Abstract
The molecular chaperone Hsp90 is abundant, ubiquitous, and catholic to biological processes in eukaryotes, controlling phosphorylation cascades, protein stability and turnover, client localization and trafficking, and ligand-receptor interactions. Not surprisingly, Hsp90 does not accomplish these activities alone. Instead, an ever-growing number of cochaperones have been identified, leading to an explosion of reports on their molecular and cellular effects on Hsp90 chaperoning of client substrates. Notable among these clients are many members of the steroid receptor family, such as glucocorticoid, androgen, estrogen and progesterone receptors. Cochaperones typically associated with the mature, hormone-competent states of these receptors include p23, the FK506-binding protein 52 (FKBP52), FKBP51, protein phosphatase 5 (PP5) and cyclophilin 40 (Cyp40). The ultimate relevance of these cochaperones to steroid receptor action depend on their physiological effects. In recent years, the first mouse genetic models of these cochaperones have been developed. This work will review the complex and intriguing phenotypes so far obtained in genetically-altered mice and compare them to the known molecular and cellular impacts of cochaperones on steroid receptors.
1. Introduction
The discovery of steroid receptors as major clients of Hsp90 occurred in the early 80s, first for the glucocorticoid receptor (GR) [1], followed in rapid succession by the progesterone (PR), estrogen (ER), androgen (AR) and mineralocorticoid (MR) receptors [2, 3]. Since then, a variety of Hsp90 cochaperone proteins have been described as components of steroid receptor complexes. These include cochaperones involved in the early stages of steroid receptor complex assembly and maturation, such as Hip, Hop, Bag-1 and Hsp40; those involved in turnover and quality control of client proteins, such as CHIP; and those found in the mature, hormone-competent binding states of the receptors, such as FKBP51, FKBP52, Cyp40 and PP5. [For comprehensive reviews of chaperones and SRs, see refs. [4, 5]]. It is typically assumed that Hsp90 machinery serves to chaperone only the steroid (type 1) branch of the nuclear receptor family, since the archetypical type 2 members, such as thyroid (TR) and retinoic acid (RAR) receptors, do not bind Hsp90 [6]. However, there are recent reports of Hsp90 interaction with the peroxisome proliferator-activated receptors, PPARα, PPARδ, and PPARγ [7, 8], aryl hydrocarbon receptor (AhR) [9], constitutive androstane receptor (CAR) [10], pregnane × receptor (PXR) [11] and vitamin D receptors (VDR) [12]. Thus, it is likely that the chaperone actions of Hsp90 machine will be essential, not only for proper functioning of the steroidal endocrine system, but for metabolic processes, as well. Deciphering the contribution of the Hsp90 machinery to physiology will obviously require, not one, but multiple genetic mouse models, subjected to diverse endocrine and metabolic stimuli and stresses. This effort is in its infancy, but important lessons are already emerging. This review will attempt to put these early reports in context by comparing the known molecular and cellular effects of each chaperone on steroid action to observed effects in vivo. [Note: a mouse genetic model of Cyp40 has not yet been described. Therefore, Cyp40 will not be covered in this review.]
2. Chaperoning steroidal endocrinology - the overarching questions
Prior to the discovery of steroid receptor-associated cochaperones, the role of Hsp90 on steroidal biology seemed simple, as its main function appeared to be protection of the receptors from degradation and promotion of the hormone-binding function. The latter role is consistent with the ATP-driven pincer or ratchet function of Hsp90 dimers that imparts conformational changes to client proteins [13, 14]. Indeed, the GR ligand-binding domain is particularly dependent on this Hsp90 function for its hormone responsiveness [15, 16]. Thus, the prediction at the time would have been that Hsp90 serves to regulate the sensitivity of organs to glucocorticosteroids (GCs). With the discovery of cochaperones that bind mature steroid receptor heterocomplexes, this prediction became too simplistic. The first SR cochaperone to be discovered was FKBP52 [17], followed by FKBP51 [18], Cyp40 [19], PP5 [20], and most recently FKBPL [21]. These proteins directly bind Hsp90 via their respective tetratricopeptide repeat (TPR) domains, entering into SR complexes at the final stage of assembly. Interestingly, because the Hsp90 dimer generates only one TPR acceptor site [4], this means that multiple, distinct steroid receptor heterocomplexes must exist in vivo based on TPR protein composition. These observations pose several overarching questions. Does each TPR protein have a unique role in steroid receptor signaling? Are these unique roles the same across all steroid receptors? Or do some TPRs preferentially regulate one receptor over another? Last and most important, do the TPRs selectively control steroidal physiology? Partial answers to all of these questions are emerging (see below and Table 1).
3. Hsp90
3.1. Molecular Actions & Predicted Endocrinology
To date, chaperoning by the Hsp90 machinery has been attributed to all classical steroid receptors, several additional nuclear receptors, AhR, and more recently PPARα and PPARγ. On this basis alone, the predicted roles of Hsp90 in steroidal endocrinology are expected to be profound and widespread, most likely controlling processes such as fertility and reproduction, sexual differentiation and organogenesis, and lipid and drug metabolism. Yet proof of these predictions in mouse models has been elusive due to the ubiquitous and pleiotropic actions of Hsp90 in numerous other cellular processes. By most accounts, Hsp90 comprises 1–2% of total cellular protein in unstressed mammalian cells, rising to 4% following stress. Estimates of Hsp90 client substrates are now approaching 300 [see Picard laboratory website for comprehensive list of Hsp90 interactors: http://www.picard.ch/downloads/downloads.htm]. Not surprisingly, early cell-based efforts to eliminate Hsp90 expression typically resulted in cell death. A notable exception was achieved in yeast, when Picard and colleagues were able to obtain a viable strain with 20-fold reduction of Hsp90 [22]. In this strain, steroidal activation of GR transcriptional activity was severely reduced. On the whole, however, the conventional wisdom has been that ablation genetics of Hsp90 isoforms in the mouse would be a biological dead end, or an uninterpretable morass. Luckily, conventional wisdom is often wrong.
3.2. The unexpected divergent phenotypes of Hsp90α and β knockout mice
In contrast to yeast, mammalian Hsp90 is encoded by two genes, Hsp90α and Hsp90β, which are thought to be functionally similar and redundant [23]. Their primary amino acid sequences are 86% identical and they are mutually expressed in all tissues of the mouse, with the exception of heart and muscle, which have greatly reduced levels of Hsp90α compared to Hsp90β [24]. Although Hsp90β is constitutively expressed, Hsp90α expression is highly-inducible by heat shock and other forms of stress [23]. Most in vitro studies show that each isoform displays the same preferences for cochaperones and clients, with the exception that some client substrates, such as c-Src and A-raf, preferentially bind Hsp90α following heat shock [25]. However, some cellular processes appear to specifically require Hsp90α, such as caspase-2 activation [26], maturation of metalloprotease 2 in the extracellular matrix and invasiveness of some metastatic cancers [27]. Taken as a whole, these in vitro parameters would have predicted relatively similar phenotypes for mice deficient in Hsp90α or Hsp90β. Surprisingly, this was not the case. The Hsp90β knockout mouse displays early embryonic lethality [28]. Yet, the only defect identified in Hsp90α-deficient mice occurs in adult males which exhibit a failure of spermatogenesis [24]. In the case of Hsp90β, lethality occurs at embryonic day 9, due to an inability of the embryo to develop a placenta, leading to a failure of implantation and death within 24 hours. This result is interesting in several ways. First, although Hsp90β is ubiquitously expressed in the day 9 embryo, the only discernible defect was a failure of placental development. Second, the allantois layer responsible for the implantation defect in these mutants also expresses Hsp90α, yet failure still occurred, suggesting that Hsp90α cannot compensate for this crucial developmental step. In contrast to Hsp90β, both male and female Hsp90α knockout mice are viable and phenotypically normal into adulthood, with the exception of sterility in male mice [24]. The specific defect in males appears to be an arrest of spermatogenesis at the pachytene stage of meiosis I, possibly due to reduced levels of meiotic regulators of chromosomal synaptonemal complexes. Yet, given the prominent role of androgens in spermatogenesis and the overwhelming in vitro evidence for Hsp90 control of AR, it seems likely that an AR defect contributes to male infertility in these mice.
