Abstract
The ubiquitous mitochondrial J-protein Jac1, called HscB in Escherichia coli, and its partner Hsp70 play a critical role in the transfer of Fe-S clusters from the scaffold protein Isu to recipient proteins. Biochemical results from eukaryotic and prokaryotic systems indicate that formation of the Jac1-Isu complex is important for both targeting of the Isu for Hsp70 binding and stimulation of Hsp70’s ATPase activity. However, in apparent contradiction, we previously reported that an 8 fold decrease in Jac1’s affinity for Isu1 is well tolerated in vivo, raising the question as to whether the Jac1:Isu interaction actually plays an important biological role. Here we report the determination of the structure of Jac1 from Saccharomyces cerevisiae. Taking advantage of this information and recently published data from the homologous bacterial system, a total of eight surface exposed residues were determined to play a role in Isu binding, as assessed by a set of biochemical assays. A variant having alanines substituted for these eight residues was unable to support growth of a jac1-Δ strain. However, replacement of three residues caused partial loss of function, resulting in a significant decrease in the Jac1:Isu1 interaction, a slow growth phenotype and a reduction in the activity of Fe-S cluster containing enzymes. Thus, we conclude that the Jac1:Isu1 interaction plays an indispensible role in the essential process of mitochondrial Fe-S cluster biogenesis.
Introduction
Iron sulfur (Fe-S) clusters are prosthetic groups required for the function of proteins involved in numerous, often essential, cellular activities, including redox reactions, enzymatic catalysis and electron transport, as well as regulatory functions 1. Although Fe-S clusters can be chemically reconstituted, a set of highly specialized proteins is dedicated to Fe-S cluster biogenesis in living cells. Mitochondria and bacteria have a similarly organized Fe-S cluster biogenesis pathway, carried out by homologous proteins. The central component of the pathway is a scaffold protein on which a Fe-S cluster is assembled, before its transfer to a recipient apo-protein. Mitochondria of the yeast Saccharomyces cerevisiae, the organism used in the work presented here, contains two very highly conserved, functionally redundant, scaffold proteins, Isu1 and Isu2 2, which are 83% identical in sequence and hereafter collectively referred to as Isu. The homologous scaffold in prokaryotes is called IscU.
The process of Fe-S cluster biogenesis can be divided into two steps, cluster formation on Isu and cluster transfer from Isu to a recipient apo-protein. Assembly requires the enzymatic function of the cysteine desulfurase Nfs1 to provide the needed sulfur. Additional proteins, Yah1 and Yfh1, also play critical roles. Yah1, an essential ferrodoxin, most likely provides electrons needed for the reduction of sulfur 1. The role played by the iron-binding protein Yfh1, the yeast frataxin homologue, is not yet well understood. However, it has been implicated as an iron source 3 and/or a regulatory element of Fe-S assembly complex 4. A specialized Hsp70 molecular chaperone system is central to the transfer process. This system is comprised of the Hsp70 Ssq1 and its J-protein co-chaperone Jac1, as well as the nucleotide release factor Mge1. Isu is the only known client protein for the Jac1/Ssq1 pair 5. Similarly, in Escherichia coli, IscU, is the only client for the Hsp70 HscA and J-protein HscB pair 6.
Jac1, like all J-proteins, contains a J-domain of approximately 70 amino acids 7; 8. The universally conserved function of the J-domain, which requires the presence of the invariant histidine:proline:aspartic acid (HPD) motif, is stimulation of the ATPase activity of its partner Hsp70. Such activity is critical for Jac1 function, as a variant in which conserved HPD residues were replaced by three alanines did not efficiently stimulate the ATPase activity of Ssq1 in vitro 9 and was unable to rescue the lethality caused by the absence of Jac1 in vivo 10. Stimulation of the ATPase activity of an Hsp70, which is effected by binding of a client protein in Hsp70’s peptide binding cleft, as well as the J-domain of its partner J-protein, is critical because when ATP is bound to an Hsp70, client protein binding and release occur very rapidly and thus interaction with a client protein is very transient 11. Upon hydrolysis of ATP to ADP, Hsp70 undergoes conformational changes that allow stable binding of client protein by preventing its fast release. Since some J-proteins, including Jac1, are able to bind client protein on their own, it has been suggested that a critical function of J-proteins is the targeting of client protein to Hsp70 12. Such a mechanism is very attractive, as J-protein dependent client protein targeting would allow orchestration of two events (i) interaction of client protein within the binding cleft of Hsp70 and (ii) stimulation of the Hsp70’s ATPase activity.
