Abstract
The protein alpha-synuclein is considered to play a major role in the etiology of Parkinson’s disease. Because it is found in a classic amyloid fibril form within the characteristic intra-neuronal Lewy body deposits of the disease, aggregation of the protein is thought to be of critical importance, but the context in which the protein undergoes aggregation within cells remains unknown. The normal function of synucleins is poorly understood, but appears to involve membrane interactions, and in particular reversible binding to synaptic vesicle membranes. Structural studies of different states of alpha-synuclein, in the absence and presence of membranes or membrane mimetics, have led to models of how membrane-bound forms of the protein may contribute both to functional properties of the protein, as well as to membrane-induced self-assembly and aggregation. This article reviews this area, with a focus on a particular model that has emerged in the past few years.
Introduction
Alpha-synuclein (α-synuclein, aS) is a small (140-residue, 14.5kDa), soluble presynaptic protein that is highly conserved in vertebrates and has been implicated in Parkinson’s disease (PD). Several mutations (A53T, A30P, and most recently E46K) in the human aS gene, designated SNCA, (1–3) as well as duplications and triplications of the gene (4, 5) have been found to be associated with rare familial Parkinson’s. In addition, Lewy bodies, protein deposits that are found intra-neuronally in Parkinson’s brains, are composed largely of β-sheet-rich alpha-synuclein amyloid fibrils (6). In light of these findings, much effort has been focused on clarifying the links between alpha-synuclein and Parkinson’s.
Since the original discovery of a synuclein protein in the electric organ synapses of the electric ray Torpedo californica (7), a relationship between synucleins and membranes has been apparent. In both ray and rat, aS was found to localize to the presynaptic membrane as well as to a region of the nuclear envelope (hence the name synuclein). Furthermore, the N-terminal domain of aS includes 7 imperfect 11-residue repeats, each containing a variant of the consensus 6-residue sequence KTKEGV, which are similar to repeats found in the exchangeable apolipoproteins and are consistent with a class A2 amphipathic alpha helices (8) suggesting a lipid-binding activity for the N-terminal domain. aS was shown to associate with synaptic vesicle preparations (9, 10), and to bind to synthetic phospholipid vesicles containing negatively-charged phospholipids (11, 12), although the association was found to be relatively weak, an observation that was subsequently confirmed in more detail (13, 14).
Presently, the normal function of alpha-synuclein remains poorly understood, although it has been linked with synaptic plasticity (15) and learning (8), neurotransmitter release (16, 17) and maintenance of synaptic vesicle pools (18, 19). The function of aS may be linked to its interesting structural properties. In dilute aqueous solutions in vitro synuclein adopts a natively unfolded, or intrinsically disordered, structural ensemble (20). However, even in this generally unstructured form, the N-terminal lipid-binding domain of aS displays a slight preference for helical structure (21). Upon binding to negatively charged lipid membranes or membrane-mimetic detergent micelles, this N-terminal domain adopts a highly helical structure (11, 21). The micelle-bound form of aS consists of two non-contacting antiparallel helices in the N-terminal domain with a short break around residues 38–44, and a flexible conformation in the C-terminal domain (22–27).
The aggregation mechanisms and propensities of aS have been studied in detail, but a consensus view of the aggregation mechanism still eludes researchers. The PD-linked mutations A30P and A53T both increase the rate of aS aggregation in vitro (28, 29), although A53T aS forms fibrils faster than wild-type while A30P aS forms fibrils more slowly (30), suggesting that it is the acceleration of oligomerization, and not mature fibril formation, which may be the common feature of PD-linked mutants. E46K aS was also found to fibrillize more rapidly (31). Since A30P was found to reduce local helical propensity and A53T to increase local β-sheet propensity, it was postulated that this change in local peptide properties was responsible for the increased likelihood to form β-sheet-rich aggregates in those mutants (32).
An additional hypothesis suggested that long-range interactions involving the central hydrophobic NAC region of alpha-synuclein may influence oligomerization of the protein (32). Early NMR residual dipolar coupling (RDC) data suggested that C- to N-terminal long-range interactions were lost in the A30P and A53T mutants (33), exposing the NAC region. However, subsequent studies indicated that the E46K mutation increases such contacts, and that they are unaffected by the A30P and A53T mutations. Other conditions that increase the compactness of regions of the protein also lead to enhanced aggregation (34) whereas variants or conditions that reduce long-range interactions are associated with reduced aggregation (35, 36), Thus, it seems that long-range contacts may not be protective against aS aggregation and might even facilitate the process (37).
Membrane interactions have also been implicated in alpha-synuclein aggregation. The rate of fibrillization of wild-type aS was found to be increased in the presence of lipids and detergents (38–42), at least under some circumstances. The detailed mechanisms by which membranes enhance the aggregation of aS remain unclear, but a potential role for helical intermediates in this process has been suggested both by recent NMR studies of vesicle-binding modes of aS and disease-linked aS mutants (43, 44) as well as by a study of trifluoroethanol (TFE) induced aS aggregation (45).
Membrane associated folded and functional states
Understanding the normal function of alpha-synuclein is important for a number of reasons, including an appreciation of its normal role in synaptic (and possibly other) pathways, a better knowledge of different contexts in which to potentially intervene in the behavior of the protein in vivo, and the remaining possibility that alterations in the normal function of the protein may play a role in PD, highlighted by recent work showing that removal of all synuclein family members in mice leads to an age-dependent neurodegeneration and a decreased life span (17). The normal membrane-binding modes of alpha-synuclein are considered to play a critical role in the protein’s function making studies of membrane-bound states of considerable interest. Of course, the possible role for membranes in facilitating aS aggregation in vivo adds to the importance of understanding how the protein interacts with membranes.
