Abstract
BACKGROUND
We recently reported that aldosterone-induced cellular senescence via an increase in p21, a cyclin-dependent kinase (CDK) inhibitor, in rat kidney and cultured human proximal tubular cells. In the present study, we investigated the contribution of aldosterone to the renal p21 expression and senescence during the development of angiotensin II (AngII)-induced hypertension.
METHODS
Mice received 1% salt in drinking water and vehicle or AngII, and were divided into five groups: 1, vehicle; 2, AngII; 3, AngII+olmesartan; 4, AngII+eplerenone; and 5, AngII+hydralazine.
RESULTS
Plasma aldosterone levels were increased by AngII infusion. Eplerenone further elevated the plasma aldosterone level, but olmesartan and hydralazine did not. AngII group showed significant increase in blood pressure compared to vehicle. Olmesartan and hydralazine, but not eplerenone, suppressed the AngII-salt hypertension. The increase in urinary protein excretion by AngII-salt was suppressed only by olmesartan. AngII with high salt induced a greater expression of p21 mRNA in the kidney than vehicle. Olmesartan abolished the increase in p21 expression, whereas neither eplerenone nor hydralazine affected it. AngII with high salt did not change the expression of p16, another CDK inhibitor. The mice lacking p21 showed identical changes on blood pressure and albuminuria in response to AngII with high salt compared to wild type.
CONCLUSION
These results suggest that aldosterone does not predominantly contribute to renal p21 expression and senescence during the development of AngII-salt hypertension, and that the increase in p21 in the kidney is not likely involved in the development of hypertension and albuminuria.
Keywords: aldosterone, angiotensin II, blood pressure, hypertension, p21
Cell senescence is characterized by irreversible growth arrest, which is regulated by telomerase, cyclin, cyclin-dependent kinase (CDK) and CDK inhibitors, such as p21 that inhibits cyclin–CDK2 or –CDK4 interaction,1 and is known as one of the fates of in vitro-cultured cells.2,3 Considerable studies currently have demonstrated that senescent cells could be detected in vivo even if animals are relatively young,4–6 and the in vivo cell senescence is implicated in the development of end organ disease.
Angiotensin II (AngII) is a vasoactive peptide that induces many (patho)physiological responses, such as vasoconstriction, sodium reabsorption, and inflammation. One of the major physiological roles of AngII is to stimulate the synthesis of aldosterone in the zona glomerulosa of the adrenal gland. Several studies have reported that the secreted endogenous aldosterone plays a role in the renal (patho)physiology in high AngII models.7–10 Ren2 transgenic rat model, a model that has high AngII levels, showed a significant increase in the number of kidney cells expressing p16, another CDK inhibitor that inhibits cyclin–CDK4 and –CDK6 interaction,1 and that the upregulation of p16 was attenuated by a hypotensive dose of an angiotensin type 1 (AT1) receptor antagonist.11 However, at this time, there is no direct in vivo evidence that AngII per se contributes to cell senescence in the kidney.
We recently demonstrated that aldosterone/mineralocorticoid receptor (MR) stimulation induced reactive oxygen species/SIRT1/p53/p21, but not blood pressure-dependent pathways in the proximal tubular cells of the kidney.12 This study also revealed that cellular senescence decreased the innate ability of tubules to protect against pathological factors and accelerated inflammatory and fibrotic factors via p21. Likewise, a recent report showed that the expression of p16 was increased in the kidneys of deoxycorticosterone acetate-salt-induced hypertensive rats.11 These studies suggest that MRs stimulated by exogenously injected ligands, such as aldosterone and deoxycorticosterone acetate, induced CDK inhibitors upregulation and cell senescence in the kidney.
Taken together, we hypothesized that the increases in endogenous aldosterone levels induce renal cell senescence during the development of AngII-salt-dependent hypertension. To address this hypothesis, we chronically infused AngII into mice receiving high salt in their drinking water and treated mice with eplerenone to block the aldosterone/MR interaction, olmesartan as an AT1 antagonist, or hydralazine to eliminate the contribution of high blood pressure, and evaluated the renal senescence.
