Abstract
Epstein-Barr virus (EBV) infection leads to Hodgkin’s disease (HD) in some immunocompetent hosts. The malignant Reed-Sternberg cells of HD only express a limited array of subdominant EBV antigens to evade preexisting immune responses to EBV. The EBV-encoded latent membrane proteins (LMP1 and LMP2), which are expressed by HD and various EBV-associated malignancies, have been proposed as a potential target for CTL-based therapy. However, the precursor frequency for LMP-specific CTL is generally low in healthy EBV-infected hosts, and immunotherapy based on these antigens is often compromised by the poor immunogenicity and the oncogenic potential. In the present study, we report that transitively expressing an inhibitor of A20, a key negative regulator of inflammatory signaling pathways, together with the LMP antigens (truncated LMP1 and full-length LMP2) greatly enhances maturation and cytokine production of human (h) monocyte-derived dendritic cells (DCs). As a consequence, LMP1/2-expressed, A20-silenced hDCs have an enhanced potency to prime LMP-specific T cell response. When the in vitro primed T cells are adoptively transferred into tumor-xenografted, severe combined immunodeficient (SCID) mice, some of the xenografted tumors approach complete regression. Thus, the study may provide an available resource of LMP-specific T cells for T cell immunotherapy.
Keywords: A20, dendritic cells, cytotoxic T lymphocyte, Epstein-Barr virus, latent membrane proteins
Introduction
DCs are professional antigen-presenting cells (APCs) with key regulatory roles in maintaining tolerance to self-antigens and in activating innate and adaptive immunity1. These cells express abundant pattern-recognition receptors (PRR) to recognize conserved microbial structures such as lipopolysaccharide (LPS), unmethylated bacterial DNA (CpG), and RNA2–4 for activating NF-kB and other signaling pathways2–3. NF-kB activation promotes APCs to express higher levels of costimulatory molecules and proinflammatory cytokines, and to present MHC-restricted peptides to T cells for inducing both primary and secondary immune responses2–3. Regulation of NF-kB signaling is critical, not only for APCs to stimulate immune responses to viral and bacterial infections, but also to avoid inducing an immune response to self-tissue, including tumor tissue. There is now substantial evidence that DCs are equipped with key regulatory mechanisms to attenuate NF-kB signaling and prevent their differentiation to APCs for maintaining self-tolerance5–6.
A20, a zinc finger ubiquitin-modifying enzyme, is defined as a negative regulator of NF-kB signaling7–10. A20 inhibits several key upstream signal transduction pathways of NF-kB induced by Toll-like receptor (TLR), the tumor necrosis factor receptor (TNFR), and retinoic acid-inducible gene I (RIG-I) in a feedback manner7–11 by ubiquitination or deubiquitination of receptor interacting protein (RIP), TNFR-associated factor (TRAF) 6, and other molecules for either promoting target protein degradation or regulating interaction of the target proteins with other signaling molecules7–11. Because TRAF6 is a common signaling component that is shared by all the members of the NF-kB family, A20 suppresses both MyD88-dependent and MyD88-independent NF-kB-signaling pathways. A20-deficient mice develop severe inflammation in multiple organs, are neonatally lethal, and highly hypersensitive to LPS and TNF7–8, 10. A20-deficient Mфs exhibit prolonged NF-κB activity7, 10. Our recent study further explored that A20 negatively regulates the maturation, inflammatory cytokine production, and immunostimulatory potency of DCs6. These results indicate an essential role of A20 in the maintenance of self-tolerance by regulating inflammatory signaling.
EBV, a human γ herpes virus with tropism for B cells, has been implicated in a variety of human tumorigenesis. In immunosuppressive patients, EBV-associated lymphoproliferative diseases express all of the EBV latent antigens12, which make CTL-based adoptive immunotherapy extremely effective in confining the diseases13–14. However, in immunocompetent hosts, EBV-associated malignancy only expresses subdominant EBV antigens, primarily, LMP1 and LMP212, 15–16. Although LMP antigens are conserved between viral strains and among Hodgkin lymphoma biopsy samples17–19, these subdominant antigens have poor immunogenicity and possess oncogenic potential20–21. Furthermore, the precursor frequency of LMP-specific CTLs is generally low in healthy virus-carriers20, 22. Thus, generation of LMP-specific CTL for adoptive immunotherapy represents a big challenge. In the present study, we report that transitively expressing an A20 inhibitor together with LMP genes (truncated LMP1 and full-length LMP2) greatly enhances maturation and cytokine production of hDCs. Consequently, LMP1/2-expressing, A20-silenced hDCs have an enhanced potency to prime an LMP-specific T cell response. When the in vitro primed T cells are adoptively transferred into tumor-xenografted SCID mice, some of the xenografted tumors approach complete regression. Hence, the study may provide an available approach to generate LMP-specific CTLs for adoptive immunotherapy.
Results
Construction and characterization of pshA20-LMP1/2
Potential EBV antigen targets for T cell therapy are limited to the subdominant antigens such as LMP1 and LMP2 which are expressed by the malignant tumors23–25. However, LMP-pulsed or expressed DCs may not prime a potent CTL response, as DCs are built-in with an inherent negative regulation mechanism to limit their maturation and immunostimulatory activity for homeostasis and immune tolerance. A20, a zinc finger ubiquitin-modifying enzyme, was reported to negatively regulate DC maturation and cytokine production. A20 silencing greatly promoted DCs to induce anti-tumor immunity. Thus, we constructed a co-expression vector pshuttleX-A20shRNA-ΔLMP1/LPM2 (pshA20-LMP1/2), which expresses the A20 shRNA under the control of the U6 promoter and a LMP1 truncate/LMP2 under the control of CMV promoter based on the fact that a full-length LMP1 is toxic when expressed at high levels26. The control vector pshuttleX-ΔLMP1-LMP2 (pLMP1/2) only expresses the LMP1 truncate and LMP2 (Fig. 1A). The recombinant vectors were transfected into hDCs by nucleofection which delivered a reporter vector pmaxGFP into hDCs with 92% efficiency (Supplemental Fig. 1A). Expression of the LMP1/2 in the pshA20-LMP1/2-transfected hDCs were confirmed by western blot at the protein level (Fig. 1B) and by qPCR at the mRNA level (Fig. 1C). Efficiency of pshA20-LMP1/2 transfecting hDCs, which is close to ~69%, was determined by ICS analysis of intracellular LMP1 expression (Supplemental Fig. 1B). To test if pshA20-LMP1/2 reduces A20 expression in the transfected hDCs, differently nucleofected or Mock hDCs were stimulated with LPS overnight, given that A20 is an inducible, feedback regulator of TLR and TNFR-mediated signaling6–7. qPCR assay explored that pshA20-LMP1/2 transfected hDCs express a drastically lower level of A20 mRNA in comparison with the control DCs (Fig. 1D, upper). Western Blot further demonstrated that pshA20-LMP1/2 transfected hDCs expressed a reduced level of A20 protein (Fig. 1D, lower). As LMP1 is known to induce A20 expression27, we also examined the A20 level in unstimulated, differently transfected hDCs by qPCR and western blot. We did not find that pLMP1/2-transfected hDCs expressed any enhanced level of A20 on both mRNA level and protein level (Supplemental Fig. 2), suggesting the truncated LMP1 does not maintain the function of the full-length LMP1. These results demonstrate pshA20-LMP1/2 transfected hDCs efficiently express the EBV subdominant antigens ΔLMP1 and LMP2, but inefficiently express the negative regulator A20 in response to LPS stimulation.
Fig 1. The construction and characterization of pshA20-LMP1/2.
