiological data indicate that the oral signal for ethanol in taste-sensitive neurons of the NTS in the P line differs from that of a nonselected WI control line (Lemon, Wilson & Brasser 2011).
While all lines exhibited an orosensory attraction to sucrose, evidenced by a monotonic increase in lick responses and trials sampled with rising concentration, P rats displayed significantly higher short-term lick rates to sucrose than both WI control and alcohol-nonpreferring (NP) rats. This enhanced orosensory-mediated responding for sucrose in the P line concurs with findings of elevated long-term intake of both sucrose and saccharin in several independently selected alcohol-preferring rat lines (P, AA, HAD, WHP) compared with their divergent –nonpreferring lines (NP, ANA, LAD, WLP; Dyr & Kostowski 2000; Sinclair et al. 1992; Stewart et al. 1994, 1998; Woods et al. 2003), although the nature of the sucrose concentration-response curve differs in long-term intake tests (i.e., inverted U-shape; Stewart et al. 1994) presumably due to postabsorptive caloric feedback at high sucrose concentrations. Ethanol and sweet stimuli are known to activate overlapping gustatory receptor and neural circuits (Blednov et al. 2008; Brasser et al. 2010; Hellekant et al. 1997; Kiefer & Mahadevan 1993; Kiefer et al. 1990; Lemon et al. 2004; Scinska et al. 2000), suggesting genetic variation in specific substrates along peripheral or central taste pathways may mediate the elevated chemosensory attraction to both ethanol and sweeteners in the P line. The greater oral avidity for sucrose in P rats relative to NP and WI lines parallels the heightened sweet taste preference observed in certain subtypes of human alcoholics as well as individuals at genetic risk for alcoholism (Kampov-Polevoy, Garbutt & Janowsky 1997; Kampov-Polevoy et al. 2003; Mennella et al. 2010; Pepino & Mennella 2007; Wronski et al. 2007) and supports this phenotype as a potential biomarker for vulnerability to alcoholism involving a familial or genetic component. These findings also emphasize that elevated sweet preference precedes, and is not simply a consequence of, nutritional or sensory alterations associated with chronic alcohol consumption.
All lines displayed a concentration-dependent sensory avoidance of quinine, with lick rates and number of trials sampled decreasing, and latencies to respond increasing, with rising quinine concentration. P rats exhibited an overall higher lick rate, quicker latency to sample, and increased trial sampling frequency during quinine testing relative to NP and WI lines, however, this effect was not specific to quinine trials but was observed for vehicle trials as well. Following standardization for individual variation in vehicle responses, minimal differences existed among lines in quinine avoidance, with WI rats showing slightly greater avoidance than both P and NP lines at low to intermediate quinine concentrations. These data indicate no reliable association between genetically influenced alcohol preference and chemosensory responses to quinine. It has likewise been shown that P and NP lines display no difference in intake of the bitter substance sucrose octaacetate (Stewart et al. 1994). The lack of a clear relationship between oral behavioral responsiveness to ethanol and bitter tastants is consistent with several neurophysiological reports to date that have not found a relationship between neural taste responses elicited by ethanol and bitter stimuli in gustatory circuits of P or WI rats (Lemon et al. 2011), outbred Sprague-Dawley rats (Lemon et al. 2004; Lemon & Smith 2005), C57BL/6J mice (Brasser et al. 2010) and nonhuman primates (Hellekant et al. 1997). Although conditioned taste aversions to alcohol cross-generalize to mixtures of sucrose and quinine in rats (Kiefer & Mahadevan 1993) and to either tastant alone in C57BL/6J mice (Blizard 2007), it is possible that generalization may be occurring between an aversive somatosensory component of ethanol and non-preferred taste stimuli. Ethanol is known to directly stimulate peripheral and central trigeminal pathways (Carstens et al. 1998; Danilova & Hellekant 2002), which process noxious chemical and thermal input from the oral cavity, including activation of sensory nociceptors sensitive to capsaicin [transient receptor potential channel vanilloid receptor 1 (TRPV1); Trevisani et al. 2002]. Direct manipulation of the trigeminal system via knockout of the TRPV1 receptor also reduces ethanol chemosensory avoidance (Ellingson et al. 2009) and increases ethanol preference (Blednov & Harris 2009) in mice, suggesting decreased sensitivity to ethanol’s trigeminal properties may enhance its intake.