METHODS
Animal preparation
All experimental procedures were performed under the guidelines for the care and use of animals established by Kagawa University (Kagawa, Japan). The experiments were performed on male C57Bl/6J mice (CLEA, Tokyo, Japan). The 6-week-old C57Bl/6J mice weighing 20–23 g were randomly divided into the following five groups and were maintained throughout the 6-week experimental period: group 1, vehicle (saline, subcutaneous (s.c.), n = 10); group 2, AngII (20 ng/min,13,14 s.c., n = 9; Sigma, St Louis, MO); group 3, AngII + olmesartan (7.2 mg/kg/day, p.o., n = 10); group 4, AngII + eplerenone (250 mg/kg/day, p.o., n = 9); group 5, AngII + hydralazine (50 mg/kg/day, p.o., n = 9). All groups received 1% NaCl in their drinking water throughout the experimental period. Mice were anesthetized with isoflurane and osmotic minipumps were implanted s.c. at the dorsum of the neck to infuse the vehicle or AngII. The doses of drugs were determined based on results from previous studies.12,15–18
Systolic blood pressure was measured in the conscious state by tail-cuff plethysmography (BP-98A; Softron, Tokyo, Japan) at weeks 0, 2, 4, and 6. Twenty-four-hour urine samples were collected starting after a 24-h acclimatization period in their metabolic cages at week 6. Mice were killed by an excessive dose of sodium pentobarbital. Kidneys were perfused by chilled sterile phosphate-buffered saline solution, and frozen in Tissue-Tek O.C.T. compound (Sakura Finetechnical, Tokyo, Japan) for senescence-associated β-galactosidase (SABG) staining.12 The remaining kidneys were snap-frozen in liquid nitrogen and stored at −80 °C until mRNA was extracted.
In a separate set of experiments, male 6-week-old p21-knockout (p21-KO) mice and wild-type control littermates (Jackson Laboratory, Bar Harbor, ME) were infused with AngII (20 ng/min, s.c., n = 3, respectively) or vehicle (n = 3, respectively) through the minipump. Mice were given 1% NaCl in their drinking water. Their blood pressures were measured by a telemetry system (see below). Twenty-four-hour urine samples were collected starting after a 24-h acclimatization period in their metabolic cages at week 3.
SABG staining
Kidney cryosections (16 μm) were washed by phosphate-buffered saline and fixed by 0.5% glutaraldehyde for 15 min. After that, the sections were immersed into β-gal staining solutions (pH 6.0) containing 1 mg/ml 5-bromo-4-chloro-3-indlyl β-D-galactopyranoside, 5 mmol/l potassium ferrocyanide, 150 mmol/l NaCl, 2 mmol/l MgCl2, 0.01% sodium deoxycholate, and 0.02% Nonidet-40 for 12 h at 37 °C.5,19,20 Kidney sections from a 14-month-old mouse were used as a positive control for staining.20
Real-time reverse transcription-PCR
The mRNA expressions of β-actin, p53, p21, p16, and SIRT1 were analyzed by real-time reverse transcription-PCR using a LightCycler FastStart DNA Master SYBR Green I kit (Applied Biosystems, Foster City, CA).21 Briefly, complementary DNA was initially denatured at 95 °C for 30 s, and then amplified by PCR for 40 cycles (95 °C for 15 s, 60 °C for 40 s).21 The oligonucleotide primer sequences of β-actin, p53, p21, and SIRT1 were described previously.12 The primer sequences for p16 were: p16 forward, 5′-CACTTCTCACCTCGCTTGTCACA-3′, reverse, 5′-CCAAGAACCTGCGACCCATGCT-3′. All data are expressed as relative differences to vehicle-infused rats after normalization for β-actin expression.
Telemetry blood pressure measurements
Telemetry transmitters (Data Sciences, St Paul, MN) were implanted according to the manufacturer’s specifications into male p21-KO and wild-type mice while under isoflurane anesthesia. In brief, the right carotid artery was briefly occluded to allow insertion of the transmitter catheter. The catheter was tied by a 6–0 silk suture. The transmitter body (TA11PA-C10) was placed at the s.c. space between the skin and left abdominal muscle. Mice were allowed to recover from surgery and returned to individual housing for at least 1 week before initiation of data acquisition. Mice were given 3 mmol/l of acetaminophen in their drinking water for 3 days after surgery. The individual mice cages were placed on top of the telemetry receivers and arterial pressure waveforms were continuously recorded throughout the 3-week experimental period.