4. p23
4.1. Molecular Actions & Predicted Endocrinology
The p23 cochaperone was first isolated from PR and GR heterocomplexes [29, 30]. Since then it has been shown to be an common component of all steroid receptors and AhR, entering these complexes in the final stages of assembly of mature, ligand-competent receptors [for review of SR assembly see refs. [31, 32]]. In cell-free extracts, the binding of p23 to Hsp90 requires the ATP-bound state of Hsp90 which serves to stabilize the p23/Hsp90 interaction with SRs, promoting the hormone-binding function and preventing SR degradation. Cell-based studies, however, paint a more complicated picture with p23 being either stimulatory [33] or inhibitory [34] of GR activity, stimulatory of ER [35], and inhibitory of TR, AR and MR [33]. These facts alone make predictions of its physiological contributions difficult. Yet, p23 is also reported to have enzymatic activity as a cytosolic prostaglandin E synthase (cPGES) [36], and has recently been implicated in telomere length maintenance by promotion of telomerase extension activity [37]. Both of these potential functions appear to be intrinsic properties of p23 not mediated by interaction with Hsp90.
4.2. Perinatal lethality of p23 null mice may be GR mediated
Because of its ubiquitous interaction with SRs, the p23 knock out mice, if viable, was expected to have a severe phenotype. The first p23 null mouse was made by the Picard laboratory [38]. The null animals exhibited perinatal lethality that principally results from defective lung development, characterized by markedly reduced airspaces and reduced expression of surfactant genes. Null mice also showed underdevelopment of whole-body skin. These phenotypes are remarkably similar to the atelectatic lungs and skin defects seen in GR null mice [39, 40]. Like p23 null mice, defective lung development in the GR KO mice also leads to perinatal lethality. Not surprisingly, analysis of p23 KO mouse embryonic fibroblast (MEF) cells showed reduced GR activity. Two other laboratories have also generated p23 null mice that demonstrated abnormal skin, underdeveloped lungs, reduced expression of GR markers and perinatal lethality [41, 42]. The p23 KO mice generated by Nakatani and coworkers showed reduced levels of prostaglandin PGE(2) in lung tissue, suggesting that the absence of prostaglandin E synthase activity of p23 may be a contributing factor. However, a more recent study by the same laboratory in p23 KO cells showed elevated secretion of PGE(2) into the media due to decreased expression of a PGE(2) degrading enzyme [43]. In the studies by Lovgren and coworkers [42], no effect of p23 deficiency was found on prostaglandin biosynthesis in tissues or cells. Moreover, mice with knockout of PGE(2) receptors, including the EP4 receptor expressed in lung, have not manifested lung defects [44]. Therefore, it is not likely that the purported cPGES activity of p23 could single-handedly account for the lethal lung defect in null mice. Given the well-established role of glucocorticoids in promotion of perinatal lung development [45], the more likely cause is abrogated GR responsiveness during this critical event in postnatal survival. It is also likely that if p23 null mice could survive, other p23-regulated ligand responses in adult mice would be uncovered. Recently, transgenic mice ubiquitously over-expressing p23 have been generated [46]. Early results in the transgenics showed abnormal kidney function similar to human hydronephrosis that correlated with increased expression of cytochrome P450 genes typically regulated by AhR. This approach, as well as generation of tissue-specific ablation of p23, will be necessary for full dissection of its contribution to physiology.
5. FKBP52
5.1. Molecular Actions & Predicted Endocrinology
FKBP52 is a TPR-containing immunophilin with peptidyl-prolyl cis-trans isomerase (PPIase) activity that is inhibited by the binding of FK506 [47]. It was first discovered by Faber and colleagues as a component of the untransformed PR complex [48] and has since been found to interact with all SR receptors [17, 49, 50]. Numerous cell-based studies uniformly point to FKBP52 as a positive regulator of SR transcriptional activity [51–53], with the possible exception of ER which appears to be less sensitive to its actions [54]. Interestingly, FKBP52 is not a global regulator of SR transcriptional activity, as some GR-regulated genes are not affected by the loss of this protein [55]. The nature and extent of SR- and gene-specific effects of FKBP52 await comprehensive gene array studies. Similarly, the exact mechanism of its potentiating activity on SRs has remained elusive. Its intrinsic PPIase activity is not required for GR, since enzymatically dead mutants of FKBP52 still exert positive effects on the GR [56]. However, full AR activity does require the PPIase function of FKBP52 [57]. The potentiation effect may be due its role in controlling intracellular trafficking of SRs. Hormone-induced translocation of GR and MR correlates with a swapping of FKBP51 for FKBP52 and concomitant recruitment of the motor protein dynein [53, 58]. The intrinsic subcellular location of SRs may also be controlled by FKBP51 and FKBP52, as GR complexes which primarily reside in the cytoplasm of intact cells preferentially interact with FKBP51, while PR complexes located in the nucleus preferentially bind FKBP52 [59]. Thus, the overall positive effect of FKBP52 on SRs may simply result from its ability to promote nuclear localization of receptors.
5.2. Tissue-specific regulation of organogenesis and fertility
Prior to generation of FKBP52 null mice, dramatic effects on steroidal endocrinology were predicted, including embryonic/neonatal lethality due to reduced GR activity, and reduced fertility due to defective PR, AR and ER responses. Interestingly, both of these overall effects have been seen. FKBP52 null mice were independently generated by the laboratories of Smith [57] and Shou [54]. Approximately 50% of the Smith KO mice propagated on the C57BL/6J background were embryonic lethals, while the ~30% of the original Shou mice propagated on 129SvEv background died during development. However, recent backcrossing of the Shou strain with C57BL/6J has increased the percent of lethal embryos [60]. Although this suggests that genetic background contributes to the penetrence of FKBP52 action, at present no other cause for embryonic death has been identified.
In both strains of mice, surviving FKBP52 null animals grew into healthy adults except for reduced fertility in males and females. In the Smith strain, null males demonstrated ambiguous external genitalia and greatly reduced (dysgenic) prostate mass [57]. Null Shou mice also showed dysgenic prostate, but the genital defect was identified as a cleft in the ventral surface of the penis, a common birth defect in humans known as hypospadias [61]. Further studies by Shou and colleagues during embryonic development showed that hypospadias occurred at E18.5 due to a failure of fusion of epithelial cell layers at the ventral aspect of the penis. Interestingly, in WT developing males FKBP52 expression was highest in the lead epithelial cells destined to make contact at the site of fusion. All of these properties pointed to a defect of AR signaling as the underlying culprit. Using cells with knockdown expression of FKBP52, the Smith laboratory showed reduced AR transcriptional activity at reporter genes, and that AR activity required the PPIase function of FKBP52 [57]. In MEF cells derived from FKBP52KO mice, the Shou laboratory showed a similar loss of AR activity at reporter genes and at endogenous AR-induced genes controlling sexual dimorphism and cell growth [61, 62]. Last and most important, supplementation of gestating Shou strain WT female mice with testosterone resulted in partial rescue of the hypospadias defect in null male embryos [62], demonstrating that FKBP52 KO males do indeed have reduced androgen responsiveness in vivo.
