Abstract
Purpose
To prospectively monitor progressive changes of retinal ganglion cell (RGC) function in early glaucoma using the pattern electroretinogram (PERG).
Methods
Fifty-nine patients enrolled as glaucoma suspects were observed untreated over an average of 5.7 ± 1.4 years, during which they were tested with PERG (PERGLA paradigm) and Standard Automated Perimetry (SAP) two times per year. PERG amplitude and phase were normalized for physiological age-related changes, and linear regressions fitted to the data to calculate progression slopes (signal), slope SE (noise), and corresponding signal-to-noise ratios (SNR = slope ÷ SE). Linear regressions were also used to fit SAP global indices mean deviation (MD) and pattern standard deviation (PSD).
Results
On average, progression slopes of PERG amplitude/phase were skewed toward negative values, their mean being significantly (P<0.01) different from zero. In contrast, mean slopes of SAP-MD and PSD were not significantly different from zero. SNRs were higher for PERG than SAP (P<0.01). A substantial number of eyes displayed significant (P < 0.05) progression of PERG amplitude (15–20%) or PERG phase (16–25%). Fewer eyes displayed significant progression of SAP-MD (0–2%) or SAP-PSD (4–8%).
Conclusions
The PERG displayed clear longitudinal loss of signal (diminished amplitude, phase delay, or both) in a substantial number of eyes of patients, indicating progressive deterioration of RGC function. Progression of SAP global indices MD and PSD was found in a relatively smaller number of eyes. It remains to be established whether PERG progression has predictive value for developing visual dysfunction.
Keywords: retinal ganglion cell function, pattern electroretinogram, glaucoma, progression, visual field
Introduction
The pattern electroretinogram (PERG) reflects the electrical activity of retinal ganglion cells (RGC), 1, 2 and has been extensively used for detecting loss of RGC function in glaucoma. 3–5 Cross-sectional studies have shown that the PERG is frequently altered in glaucoma suspects and early manifest glaucoma patients compared to normal controls.6–10 Longitudinal studies have reported progressive PERG amplitude reduction preceding progression of field loss on Standard Automated Perimetry (SAP) in patients with manifest glaucoma 11 or predicting conversion to manifest visual field defects in patients with ocular hypertension (OHT).12, 13
The present study reports results obtained a longitudinal cohort of glaucoma suspects that have been observed untreated for about 6 years. Glaucoma suspects did not have reproducible visual field defects, but most of them had suspicious appearance of the optic nerve head and normal IOP that could be associated with other risk factors for glaucoma.14 A large overlap can exist between findings in patients with early glaucoma and glaucoma suspects. Identifying individuals who are at a high risk of developing glaucomatous damage may provide a rationale for timely therapy to prevent the disease. We tested the hypothesis that a subpopulation of glaucoma suspects would display significant progressive losses of PERG signal, which would represent an indication of ongoing RGC dysfunction or death. Preliminary results have been previously published in abstract form (Ventura L, Venzara F & Porciatti V. (2007). Rates of visual field and PERG changes over 4 years in early glaucoma. Invest Ophthalmol Vis Sci 48, E-Abstract 221).
Methods
Subjects
Fifty-nine patients enrolled as glaucoma suspects (GS) were tested approximately two times per year with PERG as well as with Humphrey Perimeter Central 24-2 program SITA standard (SAP) over 5.7 ± 1.4 years. All patients received at least 5 PERG/SAP tests over a follow up period of at least 3 years (range 3–8 years) in one or both eyes. One hundred and eight eyes which met eligibility criteria contributed measurements to the analysis (Table I).
Table I.
Summary of characteristics of the study patients
| N= | % | |
|---|---|---|
| Study subjects | 59 | 100 |
| Males | 28 | 47 |
| Hispanic Ethnicity | 24 | 41 |
| African American | 16 | 27 |
| Right eyes eligible | 56 | 95 |
| Left eyes eligible | 52 | 88 |
| Mean | SD | |
| Age (years) | 54 | 9 |
| Baseline OD SAP-MD (dB) | −0.7 | 1.5 |
| Baseline OS SAP-MD (dB) | −0.9 | 1.8 |
| Baseline OD SAP-PSD (dB) | 1.9 | 0.8 |
| Baseline OS SAP-PSD (dB) | 1.8 | 0.6 |
| Baseline OD IOP (mm Hg) | 14.9 | 3.2 |
| Baseline OS IOP (mm Hg) | 14.9 | 2.6 |
| Baseline OD C/D | 0.51 | 0.16 |
| Baseline OS C/D | 0.51 | 0.16 |
| Follow up time (years) | 5.7 | 1.4 |
| Number of PERG measured per person | 9.6 | 2.5 |
| Number of SAP measured per person | 8.9 | 2.3 |
Eligibility was determined through a detailed medical and ocular history and a comprehensive eye examination. Eye examination included Best Corrected Visual Acuity (BCVA), refraction at distance and near, IOP with Goldmann applanation tonometry, corneal pachymetry (DGH 500 Pachette), gonioscopy, dilated fundus examination, stereophotographs of the optic disc, and SAP. Cataractous changes allowed were of the nuclear sclerotic type and were limited to a mild grade and allowing a BCVA ≥ 20/25. Clinical and demographic information were obtained as specified in the OHTS study design. 15 Subjects met the following inclusion criteria: BCVA ≥ 20/25 (in eyes contributing measurements), normal SAP according to the OHTS criteria 15 (reliability <15% on all indices, normality >5% on all global indices in two consecutive sessions 6 months apart), and glaucomatous optic disc appearance (C/D > 0.5, C/D asymmetry ≥ 0.2, localized thinning of the disc, splinter disc hemorrhages) or increased IOP (> 21 mm Hg). None of the patients received IOP-lowering medications at any point during the follow up period. For all subjects, exclusion criteria were the presence of ocular or systemic diseases that may cause non-specific PERG abnormality such as age-related macular degeneration, diabetes, Parkinson’s disease, and multiple sclerosis. Eyes with visual acuity lower than 20/25 (n=7) or previous intraocular surgery, except for uncomplicated cataract extraction (n=2), were excluded. Eyes with myopia higher than 6 D (n=1) were also excluded because the PERG may be nonspecifically reduced in high myopia. 16 None of the excluded eyes had glaucoma.
