Abstract
Purpose
Knowledge of transporters responsible for the renal secretion of creatinine is key to a proper interpretation of serum creatinine and/or creatinine clearance as markers of renal function in cancer patients receiving chemotherapeutic agents.
Experimental Design
Creatinine transport was studied in transfected HEK293 cells in vitro and in wildtype mice and age-matched organic cation transporter 1 and 2-deficient [Oct1/2(−/−)] mice ex vivo and in vivo. Clinical pharmacogenetic and transport inhibition studies were done in two separate cohorts of cancer patients.
Results
Compared to wildtype mice, creatinine clearance was significantly impaired in Oct1/2(−/−) mice. Furthermore, creatinine inhibited organic cation transport in freshly-isolated proximal tubules from wild-type mice and humans, but not in those from Oct1/2(−/−) mice. In a genetic-association analysis (n=590), several polymorphisms around the OCT2/SLC22A2 gene locus, including rs2504954 (P=0.000873), were significantly associated with age-adjusted creatinine levels. Furthermore, in cancer patients (n=68), the OCT2 substrate cisplatin caused an acute elevation of serum creatinine (P=0.0083), consistent with inhibition of an elimination pathway.
Conclusions
Collectively, this study shows that OCT2 plays a decisive role in the renal secretion of creatinine. This process can be inhibited by OCT2 substrates, which impair the usefulness of creatinine as a marker of renal function.
Keywords: OCT2, Creatinine, Cisplatin
INTRODUCTION
The estimation of kidney function is a central requirement in the diagnosis of renal diseases in medical practice. Normally, kidney function is assessed by measuring glomerular filtration rate (GFR). For this reason, an exact determination of GFR and its changes over time are mandatory to make opportune decisions about diagnosis, prognosis, and treatment of renal diseases. In clinical practice, GFR is routinely estimated by the determination of serum creatinine, and, if more exact data are needed, by the measurement of creatinine clearance. Since serum levels of creatinine are determined by its metabolic generation, renal excretion (glomerular filtration, tubular secretion, and reabsorption), and extrarenal elimination (1), a detailed knowledge of these factors is of extreme importance for the appropriate clinical interpretation of creatinine serum concentration and clearance measurements.
It is well known that creatinine is freely filtered by the glomerulus, but also actively secreted by the proximal tubule from the peritubular capillaries in small amounts such that creatinine clearance overestimates actual GFR by 10–20% (2). The histamine receptor antagonist cimetidine has been used successfully to increase the accuracy of GFR estimation by suppressing creatinine secretion (3). This effect of cimetidine on creatinine secretion has been attributed to competition with specific transporters expressed on the basolateral membrane of renal proximal tubule cells. These transporters are supposed to mediate the first step in renal creatinine secretion, which is basolateral uptake in proximal tubules cells. Even though identification of the transporters involved in the renal secretion of creatinine is highly relevant both for clinical practice in humans and also for experimental analysis in animal models, knowledge in this area is still scant. In a previous study (4), we observed in mice treated with cisplatin an acute increase in serum creatinine within 1 day of cisplatin administration. Since cisplatin is taken up in renal proximal tubular cells by organic cation transporters (OCTs) (4–10), and tubular damage as the originating cause should not have occurred by that time, this finding supports the idea that the increased serum creatinine is associated with inhibition of creatinine secretion by OCTs.
In order to define the role of OCTs in creatinine secretion, we here investigated (i) the in vitro transport of creatinine in cells transfected with murine or human OCTs; (ii) the ex vivo interaction of creatinine with the uptake of organic cations in freshly-isolated proximal tubules from mice and humans; (iii) the in vivo creatinine clearance in wild-type mice and in mice with genetic deletion of Oct1 and Oct2 [Oct1/2(−/−) mice]; (iv) the creatinine uptake in kidney of wild-type and Oct1/2(−/−) mice; (v) the effects of genetic variants in OCT genes on serum creatinine in humans; and (vi) the effects of acute competitive inhibition of creatinine secretion by the OCT2 substrate cisplatin in humans.
PATIENTS AND METHODS
Accumulation of creatinine in cell lines
HEK293 cell lines stably transfected with human OAT1, OAT2, OAT3, OCT1, OCT2, OCT3, mOat3, mOct1, mOct2 or an empty pcDNA vector were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and G418 sulfate (400–800 μg/ml) at 37°C under 5% CO2 and 95% humidity. Uptake studies and cell transfections were performed as described in detail elsewhere (11). Functional overexpression of transporters was confirmed by assessing the uptake of p-aminohippuric acid (5 μM at 30 min) for hOAT1 (7.3-fold increased versus cells transfected with an empty vector) and hOAT2 (2.4-fold), methotrexate (10 μM at 30 min) for hOAT3 (4.2-fold) and mOat3 (5.1-fold), and tetraethylammonium (5 μM at 5 min) for hOCT1 (4.6-fold), hOCT2 (62-fold), mOct1 (28-fold), mOct2 (30-fold), and hOCT3 (2.7-fold).