Other analyses
Urinary protein and albumin excretion were determined using commercially available assay kits (microTP-test; Wako, Osaka, Japan, and ELISA kit; Shibayagi, Gunma, Japan, respectively). Plasma potassium concentration was measured by an electrode in a Hitachi automatic analyzer (7020; Hitachi, Tokyo, Japan). Plasma aldosterone concentration was analyzed by a commercially available kit (SPACK-S aldosterone kit; TFB, Tokyo, Japan) while creatinine concentrations were measured using colorimetric Jaffé assay kits (Creatinine-test; Wako).
Statistical analysis
Results are expressed as means ± s.e. Statistical significance was assessed using analysis of variance, followed by the Turkey–Kramer test. A value of P < 0.05 was considered statistically significant.
RESULTS
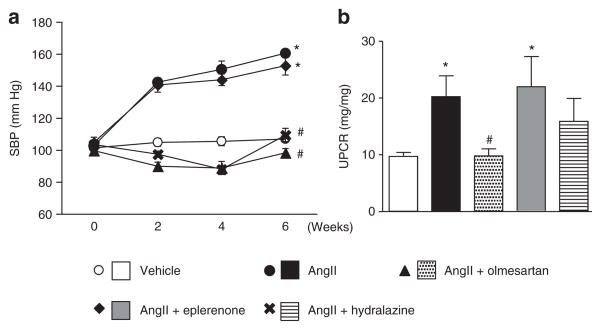
AngII-salt treatment increased the plasma aldosterone level 1.7-fold compared to vehicle infusion (vehicle: 273 ± 36 pg/ml, AngII: 461 ± 79 pg/ml, P < 0.05), indicating that the dosage of AngII was appropriate to increase endogenous aldosterone secretion. The plasma aldosterone levels in AngII-salt-treated mice were similar with those of aldosterone-salt-treated rats (Supplementary Figure S1 online), an animal model that have been shown the renal cell senescence.12 Neither olmesartan nor hydralazine affected the increased plasma aldosterone level in AngII-salt-treated mice (476 ± 61 and 417 ± 107 pg/ml, respectively). On the other hand, eplerenone further increased the plasma aldosterone level (1,808 ± 259 pg/ml, P < 0.001), indicating that eplerenone competed with aldosterone on MRs. AngII-salt treatment significantly increased blood pressure compared to vehicle infusion (Figure 1a). Treatment with olmesartan and hydralazine prevented the blood pressure elevation in AngII-salt-treated mice whereas eplerenone demonstrated only a limited effect on blood pressure. There was no difference in plasma potassium levels between the groups at week 6 (data not shown). The urinary protein excretion at week 6 was significantly greater in untreated AngII-salt-treated animals (Figure 1b). Treatment with olmesartan abolished the increase in proteinuria in AngII-salt-treated mice, whereas treatment with eplerenone did not affect the protein excretion into urine. Hydralazine tended to suppress the proteinuria but the difference did not reach statistical significance.
Figure 1.
Systolic blood pressure (SBP) and urinary protein/creatinine ratio (UPCR). (a) Angiotensin II (AngII)-salt treatment elevated the blood pressure in mice. The hypertension was suppressed by olmesartan or hydralazine, but not by eplerenone. (b) Protein excretion in the urine was increased by AngII-salt. The increase in proteinuria was suppressed by olmesartan, but not by eplerenone or hydralazine. *P < 0.05 vs. vehicle, #P < 0.05 vs. AngII.
