Abstract
Disseminated metastasis accounts for over 90% of breast cancer deaths. Recently, elevated serum levels of a glycoprotein known as chitinase-3 like-protein-1 (CHI3L1) has been correlated with poor prognosis and shorter survival of patients with metastatic breast cancer. In this study, we show that there are increased levels of CHI3L1 in plasma of tumor-bearing mice and that both tumor cells and immune cells express and secrete CHI3L1. However, the biological and physiological functions of CHI3L1 are still unclear. We demonstrate that while CHI3L1 has an inhibitory role in the expression of interferon-gamma (IFN-γ), CHI3L1 up-regulates pro-inflammatory mediators, C-chemokine ligand 2 (CCL2), Chemokine CX motif ligand 2 (CXCL2) and matrix metalloproteinase-9 (MMP-9) all of which contribute to tumor growth and metastasis. We found that in vitro inhibition of CHI3L1 by siRNA suppressed the production of CCL2, CXCL2 and MMP-9 by macrophages. In vivo treatment of mammary tumor-bearing mice with chitin (β-(1–4)-poly-N-acetyl D-glucosamine), a TH1 adjuvant and a ligand for CHI3L1, promoted immune effector functions with increased production of IFN-γ and decreased CCL2, CXCL2 and MMP-9 expression. In vivo administration of chitin to mammary tumor-bearing mice significantly decreased lung metastasis. These studies show that CHI3L1 plays a role in tumor progression and that chitin can inhibit the pleiotropic effects of CHI3L1 giving support to the idea that CHI3L1 is a useful therapeutic target for treatment of breast cancer.
Keywords: chitinase-3-like-1 protein, metastasis, immunosuppression, chitin, CCL2, CXCL2 and MMP-9
Introduction
Chitinase like proteins (CLPs) are upregulated in a number of human cancers and in non-neoplastic diseases characterized by inflammation and tissue remodeling 1. Increased serum levels of CHI3L1 is associated with disease severity, poorer prognosis, and shorter survival in cancers of the breast, colon, prostate, ovaries, brain, thyroid, lung, and liver1. CHI3L1 (aka YKL-40, BRP-39) is a non-enzymatic member of chitinase family of glycoproteins2, and is produced by macrophages, neutrophils, activated chondrocytes, smooth muscle cells and tumor cells3, 4. The expression of chitinases and CLPs has been associated with the TH2 inflammatory response 5–7. Specifically, Kzhyshkowska et al. demonstrated a direct effect of TH2 cytokines on the stimulation of Stabilin-1-CLP expression7.
Tumor progression is often skewed towards a TH2 immune response with downregulation of IFN-γ8–10. Furthermore, proinflammatory chemokines and matrix degrading enzymes are upregulated in many types of cancers including breast cancer and are known to aid in tumor growth and metastasis11–14. Our previous studies have shown that production of CCL2 and MMP-9 is increased in mammary tumor-bearing mice while the production of IFN-γ is downregulated15, 16. Recent studies have shown that alveolar macrophages exposed to recombinant CHI3L1 produce higher levels of the proinflammatory mediators MMP-9, CCL2, CCL3 and CXCL2.17. There are no reported studies to date correlating the expression of CHI3L1 with upregulation of these proinflammatory molecules in tumor-bearing hosts. We demonstrate that there are elevated levels of CHI3L1 in the sera of tumor-bearing mice and that it is one of the factors that induce CCL2, CXCL2 and MMP-9 production. Since CHI3L1 levels are increased in tumor hosts and appear to have a pleiotropic role in the production of chemokines and MMPs, we explored the role of CHI3L1 in immune modulation and tumor metastasis and assessed if inhibition of CHI3L1 improves the prognosis.
Chitin, a TH1 adjuvant and ligand of CHI3L1, affects immune responses depending on the size of its particles. Shibata et al. and others have shown that chitin (1–10 μm) induces activation of M1 type macrophages with production of cytokines that provide host resistance to microbial infections6, 18, 19. Furthermore it has been shown that phagocytosis of 1 μm polystyrene microparticles or 1–10 μm microparticles of chitosan (de-acetylated chitin) or latex beads does not activate macrophages indicating that “chitin” chemical structure is required for M1 macrophage activation18. In contrast, chitin beads (>50 μm) induce M2 type macrophages and promote allergic response20, 21. Although chitin treatment affects the immune response, it is not known whether treatment with chitin also has an effect on CHI3L1 and chemokine expression. In vivo treatment of tumor-bearing mice with chitin decreases the production of CCL2, CXCL2, MMP-9 and increases the production of IFN-γ. More importantly, treatment with chitin significantly reduced tumor growth and metastasis.
Materials and Methods
Mice and cell lines
8–12 week-old BALB/c mice used in these studies (Charles River Laboratories) were cared for and used according to the guidelines of the National Institutes of Health. DA-3 and DA-3 mammary tumor cell lines expressing firefly luciferase (DA-3 LUC) provided by Dr. Diana Lopez (University of Miami, Miami, FL.) were maintained in DMEM/high glucose media with supplements16. These tumor cells express MMP-9, granulocyte macrophage growth factor (GMCSF) and vascular endothelial growth factor (VEGF) but not CCL2. 4T1-luc-A4 mammary tumor cells22 (Caliper Life Sciences, Hopkinton, MA) were maintained in RPMI with 10% FCS. Tumor cells were implanted in BALB/c mice by subcutaneous injection of 1×106 DA-3 or 1×105 4T1 tumor cells in the lower right ventral quadrant and 5-wk tumor bearers were used unless otherwise specified. These tumors metastasize to the lung, liver, and bone but not to the spleen. All animal experiments were approved by the Institutional Animal Care and Use Committee of Florida Atlantic University.
Chitin treatment
Chitin (1–10 μm) was prepared as described previously18, 23, 24. In vitro cultures contained 10 μg/mL chitin. Normal and tumor-bearing mice were treated by intraperitoneal injection with chitin (1 mg/mouse) starting 3 days post-tumor implantation and at every third day for 5 weeks. Mice were imaged for tumor growth at 3, 10 and 21 days post-chitin treatment using a bioluminescence optical imager (IVIS Lumina LTE, Caliper Life Sciences, Xenogen). Inflammatory mediators were assayed at 5 weeks post-tumor implantation.