Subsequent to early studies using optical spectroscopies such as circular dichroism (CD), which demonstrated a large-scale disorder-to-helix transition in aS upon binding of the protein to vesicles of different types (11, 12, 46), NMR studies of the protein in the presence of membrane-mimetic sodium dodecyl sulfate (SDS) micelles revealed that the N-terminal lipid-binding domain of the protein (consisting approximately of residues 1–100) adopts a helical structure made up of two curved antiparallel helices connected by an extended linker of residues 38 to 44 (22–24). Based on NMR chemical shifts of amide resonances, as increasing concentrations of detergent are added to solutions containing aS the protein folds into the broken-helix conformation through a third, intermediate state, which may consist of more than one aS molecule bound to a micelle or a non-helical conformation (24). Evidence for such an intermediate has also been recently provided by small angle x-ray scattering (SAXS) in combination with other methods (47).
In the micelle-bound broken-helix state, the region from alanine 30 to valine 37 was found to have higher mobility as measured by RDCs (24) and lower helical propensity as measured by the difference of Cα chemical shift from the random coil value (22–24). These data are consistent with some degree of instability and/or helix fraying at the C-terminal end of the first helix. There is also an apparent kink or instability in the second of two helices around position 65. Both of the two micelle-bound helices remain on the surface of the micelle, and no evidence of any trans-micelle orientations were observed in NMR paramagnetic relaxation enhancement (PRE) studies (27). The same studies indicated that the helices may penetrate as deep as the C3 or C4 carbons of the detergent acyl chain, consistent with an estimate, based on an evaluation of the electrostatic surface potential of alpha-synuclein, that the apolar faces of the helices that interact with the micelle surface may insert past the charged headgroups (24). Meanwhile, many of the positively-charged sidechains are facing outward from the helices, possibly lying on the surface of the micelle and interacting with the negatively charged headgroups.
While an SDS micelle has a diameter of around 5 nm, synaptic vesicles are approximately ten times larger. Thus, the relevance of the broken-helix micelle-bound structure of aS to the conformation adopted by the protein when bound to synaptic vesicles has remained controversial. It was noted that the small size of the spheroidal micelles may dictate the broken-helix or “horseshoe” conformation by requiring the protein to adhere to the micelle surface (22). Indeed, studies using pulsed ESR (electron spin resonance) distance measurements demonstrated that increasing the size of the micelle to which the protein was bound leads the two helices to splay further apart (26), supporting the notion that micelle topology profoundly influences the relative arrangement of the two micelle-bound helices. The protein-vesicle complex is too large for the type of detailed NMR studies that were used to characterize the micelle-bound state, although an initial study was able to determine that in both contexts (micelle- and vesicle-bound) the same regions of the protein becomes helical (the N-terminal lipid-binding domain) and the same regions remain disordered (the acidic C-terminal tail consisting of the C-terminal 40 residues of the protein) (21). More recently pulsed dipolar ESR measurements of long intramoleculear distances (up to 90 Ångstroms) in aS bound to vesicles as well as bicelles (disk-shaped lipid bilayer structures comprised of long-chain phospholipids surrounded by a layer of short-chain detergent molecules (48)) have been used to characterize the conformation of vesicle-bound synuclein and the lipid-binding domain of the protein was found to adopt a single extended helix, in which the two separate helices of the micelle-bound broken-helix state fuse into one longer helix, with the linker region converting from an extended to a helical conformation (49, 50). ESR studies combining shorter distance measurements with computational modeling reached a similar conclusion (51), as did complementary studies using single-molecule Förster resonance energy transfer (smFRET) distance measurements (52, 53).
Despite these observations that aS adopts the extended-helix conformation when bound to lipid vesicles, it appears that the micelle-bound structure of aS remains relevant to the physiological context of synaptic vesicles and the presynaptic membrane. Several studies have noted that the broken-helix state can be observed even in the presence of lipid vesicles (54, 55) while others have noted that under some circumstances both states (extended- and broken-helix) can co-exist in the context of vesicles (49, 56, 57) suggesting that the conversion between the two states is pre-encoded into the primary sequence of the protein. Based on our initial observations of the broken- and extended-helix states, we proposed a model in which the uniquely apposed membrane topology of fusing or budding vesicles could provide a scaffold for the broken-helix conformation of alpha-synuclein to act as a bridge between different membranes (49, 58). In this model, one of the two helices of aS would bind to the plasma membrane, and the other to a docked or budding synaptic vesicle. The protein could function to anchor the vesicle to the membrane or even possibly to sense the extent of budding/fusion. This model was inspired in part by observations that synuclein expression can lead to a clustering of vesicles in yeast (59) and to a stabilization of docked vesicles in chromafin cells (60). Several reports suggesting that the N-terminal and C-terminal regions of the lipid-binding domain of aS exhibit different membrane-binding affinities (43, 61, 62) are also consistent with a potential bridging function for the protein.
The observation that aS interacts with and inhibits phospholipase D (PLD), which catalyzes the hydrolysis of phosphatidylcholine to phosphatidic acid (63) also suggests that placing synuclein at the site of docked vesicles could serve to influence the membrane remodeling processes involved in vesicle fusion or budding (although PLD regulation by aS has been contested in recent work (64)). PLD is implicated in the regulation of secretory vesicle budding or fusion through its generation of phosphatidic acid (65, 66), which can play a role in modulating membrane curvature and also regulates phosphatidylinositol-4-phosphate 5-kinase activity (67). The interaction of aS with PLD appears to require the helical, membrane-bound form of aS and involve both the helical domain and the more highly disordered C-terminal tail (68). Because PLD is likely to act on the plasma membrane and is not known to be associated with synaptic vesicles, regulation of PLD activity by aS is consistent with the idea, depicted in our model, that aS is able to contact both the vesicle and plasma membranes in some contexts.