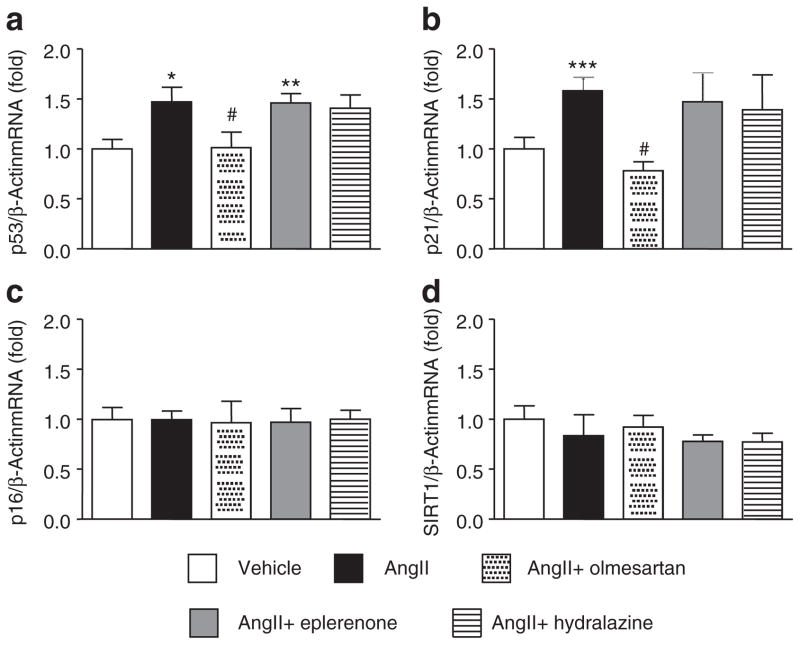
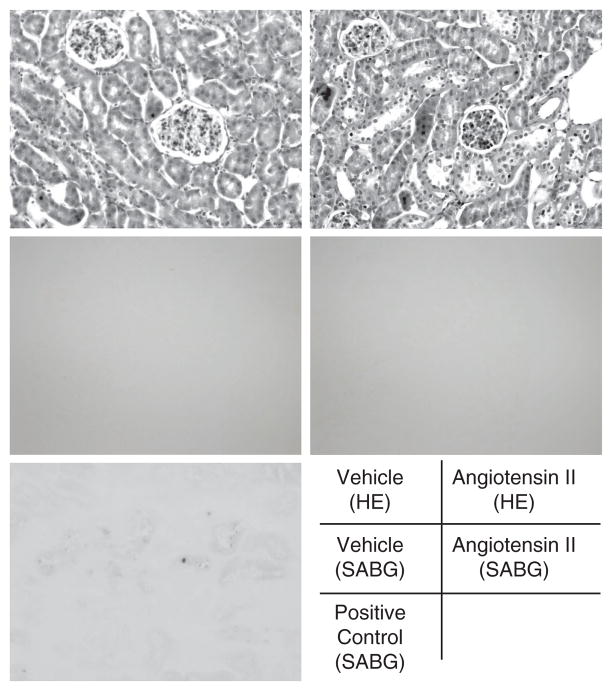
The expression of senescence-associated molecules was measured in the kidneys of animals at week 6. AngII-salt treatment increased the expression of p53 and p21 in the kidneys (Figure 2a,b). Treatment with olmesartan normalized this increased expression of p53 and p21, but neither eplerenone nor hydralazine affected the AngII-salt-induced increase in the expression of renal p53 and p21. None of the treatments changed the expression of p16 or SIRT1 in the kidneys (Figure 2c,d). Since two molecules that are associated with cell cycle arrest were increased by AngII-salt treatment, we next evaluated the cell senescence in the kidney of AngII-salt-treated mice by using SABG staining. However, we failed to detect any SABG-positive blue staining in any section of AngII-salt-treated animals (Figure 3), whereas there was positive staining in the kidney section of the 14-month old mouse.
Figure 2.
mRNA expression of senescence-associated genes. Angiotensin II (AngII)-salt treatment (a) upregulated p53 and (b) p21 in the kidney. Neither eplerenone nor hydralazine affected these increased p53 and p21 expression levels. Olmesartan inhibited the increase in p53 and p21 by AngII-salt. There was no effect of any treatment on (c) p16 and (d) SIRT1 expression in the kidney. *P < 0.05 vs. vehicle, #P < 0.05 vs. AngII.
Figure 3.
Senescence-associated β-galactosidase (SABG) staining. Angiotensin II (AngII)-salt treatment did not augment the staining for cell senescence. The kidney cryosections of a 14-month-old mouse were used as a positive control. Separated kidney sections were stained with hematoxylin–eosin (HE: top two pictures).