Purification of cell subpopulations
Subpopulations of splenic cells, i.e., T cells (CD90.2+), NK cells (CD49b+), B cells (B220+), macrophages (F4/80) and myeloid derived cells (CD11b+GrhighLy6G+ or CD11b+GrlowLy6G−) (MDCs) were purified by Miltenyi (Miltenyi Biotec., Auburn, CA) magnetic bead separation using anti-CD90.2, anti-B220, or cocktail of anti-CD11b, Gr, Ly6G -coated microbeads as described previously16, 25. Macrophages and NK cells were purified by indirect labeling with PE anti-F4/80 and PE anti-CD49b, respectively (BioLegend, San Diego, CA) followed by anti-PE microbeads (Miltenyi Biotec). These subpopulations were then isolated by positive selection using Miltenyi AutoMACS (Miltenyi). The purity of these subpopulations was >90% as determined by flow cytometry and the cells were routinely >95% viable by Trypan blue exclusion.
Cell culture
Purified splenic macrophages, T, B and NK cells, and MDCs, all at 2 × 106 cells/mL were cultured for 18 hrs in complete media as described previously16. T lymphocytes were stimulated with either 10 μg/mL Con A, or a mixture of 10ng/mL PMA and 1.5μM ionomycin, NK cells were stimulated with a mixture of PMA and ionomycin, while macrophages, MDCs and B cells were stimulated with 1 μg/mL LPS (all mitogens from Sigma Chemical Co., St. Louis, MO) and growth media (GM) control was included in all of the cultures. These three subpopulations were also cultured with either 1 ng/mL or 5 ng/mL recombinant murine CHI3L1 (rmCHI3L1) (R&D Systems, Minneapolis, MN) for 18 hrs and cell-free supernatants were collected and stored at −80°C until use.
The DA-3 mammary tumor cells were cultured for 4 days in DMEM/high glucose, 10% FCS (Hyclone Laboratories, Logan, UT), 100 U/mL penicillin, 100 mg/mL streptomycin and OPI media supplement (Sigma Chemical, St.Louis, MO). 4T1 Luc cells were cultured in RPMI with 10% FCS. Total RNA and protein was then isolated and stored at −80°C until qRT-PCR and Western blotting analyses.
siRNA treatment
Macrophages (107) were plated into 6-well plates and allowed to adhere for 1 hour. CHI3L1 specific siRNA or scrambled siRNA was administered using the Interferin transfection reagent according to the manufacturer's instructions (PolyPlus, Illkirch, France). Briefly, 50 nM of either CHI3L1 siRNA SmartPool (Dharmacon, ThermoFisher, Pittsburgh, PA) or non-Target plus 1 and non-Target plus 2 (Dharmacon) complexed to Interferin transfection reagent were added to each of the wells and incubated for 12 hours at 37°C in CO2 incubator. Complete media was then added and incubated for an additional 48 hours. Cell-free supernatants and purified RNA were analyzed by ELISA and qRT-PCR, respectively.
RNA analyses
qRT-PCR was performed for CHI3L1 expression in splenocytes and macrophages using total RNA isolated with the Qiagen Mini Kit (Qiagen Inc., Valencia, CA), and cDNA was prepared with Qiagen QuantiTect kit (Qiagen). CHI3L1 gene expression was detected by SYBR Green qPCR analysis using SYBR RT2 qPCR primers from SA Bioscience (Qiagen Inc., proprietary primers, sequences not disclosed). PCR cycles were as follows: initial denaturation at 95°C for 10 min; 95°C for 15 s and annealing at 60°C for 1 min through 40 cycles. Samples were amplified using the Stratagene Mx 3005P cycler and assays were performed in triplicates.
Immunofluorescence
To localize the expression of CHI3L1, DA-3 mammary tumor cells (105 cells) or splenic macrophages from normal and tumor bearing mice (1×106 cells) were plated on confocal cover glass, post-fixed in 4% paraformaldehyde, blocked in 4% BSA and labeled with rat anti-CHI3L1 (R&D Systems) followed by incubation in donkey anti-rat IgG conjugated to AlexaFluor 488 (Molecular Probes, Eugene, OR). For co-localization of intracellular chitin, FITC-chitin binding probe (1:500) (New England BioLabs, Ipswich, MA) was combined with rat anti-CHI3L1 followed by AlexaFluor 568 conjugated to anti-rat secondary antibody (Molecular Probes). To visualize nuclei, cells were mounted with Vectashield (Vector Laboratories, Burlingame, CA) and examined by confocal microscopy (Carl Zeiss LSM 700, Microimaging, Inc., Thornwood, NY).
Cytokine ELISA
Cell culture supernatants and sera from control and mammary tumor bearers were analyzed for protein expression by ELISA for CCL2, CXCL2 and IFN-γ (BD Biosciences, San Jose, CA), MMP-9 and CHI3L1 (R&D Systems) according to the manufacturer's instructions. Absorbance at 450 nm with wavelength correction at 570 nm was measured with a Tecan SLT Rainbow Reader (Lab Instruments, Research Triangle Park, NC) and OD values of samples were converted to picograms against a standard curve plotted from known quantities of recombinant murine cytokines.
Determination of metastasis
Mice were injected intraperitoneally with 2.5 mg of D-luciferin (Xenogen) dissolved in 100 μL of 0.9% saline, anesthetized with isofluorane and imaged using a bioluminescence optical imager (IVIS Lumina LTE). Maximal luciferase signals were quantified using Living Image 2.5 (Xenogen) image analysis software. Luciferase signal is reported as photons/sec. Tissue-specific metastasis of 4T1 tumors was measured ex vivo by luminescence immediately after euthanasia by cervical dislocation on day 45.
