Abstract
We investigated the in vivo priming of IL-17+ autoreactive T cells in experimental autoimmune uveitis-prone C57BL/6 (B6) and B10RIII mice using a combination of approaches, including limiting dilution assay. High numbers of in vivo primed IL-17+ interphotoreceptor retinoid-binding protein (IRBP)-specific T cells were found in mice immunized with a uveitogenic peptide emulsified in CFA, but not the same peptide emulsified in IFA. Both in vitro and in vivo, at least part of the effect of mycobacterial antigen in CFA could be replaced by TLR2 or TLR4 ligands. TCR-δ−/− mice immunized with IRBP peptide in CFA generated significantly lower numbers of IL-17+ T cells than immunized wild-type B6 mice. Administration of a small number of activated γδ T cells to TCR-δ−/− mice significantly increased the number of IL-17+, but not IFN-γ+, IRBP-specific T cells in these mice. γδ T cells from CFA- or IFA plus TLR ligand-treated mice were activated and injection of naïve TCR-δ−/− mice with γδ T cells from TLR ligand-treated, but not untreated, B6 mice promoted the in vivo priming of IL-17+ IRBP-reactive T cells. In conclusion, in vivo priming of IL-17+ uveitogenic T cells in mice is significantly affected by TLR ligation, and is also influenced by activated γδ T cells.
Keywords: autoimmunity, EAU, Interleukin-17, Th17, uveitis
1. Introduction
Th17 cells are defined by their production of IL-17 and IL-22, and to a lesser extent, tumor necrosis factor (TNF-α) and IL-6 [1]. Recent studies have shown that Th17 cells contain major pathogenic populations in autoimmune diseases [2–6]. An understanding of the immunologic conditions that regulate the differentiation and activation of this autoreactive T cell population is therefore of importance in understanding the pathogenesis of autoimmune diseases. As the frequencies of in vivo primed autoreactive T cells are very low (< 1 per 10,000 responder T cells in immunized rodents) [7, 8], many currently used assays determine Th17 responses by relying on in vitro expanded T cells, which requires controlled culture conditions. Th17 cells only become the dominant cell type among in vitro activated autoreactive T cells under “Th17 polarizing” conditions (culture medium containing IL-23) [6, 9]. In addition, Th17 responses are easily inhibited by a number of pro-inflammatory cytokines, including IL-12 [10], IFN-γ [2, 11], IL-4 [2, 11], and IL-2 [12]. Moreover, the in vitro expansion of in vivo primed T cells is affected by culture conditions, including antigen dose [13–15], the affinity of the T cell receptor (TCR) for antigen [16–18], the duration of the T cell-antigen presenting cell (APC) interaction [19], and the cytokines present, such as IL-2 and IL-23, which polarize activated T cells and prevent activated T cell death [20–24]. The adjustment of all of these parameters so that they faithfully reflect what happens in vivo is difficult at best. To avoid this problem, in this study, we used a combination of ex vivo and in vitro assays and estimated numbers of in vivo primed Th1 and Th17 autoreactive T cells before and after in vitro expansion using the limiting dilution assay (LDA), currently the only assay that can directly estimate the number and frequency of in vivo primed T cells [8, 25, 26]. Our goal was to determine the environmental and cell-cell interaction factors that affect the in vivo priming of Th1 and Th17 autoreactive T cells in experimental autoimmune uveitis (EAU). The results showed that the mycobacterial antigens in complete Freund’s adjuvant (CFA) were a crucial factor in promoting the in vivo priming of Th17 autoreactive T cells and that part of the mycobacterial effect could be replaced by synthetic TLR2 and TLR4 ligands, both in vitro and in vivo. We also found that, in the absence of γδ T cell participation, as in TCR-δ−/− mice, Th17 responses were significantly compromised but could be restored when recipient TCR-δ−/− mice were injected with a small number of activated γδ T cells. In conclusion, the intensity of the Th17 response in EAU appears to be regulated by TLR2 and TLR4 ligation, and γδ T cells are actively involved in the in vivo priming of Th17 autoreactive T cells.
2. Materials and Methods
2.1 Animals and reagents
Female C57BL/6 (B6), B10RIII, and TCR-δ−/− mice (all 12- to 14-weeks-old) were purchased from Jackson Laboratory (Bar Harbor, ME) and were housed and maintained in the animal facilities of the University of Southern California. Institutional approval was obtained and institutional guidelines regarding animal experimentation followed. Recombinant murine IL-23 was purchased from R & D. (Minneapolis, MN). FITC-conjugated anti-mouse IFN-γ and anti-mouse IL-17 antibodies were purchased from Biolegend (San Diego, CA), while all other antibodies were from BD Bioscience (La Jolla, CA). The synthetic TLR agonists Pam3CSK (TLR2), Poly IC (TLR3), LPS (TLR4), and CpG1826 (TLR9) were purchased from InvivoGen (San Diego, CA).
2.2 Immunization procedures and in vitro stimulation of in vivo primed T cells
Mice were immunized subcutaneously over 6 spots at the tail base and on the flank with 200 μl of emulsion containing uveitogenic peptide. The uveitogenic peptide used for B6 and TCR-δ−/− mice was IRBP1-20 [amino acids 1-20 of human interphotoreceptor retinoid-binding protein (IRBP), 150 μg/mouse] and that for B10RIII mice was IRBP161-180 (amino acids 161–180 of human IRBP, 100 μg/mouse) (Sigma, St. Louis, MO). The peptides were emulsified in either complete Freund’s adjuvant (CFA), incomplete Freund’s adjuvant (IFA) (Sigma, St. Louis, MO), or IFA containing TLR ligands. The doses of TLR ligands used for in vivo immunization were 75 μg of Pam3CSK (TLR2), 100 μg of Poly IC (TLR3), 50 μg of purified LPS (TLR4), and 50 μg of CpG1826 (TLR9). Unless otherwise stated, all immunized mice were injected intraperitoneally with a single dose of PTX (200 ng).
At day 13 post-immunization, T cells were isolated from lymph node cells and spleen cells by passage through a nylon wool column, then 1 × 107 cells in 2 ml of RPMI 1640 medium (Cellgro, VA, USA) containing 10% fetal calf serum in each well of a 6-well plate (Costar) were stimulated for 48 h with 1 μg/ml of anti-CD3 antibody or 10 μg/ml of IRBP1-20 in the presence of 1 × 107 irradiated syngeneic spleen cells (APCs) in the presence of either IL-12 or IL-23 (10 ng/ml), then activated T cell blasts were separated by Ficoll gradient centrifugation and cultured for another 72 h in the same medium used for stimulation without the peptide.
