Abstract
The liver plays a central role in ethanol metabolism and oxidative stress is implicated in alcohol-mediated liver injury. β-Catenin regulates hepatic metabolic zonation and adaptive response to oxidative stress. We hypothesized that β-catenin regulates the hepatic response to ethanol ingestion. Female liver-specific β-catenin knockout (KO) mice and wild type (WT) littermates were fed the Lieber-Decarli liquid diet (5% ethanol) in a pair-wise fashion. Liver histology, biochemistry, and gene expression studies were performed. Plasma alcohol and ammonia levels were measured using standard assays. Ethanol-fed KO mice exhibited systemic toxicity and early mortality. KO mice exhibited severe macrovesicular steatosis and five to six-fold higher serum ALT and AST levels. KO mice had modest increase in hepatic oxidative stress, lower expression of mitochondrial superoxide dismutase (SOD-2), and lower citrate synthase activity, the first step in the tricarboxylic acid cycle. N-Acetyl cysteine (NAC) did not prevent ethanol-induced mortality in KO mice. In WT livers, β-catenin was found to co-precipitate with FoxO3, the upstream regulator of SOD-2. Hepatic alcohol dehydrogenase and aldehyde dehydrogenase activities and expression were lower in KO mice. Hepatic cytochrome P450 2E1 protein levels were upregulated in ethanol-fed WT mice but were nearly undetectable in KO mice. These changes in ethanol-metabolizing enzymes were associated with 30-fold higher blood alcohol levels in KO mice.
Conclusion
β-catenin is essential for hepatic ethanol metabolism and plays a protective role in alcohol-mediated liver steatosis. Our results strongly suggest that integration of these functions by β-catenin is critical for adaptation to ethanol ingestion in vivo.
Keywords: Wnt pathway, Alcohol dehydrogenase, Cyp2E1, SOD-2, oxidative stress
Introduction
The liver plays an essential role in metabolizing ingested ethanol.(1) Excessive alcohol ingestion can lead to fatty liver (steatosis), inflammation and fibrosis (steatohepatitis), and development of cirrhosis.(Reviewed in (2)) Alcohol-related liver injury is a cause for significant morbidity and mortality around the world.((3); reviewed in(4)). The pathogenesis of ethanol-induced liver injury is complex and involves, among others, gut-derived lipopolysaccharide, cytokines, the innate immune system, and oxidative stress and the interactions of these factors with intracellular signaling pathways (reviewed in (5–9)). Few effective treatments exists for alcohol-related liver disease, making it imperative to understand its pathogenesis so that better treatments can be developed.(10)
In the liver, the first step in the metabolism of alcohol takes place via the alcohol dehydrogenase (ADH) family of enzymes, of which ADH 1A/B/C is the predominant form in the liver.(11) Acetaldehyde formed by the action of ADH is then metabolized to acetate via aldehyde dehydrogenase (ALDH) enzymes, of which ADH2 is the most abundant isoform in the liver. An alternative pathway of metabolism in the liver takes place via the microsomal cytochrome P450 2E1 enzymes (Cyp2E1), which is upregulated with chronic alcoholic ingestion.(12, 13) Cyp2E1 is an important source of reactive oxygen species generation and contributor to oxidative stress in the liver.(14)
Activity of the key alcohol metabolizing enzymes, ADH and Cyp2E1, is zonated across the liver lobule and is more prominent in the perivenous (zone 3) hepatocytes.(15, 16) Recently, β-catenin was shown to be the master regulator of hepatic metabolic zonation.(17, 18) Others and we have previously reported that β-catenin regulates expression of Cyp2E1, the loss of which makes β-catenin KO mice resistant to acetaminophen-induced hepatotoxicity.(19–21) On the other hand, liver-specific loss of β-catenin leads to increased susceptibility to steatohepatitis on the methionine choline-deficient diet model of liver injury.(22) In addition to its metabolic role, β-catenin has also been implicated in the response to oxidative stress.(23) Since alcohol metabolism generates oxidative stress in the liver, we hypothesized that β-catenin may regulate the coordinated response of the liver to alcohol-metabolism and the associated increase in oxidative stress. Thus, this study was undertaken to determine the effect of hepatocyte-specific loss of β-catenin on ethanol metabolism and alcohol-mediated liver injury in vivo in a murine model using the Lieber-DeCarli ethanol diet.