In addition, to visualize metastatic foci in the lungs of DA-3 mammary tumor bearers, a solution of 15% India ink was injected intratracheally into euthanized mice. The lungs were excised, rinsed in PBS, immersed in Fekete's solution (~50% ethanol, 8% formaldehyde and 4% acetic acid) for 24 hours and photographed by digital camera.
Statistical analyses
Results are expressed as group means ± SD. Statistical analyses were performed using GraphPad Prism 3 software (LaJolla, CA). Statistical comparisons of paired groups were determined by Student's t tests. Values of p < 0.05 were considered statistically significant.
Results
CHI3L1 is expressed by murine mammary tumor cells
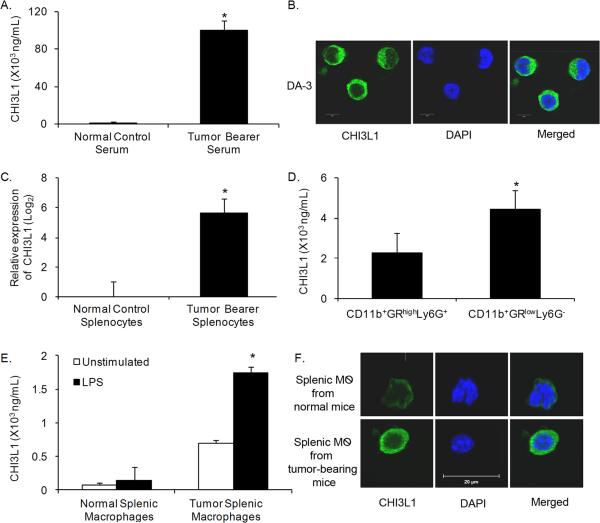
Patients with solid tumors have elevated levels of CHI3L1 in their plasma26. Similarly, we found elevated plasma levels of CHI3L1 in DA-3 mammary tumor bearers (Fig. 1A). Although the DA-3 tumor cells express this molecule as determined by immunofluorescence analysis (Fig. 1B) it does not account for the high levels of CHI3L1 in circulation. Therefore splenic cells were assessed for expression of CHI3L1 by qRT-PCR. There was a 6-fold increase in mRNA expression of CHI3L1 in tumor bearer's splenic cells compared to those of normal mice (Fig. 1C).
Figure 1.
CHI3L1 is upregulated in mammary tumor-bearing mice. (A) Serum was collected from normal and 5-week tumor-bearing mice and analyzed for CHI3L1 expression by ELISA. (B) CHI3L1 expression in DA-3 mammary tumor cells was localized by immunofluorescence using confocal microscopy, scale bar = 20 μM. (C) Purified total RNA from splenocytes of normal and 5-week DA-3 tumor bearers was used to measure expression of CHI3L1 by qRT-PCR. (D–E) Purified myeloid derived cells (D) or macrophages (E) were cultured overnight and cell-free supernatants were analyzed for CHI3L1 expression by ELISA. (F) Purified macrophages from normal and 5-week DA-3 tumor bearers were examined by immunolocalization of CHI3L1 expression and visualized by confocal microscopy, scale bar = 20 μM. For all experiments, N = 8 mice/group, *p<0.0005, Student's t-test. 159×144mm (300 × 300 DPI)
CHI3L1 is highly expressed in myeloid and macrophage populations, but not in NK cells or T lymphocytes
To clearly delineate specific subpopulations of cells contributing to CHI3L1 expression, purified splenic subpopulations of myeloid cells, macrophages, B, NK and T cells from normal and tumor-bearing mice were analyzed for CHI3L1 protein expression. Monocyte precursor subpopulations (CD11b+GRlowLy6G−) express higher constitutive levels of CHI3L1 compared to the granulocytic precursor cells (CD11b+GRhighLy6G+) (Fig. 1D). Since the monocyte precursors (CD11b+GRlowLy6G−) had higher CHI3L1 expression levels, we focused our attention on this subpopulation. Our studies also show higher constitutive production of CHI3L1 by splenic macrophages (F4/80+) from mammary tumor-bearing mice compared to LPS-stimulated macrophages from normal controls (Fig. 1E). Macrophages from tumor bearers secreted significantly more (1500 ng/mL) CHI3L1 upon LPS stimulation compared to LPS-stimulated normal controls (200 ng/mL) and CHI3L1 was found to localize to the cytoplasm of these cells (Fig. 1F). B cells from tumor bearers produced ~580 ng/mL CHI3L1 while those from normal mice produce only 93 pg/mL (data not shown). In contrast, NK and T cells did not produce CHI3L1 even upon mitogenic stimulation (data not shown).
CHI3L1 induces macrophages to secrete CCL2, CXCL2 and MMP-9
Our previous studies showed elevated levels of MMP-9 and proinflammatory chemokines in mammary tumor-bearing mice15, 16. Since CHI3L1 levels are elevated in DA-3 mammary tumor-bearing mice, we determined if CHI3L1 has downstream effects on levels of inflammatory chemokines and matrix degrading enzymes. In vitro treatment of splenic macrophages from normal mice with rmCHI3L1 alone (1 ng/mL or 5 ng/mL) or in combination with LPS resulted in dose-dependent increases in the production of CCL2 (Fig. 2A–B), CXCL2 (Fig. 2C–D) and MMP-9 (Fig. 2E–F).
Figure 2.
CHI3L1 increases the expression of CCL2, CXCL2 and MMP-9. Macrophages from normal mice were cultured overnight with either 1 ng/mL or 5 ng/mL rmCHI3L1 alone (A,C,E) or in combination with LPS (1 μg/mL) (B,D,F) and cell-free supernatants were analyzed by ELISA for CCL2 (A–B) ; CXCL2 (C–D) or MMP-9 (E–F). N= 10 mice/group, (A–B) *p<0.0015, **p<0.0001, (CD)*p<0.0005, ** p<0.0002, and (E–F) *p<0.004, **p<0.0016.