2.3 Limiting dilution analysis (LDA)
B6 and B10RIII mice were immunized with IRBP1-20 or IRBP161-180, respectively, emulsified in either CFA, IFA, or IFA containing TLR ligands. The spleen and draining lymph nodes removed 14 days later and a single-cell suspension prepared. T cells were enriched by nylon wool adherence and seeded in 24 replicates in two sets of 96-well flat-bottomed culture plates containing irradiated spleen cells (1 × 105 per well), under Th1 or Th17 polarizing conditions, with one set of plates containing an optimal dose of immunizing peptide (10 μg/ml). Based on preliminary LDA estimates of IRBP-reactive cell frequencies, the number of T cells seeded in each well varied from 3 × 103 to 2 × 105. Forty eight hours later, a fraction of the culture supernatants was harvested and analyzed for IFN-γ or IL-17 production. The plates were then pulsed with 0.5 μCi of [3H]thymidine/well for 6 h, harvested, and assessed for isotope incorporation. Preliminary results showed that the frequencies of responder T cells measured by either cytokine production or proliferation were very similar, so, in later studies, only cytokine (IL-17 or IFN-γ) production was measured.
Activation of IRBP1-20-specific T cells was estimated by comparing the proliferation and IFN-γ and IL-17 production of graded numbers (3,000–200,000/well) of T cells in the presence and absence of IRBP peptide under polarizing conditions. Twenty-four replicate wells were used for each cell density. After 44 h of incubation, the plates were centrifuged and 50 μl of supernatant harvested for lymphokine assays and the rest of the cultures pulsed with 3H-thymidine for 6 h, harvested, and counted in a β-counter. Positive microcultures were defined as those in which lymphokine activity or incorporated thymidine exceeded the mean activity in control cultures (no responders) by more than three standard deviations [27–29]. The frequency of responder T cells was obtained by the minimum estimates of precursor frequency calculated using a program developed to analyze the LDA data acquired [8, 30]. This program uses the Poisson distribution to calculate the frequency of responder T cells with 99% confidence limits.
2.4 Cell proliferation and cytokine assays
Enriched T cells (3 × 104 cells/well) from the draining lymph nodes and spleens prepared by nylon wool adherence were cultured at 37° C for 48 h in 96-well microtiter plates with irradiated syngeneic spleen APCs (1 × 105) in the presence or absence of IRBP1-20, then a fraction of the culture supernatant was analyzed for IL-17 production using ELISA kits (R & D). The plates were then pulsed for 6 h with 0.5 μCi of [3H]thymidine/well and the cells assessed for isotope incorporation (Packard). The proliferative response was expressed as the mean cpm ± standard deviation (SD) of triplicate determinations.
2.5 Induction of EAU
For active induction of EAU, B10RIII mice were immunized subcutaneously with 100 μl of an emulsion containing 100 μg of IRBP161-180 peptide emulsified in CFA, IFA (Difco, Detroit, MI), or IFA containing TLR ligands, distributed over six spots on the tail base and flank. Concurrently, 200 ng of pertussis toxin (PTX) (Sigma, St. Louis, MO) was injected intraperitoneally.
2.6. Scoring of EAU
The mice were examined three times a week for clinical signs of EAU by indirect fundoscopy. The pupils were dilated using 0.5% tropicamide and 1.25% phenylephrine hydrochloride ophthalmic solutions and fundoscopic grading of disease performed using the scoring system described previously [31]. For histopathological evaluation, whole eyes were collected at the end of the experiment and immersed for 1 h in 4% glutaraldehyde in phosphate buffer, pH 7.4, then were transferred to 10% formaldehyde in phosphate buffer until processed. The fixed and dehydrated tissues were embedded in methacrylate, then 5 μm sections were cut through the pupillary-optic nerve plane and stained with hematoxylin and eosin. Presence or absence of disease was evaluated blind by examining six sections cut at different levels for each eye. Disease was graded pathologically based on cellular infiltration and structural changes [32].
2.7 Intracellular staining and FACS analysis
For intracellular staining, T cells (2 × 105 in 100 μl) were exposed to 50 ng/ml of PMA, 1 μg/ml of ionomycin, and 1 μg/ml of brefeldin A (Sigma-Aldrich) for 4 h, then were washed, fixed, permeabilized overnight with Cytofix/Cytoperm buffer (eBioscience), and intracellularly stained with antibodies against IFN-γ and IL-17 and analyzed on a FACScalibur flow cytometer.
2.8 Statistical analysis
Experiments were repeated at least twice, usually three or more times. Experimental groups were typically composed of four mice. The figures show data from a representative experiment. Differences between the values for different groups were examined by the two-tailed t test. Statistical analyses of clinical scores were performed using one-way ANOVA with Tukey post hoc analysis. A P value < 0.05 was considered as significant.