Materials and Methods
Animal genotypes, dietary intervention, and NAC treatment
Liver-specific β-catenin KO mice were generated as previously described.(19) Female KO mice (Ctnnb1−/−, Cre+/−) and WT littermates (Ctnnb1loxp/loxp;Cre−/−; or Ctnnb1loxp/−,Cre−/−; or Ctnnb1loxp/−;Cre+/−) were between the ages of 8–12 weeks at the start of the experiments. All three WT genotypes were used in the experiments as controls and showed indistinguishable phenotype amongst them on both diets. Mice were maintained in 12 hour light-dark cycles and had free access to the diets. The high-fat Lieber-DeCarli liquid diet (5% final ethanol concentration) was used with a 6-d ramp up period (2 d of control diet; 2 d of 1.8% ethanol; 2 days at 3.4% ethanol; then 5% ethanol for 1, 6, or 22 days). The control group received an isocaloric maltodextrin-containing diet in a pair-fed fashion. For collection of blood for plasma ethanol and ammonia levels, mice were fed the high fat Lieber-DeCarli liquid diet for 7 days (6 days of ramp-up followed by 1 day of 5% ethanol) and blood was collected at the end of the dark cycle at 7AM. The University of Pittsburgh Institutional Animal Care and Use Committee approved the study.
Other reagents and methods are described in the Supplementary Methods section.
Results
KO mice exhibit systemic toxic effects and rapid mortality on ethanol ingestion
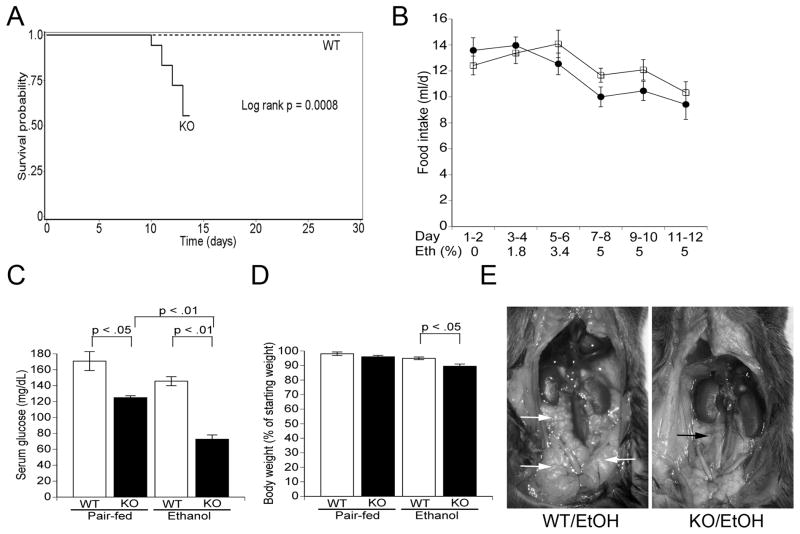
During the ethanol ramp-up period, both genotypes had similar food intake, weight change, and exhibited normal behavior. However, KO mice began to exhibit signs of acute illness between 3–7 days after initiating the 5% ethanol diet, characterized by weight loss, absence of grooming, and decreased activity. These changes were followed by death or distress necessitating euthanasia within 48 h. Fifty percent of KO mice died within the first 6 days of initiating 5% ethanol-diet while none died in the WT/ethanol group (Figure 1A). Food intake was similar in the two ethanol-fed groups except just before death in the KO group (Figure 1B). To avoid confounding results from animals in extremis, we sacrificed the remaining mice after day 6 on 5% ethanol and the experiments described below were performed on these mice. Pair-fed KO and WT mice appeared healthy and gained weight (data not shown). Ethanol-fed KO mice were hypoglycemic with 2-fold lower blood glucose levels than WT mice (Figure 1C) and had 10% lower body weight (Figure 1D). Ethanol-fed KO mice had cachexia and severely depleted intra-abdominal fat compared with the WT/ethanol group, likely representing a baseline defect in energy homeostasis and ethanol-induced acute illness and decreased food intake in KO mice (Figure 1E and Supplementary Figure S1; (24)). There was no difference in body temperature between the groups. We conclude from these results that KO mice are highly susceptible to systemic toxicity and death after short exposure to ethanol ingestion.
Figure 1. KO mice exhibit systemic effects and increased mortality on alcohol diet.
A. Kaplan-Meier survival curve showing increased mortality in ethanol-fed KO mice. B. Food intake in ethanol-fed WT (open squares) and KO (closed circles) mice. C. Serum glucose levels at the time of sacrifice. Data include only KO mice alive on day 6 of 5% ethanol treatment (n = 5/group). D. Percentage weight change on the experimental diets. E. Necropsy showing intraabdominal fat in ethanol-fed mice. Note the abundant intraabdominal fat (white arrows) in the WT animal compared with almost complete loss of visceral adipose tissue in the KO mouse (black arrow).