FKBP52 null females from both strains showed remarkably similar phenotypes. In each case, null females were found to be sterile from a complete failure of the uterus to support implantation [54, 63]. Lack of uterine receptivity was primarily due to abrogation of PR responsiveness, as determined by reduced hormone activation of PR in FKBP52 KO MEF cells and by reduced expression of progesterone-induced genes in mutant uteri. In contrast, the uterine estrogenic response was unaffected in null females, as estradiol induction of uterine weight and lactoferrin gene expression were normal. Because two isoforms of PR are known to exist, PR-A and PR-B, Shou and colleagues tested whether FKBP52 might specifically regulate one isoform over the other [54]. Results showed a preference of PR-A for interaction with FKBP52 and a greater influence of FKBP52 on PR-A activity in MEF cells. This was confirmed in vivo, as only PR-A induced uterine genes (calcitonin and amphiregulin) but not a PR-B gene (histidine decarboxylase) were reduced in null females. Interestingly, each strain also showed little to no effect of FKBP52 loss on ovulation rates, even though this response is also known to require progesterone stimulation. Similarly, progesterone induction of mammary gland alveologenesis was only marginally reduced in Shou strain null females [54]. Thus, like male FKBP52 null mice, the role of FKBP52 in control of female steroidal physiology appears to be highly receptor- and tissue-specific.
Followup studies by the Dey laboratory have expanded our understanding of how FKBP52 contributes to uterine biology. Implantation failure in null females was found to be dependent on genetic background and stage of pregnancy [64]. For example, progesterone supplementation was able to rescue implantation in CD1 null females, but not in null females on a mixed C57BL/129 background. Endometriosis is a condition of ectopic growth of uterine endometrial cells that typically arises from excessive estrogenic stimulation or insufficient stimulation by progestins. Not surprisingly, FKBP52 null females were more susceptible to ectopic endometrial growth, while human samples of endometriosis had greatly reduced levels of FKBP52 [65], showing that FKBP52 promotion of PR action is essential to protection against this condition. Lastly, FKBP52 was also found to protect the uterus against oxidative stress, in this case by a mechanism that does not involve PR, but rather by FKBP52 effects on a unique anti-oxidant protein, peroxiredoxin-6 [66].
5.3. A positive-regulator of hepatic GR-induced gluconeogenesis
Numerous cell-based studies have shown that FKBP52 is required for full activity of GR. For this reason, a GR phenotype was expected in FKBP52 null mice, perhaps even neonatal lethality. Although it is tempting to ascribe the high rate of embryonic lethals seen in FKBP52 null mice to a failure of GR, at present no direct evidence for this exists. Certainly, the FKBP52 null mice do not appear to die from the atelectasia typically seen in GR KO mice. Instead, it is more likely that FKBP52 null mice have subtle GR defects, such as reduced GC sensitivity at peripheral organs, especially under conditions of stress. This hypothesis was recently tested by determining the effects of FKBP52 loss on GR induction of gluconeogenic genes and by subjecting FKBP52 (+/−) mice to the dietary stress of a high-fat diet [60]. FKBP52 (+/−) mice were more susceptible than WT litter-mates to the adverse effects of this diet, including hyperglycemia, hyperinsulinemia, and fatty liver (steatosis). Interestingly, the mutant mice also showed elevated levels of circulating corticosterone but reduced expression of hepatic gluconeogenic genes, suggesting a state of GC resistance that arises, at least in part, from insensitivity of hepatic GR. Under these conditions, it is likely that reduced GR hepatic gluconeogenic activity may actually exacerbate hepatic fatty buildup because the excess 3-carbon compounds of the high-fat diet are shunted to lipogenic pathways. These results are the first in vivo data for alteration of GR-regulated physiology in cochaperone-deficient mice. Much is clearly left to be discovered, as it is likely that FKBP52 modulates GR responses, not only at other metabolic organs (e.g., muscle, adipose), but wherever GR and FKBP52 are co-expressed.
6. FKBP51
6.1. Molecular Actions & Predicted Endocrinology
Although FKBP51 is structurally similar to FKBP52, containing TPR domains and PPIase activity that is inhibited by FK506, it tends to have inhibitory effects on SRs, with the exception of AR where it is stimulatory [67]. Not only is the FKBP51 interaction with PR and GR complexes inhibitory of their respective activities, but each receptor will upregulate FKBP51 expression in what appears to be a negative feedback mechanism to suppress hyperactivity [68]. Thus, the reasonable prediction in FKBP51 null mice has been for phenotypes resembling GR or PR hypersensitivity. In contrast, AR-mediated physiology was expected to be suppressed, similar to that seen in FKBP52 null males.
6.2. The FKBP1 paradox: no role in male fertility yet promotion of prostate cancer cell growth
Detailed histological and fertility studies by the Smith, Dey and Shou groups have yet to uncover any defects in male or female FKBP51 null mice [57, 61, 63]. Although this was not that surprising with respect to female fertility, where elevated PR-mediated responses might not lead to loss of function, it was surprising in males. Several cell-based studies have demonstrated that FKBP51 is essential for AR function, including its ability to bind hormone and transcriptional activity [69–71]. Moreover, AR, like GR and PR, also directly upregulates expression of FKBP51, presumably as a feed-forward mechanism to potentiate its activity [69, 72–77]. In contrast to its apparent neutrality in male fertility, FKBP51 is a powerful positive regulator of androgen-mediated growth of prostate cancer cells [70, 71]. In addition, in vivo xenograph studies in mice showed that elevated FKBP51 expression correlates with progression of prostate cancer from the relatively benign androgen-dependent state to the malignant androgen-independent state [75, 78]. Lastly, analysis of human prostate cancer tissues showed a remarkable correlation with high levels of FKBP51 [71]. Taken as a whole, it may be that FKBP51 is not an important regulator of AR-mediated sexual differentiation and function, but may preferentially regulate AR-mediated cell growth. Confirmation of this hypothesis will require studies in older null male mice with or without treatment to induce prostate cancer, or the development of transgenic mice with prostate-specific over-expression of FKBP51.
6.3. A contributor to the adverse neuroendocrine effects of chronic stress
Recently, FKBP51 has received a lot of attention for its the potential role in neuroendocrine control of behavior and the onset of psychological disorders, such as post-traumatic stress disorder (PTSD) and major depression [79]. Historically, high levels of glucocorticoids have been viewed as contributory to these disorders. Yet, evidence now exists that high GC levels may reflect a state of GC resistance that actually promotes increased sensitivity to psychosocial stress. Not surprisingly, several studies of psychosocial stress have implicated genetic variations or increased expression of FKBP51 as a potential cause [80–83]. This concept was recently tested in FKBP51 null mice by Schmidt and colleagues [84]. Null mice subjected to three weeks of chronic social stress showed a less vulnerable phenotype that included a reduced response to and enhanced recovery from acute stress episodes. Interestingly, stressed KO mice also showed reduced adrenal gland weight and lower levels of basal corticosterone, indicating that enhanced GR activity due to FKBP51 loss is either increasing negative-feedback regulation of GC secretion, or enhancing neurological GC responsiveness, or both.