3. Results
3.1 Immunization converts Th1 responses to Th17 responses in C57BL/6 mice
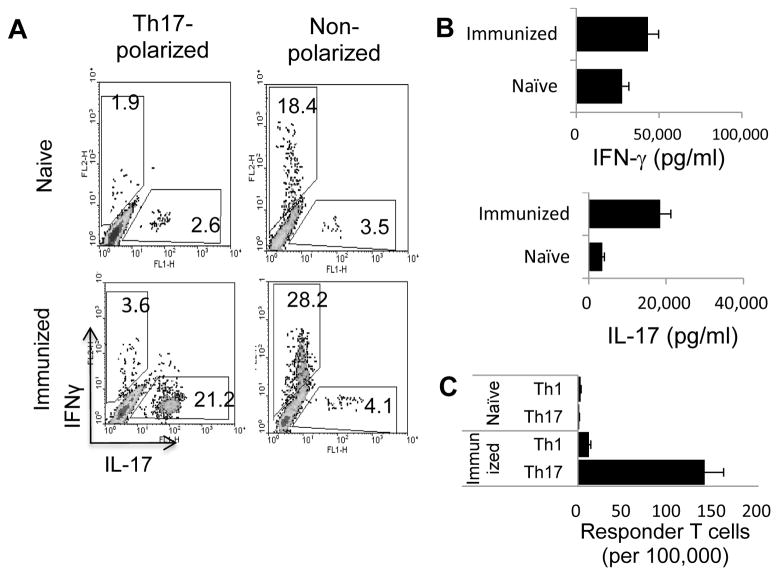
Splenic T cells prepared from naïve B6 mice were stimulated with a mitogenic dose of anti-CD3 antibodies (1 μg/ml) and syngeneic APCs (irradiated spleen cells) under either non-polarizing conditions or polarizing conditions (culture medium supplemented with IL-12 or IL-23, respectively), favoring either Th1 or Th17 reactivity. Th1 and Th17 responses were assessed by cytofluorimetric determination of IFN-γ+ and IL-17+ T cells and ELISA analysis of IFN-γ and IL-17 production by activated T cells. Fig. 1A shows that IL-17+ cells were 10 times more frequent (21.2%) among from activated splenic T cells from immunized mice than among those from in naïve mice (2.6%), whereas the frequency of IFN-γ+ T cells was increased less than two-fold (28.2% versus 18.4%). Cytokine assays (Fig. 1B) showed that T cells from immunized mice produced 6 times as much IL-17 as those from naïve mice (from 3,000 to 18,000 pg/ml), whereas amounts of IFN-γ, which were already high in untreated mice, increased less than 2-fold by immunization (from 27,000 to 43,282 pg/ml). To directly determine the frequency of in vivo primed T cells, we also performed an LDA assay. The results (Fig. 1C) showed that, in naïve mice, the average frequency of Th1 responder T cells generated by treatment with anti-CD3 antibody was approximately 3 per 100,000 responder T cells and the frequency of Th17 responder T cells was consistently lower in the range of 1–2 per 100,000 responder T cells, whereas, in immunized mice, the frequency of IL-17-producing T cells increased >40-fold to approximately 140 per 100,000 responder T cells, whereas IFN-γ-producing cells increased only 4-fold (12.3 cells per 100,000 responder T cells), indicating that the immunization procedure promotes Th17 responses more than Th1 responses.
Fig. 1. Comparison of anti-CD3 antibody-induced Th1 and Th17 responses in naïve and immunized B6 mice.
A). Splenic T cells from naïve and IRBP1-20/CFA-immunized mice enriched by passage through nylon wool were stimulated for 48 h with a mitogenic dose of anti-CD3 antibody (1 μg/ml) under Th17 polarizing or non-polarizing conditions and the activated T cells separated by Ficoll gradient centrifugation on day 3 and intracellularly stained with phycoerythrin (PE)-conjugated anti-IFN-γ antibodies and FITC-conjugated anti-IL-17 antibodies, followed by FACS analysis on Day 5. (B) IFN-γ and IL-17 levels in the culture supernatants at 48 h assessed by ELISA. (C) Responder T cell numbers evaluated by LDA. The results shown are representative of those from >5 experiments.
3.2 Mycobacterial antigens in CFA promote the generation of IL-17+ T cells in immunized mice
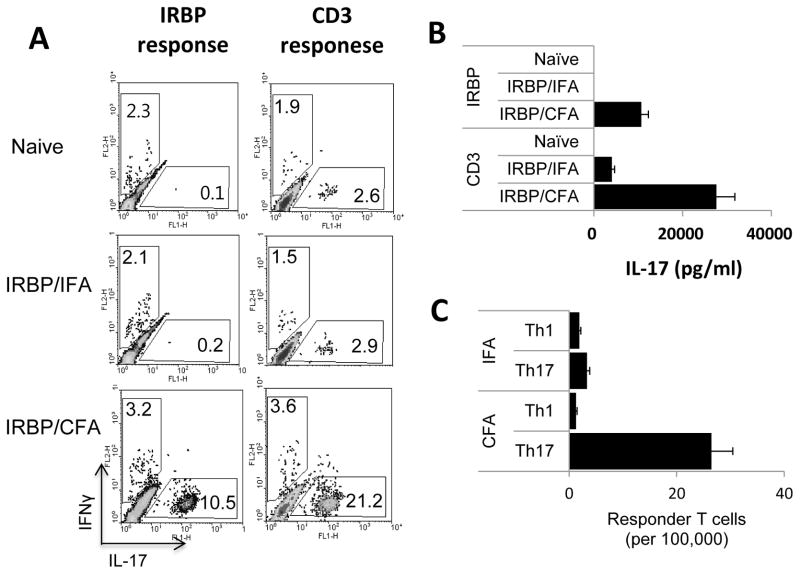
To determine the factors responsible for the enhanced generation of IL-17+ T cells, B6 mice were immunized with IRBP1-20 in either IFA or CFA, with non-immunized mice as control. T cells from naïve or immunized mice were then stimulated in vitro with either IRBP1-20 (10 μg/ml) or anti-CD3 antibody (1 μg/ml) under Th17 polarizing conditions. The frequency of IL-17+ T cells among the activated responder T cells was then determined by intracellular staining and cytokine production was measured by ELISA. As shown in Fig. 2A, mice immunized with IRBP1-20 in CFA contained a significantly higher percentage of IL-17+ T cells after stimulation with either immunizing antigen (left panel) or anti-CD3 antibodies (right panel), whereas T cells from mice immunized with IRBP1-20/IFA (center row) or naïve mice (top row) generated very few. Mice immunized with IRBP1-20/CFA also produced significantly greater amounts of IL-17 than mice immunized with IRBP1-20/IFA (Fig. 2B). These results show that components in CFA promote the in vivo activation of IL-17+ T cells. As an approach to investigating the underlying mechanism, we measured the frequency of in vivo primed IFN-γ+- and IL-17+ T cells specific for the immunizing peptide by LDA. As shown in Fig. 2C, the frequency of IRBP1-20-specific Th17 responder T cells among the T cells from IRBP1-20/CFA immunized mice was much higher than that in IRBP1-20/IFA-immunized mice, and more than 10 times higher than that of IRBP1-20-specific Th1 responder T cells.
Fig. 2. Numbers of IL-17+ cells are greatly increased in B6 mice immunized with IRBP1-20 in CFA, but not IRBP1-20 in IFA.
(A) B6 mice were immunized with the uveitogenic peptide IRBP1-20 in IFA or CFA, then splenic T cells were stimulated for 48 h with either IRBP1-20 (left panels) or anti-CD3 antibody (right panels) and the activated T cells stained with PE-conjugated anti-IFN-γ antibodies and FITC-conjugated anti-IL-17 antibodies, followed by FACS analysis. (B) IL-17 levels in the culture supernatants at 48 h assessed by ELISA. (C) Frequencies of IFN-γ+ and IL-17+ IRBP1-20 -specific T cells determined by LDA. The results shown are representative of those from >5 experiments. The differences between the CFA and IFA groups were highly significant (P ≤ 0.01) in all three assays.