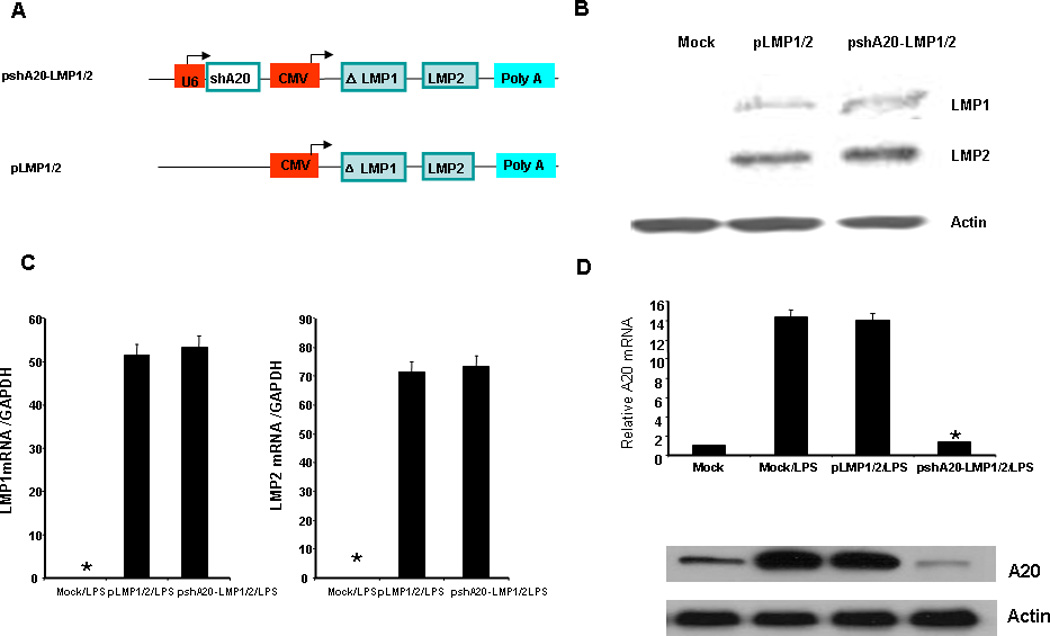
A. schematic representation of pshA20-LMP1/2 and control vector pLMP1/2. B. monocyte-derived DCs were nucleofected on day five with different vectors and matured with LPS (100 ng/ml) overnight. LMP1 and LMP2 expression in the DCs were analyzed 24 hours after nucleofection by western blot analysis. The antibody against EBV LMP1 or LMP2 was purchased from Santa Cruz Biotech (San Diego, CA). C. LMP1 and LMP2 mRNA levels were analyzed by qPCR 12hr after nucleofection. D. A20 mRNA level was analyzed by qPCR and data were normalized with mock DC A20 mRNA 24hr after nucleofection (upper). A20 protein expression was analyzed by western blot 24 hrs after nucleofection (lower). The data are representative of two independent experiments. *P < 0.01, pshA20-LMP1/2/LPS DCs vs. Mock/LPS DCs.
pshA20-LMP1/2 transfection enhances hDC maturation and cytokine production
We next tested maturation status of pshA20-LMP1/2-nucleosfected DCs in response to LPS stimulation by examining expression of costimulatory and MHC molecules via flow cytometric analysis. As shown in Fig. 2A, compared with pLMP1/2-transfected or Mock DCs, pshA20-LMP1/2-transfected DCs expressed significantly higher levels of CD80, CD86, MHC class-II molecule HLA-DR, and slightly higher levels of CD83 and chemokine receptor CCR7 at the single-cell level, although CD40 expression by differently transfected DCs or Mock DCs was very similar. Furthermore, we tested inflammatory cytokine production by differently transfected or Mock DCs. As shown in Fig. 2B, pshA20-LMP1/2-transfected DCs produced significantly higher levels of IL-6, TNF-α, and IL-12 than the control DCs in response to LPS stimulation. We also found that pshA20-LMP1/2-transfected DCs produced a higher level of IL-10, which is consistent with our previous studies in mouse models28–29. The results suggest that pshA20-LMP1/2 transfection endows DCs with enhanced maturation and activation due to the silencing of the negative regulator A20.
Fig 2. pshA20-LMP1/2-nucleofected hDCs exhibit more mature phenotypes.
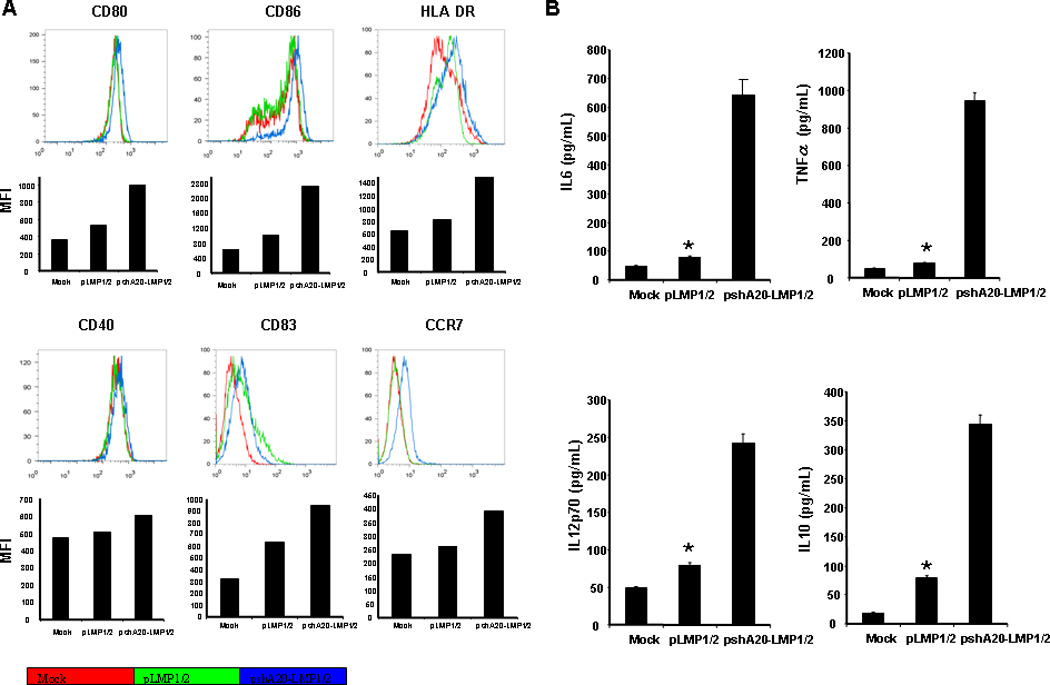
Monocyte-derived DCs were nucleofected with pshA20-LMP1/2 or pLMP1/2 on day 5 then stimulated with LPS o.n. Expression of costimulatory and MHC molecules was analyzed by flow cytometry (A). The culture media were harvested for analysis of cytokine production by ELISA (B). The data are representative of three independent experiments. *P < 0.01, pLMP1/2 DCs vs. pshA20-LMP1/2 DCs.
