Abstract
A large body of evidence demonstrates that angiotensin II and angiotensin receptors are required for the pathogenesis of experimental lung fibrosis. Angiotensin has a number of profibrotic effects on lung parenchymal cells that include the induction of growth factors for mesenchymal cells, extracellular matrix molecules, cytokines and increased motility of lung fibroblasts. Angiotensin is also proapoptotic for lung epithelial cells, and is synthesized by a local system (i.e. entirely within the lung tissue) after lung injury by a variety of agents of both xenobiotic and endogenous origins. Recent evidence shows that the counterregulatory molecule angiotensin 1-7, the product of the enzyme ACE-2, inhibits epithelial cell apoptosis and thus acts as an antifibrotic epithelial survival factor. This manuscript reviews the evidence supporting a role for angiotensin in lung fibrogenesis and discusses the signalling mechanisms underlying its action on lung parenchymal cells important in the pathogenesis of pulmonary fibrosis.
In vivo evidence implicating ANGII in lung fibrogenesis
Pulmonary fibrosis results from injury to the lung and an ensuing fibrotic response that leads to thickening of the alveolar walls and the obliteration of alveolar air spaces. If the etiology is unknown, the condition is designated as idiopathic pulmonary fibrosis (IPF) [1]. There are also various chemical toxins and other injuries known to cause pulmonary fibrosis, for example the antineoplastic agent bleomycin, the antiarrhythmic agent amiodarone, radiation, silicon dust and asbestos [2]. The main histological features of the fibrotic lung are persistent and unrepaired epithelial damage, proliferation and accumulation of fibroblasts and myofibroblasts, and increased collagen deposition [3]. This section will discuss in vivo evidence implicating ANGII in lung fibrogenesis.
The angiotensin system consists of angiotensinogen (AGT), an aspartyl protease such as renin or cathepsin D, angiotensin-converting enzyme (ACE), angiotensin II (ANGII) and angiotensin II type 1 and type 2 receptors (AT1, AT2). A recently discovered counterregulatory axis is composed of ACE-2, its product angiotensin 1-7 (ANG1-7) and the ANG1-7 receptor mas [4, 5]. There is significant in vivo evidence suggesting that the angiotensin system is involved in pulmonary fibrosis. The evidence includes genetic studies of ANG system gene polymorphisms in patients with lung fibrosis, demonstrations of activated ANG system genes and protein products in lung biopsy specimens from patients with lung fibrosis and a variety of animal model studies. The next section however, will discuss only the data from animal models.
In a variety of animal models, angiotensin system antagonists block experimental lung fibrosis. There is a substantial body of in vivo studies which demonstrated that ANGII plays a very important role in lung fibrogensis by blocking ANGII synthesis or its functions. Recently, we demonstrated that coadministration of antisense oligonucleotides against AGT mRNA, by blockade of the synthesis of lung-derived AGT, prevents bleomycin-induced lung cell apoptosis and lung fibrogenesis [6]. Furthermore, application of ACE inhibitors to inhibit ANGII production has been shown to attenuate experimental pulmonary fibrosis in animal models induced by various agents. For example, the ACE inhibitors Captopril [7], Enalapril [8], Lisinopril [9] and Perindopril [10] exerted inhibitory effects on bleomycin-, γ irradiation-, amiodarone- and paraquat- induced pulmonary fibrosis in rats, and on hyperoxia-induced chronic lung disease (CLD) in neonatal rats [11]. Moreover, the AT1 receptor-selective antagonists Candesartan [12] and Losartan [13,14] as well as the AT2 receptor-selective antagonist PD-123319 [15] were shown to have similar effects on radiation-induced lung fibrosis and bleomycin-induced lung fibrosis in rats and mice. In addition, ACE-2 overexpression by a lentiviral vector in the lung or systemic delivery of purified ACE-2 attenuated bleomycin-induced pulmonary fibrosis in rats and mice [1,16]. Together, these results support the contention that ANGII plays an important role in lung fibrogenesis via both AT1 and AT2 receptors.