3.3 The effect of mycobacterial antigen in CFA can be partially substituted by synthetic TLR ligands
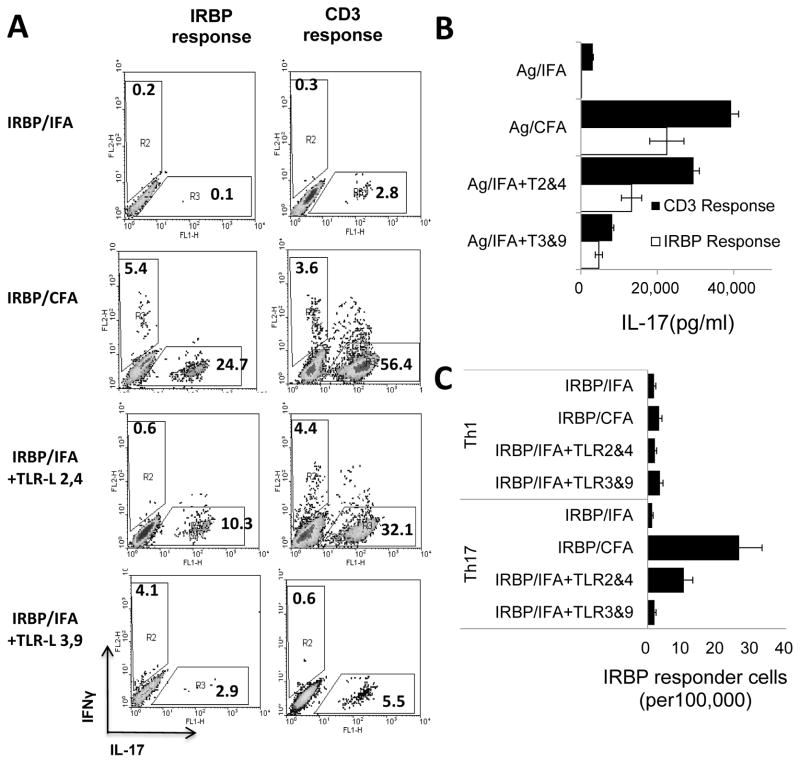
Since the only difference between IFA and CFA is the presence of mycobacteria in CFA, we examined whether the effect of the mycobacteria could be replaced by synthetic TLR ligands. Groups of B6 mice (n=4) were immunized with IRBP1-20/CFA, IRBP1-20/IFA, or IRBP1-20/IFA containing combinations of TLR2 and TLR4 ligands or TLR3 and TLR9 ligands, then the in vivo primed T cells were stimulated with immunizing antigen and APCs under Th17 polarizing conditions and the frequency of IL-17+ cells measured by intracellular staining 5 days after stimulation. As shown in Fig. 3A, the frequency of IL-17+ T cells was significantly higher in mice immunized with IRBP1-20 in CFA than in mice immunized with IRBP1-20/IFA. In addition, mice immunized with IRBP1-20 in IFA containing TLR2 and TLR4 ligands also contained a significantly higher percentage of IL-17+ IRBP1-20 -specific T cells than mice immunized with IRBP1-20/IFA, while mice immunized with the same procedure but using the TLR3 and TLR9 ligands did not. Cytokine assays showed that T cells from mice immunized with IRBP1-20 in IFA containing TLR2 and TLR4 ligands, but not TLR3 and TLR9 ligands, produced significantly higher amounts of IL-17 than those from mice immunized with IRBP1-20/IFA only (Fig. 3B). The results of an LDA assay (Fig. 3C) were consistent with the above results and showed that IRBP1-20/IFA-immunized mice generated the lowest numbers of Th17 cells (2 per 100,000 T cells) and the use of CFA resulted in a >10-fold increase in Th17 reactive T cells (26 in 100,000). Again, immunization with IRBP1-20 in IFA containing TLR2 and TLR4 ligands resulted in a significant increase in Th17 T cells, whereas immunization with IRBP1-20 in IFA containing TLR3 and TLR9 ligands did not.
Fig. 3. IFA containing synthetic TLR ligands enhances the in vivo priming of IL-17+ T cells in B6 mice.
(A) Determination of IL-17+ cells by intracellular staining. Groups of mice (n=4) were immunized with IRBP1-20/CFA, IRBP1-20/IFA, or IRBP1-20/IFA containing TLR2 and TLR4 ligands or TLR3 and TLR9 ligands. The in vivo primed T cells were then stimulated with IRBP1-20 or anti-CD3 antibodies in the presence of syngeneic APCs for 5 days under Th17 polarized conditions and activated T cells stained with PE-conjugated anti-IFN-γ antibodies and FITC-conjugated anti-IL-17 antibodies, followed by FACS analysis. (B) IL-17 levels in the culture supernatants assessed by ELISA 48H after stimulation. (C) LDA results showing the number of IFN-γ (Th1) or IL-17+ (Th17) T cells among 100,000 in vivo primed responder T cells. The results shown are representative of those in more than three experiments.
3.4 TLR2 and TLR4 ligands enhance the ability of IRBP161-180 to induce EAU in B10RIII mice in the absence of CFA
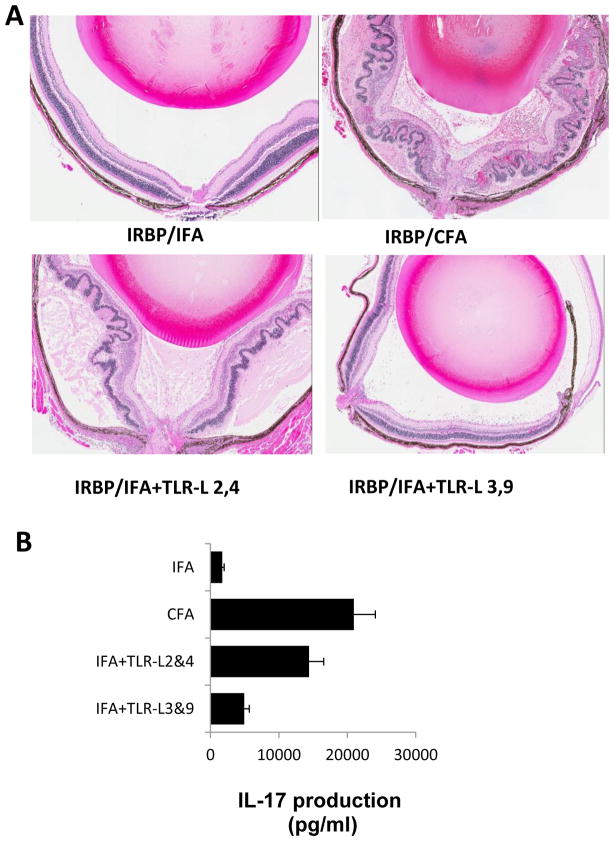
To determine the in vivo influence of TLR ligands on induction of autoreactive T cells and EAU, EAU-prone B10RIII mice were immunized with the uveitogenic peptide IRBP161-180 emulsified in IFA, CFA, or IFA containing TLR ligands. Starting from 10 days after immunization, EAU development was monitored by fundoscopy followed by pathologic examination. As shown in Fig. 4A, mice immunized with IRBP161-180/CFA developed EAU, whereas mice immunized with IRBP161-180/IFA did not. Interestingly, mice immunized with IRBP160-181 in IFA containing TLR2 and TLR4 ligands developed mild, but significant, EAU and the EAU induced by immunization with IRBP160-181 in IFA containing TLR3 and TLR9 ligands was hardly detectable. As shown in Fig. 4B, ELISA assays showed that T cells isolated from mice immunized with IRBP160-181 in IFA containing TLR2 and TLR4 ligands produced significantly increased amounts of IL-17 compared to mice immunized with IRBP161-180/IFA.