pshA20-LMP1/2 transfection enhances stimulatory activity of hDCs in priming autologous CD8+ T cells in vitro
To test if pshA20-LMP1/2 transfection enhances the stimulatory potency of hDCs in priming LMP-specific T cells, hDCs differentiated from HLA-A2+ healthy buffy coats were nucleofected with pshA20-LMP1/2, pLMP1/2, or Mock followed by LPS stimulation. Autologous human lymphocytes were cocultured with differently transfected or Mock DCs for 2–3 weeks to prime or activate LMP-specific CTLs. In cocultures with pshA20-LMP1/2-transfected DCs, 5.22% or 22.5% of CD8+ T cells were found positive for the LMP1 or LMP2-tetramer staining, compared with only 2.98% or 13.0% of LMP1 or LMP2-positive CD8+ T cells in cocultures with pLMP1/2-transfected DCs, and a background level of LMP1 or LMP2- positive CD8+ T cells in coculture with Mock DCs (Fig. 3A). Additionally, ICS assay showed that pshA20-LMP1/2 DCs substantially improved LMP-specific CD8+ CTL responses, for after coculture with pshA20-LMP1/2 DCs, 10.95% of CD8+ T cells turned to IFN-γ-positive and 22.25% of CD8+ T cells TNF-α-positive, whereas coculture with pLMP1/2 DCs only rendered 1.88 % of CD8+ T cells IFN-γ-positive and 5.71% of CD8+ T cells to express TNF-α. Mock DCs activated the cocultured CD8+ T cells to express IFN-γ (0.93%) or TNF-α (1.23%) with much lower efficiency (Fig. 3B). ELISPOT assays further supported that an increased frequency of T cells from coculture with pshA20-LMP1/2 DCs produced IFN-γ in response to re-stimulation of LMP1 MHC-I peptide, LMP2 MHC-I peptide, or a mixture of these peptides compared with those from coculture with pLMP1/2 DCs or Mock DCs (Fig. 3C). In comparison to tetramer analyses, IFN-γ ELISPOT assays seem to underestimate the frequency of epitope-specific T cells. The reason may partially be owed to that only a certain percentage of tetramer-positive T cells secrete IFN-γ after specific stimuation30–31. Furthermore, a low concentration of LMP peptides were used for pulsing autologous DCs for in vitro restimulation, which may also contribute to the low frequency of epitope-specific T cells detected in these ELISPOT assays. ELISPOT analysis of LMP-specific CTL activation from different HLA-A2+ healthy buffy coats was summarized in Table 1.
Fig 3. pshA20-LMP1/2-nucleofected DCs have an enhanced ability to prime or activate LMP1/2-specific effector T cell responses.
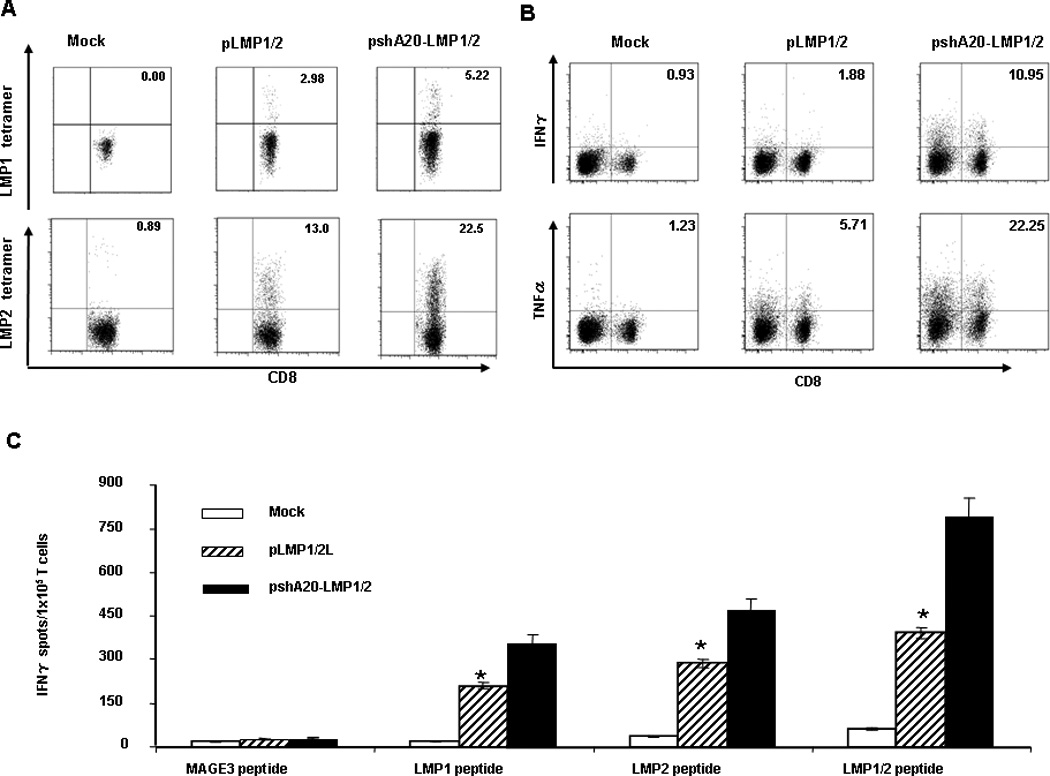
Autologous HLA-A2+ lymphocytes were co-cultured with the nucleofected DCs for 2–3 weeks with weekly re-stimulation. Priming of CD8+ T cells were assessed by LMP1- or LMP2-tetramer staining (A), ICS assay (B), and IFN-γ ELISPOT (C). The data are representative of the analyzed 5 buffy coats. *P < 0.05, pshA20-LMP1/2 DCs vs. pLMP1/2 DCs.
Table1.
IFN-γ ELISPOT analysis of CTL priming from different HLA A2 buffy coats
| Peptide | MAGE3 | LMP1 | LMP2 | LMP1+LMP2 |
|---|---|---|---|---|
| Donor A | ||||
| Mock | 15 ± 1.6 | 36 ± 3.1 | 41 ± 3.2 | 72 ± 6 |
| pLMP1/2 | 23 ± 2 | 142 ± 8 | 156 ± 6 | 298 ± 11 |
| pshA20-LMP1/2 | 36 ± 1.2 | 265.3 ± 14 | 322 ± 12 | 549 ± 25 |
| Donor B | ||||
| Mock | 8 ± 0.3 | 12 ± 1 | 34 ± 4 | 76 ± 5 |
| pLMP1/2 | 10 ± 1 | 25 ± 1.2 | 72.3 ± 2.5 | 128 ± 15 |
| pshA20-LMP1/2 | 13.3 ± 0.6 | 88 ± 5 | 103 ± 8 | 195 ± 4.5 |
| Donor C | ||||
| Mock | 52 ± 4 | 73 ± 2.5 | 60 ± 2.1 | 82 ± 8.3 |
| pLMP1/2 | 42 ± 7 | 181 ± 16 | 269 ± 15 | 486 ± 12.5 |
| pshA20-LMP1/2 | 48 ± 6.3 | 276 ± 10 | 526 ± 22 | 729 ± 18 |
| Donor D | ||||
| Mock | 28 ± 1.4 | 34 ± 4 | 63 ± 8 | 72 ± 0.5 |
| pLMP1/2 | 21 ± 3 | 73.3 ± 2.2 | 158 ± 3 | 194 ± 6 |
| pshA20-LMP1/2 | 17 ± 0.5 | 126 ± 7 | 203 ± 13.3 | 362 ±14 |
| Donor E | ||||
| Mock | 6.6 ± 1.3 | 16 ± 3 | 29 ± 1.3 | 45 ± 3 |
| pLMP1/2 | 4 ± 2 | 38 ± 5 | 89 ± 7 | 102 ± 6 |
| pshA20-LMP1/2 | 17 ± 4 | 71 ± 1.2 | 176 ± 6 | 284 ± 8 |
Numbers are mean ± SD of interferon γ (IFNγ) spot-forming cells/105 autologous T cells cocultured with different DCs.
We next determined whether activated T cells possess tumor lytic function by using LMP peptide-pulsed tumor cells or EBV-transformed, autologous B-lymphoblastoid cell lines (LCLs) as target cells for CTL assays. As shown in Fig. 4A, while T cells activated by Mock DCs barely showed any cytolytic activity to LMP1/LMP2-pulsed, HLA-A2+ lymphoblastoma cell line T2 or EBV-transformed LCLs, and T cells activated by pLMP1/2 DCs exhibited low cytolytic activities to these tumor target cells, T cells from the coculture with pshA20-LMP1/2 DCs displayed strong CTL activities against either LMP1/2 peptide-pulsed T2 lymphoblastoma cells or EBV-transformed, HLA-A2 restricted LCL. The tumor cytolytic activity was LMP antigen-specific, as the activated lymphocytes by pshA20-LMP1/2 DCs or pLMP1/2 DCs only had a background cytolytic activity against Mage3-pulsed T2 or HLA-A2+ renal carcinoma A498 cells (data not shown). It has been known that pretreatment with anti-inflammatory cytokine IL-10 promotes tolerogenic function of hDCs32. We further tested whether incubation of pshA2-LMP1/2 DCs with 10 ng/ml of IL-10 could ablate its superior immunostimulatory activity. We found that although pretreatment with IL-10 reduced either pshA20-LMP1/2 DCs or pLMP1/2 DCs to activate CTL response against LMP1/LMP2-pulsed T2 cells, T cells primed by IL-10-treated pshA20-LMP1/2 DCs still displayed higher cytotoxicity against the tumor cell line compared with those activated by IL-10-treated pLMP1/2 DCs (Fig. 4B). Repeated experiments from different donors showed similar results. Collectively, these results indicate that pshA20-LMP1/2 DCs have enhanced immunostimulatory ability to prime EBV subdominant antigen-specific CTLs.