6.4. Resistance to diet-induced adiposity in FKBP51 null mice?
With respect to metabolism, FKBP51 null mice would be expected to have elevated GR activity, leading to hyperglycemia, muscle wasting and excess lipolysis, especially at peripheral adipose depots. To date no studies on metabolism in the FKBP51 mice have been published. We have recently undertaken such as study by subjecting FKBP51 KO mice to hormonal treatments and a high-fat diet (Warrier M. et al., unpublished data). Null mice treated with dexamethasone agonist showed increased expression of GR markers in liver, muscle and adipose. Null mice subjected to a high fat diet were dramatically resistant to weight gain, especially adipose mass, suggesting elevated GR induction of lipolysis. However, serum glucose, fatty acid and triglyceride levels were very low in diet challenged null mice, suggesting an additional phenotype of heightened energy expenditure. The latter metabolic effects may arise from FKBP51 targets other than GR. Indeed, there is recent evidence that FKBP51 can act as a scaffolding protein that promotes Akt dephosphorylation by the PHLPP phosphatase [85]. Since Akt activation is critical to insulin action, most notably, promotion of glucose uptake [86], a GR-independent role for FKBP51 appears likely in the null mice. Yet, reduced levels of serum corticosterone were observed in the null mice, consistent with a state of GC hypersensitivity at peripheral organs, or increased feed-back suppression at the hypothalamus-pituitary-adrenal (HPA) axis, as shown by Hartmann et al. [84].
7. PP5
7.1. Molecular Actions & Predicted Endocrinology
Protein phosphatase 5 is a unique member of the phosphatase family due to presence of TPR domains which allow for binding to Hsp90 [87, 88]. To date, association of PP5 with SRs has only been demonstrated for GR and ER. Indeed, a side-by-side comparison of co-expressed GR and PR found a distinct preference of PP5 (and FKBP51) for GR but no detectable interaction with PR, which preferred FKBP52 [59]. More recently, a TPR peptide derived from PP5 was shown to alter the activities of PPARα and PPARγ [8], but direct binding by intact PP5 has yet to be published. Because PP5 also affects a host of non-SR responses, including MAPK-mediated growth, DNA damage repair and regulation of ion channels, interpretation of results in mouse models may be difficult. Indeed, because of its pleiotropy, most investigators predicted that the PP5 null mouse would be an embryonic lethal. Surprisingly, PP5 null mice were recently generated [89], but little work on altered physiology has been done. However, studies in PP5 KO MEF cells derived from these mice have confirmed the role of PP5 in DNA damage repair and cell cycle arrest by attenuating the activities of two checkpoint kinases, ataxia telangiectasia mutated (ATM) kinase and ATM-and-Rad3 related (ATR) kinase [89]. In a different approach, transgenic mice with cardiac myocyte-specific over-expression of PP5 have been produced, demonstrating that PP5 contributes to β-adrenergic contractility of the heart [90]. With regard to PP5 control of steroidal physiology, direct tests in mice have yet to be done, but two promising directions can be highlighted.
7.2. A role for PP5 in breast cancer?
Although a case can be made for PP5 contribution to many types of cancer, its potential role in breast cancer is perhaps the most compelling. In addition to binding ER via Hsp90, PP5 is known to be upregulated by estradiol in MCF-7 breast cancer cells and to have a functional estrogen response element in its promoter [91, 92]. Interestingly, estradiol-mediated upregulation of PP5 interferes with glucocorticoid-induced arrest of cell growth [92] due to PP5-mediated dephosphorylation and inhibition of GR [93]. It is likely, however, that ER induction of PP5 stimulates additional pro-growth pathways, although these have not yet been identified. Circumstantial evidence is growing. PP5 over-expression increased growth rates of MCF-7 cells [91]. In xenograph models of breast cancer, over-expression of PP5 accelerated tumor growth in response to estrogen [94]. Immunostaining of human breast cancer tissue has revealed high levels of PP5 in ductal carcinoma in situ, and in invasive ductal carcinoma with or without metastases [95]. Moving forward it will be interesting to see if transgenic over-expression of PP5 to mammary epithelia of the mouse will produce either spontaneous or induced breast tumors with heightened sensitivity to estrogens.
7.3. Does PP5 control PPARγ induction of adipogenesis?
Ever since Chen & Cohen discovered that PP5 phosphatase activity can be activated by polyunsaturated fatty acids (e.g., arachidonic acid), an intriguing potential role for PP5 in regulation of lipid metabolism has existed [96–98]. This idea was bolstered by the aforementioned interaction of PPARs with Hsp90. We have pursued this concept by asking whether PP5 contributes to the adipogenic properties of PPARγ (Hinds T. et al., unpublished data). Wild-type and PP5KO MEF cells were used to show that PP5-mediated dephosphorylation of PPARγ at serine 112 is required for rosiglitazone activation of PPARγ. In the absence of PP5, PPARγ could not induce expression of pro-adipogenic genes, such as aP2 and CD36. Moreover, GR activity at lipolytic genes was highly elevated in PP5KO cells, due to hyperphosphorylation at serines 212 and 234. The net effect of PP5 actions on both targets was a near complete blockade of adipogenic differentiation and lipid accumulation. Studies to confirm these mechanisms in PP5 null mice are underway.
8. Conclusion
Although the studies described above are low in number, it is already clear that mouse genetic models can provide greatly needed insights into how the Hsp90 chaperone machine contributes to steroidal control of physiology (Fig. 1). As we move forward, the physiological effects obtained will inform how we interpret existing molecular and cellular mechanisms. In turn, new molecular discoveries can be tested in vivo using the already established mouse models and new ones to be developed. Until then, a number of perplexing issues remain that are worth highlighting.
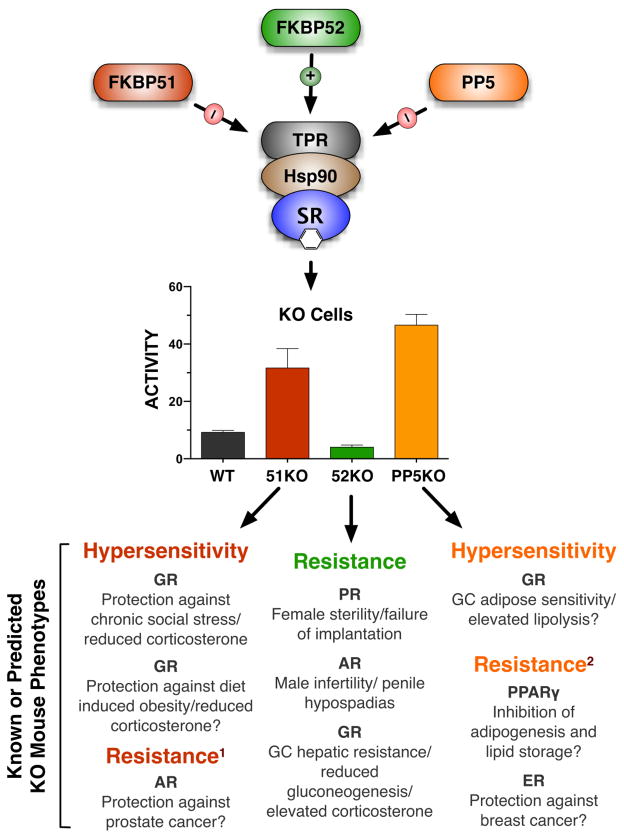
Fig. 1.
Hsp90 TPR cochaperones differentially modulate steroid receptor activity and tissue-specific endocrine physiology. In cell-based studies, FKBP52 is typically a positive regulator of SR transcriptional activity, while FKBP51 and PP5 are negative regulators. Assuming that this relationship holds true in the mouse, phenotypes consistent with steroidal resistance or steroidal hypersensitivity are therefore expected. The limited studies so far reported in genetically-ablated mice generally support this model. Exceptions to these functional relationships include: positive regulation of AR by FKBP51 leading to resistance1; positive regulation of PPARγ by PP5 leading to resistance2; regulation of ER by PP5 is still not clear, yet PP5 is a highly induced target gene of ER, suggesting an overall potential phenotype of resistance in PP5 null mice2.