The study followed the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the University of Miami. Informed written consent was obtained by all subjects after the nature of the test and possible risks were explained in detail.
PERG recording
The PERG was simultaneously recorded from both eyes according to a paradigm optimized for glaucoma detection (PERGLA), 17 which has been reported to have low test-retest variability. 8, 17–20 Retinal signals were recorded from skin electrodes taped on the lower eyelids. Subjects were fitted with the appropriate add, and were instructed to fixate on a target at the center of a pattern stimulus placed at a viewing distance of 30 cm. Subjects did not receive dilating drops, and were allowed to blink freely. The pattern stimulus consisted of horizontal gratings (1.7 cycles/degree, 25 degree diameter circular field, 98% contrast, 40 cd/m² mean luminance), reversing in counterphase 16.28 times/s). The commercial instrument we used is conceived in such a way that a circular window is cut at the pole of a half sphere through which the pattern stimulus (a CRT monitor) is presented.17 This way the stimulus display is prevented from possible reflections originating from ambient light sources. 21 Electrical signals were band-pass filtered (1–30 Hz), amplified (100,000 fold), and averaged in synchrony to the reversal period. During signal acquisition, sweeps contaminated by eye blinks or gross eye movements were automatically rejected over a threshold voltage of 25 µV. Two successive responses of 300 artifact-free sweeps each were recorded, separated by a brief pause. The first 30 sweeps of each response were rejected to allow steady-state conditions. The software allowed visual inspection of the two consecutive responses superimposed to check for consistency, and then computed the final PERG waveform (600 artifact-free sweeps). The total testing time (including electrode placement, insertion of required trial lenses for 30 cm distance to reach J1+ visual acuity, and actual PERG recording) was about 10 minutes. Since the PERG was recorded in response to relatively fast alternating gratings, the response waveforms were typically sinusoidal-like, with a temporal period corresponding to the reversal rate (examples in refs 17, 22). PERG waveforms were automatically analyzed in the frequency domain by Discrete Fourier Transform (DFT) to isolate the frequency component at the contrast-reversal rate (16.28 Hz), and compute its amplitude in µV and phase delay in π rad relative to the stimulus reversal period. Decreasing phase values are analogues to latency delays (31 ms per 1.0 π rad). We periodically checked with a photometer (Gamma Scientific DR-2000-1, S Diego, CA) that the stimulus luminance did not change over the follow up period. Several operators (n=5) alternated/overlapped in PERG recording over the follow up period. All operators received a brief training by one of us (VP) and were supervised for one month by an experienced operator. Each subject participating in the study was tested by at least 3 operators during the follow up period.
Analysis of PERG changes with time
The PERG is known to display physiological changes with age (see ref. 17 for references and discussion). Physiological PERG changes are due in part to age-related neural losses and in part to age-related optical factors (reduced luminance and contrast due to senile miosis and light scatter of the lens). Previous normative data have been obtained in a mixed population of 114 normal controls 7 (age range, 22–85 years; cataractous changes allowed were of the early nuclear sclerotic type and allowing BCVA of ≥ 20/20; patients with axial myopia >5 diopters were excluded. In that study, physiological changes of PERG amplitude and phase with age could be well described by linear regression functions. The regression of amplitude with age was linear on log/log coordinates, whereas that of phase with age was linear on linear scales. The equations of the regression lines were: log (Amplitude) = 0.632 − 0.389 · log (Age); Phase= 1.99 − 0.0032 · Age; residuals had normal distribution.7, 17 From these equations it is therefore possible to calculate, for each age of participants, the expected amplitude/phase. Present experimental data were normalized for physiological age-related changes by expressing them as deviations from age-specific normal values [PERG amplitude change (%) = (measured µV−predicted µV) ÷ predicted µV · 100]; [PERG phase change (ms) = (measured π rad − predicted π rad) · 31 ms/π rad]. The rate of change of PERG amplitude and phase with time was evaluated by linear regression analysis. Linear regression analyses were also performed on SAP global indices MD and PSD, which represent standard benchmarks of linear progression.23
Statistics
Longitudinal measurements PERG amplitude (% deviation from age-specific norms), PERG phase (ms delay from age-specific norms) and SAP MD/PSD were analyzed by simple linear regression. Regression slopes, SE of slopes, and P-values were calculated. Right eyes and left eyes were evaluated separately since progression may occur at different rates in the two eyes 24. Each eye was characterized as demonstrating progression, “improvement”, or no change, for both PERG and SAP measurements, based on the statistical significance and sign of the respective slopes with time.
For a given number of observations in a longitudinal series, the ability to detect a significant change (ie, progression) depends on both the rate of change (slope of linear regression) and its standard error (SE). We defined the regression slope as signal, and its SE as noise. The regression slope divided by its SE represents the Signal-to-Noise Ratio (SNR). The SNR provides a normalizing index to compare measurements having different scales25 such as PERG amplitude/phase and SAP MD/PSD. The strength of association between SNR of PERG and SAP-MD slopes was measured with Spearman’s non-parametric rho to minimize the influence of outliers. Patients were classified into those which exhibited worsening PERG measurements over time as determined by significant linear regression, and those which did not. Multivariate logistic regression was used to investigate the association between PERG progression and baseline variables (at both patient and ocular level). For the analysis of the latter, the worst eyes only were included assuming that they are stronger candidate predictors than better eyes for progression in glaucoma suspects.