Actions of Angiotensin on Lung Parenchymal Cells
The angiotensin system is known to be activated after tissue injury to promote tissue repair and, when in excess, tissue fibrosis. ANGII, the major effector peptide of this system, is now recognized as a growth factor that regulates cell growth and fibrogenesis. Previous works from this laboratory have demonstrated the capacity for local (i.e., extravascular) ANGII generation within the parenchyma of the lungs [17]. There is an increase of ANG peptides and AT1 and AT 2 receptor expression in lung tissue from patients with IPF [17,18]. Lung alveolar epithelial cells and myofibroblasts have been identified as the main local sources of this angiotensin generation [17]. Here, we summarize the wide variety of ANGII functions acting on lung parenchymal cells and discuss them in relation to the fibrotic cascade.
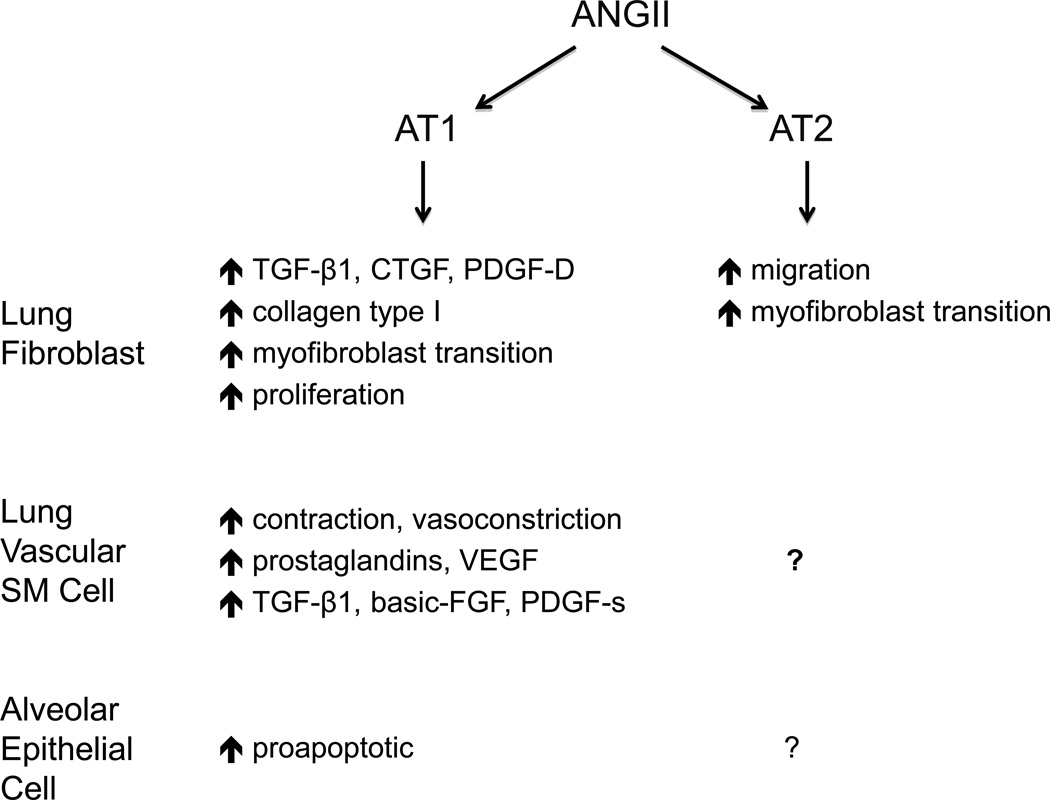
First, ANGII is mitogenic for human lung fibroblasts through AT1 and AT2 receptors [18]. The role of ANGII as a potent inducer of DNA synthesis and fibroblast proliferation has been widely studied [19]. It has been shown (see Figure 1) that both AT1 and AT2 receptors mediate ANGII signalling on fibroblast cell cycle and migration via phosphorylation of the mitogen-activated protein kinases p38 and p42/44 [18,19]. Furthermore, the effect of ANGII on human lung fibroblasts promotes extracellular matrix (ECM) synthesis [1,19]. Type I collagen is a principal matrix protein in the lung interstitium. Excessive collagen type I, mainly synthesized by activated fibroblasts, has been largely recognized in the pathogenesis of fibrosis and causes thickened alveolar walls and reduction of lung compliance. It has been demonstrated that ANGII signalling induces procollagen production in normal fibroblasts, whereas AT1 receptor antagonists abrogate the constitutively higher collagen synthesis exhibited by fibroblasts isolated from fibrotic human lung tissue [1]. ECM synthesis mediated by ANGII has been ascribed not only to AT1 receptor activation but also to the autocrine action of transforming growth factor-β1 (TGF-β1) [1,19] and connective tissue growth factor (CTGF) [20]. Both growth factors contribute by themselves to the development of lung fibrosis, in part by acting on human fibroblasts to enhance their differentiation to myofibroblasts and collagen synthesis. In vitro studies have demonstrated that these pro-fibrotic effects induced by TGF-β1 and CTGF, as well as the collagen synthesis triggered by oxidant stress, are abrogated through the blockade of angiotensin receptors [1,19,21].
Figure 1.