Transport of creatinine in freshly isolated proximal tubules
Human kidney samples were obtained from patients undergoing tumor nephrectomy. Pieces of normal kidney tissue surrounding the tumor were used. The procedure was approved by the ethics commission of the Universitätsklinikum Münster, and written consent was obtained from each patient. Renal tissue was transferred into chilled HCO3− free phosphate buffer immediately after nephrectomy. Proximal tubules from mice or humans were isolated for functional analyses using the procedure customary in our laboratory (12). Briefly, thin corticomedullary slices were cut from small human kidney pieces (~5–7 mm3 each) or mouse kidneys using a scalpel and immediately transferred into a dissection dish containing minimum Eagle's culture medium and 5 mM glycine at 4 °C. Single proximal tubules were mechanically isolated using fine forceps under stereo microscopic observation and transferred to a perfusion chamber, and fixed by two holding pipettes closing the lumina at the ends of the tubule segment for microfluorimetric measurements. In this way, only the basolateral side of the tubules, where organic cation transporters are located, could be reached by the experimental solutions.
Fluorescence measurements
As substrate for OCTs, the fluorescent organic cation 4-(4-(dimethylamino)styryl)-N-methylpyridinium (ASP+; Molecular Probes, Leiden, The Netherlands) at a concentration of 1 μM was used. First, the influence of creatinine on ASP+ transport has been evaluated in HEK293 cells stably transfected with hOCT2. These measurements were performed at 37°C using a fluorescent plate reader (Infinity M200, Tecan, Crailsheim, Germany), according to the method described by us before (13). Briefly, hOCT2 expressing cells confluently grown on 96-well-microplates (Nunclon 96 Flat bottom, Nunc, Wiesbaden, Germany) were excited with monochromatic light of 450 nm and fluorescence emission, filtered by a second monochromator at 590 nm, was finally measured by a fluorescence detector. Fluorescence was measured dynamically in each well before and after ASP+ injection. ASP+ uptake was quantified by linear regression of the initial fluorescence increase. For the ASP+-uptake experiments, HEK293 cells were stably transfected with hOCT2 and selected as described earlier (14), and cultivated at 37°C in DMEM containing 3.7 g/l NaHCO3, 1.0 g/l D-glucose, and 2 mM L-glutamine, 10% heat-inactivated fetal calf serum, 100,000 U/l penicillin, 100 mg/l streptomycin, and 0.8 mg/ml geneticin, as described. Experiments with freshly isolated proximal tubules from mice or patients were performed as already described by us for human and rat tubules, respectively.12 Briefly, dynamic fluorescence microscopy with isolated tubules was performed in the dark at 37°C with an inverted microscope (Axiovert 135, Zeiss, Oberkochen, Germany) equipped with a 100 × 1.45 oil-immersion objective. Data acquisition and analysis were performed with Metafluor Software (Visitron Systems, Puchheim, Germany). Excitation light (488 nm) from a polychromator system (VisiChrome, Visitron Systems, Puchheim, Germany) was reflected to the tubules by a dichroic mirror (560 nm) and emission was detected after passing an emission filter (575 – 640 nm) by a Photometrics CoolSNAPEZ digital camera (Roper Scientific, Tuscon, AZ). In all fluorescent measurements, the initial linear slope of cellular fluorescence increase during the first 10 to 40 s was used as transport parameter. This initial fluorescence increase represents directly the ASP+ uptake across plasma membranes via OCTs and is not significantly influenced by exit of ASP+ from the cells, intracellular compartmentalization and bleaching of the dye (15). Results of drug interaction are expressed as percentage of control experiments performed on the same day with tubules from the same animal or patient. In some experiments with isolated mouse proximal tubules, the effect of ketamine on the ASP+ transport was also tested.
Animals and assessment of renal parameters
Adult (7–12 weeks old) male wild-type and Oct1/2(−/−) mice on an FVB background were licensed from Taconic (Hudson, NY). All animals were housed and handled in accordance with the Institutional Animal Care and Use Committee of St. Jude Children's Research Hospital or approved by a governmental committee on animal welfare of the University of Münster and were performed in accordance with national animal protection laws. Animals were housed in a temperature-controlled environment with a 12-hour light cycle and were given a standard diet and water ad libitum. For experiments involving collection of urine, Oct1/2(−/−) and wild-type animals were single-housed in metabolic cages in a temperature-controlled environment with a 12-hour reverse light cycle. After the animals had acclimated to metabolic cages, a 24-hour baseline urine sample was collected 16 hours after the onset of light. Blood collected from the retro-orbital plexus and 24-h urine were used for analysis of creatinine content. Levels of serum and urinary creatinine were determined using a Vetscan autoanalyzer (Abaxis, Union City, CA).