Ethanol-fed KO mice exhibit striking hepatic steatosis
Both groups of KO mice had lower liver weight (Supplementary Figure S2). However, only pair-fed KO mice had lower liver:body weight ratio compared with the corresponding WT group (Supplementary Figure S3). On microscopic examination of the liver, ethanol-fed KO mice exhibited severe micro- and macrovesicular steatosis in all 3 zones of the liver lobule. In contrast, WT mice developed only mild, predominantly zone 2 microvesicular steatosis (Figure 2A, upper panel). Similarly, Oil red O staining for neutral lipids confirmed the presence of increased hepatic steatosis in the KO/ethanol group (Figure 2A, bottom panel). KO mice had approximately 5-fold higher ALT and AST levels than WT mice on the ethanol diet (Figure 2B and C). Biochemical assays revealed higher liver triglyceride and cholesterol levels in the KO/ethanol group compared with WT mice (Figure 2D and E). Serum triglyceride and total cholesterol levels were similar in WT and KO mice (data not shown). Thus, these results show that KO mice develop severe liver steatosis and moderate transaminase elevation on ethanol ingestion in a time period that causes only mild lipid accumulation and no change in liver injury tests in WT mice.
Figure 2. Alcohol-fed KO mice develop severe liver steatosis.
A. Liver sections stained with H&E or Oil Red O (ORO) from the four experimental groups as indicated. B. Serum ALT levels. C. Serum AST levels. D. Liver triglyceride levels. E. Liver total cholesterol levels. (n = 5–7/group).
Ethanol ingestion increases hepatic oxidative stress in KO mice
Increased hepatic oxidative stress is an important mechanism of ethanol-mediated liver injury and lipid peroxidation is used as an indicator of oxidative stress in tissues. Therefore, we performed assay for malondialdehyde (MDA) levels as an indicator of lipid peroxidation in the liver. KO mice had higher hepatic MDA levels than WT mice on the ethanol diet (Figure 3A). Since a supply of reducing equivalents is essential in the face of oxidative stress, we measured hepatic NADPH levels. Ethanol-fed KO mice had 2-fold lower hepatic NADPH levels than corresponding WT mice (Figure 3B and Supplementary Figure S4). Thus, the oxidative stress generated in KO livers by ethanol ingestion likely depletes hepatic NADPH, an important donor of reducing equivalents in antioxidant defense pathways.
Figure 3. Increased hepatic oxidative stress in ethanol-fed KO mice.
A. Hepatic MDA levels. B. Hepatic NADPH levels. (n = 5/group).
Changes in mitochondrial function in ethanol-fed KO mice
Mitochondrial dysfunction resulting from hepatic oxidative stress can mediate alcohol-induced liver injury. To determine if the increased oxidative stress in ethanol-fed KO mice was associated with mitochondrial dysfunction we assayed activities of key enzymes in isolated mitochondria from freshly harvested liver tissue (Figure 4). KO mice had no change in complex I, II, and IV activities but had lower activity of citrate synthase, the first enzyme of the tricarboxylic acid (TCA) cycle, than the corresponding WT groups. Additionally, activity of aconitase, which catalyzes the second step in the TCA cycle, was lower in pair-fed KO mice and in both ethanol-fed groups. Citrate synthase activity is known to be susceptible to oxidative damage from peroxyl radicals.(25) We conclude from these results that ethanol-fed KO mice have mitochondrial dysfunction associated with increased oxidative stress.
Figure 4. Changes in mitochondrial function in KO mice.
A. Complex I activity. B. Complex II activity. C. Complex IV activity. D. Citrate synthase activity. E. Aconitase activity. n = 4–5/group.
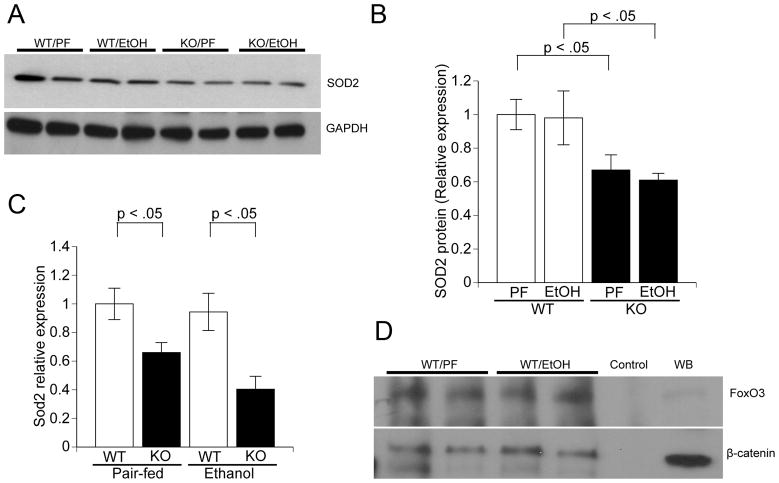
Hepatic SOD-2 expression is lower in KO mice
β-Catenin has been implicated in the oxidative stress response via binding to FOXO transcription factors and regulating expression of antioxidant genes.(23) Manganese superoxide dismutase (SOD-2) is a mitochondrial enzyme that is critical for protection against oxidative stress. We hypothesized that β-catenin participates in protection against alcohol-mediated oxidative stress by regulating expression of SOD2. Western blot analysis showed significantly lower SOD-2 protein levels in KO mice in both treatment groups (Figure 5A and B). Real-time PCR analysis showed that expression of Sod2 was lower in KO mice, suggesting transcriptional regulation by β-catenin (Figure 5C). Expression of Sod2 is upregulated by the Forkhead transcription factor FoxO3a. Therefore, we performed immunoprecipitation studies in WT livers and found protein-protein interaction between FoxO3 and β-catenin (Figure 5D). Expression of two other targets of FoxO3, Cdkn1b and GADD45, were lower in KO mice (Supplementary Figure S5). We conclude from these results that β-catenin transcriptionally regulates the critical mitochondrial oxidative stress response protein SOD-2 via binding to its upstream regulator FoxO3.