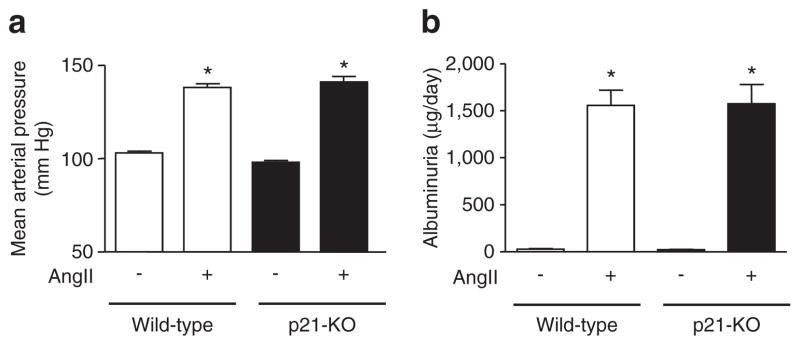
Since AngII-salt treatment increased the renal p21 expression level and olmesartan suppressed it, we next analyzed the role of p21 in the AngII-salt-treated mice. The mice lacking functional p21 were infused with AngII for 3 weeks and the changes in blood pressure and albuminuria were measured. The p21-KO animals showed identical changes in blood pressure (Figure 4a), pulse pressure, and diurnal deviation of blood pressure (data not shown) in response to AngII-salt treatment compared to wild-type animals. The albuminuria at week 3 after AngII-salt treatment was almost at the same level between wild-type and p21-KO mice (Figure 4b). The expression of p21 in the kidney was significantly increased by 3-week AngII-salt treatment (vehicle; 1.00 ± 0.25-fold, and AngII: 2.79 ± 0.25-fold, P≤0.01) in wild-type mice.
Figure 4.
Blood pressure and albuminuria in p21-knockout (KO) mice. The hypertensive responses to angiotensin II (AngII)-salt for 3 weeks were similar between (a) wild-type and p21-KO mice. (b) AngII-salt treatment exaggerated the urinary excretion of albumin at week 3, whereas there was no difference in the responses to AngII-salt between wild-type and p21-KO mice. *P < 0.05 vs. vehicle, #P < 0.05 vs. AngII.
DISCUSSION
AngII itself induces various physiological responses. However, a part of the effect by AngII is mediated through aldosterone secreted from the adrenal zona glomerulosa.7–10 In the present study, the plasma aldosterone levels after 6 weeks of AngII-salt treatment almost doubled compared to vehicle-infused mice and was at the similar level with that of aldosterone-salt rats. Eplerenone further increased the plasma aldosterone levels in mice, indicating that the dosages of AngII and eplerenone were sufficient for the purpose of the study (to increase endogenous aldosterone secretion and to block MR). However, eplerenone, even with 2.5 times higher dosage than that in our previous study,12 only affected the plasma aldosterone level and did not have any effect on the other parameters in the present study (blood pressure, plasma potassium, proteinuria, and senescence-associated markers). Olmesartan suppressed all changes induced by AngII-salt treatment. These data suggest that AngII/AT1 receptors, but not aldosterone/MRs, play a predominant role on the hypertension, proteinuria, and kidney p53 and p21 pathway during the development of AngII-salt hypertension in mice. The phenotypic changes of the kidney in response to AngII-salt and subsequent renal p53 and p21 upregulation are still unclear. However, our data suggest that renal p21 does not contribute to the AngII-salt-induced increase in blood pressure and albumin excretion because the responses to AngII-salt in p21-KO mice were identical with those in wild-type mice.
We have previously reported that exogenously infused aldosterone caused an increase in senescence in the kidney of rats and in human proximal tubular cells,12 which was mediated through the p21-dependent pathway. Also, there are several studies showing that AngII-induced cell senescence in the vessels in in vivo and in vitro studies.22–24 These studies proposed that AngII-induced vascular senescnece is involved in the hypertrophy, inflammation and atherosclerosis of the kidneys. Since the kidney is also a “vasculature” organ, we expected that AngII and the subsequent increase in aldosterone would induce cellular senescence in the tubules as well as the small vessels in the kidney. However, in the present study, we failed to detect any increase in SABG staining despite the increase in endogenous aldosterone levels and kidney p21 expression in mice. Because, to our knowledge, there is no more sensitive and generally accepted marker of senescence than SABG at this time, we conclude that neither AngII, at least by current dosage with 1% salt in the drinking water for 6 weeks, nor aldosterone increases the number of senescent cells in the murine kidney. We do not yet have any evidence to explain this dissociation between the present study and other previous studies including a study of ours except that the species difference (mouse vs. rat and human) may be one of the reasons for this contradiction. In general, mice, and in particular the C57Bl/6 strain, have very high resistance to mineralocorticoid compared to rats.25,26 Eplerenone prevented the development of proteinuria in AngII-infused rats,10 indicating the contribution of endogenous aldosterone on renal injury in rat; however, again, we did not observe any renoprotection in mice even by using 2.5 times greater dosage of eplerenone, indicating that there is a difference in the signal/damage response between the species. Indeed, the changes in the expression of senescence-associated molecules are different between the previous studies (rat and human) and the present study (mouse). Our previous study demonstrated that aldosterone/MR decreased SIRT1 and acetylated p53, and increased p21 and senescent cells.12 In the present study, we did not detect any changes in SIRT in the murine kidney. Likewise, Westhoff et al.11 reported that mRen2 transgenic rats, a model with high AngII and aldosterone,27,28 showed an increase in p16 in the kidney and that this increase was suppressed by losartan, another AT1 receptor antagonist. Again, we did not detect any changes in p16 in the kidney of mice. Taken together, it appears that there are differences in signaling, except p21, in the kidney in response to AngII or aldosterone between mice and rats. Another possibility is that AngII induces kidney cell senescence and that the senescent cells became undetectable because of apoptosis and washout by inflammatory cells since AngII induces apoptosis and inflammation.29–33 The relationship among cell senescence, apoptosis, inflammation, and renal lesions, such as fibrosis and albuminuria, should be determined in future studies.