Fig. 4. Synthetic TLR2 and TLR4 agonists increase the EAU-inducing activity of the uveitogenic peptide IRBP161-180 in the B10RIII mouse.
(A) B10RIII mice were immunized with 100 μg of uveitogenic peptide IRBP161-180 emulsified in IFA, CFA, or IFA containing TLR ligands as indicated. All the mice received an additional injection of PTx (200 ng/mouse) on day 0. The photographs show the histopathology of EAU in immunized mice (hematoxylin and eosin, original magnification, x200). (B) The in vivo primed T cells were stimulated with IRBP161-180 in the presence of syngeneic APCs for 5 days under Th17 polarizing conditions and IL-17 levels in the culture supernatant were assessed by ELISA.
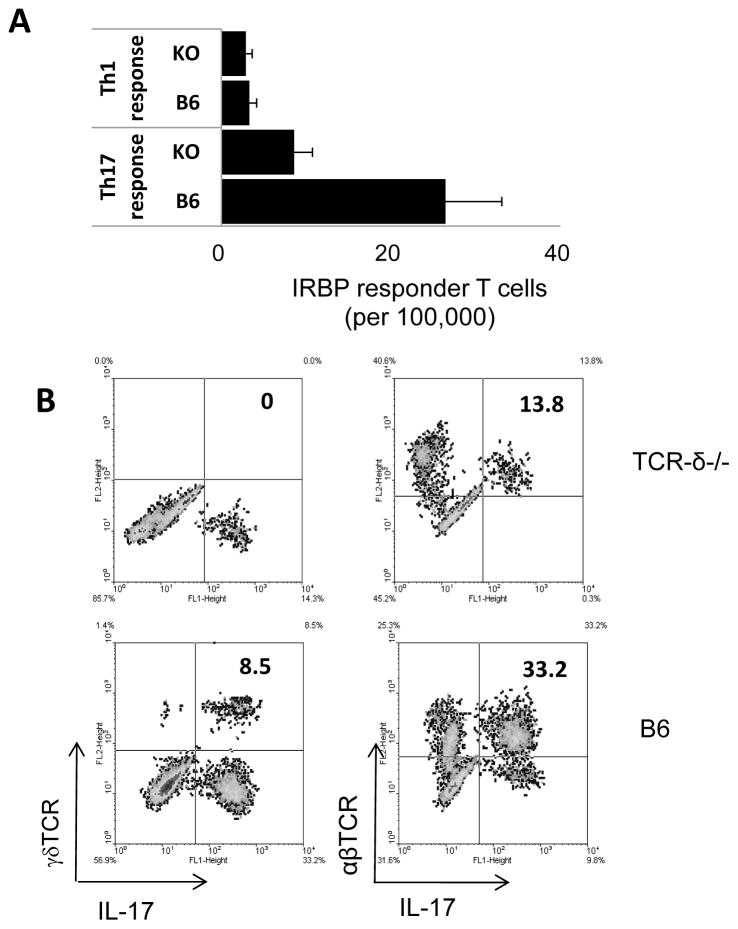
3.5 Numbers of in vivo primed IL-17+ T cells are decreased in immunized TCR-δ−/− mice compared to immunized wild-type mice
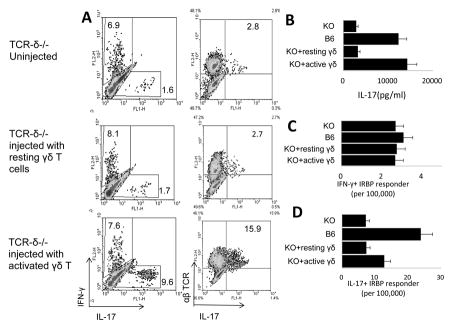
Based on the numbers of in vitro expanded T cells derived from in vivo primed T cells, it appeared that TCR-δ−/− mice generate decreased numbers of IL-17+ autoreactive T cells [33–35]. To examine this question more rigorously, we directly compared the frequencies of in vivo primed Th1 and Th17 autoreactive T cells in immunized wild-type (WT) B6 and TCRδ−/− mice, using LDA. As shown in Fig. 5A, the frequency of in vivo primed IFN-γ+ IRBP1-20-specific T cells was not significantly different in immunized TCRδ−/− mice (2.9 per 100,000) when compared to WT B6 mice (3.3 per 100,000. In contrast, the frequency of in vivo primed Th17 IRBP1-20-specific T cells was much lower in TCR-δ−/− mice (8.6 per 10,000) than in WT mice (26.5 per 100,000 responder T cells). Intracellular staining of the in vivo primed T cells expanded after stimulation with the immunizing antigen also showed that TCR-δ−/− mice generated substantially lower numbers of IL-17+ T cells compared to their B6 counterparts (Fig. 5B). However, when a small number (100,000/mouse) of activated, but not non-activated, γδ T cells was administered 3 days before immunization, the recipient mice showed a strong enhancement of the Th17 response (Fig. 6A). The results of the LDA assay (Fig. 6C and D) further emphasized these findings and showed that TCR-δ−/− mice injected with activated, but not resting, γδ T cells generated significantly increased numbers of Th17 cells, but not Th1 cells. Taken together, these observations suggest that, γδ T cells have a stronger regulatory effect on the Th17 response than the Th1 response, at least in this experimental setting.