Figure 5. KO mice have lower SOD-2 expression.
A. Western blot analysis of SOD-2 protein levels in the liver. B. Quantification of SOD-2 protein, normalized to GAPDH. Averages of four samples in each group from two independent experiments are shown. C. Real-time PCR analysis of SOD-2 expression levels in the liver (n = 5/group). D. β-catenin co-immunoprecipitates with FoxO3, the transcriptional regulator of SOD-2 expression. PF: pair-fed; EtOH: ethanol-fed; IP: immunoprecipitation; IB: immunoblot; IgG: IP performed with IgG as negative control; Input: immunoblot with indicated antibody and total liver lysate as positive control.
We then asked whether treatment with the antioxidant NAC could prevent mortality associated with ethanol ingestion in KO mice. KO mice were given NAC via twice daily intraperitoneal injection (500 mg/Kg). We found that NAC could not prevent either death (data not shown) or liver steatosis (Supplementary Figure S6) in ethanol-fed KO mice.
KO mice exhibit defective ethanol metabolism
We then asked whether KO mice exhibited differences in ethanol metabolism in vivo. To avoid the potentially confounding results obtained from acutely ill animals with severe liver steatosis, we assayed plasma alcohol levels in mice exposed to the 5% alcohol diet for only 1 day after the initial ramp-up period. At this time point, food intake and weight gain was similar between the two genotypes and KO mice had only modestly greater hepatic steatosis compared with WT mice (Supplementary Figure S7). Under these conditions, plasma alcohol levels in KO mice were 30-fold higher than WT mice (Figure 6A). We conclude that KO mice have defective hepatic ethanol metabolism that is independent of the severity of ethanol-induced liver steatosis.
Figure 6. KO mice have higher plasma alcohol and ammonia levels.
A. Serum alcohol levels. B. Serum ammonia levels. n = 5/group. ND: not determined.
β-Catenin regulates glutamine synthetase expression in the liver and KO mice develop hyperammonemia in certain conditions.(20) Consistent with those previous results, we found that in freshly collected blood samples the KO/ethanol group had significantly higher plasma ammonia levels compared the WT/ethanol group (Figure 6B). This hyperammonemia likely represents an additional source of morbidity in ethanol-fed KO mice.
Changes in major hepatic alcohol metabolizing enzymes in KO mice
Given the high blood alcohol levels in KO mice, we measured activity of the major enzymes responsible for hepatic ethanol metabolism. Both ADH and ALDH activities were lower in pair-fed KO mice. However, enzyme activities in the two ethanol-fed groups were similar (Figure 7A). The NAD/NADH ratio was similar between KO and WT mice (Supplementary Figure S8). Because of previous reports that ethanol-metabolizing enzymes have a perivenous zone-predominant expression pattern and the role of β-catenin as a transcriptional regulator, we then asked whether β-catenin regulated the expression of major genes involved in alcohol metabolism. Real-time PCR analysis showed lower expression of Adh1 and Aldh2 in KO mice (Figure 7B). Western blot analysis revealed lower ADH 1 protein levels in both groups of KO mice but ALDH2 level were lower only in ethanol-fed KO mice. (Figure 7C and D). Western blot analysis for Cyp2E1 protein levels in hepatic microsomal preparations showed increased expression in WT mice on ethanol. However, KO mice had almost no detectable levels of Cyp2E1 protein on either diet (Figure 7E and F).
Figure 7. KO mice have decreased expression of major alcohol metabolizing pathways.