Results
An example of how PERG and SAP data were analyzed is shown for a patient who received repeated testing (n=14) over 6.7 years (Fig. 1). The patient was a Caucasian female with vertical cup to disc =0.5 in each eye, − 4.0 diopters of myopia in each eye and corrected visual acuity of 20/20 in each eye. The IOP, averaged of the follow up period, was 14.9 ±2.2 mm Hg in the right eye and 14.3 ±1.6 mm Hg in the left eye.
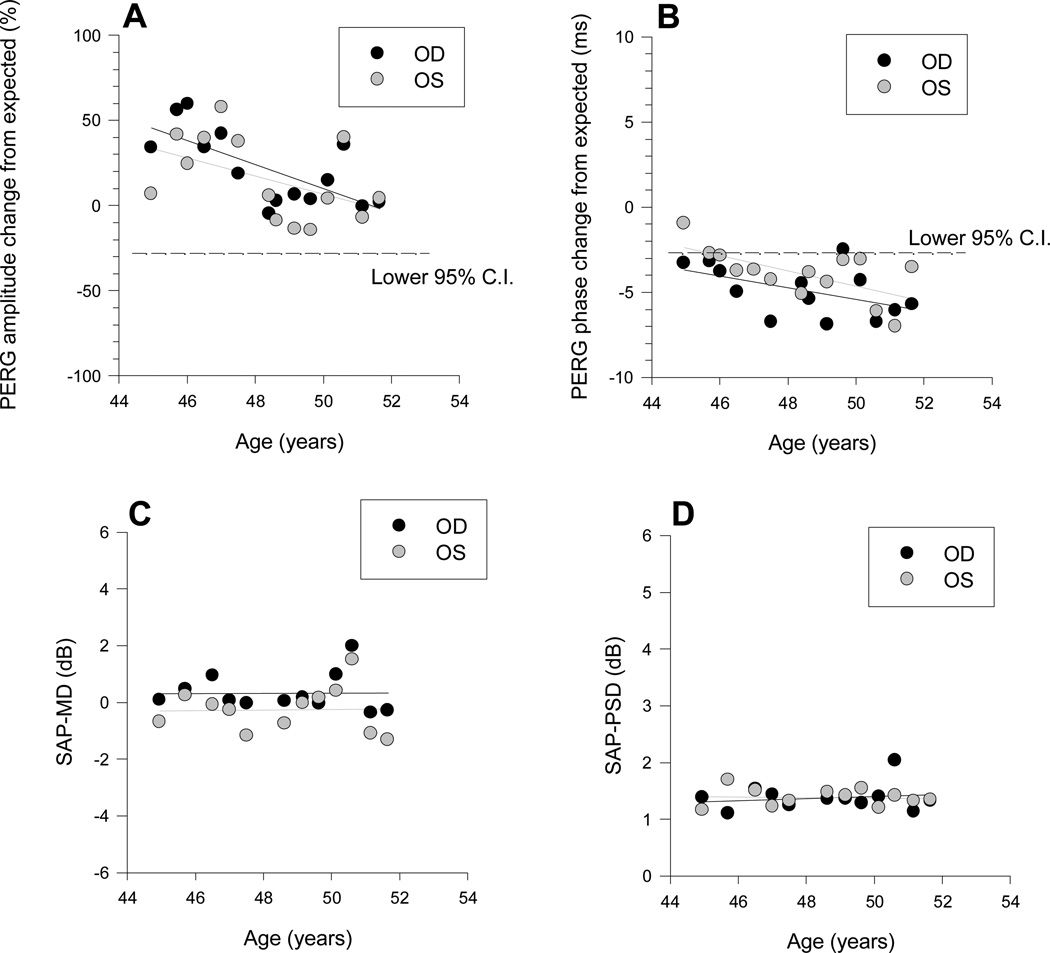
Figure 1.
Example of PERG and SAP changes over time in an individual patient. PERG amplitude data (A) are expressed a % deviation from age-specific norms. PERG phase data (B) are expressed as delay in milliseconds from age- specific norms. Black symbols: right eye, grey symbols: left eye. The dashed lines in A and B represent the lower 95% confidence intervals of normal controls. E, F: Longitudinal measurements of SAP-MD (E) and SAP-PSD (F). In all panels, data were fitted with linear regression lines.
The PERG amplitude (Fig.1A) was within the normal limits in each eye during the entire observation period; however, it showed a strong trend to a decrease with time in both eyes (OD: slope (SE) = −7.01 (2.1) %/year, P=0.005; OS: −5.3 (2.85) %/year, P=0.087). The PERG phase (Fig.1B) had a borderline delay at baseline, which tended to progress over time (OD: slope (SE) = 0.341 (0.17) ms/year, P=0.068; OS: −0.44 (0.15) ms/year, P=0.015). The SAP-MD (Fig.1C) and SAP-PSD (fig.1D) were in the normal range at baseline and did not display trends to change over the observation period. Altogether, data displayed in Fig. 1 demonstrate that in this particular patient the PERG amplitude displayed significant changes over time that were not associated to corresponding SAP changes.