Known actions of angiotensin II on lung parenchymal cells. On lung fibroblasts, angiotensin II (ANGII) has profibrotic actions on growth factor expression, extracellular matrix synthesis and migration mediated through both AT1 and AT2 receptors. On lung vascular smooth muscle (VSM) cells, ANGII promotes vasoconstriction, growth factor expression and prostaglandin synthesis primarily through AT1 receptors. ANGII is proapoptotic for alveolar lung epithelial cell (AECs). The functions of AT2 receptors on VSM and AECs, if any, are currently unknown. See text for details and references.
On the other hand, transcriptional regulation and autocrine loop systems are believed to be involved in the molecular mechanisms by which ANGII exerts its functions in lung fibrosis. For example, an ANGII-TGF-β1 crosstalk has been identified in lung fibroblasts isolated from fibrotic human lung [1]. It has been demonstrated that the transition to the myofibroblast phenotype induced by TGF-β1 results in an increase of AGT and AT1 receptor expression [1,22,23]. Recent data from our laboratory have shown that TGF-β1-induced AGT expression is caused by an increased binding of two transcription factors involved in the pathogenesis of lung fibrosis, JunD and hypoxia-inducible factor (HIF)-1α, to an AGT promoter domain close to the transcription start site [23]. Earlier work revealed that constitutive expression of active TGF-β1 by human lung myofibroblasts was downregulated by ANGII receptor blockade and was accompanied by the inhibition of collagen synthesis [1]. Therefore, local upregulation of either angiotensin or TGF-β1 expression could induce the other (i.e., an autocrine loop), and the synergy between both systems should be inhibited in order to block the progression of lung fibrotic disease.
Recently Renzoni and coworkers [22] have described an additional effect of ANGII on ECM and human lung fibroblast cells; they have observed that the increased contractility of lung fibroblasts isolated from fibrotic lungs is dependent on angiotensin signalling. ANGII stimulated the contraction of floating collagen gels and α-smooth muscle actin expression by lung fibroblasts through activation of AT1 receptors. Consequently, in addition to exerting the mitogenic and regulatory effects, ANGII can also modify cytoskeletal function and the mechanical characteristics of human lung fibroblasts.
Little is known about the potential profibrotic actions of ANGII on other lung parenchymal cells such as vascular cells or macrophages or mast cells, in part because these cells have not yet been studied from the perspective of the local ANG system in the lungs, and also because the function(s) of these cells in lung fibrosis is poorly understood.