A recent ESR study of the interconversion between the broken- and extended-helix conformations of aS in the presence of detergents showed that the detergent to protein concentration ratio determines which conformation is adopted by the protein, with higher populations of the extended-helix conformation observed at ratios above ~500:1 (50). The extended-helix state could be observed even at detergent concentrations below those required for cylindrical micelle formation in the absence of protein, indicating that the protein can alter micelle behavior and topology. This finding suggests that aS is not just a passive adapter to a preconfigured membrane topology, but may itself influence the topology of the membranes to which it binds. A membrane bending or remodeling activity of alpha-synuclein has been documented by others (69, 70) supporting a potential role for this activity of aS in the membrane remodeling required for vesicle fusion or budding. Indeed, a recent report shows that aS may interact directly with SNARE proteins and may influence the efficiency of synaptic vesicle exocytosis (17). The protein has also been implicated in endocytic pathways (71).
In addition to the above, structure-based model of alpha-synuclein function, others have been proposed. Various forms of aS, including oligomeric or protofibrillar species, have been reported to permeabilize membranes (72), suggesting a potential function for the protein as some kind of pore or channel. Indeed, a recent study provides evidence of channel formation by aS even as a monomer (73) and proposes a model of helical aS monomers partitioning from the membrane surface into a hairpin transmembrane topology in the presence of a voltage across the membrane, such as that generated in the neuron during transmission of an action potential.
Beyond its regulation of PLD, aS has been reported to preferentially interact with several proteins in its lipid-bound form, among them endosulfine-α and its close relatives the cAMP-regulated phosphoproteins ARPP-19 and ARPP-16 (74). These proteins are though to function in dopamine and other signaling networks (75), and are also regulators of protein phosphatases (76).
While the N-terminal ~100 residues of aS are usually considered to constitute the lipid-binding domain of the protein, the acidic C-terminal tail may also play a role in membrane-binding, as well as other aspects of synuclein function. In the micelle-bound structure, the C-terminal tail was found to be mostly disordered ; however, there was some heterogeneity in the NMR properties (24, 27) and a subsequent study also reported a weak vesicle interaction for residues in the C-terminal tail, which was associated with shift between trans and cis X-Pro peptide bonds among several proline residues and the ensuing increased lipid binding of the cis-containing conformations (43). Interestingly, a recent report indicates that nitration of tyrosines in the C-terminal tail of aS alters its affinity for membranes (77), again suggesting some role (either direct or indirect) for the C-terminal tail in modulating aS membrane interactions. In general, however, the C-terminal tail of aS (as well as those of the closely related family members β- and γ-synuclein) is thought to function as a protein-protein interaction motif (78), as illustrated by its role in interactions with both PLD and the neuronal v-SNARE protein synaptobrevin 2 (17), and other post-translational modifications such as phosphorylation at serine 129 or serin 87 have not thus far been shown to alter membrane binding (36, 79).
Membrane-induced aggregation
Lipids, fatty acids and detergents have been found to accelerate aS fibrillization under various circumstances (38–42, 80–83). A critical factor seems to be the ratio of protein to lipid or detergent (40, 41), with higher ratios (low concentrations of lipid or detergent) driving aggregation, while lower ratios (high concentrations of lipid or detergent) tend to prevent aggregation (84, 85). This observation suggests that one mechanism by which lipids or lipid-like molecules may facilitate aS aggregation is by confining the protein to a small and/or two dimensional surface (the surface of a vesicle or micelle), thereby increasing the effective concentration and driving aggregation through mass action (86, 87). Some support for this scenario is provided by the observation that in the case of detergents, aggregation is enhanced when the detergent:protein ratio falls below the number of detergent molecules required to form a single micelle, and inhibited once there is sufficient detergent to insure that each protein can be accommodated on separate micelle (unpublished data and (24)). However, this effect is difficult to verify directly, and additional factors such as potentially different diffusion rates on a membrane surface or in solution (88, 89) must be taken into account as well.
An additional potential contribution to membrane-induced aS aggregation may arise from the conformational changes that are induced in the protein by its membrane interactions, and that can favor intermolecular interactions leading to aggregation. Support for this model comes from observations that at concentrations that favor aggregation, detergents also appear to induce intermediate conformations of aS (24, 47, 80, 90).
Recently, we published a study aimed at assessing the role of membrane-induced structural changes, versus surface-induced mass action effects. We used fluorinated alcohols to decouple conformational changes similar to those induced by membranes from the process of binding to a surface. Several previous studies demonstrated that such cosolvents enhanced the propensity of aS to aggregate (91, 92) and could also be used to model the protein’s membrane interactions (93). Working at very low protein concentrations to maintain aS in a monomeric state and preclude aggregation, we used CD spectroscopy to analyze the changes induced in aS by varying concentrations of TFE, and to correlate these effects with changes in aggregation propensity (45). It was first shown that at very low (below a few percent by volume) and very high (above 30 percent by volume) TFE concentrations, aggregation of aS is inhibited, just as it is in the presence of very low or very high concentrations of lipids or detergent, while at intermediate TFE concentrations, aS aggregation becomes very efficient, just as it is at intermediate concentrations of lipids or detergents. The results then showed that a distinct and partially helical aS intermediate was formed at intermediate TFE concentrations, and that the maximal population of the intermediate state correlated with the maximal aggregation propensity induced by the cosolvent. This result strongly suggests that the helical structure in the intermediate state plays a role in driving the aggregation of the protein, even in the case where no mass action effect is present. A similar conclusion was achieved based on studies of aS in the presence of combinations of crowding agents with other reagents, including organic cosolvents, that favor partial folding of aS (94)
A reconstruction of the far-UV CD spectrum for this TFE-induced partially folded intermediate demonstrated that approximately 20–30 residues are likely to adopt a helical structure within the context of the intermediate, and an analysis of the PD-linked mutations in the protein revealed that only the A30P mutation affected the spectrum of the intermediate, causing a significant decrease in its helicity and suggesting that position 30 is included in the helical structure. The original study of helical propensities in the free state of aS (21) noted that the N-terminal 30–40 residues exhibited the strongest intrinsic propensity for helical structure and suggested that this region might fold first upon association of the protein with membranes. A recent study of synuclein peptide fragments binding to lipids supports this assertion (95). Furthermore, several studies have suggested the existence of partially folded membrane-bound intermediates, in which the N-terminal region is folded and helical, with the remainder of the lipid-binding domain remains unbound (43, 44, 61). Thus, it appears that the partially helical intermediate formed in the presence of TFE, which efficiently drives the aggregation of the protein, may have a corresponding membrane-induced intermediate where the N-terminal region of the protein is bound and helical, but the remainder of the lipid-binding domain remains disordered. Although the membrane-bound intermediate might be expected to bury its hydrophobic face at the protein membrane interface, the relatively low affinity of synuclein-membrane interactions may favor the formation of protein-protein interactions when two membrane-bound intermediates encounter each other. Thus, this intermediate state could drive self association of the protein on the membrane surface (perhaps in a manner further enhanced by mass action effects as discussed above), bringing the more C-terminal unstructured regions into close proximity and facilitating the nucleation of inter-molecular beta-sheet structure and the eventual formation of amyloid oligomers and fibrils. Notably, models invoking similar roles for helical intermediates in promoting the aggregation of other amyloidogenic proteins have been gaining support in recent years (96).