A. ADH and ALDH activity assays (n = 5/group). B. Real-time PCR analysis of Adh1 and Aldh2 genes in the liver (n = 5–6/group). C. Western blot analysis of ADH1 and ALDH2 proteins in the liver. GAPDH is shown as internal loading control. D. Densitometric analysis of western blot shown in Figure 7C with normalization to GAPDH. E. Representative western blot analysis showing Cyp2E1 protein levels in hepatic microsomal preparation. F. Densitometric analysis of microsomal Cyp2E1 levels.
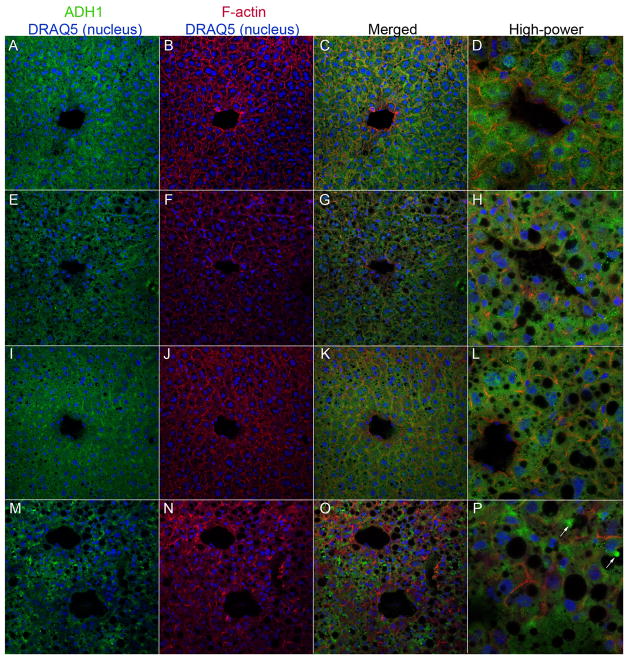
We then analyzed the expression pattern of ADH1 in liver sections by immunofluorescence microscopy (Figure 8). Pair-fed and ethanol-fed WT mice exhibited a modest perivenous-predominant staining pattern for ADH1 and diffuse cytoplasmic ADH1 staining was visible within hepatocytes (Figure 8, panels A–D and I–L, respectively). In contrast, pair-fed KO mice had less prominent ADH1 staining around the central veins (Figure 8, panels EH). Furthermore, ethanol-fed KO mice had a patchy, non-uniform ADH1 staining with several hepatocytes showing aberrantly stained globules projecting into intracellular vacuoles (Figure 8, panels M–P). Taken together, these results establish that β-catenin regulates the expression, sub-cellular and lobular localization, and activity of the major ethanol metabolizing enzymes in the liver.
Figure 8. Changes in ADH1 localization in hepatocytes in KO mice.
Frozen liver sections were triple-stained with ADH1 antibody (FITC; green), TRITC-phalloidin (for F-actin; red) and DRAQ5 (nuclear counter-stain; blue) and images were analyzed by confocal laser scanning microscopy. Panels A–D: WT/pair-fed; E–H: KO/pair-fed; I–L: WT/EtOH; M–P: KO/EtOH. (400x magnification. Images in panels D, H, L, and P are at 800x magnification). Arrows in panel P show the aberrant localization of ADH1 in vacuolated structures within hepatocytes in the KO/EtOH group.
Despite many attempts with using mouse liver tissue or the human hepatoma cell lines, Hep3B and HepG2, we could not demonstrate binding of TCF4, the transcriptional co-activator for β-catenin, to approximately 5000 bp regions spanning the ADH1A and CYP2E1 transcription start sites (Supplemental Figure S9 and data not shown). These results imply that β-catenin either regulates these genes indirectly or that it binds an enhancer outside the span of DNA targeted in our experiments (see discussion below).
Discussion
Given the growing research interest in the functional role of β-catenin in adult liver homeostasis, we undertook this study to determine whether β-catenin participates in ethanol metabolism and protection against alcohol-mediated liver pathology. β-catenin is known to associate with FoxO proteins to regulate expression of SOD and mediates adaptation to oxidative stress.(23, 26) SOD-2, which is present in the mitochondria, is critical for protection against oxidative stress. Mice with homozygous Sod-2 disruption exhibit dysfunction in multiple organs and mortality in the perinatal or early neonatal period.(27, 28) Heterozygous disruption of Sod2 causes increased oxidative stress and mitochondrial dysfunction.(29) On the other hand, overexpression of SOD-2 protects against alcohol-induced liver injury in rodents.(30) Thus, our results show conservation of the β-catenin-FoxO interaction in the mammalian liver and its relevance to the hepatic oxidative stress response. It should be noted that the Sod2 gene has multiple levels of transcriptional, epigenetic, and posttranslational regulation (reviewed recently in (31)). Therefore, regulation by β-catenin represents just one of many inputs of the complex network regulating SOD2 expression.