Angiotensin Signalling in Alveolar Epithelial Cell Survival
Apoptosis of alveolar epithelial cells (AECs) is now believed to be a critical event in the pathogenesis of pulmonary fibrosis. Support for this belief came first from animal models of lung fibrosis, in which experimental inhibition of apoptosis by either caspase inhibition [7] or deletion of genes necessary for epithelial apoptosis [24] decreased or abrogated the fibrotic response subsequent to lung injury. On the other hand, experimental induction of apoptosis primarily within AECs caused a subsequent fibrotic response, the severity of which was proportional to the severity of apoptosis induction [25]. More recently, apoptosis of AECs has become a consistent finding in biopsy specimens obtained from patients with Idiopathic Pulmonary Fibrosis [1,18].
In a variety of organs including the liver, pancreas and lungs, ANGII is known to be proapoptotic for epithelial cells [1]. In cultured lung AECs, ANGII induces apoptosis with an EC50 of about 10nM [26]; similarly, ANGII induces apoptosis of cultured endothelial cells with an EC50 of about 100nM [ibid]. These effective concentrations for apoptosis are significantly higher than the steady-state concentration of ANGII within the circulation, which rarely rises above 10pM, even in severe hypertension [1]; the higher levels of ANGII required for apoptosis induction may explain how the ANGII generated by the action of angiotensin converting enzyme (ACE) within the pulmonary vascular bed does not damage the organ in which it is generated. On the other hand, measurements of steady-state ANGII concentrations in the extravascular compartment suggest that tissue ANGII levels are likely orders of magnitude higher than those in the serum, due largely to the longer biological halflife of ANGII outside the vasculature.
In addition, evidence suggests that at least two cell types in the lung, AECs and myofibroblasts that emerge during wound healing, express ANGII de novo after lung injury. In cultured AECs, exposure to chemical toxins such as bleomycin or endogenous toxins such as Fas ligand or tumor necrosis factor (TNF)-alpha, all induce expression of AGT mRNA, AGT protein and the mature octapeptide ANGII derived from it [1]. In addition, the transition of normal lung fibroblasts to myofibroblasts upon exposure to TGF-β1 in vitro is accompanied by robust expression of AGT mRNA, protein and ANGII peptide [23]. The physiological relevance of these cell culture data is supported by the findings of AGT mRNA and immunoreactive ANG peptides in apoptotic AECs and myofibroblasts within lung sections obtained from bleomycin-injured rats [6] or biopsy specimens from patients with IPF [17]. Thus, two additional sources of local production of ANGII, at concentrations likely to be proapoptotic in the local microenvironment, are lung myofibroblasts and apoptotic AECs themselves.
Apoptosis of AECs in response to ANGII is mediated by the AT1 receptor; ANGII-induced cell loss and nuclear fragmentation in primary cultures of AECs could be blocked by the AT1-selective antagonists L-158809 but not by the AT2-selective blocker PD126055 [26]. Subsequent studies found that the AT1 selective blockers L-158809 or Losartan could also block bleomycin-induced nuclear fragmentation of cultured AECs; these data support the premise that autocrine production of ANGII mediates the apoptotic response of AECs to bleomycin [27]. When administered to mice, the AT1 blocker Losartan also significantly reduced bleomycin-induced caspase 3 activation, which supports the theory that this mechanism is also active in the intact lung in vivo.