Fig. 5. Immunized TCR-δ−/− mice generate fewer IL-17+ autoreactive T cells than immunized B6 mice.
(A) B6 and TCR-δ−/− mice were immunized with IRBP1-20/CFA, then, 13 days later, splenic T cells were enriched and assessed for Th1 and Th17 IRBP1-20-specific T cells by LDA. (B) Splenic T cells from immunized B6 and TCR-δ−/− mice were stimulated with IRBP1-20 and APCs under Th17 polarizing conditions for 5 days, then the activated T cells were separated, fixed, and stained with anti-IFN-γ, anti-IL-17, and anti-αβ TCR antibodies.
Fig. 6. Activated γδ T cells enhance the Th17 response in TCR-δ−/− mice.
(A) Three days before immunization with IRBP1-20/CFA TCR-δ−/− mice were left untreated (top panels) or were injected with 100,000 non-activated (resting) γδ T cells (center panels) or anti-CD3 antibody-activated γδ T cells (bottom panels) [33]. Fourteen days after immunization, 1.5 × 106/well splenic T cells were stimulated with immunizing peptide and APCs in 24-well plates for 2 days and IFN-γ and IL-17+ T cells among the activated T cells were assessed by FACS analysis after intracellular staining for IFN-γ and IL-17. (B) Part of the culture supernatants was collected for IL-17 measurement. (C and D) LDA results showing the numbers of IFN-γ (Th1) or IL-17+ (Th17) T cells among 100,000 in vivo primed responder T cells. The results shown are representative of those in more than three experiments. In B–D, results for immunized WT B6 and TCR-δ−/− mice are shown for comparison.
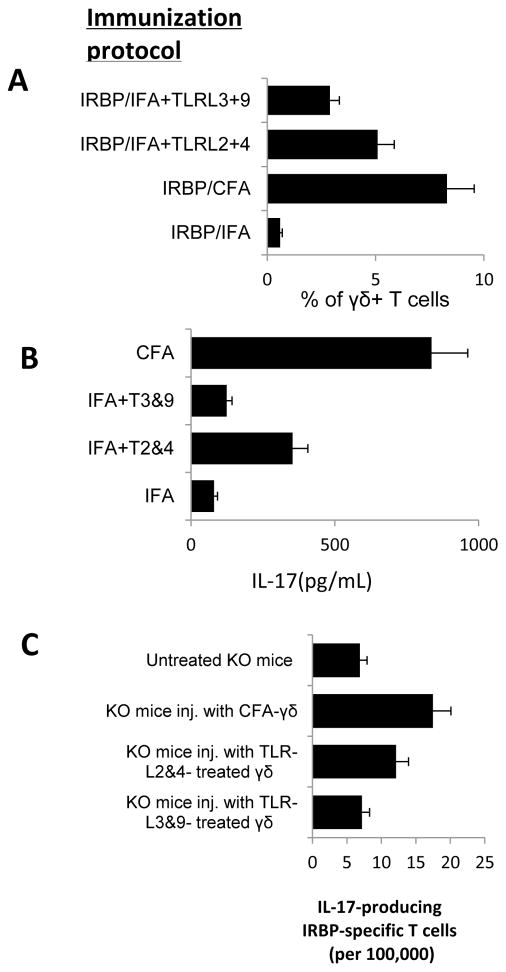
3.6 TLR ligands enhance the Th17 response by acting on γδ T cells
To determine the mechanism(s) by which TLR2 and TLR4 ligands enhance the Th17 response in EAU, we examined whether the TLR ligands that caused an enhanced Th17 response had a direct effect on dendritic cells or γδ T cells. First, we tested whether splenic APCs from IRBP1-20/CFA-immunized B6 mice had a greater stimulatory effect than those from IRBP1-20/IFA-immunized mice on the activation of in vivo primed Th17 cells from IRBP1-20/CFA-immunized mice. The results showed that the two types of APCs induced similar numbers of IL-17+ T cells as examined by intracellular staining (Fig. 7A) and by IL-17 ELISA (data not shown). We then tested whether the TLR ligands acted on γδ T cells. Mice immunized with IRBP1-20 in IFA containing TLR2 and TLR4 ligands contained increased numbers of γδ T cells compared to those immunized with IFA alone (Fig. 7A) and γδ T cells freshly isolated from mice immunized with CFA or IFA containing TLR2 and TLR4 ligands, but not those from mice immunized with IFA containing TLR3 and TLR9 ligands produced increased amounts of IL-17 (Fig. 7B). We then compared the Th17 responses in recipient TCR-δ−/− mice injected with equal numbers of γδ T cells from B6 mice immunized with IRBP1-20 in CFA, IFA, or IFA containing TLR ligands, and subsequently immunized with IRBP1-20/CFA by measuring the IRBP1-20-specific IL17+ T cells among the activated T cells after stimulation with the immunizing peptide. As shown in Fig. 7C, TCR-δ−/− mice injected with γδ T cells from IRBP1-20/CFA-immunized B6 mice contained a higher frequency of IL17+ IRBP1-20-specific T cells than those injected with γδT cells from B6 mice immunized with IRBP1-20/IFA only. TCR-δ−/− mice injected with γδ T cells from B6 mice immunized with IRBP1-20 in IFA containing TLR2 and TLR4 ligands also contained a higher frequency of IL17+ IRBP1-20-specific T cells than those injected with γδT cells from B6 mice immunized with IRBP1-20/IFA, whereas TCR-δ−/− mice injected with γδT cells from B6 mice immunized with IRBP1-20 in IFA containing TLR3 and TLR9 ligands did not.
Fig. 7. Treatment of B6 mice with a single dose of TLR ligand-activated γδ T cells rendered γδT cells acquired enhanced effect promoting the generation of IL-17+ autoreactive T cells in vivo.
(A) Splenic T cells from B6 mice immunized with IRBP1-20/IFA, IRBP1-20/CFA, or IRBP1-20/IFA containing TLRL2 and TLR4 ligands or TLRL3 and TLR9 ligands were examined for γδTCR+ T cells 14 days after immunization. (B) γδ T cells freshly isolated from B6 mice immunized with CFA or IFA containing TLR ligands 2 and TLR4 produce increased amounts of IL-17. (C) γδ T cells were isolated from B6 mice immunized with IRBP1-20/CFA, IRBP1-20/IFA, or IRBP1-20/IFA containing TLRL2 and TLR4 ligands or TLRL3 and TLR9 ligands and 100,000 isolated γδ T cells from each group were injected into four different groups of TCR-δ−/− recipient mice, then all the recipient mice were immunized with IRBP1-20/CFA, and IFN-γ+ and IL-17+ IRBP1-20 -specific responder T cells were assessed by LDA. The results shown are representative of those from three experiments.