Conclusions
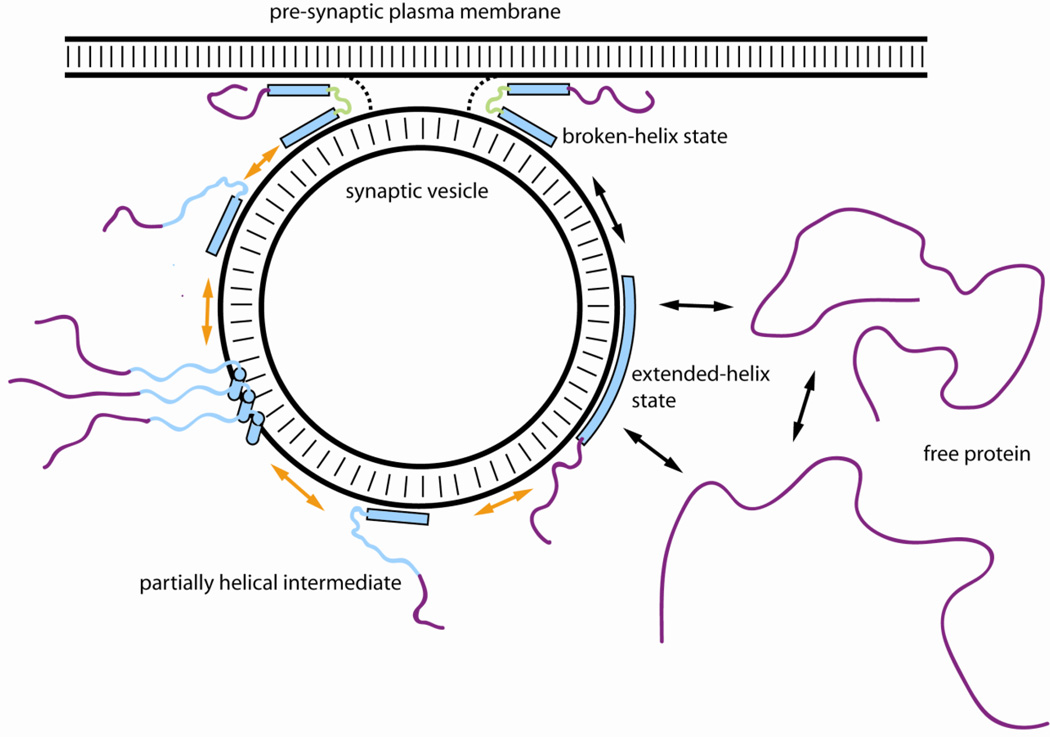
Some of the models for membrane-induced folding, function and aggregation of aS are summarized in the figure below. The highly disordered free state of the protein is proposed to exist in equilibrium with the vesicle-bound extended-helix form of the protein, which can interconvert into the broken-helix state upon the close approach of a synaptic vesicle to the plasma membrane. The broken-helix form can function as a structural support or sensor for docked vesicles, but may also play an active role in vesicle fusion either by regulation the activities of other proteins such as PLD or synaptobrevin, or by directly influencing the properties of the membranes involved in order to influence the fusion process. Under different circumstances, such as when the protein first begins to fold upon binding to membranes, or upon release of the C-terminal region of the extended-helix conformation from the membrane surface, or upon release of the second helix of the broken-helix state from the membrane-bridging configuration, an intermediate state may be formed in which the N-terminal region remains bound and helical, while the C-terminal region of the lipid-binding domain is unstructured. The helical regions of such intermediates may drive their intermolecular interactions, bringing the disordered C-terminal regions into close proximity and facilitating their aggregation into the beta-sheet rich aggregates characteristic of PD. While all aspects of these models require extensive testing before they can be considered to reflect the actual behavior of the protein in vivo, they are consistent with many existing observations regarding the properties and potential functions of aS.
Figure 1.