In summary, although exogenous aldosterone-induced renal cell senescence in rat and human proximal tubules,12 endogenous aldosterone did not contribute to renal p21 expression and senescence during the development of AngII-salt hypertension in mice. AngII/AT1 receptor stimulation changed kidney p21 expression independent of either aldosterone or high blood pressure, but the changes were not involved in albumin excretion in the urine of mice. Future studies will determine the role of renal p21 upregulation by AngII.
Supplementary Material
Acknowledgments
We are grateful to Daiichi-Sankyo Inc. for supplying olmesartan. This work was supported in part by the fund for Kagawa University Young Scientists 2010 (to D.N.), and a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (20590253).
Footnotes
Supplementary material is linked to the online version of the paper at http://www.nature.com/ajh
Disclosure: The authors declared no conflict of interest.
References
- 1.Modiano JF, Ritt MG, Wojcieszyn J. The molecular basis of canine melanoma: pathogenesis and trends in diagnosis and therapy. J Vet Intern Med. 1999;13:163–174. doi: 10.1892/0891-6640(1999)013<0163:tmbocm>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 3.Hayflick L. Living forever and dying in the attempt. Exp Gerontol. 2003;38:1231–1241. doi: 10.1016/j.exger.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Nakano M, Oenzil F, Mizuno T, Gotoh S. Age-related changes in the lipofuscin accumulation of brain and heart. Gerontology. 1995;41 (Suppl 2):69–79. doi: 10.1159/000213726. [DOI] [PubMed] [Google Scholar]
- 5.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minamino T, Komuro I. Vascular aging: insights from studies on cellular senescence, stem cell aging, and progeroid syndromes. Nat Clin Pract Cardiovasc Med. 2008;5:637–648. doi: 10.1038/ncpcardio1324. [DOI] [PubMed] [Google Scholar]
- 7.Rocha R, Stier CT, Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141:3871–3878. doi: 10.1210/endo.141.10.7711. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz RM, Graciano ML, Seth D, Awayda MS, Navar LG. Aldosterone receptor antagonism exacerbates intrarenal angiotensin II augmentation in ANG II-dependent hypertension. Am J Physiol Renal Physiol. 2007;293:F139–F147. doi: 10.1152/ajprenal.00504.2006. [DOI] [PubMed] [Google Scholar]
- 9.Ortiz RM, Graciano ML, Mullins JJ, Mitchell KD. Aldosterone receptor antagonism alleviates proteinuria, but not malignant hypertension, in Cyp1a1-Ren2 transgenic rats. Am J Physiol Renal Physiol. 2007;293:F1584–F1591. doi: 10.1152/ajprenal.00124.2007. [DOI] [PubMed] [Google Scholar]
- 10.Ortiz RM, Mamalis A, Navar LG. Aldosterone Receptor Antagonism Reduces Urinary C-Reactive Protein Excretion in Angiotensin II-Infused, Hypertensive Rats. J Am Soc Hypertens. 2009;3:184–191. doi: 10.1016/j.jash.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westhoff JH, Hilgers KF, Steinbach MP, Hartner A, Klanke B, Amann K, Melk A. Hypertension induces somatic cellular senescence in rats and humans by induction of cell cycle inhibitor p16INK4a. Hypertension. 2008;52:123–129. doi: 10.1161/HYPERTENSIONAHA.107.099432. [DOI] [PubMed] [Google Scholar]