8.1. What, no compensation?
A surprising outcome of the Hsp90α and Hsp90β null mice is the almost complete lack of compensation of one isoform for the other. This is in spite of the overwhelming in vitro data showing more overlap than differences when it comes to functionality and client substrate specificity. Although it could be argued that the normal phenotypes of both Hsp90 heterozygotes are examples of compensation, it is more likely that half-gene dosage is simply enough to avoid producing serious defects that are easy to detect. In the FKBP52 heterozygote null mice, no fertility problems were observed in males or females. Because of this, we have compared TPR protein recruitment to PR and GR complexes in FKBP52 KO cells and have found no compensatory binding by FKBP51, PP5 or Cyp40 to either receptor complex [55, 59]. Moreover, it can be inferred that if compensation is an important functional process, then compensatory increased expression of partner proteins should occur. To date, we have found no evidence for increased expression of any TPR protein in FKBP52 KO cells or tissues. Similarly, increased expression of Hsp90β was not seen in the Hsp90α null mice. These types of results reinforce the notion that in vitro experiments can be flawed. That is, without the energy-driven, compartmentalized environment of cells in an endocrine and metabolically regulated environment, interactions can occur in solution, or even in tissue culture cells, that never have an opportunity in the intact cell of an organism.
8.2. Neuroendocrine vs peripheral sensitivity
Even before the first TPR null mouse was generated, a chicken & egg dilemma existed because SR activity is necessary for peripheral organ sensitivity and for negative-feedback regulation of steroid secretion. Using GR and FKBP52 as an example, if loss of FKBP52 were to globally decrease GR activity in all tissues, what would be the balance between the HPA axis and peripheral organ sensitivity? Would lack of organ sensitivity be more that compensated for by loss of negative-feedback suppression, resulting in very high levels of secreted GCs and perhaps a phenotype indicative of over-stimulation? Or would organ insensitivity be the dominant phenotype? Moreover, extra-endocrine steroidal synthesis is also known to occur, such as production and secretion of GCs by several peripheral tissues [99]. Although this dilemma is far from resolved, the results so far obtained are intriguing. In the FKBP52 mutant mouse, reduced sensitivity of the liver to GCs appears to occur, even in the presence of elevated serum corticosterone [60]. Thus, the HPA axis is not incapable of responding to peripheral insensitivity by increasing corticosterone secretion. To what extent, if any, it is limited by reduced GR-mediated feedback suppression is still unknown. In the FKBP51 null mice, hypersensitivity to GCs at peripheral organs was expected. The results of Schmidt and colleagues [84], and our own unpublished data, showed low levels of serum corticosterone, indicative of an overall GC hypersensitivity syndrome at peripheral organs and the HPA axis. Determination of the relative contribution of each still awaits.
8.3. The tissue profile paradox
One would normally predict that the tissue expression profile of a protein would correlate with the observed phenotypes in globally ablated mice. This has not been the case in chaperone null mice. A good example of this is the FKBP52 null male, which shows penile hypospadias and dysgenic prostate, yet all other genital organs developed normally, such as testes. Testicular function was normal as measured by sperm count, although reduced motility of sperm was observed [61]. Yet, profiling of FKBP52 showed expression of the protein in all genital organs, with testes being the highest. Thus, FKBP52 cannot be a global and essential regulator AR, since that should lead to a phenotype similar to the AR null mouse, where all male genital organs are reduced or missing, leading to a feminization syndrome [100]. Yet, the question remains as to why AR functions are not affected by FKBP52 loss in the testes. As discussed above, this could arise from compensation by FKBP51, which also promotes AR activity. But increased recruitment of FKBP51 to AR in FKBP52 KO cells has been found, as well as no increase of FKBP51 expression in testes of null mice [61]. Perhaps the function of FKBP52 in the testes is to regulate another SR. If true, then tissue-specific signal pathways or processes must exist that direct TPR proteins to one receptor over another.
In summary, these early studies using mouse genetic models of chaperones are providing more unanswered questions than facts. Resolution of the major issues will require the generation of tissue-specific knockout and over-expressing mice, as well as better insights into the potential developmental, hormonal, or even metabolic variables that impact how cochaperones acting through Hsp90 regulate the tissue-specific responses to steroids.
Highlights.
The roles of Hsp90 and select cochaperones in steroidal physiology are reviewed
Mouse genetic models of Hsp90, p23, FKBP51, FKBP52 and PP5 are described in detail
Effects on glucocorticoid, androgen, and progesterone physiology have been found
Acknowledgments
This work was supported in part by National Institutes of Health grant DK70127. We apologize to those authors whose original studies were not cited due to conceptual and space constraints.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanchez ER, Toft DO, Schlesinger MJ, Pratt WB. Evidence that the 90-kDa phosphoprotein associated with the untransformed L-cell glucocorticoid receptor is a murine heat shock protein. J Biol Chem. 1985;260:12398–12401. [PubMed] [Google Scholar]
- 2.Catelli MG, Binart N, Jung-Testas I, Renoir JM, Baulieu EE, Feramisco JR, Welch WJ. The common 90-kd protein component of non-transformed ‘8S’ steroid receptors is a heat-shock protein. EMBO J. 1985;4:3131–3135. doi: 10.1002/j.1460-2075.1985.tb04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuh S, Yonemoto W, Brugge J, Bauer VJ, Riehl RM, Sullivan WP, Toft DO. A 90,000-dalton binding protein common to both steroid receptors and the Rous sarcoma virus transforming protein, pp60v-src. J Biol Chem. 1985;260:14292–14296. [PubMed] [Google Scholar]
- 4.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18(3):306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 5.Picard D. Chaperoning steroid hormone action. Trends Endocrinol Metab. 2006;17:229–235. doi: 10.1016/j.tem.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Dalman FC, Koenig RJ, Perdew GH, Massa E, Pratt WB. In contrast to the glucocorticoid receptor, the thyroid hormone receptor is translated in the DNA binding state and is not associated with hsp90. J Biol Chem. 1990;265:3615–3618. [PubMed] [Google Scholar]
- 7.Sumanasekera WK, Tien ES, Turpey R, Vanden Heuvel JP, Perdew GH. Evidence that peroxisome proliferator-activated receptor alpha is complexed with the 90-kDa heat shock protein and the hepatitis virus B X-associated protein 2. J Biol Chem. 2003;278:4467–4473. doi: 10.1074/jbc.M211261200. [DOI] [PubMed] [Google Scholar]