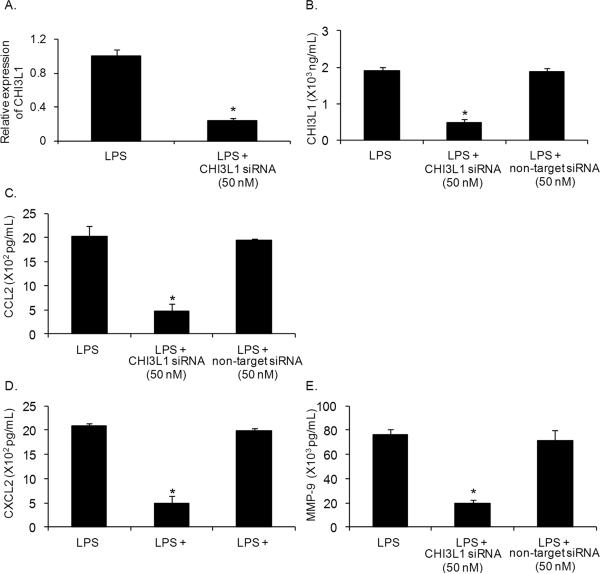
We determined if CHI3L1 gene silencing by siRNA in macrophages affects the expression of proinflammatory mediators. Splenic macrophages from mammary tumor bearers were transfected with CHI3L1 siRNA (50 nM) and evaluated 60 hrs later. qRT-PCR analysis showed a 75% reduction in CHI3L1 RNA (Fig. 3A) and a >70% reduction in protein levels as determined by CHI3L1 ELISA (Fig. 3B) compared with non-target control siRNA. Notably, silencing CHI3L1 expression resulted in >60% reduction in the production of CCL2 (Fig. 3C), CXCL2 (Fig. 3D) and MMP-9 (Fig 3E) compared to non-target siRNA control treatment of splenic macrophages from tumor-bearing mice.
Figure 3.
Silencing CHI3L1 decreases secretion of proinflammatory molecules by macrophages. (A) In vitro treatment of LPS (1 μg/mL) stimulated macrophages from 5-week DA-3 tumor bearers with 50 nM CHI3L1 siRNA analyzed by qRT-PCR for CHI3L1 gene expression and protein expression as determined by ELISA (B). (C–E) Purified macrophages from 5-week DA-3 tumor bearers were cultured with 50 nM CHI3L1 siRNA or 50 NM nontarget siRNA in the presence of LPS and cell-free culture supernatants analyzed for CCL2 (C), CXCL2 (D) and MMP-9 (E). N = 5 mice/group, *p < 0.05, Student's t-test
CHI3L1 downregulates IFN-γ
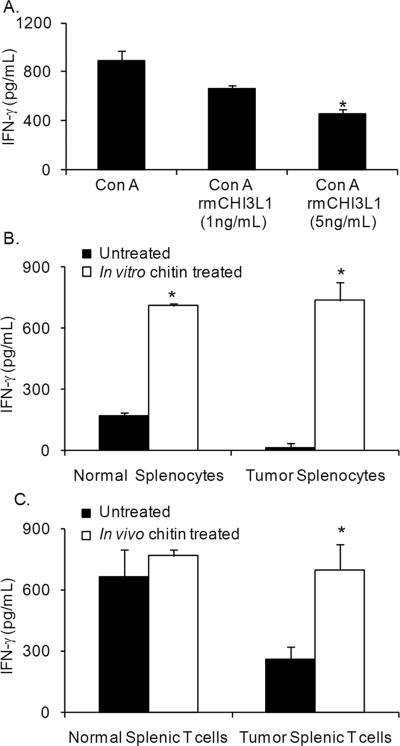
Previous studies have shown that CHI3L1 is associated with a TH2 type of allergic immune response27. Since T lymphocytes are the major producers of IFN-γ in the spleen, we tested the effects of CHI3L1 on purified splenic T lymphocytes. T cells from normal mice cultured with 5 ng/mL of rmCHI3L1 produced significantly less IFN-γ compared to untreated controls (Fig. 4A).
Figure 4.
CHI3L1 exposure inhibits IFN-γ while exposure to chitin enhances IFN-γ production by T lymphocytes. (A) Purified CD90.2+ T cells from normal mice were cultured overnight with either Con A (10 ng/mL) alone or with rmCHI3L1 (1 ng/mL or 5 ng/mL) and Con A (10 ng/mL), and cellfree culture supernatants analyzed by ELISA for IFN-γ. N = 8 mice, *p< 0.002, Student's t-test. (B) Splenocytes from normal and 5-week DA-3 tumor bearers were cultured in vitro overnight with chitin (10 μg/mL) and cell-free supernatants analyzed by IFN-γ ELISA. Data are representative of three independent experiments with N= 4 mice/group; *p < 0.002, Student's t-test. (C) Normal and 5-week DA-3 tumor bearers were treated in vivo with chitin (1 mg/mouse) and purified T lymphocytes were cultured overnight with PMA (1 ng/mL) and ionomycin (1.5 nM). Cell-free supernatants analyzed for IFN-γ by ELISA. Data were collected from three independent experiments with N= 4 mice/group; *p < 0.005, Student's t-test
Chitin, a TH1 adjuvant, increases IFN-γ production and decreases production of proinflammatory molecules
Our previous work has shown a profound downregulation of the TH1 cytokine, IFN-γ, in tumor-bearing mice. Since CHI3L1 appears to contribute to decreased IFN-γ production, we determined if in vitro treatment of splenocytes with chitin, a TH1 adjuvant, would alter production of this cytokine. In vitro treatment of splenic cells from normal and tumor-bearing mice with chitin resulted in a significant increase in IFN-γ secretion (Fig. 4B). In contrast, in vitro treatment of purified T lymphocytes with chitin microparticles had minimal effects on the production of IFN-γ (data not shown). It is possible that interaction with antigen presenting cells may be needed for purified T cells to respond in vitro as chitin is known to mediate its effects through macrophages.