The present study examined responsiveness to the prototypic trigeminal stimulant capsaicin among P, NP and WI control lines to determine whether any differences in chemosensory ethanol preference among these lines may be related to variation in oral trigeminal sensitivity. All lines were found to exhibit a concentration-dependent avoidance of capsaicin, with decreasing lick rates and trial sampling frequency, and increased latencies to initiate responding, with increasing capsaicin concentration. NP rats displayed substantially lower lick rates, fewer trials sampled, and longer latencies to respond during capsaicin testing than both P and WI lines, however, this effect was observed during both capsaicin and vehicle trials, suggesting a nonspecific lower overall motivation to respond. When responses to capsaicin were standardized relative to vehicle, similar capsaicin sensitivity was observed across lines. Nevertheless, it should be considered that the pronounced reduction of both vehicle and capsaicin responses in NP rats relative to P and WI lines during capsaicin testing could potentially reflect an increased aversive response to capsaicin that resulted in an overall behavioral suppression of lick responses. It is unlikely that the low ethanol concentration (1.5%) present in the vehicle solution for capsaicin could explain the suppression of vehicle responses in NP rats compared to P and WI lines, given that no line differences were observed in the same animals in response to ethanol concentrations ≤ to 3% (Figure 1A). The current study is the only investigation we are aware of to date that has examined differences in trigeminal responding between selectively bred alcohol-preferring and –nonpreferring rat lines. While these data do not support a clear association between genetic alcohol preference and trigeminal sensitivity as assessed via capsaicin responses in these lines, a comprehensive array of trigeminal stimuli activating multiple trigeminal receptor subtypes [i.e., transient receptor potential channel ankyrin-1 (TRPA1), transient receptor potential channel melastatin-8 (TRPM8), TRPV1, etc.] should be tested to further address this question. Additionally, no studies have yet assessed differences in peripheral or central neurophysiological responses to oral trigeminal stimulants, including ethanol, among alcohol-preferring and –nonpreferring lines. These studies are important to determine the precise mechanisms mediating ethanol chemosensory aversion in nonselected and nonpreferring lines and the absence of such avoidance in genetically preferring rats.
The alcohol-preferring P line of rats is known to gain reinforcement from ethanol independent of oral administration. P rats will self-administer ethanol intragastrically (Waller et al. 1984) as well as directly into the ventral tegmental area of the brain (Gatto et al. 1994) and have also been shown to differ from NP rats in responses to systemically administered ethanol (Bell et al. 2006, for review). Such studies provide support for postingestive reinforcing effects of ethanol in the P line, however, they do not directly test or provide data about the relative contribution of orally derived reinforcement from ethanol, which is not dichotomous with the drug’s postabsorptive effects. A very limited number of studies have attempted to directly examine sensory mediated responsiveness to ethanol driven via stimulation of oral pathways in the absence of significant postabsorptive feedback. A key prior study by Bice and Kiefer (1990) measuring taste reactivity responses to ethanol in genetically selected P and NP rats found no difference between these lines in initial ingestive orofacial responses (i.e., tongue protrusions, lateral tongue protrusions) to intra-orally infused alcohol (5–40%) or a selected concentration of sucrose (0.3 M), suggesting P and NP rats exhibit similar innate reflexive orosensory reactions to ethanol and sweet stimuli. Nevertheless, the present data indicate a large difference in self-initiated initial short-term lick responses to ethanol between the P line and both the NP and WI lines at comparable stimulus concentrations and similar durations of ethanol exposure as in prior taste reactivity studies (1 min ethanol exposure/session—Bice & Kiefer 1990; 1.6 min mean ethanol exposure/session in P rats—current study). Significant methodological differences between the taste reactivity test and the brief access assay utilized here include both the method of stimulus delivery (experimenter-administered vs. self-administered) and the measure used to index orosensory responsiveness (orofacial reactions vs. lick responses). Differences in taste-related behavior have previously been demonstrated when a stimulus is delivered via self-generated instrumental lick responses vs. experimenter-administered intraoral infusion (Fouquet et al. 2001; Yamamoto et al. 2002). Additionally, initial short-term lick rates in alcohol-naive animals more readily predict subsequent alcohol consumption than taste reactivity responses (Bice et al. 1992; Kiefer & Dopp 1989). These and other data indicate that while taste reactivity responses appear to reflect intrinsic brain mechanisms of palatability (Berridge 2000, for review), self-initiated short-term lick measures of orosensory behavior likely involve both motivational and palatability components and their corresponding brain substrates.