Fig. 4. pshA20-LMP1/2-nucleofected DCs have an enhanced ability to stimulate CTL activity.
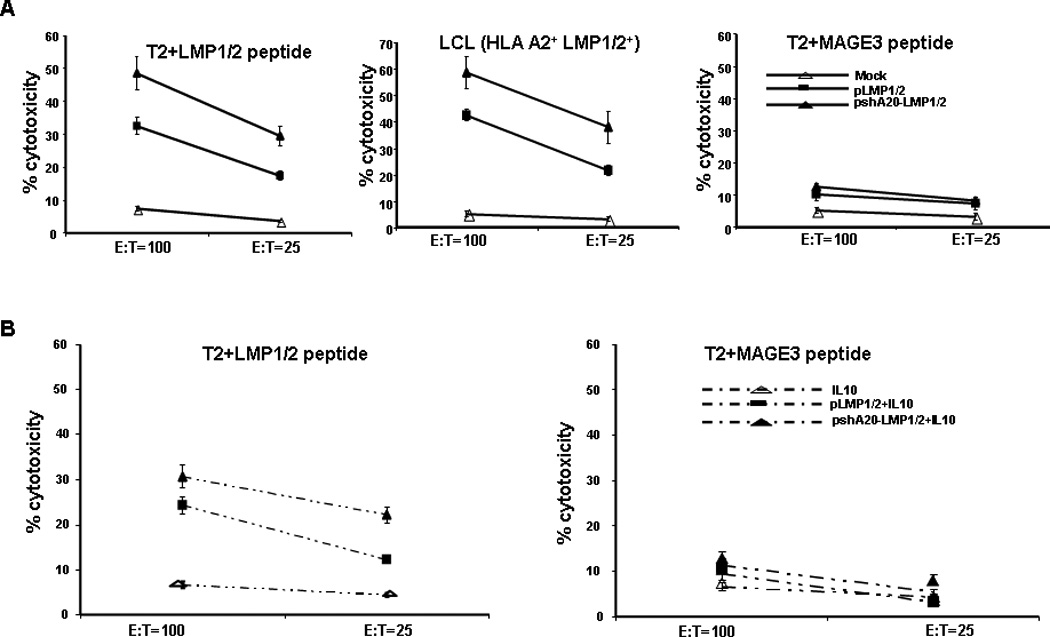
A. Autologous HLA-A2+ T cells were cocultured with differently treated DCs for two weeks. CTL activities against T2 cell line pulsed with 20 µg/ml of LMP1/LMP2 peptides (left), autologous HLA-A2+EBV+LCL (middle) or T2 cell line pulsed with 20 µg/ml MAGE3 (right) were evaluated by LDH assay. The data are representative of two analyzed buffy coats. P < 0.05 (chi-square), pshA20-LMP1/2 DCs vs. pLMP1/2 DC-primed T cells. B. The autologous HLA-A2+ T cells were co-cultured with different DCs in the absence or presence of pretreatment with IL-10 for two weeks with weekly re-stimulation. CTL activities against LMP1/2-pulsed T2 cell line or CTL activity against MAGE3- pulsed T2 cells was evaluated by LDH assay. The data are representative of two analyzed buffy coats. P < 0.05 (Chi-square), pshA20-LMP1/2 DCs vs. pLMP1/2 DC-primed T cells.
pshA20-LMP1/2 transfection enhances hDCs to prime type-I CD4+ T cell response in vitro
To further explore the mechanism by which A20-silenced hDCs provoke more potent CTL responses against LMP, we compared the CD4+ T cell response in the cocultures with different DCs, given the importance of CD4+ T cells in coordinating immune responses by production of various cytokines. We found that after coculture with pshA20-LMP1/2 DCs, 16.12% or 23.58% of CD4+ T cells produced IFN-γ or TNF-α compared with only 3.09% or 6.22% of CD4+ T cells that express IFN-γ or TNF-α after coculture with pLMP1/2 DCs, and compared with the background levels of CD4+ T cells (0.87% or 0.56%) that express the cytokines after coculture with Mock DCs (Fig. 5A). In contrast, pshA20-LMP1/2 DC does not show any advantage over pLMP1/2 DC in promoting expression of type-II cytokines by cocultured CD4+ T cells (data not shown). These results are consistent with our previously published studies that A20-silenced DCs or macrophages preferentially prime IFN-γ/TNF-α-producing T cells and provoke a type-I immune environment6, 33. More intriguingly, A20-silenced hDCs via siRNA oligo, when pulsed or treated with Hodgkin lymphoma HDLM2 tumor lysate (TL), display a large reduction in eliciting IL-10 expression by cocultured CD4+ T cells compared with control siRNA-transfected, TL-treated hDCs, as shown by ELISA analysis of IL-10 moiety in the supernatants of the primed CD4+ T cell cultures (Fig. 5B, left) or by ICS analysis of the cocultured CD4+ T cells (Fig. 5B, right). Furthermore, we tested immunosuppressive activity of these different DC-primed CD4+ T cells by coculturing the primed CD4+ T cells with naïve autologous CD4+ T cells at different ratios in the presence of anti-CD3 as described. 3H-Thymidine incorporation assay explored that the CD4+ T cells primed by A20-silenced, TL-treated DCs showed a reduced ability to inhibit naïve autologous CD4+ T cell proliferation (Fig. 5C). Hodgkin lymphoma patients are characterized by profound systemic immunosuppression34. Hodgkin lymphoma cells secrete high levels of IL-10 and TGF- β35, as confirmed by ELISA analysis of HDLM2 supernatants (Supplementary Fig. 3), to inhibit activation of type-I immune responses, versus to promote type-II or regulatory T cell responses36. These results may implicate that A20-silenced DCs not only promote type-I immunity, also have enhanced refraction to tumor-mediated differentiation of tolerogenic function.
Fig. 5. Priming of CD4+ T cells by differently transfected or Mock DCs.