Despite striking liver steatosis, ethanol-fed KO mice had relatively modest increase in oxidative stress and serum ALT/AST levels, and exhibited no survival advantage with NAC treatment. While NAC does not prevent liver steatosis, it does prevent alcohol-induced oxidative stress.(32) Therefore, factors other than oxidative stress-mediated liver injury were likely causing mortality in KO mice. We show here that the absence of hepatic β-catenin affects expression and activity of ethanol metabolizing enzymes and results in high blood alcohol levels. This defect in ethanol metabolism, along with the hyperammonemia in β-catenin KO mice resulting from loss of hepatic glutamine synthetase expression, likely results in the acute sickness and mortality soon after exposure to a 5% ethanol diet. (20)
Several hypotheses have been proposed for the rate-limiting step of the major pathway of alcohol metabolism. Some investigators have proposed that the rate of ethanol metabolism is regulated by the amount of hepatic alcohol dehydrogenase.(33) Others have suggested that the rate at which NADH is reoxidized to NAD+ represents the rate-limiting step in alcohol metabolism.(34) A third point of view proposes that there is not a single rate-limiting step in ethanol metabolism and control is shared among several steps.(35) These controversies notwithstanding, Ronis et al recently demonstrated that a significant proportion of alcohol-mediated liver injury occurs independently of alcohol metabolism.(36) Our results showing that KO mice develop severe steatosis despite significant block in hepatic ethanol metabolism are consistent with their study.
A remarkable feature of the adult liver is the zonation of metabolic function across the liver acinus (reviewed in (37)). β-catenin is a key player in establishing hepatic metabolic zonation and a regulator of the perivenous program of gene expression.(17, 18, 24) Whether alcohol metabolism is zonated is somewhat controversial and two opposing views of alcohol metabolism have been proposed. Studies with microquantitative techniques or immunohistochemistry suggested that ADH activity was maximal in the perivenous area.(38, 39) Microquantitative techniques for ADH and ALDH from the human liver similarly showed an increasing periportal to perivenous gradient, although there were gender and age related differences.(40) On the other hand, Kashiwagi et al reported no zonal differences in ADH-dependent ethanol metabolism in hemoglobin-free perfused rat liver.(41) The impact of the hepatic zonal architecture on ethanol metabolism is highlighted by the fact that Cyp2E1 is strongly perivenous in its distribution.(12, 15) Our results suggest a modest increase in perivenous ADH1 staining in WT mice, which is absent in KO livers. Furthermore, in contrast to WT mice, we found that ethanol-fed KO mice exhibited non-uniform pattern of cytoplasmic ADH staining and vacuoles within hepatocytes where there was intense, localized ADH staining. The functional significance of these findings is currently under investigation but may represent defective protein trafficking within KO hepatocytes, as previously reported for specific proteins in cells depleted of β-catenin-E-cadherin-based adherens junctions, or stress-induced autophagy.(42, 43)
We could not detect binding of TCF4, the transcriptional coactivator of β-catenin, at the ADH1A and CYP2E1 promoters. While these negative results may imply that these genes are not direct transcriptional targets of β-catenin, it is possible that β-catenin/TCF4 bind to enhancers located outside these approximately 5 Kb regions spanning the transcription start sites that were targeted by us. Hatzis et al showed that TCF4 binding sites could be located at large distances (>100Kb) and be far-upstream, intronic, or downstream of transcription start sites of target genes.(44) Similarly, there are five β-catenin responsive elements located 400 Kb upstream from its target gene MYC and align with the MYC promoter through long-range chromatin loops.(45) Thus, further studies will be needed to determine whether β-catenin directly or indirectly regulates ethanol-metabolizing genes in the liver.
In summary, we show here that liver-specific loss of β-catenin leads to defective ethanol metabolism, increased hepatic oxidative stress, mitochondrial dysfunction, and severe liver steatosis, which combine to produce rapid mortality in ethanol-fed mice. Thus, β-catenin plays a key role in integration of ethanol metabolism and oxidative stress functions of the liver.
Supplementary Material
Acknowledgments
Grant support: Funded by National Institutes of Health Grant 1K08AA017622 (JB). This material is also the result of work supported in part with resources from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development National Merit Review grant (KK), and the use of facilities at the VA Nebraska-Western Iowa Health Care System, and by NIH Grant R56DK065149 (DKS).