Creatinine clearance (CCr) was calculated according to the following equation:
| (a) |
Renal accumulation of creatinine was investigated in Oct1/2(−/−) animals by administration of [14C]creatinine (American Radiolabeled Chemicals Inc., St. Louis, MO; specific activity, 50 mCi/mmol) as i.v. bolus at a dose of 1 μCi (~20 nmol) in 100 μl saline. Kidney (homogenized in 1N sodium hydroxide solution at 1:10 wt/vol) and serum samples were obtained at 30 min after dosing (n=6 per group), and aliquots analyzed by liquid scintillation counting.
Clinical samples
Variant genotypes in or around the region of interest were determined in germline DNA samples from 590 pediatric patients treated for acute lymphoblastic leukemia at St. Jude Children's Research Hospital (Memphis, TN). Samples were amplified, labeled and hybridized to their respective chips, and analyzed as described (16, 17). The analysis was restricted to the region spanning 160500 to 160900 (kb) on chromosome 6, the locus which includes SLC22A2 and SLC22A3, the genes encoding OCT2 and OCT3, respectively. The chips covered approximately 100 different SNPs in this region. Of 590 patients, 297 had their serum creatinine levels measured more than 15 hours prior to receiving their first consolidation dose of methotrexate, while 72 had it measured between 18 and 5 hours and 14 were assayed less than 5 hours before treatment. The time of serum collection had no influence on age-adjusted serum creatinine levels (P>0.05). Parents and patients gave informed consent and assent as appropriate, and the protocols and analyses were approved by the Institutional Review Board of St. Jude Children's Research Hospital.
Acute effects of a 3-h i.v. infusion of cisplatin (100 mg/m2) on serum creatinine changes were assessed in samples retrospectively collected and described previously (18). Paired baseline and day 1 data were available from 68 patients, who were treated at the Erasmus MC – Daniel den Hoed Cancer Center (Rotterdam, the Netherlands). The study protocols were reviewed and approved by the Erasmus MC review board, and patients provided written informed consent.
Statistical analysis
Data are presented as mean values ± SEM unless stated otherwise, with n referring to the number of animals, cell monolayers or isolated tubules used in the experiments. An unpaired two sided Student's t-test, and ANOVA (with Tukey post-test) were used to demonstrate statistical significance of the effects. P<0.05 was considered statistically significant. Statistical analyses and determination of IC50 values were performed using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA).
RESULTS
In vitro transport of creatinine
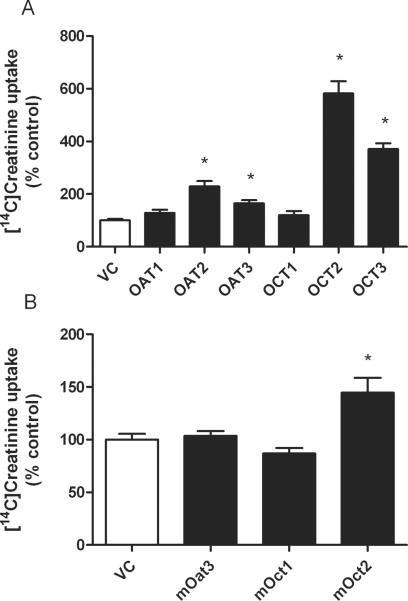
The uptake of creatinine was markedly stimulated in hOCT2- and hOCT3-expressing HEK293 cells compared to cells transfected with the null vector (5.8-fold and 3.7-fold, respectively) (Fig. 1A). A significant stimulation of creatinine uptake was also observed in hOAT2- and hOAT3-expressing HEK293 cells (2.3-fold and 1.6-fold, respectively), while the uptake by hOCT1-, and hOAT1-expressing cells was comparable to that by null vector transfected cells (Fig. 1A). Among the murine transporters investigated (mOat3, mOct1 and mOct2), only mOct2 showed a significantly higher creatinine accumulation compared to null vector transfected cells (1.4-fold) (Fig. 1B). These in vitro findings support the possibility that hOCT2 and mOct2 may be the dominant creatinine transporters in the proximal tubule in humans and in mice, respectively.
Figure 1.
Accumulation of [14C]creatinine in HEK293 cells stably transfected with human (panel A) and murine (panel B) organic ion transporters. Data are presented as mean values ± SEM. The number of experiments for each group is 6. * indicates a statistical significant difference compared with control experiments with HEK293 cells transfected with null vector (P<0.05, unpaired two-tailed t-test).