- 12.Fan YY, Kohno M, Hitomi H, Kitada K, Fujisawa Y, Yatabe J, Yatabe M, Felder RA, Ohsaki H, Rafiq K, Sherajee SJ, Noma T, Nishiyama A, Nakano D. Aldosterone/Mineralocorticoid receptor stimulation induces cellular senescence in the kidney. Endocrinology. 2011;152:680–688. doi: 10.1210/en.2010-0829. [DOI] [PubMed] [Google Scholar]
- 13.Nobuhiko A, Suganuma E, Babaev VR, Fogo A, Swift LL, Linton MF, Fazio S, Ichikawa I, Kon V. Angiotensin II amplifies macrophage-driven atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2143–2148. doi: 10.1161/01.ATV.0000145607.03879.e0. [DOI] [PubMed] [Google Scholar]
- 14.Izumiya Y, Kim S, Izumi Y, Yoshida K, Yoshiyama M, Matsuzawa A, Ichijo H, Iwao H. Apoptosis signal-regulating kinase 1 plays a pivotal role in angiotensin II-induced cardiac hypertrophy and remodeling. Circ Res. 2003;93:874–883. doi: 10.1161/01.RES.0000100665.67510.F5. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004;43:841–848. doi: 10.1161/01.HYP.0000118519.66430.22. [DOI] [PubMed] [Google Scholar]
- 16.Nishiyama A, Yoshizumi M, Hitomi H, Kagami S, Kondo S, Miyatake A, Fukunaga M, Tamaki T, Kiyomoto H, Kohno M, Shokoji T, Kimura S, Abe Y. The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats. J Am Soc Nephrol. 2004;15:306–315. doi: 10.1097/01.asn.0000108523.02100.e0. [DOI] [PubMed] [Google Scholar]
- 17.Koh PJ, Koitka A, Cooper ME, Allen TJ. Eplerenone does not attenuate diabetes-associated atherosclerosis. J Hypertens. 2009;27:1431–1438. doi: 10.1097/HJH.0b013e32832bd284. [DOI] [PubMed] [Google Scholar]
- 18.Deuchar GA, McLean D, Hadoke PW, Brownstein DG, Webb DJ, Mullins JJ, Chapman K, Seckl JR, Kotelevtsev YV. 11β-hydroxysteroid dehydrogenase type 2 deficiency accelerates atherogenesis and causes proinflammatory changes in the endothelium in apoe−/− mice. Endocrinology. 2011;152:236–246. doi: 10.1210/en.2010-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melk A, Kittikowit W, Sandhu I, Halloran KM, Grimm P, Schmidt BM, Halloran PF. Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int. 2003;63:2134–2143. doi: 10.1046/j.1523-1755.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 21.Du J, Fan YY, Hitomi H, Kiyomoto H, Kimura S, Kong CZ, Noma T, Kohno M, Nishiyama A, Nakano D. Mineralocorticoid receptor blockade and calcium channel blockade have different renoprotective effects on glomerular and interstitial injury in rats. Am J Physiol Renal Physiol. 2009;297:F802–F808. doi: 10.1152/ajprenal.00197.2009. [DOI] [PubMed] [Google Scholar]
- 22.Kunieda T, Minamino T, Nishi J, Tateno K, Oyama T, Katsuno T, Miyauchi H, Orimo M, Okada S, Takamura M, Nagai T, Kaneko S, Komuro I. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–960. doi: 10.1161/CIRCULATIONAHA.106.626606. [DOI] [PubMed] [Google Scholar]
- 23.Min LJ, Mogi M, Iwanami J, Li JM, Sakata A, Fujita T, Tsukuda K, Iwai M, Horiuchi M. Cross-talk between aldosterone and angiotensin II in vascular smooth muscle cell senescence. Cardiovasc Res. 2007;76:506–516. doi: 10.1016/j.cardiores.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Min LJ, Mogi M, Iwanami J, Li JM, Sakata A, Fujita T, Tsukuda K, Iwai M, Horiuchi M. Angiotensin II type 2 receptor deletion enhances vascular senescence by methyl methanesulfonate sensitive 2 inhibition. Hypertension. 2008;51:1339–1344. doi: 10.1161/HYPERTENSIONAHA.107.105692. [DOI] [PubMed] [Google Scholar]