Biochemical results, including the requirement for the simultaneous presence of HscB, HscA and ATP in a reconstituted E. coli system for transfer of a Fe-S clusters to an apo-protein, supports such a targeting scenario 13. In addition, the presence of both Jac1/HscB and Isu/IscU is required to observe robust stimulation of Hsp70 ATPase activity in vitro supporting the idea of targeting 14; 15. However, the physiological importance of the targeting mechanism remains to be demonstrated in vivo for most Hsp70:J-protein pairs, including the Jac1/Ssq1 and HscA/HscB pairs. The first step of targeting, and very central to the model, is the binding of the J-protein to client protein 11. Verification of the importance of such an interaction under physiological conditions requires identification of the residues critical for the Jac1-Isu1 interaction, followed by testing of the importance of such residues for Fe-S cluster biogenesis in vivo.
The presence of only a single Fe-S cluster biogenesis system in eukaryotes makes the Ssq1/Jac1/Isu system essential for S. cerevisiae and thus a useful model system in which to test these ideas 16. Previously we reported 17 that the C-terminal domain (C-domain) of Jac1 is sufficient for Isu1 binding and identified several conserved charged residues on the surface of the C-domain involved in the Isu1 interaction. Replacement of these residues by alanines resulted in an approximately 8 fold decrease in the affinity of Jac1 for Isu1 17. However, when the variant was expressed as the only Jac1 in the cell, no growth defect was observed. Recently, detailed biochemical and biophysical studies of bacterial HscB identified a set of hydrophobic residues, located on the surface of the C-domain in close proximity to the charged region, as playing a significant role in the HscB:IscU interaction 18; 19. However, the physiological function of these newly identified residues has not been tested, because in bacterial cells the presence of an alternative redundant pathway of Fe-S cluster biogenesis hampers such studies 20. Utilizing newly obtained structural information of S. cerevisiae Jac1, we now report a combined genetic, biochemical and physiological analysis of the importance of residues of Jac1 that play a role in the interaction with Isu. Our results support the targeting model, as they indicate that the interaction between Jac1 and Isu is indispensible in vivo.
Results and Discussion
X-ray structure of Jac1 from Saccharomyces cerevisiae
To gain Jac1 structural information, we purified recombinant Jac1 protein encompassing residues 10–184, which we call Jac1-C1. As Jac1 is synthesized as a preprotein with a 9 amino acids pre-sequence at its N-terminus, which is removed upon translocation into mitochondria 10, Jac1-C1 contains all of the residues found in the mature form of the protein. Jac1-C1 crystallized with two molecules (designated A and B) in the asymmetric unit and diffracted X-rays to a resolution of 2.13 Å. However, the complete structure could not be determined because some segments were not well resolved (see Supplementary Information). Therefore, we prepared a collection of truncation variants of Jac1 to find proteins that yielded improved crystal quality (see Supplementary Information). The best results were obtained for variant Jac1-C2 (residues 5–182). Similar to Jac1-C1, Jac1-C2 crystallized with two molecules (A and B) in the asymmetric unit and diffracted X-rays to 1.85 Å resolution. The structure was determined for residues 8–181 (A) and 12–176 (B), but segments consisting of residues 47–60 and 95–98 (B) were not resolved. The combined models provided good structural information for residues 10–46 and 56–181 of Jac1 (Fig. 1). Atomic coordinates and structure factors were deposited into the Protein Data Bank as 3UO2 and 3UO3 for Jac1-C1 and Jac1-C2, respectively. The overall structure of Jac1 resembles previously reported structures of its orthologs: HscB from E. coli 21 (PDB id: 1FPO), HscB from Vibrio cholerae (PDB id: 3HHO) and HscB from Homo sapiens 22 (PDB id: 3BVO) with which it shares 29%, 31% and 28% sequence identity, respectively. The structure of Jac1 is L-shaped and consists of 2 distinct α-helical domains (Fig. 1); the N-terminal J-domain (residues 11–84) and the C-terminal Isu binding C-domain (residues 101–184). These two domains are connected by a flexible linker (residues 85–100).
Fig. 1. Saccharomyces cerevisiae Jac1 protein structure.