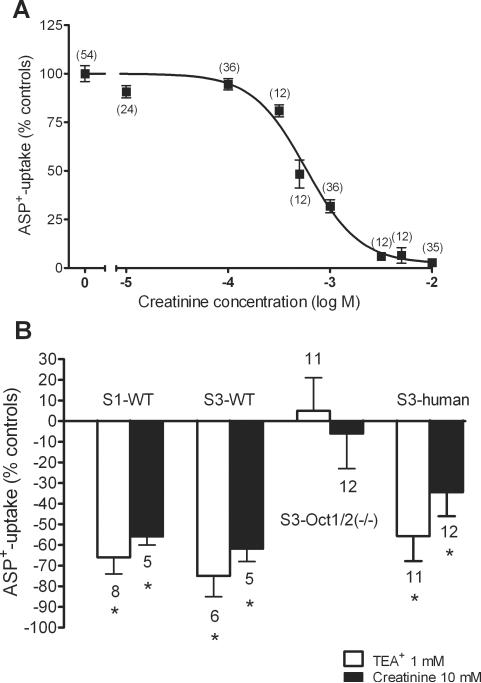
The uptake of the fluorescent organic cation ASP+ in hOCT2-expressing HEK293 cells was inhibited by creatinine in a concentration dependent manner, with an IC50 of 580 μM (Fig. 2A). Since at a concentration of 10 mM creatinine was able to completely inhibit ASP+ uptake, we used this concentration to investigate the interaction of creatinine with the transport of organic cations in renal proximal tubules freshly isolated from mice or from “normal” renal tissue of humans undergoing tumor nephrectomy. At this concentration, creatinine was able to significantly inhibit the initial ASP+ uptake across the basolateral membrane from S1 and also S3 segments of mouse proximal tubules (−56±4%, n=5 and −62±6%, n=5, respectively) (Fig. 2B). The prototypical organic cation TEA+, at a concentration of 1 mM, significantly inhibited ASP+-uptake in these segments by −66±8% (n=8) and −75±10% (n=6), respectively (Fig. 2B). Conversely, in S3 segments from male Oct1/2(−/−) mice, which only express Oct3 at very low levels (19), neither TEA+ nor creatinine significantly inhibited ASP+-transport (5±16%, n=11 and −6±17%, n=12, respectively) (Fig. 2B), again confirming an interaction of creatinine with mOct2. In human S3-segments, initial ASP+-uptake across the basolateral membrane could be significantly inhibited by 1 mM TEA+ (−56±12%, n=11), and also by 10 mM creatinine (−34±12%, n=12) (Fig. 2B), suggesting that also in humans renal creatinine secretion is mediated by OCT2, the most highly-expressed OCT isoform in human proximal tubules (20, 21).
Figure 2.
Concentration-response curves for the inhibition of initial ASP+-uptake by creatinine in HEK 293 cells stably expressing hOCT2 (panel A). Values are means ± SEM expressed as % ASP+-uptake in the absence of creatinine with number of observation in parentheses. IC50 value of ASP+-uptake inhibition by creatinine determined from this curve was 580 μM. Effect of 1 mM TEA+ (white columns) and 10 mM creatinine (black columns) on the uptake of ASP+ in freshly isolated S1 and S3 segments of proximal tubules from wild-type mice (panel B). The effects of these concentrations of TEA+ and creatinine on ASP+-uptake in S3 segments from Oct1/2(−/−) mice and from humans is also shown. Data are presented as mean values ± SEM. The number of mouse or human tubules for each group is indicated above the column. * indicates a statistical significant difference compared with control experiments in the presence of ASP+ only (P<0.05, unpaired two-tailed t-test).
Creatinine clearance in transporter-deficient mice
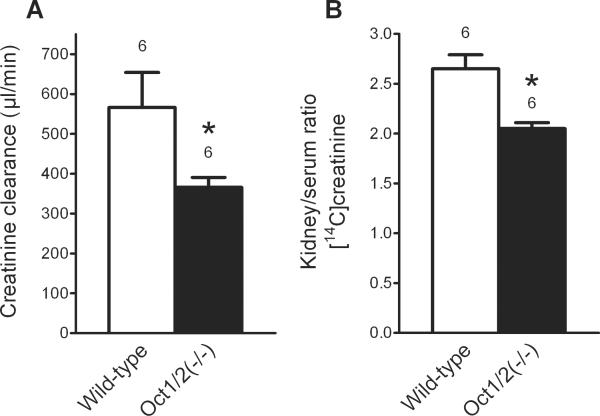
The creatinine clearance in male wild-type mice was significantly higher than that observed in male Oct1/2(−/−) mice (566±88 μl/min, n=6 versus 366±25 μl/min, n=6, respectively) (Fig. 3A). Moreover, the renal accumulation of exogenous creatinine in male wild-type mice was significantly higher than that in male Oct1/2(−/−) mice after correction for serum creatinine (kidney/serum ratio of creatinine, 2.05±0.067, n=6 versus 2.65±0.140, n=6, respectively) (Fig. 3B). These changes occurred without any substantial alterations in the renal expression of other transporter genes [Slc22a6 (Oat1), Slc22a7 (Oat2), Slc22a8 (Oat3), and Slc47a1 (Mate1)] in the Oct1/2(−/−) mice (Suppl. Fig. S1).
Figure 3.
Creatinine clearance (panel A) and accumulation of exogenous [14C]creatinine (panel B) in male wild-type and Oct1/2(−/−) mice. Data are presented as mean values ± SEM. The number of animals for each group is indicated above the column. * indicates a statistical significant difference compared with wild-type mice (P<0.05, unpaired two-tailed t-test).