4. Discussion
In this study, we added LDA as a quantitative assay system for detecting IRBP-specific autoreactive T cells because we wished to directly estimate the frequency of in vivo primed Th1 and Th17 autoreactive T cells. As the frequencies of in vivo primed autoreactive T cells were much lower (<1 in 10,000 responder T cells) than the minimal levels required for antibody staining [7, 8], direct counting of the number of in vivo primed responder T cells exploiting binding assays using peptide-MHC multimers [36–38] was not possible. LDA can directly assess a low number of in vivo primed T cells, even though this technique may fail to detect responder T cells in a state of anergy [36]. In this study, the LDA results were very reproducible in assessing in vivo primed Th17 autoreactive T cells. In comparison with other assays, the LDA assay is particularly reliable and variations between assays are smaller than those seen in cytokine assays or using intracellular staining. In addition, the results obtained using LDA based on cytokine or proliferation assays were comparable.
A number of autoimmune diseases have been experimentally induced in rodents by immunization of disease-prone animals with defined pathogenic antigens; however, the immunizing antigen has to be emulsified in CFA, which contains killed R37a mycobacteria [39–41]. To investigate the factors that control the activation of Th17 autoreactive T cells and the role of γδ T cells in the in vivo priming and in vitro activation of autoreactive T cells, we determined the number of activated Th1 and Th17 autoreactive T cells in B6 mice immunized with the uveitogenic peptide IRBP1-20 emulsified in either IFA or CFA and showed that immunization of mice with the uveitogenic peptide IRBP1-20 induced significantly increased numbers of IFN-γ or IL-17+ uveitogenic T cells, but only when the antigen was emulsified in CFA and not IFA. The mycobacterial components in CFA could be partially replaced by a combination of TLR2 or TLR4 ligands, whereas the effects of TLR3 and TLR9 agonists were modest.
Recent studies have shown that reciprocal interactions between the innate and adaptive immune systems play a major role in regulating the immune response [42–45]. We previously reported that initiation and progression of EAU are closely associated with increased activation of γδ T cells and that changes in the number and activation status of γδ T cells significantly affect the response of uveitogenic T cells [6, 33–35], indicating that γδ T cells play a regulatory role in this autoimmune disease. To confirm these findings and determine whether activation of γδ T cells enhances the in vivo priming of autoreactive T cells, we compared the numbers of in vivo primed Th1 and Th17 autoreactive T cells in immunized B6 and TCR-δ−/− mice and determined whether injection of TCR-δ−/− mice with a small number of γδ T cells affected the number of autoreactive T cells generated in the recipient mice. Our results showed that TCR-δ−/− mice generated only a few autoreactive T cells compared to their B6 counterparts. Injection of a small number of activated, but not resting, γδ T cells, significantly restored the ability of TCR-δ−/− mice to generate increased numbers of autoreactive T cells. Moreover, comparative studies showed that the injected γδ T cells had a greater effect on Th17 autoreactive T cells than Th1 autoreactive T cells. To determine the mechanism by which TLR ligands enhanced the in vivo activation of Th17 autoreactive T cells, we tested the effect of TLR ligands on dendritic cells and on γδT cells and found that γδ T cells from mice immunized with IRBP in IFA containing TLR2 and TLR4 agonists had a greater effect in promoting the in vivo generation of Th17 autoreactive T cells than γδ T cells isolated from IRBP/IFA-immunized mice. Our results support the conclusion that exposure to TLR ligands can promote the activation of Th17 autoreactive T cells, partially via γδ T cells, leading to a greater incidence of disease.
Highlights.
directly assessing the numbers of in vivo priming of Th17 cells using limiting dilution assays.
different TLR ligands may have different effect on Th1 and Th17 responses;
γd T cell activation via TLR ligands play a major role in the activation of autoreactive T cells in induction of autoimmune diseases via using CFA.
Acknowledgments
This work was supported in part by NIH grants EY018827, EY017373, and EY003040.
Abbreviations
- CFSE
carboxyfluorescein succinimidyl ester
- CFA
complete Freund’s adjuvant
- EAU
experimental autoimmune uveitis
- IFA
incomplete Freund’s adjuvant
- IRBP
interphotoreceptor retinoid-binding protein
- LDA
limiting dilution analysis
- PTX
pertussis toxin
- TLRs
Toll-like receptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of TH17 cells. Nature. 2008;453:1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 3.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 Plays an Important Role in the Development of Experimental Autoimmune Encephalomyelitis. J Immunol. 2006;177:566–73. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 5.Bettelli E, Oukka M, Kuchroo VK. TH-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 6.Peng Y, Han G, Shao H, Wang Y, Kaplan HJ, Sun D. Characterization of IL-17+ Interphotoreceptor Retinoid-Binding Protein-Specific T Cells in Experimental Autoimmune Uveitis. Invest Ophthalmol Vis Sci. 2007;48:4153–61. doi: 10.1167/iovs.07-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen JA, Essayan DM, Zweiman B, Lisak RP. Limiting dilution analysis of antigen-reactive lymphocytes isolated from the central nervous system of Lewis rats with experimental allergic encephalomyelitis. Cell Immunol. 1987;108:203–16. doi: 10.1016/0008-8749(87)90204-8. [DOI] [PubMed] [Google Scholar]
- 8.Sun D, Wilson DB, Cao L, Whitaker JN. The role of regulatory T cells in Lewis rats resistant to EAE. J Neuroimmunol. 1997;78:69–78. doi: 10.1016/s0165-5728(97)00176-8. [DOI] [PubMed] [Google Scholar]