Longitudinal PERG and SAP changes
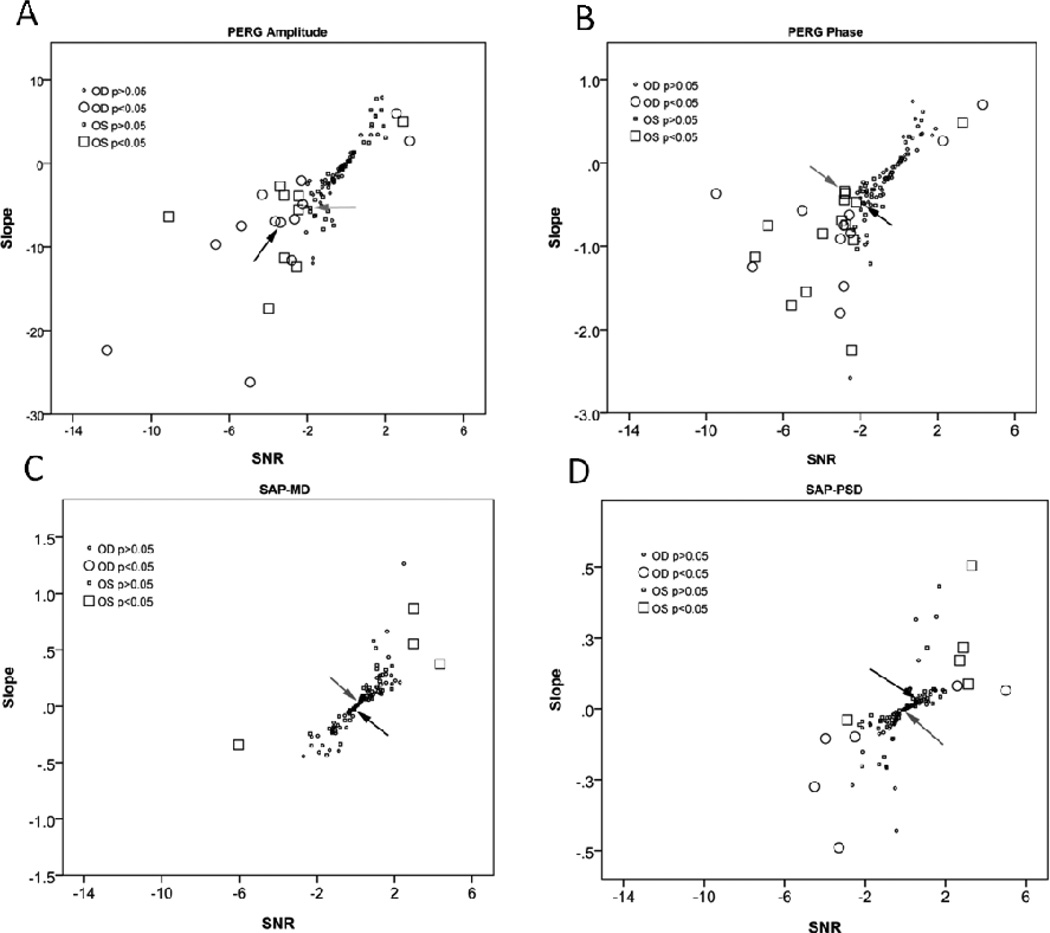
Linear regression analysis was used to evaluate progression slopes for age-normalized PERG amplitude/phase and SAP-MD/PSD, together with corresponding SE of slopes. For each measure, a signal-to-noise ratio (SNR) was calculated by dividing regression slopes (signal) by their SE (noise). SNRs provide a means to normalize slopes of different measures. To document progression of dysfunction, pertinent measures are the rate of change with time (slope of the linear regression) and the SNR of the measurement. In Figure 2 slopes of PERG amplitude and phase, as well as of SAP-MD and PSD, as plotted as a function of corresponding SNR. In Fig. 2 A and B it is readily apparent that there was a large spread of PERG slope values. Most slopes clustered around zero and were not statistically significant; however for both amplitude and phase the distribution of slopes was clearly skewed towards negative values. For each data point, the statistical significance was calculated according to the t-distribution (two tailed). For PERG amplitude/phase, significant negative slopes were dubbed as “progressed”, and positive slopes as “improved”. As expected, significant slopes were associated with higher SNRs. For both amplitude and phase, the proportion of PERG progressing slopes substantially exceeded that of improving slopes. The proportions of eyes with significant slopes were: PERG amplitude progressing eyes: OD, 20%; OS, 15%. PERG amplitude improving eyes were: OD, 4%; OS, 2%. PERG phase progressing eyes were: OD, 16%; OS, 25%. PERG phase improving eyes were: OD, 4%; OS, 2%.
Figure 2.
Linear regression slopes calculated on individual eyes for A: PERG amplitude (% change from age-specific normal/year), B: PERG phase (ms delay from age-specific normal/year), C: SAP-MD (dB/year), and D: SAP-PSD (dB/year) as a function of corresponding signal to noise ratios (SNR). SNR is defined as regression slope divided by the slope standard error. Larger symbols mean that the regression slope was significant at P < 0.05 or less. Arrows indicate slopes corresponding to the example patient shown in Figure 1.
Figure 2 C and D displays slopes of SAP-MD and SAP-PSD against corresponding SNRs. For both MD and PSD, the distribution of slopes was virtually symmetrical around zero. Similar to PERG, the statistical significance of SAP slopes was calculated for individual eyes according to the t-distribution (two tails). For SAP-MD, significant negative slopes were dubbed as “progressed”, and positive slopes as “improved”. For SAP-PSD, positive slopes were dubbed as “progressed” whereas negative slopes were dubbed as “improved”. For both SAP MD and PSD, the proportion of progressing slopes was small and was very similar to that of improving slopes (SAP-MD progressing eyes were: OD, 0%; OS, 2%. SAP-MD improving eyes were: OD, 0%; OS, 6%. SAP-PSD progressing eyes were: OD, 4%; OS, 8%. SAP-PSD improving eyes were: OD, 7%; OS, 2%. A summary of regression slopes for both PERG and SAP is shown in Table II. Corresponding SNRs are shown in Table III.