- 8.Sumanasekera WK, Tien ES, Davis JWn, Turpey R, Perdew GH, Vanden Heuvel JP. Heat shock protein-90 (Hsp90) acts as a repressor of peroxisome proliferator-activated receptor-alpha (PPARalpha) and PPARbeta activity. Biochemistry. 2003;42:10726–10735. doi: 10.1021/bi0347353. [DOI] [PubMed] [Google Scholar]
- 9.Chen HS, Singh SS, Perdew GH. The Ah receptor is a sensitive target of geldanamycin-induced protein turnover. Arch Biochem Biophys. 1997;348:190–198. doi: 10.1006/abbi.1997.0398. [DOI] [PubMed] [Google Scholar]
- 10.Yoshinari K, Kobayashi K, Moore R, Kawamoto T, Negishi M. Identification of the nuclear receptor CAR:HSP90 complex in mouse liver and recruitment of protein phosphatase 2A in response to phenobarbital. FEBS Lett. 2003;548:17–20. doi: 10.1016/s0014-5793(03)00720-8. [DOI] [PubMed] [Google Scholar]
- 11.Squires EJ, Sueyoshi T, Negishi M. Cytoplasmic localization of pregnane × receptor and ligand-dependent nuclear translocation in mouse liver. J Biol Chem. 2004;279:49307–49314. doi: 10.1074/jbc.M407281200. [DOI] [PubMed] [Google Scholar]
- 12.Angelo G, Lamon-Fava S, Sonna LA, Lindauer ML, Wood RJ. Heat shock protein 90beta: a novel mediator of vitamin D action. Biochem Biophys Res Commun. 2008;367:578–583. doi: 10.1016/j.bbrc.2007.12.179. [DOI] [PubMed] [Google Scholar]
- 13.Buchner J. Hsp90 & Co. - a holding for folding. Trends Biochem Sci. 1999;24:136–141. doi: 10.1016/s0968-0004(99)01373-0. [DOI] [PubMed] [Google Scholar]
- 14.Grenert JP, Sullivan WP, Fadden P, Haystead TA, Clark J, Mimnaugh E, Krutzsch H, Ochel HJ, Schulte TW, Sausville E, Neckers LM, Toft DO. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem. 1997;272:23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 15.Bresnick EH, Dalman FC, Sanchez ER, Pratt WB. Evidence that the 90-kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. J Biol Chem. 1989;264:4992–4997. [PubMed] [Google Scholar]
- 16.Scherrer LC, Dalman FC, Massa E, Meshinchi S, Pratt WB. Structural and functional reconstitution of the glucocorticoid receptor-hsp90 complex. J Biol Chem. 1990;265:21397–21400. [PubMed] [Google Scholar]
- 17.Tai PK, Maeda Y, Nakao K, Wakim NG, Duhring JL, Faber LE. A 59-kilodalton protein associated with progestin, estrogen, androgen, and glucocorticoid receptors. Biochemistry. 1986;25:5269–5275. doi: 10.1021/bi00366a043. [DOI] [PubMed] [Google Scholar]
- 18.Smith DF, Baggenstoss BA, Marion TN, Rimerman RA. Two FKBP-related proteins are associated with progesterone receptor complexes. J Biol Chem. 1993;268:18365–18371. [PubMed] [Google Scholar]
- 19.Ratajczak T, Carrello A, Mark PJ, Warner BJ, Simpson RJ, Moritz RL, House AK. The cyclophilin component of the unactivated estrogen receptor contains a tetratricopeptide repeat domain and shares identity with p59 (FKBP59) J Biol Chem. 1993;268:13187–13192. [PubMed] [Google Scholar]
- 20.Chen MS, Silverstein AM, Pratt WB, Chinkers M. The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J Biol Chem. 1996;271:32315–32320. doi: 10.1074/jbc.271.50.32315. [DOI] [PubMed] [Google Scholar]
- 21.McKeen HD, McAlpine K, Valentine A, Quinn DJ, McClelland K, Byrne C, O’Rourke M, Young S, Scott CJ, McCarthy HO, Hirst DG, Robson T. A novel FK506-like binding protein interacts with the glucocorticoid receptor and regulates steroid receptor signaling. Endocrinology. 2008;149:5724–5734. doi: 10.1210/en.2008-0168. [DOI] [PubMed] [Google Scholar]
- 22.Picard D, Khursheed B, Garabedian MJ, Fortin MG, Lindquist S, Yamamoto KR. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990;348:166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- 23.Sreedhar AS, Kalmar E, Csermely P, Shen YF. Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett. 2004;562:11–15. doi: 10.1016/s0014-5793(04)00229-7. [DOI] [PubMed] [Google Scholar]
- 24.Grad I, Cederroth CR, Walicki J, Grey C, Barluenga S, Winssinger N, De Massy B, Nef S, Picard D. The Molecular Chaperone Hsp90alpha Is Required for Meiotic Progression of Spermatocytes beyond Pachytene in the Mouse. PLoS One. 2010;5:e15770. doi: 10.1371/journal.pone.0015770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taherian A, Krone PH, Ovsenek N. A comparison of Hsp90alpha and Hsp90beta interactions with cochaperones and substrates. Biochem Cell Biol. 2008;86:37–45. doi: 10.1139/o07-154. [DOI] [PubMed] [Google Scholar]
- 26.Bouchier-Hayes L, Oberst A, McStay GP, Connell S, Tait SW, Dillon CP, Flanagan JM, Beere HM, Green DR. Characterization of cytoplasmic caspase-2 activation by induced proximity. Mol Cell. 2009;35:830–840. doi: 10.1016/j.molcel.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eustace BK, Sakurai T, Stewart JK, Yimlamai D, Unger C, Zehetmeier C, Lain B, Torella C, Henning SW, Beste G, Scroggins BT, Neckers L, Ilag LL, Jay DG. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat Cell Biol. 2004;6:507–514. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- 28.Voss AK, Thomas T, Gruss P. Mice lacking HSP90beta fail to develop a placental labyrinth. Development. 2000;127:1–11. doi: 10.1242/dev.127.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Smith DF, Faber LE, Toft DO. Purification of unactivated progesterone receptor and identification of novel receptor-associated proteins. J Biol Chem. 1990;265:3996–4003. [PubMed] [Google Scholar]
- 30.Bresnick EH, Dalman FC, Pratt WB. Direct stoichiometric evidence that the untransformed Mr 300,000, 9S, glucocorticoid receptor is a core unit derived from a larger heteromeric complex. Biochemistry. 1990;29:520–527. doi: 10.1021/bi00454a028. [DOI] [PubMed] [Google Scholar]
- 31.Pratt WB, Galigniana MD, Morishima Y, Murphy PJ. Role of molecular chaperones in steroid receptor action. Essays Biochem. 2004;40:41–58. doi: 10.1042/bse0400041. [DOI] [PubMed] [Google Scholar]
- 32.Smith DF, Toft DO. Minireview: the intersection of steroid receptors with molecular chaperones: observations and questions. Mol Endocrinol. 2008;22(10):2229–2240. doi: 10.1210/me.2008-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freeman BC, Felts SJ, Toft DO, Yamamoto KR. The p23 molecular chaperones act at a late step in intracellular receptor action to differentially affect ligand efficacies. Genes Dev. 2000;14:422–434. [PMC free article] [PubMed] [Google Scholar]
- 34.Wochnik GM, Young JC, Schmidt U, Holsboer F, Hartl FU, Rein T. Inhibition of GR-mediated transcription by p23 requires interaction with Hsp90. FEBS Lett. 2004;560:35–38. doi: 10.1016/S0014-5793(04)00066-3. [DOI] [PubMed] [Google Scholar]
- 35.Oxelmark E, Roth JM, Brooks PC, Braunstein SE, Schneider RJ, Garabedian MJ. The cochaperone p23 differentially regulates estrogen receptor target genes and promotes tumor cell adhesion and invasion. Mol Cell Biol. 2006;26:5205–5213. doi: 10.1128/MCB.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanioka T, Nakatani Y, Semmyo N, Murakami M, Kudo I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J Biol Chem. 2000;275:32775–32782. doi: 10.1074/jbc.M003504200. [DOI] [PubMed] [Google Scholar]
- 37.Toogun OA, Zeiger W, Freeman BC. The p23 molecular chaperone promotes functional telomerase complexes through DNA dissociation. Proc Natl Acad Sci U S A. 2007;104:5765–5770. doi: 10.1073/pnas.0701442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grad I, McKee TA, Ludwig SM, Hoyle GW, Ruiz P, Wurst W, Floss T, Miller CAr, Picard D. The Hsp90 cochaperone p23 is essential for perinatal survival. Mol Cell Biol. 2006;26:8976–8983. doi: 10.1128/MCB.00734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 40.Bayo P, Sanchis A, Bravo A, Cascallana JL, Buder K, Tuckermann J, Schutz G, Perez P. Glucocorticoid receptor is required for skin barrier competence. Endocrinology. 2008;149:1377–1388. doi: 10.1210/en.2007-0814. [DOI] [PubMed] [Google Scholar]