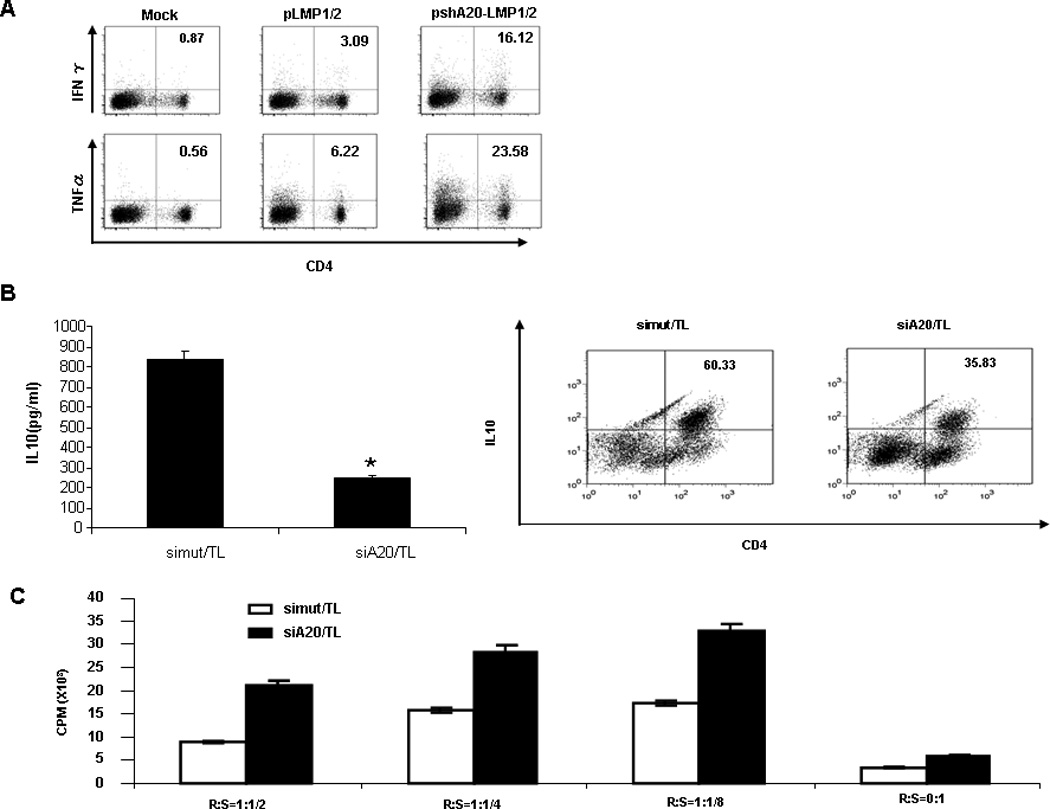
A. Autologous T cells were cocultured with pshA20-LMP1/2-DCs, pLMP1/2-DCs, or Mock DCs for two weeks. Priming of CD4+ T cells were assessed by ICS assay. B. hDCs were transfected with A20 siRNA (siA20) or mutant A20 siRNA (siMut) and pulsed with HDLM2 tumor lysates (5:1) for 48 hrs. After 6 hr of LPS stimulation, the hDCs were cocultured with autologous CD4+ T cells for 7–9 days. IL-10 expression in the cocultured CD4+ T cells was determined by ELISA (left) and ICS (right). C. The primed CD4+ T cells by TL-pulsed, siRNA-transfected DCs were cocultured with naïve autologous CD4+ T cells (CD4+CD45RA+) at varying ratios in anti-CD3-coated 96 well plates. T cell proliferation was determined by 3H-Thymidine incorporation assay. *p < 0.05, siA20/TL vs. siMut/TL.
As shown in Fig. 2B, pshA20-LMP1/2-transfected DCs not only produce higher levels of proinflammatory cytokines, they also produce an elevated amount of anti-inflammatory cytokine IL-10. Hence, we did down-regulation of IL-10 or its signaling in hDCs by IL-10 siRNA or IL-10 receptor (IL-10R) siRNA oligos. We found that down-regulation of IL-10 or its signaling by the siRNAs does not significantly impact DC expression of IL-12 (Supplementary Fig. 4A), a key cytokine in the activation of CTL responses37, which is consistent with the previously reported results from Breckpot et al.38. However, downregulation of IL-10 or IL-10R with siRNA significantly enhanced IL-12 expression in TL-treated hDCs (Supplementary Fig. 4A), which is coherent with an earlier study that autocrine production of IL-10 mediates defective IL-12 production in tumor-associated macrophages39. We further found that down-regulation of IL-10 also reduced TL-pulsed hDC to induce IL-10-expressed CD4+ T cells albeit with a lesser extent than down-regulation of A20 alone (Supplementary Fig. 4B).
pshA20-LMP1/2 DC-activated T cells have improved control of tumor growth in vivo
To assess whether pshA20-LMP1/2 DC-activated T cells could effectively control the growth of EBV+ lymphomas in vivo, we used a SCID mouse xenograft model. 5 to 6 week-old sublethally irradiated SCID mice (6 mice per group) were implanted with FFLuc-labeled EBV+ lymphomas in the peritoneal cavity and monitored for tumor growth by examining light emission of implanted tumor cells. Once tumor bioluminescence in the tumor-inoculated mice was found progressively increasing, which usually occurred at day 4–5 after tumor injection, mice were treated intraperitoneally with in vitro primed autologous T cells (1×107) by differently transfected DCs or Mock DCs at days 5 and 12 post tumor cell inoculation. Based on 22% of CD8+ T cells present 2 – 3 weeks after coculture (Supplemental Fig. 5) and 5.22% or 22.5% of the CD8+ T cells LMP1 or LMP2-positive (Fig. 3A), in the pshA20-LMP1/2 DC group, a ~5.0×105 of LMP2+ and ~1.2×105 of LMP1+ CD8+ T cells were used for adaptive transfer each time. As shown in Fig. 6A & B, the mice treated with Mock DC-primed T cells exhibited progressively increased tumor bioluminescence and the mice treated with pLMP1/2 DC-primed T cells displayed slightly decreased tumor bioluminescence, whereas the mice treated with pshA20-LMP1/2 DC-primed T cells had dramatically reduced tumor bioluminescence during the observed period and some of them achieved nearly complete extinguishment of tumor bioluminescence by day 28 after tumor implantation. Accordingly, treatment of pshA20-LMP1/2 DC-primed T cells also contributed to an improved survival compared with mice receiving pLMP1/2 DC- or Mock DC-primed T cells. Fig. 6C showed that all the mice treated with pshA20-LMP1/2 DC-primed T cells survived during the observed period (more than 50 days after tumor injection). Hence, pshA20-LMP1/2 DC-primed T cells can control tumor growth in vivo with a higher efficiency.
Fig. 6. CTLs primed by pshA20-LMP1/2 exhibit enhanced anti-tumor immunity.
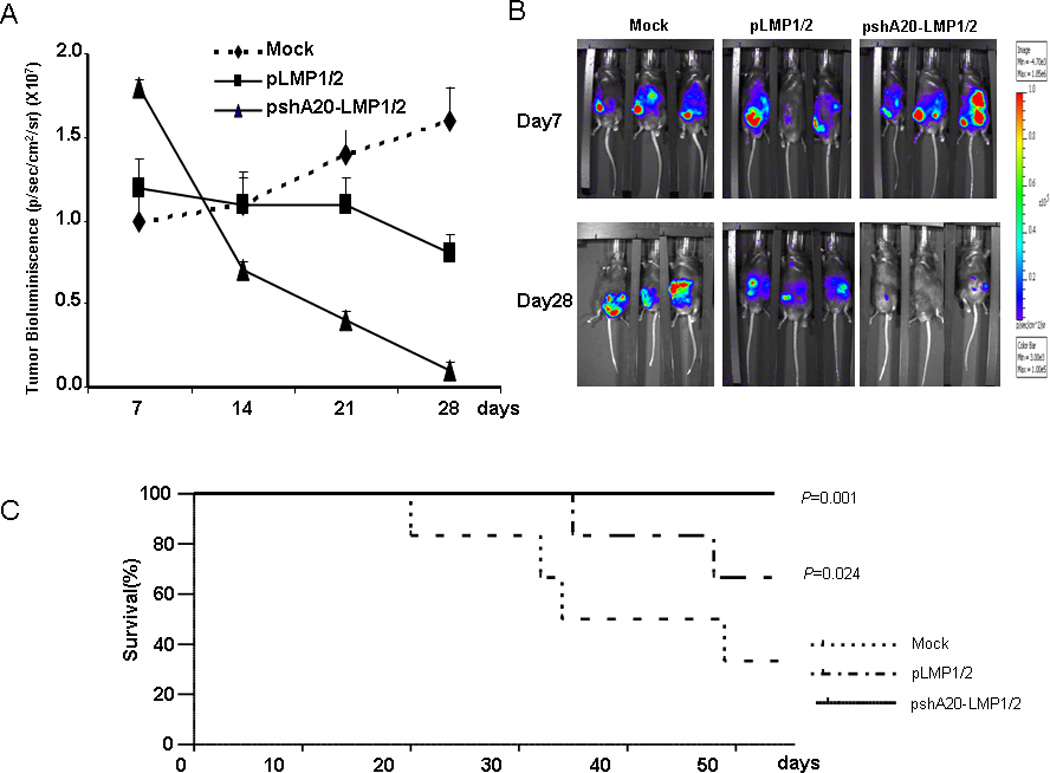
T cells primed by different DCs were injected intraperitoneally into SCID mice bearing EBV+ lymphoma labeled with FFLuc at day 5 and 12 after intraperitoneal tumor implantation. Tumor growth was measured weekly as maximum p/s/cm2/sr. Lines represent the average light emission ± SD (A). Pictures of 3 representative mice per group at day 7 and day 28 (B). The survival curve for SCID mice bearing EBV+ lymphoma that received CTLs primed by differently transfected or Mock DCs (C).