Clinical studies
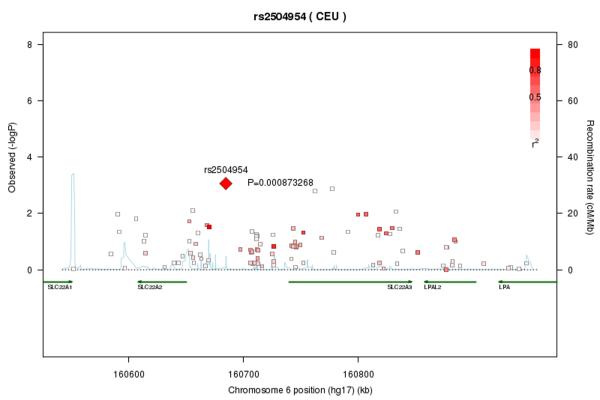
An exploratory analysis on samples from 590 children showed that serum creatinine levels were significantly dependent on age (Suppl. Fig. S2), but not on racial ancestry (P=0.42) or sex (P=0.41). Focussing specifically on chromosome 6 in the region bearing the genetic information for hOCTs and, based on data obtained in the mice, on male subjects (n=214), we found one intergenic single-nucleotide polymorphism (rs2504954) that was significantly associated with serum creatinine (P=0.000873), and that was in strong linkage with various single nucleotide polymorphisms within the OCT2 and OCT3 genes SLC22A2 and SLC22A3, respectively (Fig. 4). The rs2504954 exhibits the variation G or T, and the G allele (found in 27% of whites) was associated with higher creatinine levels.
Figure 4.
Association of serum creatinine levels with single nucleotide polymorphisms (SNPs) located in or near the OCT2 gene, SLC22A2. Each SNP is individually plotted according to its chromosomal location versus the P-value of its association with serum creatinine levels. The SNP exhibiting the smallest P-value is shown as a diamond and labeled with its corresponding rs number (rs2504954) and P-value (P=0.000873). The remaining SNPs are indicated as boxes whose color intensities are in direct proportion to the degree of linkage disequilibrium (LD) between the corresponding SNP and rs2504954, relative to CEU/HapMap (release 21). The light blue lines represent the magnitude of the recombination rate observed along the indicated chromosomal stretch. The green arrows indicate RNA transcripts.
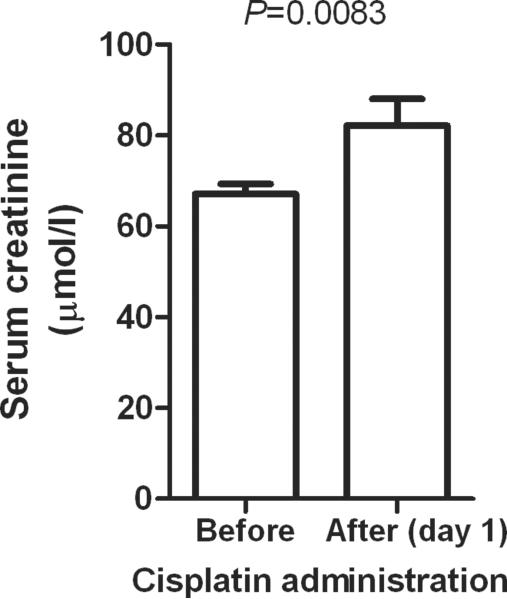
In order to provide further evidence for the involvement of OCT2 in creatinine secretion, we next assessed the potential influence of cisplatin, a known substrate and inhibitor of OCT2 (see Suppl. Fig. S3), on serum creatinine levels. We found that serum creatinine significantly increased on average by 24.3±7.80% (P=0.0083) in adult cancer patients following cisplatin administration compared with baseline values (Fig. 5). This percentage is in the same order of magnitude as the contribution of tubular secretion of creatinine to creatinine clearance in humans (2).
Figure 5.
Changes in serum creatinine measured at baseline and after the first day of a 3-h i.v. infusion of cisplatin at a dose of 100 mg/m2 in 68 adult cancer patients. Data are presented as mean values (bars) ± SEM (error bars).
DISCUSSION
The exact knowledge of renal creatinine handling is of pivotal importance for an appropriate interpretation of data on serum creatinine concentration and creatinine clearance in the assessment of kidney function in clinical practice. In fact, because of tubular secretion, estimation of GFR by creatinine clearance determination results in an overestimation. Moreover, in renal diseases accompanied by diminished GFR, the contribution of tubular creatinine secretion to creatinine clearance may be dramatically increased and when equalling creatinine clearance with GFR, this may result in a profound overestimation of GFR (2, 22, 23). In other clinical settings (for example, de-compensated heart disease or uncontrolled diabetes mellitus), a primary reabsorption of creatinine by renal tubules was observed (2, 24). Finally, administration of cationic drugs which are substrates of organic cation transporters may interfere competitively with renal creatinine secretion and consequently could falsely indicate a reduced GFR.
Since in the attempt to understand the mechanisms and to develop new therapeutic concepts of kidney diseases, preclinical studies with mouse models of renal pathology have been increasingly used, accurate evaluation of the GFR also in these animal models is a critical parameter for translational drug development (2). For this reason, increasing attention has recently been paid to the assessment of the accuracy of renal function determination in mice.