Enhanced sensory-driven behavioral attraction to alcohol and sweet stimuli in alcohol-preferring P rats compared to their –nonpreferring and nonselected control lines may potentially be mediated by variation in neurobiological substrates at any point along pathways that process orosensory information, from variation in peripheral taste receptor distribution/sensitivity to differences in downstream limbic or cortical mechanisms activated by oral stimulation. A recent neurophysiological study examining activation of taste neurons in the NTS of selectively bred P and WI control lines in response to ethanol applied to the tongue and palate indicates that differences in the processing of oral ethanol signals between these lines occurs as early as the brain stem (Lemon et al. 2011). While oral ethanol stimulation in both lines similarly and most strongly activated NTS neurons with heightened sensitivity to sucrose, only in P rats did ethanol also produce concentration-dependent activation of neurons with low sucrose sensitivity as well as evoke a unique across-neuron response pattern that was not clearly associated with any prototypic taste quality class. Responses of NTS neurons in P and WI lines to a concentration series of sucrose, however, did not differ at the level of the NTS (Lemon et al. 2011). These latter data suggest that differences in behavioral chemosensory responses to sucrose and to ethanol between these lines may also be mediated by variation in neural substrates in downstream gustatory pathways. A proportion of oral alcohol-responsive neurons in the brain stem directly project to limbic forebrain areas including the nucleus accumbens (Cho & Li 2007) and oral ethanol self-administration in rodents has been shown to immediately elevate accumbal dopamine levels prior to ethanol itself reaching the brain (Doyon et al. 2003). Similarly, oral sucrose stimulation via sham-feeding produces an immediate concentration-dependent increase in dopamine release in the nucleus accumbens (Hajnal et al. 2004), which is attenuated by selective damage to limbic taste projections (Norgren et al. 2006). Alcohol-preferring P rats are known to display innate differences in mesolimbic DA system function compared to both NP and WI control lines (Bell et al. 2006, for review), including a more pronounced increase in extracellular accumbal DA levels than W rats with oral ethanol administration (Weiss et al. 1993). These findings raise the possibility that genetic variation in limbic substrates activated by appetitive oral inputs may also potentially contribute to the elevated chemosensory avidity for ethanol and sweet stimuli in P rats relative to NP and nonselected WI lines.
Overall, the present findings warrant further investigation of the brain pathways activated by ethanol orosensory input and how this information is integrated with ethanol’s postingestive effects to promote and maintain alcohol ingestion. Not only may elevated initial sensitivity to the sensory reinforcing properties of ethanol serve as a permissive factor for further intake, but ethanol’s chemosensory cues may become powerful mediators of ethanol craving and relapse after chronic association with the drug’s postabsorptive effects. Although it is now well-established that genetic factors contribute to variation in sweet and bitter taste perception and oral preferences (Reed, Tanaka & McDaniel 2006, for review), a more thorough understanding of the genetic basis of differential sensory responses to alcohol is necessary to advance knowledge of the mechanisms that regulate propensity for alcohol intake.
Acknowledgements
This research was supported by NIH Grants AA015741 (SMB) and AA015512 (Indiana Alcohol Research Center).
Footnotes
The authors declare no conflict of interest.
Authors Contribution
SMB and CHL were responsible for the study concept and design. BCS, MJK and JJO contributed to the acquisition of animal data. MJK performed the blood ethanol analysis. SMB and BCS were responsible for data analysis and interpretation of findings and drafted the manuscript. CHL provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
References
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Bice PJ, Kiefer SW. Taste reactivity in alcohol preferring and nonpreferring rats. Alcohol Clin Exp Res. 1990;14:721–727. doi: 10.1111/j.1530-0277.1990.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Bice PJ, Kiefer SW, Elder NB. Evaluating the palatability of alcohol for rats with measures of taste reactivity, consumption, and lick rate. Alcohol. 1992;9:381–387. doi: 10.1016/0741-8329(92)90036-a. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Harris RA. Deletion of vanilloid receptor (TRPV1) in mice alters behavioral effects of ethanol. Neuropharmacology. 2009;56:814–820. doi: 10.1016/j.neuropharm.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 2008;7:1–13. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA. Sweet and bitter taste of ethanol in C57BL/6J and DBA2/J mouse strains. Behav Genet. 2007;37:146–159. doi: 10.1007/s10519-006-9121-4. [DOI] [PubMed] [Google Scholar]