Discussion
Adoptive transfer of EBV-specific CTLs is extremely effective in treating EBV-associated malignant lymphomas caused by hemopoietic stem cell transplantation in highly immunosuppressed patients13–14, 40. The reason is attributed to these lymphoma cells expressing all of the latent EBV proteins including the immunodominant EBV nuclear Ag (EBNA) 3 proteins, which are targeted by a majority of EBV-CTL populations. However, EBV infection leads to HD in some immunocompetent hosts and the malignant Reed-Sternberg cells of HD develop a multiplicity of immune evasion mechanisms, including the down-regulation of the immunodominant EBV nuclear antigens, to evade preexisting immune responses to EBV. Nevertheless, the tumor cells continue to express the immunologically subdominant viral latency proteins such as LMP1 and LMP2. To generate CTLs against the LMP antigen for the adoptive immunotherapy of EBV-associated malignancies, Gahn et al. found that human monocyte-derived DCs transduced by recombinant adenoviruses encoding LMP2A effectively directed the generation of LMP2A-specific CTLs with much higher efficiency than autologous LCLs. Considering that only small amounts of patient peripheral blood can be collected to produce LMP-expressing DCs, Bollard et al. devised an initial activation of LMP2-specific CTLs by LMP2-transfected DCs followed by stimulation with LCLs overexpressing LMP2 to produce large numbers of LMP2-specfic CTLs, which are demonstrated to contain both CD4+ and CD8+ T cells and recognize multiple LMP2-epitopes23. Furthermore, Bollard et al. used these in vitro activated autologous and donor-derived CTL lines to treat a patient with multiple relapsed EBV positive HD after multiple chemotherapeutic and radiotherapy regimens and rendered the treated patient to remain in complete remission 5 years post-allogenic stem cell transplantation15. The same group also observed that in vitro activated, LMP2-specific CTLs could expand and persist for a long time after transfer into patients and had substantial antitumor activity in the patient recipients41. As another potential target for HD immunotherapy, the full-length LMP1 is toxic when expressed at high levels, which has prevented DCs from being used for CTL stimulation. Gottschalk et al. therefore defined that an inactive, non toxic LMP1 mutant (ΔLMP1) could be expressed in DCs via an adenoviral vector, and the ΔLMP1-expressed DCs enabled the activation and expansion of polyclonal LMP1-specific CTLs26. Collectively, these studies highlighted that LMP-specific CTLs can be generated in vitro and both autologous and donor-derived CTLs can be used for adoptive immunotherapy to combat against EBV-associated malignancies to a certain extent.
Given the fact that DCs are equipped with multiple negative regulatory mechanisms in the maintenance of self-tolerance42, we coexpressed a shRNA of A20, a key negative regulator of proinflammatory signaling pathways, together with LMP1/LMP2 to promote the ability of hDCs to activate LMP-specific CTLs. We found that pshA20-LMP1/2-transfected DCs exhibit more mature phenotypes and produced higher levels of inflammatory cytokines in response to stimulation of a TLR-ligand. As a consequence, the pshA20-LMP1/2-transfected DCs primed more potent CTL responses, as manifested by higher frequencies of CD8+ T cells LMP tetramer-positive, and large percentages of CD8+ T cells to express IFN-γ or TNF-α. Furthermore, the CTLs activated by pshA20-LMP1/2-transfected DCs exhibited stronger cytotoxicity against EBV+ tumor cells, and functioned more efficiently in controlling EBV-transformed tumors after adoptive transfer into SCID mice. These results suggested that silencing the negative regulator A20 can boost the ability of hDCs to prime the LMP-specific CTL response. The experimental results are also consistent with our previously reported studies in which A20-silenced murine DCs displayed a superior immunostimulatory function.6, 28.
Overall, the study demonstrated that transient expression of an A20 inhibitor enhanced the efficiency of LMP-expressing hDCs to prime the LMP-specific CTL response. The finding potentially provides an approach which allows small numbers of patient DCs to operate as APCs for activation of LMP-specific CTLs more efficiently. This strategy is also likely to benefit for generation of other tumor-specific CTLs for adoptive immunotherapy, especially when weak tumor antigens are targeted
Materials and Methods
Construction of expression vectors
The control vector pLMP1/2 expresses the truncated EBV LMP1 (ΔLMP1) and a full-length LMP2 with an IRES (internal ribosome entry site) link under the control of CMV promoter. To construct the co-expression vector pshA20-LMP1/2, which additionally expresses a small hairpin shRNA of human A20 (shA20) under the control of the U6 promoter, synthetic shA20 oligo duplex (5′-GATCCCCCCCATGCACCGATACACACTTTCAAGAGAAGTGTGTATCGGTGCATGGTTTTTGGAAA-3’) was cloned into pSilencer2.0-U6 (Ambion). The U6 RNA polymerase promoter-shA20 fragment was generated by PCR and cloned into the Spe-I-digested plasmid pLMP1/2. The sequences of the inserts in the plasmid were confirmed by DNA sequencing (Lone Star Labs, Inc, Houston, TX).
Generation and transfection of human monocyte-derived DCs
Human monocyte-derived DCs were prepared from peripheral blood mononuclear cells (PBMCs) of buffy coat (Gulf Coast Regional Blood Center, Houston, TX) as described in our previous studies43–45. For hDC transfection, the monocyte-derived DCs were harvested for nucleofection with different siRNAs or expression vectors according to the manufacturer’s instruction (Amaxa Inc., Gaithersburg, MD) for further studies.
Quantitative PCR (q-PCR) assay
For qPCR analyses of the nucleofected DCs, the qPCR primers were synthesized as below: human A20 primers (5’-CACGCTCAAGGAAACAGACA-3’; 5’-CATGGGTGTGTCTGTGGAAG-3’); LMP1 primers (5’-TGAACACCACCACGATGACT-3’; 5’-GTGCGCCTAGGTTTTGAGAG-3’); LMP2 primers (5’-TCCATCTGCTTCTGGCTCTT-3’; 5’-ATGAGTCATCCCGTGGAGAG-3’).
Tetramer and intracellular staining (ICS)
For tetramer staining, an HLA-A2-restricted EBV LMP1 peptide (YLQQNWWTL)26, 46, an HLA-2-restricted LMP2 peptide (CLGGLLTMV)23, and a control human melanoma antigen MAGE3 peptide (FLWGPRALV) were synthesized and purified by HPLC to >95% purity by Genemed Synthesis Inc. (South San Francisco, CA, USA)47. Human LMP1/HLA-A2 tetramer and human LMP2/HLA-A2 tetramer were synthesized at the Baylor College of Medicine Tetramer Core Facility (Houston, TX, USA). For ICS assay, the co-cultured T cells were re-stimulated for six hours in the presence of the protein transport inhibitor GolgiPlug (BD Biosciences, Erembodgem, Belgium). After cell surface staining, cells were permeabilized with BD perm/wash solution, and then stained with specific monoclonal antibodies (BD Bioscience).