- 25.Nakano D, Itoh C, Ishii F, Kawanishi H, Takaoka M, Kiso Y, Tsuruoka N, Tanaka T, Matsumura Y. Effects of sesamin on aortic oxidative stress and endothelial dysfunction in deoxycorticosterone acetate-salt hypertensive rats. Biol Pharm Bull. 2003;26:1701–1705. doi: 10.1248/bpb.26.1701. [DOI] [PubMed] [Google Scholar]
- 26.Nakano D, Kwak CJ, Fujii K, Ikemura K, Satake A, Ohkita M, Takaoka M, Ono Y, Nakai M, Tomimori N, Kiso Y, Matsumura Y. Sesamin metabolites induce an endothelial nitric oxide-dependent vasorelaxation through their antioxidative property-independent mechanisms: possible involvement of the metabolites in the antihypertensive effect of sesamin. J Pharmacol Exp Ther. 2006;318:328–335. doi: 10.1124/jpet.105.100149. [DOI] [PubMed] [Google Scholar]
- 27.Whaley-Connell A, Habibi J, Wei Y, Gutweiler A, Jellison J, Wiedmeyer CE, Ferrario CM, Sowers JR. Mineralocorticoid receptor antagonism attenuates glomerular filtration barrier remodeling in the transgenic Ren2 rat. Am J Physiol Renal Physiol. 2009;296:F1013–F1022. doi: 10.1152/ajprenal.90646.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lastra G, Habibi J, Whaley-Connell AT, Manrique C, Hayden MR, Rehmer J, Patel K, Ferrario C, Sowers JR. Direct renin inhibition improves systemic insulin resistance and skeletal muscle glucose transport in a transgenic rodent model of tissue renin overexpression. Endocrinology. 2009;150:2561–2568. doi: 10.1210/en.2008-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie MJ, Chang H, Wang YY, Zhang L, Song Z, Guo WG, Wang T, Che HL, Yu ZB. Evidence that apoptotic signalling in hypertrophic cardiomyocytes is determined by mitochondrial pathways involving protein kinase Cd. Clin Exp Pharmacol Physiol. 2010;37:1120–1128. doi: 10.1111/j.1440-1681.2010.05447.x. [DOI] [PubMed] [Google Scholar]
- 30.Morisada N, Nomura M, Nishii H, Furuno Y, Sakanashi M, Sabanai K, Toyohira Y, Ueno S, Watanabe S, Tamura M, Matsumoto T, Tanimoto A, Sasaguri Y, Shimokawa H, Kusuhara K, Yanagihara N, Shirahata A, Tsutsui M. Complete disruption of all nitric oxide synthase genes causes markedly accelerated renal lesion formation following unilateral ureteral obstruction in mice in vivo. J Pharmacol Sci. 2010;114:379–389. doi: 10.1254/jphs.10143fp. [DOI] [PubMed] [Google Scholar]
- 31.Hale TM, Bushfield TL, Woolard J, Pang JJ, Rees-Milton KJ, Adams MA. Changes critical to persistent lowering of arterial pressure in spontaneously hypertensive rat occur early in antihypertensive treatment. J Hypertens. 2011;29:113–122. doi: 10.1097/HJH.0b013e32833fb7cb. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto C, Fukuda N, Jumabay M, Saito K, Matsumoto T, Ueno T, Soma M, Matsumoto K, Shimosawa T. Protective effects of statin on cardiac fibrosis and apoptosis in adrenomedullin-knockout mice treated with angiotensin II and high salt loading. Hypertens Res. 2011;34:348–353. doi: 10.1038/hr.2010.243. [DOI] [PubMed] [Google Scholar]
- 33.Fabris B, Candido R, Bortoletto M, Toffoli B, Bernardi S, Stebel M, Bardelli M, Zentilin L, Giacca M, Carretta R. Stimulation of cardiac apoptosis in ovariectomized hypertensive rats: potential role of the renin-angiotensin system. J Hypertens. 2011;29:273–281. doi: 10.1097/HJH.0b013e328340d0d3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.