Table II.
Slopes of linear regressions fitting PERG amplitude/phase and SAP-MD/PSD measurements performed during the observation period in 59 untreated glaucoma suspects.
| Regression Slopes Subjects, N=59 |
Mean Slope |
SD | p-value (difference of mean slope from zero |
+95% CI Mean Slope |
−95% CI Mean Slope |
Mean # observations per regression |
SD |
|---|---|---|---|---|---|---|---|
| OD-PERG Amplitude (% difference from normal/yr) | −3.067 | 5.855 | <0.001 | −1.499 | −4.635 | 9.5 | 2.6 |
| OD PERG Phase (ms delay/year) | −0.296 | 0.593 | <0.001 | −0.137 | −0.455 | 9.5 | 2.6 |
| OD SAP-MD (dB/year) | 0.038 | 0.274 | .298 | 0.112 | −0.035 | 8.8 | 2.4 |
| OD SAP-PSD (dB/year) | −0.028 | 0.134 | .125 | 0.008 | −0.064 | 8.8 | 2.3 |
| OS-PERG Amplitude (% difference from normal/yr) | −1.903 | 4.961 | .008 | −0.522 | −3.284 | 9.6 | 2.5 |
| OS PERG Phase (ms delay/year) | −0.409 | 0.548 | <0.001 | −0.257 | −0.562 | 9.6 | 2.5 |
| OS SAP-MD (dB/year) | 0.064 | 0.257 | .077 | 0.136 | −0.007 | 8.9 | 2.3 |
| OS SAP-PSD (dB/year) | 0.009 | 0.128 | .609 | 0.045 | −0.026 | 8.9 | 2.2 |
Table III.
Signal to Noise Ratios (SNR=regression slope ÷ slope SE) for PERG amplitude/phase and SAP-MD/PSD calculated for 59 untreated glaucoma suspects.
| Signal to Noise Ratios (SNR) Subjects, N=59 |
Mean SNR |
SD | +95% CI Mean SNR |
−95% CI Mean SNR |
|---|---|---|---|---|
| OD-PERG Amplitude | −1.20 | 2.35 | −1.83 | −0.57 |
| OD PERG | −1.07 | 2.15 | −1.64 | −0.49 |
| OD SAP-MD (dB/year) | 0.21 | 1.20 | −0.11 | 0.53 |
| OD SAP-PSD (dB/year) | −0.26 | 1.55 | −0.67 | 0.16 |
| OS-PERG Amplitude | −0.73 | 1.91 | −1.26 | −0.20 |
| OS PERG Phase | −1.53 | 1.90 | −2.06 | −1.00 |
| OS SAP-MD (dB/year) | 0.23 | 1.54 | −0.20 | 0.66 |
| OS SAP-PSD (dB/year) | 0.09 | 1.40 | −0.30 | 0.48 |
Association between PERG and SAP slopes, and between slopes of the two eyes
Correlations between PERG amplitude/phase and SAP MD/PSD have been calculated using SNRs, which provides a normalizing index for measurements with different scales. Only a small fraction of eyes (0–3%) displayed concurrent progression in PERG and SAP. As the great majority of eyes (64–80%) displayed insignificant longitudinal changes in both PERG amplitude/phase and SAP-MD/PSD, all correlations between PERG and SAP were not significant (Table IV).
Table IV.
Correlations (Spearman’s) between normalized progression slopes (signal-to-noise ratio: SNR) of PERG amplitude/phase and SAP MD/PSD. For each variable, SNR is defined as progression slope divided by the corresponding slope standard error.
| OD amplitude | N=95 | MD | PSD |
|---|---|---|---|
| Rho | −0.01 | −0.11 | |
| p | 0.79 | 0.30 | |
| OD phase | N=95 | MD | PSD |
| Rho | 0.04 | 0.21 | |
| p | 0.78 | 0.13 | |
| OS amplitude | N=95 | MD | PSD |
| Rho | 0.12 | 0.03 | |
| p | 0.39 | 0.84 | |
| OS phase | N=95 | MD | PSD |
| Rho | 0.24 | −0.02 | |
| p | 0.09 | 0.90 |
Interocular correlations of SNRs were calculated for the 49 patients with both eyes contributing to the measurements. Interocular correlations were significant for both PERG and SAP (P-values ranged from 0.036 to <0.001), and ranged in strength from moderate (PERG amplitude, rho=0.64, PERG phase, rho=0.64, SAP-MD, rho= 0.70) to weak (SAP-PSD, rho=0.30).
Association between PERG changes and baseline variables
Twenty-five out of the 59 study patients were identified as having significantly worsening regression slopes for either PERG amplitude or phase. Relevant baseline characteristics (individual patient as well as ocular) were analyzed for association with PERG progression compared to the 34 patients who did not progress. The following baseline variables were allowed stepwise inclusion in a logistic regression model: gender; age; race; Hispanic ethnicity; the maximum (between the right and left eyes) of IOP, C/D ratio, SAP-pattern standard deviation; and minimum (between right and left eyes) of central corneal thickness and SAP mean deviation. Two statistically significant univariate risk factors emerged: maximum PSD (p=0.049), for which a 1 unit higher PSD was associated with a 1.8 times higher odds of PERG progression, and age (p=0.035), for which ten years greater age was associated with a 2.0 times higher odds or PERG progression. Only age was statistically significant in a multivariate model. We did not use current risk calculators such as the one available on line (http://ohts.wustl.edu/risk/calculator.html ) as the predictive models are only valid for patients that meet the inclusion criteria of OHTS, 26 and cannot be generalized to the population of glaucoma suspects investigated in the present study.