ELISA and ELISPOT assay
Levels of various proinflammatory cytokines were quantified from the supernatant of DC or T cell cultures by ELISA analysis (BD Biosciences) according to the manufacturer’s instructions. ELISPOT assays were performed as described previously6, 43. Briefly, MultiScreen-HA plates (Millipore, Bedford, MA) were incubated with the anti-human IFN-γ mAb 1-D1K (Mabtech, Stockholm,Sweden) overnight, and then blocked with RPMI 1640 supplemented with 10% human serum (Bethyl, Inc., Montgomery, TX). Primed T cells (1×105) were co-cultured with irradiated peptide-pulsed autologous DCs (4×103) in the coated plate for 20 hr at 37°C. After washing, biotinylated anti-human IFN-γ antibody 7-B6-1 (Mabtech) was added to the wells, and then incubated for 2 hr. After another wash, HRP-conjugated avidin was added to the wells and incubated for 1 hr. Finally, spots were developed by the addition of HRP substrate (Vectastain ABC Kit, Vector Laboratories). After spot development, the plate was rinsed thoroughly with ddH2O and allowed to dry. Spots were analyzed by Zellnet Consulting, Inc. (New York).
Priming of autologous T cells
In vitro priming of autologous T cells was performed as reported43–44. 1×105 human DCs nucleofected with either pshA20-LMP1/2, pLMP1/2, or PBS were harvested and then co-cultured with autologous lymphocytes at a ratio of 1:20 in the presence of recombinant human IL-2 (50 U/ml) (R&D Systems). The T cells were re-stimulated weekly with freshly prepared nucleofected DCs. After 2–3 weeks of co-culture, the lymphocytes were collected for immunological assays.
CTL assay
CD8+ CTL responses were assessed with LDH release assay (Roche Diagnostics). Briefly, DC-primed T cells were re-stimulated with 20 µg/ml LMP1 and 20 µg/ml LMP2 peptide-pulsed autologous DCs for two hours. LMP1, LMP2, or Mage3-pulsed T2 or A498, or autologous LCL served as target cells. 104 target cells and various numbers of DC-primed T cells were cocultured at 37°C in 96-well round-bottom plates. Four hours later, 100 µl of the supernatant was harvested from each well, mixed with LDH reagents, and analyzed by absorbance at OD490 in the Microwin 2000 counter. The percent lysis was defined as (experimental LDH release - spontaneous LDH release)/(maximal LDH release - spontaneous LDH release) × 100%. Spontaneous and maximal LDH releases were determined in the presence of either medium or 2% Triton-100, respectively.
Antitumor activity
To evaluate in vivo antitumor activity, autologous EBV+-LCLs were generated by transforming PBMC with EBV B95-8 virus (ATCC) and expanded for 3–4 weeks. EBV+-LCLs were transfected with FFLuc vector and selected using 2 µg/ml puromycin (Invitrogen) for 1–2 weeks. C57/SCID mice (5 to 6 weeks-old, Jackson Laboratories) were sublethally irradiated (230 cGy) and xenografted intraperitoneally with 3×106 FFLuc+ EBV+-LCLs resuspended in Matrigel (BD Biosciences)48–49. CTLs (10×106 per mouse) activated by pshA20-LMP1/2, control pLMP1/2, or Mock DC were injected intraperitoneally at day 5 and 12 after intraperitoneal tumor implantation (6 mice per group). Tumor growth was evaluated over time using the IVIS imaging system. A constant region of interest was drawn over the tumor regions and the intensity of the signal measured as total photon/sec/cm2/steradian (p/s/cm2/sr) as previously described49–50.
Statistical analysis
For statistical analysis, we used Student's t test or Chi-square test (indicated when being used), and a 95% confidence limit was taken to be significant, defined as p < 0.05. Results are typically presented as means ± standard errors (SE).
Supplementary Material
Acknowledgement
We thank many colleagues at the Center for Cell and Gene Therapy in Baylor College of Medicine for helpful suggestions and assistance. This work was supported by the Leukemia and Lymphoma Society Research Grant (to SYC) and NIH research grants (R01AI68472, R01AI084811, R01CA090427 and R01CA116677 to SYC, and R01CA100841 and R21/R33 AI08185 to XFH).
Footnotes
Conflict of interest
The authors have declared no financial or commercial conflict of interest.
References
- 1.Diebold SS. Activation of dendritic cells by toll-like receptors and C-type lectins. Handbook of experimental pharmacology. 2009;(188):3–30. doi: 10.1007/978-3-540-71029-5_1. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003;3(2):169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 4.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 5.Shen L, Evel-Kabler K, Strube R, Chen SY. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol. 2004;22(12):1546–1553. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- 6.Song XT, Evel-Kabler K, Shen L, Rollins L, Huang XF, Chen SY. A20 is an antigen presentation attenuator, and its inhibition overcomes regulatory T cell-mediated suppression. Nat Med. 2008;14(3):258–265. doi: 10.1038/nm1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5(10):1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 8.Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289(5488):2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarma V, Lin Z, Clark L, Rust BM, Tewari M, Noelle RJ, et al. Activation of the B-cell surface receptor CD40 induces A20, a novel zinc finger protein that inhibits apoptosis. J Biol Chem. 1995;270(21):12343–12346. doi: 10.1074/jbc.270.21.12343. [DOI] [PubMed] [Google Scholar]
- 10.Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430(7000):694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 11.Saitoh T, Yamamoto M, Miyagishi M, Taira K, Nakanishi M, Fujita T, et al. A20 is a negative regulator of IFN regulatory factor 3 signaling. J Immunol. 2005;174(3):1507–1512. doi: 10.4049/jimmunol.174.3.1507. [DOI] [PubMed] [Google Scholar]
- 12.Poppema S, Potters M, Visser L, van den Berg AM. Immune escape mechanisms in Hodgkin's disease. Ann Oncol. 1998;9(Suppl 5):S21–S24. doi: 10.1093/annonc/9.suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- 13.Heslop HE, Ng CY, Li C, Smith CA, Loftin SK, Krance RA, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nature medicine. 1996;2(5):551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 14.Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92(5):1549–1555. [PubMed] [Google Scholar]
- 15.Bollard CM, Gottschalk S, Huls MH, Molldrem J, Przepiorka D, Rooney CM, et al. In vivo expansion of LMP 1- and 2-specific T-cells in a patient who received donor-derived EBV-specific T-cells after allogeneic stem cell transplantation. Leuk Lymphoma. 2006;47(5):837–842. doi: 10.1080/10428190600604724. [DOI] [PubMed] [Google Scholar]
- 16.Straathof KC, Leen AM, Buza EL, Taylor G, Huls MH, Heslop HE, et al. Characterization of latent membrane protein 2 specificity in CTL lines from patients with EBV-positive nasopharyngeal carcinoma and lymphoma. J Immunol. 2005;175(6):4137–4147. doi: 10.4049/jimmunol.175.6.4137. [DOI] [PubMed] [Google Scholar]
- 17.Busson P, Edwards RH, Tursz T, Raab-Traub N. Sequence polymorphism in the Epstein-Barr virus latent membrane protein (LMP)-2 gene. J Gen Virol. 1995;76(Pt 1):139–145. doi: 10.1099/0022-1317-76-1-139. [DOI] [PubMed] [Google Scholar]
- 18.Lee SP, Thomas WA, Murray RJ, Khanim F, Kaur S, Young LS, et al. HLA A2.1-restricted cytotoxic T cells recognizing a range of Epstein-Barr virus isolates through a defined epitope in latent membrane protein LMP2. J Virol. 1993;67(12):7428–7435. doi: 10.1128/jvi.67.12.7428-7435.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray PG, Constandinou CM, Crocker J, Young LS, Ambinder RF. Analysis of major histocompatibility complex class I, TAP expression, and LMP2 epitope sequence in Epstein-Barr virus-positive Hodgkin's disease. Blood. 1998;92(7):2477–2483. [PubMed] [Google Scholar]