Our in vitro studies showed that the stable expression of hOCT2 is linked to a significant accumulation of creatinine in HEK293 cells. Interestingly, stable expression of hOCT3, hOAT2 and hOAT3 but not of hOAT1 or hOCT1 also allowed HEK293 cells to accumulate creatinine. The IC50 of 580 μM for the inhibition of the ASP+-uptake by creatinine suggests that creatinine is a low affinity substrate for hOCT2. Recently, similar findings have been obtained by Imamura et al. (25) who showed that in an in vitro system hOCT2, hOAT3, hOAT4, and hOCT3 mediate a significant uptake of creatinine and that creatinine has a low affinity for hOCT2. Of the murine transporters we investigated in vitro (mOat3, mOct1 and mOct2), only mOct2 was able to mediate a significant transport of creatinine. The interaction of creatinine with the transport by organic cations is also evident in the experiments with freshly isolated proximal tubules: in S1 and also S3 segments from wild-type mice, creatinine significantly inhibited the transport of ASP+, while this effect was not detectable in Oct1/2(−/−) mice. Since mOct1 seems not to accept creatinine as a substrate and the mOct3-mRNA expression in proximal tubules is only 3% of that of mOct2-mRNA (26), the inhibition of ASP+-uptake in wild-type mice by creatinine is principally ascribable to an interaction with mOct2. Even more importantly, creatinine showed a significant interaction also with the transport of ASP+ in freshly isolated human S3-proximal tubules at physiologically relevant levels. Since the principal isoform of OCTs in the human kidney is hOCT2 (20, 21), these findings suggest that in vivo in humans hOCT2 plays an important role in the secretion of creatinine. Such an hypothesis is strongly supported by recent studies with the antibacterial agent des-fluoro(6)-quinolone (25), which at therapeutic dosages can inhibit hOCT2 as well as certain luminal transporters such as hMATE1 and hMATE2-K, leading to a significant inhibition of tubular secretion of creatinine and consequently to elevation of serum creatinine levels.
The in vivo measurements performed in wild-type and Oct1/2(−/−) mice further demonstrated the importance of OCTs for creatinine secretion. Indeed, the absence of secretion through OCTs caused a small, but statistically significant increase in serum creatinine concentration in Oct1/2(−/−) mice compared with wild-type mice at baseline. These knockout mice do not differ with respect to urinary volume, total protein, glucose excretion, GFR (27), blood urea nitrogen, or in the expression of other solute transporters (4). Moreover, the important role of OCTs in the vectorial transport of creatinine in renal proximal tubules is underlined by the significantly lower renal creatinine accumulation in male Oct1/2(−/−) mice compared with wild-type mice upon administration of exogenous creatinine.
Curiously, our current murine data are in contradiction to recent findings observed by Eisner et al. (28), using the same animal model. These authors detected no difference in creatinine secretion between wild-type and Oct1/2(−/−) mice and suggested that the mouse Oat1 (mOat1) but not mOct2 is the murine transporter responsible for creatinine secretion (28). Their experiments were performed under ketamine (100 mg/kg, subcutaneous injection)/xylazin anesthesia. Ketamine is a weak basic compound that has been demonstrated to interact with rat and human OCTs with a Ki for the transport of the organic cation 1-methyl-4-phenylpyridinium of 23 and 207 μM for hOCT2 and rOct2, respectively (29). There are no data in the literature on plasma ketamine concentration after subcutaneous administration of the agent at a dose of 100 mg/kg. However, intraperitoneal administration of ketamine (200 mg/kg) in mice resulted in plasma ketamine concentrations of 30 and 10 mg/ml after 10 and 60 min, respectively (30). Assuming a plasma concentration of 5 mg/ml 60 min after a dose of 100 mg/kg, this would correspond to a concentration of 20 mM, which is well above the Ki for interaction with OCTs and also above the concentration producing 80% inhibition of the ASP+-uptake observed in our experiments (Suppl. Fig. S4). Under ketamine anesthesia, secretion by OCTs should be blocked and it can be speculated that for this reason Eisner et al. did not detect any difference in creatinine secretion between wild-type and Oct1/2(−/−) mice. However, it cannot be excluded that other transporters such as Oat1 could be involved in the renal secretion of creatinine in mice.
The single-nucleotide polymorphism (rs2504954) in the OCT1–3 gene region identified from our genetic association study further confirms the importance of OCTs for creatinine secretion in humans. Such a link between polymorphisms of hOCT2 and serum creatinine levels has already been observed in studies performed in adults that identified rs2279463 (located in the OCT2 gene SLC22A2) (31) and rs3127573 (near SLC22A2) (32) as significantly associated with serum creatinine. These previously identified sites on chromosome 6 were unfortunately not directly interrogated by the chips used in our analysis. Nonetheless, these findings lend further credence to the thesis that OCTs, in particular OCT2, play a role in the renal secretion of creatinine in humans.