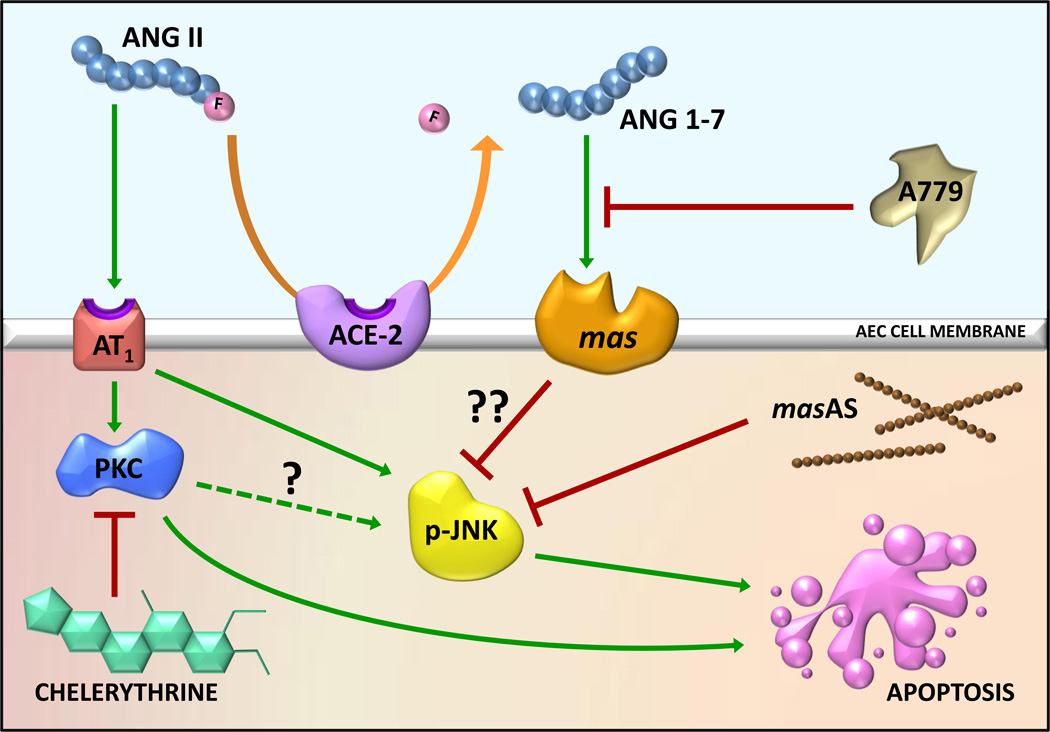
The apoptotic response of AECs to AT1 receptor activation appears to be mediated by protein kinase C (PKC, see Figure 2); the PKC inhibitor chelerythrine prevented ANGII-mediated apoptosis measured by nuclear fragmentation assay in primary cultures of rat alveolar epithelial cells [28]. Recent studies have shown that AECs also express angiotensin converting enzyme-2 (ACE-2), the homolog of ACE that cleaves a single amino acid from the c-terminal end of the octapeptide ANGII to form the heptapeptide ANG1-7 [1]. In nonpulmonary cell types, ANG1-7 binds to the specific ANG1-7 receptor mas and inhibits ANGII-induced activation of mitogen-activated protein kinases including JNK [4]. Lee et al. [29] demonstrated that JNK phosphorylation is required for apoptosis of AECs; the specific steps between PKC and p-JNK (?), if any, are currently unknown. Nonetheless, it was of high interest to determine if ANG1-7 could also inhibit AT1-induced apoptosis of AECs through the ANG1-7 receptor mas.
Figure 2.
Known actions of angiotensin II on alveolar epithelial cell survival. The octapeptide angiotensin (ANG)II induces apoptosis of alveolar epithelial cells (AECs) through the AT1 receptor; the protein kinase C (PKC) inhibitor chelerythrine blocked AEC apoptosis in response to ANGII. Moreover, the phosphorylation of JUN N-terminal Kinase (p-JNK) is a required event in AEC apoptosis in response to ANGII or bleomycin. Angiotensin converting enzyme-2 (ACE-2) acts as a survival factor by a) degrading the proapoptotic ANGII and b) producing the heptapeptide angiotensin1-7 (ANG1-7), which inhibits apoptosis by blocking JNK phosphorylation through the ANG1-7 receptor mas. Both JNK phosphorylation and apoptosis of AECs can be blocked by either the specific mas receptor antagonist A779 (D-Ala7-Ang1–7) or by antisense oligonucleotides against mas mRNA (masAS). The steps, if any, between PKC and p-JNK (?) are currently unknown. Similarly, the mechanism(s) by which mas activation inhibits JNK phosphorylation (??) in AECs are unknown but are currently being investigated. The intra-versus extracellular locations of each of the events described are currently unknown, but were depicted as shown on the basis of available data; whether ANGII accumulation, degradation to ANG1-7 and/or binding to AT1 or mas can occur intracellularly is presently unknown. See text for more detail.