- 20.Duraiswamy J, Bharadwaj M, Tellam J, Connolly G, Cooper L, Moss D, et al. Induction of therapeutic T-cell responses to subdominant tumor-associated viral oncogene after immunization with replication-incompetent polyepitope adenovirus vaccine. Cancer Res. 2004;64(4):1483–1489. doi: 10.1158/0008-5472.can-03-2196. [DOI] [PubMed] [Google Scholar]
- 21.Wang JH, Yan YW, Garrett TP, Liu JH, Rodgers DW, Garlick RL, et al. Atomic structure of a fragment of human CD4 containing two immunoglobulin-like domains. Nature. 1990;348(6300):411–418. doi: 10.1038/348411a0. [DOI] [PubMed] [Google Scholar]
- 22.Khanna R, Burrows SR, Nicholls J, Poulsen LM. Identification of cytotoxic T cell epitopes within Epstein-Barr virus (EBV) oncogene latent membrane protein 1 (LMP1): evidence for HLA A2 supertype-restricted immune recognition of EBV-infected cells by LMP1-specific cytotoxic T lymphocytes. Eur J Immunol. 1998;28(2):451–458. doi: 10.1002/(SICI)1521-4141(199802)28:02<451::AID-IMMU451>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 23.Bollard CM, Straathof KC, Huls MH, Leen A, Lacuesta K, Davis A, et al. The generation and characterization of LMP2-specific CTLs for use as adoptive transfer from patients with relapsed EBV-positive Hodgkin disease. J Immunother. 2004;27(4):317–327. doi: 10.1097/00002371-200407000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Khanna R, Burrows SR, Moss DJ. Immune regulation in Epstein-Barr virus-associated diseases. Microbiol Rev. 1995;59(3):387–405. doi: 10.1128/mr.59.3.387-405.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rickinson AB, Moss DJ. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu Rev Immunol. 1997;15:405–431. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- 26.Gottschalk S, Edwards OL, Sili U, Huls MH, Goltsova T, Davis AR, et al. Generating CTLs against the subdominant Epstein-Barr virus LMP1 antigen for the adoptive immunotherapy of EBV-associated malignancies. Blood. 2003;101(5):1905–1912. doi: 10.1182/blood-2002-05-1514. [DOI] [PubMed] [Google Scholar]
- 27.Laherty CD, Hu HM, Opipari AW, Wang F, Dixit VM. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. The Journal of biological chemistry. 1992;267(34):24157–24160. [PubMed] [Google Scholar]
- 28.Hong B, Song XT, Rollins L, Berry L, Huang XF, Chen SY. Mucosal and systemic anti-HIV immunity controlled by A20 in mouse dendritic cells. J Clin Invest. 2011;121(2):739–751. doi: 10.1172/JCI42656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song XT, Evel-Kabler K, Rollins L, Aldrich M, Gao F, Huang XF, et al. An alternative and effective HIV vaccination approach based on inhibition of antigen presentation attenuators in dendritic cells. PLoS Med. 2006;3(1):e11. doi: 10.1371/journal.pmed.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittet MJ, Zippelius A, Speiser DE, Assenmacher M, Guillaume P, Valmori D, et al. Ex vivo IFN-gamma secretion by circulating CD8 T lymphocytes: implications of a novel approach for T cell monitoring in infectious and malignant diseases. J Immunol. 2001;166(12):7634–7640. doi: 10.4049/jimmunol.166.12.7634. [DOI] [PubMed] [Google Scholar]
- 31.Tussey L, Speller S, Gallimore A, Vessey R. Functionally distinct CD8+ memory T cell subsets in persistent EBV infection are differentiated by migratory receptor expression. Eur J Immunol. 2000;30(7):1823–1829. doi: 10.1002/1521-4141(200007)30:7<1823::AID-IMMU1823>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, Agematsu K, et al. Defective IL-10 signaling in hyper-IgE syndrome results in impaired generation of tolerogenic dendritic cells and induced regulatory T cells. J Exp Med. 2011;208(2):235–249. doi: 10.1084/jem.20100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song XT, Turnis ME, Zhou X, Zhu W, Hong BX, Rollins L, et al. A Th1-inducing adenoviral vaccine for boosting adoptively transferred T cells. Mol Ther. 2011;19(1):211–217. doi: 10.1038/mt.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, et al. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103(5):1755–1762. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 35.Poppema S, van den Berg A. Interaction between host T cells and Reed-Sternberg cells in Hodgkin lymphomas. Semin Cancer Biol. 2000;10(5):345–350. doi: 10.1006/scbi.2000.0327. [DOI] [PubMed] [Google Scholar]
- 36.Fiebiger E, Meraner P, Weber E, Fang IF, Stingl G, Ploegh H, et al. Cytokines regulate proteolysis in major histocompatibility complex class II-dependent antigen presentation by dendritic cells. J Exp Med. 2001;193(8):881–892. doi: 10.1084/jem.193.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss JM, Subleski JJ, Wigginton JM, Wiltrout RH. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin Biol Ther. 2007;7(11):1705–1721. doi: 10.1517/14712598.7.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breckpot K, Aerts-Toegaert C, Heirman C, Peeters U, Beyaert R, Aerts JL, et al. Attenuated expression of A20 markedly increases the efficacy of double-stranded RNA-activated dendritic cells as an anti-cancer vaccine. J Immunol. 2009;182(2):860–870. doi: 10.4049/jimmunol.182.2.860. [DOI] [PubMed] [Google Scholar]
- 39.Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, et al. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol. 2000;164(2):762–767. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 40.Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative disorders. Annu Rev Med. 2005;56:29–44. doi: 10.1146/annurev.med.56.082103.104727. [DOI] [PubMed] [Google Scholar]
- 41.Bollard CM, Aguilar L, Straathof KC, Gahn B, Huls MH, Rousseau A, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease. J Exp Med. 2004;200(12):1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 43.Hong B, Ren W, Song XT, Evel-Kabler K, Chen SY, Huang XF. Human suppressor of cytokine signaling 1 controls immunostimulatory activity of monocyte-derived dendritic cells. Cancer Res. 2009;69(20):8076–8084. doi: 10.1158/0008-5472.CAN-09-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schroers R, Huang XF, Hammer J, Zhang J, Chen SY. Identification of HLA DR7-restricted epitopes from human telomerase reverse transcriptase recognized by CD4+ T-helper cells. Cancer Res. 2002;62(9):2600–2605. [PubMed] [Google Scholar]
- 45.Schroers R, Shen L, Rollins L, Rooney CM, Slawin K, Sonderstrup G, et al. Human telomerase reverse transcriptase-specific T-helper responses induced by promiscuous major histocompatibility complex class II-restricted epitopes. Clin Cancer Res. 2003;9(13):4743–4755. [PubMed] [Google Scholar]
- 46.Duraiswamy J, Sherritt M, Thomson S, Tellam J, Cooper L, Connolly G, et al. Therapeutic LMP1 polyepitope vaccine for EBV-associated Hodgkin disease and nasopharyngeal carcinoma. Blood. 2003;101(8):3150–3156. doi: 10.1182/blood-2002-10-3092. [DOI] [PubMed] [Google Scholar]
- 47.van der Bruggen P, Bastin J, Gajewski T, Coulie PG, Boel P, De Smet C, et al. A peptide encoded by human gene MAGE-3 and presented by HLA-A2 induces cytolytic T lymphocytes that recognize tumor cells expressing MAGE-3. Eur J Immunol. 1994;24(12):3038–3043. doi: 10.1002/eji.1830241218. [DOI] [PubMed] [Google Scholar]
- 48.De Angelis B, Dotti G, Quintarelli C, Huye LE, Zhang L, Zhang M, et al. Generation of Epstein-Barr virus-specific cytotoxic T lymphocytes resistant to the immunosuppressive drug tacrolimus (FK506) Blood. 2009;114(23):4784–4791. doi: 10.1182/blood-2009-07-230482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vera JF, Hoyos V, Savoldo B, Quintarelli C, Giordano Attianese GM, Leen AM, et al. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Mol Ther. 2009;17(5):880–888. doi: 10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savoldo B, Rooney CM, Di Stasi A, Abken H, Hombach A, Foster AE, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110(7):2620–2630. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