- Brasser SM, Mozhui K, Smith DV. Differential covariation in taste responsiveness to bitter stimuli in rats. Chem Senses. 2005;30:793–799. doi: 10.1093/chemse/bji071. [DOI] [PubMed] [Google Scholar]
- Brasser SM, Norman MB, Lemon CH. T1r3 taste receptor involvement in gustatory neural responses to ethanol and oral ethanol preference. Physiol Genomics. 2010;41:232–243. doi: 10.1152/physiolgenomics.00113.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens E, Kuenzler N, Handwerker HO. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J Neurophysiol. 1998;80:465–492. doi: 10.1152/jn.1998.80.2.465. [DOI] [PubMed] [Google Scholar]
- Cho YK, Li CS. Activation of the nucleus accumbens differentially modulates gustatory/ethanol neurons in the NST and PbN in the hamster. Soc Neurosci Abstr. 2007;713:14. [Google Scholar]
- Danilova V, Hellekant G. Oral sensation of ethanol in a primate model III: responses in the lingual branch of the trigeminal nerve of Macaca mulatta. Alcohol. 2002;26:3–16. doi: 10.1016/s0741-8329(01)00178-1. [DOI] [PubMed] [Google Scholar]
- Dotson CD, Spector AC. The relative affective potency of glycine, L-serine and sucrose as assessed by a brief-access taste test in inbred strains of mice. Chem Senses. 2004;29:489–498. doi: 10.1093/chemse/bjh051. [DOI] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1573–1582. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- Dyr W, Kostowski W. Animal model of ethanol abuse: rats selectively bred for high and low voluntary alcohol intake. Acta Pol Pharm. 2000;(57 Suppl):90–92. [PubMed] [Google Scholar]
- Ellingson JM, Silbaugh BC, Brasser SM. Reduced oral ethanol avoidance in mice lacking transient receptor potential channel vanilloid receptor 1. Behav Genet. 2009;39:62–72. doi: 10.1007/s10519-008-9232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquet N, Oberling P, Sandner G. Differential effect of free intake versus oral perfusion of sucrose in conditioned taste aversion in rats. Physiol Behav. 2001;74:465–474. doi: 10.1016/s0031-9384(01)00585-6. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regulatory Integrative Comp Physiol. 2004;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Danilova V, Roberts T, Ninomiya Y. The taste of ethanol in a primate model: I. Chorda tympani nerve response in Macaca mulatta. Alcohol. 1997;14:473–484. doi: 10.1016/s0741-8329(96)00215-7. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy A, Garbutt JC, Janowsky D. Evidence of preference for a high-concentration sucrose solution in alcoholic men. Am J Psychiatry. 1997;154:269–270. doi: 10.1176/ajp.154.2.269. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Khalitov E. Family history of alcoholism and response to sweets. Alcohol Clin Exp Res. 2003;27:1743–1749. doi: 10.1097/01.ALC.0000093739.05809.DD. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Badia-Elder N, Bice PJ. Taste reactivity in high alcohol drinking and low alcohol drinking rats. Alcohol Clin Exp Res. 1995;19:279–284. doi: 10.1111/j.1530-0277.1995.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Bice PJ, Orr MR, Dopp JM. Similarity of taste reactivity responses to alcohol and sucrose mixtures in rats. Alcohol. 1990;7:115–120. doi: 10.1016/0741-8329(90)90071-j. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Dopp JM. Taste reactivity to alcohol in rats. Behav Neurosci. 1989;103:1318–1326. doi: 10.1037//0735-7044.103.6.1318. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Mahadevan RS. The taste of alcohol for rats as revealed by aversion generalization tests. Chem Senses. 1993;18:509–522. [Google Scholar]
- Lemon CH, Brasser SM, Smith DV. Alcohol activates a sucrose-responsive gustatory neural pathway. J Neurophysiol. 2004;92:536–544. doi: 10.1152/jn.00097.2004. [DOI] [PubMed] [Google Scholar]
- Lemon CH, Smith DV. Neural representation of bitter taste in the nucleus of the solitary tract. J Neurophysiol. 2005;94:3719–3729. doi: 10.1152/jn.00700.2005. [DOI] [PubMed] [Google Scholar]