Since in vitro treatment with chitin enhanced the IFN-γ secretion by splenic cells, we tested if in vivo treatment with chitin has similar effects on IFN-γ production. Significantly higher levels of IFN-γ were produced by T cells from in vivo chitin treated tumor bearers and these levels were, comparable to those found in normal untreated mice (Fig. 4C). However, chitin treatment of normal mice resulted in only slightly enhanced IFN-γ production, suggesting that the levels of this cytokine are already maximal.
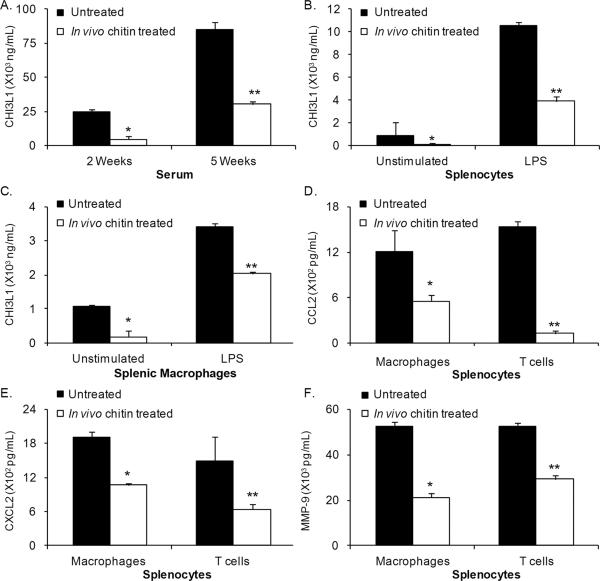
We also tested the effects of chitin treatment on CHI3L1 expression. A significant reduction in CHI3L1 was observed in serum of tumor-bearing mice treated with chitin (Fig. 5A). In addition, CHI3L1 expression by splenic cells (Fig. 5B) and splenic macrophages (Fig. 5C) was decreased in chitin-treated tumor bearers. Since in vivo chitin treatment impacted CHI3L1 expression, we assessed the effect of this treatment on production of CCL2, CXCL2 and MMP-9 in splenic cells, and observed significant reductions in the levels of these molecules (Figs. 5D–F).
Figure 5.
In vivo treatment with chitin decreases CHI3L1, CCL2, CXCL2 and MMP-9 expression in DA-3 tumor-bearing mice. (A) Serum from 2- and 5-week DA-3 tumor bearers either untreated or chitin treated (1 mg/mouse) was analyzed for CHI3L1 expression by ELISA. (B–C) Splenocytes (B) and splenic macrophages (C) from untreated or chitin treated 5-week DA-3 tumor-bearing mice were cultured overnight in the presence or absence of LPS (1 μg/mL) and cell-free supernatants were analyzed for CHI3L1 by ELISA. (D–F) Purified macrophages and T cells from spleens of 5-week DA-3 tumor-bearing mice, untreated, or chitin treated were cultured overnight with mitogens as described, and cell-free supernatants were analyzed by ELISA for CCL2 (D), CXCL2 (E) and MMP-9 (F). Data shown are the results of three independent experiments with N= 4 mice/group; *p<0.003, **p<0.002, Student's t-test
In vivo treatment with chitin decreases pulmonary metastasis
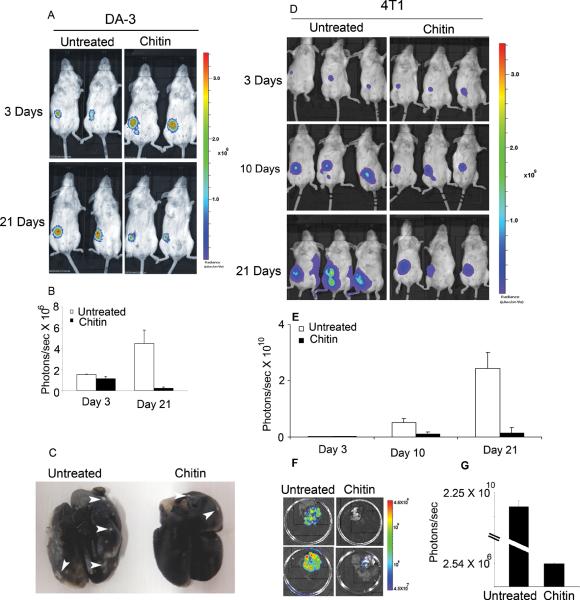
We have shown that administration of chitin decreases MMP-9 secretion and increases IFN-γ production. Since IFN-γ can exert anti-proliferative effects on tumor cells, the effect of chitin treatment on tumor growth was evaluated. In vivo chitin treatment of DA-3 luc mammary tumor bearers caused a reduction in tumor cell proliferation compared to untreated mice by 21 days post-tumor cell inoculation (Fig. 6A). These results were validated by quantification of bioluminescent signals (Fig. 6B). To confirm that this type of response is not unique to our tumor model, we tested the effects of chitin administration using 4T1 mammary tumor cells (Fig. 6D). While the untreated mice developed large tumors (2.4 × 1010 photons), chitin-treated mice had tumors that were significantly smaller (<0.5 × 109 photons) (Fig. 6E).
Figure 6.
Treatment with chitin decreases tumor growth and metastasis. Mice bearing either DA-3 luc cells (A–C) or 4T1 luc cells (D–G) were treated in vivo with chitin and analyzed for tumor growth and metastasis by in vivo imaging system. (A) Ventral image of untreated and chitin-treated DA-3 tumor-bearing mice showing 2 out of 5 mice/treatment group. (B) Quantification of tumor-specific bioluminescence indicates decreased tumor growth at 21 days post-tumor implantation in the chitin treated group. (C) Digital image of excised lungs from untreated control and chitin treated DA-3 tumor bearers showing decreased metastasis in chitin treated mice, as analyzed by India Ink staining. (D) Ventral image of untreated or chitin-treated 4T1 mammary tumor-bearing mice showing 3 out of 5 mice/group at 3 different time points. (E) Quantification of tumor-specific bioluminescence signal indicates decreased tumor growth in the chitin treated group. (F) Ex vivo lungs from 4T1 tumor bearers show decreased bioluminescence signal in chitin treated mice. (G) Quantification of bioluminescence (photons/sec) demonstrates more than 1000-fold decrease in signal with chitin treatment
To determine if decreased MMP-9 levels with chitin treatment subsequently reduces tumor metastasis, we examined pulmonary tissue from untreated and chitin treated tumor-bearing mice. In vivo treatment of DA-3 mammary tumor bearers and subsequent analyses by India Ink revealed a significant reduction in metastasis in the chitin treated mice compared to untreated controls which had multiple metastatic foci (Fig. 6C). Similarly, chitin treatment of 4T1 tumor bearers decreased pulmonary metastasis as detected by ex vivo imaging (Fig. 6F). Quantification of bioluminescent signals by photon analysis measured a greater than 1000-fold decrease in the lungs of chitin treated mice (Fig. 6G).