In recent studies published elsewhere [30] we found that ANG1-7 could inhibit JNK phosphorylation in AECs exposed to either ANGII or bleomycin (Fig.2). ANG1-7 could also inhibit AEC apoptosis stimulated by either of these inducers, as measured by caspase-3 or -9 activation or by nuclear fragmentation assay. Moreover, the inhibition by ANG1-7 was exerted through the ANG1-7 receptor mas; this was shown by blockage of the inhibition with the specific mas receptor blocker A779 or with antisense nucleotides against mas mRNA [30]. The pathways by which activation of mas inhibits JNK phosphorylation (?? in Fig.2), and therefore apoptosis of AECs, are currently under investigation. Our current working hypothesis contends that activation of mas might induce a JNK phosphatase which, under normal conditions in which ANG1-7 is much more abundant than ANGII (see 30), constitutively dephosphorylates JNK as a cell survival mechanism. Evaluation of this working hypothesis and identification of which, if any, JNK phosphatases might be activated by mas is currently under investigation in our laboratory. The cellular locations of most of the event depicted in Figure 2 were drawn on the basis of the limited data currently available, but may ultimately be found to occur in intra- or extracellular locations other than those depicted.
Summary and Future Perspectives
A large body of literature has shown that angiotensin II (ANGII) stimulates expression of TGF-β1 and α1 collagen by lung fibroblasts. In the human fibrotic lung, ANGII is synthesized by lung myofibroblasts and dying lung epithelial cells. Cell culture and mouse studies have shown that ANGII receptor AT1 mediates alveolar epithelial apoptosis through JNK phosphorylation. Importantly, ANG1-7, the product of the counteregulatory enzyme ACE-2 that degrades ANGII, inhibits JNK phosphorylation and apoptosis of lung epithelial cells. Moreover, the specific ANG1-7 receptor mas mediates the antiapoptotic effects of ANG1-7. These data offer new hypotheses regarding the potential of ANG1-7, or mimicks of this peptide, in the control of apoptotic lung injury and fibrogenesis. Activators of mas or other inhibitors of JNK phosphorylation would be expected to block both lung epithelial apoptosis and the subsequent fibrotic response. Both these and related hypotheses will be interesting topics for future inquiry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Uhal BD, Kim JK, Li X, Molina-Molina M. Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages. Curr. Pharm. Des. 2007;13:1247–1256. doi: 10.2174/138161207780618885. [DOI] [PubMed] [Google Scholar]
- 2.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal. Immunol. 2009;2:103–121. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann. Intern. Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 4.Burrell LM, Johnston CI, Tikellis C, Cooper ME. ACE2, a new regulator of the renin-angiotensin system. Trends. Endocrinol. Metab. 2004;15:166–169. doi: 10.1016/j.tem.2004.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Shu R, Filippatos G, Uhal BD. Apoptosis in lung injury and remodeling. J. Appl. Physiol. 2004;97:1535–1542. doi: 10.1152/japplphysiol.00519.2004. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Zhuang J, Rayford H, Zhang H, Shu R, Uhal BD. Attenuation of bleomycin-induced pulmonary fibrosis by intratracheal administration of antisense oligonucleotides against angiotensinogen mRNA. Curr. Pharm. Des. 2007;13:1257–1268. doi: 10.2174/138161207780618867. [DOI] [PubMed] [Google Scholar]