Discussion
The PERG has long been considered an important indicator of RGC function in glaucoma. The recent introduction of recording paradigm (PERGLA) with relatively low test-retest and operator-dependent variability 17–20 encouraged the use of PERG for longitudinal studies.
Here we asked the question of whether the PERG displayed measurable longitudinal changes in patients with suspicion of glaucoma. Suspicion was primarily based on glaucomatous appearance of the optic disc, which could be associated with other risk factors for glaucoma. The great majority of patients had normal IOP. SITA SAP MD and PSD were used as a standard benchmark of visual field damage to exclude that patients had manifest glaucoma based on the OHTS criteria. SAP MD and PSD were analyzed with the same straightforward linear regression method as the age-normalized PERG amplitude and phase. Outcome measures included slopes of the linear regressions as well as signal-to-noise ratios (SNR=slope÷slope SE) to compare different outcome measures on a normalized scale.
It was important to control that longitudinal PERG changes were not merely due to progressive failure of the recording system components (eg, reduction of stimulus luminance and/or contrast with time, amplifiers' gain), or to non-specific biological changes (eg, physiological aging, development of cataracts). The luminance/contrast of the PERG stimulus as well as the gain of the amplifiers did not change over the study period. For all patients, the visual acuity was ≥ 20/25 and did not change over the study period. PERG amplitude and phase data were age-normalized by expressing them as changes from age-specific normal values based on previous studies on physiological, age-related PERG changes determined using the same technique and recording apparatus.7
Our results show that the distribution of age-normalized PERG amplitude/phase slopes was significantly skewed towards negative values (ie, the mean slope was significantly less than zero). In individual eyes, the great majority of PERG slopes did not show significant changes (amplitude: 77–83%, phase: 80–92%). In a substantial number of eyes (PERG amplitude: 15–20%, PERG phase: 16–25%), however, negative slopes were significant. Fewer significant positive slopes were also detectable (PERG amplitude: 2–4% of eyes, PERG phase: 2–4%). That the distribution of PERG slopes included a majority of unchanging slopes as well as some others with positive values, suggests that the tail of significant negative slopes was not merely the result of an overall shift of slopes towards negative values due to unaccounted effects of aging and instrument deterioration resulting in reduced PERG signal. Finally, significant negative slopes of PERG amplitude and phase were often dissociated; that one component of the PERG did change while the other did not, represents an internal control for non-specific progression of RGC dysfunction.
PERG amplitude and phase represent different aspects of RGC function.5 PERG amplitude reduction may occur because of lost RGCs, dysfunctional RGCs, or a combination of both conditions. PERG phase reductions (delay in response latency) may mean that active RGCs respond in a slower fashion. Phase becomes progressively delayed with physiological aging 17 and may be further delayed in patients with early glaucoma.7 It is important to note that phase is not delayed when the stimulus contrast is artificially deteriorated in such a way as to simulate cataracts that substantially reduce visual acuity.5 Thus, the cases of phase progression in our study are unlikely to be due to the development of incipient cataracts.
The distribution of SAP-MD and SAP-PSD slopes was virtually symmetrical around zero, and few eyes displayed significant progression (SAP-MD, 1%; SAP-PSD, 5%). A few eyes also displayed significant improving slopes (SAP-MD, 3%; SAP-PSD, 5%). A small number of either progressing or improving slopes was expected based on normal statistical variability. We cannot exclude that other visual field modalities might have detected a larger number of progressing eyes. FDT, SWAP9, 27 and microperimetry 28 were not used, and neither were methodologies such as GPA software or the visual field index.29 This study was not designed to compare visual field modalities with respect to detection of incident glaucoma or progression of disease.
Progressive deterioration of PERG signal associated with normal standard visual field has been reported before in patients with ocular hypertension. 13, 30 Bode et al, 2001 13 monitored for 10 years 64 OHT patients (IOP >25 mm Hg or >/=23 mm Hg with additional risk factors but normal optic disk) and reported that the PERG amplitude detected glaucoma patients 4 years before visual field changes occurred, with a sensitivity/specificity of 75%/76%. In keeping with these previous PERG studies, the present study showed progressive PERG changes in a subset of patients not associated with SAP changes. Different from previous studies, 13, 30 however, only a small minority of present patients had IOP higher than 21 mm Hg, and most of them had suspicious optic disc. In a longitudinal study of patients with manifest primary open-angle glaucoma who subsequently showed progression on SAP, Bayer and Erb, 200211 reported that transient PERG displayed progressive deficits before SAP progression. SWAP and FDT also displayed progressive deficits in a slightly higher number of eyes compared to PERG. It should be considered that patients studied by Bayer and Erb, 2002 11 were tested in a more advanced stage of the disease. It has been suggested that the PERG signal, while very sensitive to RGC dysfunction in early glaucoma, tends to saturate relatively early during disease progression, so that relatively lesser changes compared to other tests may be expected.31
That in present study the PERG was more frequently altered than SAP global indices may be simply due to relatively smaller test-retest variability of PERG resulting in higher SNRs. A possibility is that at these early stages of the disease the PERG becomes altered before SAP; however the PERG may not be able to show progressive changes at later stages of the disease, at which the limited dynamic range of PERG does not allow further reduction of the signal. Other causes for differences between PERG and SAP progressions should also be taken into account. In particular, the PERG has a strong macular over-representation due to the highest RGC density in the macular region compared to extramacular regions.5, 32–34 In contrast, for SAP the macular region is relatively less represented than extramacular regions, and this is reflected in the calculations of MD and PSD global indices. Furthermore, while the PERG provides a measure of RGC electrical responsiveness to high-contrast stimuli at the retinal level, SAP provides an assessment of the psychophysical contrast threshold for the entire visual pathway. This includes losses due to primary damage occurring at the RGC level as well as secondary trans-synaptic damage at the thalamic and cortical levels. 35–37 Visual sensitivity may be influenced by remodeling and compensatory mechanisms at the cortical level. 38
In conclusion, in our patient cohort of glaucoma suspects, the PERG displayed clear longitudinal loss of signal (diminished amplitude, phase delay, or both) in a substantial number of eyes. Longitudinal losses of PERG signal were unlikely to originate from either technical failure of the recording instrument or non-specific biological causes such as physiological aging and increased lens scattering. The pathophysiological significance of progressive PERG changes was not obvious. Multivariate analysis revealed weak effects of baseline older age and baseline higher SAP-PSD as greater odds for PERG progression. Previous studies have reported progressive PERG amplitude reduction preceding conversion to glaucomatous visual field defects in ocular hypertensive patients, 12,13 or preceding progression of field loss on SAP in patients with manifest glaucoma. 11 While this is also a possibility in our group of glaucoma suspects, further follow up will be required to determine whether these PERG losses will have served as predictors of future visual field loss. This represents a crucial issue for clinicians in order to establish appropriate management of the patient.