Discussion
Abnormal expression of proteins either by the tumor itself or by the stromal cells confers advantage to the tumor by inducing angiogenesis, stimulating proliferation and suppression of antitumor immune responses. CHI3L1 is a glycoprotein that has been correlated with disease severity, poorer prognosis and shorter survival times in many types of cancer1, 26. However, the biological role of CHI3L1 in breast cancer has not yet been clearly elucidated. In the present study we used an in vivo mouse mammary tumor model mimicking CHI3L1 expression in breast cancer patients to demonstrate a key role for CHI3L1 in inducing immunosuppression and metastasis.
Although CHI3L1 is expressed in breast tumors, there is lack of its expression in many human breast cancer cell lines1. Using DA-3 and 4T1 mouse mammary tumor cell lines endogenously expressing CHI3L1, we detect high levels of CHI3L1 in circulation as seen in breast cancer patients. We also show that CHI3L1 is one of the molecules contributing to the immune suppression in mice bearing mammary tumors.
Immunosuppression in breast cancer patients is often accompanied by downregulation of IFN-γ and low levels of this cytokine have been correlated with tumor progression16, 28, 29, 26, 30. Our studies show for the first time a direct correlation between CHI3L1 expression and downregulation of IFN-γ in tumor-bearing mice. Although T cells from tumor bearers do not produce CHI3L1, tumor-derived or immune cell-derived CHI3L1 may be acting on T cells to dowregulate IFN-γ. Using mice with BRP-39 (aka CHI3L1) null mutation, Lee et al. demonstrated that CHI3L1 plays a critical role in TH2 inflammation with decreased TH2 cytokine production5 in an asthma model. These and our studies suggest that CHI3L1 may be involved in inducing pro-TH2 type of response with decreased TH1 cytokine production. Ongoing studies in our laboratory are delineating the mechanisms in suppression of TH1 cytokine production by CHI3L1.
In contrast to decreased IFN-γ, the production of CCL2, CXCL2 and MMP-9 is increased in tumor bearing-mice. While some have shown that CHI3L1 induces CCL2, CXCL2 and MMP-9 by alveolar macrophages from smokers17, others have shown that CHI3L1 suppresses cytokine-induced secretion of IL-8, the homolog of CXCL2 by articular chondrocytes or skin fibroblasts31. In this study we show that splenic macrophages treated with rmCHI3L1 produce appreciable levels of CCL2, CXCL2 and MMP-9. CHI3L1-induced expression of CCL2 and CXCL2 may promote tumor growth through increased angiogenic potential while high levels of MMP-9 could enable metastasis in mice bearing mammary tumors. It was recently shown that ectopic expression of YKL-40 (aka CHI3L1) in human breast and colon cancer cell lines induces an angiogenic phenotype with increased tumor growth in xenograft models32. Since CHI3L1 increases the production of these proinflammatory molecules, we tested to see if silencing CHI3L1 would affect their production. In vitro silencing using CHI3L1 siRNA significantly decreased the expression of CCL2, CXCL2 and MMP-9 by macrophages lending support to the idea that inhibition of CHI3L1 may improve prognosis for tumor bearers.
Blocking cellular receptors through use of ligands, receptor antagonists or antibodies to inhibit various pathways has been reported in many different systems33–35. For example, Faibish et al. used monoclonal anti-YKL-40 antibodies to inhibit angiogenesis in U87 xenograft brain tumor model36. In this study we used chitin, one of the known molecules binding to chitinases and chitin-like molecules37, and show that administration of chitin to tumor-bearing mice reduces tumor growth and metastasis. Chitin could affect tumor growth either directly by binding to CHI3L1 and/or indirectly by activating immune responses.
Chitin, a nontoxic, nonallergenic and biodegradable compound with powerful immune effects18, 19, 38, 39 may affect tumor growth indirectly by its effects on the immune system. Shibata et al. have shown a shift in immune response from TH2 to TH1 with increased production of IFN-γ in allergen induced chitin treated mice 39, 40. In this study, we show that administration of chitin to tumor-bearing mice restored T cell production of IFN-γ levels comparable to those in normal mice. It is important to emphasize that these effects on T lymphocyte production of IFN-γ were only observed in vivo. This may be partly due to the fact that chitin mediates its effects through macrophages by a mechanism involving IL-1241.
It could be argued that other chitinase-like proteins could also participate in immunosuppression in tumor bearers. Thus, tumor progression in chitin treated mice may have been influenced by the binding of chitin to chitinases that have enzymatic activity, as well as to chitinase-like proteins that like CHI3L1 lacking the enzymatic activity. These interactions could affect the type of immune response generated with a shift from pro-tumorigenic TH2 type to anti-tumorigenic TH1 type of response to limit tumor growth and metastasis. However, the present study demonstrates that intracellular chitin microparticles co-localize with CHI3L1 (Supplement Fig.1) suggesting chitin-CHI3L1 interaction plays a role in the anti-tumor immune response generated.