- Lemon CH, Wilson DM, Brasser SM. Differential neural representation of oral ethanol by central taste-sensitive neurons in ethanol-preferring and genetically heterogeneous rats. J Neurophysiol. 2011 doi: 10.1152/jn.00580.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng L, Hawkins TD, Li TK. New Strains of Rats with Alcohol Preference and Non-preference. In: Thurman RG, Williamson JR, Drott H, Chance B, editors. Alcohol and Aldehyde Metabolizing Systems. Vol. III. New York: Academic Press; 1977. pp. 537–544. [Google Scholar]
- Mennella JA, Pepino MY, Lehmann-Castor SM, Yourshaw LM. Sweet preferences and analgesia during childhood: effects of family history of alcoholism and depression. Addiction. 2010;105:666–675. doi: 10.1111/j.1360-0443.2009.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, McBride WJ, Lumeng L, Li TK. Operant responding for oral ethanol in the alcohol-preferring P and alcohol-nonpreferring NP lines of rats. Alcohol. 1989;6:127–131. doi: 10.1016/0741-8329(89)90037-2. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li TK. Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol. 1986;3:331–336. doi: 10.1016/0741-8329(86)90010-8. [DOI] [PubMed] [Google Scholar]
- Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiol Behav. 2006;89:531–535. doi: 10.1016/j.physbeh.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuni Y. Response of chorda tympani fibers of the rat to pungent spices and irritants inpungent spices. Shikwa Gakuho. 1977;77:1323–1349. [PubMed] [Google Scholar]
- Olney JJ, Castro N, Dudley J, Pipkin J, Herr DR, Walls SM, Harris GL, Brasser SM. Microstructural characteristics of oral alcohol consumption in selectively bred ethanol-preferring (P) rats given intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2010;34:81A. [Google Scholar]
- Pepino MY, Mennella JA. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol Clin Exp Res. 2007;31:1891–1899. doi: 10.1111/j.1530-0277.2007.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DR, Tanaka T, McDaniel AH. Diverse tastes: Genetics of sweet and bitter perception. Physiol Behav. 2006;88:215–226. doi: 10.1016/j.physbeh.2006.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinehart-Doty JA, Schumm J, Smith JC, Smith GP. A non-taste cue of sucrose in short-term taste tests in rats. Chem Senses. 1994;19:425–431. doi: 10.1093/chemse/19.5.425. [DOI] [PubMed] [Google Scholar]
- Scinska A, Koros E, Habrat B, Kukwa A, Kostowski W, Bienkowski P. Bitter and sweet components of ethanol taste in humans. Drug Alcohol Depend. 2000;60:199–206. doi: 10.1016/s0376-8716(99)00149-0. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Kampov-Polevoy A, Stewart R, Li TK. Taste preferences in rat lines selected for low and high alcohol consumption. Alcohol. 1992;9:155–160. doi: 10.1016/0741-8329(92)90027-8. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Murphy JM, Lumeng L, Li TK. Intake of saccharin solution in selectively-bred high and low alcohol drinking (HAD and LAD) lines of rats and in the F2 progeny of HAD and LAD rat crosses. Alcohol Clin Exp Res. 1998;(22 Suppl):55A. [Google Scholar]
- Stewart RB, Russell RN, Lumeng L, Li TK, Murphy JM. Consumption of sweet, salty, sour, and bitter solutions by selectively bred alcohol-preferring and alcohol-nonpreferring lines of rats. Alcohol Clin Exp Res. 1994;18:375–381. doi: 10.1111/j.1530-0277.1994.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, Owen D, Smith GD, Randall AD, Harrison S, Bianchi A, Davis JB, Geppetti P. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- Waller MB, McBride WJ, Gatto GJ, Lumeng L, Li TK. Intragastric self-infusion of ethanol by ethanol-preferring and -nonpreferring lines of rats. Science. 1984;225:78–80. doi: 10.1126/science.6539502. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Woods JE, McKay PF, Masters J, Seyoum R, Chen A, La Duff L, Lewis MJ, June HL. Differential responding for brain stimulation reward and sucrose in high-alcohol-drinking (HAD) and low-alcohol-drinking (LAD) rats. Alcohol Clin Exp Res. 2003;27:926–936. doi: 10.1097/01.ALC.0000071920.53470.C1. [DOI] [PubMed] [Google Scholar]
- Wronski M, Skrok-Wolska D, Samochowiec J, Ziolkowski M, Swiecicki L, Bienkowski P, Korkosz A, Zatorski P, Kukwa W, Scinska A. Perceived intensity and pleasantness of sucrose taste in male alcoholics. Alcohol Alcohol. 2007;42:75–79. doi: 10.1093/alcalc/agl097. [DOI] [PubMed] [Google Scholar]
- Yamamoto J, Fresquet N, Sandner G. Conditioned taste aversion using four different means to deliver sucrose to rats. Physiol Behav. 2002;75:387–396. doi: 10.1016/s0031-9384(01)00671-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Neural substrates for the processing of cognitive and affective aspects of taste in the brain. Arch Histol Cytol. 2006;69:243–255. doi: 10.1679/aohc.69.243. [DOI] [PubMed] [Google Scholar]