Abbreviations
- KO
Knockout
- WT
Wild type
- SOD-2
superoxide dismutase
- ADH
alcohol dehydrogenase
- ALDH
aldehyde dehydrogenase
- Cyp2E1
Cytochrome P450 2E1
- MDA
malondialdehyde
- NADPH
nicotinamide adenine dinucleotide phosphate-reduced
- NAC
N-acetyl cysteine
- TCA
tricarboxylic acid
- FOXO
Forkhead box
- NAD
nicotinamide adenine dinucleotide
Footnotes
Disclosures: None.
References
- 1.Lieber CS. Metabolism and metabolic effects of alcohol. Med Clin North Am. 1984;68:3–31. doi: 10.1016/s0025-7125(16)31238-x. [DOI] [PubMed] [Google Scholar]
- 2.Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Yang AL, Vadhavkar S, Singh G, Omary MB. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168:649–656. doi: 10.1001/archinte.168.6.649. [DOI] [PubMed] [Google Scholar]
- 4.Mandayam S, Jamal MM, Morgan TR. Epidemiology of alcoholic liver disease. Semin Liver Dis. 2004;24:217–232. doi: 10.1055/s-2004-832936. [DOI] [PubMed] [Google Scholar]
- 5.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller AM, Horiguchi N, Jeong WI, Radaeva S, Gao B. Molecular Mechanisms of Alcoholic Liver Disease: Innate Immunity and Cytokines. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2010.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao B, Seki E, Brenner D, Friedman SL, Cohen J, Nagy LE, Szabo G, et al. Innate immunity and alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011 doi: 10.1152/ajpgi.00537.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen JI, Chen X, Nagy LE. Redox signaling and the innate immune system in alcoholic liver disease. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barve A, Khan R, Marsano L, Ravindra KV, McClain C. Treatment of alcoholic liver disease. Ann Hepatol. 2008;7:5–15. [PubMed] [Google Scholar]
- 11.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29:245–254. [PMC free article] [PubMed] [Google Scholar]
- 12.Lieber CS, DeCarli LM. Ethanol oxidation by hepatic microsomes: adaptive increase after ethanol feeding. Science. 1968;162:917–918. doi: 10.1126/science.162.3856.917. [DOI] [PubMed] [Google Scholar]
- 13.Lieber CS, Teschke R, Hasumura Y, Decarli LM. Differences in hepatic and metabolic changes after acute and chronic alcohol consumption. Fed Proc. 1975;34:2060–2074. [PubMed] [Google Scholar]
- 14.Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 15.Buhler R, Lindros KO, Nordling A, Johansson I, Ingelman-Sundberg M. Zonation of cytochrome P450 isozyme expression and induction in rat liver. Eur J Biochem. 1992;204:407–412. doi: 10.1111/j.1432-1033.1992.tb16650.x. [DOI] [PubMed] [Google Scholar]
- 16.Sokal EM, Collette E, Buts JP. Continuous increase of alcohol dehydrogenase activity along the liver plate in normal and cirrhotic human livers. Hepatology. 1993;17:202–205. [PubMed] [Google Scholar]
- 17.Benhamouche S, Decaens T, Godard C, Chambrey R, Rickman DS, Moinard C, Vasseur-Cognet M, et al. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev Cell. 2006;10:759–770. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Burke ZD, Reed KR, Phesse TJ, Sansom OJ, Clarke AR, Tosh D. Liver zonation occurs through a beta-catenin-dependent, c-Myc-independent mechanism. Gastroenterology. 2009;136:2316–2324. e2311–2313. doi: 10.1053/j.gastro.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 19.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 20.Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology. 2006;43:817–825. doi: 10.1002/hep.21131. [DOI] [PubMed] [Google Scholar]
- 21.Braeuning A, Sanna R, Huelsken J, Schwarz M. Inducibility of drug-metabolizing enzymes by xenobiotics in mice with liver-specific knockout of Ctnnb1. Drug Metab Dispos. 2009;37:1138–1145. doi: 10.1124/dmd.108.026179. [DOI] [PubMed] [Google Scholar]
- 22.Behari J, Yeh TH, Krauland L, Otruba W, Cieply B, Hauth B, Apte U, et al. Liver-specific beta-catenin knockout mice exhibit defective bile acid and cholesterol homeostasis and increased susceptibility to diet-induced steatohepatitis. Am J Pathol. 2010;176:744–753. doi: 10.2353/ajpath.2010.090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–1184. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Fergusson MM, Wu JJ, Rovira II, Liu J, Gavrilova O, Lu T, et al. Wnt signaling regulates hepatic metabolism. Sci Signal. 2011;4:ra6. doi: 10.1126/scisignal.2001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chepelev NL, Bennitz JD, Wright JS, Smith JC, Willmore WG. Oxidative modification of citrate synthase by peroxyl radicals and protection with novel antioxidants. J Enzyme Inhib Med Chem. 2009;24:1319–1331. doi: 10.3109/14756360902852586. [DOI] [PubMed] [Google Scholar]