Chitin has been reported to have size-dependent adjuvant effects on host immune responses; larger particles are considered to induce innate immunity with eosinophilia and M2 macrophage activation supporting TH2/allergic responses20, 21, 23. Our studies show small size chitin particles (1–10 μM) induce a TH1 response while similar size phagocytosable latex beads did not affect the levels of CCL2, CXCL2 and MMP-9 production by splenocytes isolated from tumor-bearing mice (data not shown). These findings suggest that not all phagocytosable particles alter CHI3L1-mediated inflammation. It remains to be determined if the reduction of CHI3L1 expression by chitin is size-dependent.
The effect of chitin on tumor growth and metastasis may also be mediated directly through blocking CHI3L1 activity. We show that the levels of CCL2, CXCL2 and MMP-9 from tumor bearers were decreased in mice treated with chitin by more than 50% compared to untreated controls. Metastasis of tumors is preceded by increased expression of matrix metalloproteinases12 and our previous studies showed an upregulation of MMP-9 in mammary tumor-bearing mice15. Various small molecular weight inhibitors of MMPs have been used to block its activity, but these treatments result in adverse side effects42. Our studies show for the first time that in vivo treatment of mammary tumor-bearing mice with chitin decreases MMP-9 secretion and more importantly metastasis to the lung was decreased significantly.
Studies from both human cancers and animal models indicate that immunosuppression with increased proangiogenic and matrix degrading molecules correlate with tumor growth, angiogenesis and metastasis13, 30, 43. The present findings indicate that CHI3L1 plays a role in these processes, and that treatment with chitin significantly reduces these effects. Future therapies in our studies include combined targeted immunotherapies with anti-CHI3L1 antibodies to decrease the levels of protumorigenic molecules while enhancing anti-tumor immune response to limit breast cancer growth and metastasis.
Supplementary Material
Acknowledgments
We thank Drs. Diana Lopez and Eli Gilboa (University of Miami) and Dr. Mahayar Nouri-Shirazi (Florida Atlantic University) for critical discussion and reading of the manuscript.
Grant Support NIH/NCI grants to Vijaya Iragavarapu-Charyulu: NIH R15 CA135513-01 and R15 CA135513-01-OS1
Footnotes
No financial disclosure or conflict of interest
References Cited
- 1.Coffman FD. Chitinase 3-Like-1 (CHI3L1): a putative disease marker at the interface of proteomics and glycomics. Crit Rev Clin Lab Sci. 2008;45:531–62. doi: 10.1080/10408360802334743. [DOI] [PubMed] [Google Scholar]
- 2.Kzhyshkowska J, Gratchev A, Goerdt S. Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomark Insights. 2007;2:128–46. [PMC free article] [PubMed] [Google Scholar]
- 3.Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268:25803–10. [PubMed] [Google Scholar]
- 4.Rehli M, Krause SW, Andreesen R. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics. 1997;43:221–5. doi: 10.1006/geno.1997.4778. [DOI] [PubMed] [Google Scholar]
- 5.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, Humbles A, Kearley J, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009;206:1149–66. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CG, Da Silva CA, Lee JY, Hartl D, Elias JA. Chitin regulation of immune responses: an old molecule with new roles. Curr Opin Immunol. 2008;20:684–9. doi: 10.1016/j.coi.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kzhyshkowska J, Mamidi S, Gratchev A, Kremmer E, Schmuttermaier C, Krusell L, Haus G, Utikal J, Schledzewski K, Scholtze J, Goerdt S. Novel stabilin-1 interacting chitinase-like protein (SI-CLP) is up-regulated in alternatively activated macrophages and secreted via lysosomal pathway. Blood. 2006;107:3221–8. doi: 10.1182/blood-2005-07-2843. [DOI] [PubMed] [Google Scholar]
- 8.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura T, Iwakabe K, Sekimoto M, Ohmi Y, Yahata T, Nakui M, Sato T, Habu S, Tashiro H, Sato M, Ohta A. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med. 1999;190:617–27. doi: 10.1084/jem.190.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corthay A, Skovseth DK, Lundin KU, Rosjo E, Omholt H, Hofgaard PO, Haraldsen G, Bogen B. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–83. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001;11:S37–43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 13.Balkwill F. Chemokine biology in cancer. Semin Immunol. 2003;15:49–55. doi: 10.1016/s1044-5323(02)00127-6. [DOI] [PubMed] [Google Scholar]
- 14.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owen JL, Iragavarapu-Charyulu V, Gunja-Smith Z, Herbert LM, Grosso JF, Lopez DM. Up-regulation of matrix metalloproteinase-9 in T lymphocytes of mammary tumor bearers: role of vascular endothelial growth factor. J Immunol. 2003;171:4340–51. doi: 10.4049/jimmunol.171.8.4340. [DOI] [PubMed] [Google Scholar]
- 16.Owen JL, Lopez DM, Grosso JF, Guthrie KM, Herbert LM, Torroella-Kouri M, Iragavarapu-Charyulu V. The expression of CCL2 by T lymphocytes of mammary tumor bearers: role of tumor-derived factors. Cell Immunol. 2005;235:122–35. doi: 10.1016/j.cellimm.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 17.Letuve S, Kozhich A, Arouche N, Grandsaigne M, Reed J, Dombret MC, Kiener PA, Aubier M, Coyle AJ, Pretolani M. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J Immunol. 2008;181:5167–73. doi: 10.4049/jimmunol.181.7.5167. [DOI] [PubMed] [Google Scholar]
- 18.Shibata Y, Metzger WJ, Myrvik QN. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: mannose receptor-mediated phagocytosis initiates IL-12 production. J Immunol. 1997;159:2462–7. [PubMed] [Google Scholar]
- 19.Da Silva CA, Chalouni C, Williams A, Hartl D, Lee CG, Elias JA. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J Immunol. 2009;182:3573–82. doi: 10.4049/jimmunol.0802113. [DOI] [PubMed] [Google Scholar]