The exact identification of the molecular entities responsible for this transport is of prominent importance to furnish a rational basis for the correct interpretation of creatinine as marker of renal glomerular function not only in translational medicine, but also in clinical practice. Since organic cation transporters are polyspecific membrane transporters, they can interact with a multitude of substances, among which many are drugs of common use. Drug-creatinine interactions at the transporter level may be the reason for changes of creatinine serum concentration independent from changes of renal glomerular function. Moreover, the expression and activity of these transporters can change under specific pathological situations. For example, OCT2 seems to be down-regulated in chronic renal failure, at least in a rat model (19). Therefore, transporter-drug interaction and regulation of transporter activity in different pathophysiological situations may be responsible for changes in renal creatinine secretion and thus, in serum creatinine concentrations, without changes in glomerular filtration. From experiments in animal models it is known that under some pathological situations the expression of OCTs is up- or downregulated. For example, in kidneys of rats with liver cirrhosis, OCT2 is significantly upregulated (33). Intriguingly, patients with compensated cirrhosis have been shown to have a significant tubular secretion of creatinine (34), which is consistent with an increased expression of OCT2.
In conclusion, our current data demonstrate that the organic cation transporter OCT2 plays a decisive role in the tubular secretion of creatinine. This finding should be taken into consideration when using serum creatinine or creatinine clearance to evaluate renal function in rodents and humans.
Supplementary Material
Statement of Translational Relevance.
Exact knowledge of renal handling of creatinine is of pivotal importance for an appropriate interpretation of data on serum creatinine concentration and creatinine clearance in the assessment of kidney function in cancer patients receiving chemotherapeutic agents. Indeed, because of tubular secretion, the use of creatinine clearance to estimate filtration results in substantial overestimation of GFR. Moreover, in renal diseases accompanied by diminished GFR, the contribution of tubular creatinine secretion to creatinine clearance may be dramatically increased and when equalling creatinine clearance with GFR, this may result in a profound overestimation of GFR. We found that creatinine clearance is significantly influenced by organic cation transporters in mice and humans. Using an array of technologies, including transfected cell models, transporter-deficient mice, genetic-association analyses, and drug-creatinine interaction studies in patients, we found that OCT2 is a dominant transporter involved in the renal secretion of creatinine. Our study is of direct human relevance because interference with OCT2 function by agents like cisplatin may compromise the utility of serum creatinine analysis as a measure of renal function in mammalians.
Acknowledgments
The expert help of U. Neugebauer, B. Gelschefarth, A. Gibson and C. Smith is gratefully acknowledged. This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG CI 107/4-1) to G.C. and E.S., the American Lebanese Syrian Associated Charities (ALSAC), USPHS Cancer Center Support Grant 3P30CA021765, and the National Institutes of Health grants NCI 5R01CA151633-01 to A.S. and U01 GM92666 to M.V.R.
Footnotes
Disclosure of Potential Conflicts of Interest The authors have no conflicts of interest to disclose.
References
- 1.Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. 2009;20:2305–13. doi: 10.1681/ASN.2009020171. [DOI] [PubMed] [Google Scholar]
- 2.Breyer MD, Qi Z. Better nephrology for mice--and man. Kidney Int. 2010;77:487–9. doi: 10.1038/ki.2009.544. [DOI] [PubMed] [Google Scholar]
- 3.Olsen NV, Ladefoged SD, Feldt-Rasmussen B, et al. The effects of cimetidine on creatinine excretion, glomerular filtration rate and tubular function in renal transplant recipients. Scand J Clin Lab Invest. 1989;49:155–9. doi: 10.3109/00365518909105415. [DOI] [PubMed] [Google Scholar]
- 4.Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86:396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciarimboli G, Deuster D, Knief A, et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am J Pathol. 2010;176:1169–80. doi: 10.2353/ajpath.2010.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciarimboli G, Ludwig T, Lang D, et al. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol. 2005;167:1477–84. doi: 10.1016/S0002-9440(10)61234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filipski KK, Loos WJ, Verweij J, Sparreboom A. Interaction of cisplatin with the human organic cation transporter 2. Clin Cancer Res. 2008;14:3875–80. doi: 10.1158/1078-0432.CCR-07-4793. [DOI] [PubMed] [Google Scholar]
- 8.Yokoo S, Yonezawa A, Masuda S, et al. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol. 2007;74:477–87. doi: 10.1016/j.bcp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Yonezawa A, Masuda S, Nishihara K, et al. Association between tubular toxicity of cisplatin and expression of organic cation transporter rOCT2 (Slc22a2) in the rat. Biochem Pharmacol. 2005;70:1823–31. doi: 10.1016/j.bcp.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Yonezawa A, Masuda S, Yokoo S, Katsura T, Inui K. Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1-3 and multidrug and toxin extrusion family) J Pharmacol Exp Ther. 2006;319:879–86. doi: 10.1124/jpet.106.110346. [DOI] [PubMed] [Google Scholar]