Summary of some of the models presented in this manuscript for the membrane-induced structure, function and aggregation of the Parkinson’s disease linked protein alpha-synuclein. Poorly structured protein regions are depicted as solid lines, while helical regions are depicted as filled bars or cylinders. Membrane bilayers are indicated as double black lines with hashes. The unbound, intrinsically disordered state of aS is depicted as existing in equilibrium between less compact and more compact conformations, which are also in equilibrium with the vesicle-bound extended-helix state. The latter can convert to the broken-helix state upon approach of the vesicle to another membrane, such as the pre-synaptic plasma membrane. Transitions between these conformations, which are all considered to occur as part of the normal function of the protein, are indicated by black double arrows. The potential activity of the broken helix state (possibly through the action of the linker region) in modulating membrane properties during membrane fusion is indicate by the green color of the linker region and the dotted lines between the vesicle and plasma membranes. Either the extended- or broken-helix states may be able to convert to a membrane-bound partially helical intermediate by the release of the C-terminal region of the lipid-binding domain from the membrane surface. Intermolecular association of these intermediates, driven by the membrane-associated N-terminal helices, may bring the disordered regions into close proximity, facilitating intermolecular beta-sheet formation leading to amyloid oligomer and fibril formation. These transitions, which are considered to occur as part of the pathological behavior of the protein, are indicated by orange double arrows.
Highlights.
Membrane binding by synuclein is associated with normal function
Membrane binding by synuclein is associated with aggregation
Membranes induce both broken- and extended-helix states and both may be functional
Membranes may induce partially helical intermediates that drive aggregation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 2.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 3.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 4.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha- Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 5.Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 6.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 9.Irizarry MC, Kim TW, McNamara M, Tanzi RE, George JM, Clayton DF, Hyman BT. Characterization of the precursor protein of the non-A beta component of senile plaques (NACP) in the human central nervous system. J Neuropathol Exp Neurol. 1996;55:889–895. doi: 10.1097/00005072-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okochi M, Leimer U, van Der Putten H, Probst A, Kremmer E, Kretzschmar HA, Haass C. Subcellular localization of wild-type and Parkinson's disease-associated mutant alpha -synuclein in human and transgenic mouse brain. J Neurosci. 2000;20:6365–6373. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 12.Jo E, McLaurin J, Yip CM, St George-Hyslop P, Fraser PE. alpha-Synuclein membrane interactions and lipid specificity. J Biol Chem. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 13.Rhoades E, Ramlall TF, Webb WW, Eliezer D. Quantification of alpha-synuclein binding to lipid vesicles using fluorescence correlation spectroscopy. Biophys J. 2006;90:4692–4700. doi: 10.1529/biophysj.105.079251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bussell R, Jr, Eliezer D. Effects of Parkinson's disease-linked mutations on the structure of lipid-associated alpha-synuclein. Biochemistry. 2004;43:4810–4818. doi: 10.1021/bi036135+. [DOI] [PubMed] [Google Scholar]
- 15.Watson JB, Hatami A, David H, Masliah E, Roberts K, Evans CE, Levine MS. Alterations in corticostriatal synaptic plasticity in mice overexpressing human alpha-synuclein. Neuroscience. 2009;159:501–513. doi: 10.1016/j.neuroscience.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 17.Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 20.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 21.Eliezer D, Kutluay E, Bussell R, Jr, Browne G. Conformational properties of alpha-synuclein in its free and lipid- associated states. J Mol Biol. 2001;307:1061–1073. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- 22.Bussell R, Jr, Eliezer D. A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J Mol Biol. 2003;329:763–778. doi: 10.1016/s0022-2836(03)00520-5. [DOI] [PubMed] [Google Scholar]
- 23.Chandra S, Chen X, Rizo J, Jahn R, Sudhof TC. A broken alpha -helix in folded alpha - Synuclein. J Biol Chem. 2003;278:15313–15318. doi: 10.1074/jbc.M213128200. [DOI] [PubMed] [Google Scholar]
- 24.Ulmer TS, Bax A, Cole NB, Nussbaum RL. Structure and dynamics of micelle-bound human alpha-synuclein. J Biol Chem. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 25.Bisaglia M, Tessari I, Pinato L, Bellanda M, Giraudo S, Fasano M, Bergantino E, Bubacco L, Mammi S. A Topological Model of the Interaction between alpha-Synuclein and Sodium Dodecyl Sulfate Micelles. Biochemistry. 2005;44:329–339. doi: 10.1021/bi048448q. [DOI] [PubMed] [Google Scholar]
- 26.Borbat P, Ramlall TF, Freed JH, Eliezer D. Inter-helix distances in lysophospholipid micelle-bound alpha-synuclein from pulsed ESR measurements. J Am Chem Soc. 2006;128:10004–10005. doi: 10.1021/ja063122l. [DOI] [PubMed] [Google Scholar]