- 41.Nakatani Y, Hokonohara Y, Kakuta S, Sudo K, Iwakura Y, Kudo I. Knockout mice lacking cPGES/p23, a constitutively expressed PGE2 synthetic enzyme, are peri-natally lethal. Biochem Biophys Res Commun. 2007;362:387–392. doi: 10.1016/j.bbrc.2007.07.180. [DOI] [PubMed] [Google Scholar]
- 42.Lovgren AK, Kovarova M, Koller BH. cPGES/p23 is required for glucocorticoid receptor function and embryonic growth but not prostaglandin E2 synthesis. Mol Cell Biol. 2007;27:4416–4430. doi: 10.1128/MCB.02314-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakatani Y, Hokonohara Y, Tajima Y, Kudo I, Hara S. Involvement of the constitutive prostaglandin E synthase cPGES/p23 in expression of an initial prostaglandin E2 inactivating enzyme, 15-PGDH. Prostaglandins Other Lipid Mediat. 2011;94:112–117. doi: 10.1016/j.prostaglandins.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 45.Bolt RJ, van Weissenbruch MM, Lafeber HN, Delemarre-van de Waal HA. Glucocorticoids and lung development in the fetus and preterm infant. Pediatr Pulmonol. 2001;32:76–91. doi: 10.1002/ppul.1092. [DOI] [PubMed] [Google Scholar]
- 46.Lee J, Kim HJ, Moon JA, Sung YH, Baek IJ, Roh JI, Ha NY, Kim SY, Bahk YY, Lee JE, Yoo TH, Lee HW. Transgenic overexpression of p23 induces spontaneous hydronephrosis in mice. Int J Exp Pathol. 2011;92:251–259. doi: 10.1111/j.1365-2613.2011.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies TH, Sanchez ER. Fkbp52. Int J Biochem Cell Biol. 2005;37(1):42–47. doi: 10.1016/j.biocel.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 48.Nakao K, Myers JE, Faber LE. Development of a monoclonal antibody to the rabbit 8.5S uterine progestin receptor. Can J Biochem Cell Biol. 1985;63:33–40. doi: 10.1139/o85-005. [DOI] [PubMed] [Google Scholar]
- 49.Renoir JM, Radanyi C, Faber LE, Baulieu EE. The non-DNA-binding heterooligomeric form of mammalian steroid hormone receptors contains a hsp90-bound 59-kilodalton protein. J Biol Chem. 1990;265:10740–10745. [PubMed] [Google Scholar]
- 50.Sanchez ER, Faber LE, Henzel WJ, Pratt WB. The 56–59-kilodalton protein identified in untransformed steroid receptor complexes is a unique protein that exists in cytosol in a complex with both the 70- and 90-kilodalton heat shock proteins. Biochemistry. 1990;29:5145–5152. doi: 10.1021/bi00473a021. [DOI] [PubMed] [Google Scholar]
- 51.Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, Gaber R, Picard D, Smith DF. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003;22:1158–1167. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- 53.Davies TH, Ning YM, Sanchez ER. Differential Control of Glucocorticoid Receptor Hormone-Binding Function by Tetratricopeptide Repeat (TPR) Proteins and the Immunosuppressive Ligand FK506. Biochemistry. 2005;44:2030–2038. doi: 10.1021/bi048503v. [DOI] [PubMed] [Google Scholar]
- 54.Yang Z, Wolf IM, Chen H, Periyasamy S, Chen Z, Yong W, Shi S, Zhao W, Xu J, Srivastava A, Sanchez ER, Shou W. FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor a isoform. Mol Endocrinol. 2006;20:2682–2694. doi: 10.1210/me.2006-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolf IM, Periyasamy S, Hinds TD, Jr, Yong W, Shou W, Sanchez ER. Targeted ablation reveals a novel role of FKBP52 in gene-specific regulation of glucocorticoid receptor transcriptional activity. J Steroid Biochem Mol Biol. 2009;113:36–45. doi: 10.1016/j.jsbmb.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riggs DL, Cox MB, Tardif HL, Hessling M, Buchner J, Smith DF. Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling. Mol Cell Biol. 2007;27:8658–8669. doi: 10.1128/MCB.00985-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheung-Flynn J, Prapapanich V, Cox MB, Riggs DL, Suarez-Quian C, Smith DF. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol. 2005;19:1654–1666. doi: 10.1210/me.2005-0071. [DOI] [PubMed] [Google Scholar]
- 58.Galigniana MD, Erlejman AG, Monte M, Gomez-Sanchez C, Piwien-Pilipuk G. The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events. Mol Cell Biol. 2010;30:1285–1298. doi: 10.1128/MCB.01190-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banerjee A, Periyasamy S, Wolf IM, Hinds TDJ, Yong W, Shou W, Sanchez ER. Control of glucocorticoid and progesterone receptor subcellular localization by the ligand-binding domain is mediated by distinct interactions with tetratricopeptide repeat proteins. Biochemistry. 2008;47:10471–10480. doi: 10.1021/bi8011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warrier M, Hinds TDJ, Ledford KJ, Cash HA, Patel PR, Bowman TA, Stechschulte LA, Yong W, Shou W, Najjar SM, Sanchez ER. Susceptibility to diet-induced hepatic steatosis and glucocorticoid resistance in FK506-binding protein 52-deficient mice. Endocrinology. 2010;151:3225–3236. doi: 10.1210/en.2009-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yong W, Yang Z, Periyasamy S, Chen H, Yucel S, Li W, Lin LY, Wolf IM, Cohn MJ, Baskin LS, Sanchez ER, Shou W. Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J Biol Chem. 2007;282:5026–5036. doi: 10.1074/jbc.M609360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen H, Yong W, Hinds TDJ, Yang Z, Zhou Y, Sanchez ER, Shou W. Fkbp52 regulates androgen receptor transactivation activity and male urethra morphogenesis. J Biol Chem. 2010;285:27776–27784. doi: 10.1074/jbc.M110.156091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF, Dey SK. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A. 2005;102:14326–14331. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tranguch S, Wang H, Daikoku T, Xie H, Smith DF, Dey SK. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest. 2007;117:1824–1834. doi: 10.1172/JCI31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hirota Y, Tranguch S, Daikoku T, Hasegawa A, Osuga Y, Taketani Y, Dey SK. Deficiency of immunophilin FKBP52 promotes endometriosis. Am J Pathol. 2008;173:1747–1757. doi: 10.2353/ajpath.2008.080527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirota Y, Acar N, Tranguch S, Burnum KE, Xie H, Kodama A, Osuga Y, Ustunel I, Friedman DB, Caprioli RM, Daikoku T, Dey SK. Uterine FK506-binding protein 52 (FKBP52)-peroxiredoxin-6 (PRDX6) signaling protects pregnancy from overt oxidative stress. Proc Natl Acad Sci U S A. 2010;107:15577–15582. doi: 10.1073/pnas.1009324107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stechschulte LA, Sanchez ER. FKBP51-a selective modulator of glucocorticoid and androgen sensitivity. Curr Opin Pharmacol. 2011;11:332–337. doi: 10.1016/j.coph.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144:2380–2387. doi: 10.1210/en.2003-0092. [DOI] [PubMed] [Google Scholar]
- 69.Febbo PG, Lowenberg M, Thorner AR, Brown M, Loda M, Golub TR. Androgen mediated regulation and functional implications of fkbp51 expression in prostate cancer. J Urol. 2005;173:1772–1777. doi: 10.1097/01.ju.0000155845.44729.ba. [DOI] [PubMed] [Google Scholar]