- 11.Baker SD, Verweij J, Cusatis GA, et al. Pharmacogenetic pathway analysis of docetaxel elimination. Clin Pharmacol Ther. 2009;85:155–63. doi: 10.1038/clpt.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlatter E, Schafer JA. Electrophysiological studies in principal cells of rat cortical collecting tubules. ADH increases the apical membrane Na+-conductance. Pflugers Arch. 1987;409:81–92. doi: 10.1007/BF00584753. [DOI] [PubMed] [Google Scholar]
- 13.Wilde S, Schlatter E, Koepsell H, et al. Calmodulin-associated post-translational regulation of rat organic cation transporter 2 in the kidney is gender dependent. Cell Mol Life Sci. 2009;66:1729–40. doi: 10.1007/s00018-009-9145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busch AE, Karbach U, Miska D, et al. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol Pharmacol. 1998;54:342–52. doi: 10.1124/mol.54.2.342. [DOI] [PubMed] [Google Scholar]
- 15.Ciarimboli G, Koepsell H, Iordanova M, et al. Individual PKC-phosphorylation sites in organic cation transporter 1 determine substrate selectivity and transport regulation. J Am Soc Nephrol. 2005;16:1562–70. doi: 10.1681/ASN.2004040256. [DOI] [PubMed] [Google Scholar]
- 16.Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–64. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 17.Trevino LR, Shimasaki N, Yang W, et al. Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. J Clin Oncol. 2009;27:5972–8. doi: 10.1200/JCO.2008.20.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jongh FE, Gallo JM, Shen M, Verweij J, Sparreboom A. Population pharmacokinetics of cisplatin in adult cancer patients. Cancer Chemother Pharmacol. 2004;54:105–12. doi: 10.1007/s00280-004-0790-5. [DOI] [PubMed] [Google Scholar]
- 19.Lee WK, Reichold M, Edemir B, et al. Organic cation transporters OCT1, 2, and 3 mediate high-affinity transport of the mutagenic vital dye ethidium in the kidney proximal tubule. Am J Physiol Renal Physiol. 2009;296:F1504–13. doi: 10.1152/ajprenal.90754.2008. [DOI] [PubMed] [Google Scholar]
- 20.Motohashi H, Sakurai Y, Saito H, et al. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol. 2002;13:866–74. doi: 10.1681/ASN.V134866. [DOI] [PubMed] [Google Scholar]
- 21.Pietig G, Mehrens T, Hirsch JR, et al. Properties and regulation of organic cation transport in freshly isolated human proximal tubules. J Biol Chem. 2001;276:33741–6. doi: 10.1074/jbc.M104617200. [DOI] [PubMed] [Google Scholar]
- 22.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 23.Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–8. doi: 10.1038/ki.1985.205. [DOI] [PubMed] [Google Scholar]
- 24.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–53. [PubMed] [Google Scholar]
- 25.Imamura Y, Murayama N, Okudaira N, et al. Prediction of fluoroquinolone-induced elevation in serum creatinine levels: a case of drug-endogenous substance interaction involving the inhibition of renal secretion. Clin Pharmacol Ther. 2011;89:81–8. doi: 10.1038/clpt.2010.232. [DOI] [PubMed] [Google Scholar]
- 26.Holle SK, Ciarimboli G, Edemir B, et al. Properties and regulation of organic cation transport in freshly isolated mouse proximal tubules analyzed with a fluorescence reader-based method. Pflugers Arch. 2011;462:359–69. doi: 10.1007/s00424-011-0969-7. [DOI] [PubMed] [Google Scholar]
- 27.Jonker JW, Wagenaar E, Van Eijl S, Schinkel AH. Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol Cell Biol. 2003;23:7902–8. doi: 10.1128/MCB.23.21.7902-7908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisner C, Faulhaber-Walter R, Wang Y, et al. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int. 2010;77:519–26. doi: 10.1038/ki.2009.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amphoux A, Vialou V, Drescher E, et al. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50:941–52. doi: 10.1016/j.neuropharm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Sato Y, Kobayashi E, Hakamata Y, et al. Chronopharmacological studies of ketamine in normal and NMDA epsilon1 receptor knockout mice. Br J Anaesth. 2004;92:859–64. doi: 10.1093/bja/aeh144. [DOI] [PubMed] [Google Scholar]
- 31.Kottgen A, Pattaro C, Boger CA, et al. New loci associated with kidney function and chronic kidney disease. Nat Genet. 2010;42:376–84. doi: 10.1038/ng.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chambers JC, Zhang W, Lord GM, et al. Genetic loci influencing kidney function and chronic kidney disease. Nat Genet. 2010;42:373–5. doi: 10.1038/ng.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez-Parra M, Telleria N, Titos E, et al. Gene expression profiling of renal dysfunction in rats with experimental cirrhosis. J Hepatol. 2006;45:221–9. doi: 10.1016/j.jhep.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Sansoe G, Ferrari A, Castellana CN, et al. Cimetidine administration and tubular creatinine secretion in patients with compensated cirrhosis. Clin Sci (Lond) 2002;102:91–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.