- 20.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–6. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kogiso M, Nishiyama A, Shinohara T, Nakamura M, Mizoguchi E, Misawa Y, Guinet E, Nouri-Shirazi M, Dorey CK, Henriksen RA, Shibata Y. Chitin particles induce size-dependent but carbohydrate-independent innate eosinophilia. J Leukoc Biol. doi: 10.1189/jlb.1110624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JB, Urban K, Cochran E, Lee S, Ang A, Rice B, Bata A, Campbell K, Coffee R, Gorodinsky A, Lu Z, Zhou H, et al. Non-invasive detection of a small number of bioluminescent cancer cells in vivo. PLoS One. 5:e9364. doi: 10.1371/journal.pone.0009364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishiyama A, Tsuji S, Yamashita M, Henriksen RA, Myrvik QN, Shibata Y. Phagocytosis of N-acetyl-D-glucosamine particles, a Th1 adjuvant, by RAW 264.7 cells results in MAPK activation and TNF-alpha, but not IL-10, production. Cell Immunol. 2006;239:103–12. doi: 10.1016/j.cellimm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Strong P, Clark H, Reid K. Intranasal application of chitin microparticles down-regulates symptoms of allergic hypersensitivity to Dermatophagoides pteronyssinus and Aspergillus fumigatus in murine models of allergy. Clin Exp Allergy. 2002;32:1794–800. doi: 10.1046/j.1365-2222.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 25.Torroella-Kouri M, Silvera R, Rodriguez D, Caso R, Shatry A, Opiela S, Ilkovitch D, Schwendener RA, Iragavarapu-Charyulu V, Cardentey Y, Strbo N, Lopez DM. Identification of a subpopulation of macrophages in mammary tumor-bearing mice that are neither M1 nor M2 and are less differentiated. Cancer Res. 2009;69:4800–9. doi: 10.1158/0008-5472.CAN-08-3427. [DOI] [PubMed] [Google Scholar]
- 26.Johansen JS, Christensen IJ, Riisbro R, Greenall M, Han C, Price PA, Smith K, Brunner N, Harris AL. High serum YKL-40 levels in patients with primary breast cancer is related to short recurrence free survival. Breast Cancer Res Treat. 2003;80:15–21. doi: 10.1023/A:1024431000710. [DOI] [PubMed] [Google Scholar]
- 27.Lee CG, Elias JA. Role of breast regression protein-39/YKL-40 in asthma and allergic responses. Allergy Asthma Immunol Res. 2:20–7. doi: 10.4168/aair.2010.2.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–61. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Tunon I, Ricote M, Ruiz AA, Fraile B, Paniagua R, Royuela M. Influence of IFN-gamma and its receptors in human breast cancer. BMC Cancer. 2007;7:158. doi: 10.1186/1471-2407-7-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elsasser-Beile U, von Kleist S, Sauther W, Gallati H, Monting JS. Impaired cytokine production in whole blood cell cultures of patients with gynaecological carcinomas in different clinical stages. Br J Cancer. 1993;68:32–6. doi: 10.1038/bjc.1993.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling H, Recklies AD. The chitinase 3-like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumour necrosis factor-alpha. Biochem J. 2004;380:651–9. doi: 10.1042/BJ20040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao R, Hamel K, Petersen L, Cao QJ, Arenas RB, Bigelow C, Bentley B, Yan W. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene. 2009;28:4456–68. doi: 10.1038/onc.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Das AM, Seideman J, Griswold D, Afuh CN, Kobayashi T, Abe S, Fang Q, Hashimoto M, Kim H, Wang X, Shen L, et al. The CC chemokine ligand 2 (CCL2) mediates fibroblast survival through IL-6. Am J Respir Cell Mol Biol. 2007;37:121–8. doi: 10.1165/rcmb.2005-0253OC. [DOI] [PubMed] [Google Scholar]
- 34.Wickremasinghe MI, Thomas LH, O'Kane CM, Uddin J, Friedland JS. Transcriptional mechanisms regulating alveolar epithelial cell-specific CCL5 secretion in pulmonary tuberculosis. J Biol Chem. 2004;279:27199–210. doi: 10.1074/jbc.M403107200. [DOI] [PubMed] [Google Scholar]
- 35.Cousens LP, Orange JS, Su HC, Biron CA. Interferon-alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. Proc Natl Acad Sci U S A. 1997;94:634–9. doi: 10.1073/pnas.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faibish M, Francescone R, Bentley B, Yan W, Shao R. A YKL-40-neutralizing antibody blocks tumor angiogenesis and progression: a potential therapeutic agent in cancers. Mol Cancer Ther. 2011;10:742–51. doi: 10.1158/1535-7163.MCT-10-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ober C, Chupp GL. The chitinase and chitinase-like proteins: a review of genetic and functional studies in asthma and immune-mediated diseases. Curr Opin Allergy Clin Immunol. 2009;9:401–8. doi: 10.1097/ACI.0b013e3283306533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, He CH, Takyar S, Elias JA. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shibata Y, Foster LA, Metzger WJ, Myrvik QN. Alveolar macrophage priming by intravenous administration of chitin particles, polymers of N-acetyl-D-glucosamine, in mice. Infect Immun. 1997;65:1734–41. doi: 10.1128/iai.65.5.1734-1741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibata Y, Foster LA, Bradfield JF, Myrvik QN. Oral administration of chitin down-regulates serum IgE levels and lung eosinophilia in the allergic mouse. J Immunol. 2000;164:1314–21. doi: 10.4049/jimmunol.164.3.1314. [DOI] [PubMed] [Google Scholar]
- 41.Shibata Y, Foster LA, Kurimoto M, Okamura H, Nakamura RM, Kawajiri K, Justice JP, Van Scott MR, Myrvik QN, Metzger WJ. Immunoregulatory roles of IL-10 in innate immunity: IL-10 inhibits macrophage production of IFN-gamma-inducing factors but enhances NK cell production of IFN-gamma. J Immunol. 1998;161:4283–8. [PubMed] [Google Scholar]
- 42.Suzuki S, Okawa Y, Okura Y, Hashimoto K, Suzuki M. In: Immunoadjuvant Effect of Chitin and Chitosan. Hirano S, TS, editors. Japan Soc Chitin Chitosan; Chitin and Chitosan Sapporo: 1982. pp. 210–2. [Google Scholar]
- 43.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.