- 9.Iezzi G, Sonderegger I, Ampenberger F, Schmitz N, Marsland BJ, Kopf M. CD40-CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc Natl Acad Sci USA. 2009;106:876–81. doi: 10.1073/pnas.0810769106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 Promotes a Distinct CD4 T Cell Activation State Characterized by the Production of Interleukin-17. Journal of Biological Chemistry. 2003;278:1910–4. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 11.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annu Rev Immunol. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 12.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–91. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanzavecchia A, Sallusto F. Dynamics of T Lymphocyte Responses: Intermediates, Effectors, and Memory Cells. Science. 2000;290:92–7. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 14.Bluestone JA. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995;2:555–9. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 15.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591–6. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valitutti S, Müller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide- MHC complexes. Nature. 1995;375:148–51. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 17.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–6. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 18.Wulfing C, Rabinowitz JD, Beeson C, Sjaastad MD, McConnell HM, Davis MM. Kinetics and Extent of T Cell Activation as Measured with the Calcium Signal. J Exp Med. 1997;185:1815–25. doi: 10.1084/jem.185.10.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 20.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:3810–5. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow YH, Chiang BL, Lee YL, et al. Development of Th1 and Th2 populations and the nature of immune response to hapatitis B virus DNA can be modulated by codilivery of various cytokine genes. J Immunol. 1998;160:1320–9. [PubMed] [Google Scholar]
- 22.Del Pozo MA, Sánchez-Mateos P, Sánchez-Madrid F. Cellular polarization induced by chemokines: A mechanism for leukocyte recruitment. Immunol Tod. 1996;17:127–31. doi: 10.1016/0167-5699(96)80604-9. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura T, Kamogawa Y, Bottomly K, Flavell RA. Polarization of IL-4- and IFN-γ-producing CD4+ T cells following activation of naive CD4+ T cells. J Immunol. 1997;158:1085–94. [PubMed] [Google Scholar]
- 24.Yi T, Chen Y, Wang L, et al. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009;114:3101–12. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fazekas dS. The evaluation of limiting dilution assays. J Immunol Methods. 1982;49:R11–R23. doi: 10.1016/0022-1759(82)90269-1. [DOI] [PubMed] [Google Scholar]
- 26.Olsson T, Wang WZ, Bo H, et al. Autoreactive T lymphocytes in multiple sclerosis determined by antigen-induced secretion of interferon. J Clin Invest. 1990;86:981–5. doi: 10.1172/JCI114800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerottini JC, MacDonald HR. Limiting dilution analysis of alloantigen-reactive T lymphocytes. V. Lyt phenotype of cytolytic T lymphocyte precursors reactive against normal and mutant H-2 antigens. J Immunol. 1981;126:490–6. [PubMed] [Google Scholar]
- 28.Kelso A, MacDonald HR, Smith KA, Cerottini JC, Brunner KT. Interleukin 2 enhancement of lymphokine secretion by T lymphocytes: analysis of established clones and primary limiting dilution microcultures. J Immunol. 1984;132:2932–8. [PubMed] [Google Scholar]
- 29.Romero P, Rod Dunbar P, Valmori D, et al. Ex Vivo Staining of Metastatic Lymph Nodes by Class I Major Histocompatibility Complex Tetramers Reveals High Numbers of Antigen-experienced Tumor-specific Cytolytic T Lymphocytes. J Exp Med. 1998;188:1641–50. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun D, Whitaker JN, Wilson DB. Regulatory T cells in experimental allergic encephalomyelitis. I. Frequency and specificity analysis in normal and immune rats of a T cell subset that inhibits disease. Int Immunol. 1999;11:307–15. doi: 10.1093/intimm/11.3.307. [DOI] [PubMed] [Google Scholar]
- 31.Thurau SR, Chan CC, Nussenblatt RB, Caspi RR. Oral tolerance in a murine model of relapsing experimental autoimmune uveoretinitis (EAU): induction of protective tolerance in primed animals. Clin Exp Immunol. 1997;109:370–6. doi: 10.1046/j.1365-2249.1997.4571356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao H, Liao T, Ke Y, Shi H, Kaplan HJ, Sun D. Severe chronic experimental autoimmune uveitis (EAU) of the C57BL/6 mouse induced by adoptive transfer of IRBP1-20-specific T cells. Exp Eye Res. 2006;82:323–31. doi: 10.1016/j.exer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Nian H, Shao H, O’Brien BA, Born WK, Kaplan HJ, Sun D. Activated γδ cells promote the activation of uveitogenic T cells and exacerbate EAU development. Invest Ophthalmol Vis Sci. 2011;52:5920–7. doi: 10.1167/iovs.10-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nian H, Shao H, Zhang G, et al. Regulatory effect of T cells on IL-17+ uveitogenic T cells. Invest Ophthalmol Vis Sci. 2010;51:4661–7. doi: 10.1167/iovs.09-5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui Y, Shao H, Lan C, et al. Major Role of γδ T Cells in the Generation of IL-17+ Uveitogenic T Cells. J Immunol. 2009;183:560–7. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMichael AJ, O’Callaghan CA. A new look at T cells. J Exp Med. 1998;187:1367–71. doi: 10.1084/jem.187.9.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abastado J-P, Lone Y-C, Casrouge A, Boulot G, Kourilsky P. Dimerization of soluble major histocompatibility complex-peptide complexes is sufficient for activation of T cell hybridoma and induction of unresponsiveness. J Exp Med. 1995;182:439–47. doi: 10.1084/jem.182.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allan DSJ, Colonna M, Lanier LL, et al. Tetrameric complexes of human histocompatibility leukocyte antigen (HLA)-G bind to peripheral blood myelomonocytic cells. J Exp Med. 1999;189:1149–55. doi: 10.1084/jem.189.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welch AM, Holda JH, Swanborg RH. Regulation of experimental allergic encephalomyelitis II. Appearance of suppressor cells during the remmision phase of the disease. J Immunol. 1980;125:186–9. [PubMed] [Google Scholar]
- 40.Sun D, Whitaker JN, Huang Z, et al. Myelin Antigen-Specific CD8+ T cells are Encephalitogenic and Produce Severe Disease In C57BL/6 Mice. J Immunol. 2001;166:7579–87. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- 41.Sun D, Zhang Y, Wei B, Peiper SC, Shao H, Kaplan HJ. Encephalitogenic activity of truncated myelin oligodendrocyte glycoprotein (MOG) peptides and their recognition by CD8+ MOG-specific T cells on oligomeric MHC class I molecules. Int Immunol. 2003;15:261–8. doi: 10.1093/intimm/dxg023. [DOI] [PubMed] [Google Scholar]
- 42.Strominger JL. Bacterial cell walls, innate immunity and immunoadjuvants. Nat Immunol. 2007;8:1269–71. doi: 10.1038/ni1207-1269. [DOI] [PubMed] [Google Scholar]
- 43.Fearon DT, Locksley RM. The Instructive Role of Innate Immunity in the Acquired Immune Response. Science. 1996;272:50–4. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 44.Dong Kim K, Zhao J, Auh S, et al. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13:1248–52. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–50. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]