Acknowledgments
Financial support: NIH-NEI RO1 EY014957, NIH center grant P30-EY014801, unrestricted grant to Bascom Palmer Eye Institute from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure: LMV, none, IG, none, WJF, none, VP, none.
References
- 1.Maffei L, Fiorentini A. Electroretinographic responses to alternating gratings before and after section of the optic nerve. Science. 1981;211:953–955. doi: 10.1126/science.7466369. [DOI] [PubMed] [Google Scholar]
- 2.Zrenner E. The physiological basis of the pattern electroretinogram. In: Osborne N, Chader G, editors. Progress in Retinal Research. Oxford: Pergamon Press; 1990. pp. 427–464. [Google Scholar]
- 3.Ventura LM, Sorokac N, De Los Santos R, Feuer WJ, Porciatti V. The relationship between retinal ganglion cell function and retinal nerve fiber thickness in early glaucoma. Invest Ophthalmol Vis Sci. 2006;47:3904–3911. doi: 10.1167/iovs.06-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bach M, Hoffmann MB. Update on the pattern electroretinogram in glaucoma. Optom Vis Sci. 2008;85:386–395. doi: 10.1097/OPX.0b013e318177ebf3. [DOI] [PubMed] [Google Scholar]
- 5.Porciatti V, Ventura LM. Physiologic significance of steady-state pattern electroretinogram losses in glaucoma: clues from simulation of abnormalities in normal subjects. J Glaucoma. 2009;18:535–542. doi: 10.1097/IJG.0b013e318193c2e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayer AU, Maag KP, Erb C. Detection of optic neuropathy in glaucomatous eyes with normal standard visual fields using a test battery of short-wavelength automated perimetry and pattern electroretinography. Ophthalmology. 2002;109:1350–1361. doi: 10.1016/s0161-6420(02)01100-4. [DOI] [PubMed] [Google Scholar]
- 7.Ventura LM, Porciatti V, Ishida K, Feuer WJ, Parrish RK., 2nd Pattern electroretinogram abnormality and glaucoma. Ophthalmology. 2005;112:10–19. doi: 10.1016/j.ophtha.2004.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowd C, Vizzeri G, Tafreshi A, Zangwill LM, Sample PA, Weinreb RN. Diagnostic accuracy of pattern electroretinogram optimized for glaucoma detection. Ophthalmology. 2009;116:437–443. doi: 10.1016/j.ophtha.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tafreshi A, Racette L, Weinreb RN, et al. Pattern Electroretinogram and Psychophysical Tests of Visual Function for Discriminating Between Healthy and Glaucoma Eyes. American journal of ophthalmology. 2010;149:488–495. doi: 10.1016/j.ajo.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowd C, Tafreshi A, Zangwill LM, Medeiros FA, Sample PA, Weinreb RN. Pattern electroretinogram association with spectral domain-OCT structural measurements in glaucoma. Eye (Lond) 2011;25:224–232. doi: 10.1038/eye.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayer AU, Erb C. Short wavelength automated perimetry, frequency doubling technology perimetry, and pattern electroretinography for prediction of progressive glaucomatous standard visual field defects. Ophthalmology. 2002;109:1009–1017. doi: 10.1016/s0161-6420(02)01015-1. [DOI] [PubMed] [Google Scholar]
- 12.Bach M, Unsoeld AS, Philippin H, et al. Pattern ERG as an early glaucoma indicator in ocular hypertension: a long-term, prospective study. Invest Ophthalmol Vis Sci. 2006;47:4881–4887. doi: 10.1167/iovs.05-0875. [DOI] [PubMed] [Google Scholar]
- 13.Bode SF, Jehle T, Bach M. Pattern electroretinogram in glaucoma suspects: new findings from a longitudinal study. Invest Ophthalmol Vis Sci. 2011;52:4300–4306. doi: 10.1167/iovs.10-6381. [DOI] [PubMed] [Google Scholar]
- 14.Prum BE, Friedman DS, Gedde SJ, et al. Primary Open-Angle Glaucoma Suspect. American Academy of Ophthalmology, Preferred Practice Patterns Guidelines. 2010 [Google Scholar]
- 15.Gordon MO, Kass MA. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–583. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 16.Oner A, Gumus K, Arda H, Karakucuk S, Mirza E. Pattern electroretinographic recordings in eyes with myopia. Eye Contact Lens. 2009;35:238–241. doi: 10.1097/ICL.0b013e3181b343d9. [DOI] [PubMed] [Google Scholar]