- 70.Ni L, Yang CS, Gioeli D, Frierson H, Toft DO, Paschal BM. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells. Mol Cell Biol. 2010;30:1243–1253. doi: 10.1128/MCB.01891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Periyasamy S, Hinds TJ, Shemshedini L, Shou W, Sanchez ER. FKBP51 and Cyp40 are positive regulators of androgen-dependent prostate cancer cell growth and the targets of FK506 and cyclosporin A. Oncogene. 2010;29:1691–1701. doi: 10.1038/onc.2009.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu W, Zhang JS, Young CY. Silymarin inhibits function of the androgen receptor by reducing nuclear localization of the receptor in the human prostate cancer cell line LNCaP. Carcinogenesis. 2001;22:1399–1403. doi: 10.1093/carcin/22.9.1399. [DOI] [PubMed] [Google Scholar]
- 73.Vanaja DK, Mitchell SH, Toft DO, Young CY. Effect of geldanamycin on androgen receptor function and stability. Cell Stress Chaperones. 2002;7:55–64. doi: 10.1379/1466-1268(2002)007<0055:eogoar>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clegg N, Eroglu B, Ferguson C, Arnold H, Moorman A, Nelson PS. Digital expression profiles of the prostate androgen-response program. J Steroid Biochem Mol Biol. 2002;80:13–23. doi: 10.1016/s0960-0760(01)00167-4. [DOI] [PubMed] [Google Scholar]
- 75.Velasco AM, Gillis KA, Li Y, Brown EL, Sadler TM, Achilleos M, Greenberger LM, Frost P, Bai W, Zhang Y. Identification and validation of novel androgen-regulated genes in prostate cancer. Endocrinology. 2004;145:3913–3924. doi: 10.1210/en.2004-0311. [DOI] [PubMed] [Google Scholar]
- 76.Magee JA, Chang LW, Stormo GD, Milbrandt J. Direct, androgen receptor-mediated regulation of the FKBP5 gene via a distal enhancer element. Endocrinology. 2006;147:590–598. doi: 10.1210/en.2005-1001. [DOI] [PubMed] [Google Scholar]
- 77.Makkonen H, Kauhanen M, Paakinaho V, Jaaskelainen T, Palvimo JJ. Long-range activation of FKBP51 transcription by the androgen receptor via distal intronic enhancers. Nucleic Acids Res. 2009;37:4135–4148. doi: 10.1093/nar/gkp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amler LC, Agus DB, LeDuc C, Sapinoso ML, Fox WD, Kern S, Lee D, Wang V, Leysens M, Higgins B, Martin J, Gerald W, Dracopoli N, Cordon-Cardo C, Scher HI, Hampton GM. Dysregulated expression of androgen-responsive and nonresponsive genes in the androgen-independent prostate cancer xenograft model CWR22-R1. Cancer Res. 2000;60:6134–6141. [PubMed] [Google Scholar]
- 79.Drago A, De Ronchi D, Serretti A. Pharmacogenetics of antidepressant response: an update. Hum Genomics. 2009;3:257–274. doi: 10.1186/1479-7364-3-3-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Kunzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Kohnlein O, Dabitz H, Bruckl T, Muller N, Pfister H, Lieb R, Mueller JC, Lohmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Muller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 81.Ising M, Depping AM, Siebertz A, Lucae S, Unschuld PG, Kloiber S, Horstmann S, Uhr M, Muller-Myhsok B, Holsboer F. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur J Neurosci. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- 82.Kirchheiner J, Lorch R, Lebedeva E, Seeringer A, Roots I, Sasse J, Brockmoller J. Genetic variants in FKBP5 affecting response to antidepressant drug treatment. Pharmacogenomics. 2008;9:841–846. doi: 10.2217/14622416.9.7.841. [DOI] [PubMed] [Google Scholar]
- 83.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hartmann J, Wagner KV, Liebl C, Scharf SH, Wang XD, Wolf M, Hausch F, Rein T, Schmidt U, Touma C, Cheung-Flynn J, Cox MB, Smith DF, Holsboer F, Muller MB, Schmidt MV. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 85.Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, Petersen G, Lou Z, Wang L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000;106:165–169. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hinds TD, Jr, Sanchez ER. Protein phosphatase 5. Int J Biochem Cell Biol. 2008;40(11):2358–2362. doi: 10.1016/j.biocel.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Golden T, Swingle M, Honkanen RE. The role of serine/threonine protein phosphatase type 5 (PP5) in the regulation of stress-induced signaling networks and cancer. Cancer Metastasis Rev. 2008;27:169–178. doi: 10.1007/s10555-008-9125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yong W, Bao S, Chen H, Li D, Sanchez ER, Shou W. Mice lacking protein phosphatase 5 are defective in ataxia telangiectasia mutated (ATM)-mediated cell cycle arrest. J Biol Chem. 2007;282:14690–14694. doi: 10.1074/jbc.C700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gergs U, Boknik P, Buchwalow IB, Fabritz L, Grundker N, Kucerova D, Matus M, Werner F, Schmitz W, Neumann J. Modulation of cardiac contractility by serine/threonine protein phosphatase type 5. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 91.Urban G, Golden T, Aragon IV, Scammell JG, Dean NM, Honkanen RE. Identification of an estrogen-inducible phosphatase (PP5) that converts MCF-7 human breast carcinoma cells into an estrogen-independent phenotype when expressed constitutively. J Biol Chem. 2001;276:27638–27646. doi: 10.1074/jbc.M103512200. [DOI] [PubMed] [Google Scholar]
- 92.Urban G, Golden T, Aragon IV, Cowsert L, Cooper SR, Dean NM, Honkanen RE. Identification of a functional link for the p53 tumor suppressor protein in dexamethasone-induced growth suppression. J Biol Chem. 2003;278:9747–9753. doi: 10.1074/jbc.M210993200. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y, Leung DY, Nordeen SK, Goleva E. Estrogen inhibits glucocorticoid action via protein phosphatase 5 (PP5) mediated glucocorticoid receptor dephosphorylation. J Biol Chem. 2009 doi: 10.1074/jbc.M109.021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Golden T, Aragon IV, Zhou G, Cooper SR, Dean NM, Honkanen RE. Constitutive over expression of serine/threonine protein phosphatase 5 (PP5) augments estrogen-dependent tumor growth in mice. Cancer Lett. 2004;215:95–100. doi: 10.1016/j.canlet.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 95.Golden T, Aragon IV, Rutland B, Tucker JA, Shevde LA, Samant RS, Zhou G, Amable L, Skarra D, Honkanen RE. Elevated levels of Ser/Thr protein phosphatase 5 (PP5) in human breast cancer. Biochim Biophys Acta. 2008;1782:259–270. doi: 10.1016/j.bbadis.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen MX, Cohen PT. Activation of protein phosphatase 5 by limited proteolysis or the binding of polyunsaturated fatty acids to the TPR domain. FEBS Lett. 1997;400:136–140. doi: 10.1016/s0014-5793(96)01427-5. [DOI] [PubMed] [Google Scholar]
- 97.Skinner J, Sinclair C, Romeo C, Armstrong D, Charbonneau H, Rossie S. Purification of a fatty acid-stimulated protein-serine/threonine phosphatase from bovine brain and its identification as a homolog of protein phosphatase 5. J Biol Chem. 1997;272:22464–22471. doi: 10.1074/jbc.272.36.22464. [DOI] [PubMed] [Google Scholar]
- 98.Kang H, Sayner SL, Gross KL, Russell LC, Chinkers M. Identification of amino acids in the tetratricopeptide repeat and C-terminal domains of protein phosphatase 5 involved in autoinhibition and lipid activation. Biochemistry. 2001;40:10485–10490. doi: 10.1021/bi010999i. [DOI] [PubMed] [Google Scholar]
- 99.Taves MD, Gomez-Sanchez CE, Soma KK. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am J Physiol Endocrinol Metab. 2011;301:E11–24. doi: 10.1152/ajpendo.00100.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002;99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]