- 27.Bussell R, Jr, Ramlall TF, Eliezer D. Helix periodicity, topology, and dynamics of membrane-associated alpha-synuclein. Protein Sci. 2005;14:862–872. doi: 10.1110/ps.041255905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, Louis JC, Wypych J, Biere AL, Citron M. Both familial Parkinson's disease mutations accelerate alpha-synuclein aggregation. J Biol Chem. 1999;274:9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- 29.Giasson BI, Uryu K, Trojanowski JQ, Lee VM. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999;274:7619–7622. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- 30.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW, Axelsen PH, Giasson BI. The E46K mutation in alpha-synuclein increases amyloid fibril formation. J Biol Chem. 2005;280:7800–7807. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- 32.Bussell R, Jr, Eliezer D. Residual structure and dynamics in Parkinson's disease-associated mutants of alpha-synuclein. J Biol Chem. 2001;276:45996–46003. doi: 10.1074/jbc.M106777200. [DOI] [PubMed] [Google Scholar]
- 33.Bertoncini CW, Fernandez CO, Griesinger C, Jovin TM, Zweckstetter M. Familial mutants of alpha-synuclein with increased neurotoxicity have a destabilized conformation. J Biol Chem. 2005;280:30649–30652. doi: 10.1074/jbc.C500288200. [DOI] [PubMed] [Google Scholar]
- 34.McClendon S, Rospigliosi CC, Eliezer D. Charge neutralization and collapse of the C-terminal tail of alpha-synuclein at low pH. Protein Sci. 2009;18:1531–1540. doi: 10.1002/pro.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sung YH, Eliezer D. Residual Structure, Backbone Dynamics, and Interactions within the Synuclein Family. J Mol Biol. 2007;372:689–707. doi: 10.1016/j.jmb.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paleologou KE, Schmid AW, Rospigliosi CC, Kim HY, Lamberto GR, Fredenburg RA, Lansbury PT, Jr, Fernandez CO, Eliezer D, Zweckstetter M, Lashuel HA. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J Biol Chem. 2008;283:16895–16905. doi: 10.1074/jbc.M800747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rospigliosi CC, McClendon S, Schmid AW, Ramlall TF, Barre P, Lashuel HA, Eliezer D. E46K Parkinson's-linked mutation enhances C-terminal-to-N-terminal contacts in alpha-synuclein. J Mol Biol. 2009;388:1022–1032. doi: 10.1016/j.jmb.2009.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perrin RJ, Woods WS, Clayton DF, George JM. Exposure to long chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J Biol Chem. 2001;276:41958–41962. doi: 10.1074/jbc.M105022200. [DOI] [PubMed] [Google Scholar]
- 39.Lee HJ, Choi C, Lee SJ. Membrane-bound alpha-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J Biol Chem. 2002;277:671–678. doi: 10.1074/jbc.M107045200. [DOI] [PubMed] [Google Scholar]
- 40.Necula M, Chirita CN, Kuret J. Rapid anionic micelle-mediated alpha-synuclein fibrillization in vitro. J Biol Chem. 2003;278:46674–46680. doi: 10.1074/jbc.M308231200. [DOI] [PubMed] [Google Scholar]
- 41.Zhu M, Li J, Fink AL. The association of alpha-synuclein with membranes affects bilayer structure, stability, and fibril formation. J Biol Chem. 2003;278:40186–40197. doi: 10.1074/jbc.M305326200. [DOI] [PubMed] [Google Scholar]
- 42.Sharon R, Bar-Joseph I, Frosch MP, Walsh DM, Hamilton JA, Selkoe DJ. The formation of highly soluble oligomers of alpha-synuclein is regulated by fatty acids and enhanced in Parkinson's disease. Neuron. 2003;37:583–595. doi: 10.1016/s0896-6273(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 43.Bodner CR, Dobson CM, Bax A. Multiple Tight Phospholipid-Binding Modes of alpha-Synuclein Revealed by Solution NMR Spectroscopy. J Mol Biol. 2009;390:775–790. doi: 10.1016/j.jmb.2009.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bodner CR, Maltsev AS, Dobson CM, Bax A. Differential phospholipid binding of alpha-synuclein variants implicated in Parkinson's disease revealed by solution NMR spectroscopy. Biochemistry. 2010;49:862–871. doi: 10.1021/bi901723p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson VL, Ramlall TF, Rospigliosi CC, Webb WW, Eliezer D. Identification of a helical intermediate in trifluoroethanol-induced alpha-synuclein aggregation. Proc Natl Acad Sci U S A. 2010;107:18850–18855. doi: 10.1073/pnas.1012336107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human alpha-Synuclein and Parkinson's disease variants with phospholipids. Structural analysis using site-directed mutagenesis. J Biol Chem. 2000;275:34393–34398. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- 47.Giehm L, Oliveira CL, Christiansen G, Pedersen JS, Otzen DE. SDS-induced fibrillation of alpha-synuclein: an alternative fibrillation pathway. J Mol Biol. 2010;401:115–133. doi: 10.1016/j.jmb.2010.05.060. [DOI] [PubMed] [Google Scholar]
- 48.Whiles JA, Deems R, Vold RR, Dennis EA. Bicelles in structure-function studies of membrane-associated proteins. Bioorg Chem. 2002;30:431–442. doi: 10.1016/s0045-2068(02)00527-8. [DOI] [PubMed] [Google Scholar]