- 7.Wang R, Ibarra-Sunga O, Verlinski L, Pick R, Uhal BD. Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:143–151. doi: 10.1152/ajplung.2000.279.1.L143. [DOI] [PubMed] [Google Scholar]
- 8.Molteni A, Wolfe LF, Ward WF, Ts'ao CH, Molteni LB, Veno P, Fish BL, Taylor JM, Quintanilla N, Herndon B, Moulder JE. Effect of an angiotensin II receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alpha-actomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr. Pharm. Des. 2007;13:1307–1316. doi: 10.2174/138161207780618777. [DOI] [PubMed] [Google Scholar]
- 9.Mohammadi-Karakani A, Ghazi-Khansari M, Sotoudeh M. Lisinopril ameliorates paraquat-induced lung fibrosis. Clin. Chim. Acta. 2006;367:170–174. doi: 10.1016/j.cca.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Meng Y, Meng Y, Li X, Cai SX, Tong WC, Cheng YX. Perindopril and losartan attenuate bleomycin A5-induced pulmonary fibrosis in rats. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:919–924. [PubMed] [Google Scholar]
- 11.Li JJ, Xue XD. Protection of captopril against chronic lung disease induced by hyperoxia in neonatal rats. Zhongguo Dang Dai Er Ke Za Zhi. 2007;9:169–173. [PubMed] [Google Scholar]
- 12.Otsuka M, Takahashi H, Shiratori M, Chiba H, Abe S. Reduction of bleomycin induced lung fibrosis by candesartan cilexetil, an angiotensin II type 1 receptor antagonist. Thorax. 2004;59:31–38. doi: 10.1136/thx.2003.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molteni A, Wolfe LF, Ward WF, Ts'ao CH, Molteni LB, Veno P, Fish BL, Taylor JM, Quintanilla N, Herndon B, Moulder JE. Effect of an angiotensin II receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alpha-actomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr. Pharm. Des. 2007;13:1307–1316. doi: 10.2174/138161207780618777. [DOI] [PubMed] [Google Scholar]
- 14.Chen N, Li JJ, Xue XD. Effect of losartan on lung fibrosis in neonatal rats with hyperoxia-induced chronic lung disease. Zhongguo Dang Dai Er Ke Za Zhi. 2007;9:591–594. [PubMed] [Google Scholar]
- 15.Li X, Molina-Molina M, Abdul-Hafez A, Uhal V, Xaubet A, Uhal BD. Angiotensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295:178–185. doi: 10.1152/ajplung.00009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shenoy V, Ferreira AJ, Qi Y, Fraga-Silva RA, Diez-Freire C, Dooies A, Jun JY, Sriramula S, Mariappan N, Pourang D, Venugopal CS, Francis J, Reudelhuber T, Santos RA, Patel JM, Raizada MK, Katovich MJ. The angiotensin-converting enzyme 2/angiogenesis-(1-7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2010;182:1065–1072. doi: 10.1164/rccm.200912-1840OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Molina-Molina M, Abdul-Hafez A, Ramirez J, Serrano-Mollar A, Xaubet A, Uhal BD. Extravascular sources of lung angiotensin peptide synthesis in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L887–L895. doi: 10.1152/ajplung.00432.2005. [DOI] [PubMed] [Google Scholar]
- 18.Königshoff M, Wilhelm A, Jahn A, Sedding D, Amarie OA, Eul B, Seeger W, Fink L, Günther A, Eickelberg O, Rose F. The angiotensin II receptor 2 is expressed and mediates angiotensin II signaling in lung fibrosis. Am. J. Respir. Cell Mol. Biol. 2007;37:640–650. doi: 10.1165/rcmb.2006-0379TR. [DOI] [PubMed] [Google Scholar]