- 26.Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. J Biol Chem. 2008;283:9224–9230. doi: 10.1074/jbc.M706638200. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 28.Lebovitz RM, Zhang H, Vogel H, Cartwright J, Jr, Dionne L, Lu N, Huang S, et al. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci U S A. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams MD, Van Remmen H, Conrad CC, Huang TT, Epstein CJ, Richardson A. Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J Biol Chem. 1998;273:28510–28515. doi: 10.1074/jbc.273.43.28510. [DOI] [PubMed] [Google Scholar]
- 30.Wheeler MD, Kono H, Yin M, Rusyn I, Froh M, Connor HD, Mason RP, et al. Delivery of the Cu/Zn-superoxide dismutase gene with adenovirus reduces early alcohol-induced liver injury in rats. Gastroenterology. 2001;120:1241–1250. doi: 10.1053/gast.2001.23253. [DOI] [PubMed] [Google Scholar]
- 31.Miao L, St Clair DK. Regulation of superoxide dismutase genes: implications in disease. Free radical biology & medicine. 2009;47:344–356. doi: 10.1016/j.freeradbiomed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronis MJ, Hennings L, Stewart B, Basnakian AG, Apostolov EO, Albano E, Badger TM, et al. Effects of long-term ethanol administration in a rat total enteral nutrition model of alcoholic liver disease. American journal of physiology Gastrointestinal and liver physiology. 2011;300:G109–119. doi: 10.1152/ajpgi.00145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crow KE, Cornell NW, Veech RL. The rate of ethanol metabolism in isolated rat hepatocytes. Alcohol Clin Exp Res. 1977;1:43–50. doi: 10.1111/j.1530-0277.1977.tb05765.x. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins RD, Kalant H. The metabolism of ethanol and its metabolic effects. Pharmacol Rev. 1972;24:67–157. [PubMed] [Google Scholar]
- 35.Page RA, Kitson KE, Hardman MJ. The importance of alcohol dehydrogenase in regulation of ethanol metabolism in rat liver cells. Biochem J. 1991;278 (Pt 3):659–665. doi: 10.1042/bj2780659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronis MJ, Korourian S, Blackburn ML, Badeaux J, Badger TM. The role of ethanol metabolism in development of alcoholic steatohepatitis in the rat. Alcohol. 2010;44:157–169. doi: 10.1016/j.alcohol.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jungermann K, Kietzmann T. Zonation of parenchymal and nonparenchymal metabolism in liver. Annu Rev Nutr. 1996;16:179–203. doi: 10.1146/annurev.nu.16.070196.001143. [DOI] [PubMed] [Google Scholar]
- 38.Morrison GR, Brock FE. Quantitative measurement of alcohol dehydrogenase activity within the liver lobule of rats after prolonged ethanol ingestion. J Nutr. 1967;92:286–292. doi: 10.1093/jn/92.2.286. [DOI] [PubMed] [Google Scholar]
- 39.Buehler R, Hess M, Von Wartburg JP. Immunohistochemical localization of human liver alcohol dehydrogenase in liver tissue, cultured fibroblasts, and HeLa cells. Am J Pathol. 1982;108:89–99. [PMC free article] [PubMed] [Google Scholar]
- 40.Maly IP, Sasse D. Intraacinar profiles of alcohol dehydrogenase and aldehyde dehydrogenase activities in human liver. Gastroenterology. 1991;101:1716–1723. doi: 10.1016/0016-5085(91)90412-e. [DOI] [PubMed] [Google Scholar]
- 41.Kashiwagi T, Ji S, Lemasters JJ, Thurman RG. Rates of alcohol dehydrogenase-dependent ethanol metabolism in periportal and pericentral regions of the perfused rat liver. Mol Pharmacol. 1982;21:438–443. [PubMed] [Google Scholar]
- 42.Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, Lu B, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–1752. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theard D, Steiner M, Kalicharan D, Hoekstra D, van Ijzendoorn SC. Cell polarity development and protein trafficking in hepatocytes lacking E-cadherin/beta-catenin-based adherens junctions. Mol Biol Cell. 2007;18:2313–2321. doi: 10.1091/mbc.E06-11-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hatzis P, van der Flier LG, van Driel MA, Guryev V, Nielsen F, Denissov S, Nijman IJ, et al. Genome-wide pattern of TCF7L2/TCF4 chromatin occupancy in colorectal cancer cells. Molecular and cellular biology. 2008;28:2732–2744. doi: 10.1128/MCB.02175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yochum GS. Multiple Wnt/b-catenin responsive enhancers align with the MYC promoter through long-range chromatin loops. PloS one. 2011;6:e18966. doi: 10.1371/journal.pone.0018966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.