- 49.Georgieva ER, Ramlall TF, Borbat PP, Freed JH, Eliezer D. Membrane-bound alpha-synuclein forms an extended helix: long-distance pulsed ESR measurements using vesicles, bicelles, and rodlike micelles. J Am Chem Soc. 2008;130:12856–12857. doi: 10.1021/ja804517m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Georgieva ER, Ramlall TF, Borbat PP, Freed JH, Eliezer D. The lipid-binding domain of wild type and mutant alpha-synuclein: compactness and interconversion between the broken and extended helix forms. J Biol Chem. 2010;285:28261–28274. doi: 10.1074/jbc.M110.157214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jao CC, Hegde BG, Chen J, Haworth IS, Langen R. Structure of membrane-bound alpha-synuclein from site-directed spin labeling and computational refinement. Proc Natl Acad Sci U S A. 2008;105:19666–19671. doi: 10.1073/pnas.0807826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trexler A, Rhoades E. Alpha-Synuclein binds large unilamellar vesicles as an extended helix. Biochemistry. 2009 doi: 10.1021/bi900114z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferreon AC, Gambin Y, Lemke EA, Deniz AA. Interplay of alpha-synuclein binding and conformational switching probed by single-molecule fluorescence. Proc Natl Acad Sci U S A. 2009;106:5645–5650. doi: 10.1073/pnas.0809232106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bortolus M, Tombolato F, Tessari I, Bisaglia M, Mammi S, Bubacco L, Ferrarini A, Maniero AL. Broken helix in vesicle and micelle-bound alpha-synuclein: insights from site-directed spin labeling-EPR experiments and MD simulations. J Am Chem Soc. 2008;130:6690–6691. doi: 10.1021/ja8010429. [DOI] [PubMed] [Google Scholar]
- 55.Drescher M, Veldhuis G, van Rooijen BD, Milikisyants S, Subramaniam V, Huber M. Antiparallel arrangement of the helices of vesicle-bound alpha-synuclein. J Am Chem Soc. 2008;130:7796–7797. doi: 10.1021/ja801594s. [DOI] [PubMed] [Google Scholar]
- 56.Robotta M, Braun P, van Rooijen B, Subramaniam V, Huber M, Drescher M. Direct evidence of coexisting horseshoe and extended helix conformations of membrane-bound alpha-synuclein. Chemphyschem. 2011;12:267–269. doi: 10.1002/cphc.201000815. [DOI] [PubMed] [Google Scholar]
- 57.Lokappa SB, Ulmer TS. {alpha}-Synuclein Populates Both Elongated and Broken Helix States on Small Unilamellar Vesicles. J Biol Chem. 2011;286:21450–21457. doi: 10.1074/jbc.M111.224055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eliezer D. Protein folding and aggregation in in vitro models of Parkinson’s disease: Structure and function of α -Synuclein. In: Nass R, Prezedborski S, editors. Parkinson’s Disease : molecular and therapeutic insights from model systems. New York: Academic Press; 2008. [Google Scholar]
- 59.Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet JC, McCaffery JM, Barlowe C, Lindquist S. The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci U S A. 2008;105:145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, Stefanis L, Sulzer D. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drescher M, Godschalk F, Veldhuis G, van Rooijen BD, Subramaniam V, Huber M. Spin-label EPR on alpha-synuclein reveals differences in the membrane binding affinity of the two antiparallel helices. Chembiochem. 2008;9:2411–2416. doi: 10.1002/cbic.200800238. [DOI] [PubMed] [Google Scholar]
- 62.Pfefferkorn CM, Lee JC. Tryptophan probes at the alpha-synuclein and membrane interface. J Phys Chem B. 2010;114:4615–4622. doi: 10.1021/jp908092e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jenco JM, Rawlingson A, Daniels B, Morris AJ. Regulation of phospholipase D2: selective inhibition of mammalian phospholipase D isoenzymes by alpha- and beta-synucleins. Biochemistry. 1998;37:4901–4909. doi: 10.1021/bi972776r. [DOI] [PubMed] [Google Scholar]
- 64.Rappley I, Gitler AD, Selvy PE, LaVoie MJ, Levy BD, Brown HA, Lindquist S, Selkoe DJ. Evidence that alpha-synuclein does not inhibit phospholipase D. Biochemistry. 2009;48:1077–1083. doi: 10.1021/bi801871h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDermott M, Wakelam MJ, Morris AJ. Phospholipase D. Biochem Cell Biol. 2004;82:225–253. doi: 10.1139/o03-079. [DOI] [PubMed] [Google Scholar]
- 66.Siddhanta A, Shields D. Secretory vesicle budding from the trans-Golgi network is mediated by phosphatidic acid levels. J Biol Chem. 1998;273:17995–17998. doi: 10.1074/jbc.273.29.17995. [DOI] [PubMed] [Google Scholar]
- 67.Jenkins GH, Fisette PL, Anderson RA. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J Biol Chem. 1994;269:11547–11554. [PubMed] [Google Scholar]
- 68.Payton JE, Perrin RJ, Woods WS, George JM. Structural determinants of PLD2 inhibition by alpha-synuclein. J Mol Biol. 2004;337:1001–1009. doi: 10.1016/j.jmb.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 69.Varkey J, Isas JM, Mizuno N, Jensen MB, Bhatia VK, Jao CC, Petrlova J, Voss JC, Stamou DG, Steven AC, Langen R. Membrane curvature induction and tubulation are common features of synucleins and apolipoproteins. J Biol Chem. 2010;285:32486–32493. doi: 10.1074/jbc.M110.139576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pandey AP, Haque F, Rochet JC, Hovis JS. alpha-Synuclein-induced tubule formation in lipid bilayers. J Phys Chem B. 2011;115:5886–5893. doi: 10.1021/jp1121917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ben Gedalya T, Loeb V, Israeli E, Altschuler Y, Selkoe DJ, Sharon R. Alpha-synuclein and polyunsaturated fatty acids promote clathrin-mediated endocytosis and synaptic vesicle recycling. Traffic. 2009;10:218–234. doi: 10.1111/j.1600-0854.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Volles MJ, Lee SJ, Rochet JC, Shtilerman MD, Ding TT, Kessler JC, Lansbury PT., Jr Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson's disease. Biochemistry. 2001;40:7812–7819. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- 73.Zakharov SD, Hulleman JD, Dutseva EA, Antonenko YN, Rochet JC, Cramer WA. Helical alpha-synuclein forms highly conductive ion channels. Biochemistry. 2007;46:14369–14379. doi: 10.1021/bi701275p. [DOI] [PubMed] [Google Scholar]
- 74.Woods WS, Boettcher JM, Zhou DH, Kloepper KD, Hartman KL, Ladror DT, Qi Z, Rienstra CM, George JM. Conformation-specific binding of alpha-synuclein to novel protein partners detected by phage display and NMR spectroscopy. J Biol Chem. 2007;282:34555–34567. doi: 10.1074/jbc.M705283200. [DOI] [PubMed] [Google Scholar]