- 19.Marshall RP, McAnulty RJ, Laurent GJ. Angiotensin II is mitogenic for human lung fibroblasts via activation of the type 1 receptor. Am. J. Respir. Crit. Care Med. 2000;161:1999-04. doi: 10.1164/ajrccm.161.6.9907004. [DOI] [PubMed] [Google Scholar]
- 20.Abdul-Hafez A, Shu R, Uhal BD. JunD and HIF-1α mediate transcriptional activation of angiotensinogen by TGF-β1 in human lung fibroblasts. FASEB J. 2009;23:1655–1662. doi: 10.1096/fj.08-114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain A, Wyatt AW, Wang K, Bhandaru M, Biswas R, Avram D, Föller M, Rexhepaj R, Friedrich B, Ullrich S, Müller G, Kuhl D, Risler T, Lang F. SGK1-dependent upregulation of connective tissue growth factor by angiotensin II. Kidney Blood Press. Res. 2008;31:80–86. doi: 10.1159/000119703. [DOI] [PubMed] [Google Scholar]
- 22.Renzoni EA, D, Abraham J, Howat S, Shi-Wen X, Sestini P, Bou-Charios G, Wells AU, Veeraraghavan S, Nicholson AG, Denton CP, Leask A, Pearson JD, Black CM, Welsh KI, du Bois RM. Gene expression profiling reveals novel TGFβ targets in adult lung fibroblasts. Respiratory Research. 2004;5:24. doi: 10.1186/1465-9921-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdul-Hafez A, Shu R, Uhal BD. JunD and HIF-1α mediate transcriptional activation of angiotensinogen by TGF-β1 in human lung fibroblasts. FASEB J. 2009;23:1655–1662. doi: 10.1096/fj.08-114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budinger GR, Mutlu GM, Eisenbart J, Fuller AC, Bellmeyer AA, Baker CM, Wilson M, Ridge K, Barrett TA, Lee VY, Chandel NS. Proapoptotic Bid is required for pulmonary fibrosis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4604–4609. doi: 10.1073/pnas.0507604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagimoto N, Kuwano K, Miyazaki H, Kunitake R, Fujita M, Kawasaki M, Kanika Y, Hara N. Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of FAS antigen. Am. J. Respir. Cell Mol. Biol. 1997;17:272–278. doi: 10.1165/ajrcmb.17.3.2893. [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Zagariya A, Ibarra-Sunga O, Gidea C, Ang E, Deshmukh S, Chaudhary G, Baraboutis J, Filippatos G, Uhal BD. Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am. J. Physiol. 1999;276:L885–L889. doi: 10.1152/ajplung.1999.276.5.L885. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Zhang H, Soledad-Conrad V, Zhuang J, Uhal BD. Bleomycin-induced apoptosis of alveolar epithelial cells requires angiotensin synthesis de novo. Am. J. Physiol. 2003;284:L501–L507. doi: 10.1152/ajplung.00273.2002. [DOI] [PubMed] [Google Scholar]
- 28.Papp M, Li X, Zhuang J, Wang R, Uhal BD. Angiotensin receptor subtype AT1 mediates alveolar epithelial cell apoptosis in response to ANGII. Am. J. Physiol. 2002;282:L713–L718. doi: 10.1152/ajplung.00103.2001. [DOI] [PubMed] [Google Scholar]
- 29.Lee VY, Schroedl C, Brunelle JK, Buccellato LJ, Akinci OI, Kaneto H, Snyder C, Eisenbart J, Budinger GR, Chandel NS. Bleomycin induces alveolar epithelial cell death through JNK-dependent activation of the mitochondrial death pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L521–L528. doi: 10.1152/ajplung.00340.2004. [DOI] [PubMed] [Google Scholar]
- 30.Uhal BD, Li X, Xue A, Gao X, Abdul-Hafez A. Regulation Of Alveolar Epithelial Cell Survival By The ACE-2/Angiotensin 1-7/Mas Axis. Am J Physiol. 2011;301:L269–L274. doi: 10.1152/ajplung.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]