- 17.Porciatti V, Ventura LM. Normative data for a user-friendly paradigm for pattern electroretinogram recording. Ophthalmology. 2004;111:161–168. doi: 10.1016/j.ophtha.2003.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang A, Swanson WH. A new pattern electroretinogram paradigm evaluated in terms of user friendliness and agreement with perimetry. Ophthalmology. 2007;114:671–679. doi: 10.1016/j.ophtha.2006.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fredette MJ, Anderson DR, Porciatti V, Feuer W. Reproducibility of pattern electroretinogram in glaucoma patients with a range of severity of disease with the new glaucoma paradigm. Ophthalmology. 2008;115:957–963. doi: 10.1016/j.ophtha.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowd C, Tafreshi A, Vizzeri G, Zangwill LM, Sample PA, Weinreb RN. Repeatability of pattern electroretinogram measurements using a new paradigm optimized for glaucoma detection. J Glaucoma. 2009;18:437–442. doi: 10.1097/IJG.0b013e31818c6f44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bach M, Schumacher M. The influence of ambient room lighting on the pattern electroretinogram (PERG) Doc Ophthalmol. 2002;105:281–289. doi: 10.1023/a:1021254427782. [DOI] [PubMed] [Google Scholar]
- 22.Ventura LM, Porciatti V. Pattern electroretinogram in glaucoma. Curr Opin Ophthalmol. 2006;17:196–202. doi: 10.1097/01.icu.0000193082.44938.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauhan BC, Garway-Heath DF, Goni FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92:569–573. doi: 10.1136/bjo.2007.135012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schumann J, Orgül S, Gugleta K, Dubler B, Flammer J. Interocular difference in progression of glaucoma correlates with interocular differences in retrobulbar circulation. American journal of ophthalmology. 2000;129:728–733. doi: 10.1016/s0002-9394(99)00481-x. [DOI] [PubMed] [Google Scholar]
- 25.Artes PH, Chauhan BC. Signal/noise analysis to compare tests for measuring visual field loss and its progression. Invest Ophthalmol Vis Sci. 2009;50:4700–4708. doi: 10.1167/iovs.09-3601. [DOI] [PubMed] [Google Scholar]
- 26.Mansberger SL, Medeiros FA, Gordon M. Diagnostic Tools for Calculation of Glaucoma Risk. Survey of Ophthalmology. 2008;53:S11–S16. doi: 10.1016/j.survophthal.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah NN, Bowd C, Medeiros FA, et al. Combining structural and functional testing for detection of glaucoma. Ophthalmology. 2006;113:1593–1602. doi: 10.1016/j.ophtha.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Ozturk F, Yavas GF, Kusbeci T, Ermis SS. A comparison among Humphrey field analyzer, Microperimetry, and Heidelberg Retina Tomograph in the evaluation of macula in primary open angle glaucoma. J Glaucoma. 2008;17:118–121. doi: 10.1097/IJG.0b013e31814b97fd. [DOI] [PubMed] [Google Scholar]
- 29.Casas-Llera P, Rebolleda G, Munoz-Negrete FJ, Arnalich-Montiel F, Perez-Lopez M, Fernandez-Buenaga R. Visual field index rate and event-based glaucoma progression analysis: comparison in a glaucoma population. Br J Ophthalmol. 2009;93:1576–1579. doi: 10.1136/bjo.2009.158097. [DOI] [PubMed] [Google Scholar]
- 30.Pfeiffer N, Tillmon B, Bach M. Predictive value of the pattern electroretinogram in high-risk ocular hypertension. Invest Ophthalmol Vis Sci. 1993;34:1710–1715. [PubMed] [Google Scholar]
- 31.Hood DC, Xu L, Thienprasiddhi P, et al. The pattern electroretinogram in glaucoma patients with confirmed visual field deficits. Invest Ophthalmol Vis Sci. 2005;46:2411–2418. doi: 10.1167/iovs.05-0238. [DOI] [PubMed] [Google Scholar]
- 32.Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300:5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- 33.Hess RF, Baker CL., Jr Human pattern-evoked electroretinogram. J Neurophysiol. 1984;51:939–951. doi: 10.1152/jn.1984.51.5.939. [DOI] [PubMed] [Google Scholar]
- 34.Drasdo N, Thompson DA, Arden GB. A comparison of pattern ERG amplitudes and nuclear layer thickness in different zones of the retina. Clin Vision Sciences. 1990;5:415–420. [Google Scholar]
- 35.Yucel YH, Zhang Q, Weinreb RN, Kaufman PL, Gupta N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. 2003;22:465–481. doi: 10.1016/s1350-9462(03)00026-0. [DOI] [PubMed] [Google Scholar]
- 36.Gupta N, Yucel YH. Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol. 2007;18:110–114. doi: 10.1097/ICU.0b013e3280895aea. [DOI] [PubMed] [Google Scholar]
- 37.Duncan RO, Sample PA, Weinreb RN, Bowd C, Zangwill LM. Retinotopic organization of primary visual cortex in glaucoma: Comparing fMRI measurements of cortical function with visual field loss. Prog Retin Eye Res. 2007;26:38–56. doi: 10.1016/j.preteyeres.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lam DY, Kaufman PL, Gabelt BT, To EC, Matsubara JA. Neurochemical correlates of cortical plasticity after unilateral elevated intraocular pressure in a primate model of glaucoma. Invest Ophthalmol Vis Sci. 2003;44:2573–2581. doi: 10.1167/iovs.02-0779. [DOI] [PubMed] [Google Scholar]