- 75.Dulubova I, Horiuchi A, Snyder GL, Girault JA, Czernik AJ, Shao L, Ramabhadran R, Greengard P, Nairn AC. ARPP-16/ARPP-19: a highly conserved family of cAMP-regulated phosphoproteins. J Neurochem. 2001;77:229–238. doi: 10.1046/j.1471-4159.2001.t01-1-00191.x. [DOI] [PubMed] [Google Scholar]
- 76.Gharbi-Ayachi A, Labbe JC, Burgess A, Vigneron S, Strub JM, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science. 2010;330:1673–1677. doi: 10.1126/science.1197048. [DOI] [PubMed] [Google Scholar]
- 77.Sevcsik E, Trexler AJ, Dunn JM, Rhoades E. Allostery in a disordered protein: oxidative modifications to alpha-synuclein act distally to regulate membrane binding. J Am Chem Soc. 2011;133:7152–7158. doi: 10.1021/ja2009554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sung YH, Eliezer D. Secondary structure and dynamics of micelle bound beta- and gamma-synuclein. Protein Sci. 2006;15:1162–1174. doi: 10.1110/ps.051803606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paleologou KE, Oueslati A, Shakked G, Rospigliosi CC, Kim HY, Lamberto GR, Fernandez CO, Schmid A, Chegini F, Gai WP, Chiappe D, Moniatte M, Schneider BL, Aebischer P, Eliezer D, Zweckstetter M, Masliah E, Lashuel HA. Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions. J Neurosci. 2010;30:3184–3198. doi: 10.1523/JNEUROSCI.5922-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rivers RC, Kumita JR, Tartaglia GG, Dedmon MM, Pawar A, Vendruscolo M, Dobson CM, Christodoulou J. Molecular determinants of the aggregation behavior of alpha- and beta-synuclein. Protein Sci. 2008;17:887–898. doi: 10.1110/ps.073181508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jo E, Darabie AA, Han K, Tandon A, Fraser PE, McLaurin J. alpha-Synuclein-synaptosomal membrane interactions: implications for fibrillogenesis. Eur J Biochem. 2004;271:3180–3189. doi: 10.1111/j.1432-1033.2004.04250.x. [DOI] [PubMed] [Google Scholar]
- 82.Cole NB, Murphy DD, Grider T, Rueter S, Brasaemle D, Nussbaum RL. Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. J Biol Chem. 2002;277:6344–6352. doi: 10.1074/jbc.M108414200. [DOI] [PubMed] [Google Scholar]
- 83.De Franceschi G, Frare E, Pivato M, Relini A, Penco A, Greggio E, Bubacco L, Fontana A, de Laureto PP. Structural and morphological characterization of aggregated species of alpha-synuclein induced by docosahexaenoic acid. J Biol Chem. 2011;286:22262–22274. doi: 10.1074/jbc.M110.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu M, Fink AL. Lipid binding inhibits alpha-synuclein fibril formation. J Biol Chem. 2003;278:16873–16877. doi: 10.1074/jbc.M210136200. [DOI] [PubMed] [Google Scholar]
- 85.Narayanan V, Scarlata S. Membrane binding and self-association of alpha-synucleins. Biochemistry. 2001;40:9927–9934. doi: 10.1021/bi002952n. [DOI] [PubMed] [Google Scholar]
- 86.Aisenbrey C, Borowik T, Bystrom R, Bokvist M, Lindstrom F, Misiak H, Sani MA, Grobner G. How is protein aggregation in amyloidogenic diseases modulated by biological membranes? Eur Biophys J. 2008;37:247–255. doi: 10.1007/s00249-007-0237-0. [DOI] [PubMed] [Google Scholar]
- 87.Hardt SL. Rates of diffusion controlled reactions in one, two and three dimensions. Biophys Chem. 1979;10:239–243. doi: 10.1016/0301-4622(79)85012-7. [DOI] [PubMed] [Google Scholar]
- 88.Saffman PG, Delbruck M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975;72:3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen H, Farkas ER, Webb WW. Chapter 1: In vivo applications of fluorescence correlation spectroscopy. Methods Cell Biol. 2008;89:3–35. doi: 10.1016/S0091-679X(08)00601-8. [DOI] [PubMed] [Google Scholar]
- 90.Ahmad MF, Ramakrishna T, Raman B, Rao Ch M. Fibrillogenic and non-fibrillogenic ensembles of SDS-bound human alpha-synuclein. J Mol Biol. 2006;364:1061–1072. doi: 10.1016/j.jmb.2006.09.085. [DOI] [PubMed] [Google Scholar]
- 91.Kim TD, Paik SR, Yang CH, Kim J. Structural changes in alpha-synuclein affect its chaperone-like activity in vitro. Protein Sci. 2000;9:2489–2496. doi: 10.1110/ps.9.12.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li HT, Du HN, Tang L, Hu J, Hu HY. Structural transformation and aggregation of human alpha-synuclein in trifluoroethanol: non-amyloid component sequence is essential and beta-sheet formation is prerequisite to aggregation. Biopolymers. 2002;64:221–226. doi: 10.1002/bip.10179. [DOI] [PubMed] [Google Scholar]
- 93.Munishkina LA, Phelan C, Uversky VN, Fink AL. Conformational behavior and aggregation of alpha-synuclein in organic solvents: modeling the effects of membranes. Biochemistry. 2003;42:2720–2730. doi: 10.1021/bi027166s. [DOI] [PubMed] [Google Scholar]
- 94.Munishkina LA, Fink AL, Uversky VN. Accelerated fibrillation of alpha-synuclein induced by the combined action of macromolecular crowding and factors inducing partial folding. Curr Alzheimer Res. 2009;6:252–260. doi: 10.2174/156720509788486491. [DOI] [PubMed] [Google Scholar]
- 95.Bartels T, Ahlstrom LS, Leftin A, Kamp F, Haass C, Brown MF, Beyer K. The N-terminus of the intrinsically disordered protein alpha-synuclein triggers membrane binding and helix folding. Biophys J. 2010;99:2116–2124. doi: 10.1016/j.bpj.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abedini A, Raleigh DP. A role for helical intermediates in amyloid formation by natively unfolded polypeptides? Phys Biol. 2009;6:015005. doi: 10.1088/1478-3975/6/1/015005. [DOI] [PMC free article] [PubMed] [Google Scholar]