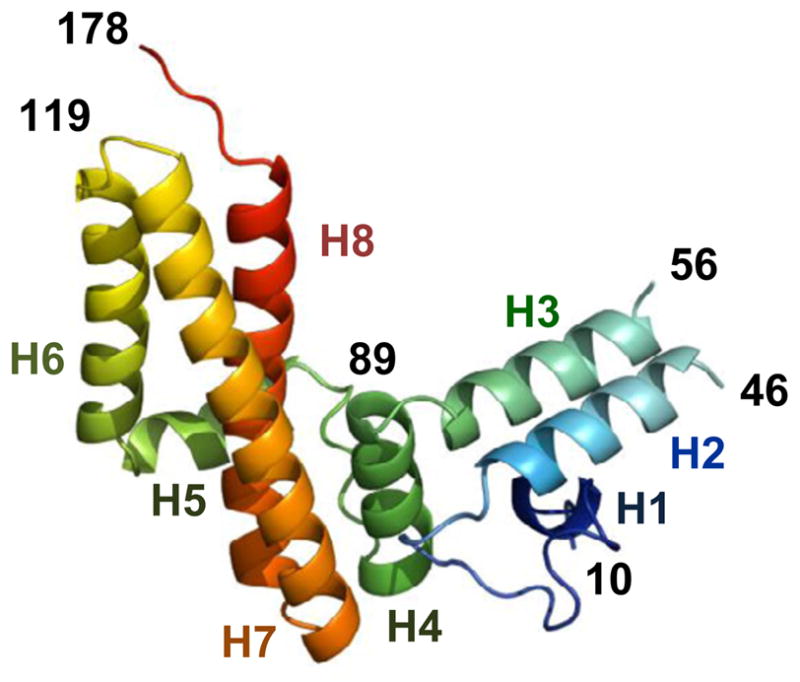
Ribbon diagram of Jac1 protein. Rainbow coloring from blue to red indicates the N- to C-terminal positions of the residues in the model. The α-helices are numbered in corresponding colors. Numbers in black correspond to residue positions. The diagram was generated using Pymol (DeLano Scientific LL).
As expected, the J-domain contains three α-helices, with helices H2 and H3 comprising an antiparallel coiled-coil connected by a loop with the conserved J-domain HPD signature motif (Suppl. Fig. 1A). An unusual feature of the J-domain structure is the disorder present at the ends of the H2 and H3 α-helices including the loop connecting these helices (Suppl. Fig. 1A), as these regions are well defined in the previously solved structures of Jac1 orthologs. The disorder present in both the Jac1-C1 and Jac1-C2 structures may reflect a higher flexibility of the Jac1 J-domain compared to that of orthologs from other species. As noted previously, based on the sequence comparison of Jac1 from S. cerevisiae and closely related yeast species with that of other species, the loop region is shorter than the one present in either bacterial (Suppl. Fig. 1B) or human orthologs 23. The linker between the J-domain and the C-domain also differs significantly between S. cerevisiae Jac1 and its orthologs. It contains an additional α-helix (residues 91–100), which is not observed in either the bacterial or human structures21; 22. Interestingly, the linker helix is ordered in molecule A of both Jac1 structures and disordered in molecule B, suggesting intrinsic flexibility of this region.
These unique structural features of Jac1’s J-domain and linker may have emerged as a result of the complex evolutionary history of mitochondrial Hsp70s and J-proteins involved in the biogenesis of Fe-S clusters in S. cerevisiae 24. In contrast to most eukaryotic species in which Jac1 orthologs partner with the multi-functional mitochondrial Hsp70, which is also involved in facilitating general protein folding and the translocation of proteins into mitochondria, Jac1 in S. cerevisiae and closely related yeast species functions with the specialized Ssq1 24. All evidence indicates that SSQ1 arose from a duplication of a gene encoding mitochondrial Hsp70, which in the course of evolution became highly specialized and now functions exclusively in the biogenesis of Fe-S clusters. We proposed previously, based on computer structure prediction and phylogenetic analysis, that the altered J-domain of Jac1 evolved as consequence of molecular co-adaptation between Jac1 and its new Hsp70 partner Ssq1 23. The unusual structural properties of the J-domain of Jac1 reported here provide strong support for such an evolutionary scenario.
In contrast to the altered structure of the J-domain, the structure of Jac1’s C-domain closely resembles those of other orthologs 21; 22. It is a three-helical bundle with a hydrophobic core formed by the interaction of nonpolar side chains from helices H6, H7 and H8 (Fig. 1). The length and orientation of these helices are almost the same as that found in orthologous structures. In addition, the physicochemical properties of residues exposed on the surface of the C-domain are conserved between Jac1 and HscB (Fig. 2A). Three acidic residues (D110, D113 and D114) on the surface of helix H6 have been shown previously to be sites for interaction with Isu 17. Surface exposed hydrophobic residues (L105, L106, L109) are situated adjacent to this negatively charged patch on helix H6. A similar hydrophobic patch is present on the equivalent surface of HscB. Detailed biochemical and biophysical studies indicated that three of these residues (L92, L96 and F153) are critical for the HscB-IscU interaction 18; 19. Interestingly, Tyr163 in the Jac1 structure, located on helix H8, occupies a site homologous to Phe153 of HscB, suggesting that it may play a role in the Jac1:Isu interaction (Fig. 2A). Considering the fast rate of Jac1 evolution, as illustrated by only 29% sequence identity between Jac1 from S. cerevisiae and Candida albicans, a relatively closely related yeast species, the described above evolutionary conservation of both charged and hydrophobic regions is quite remarkable.
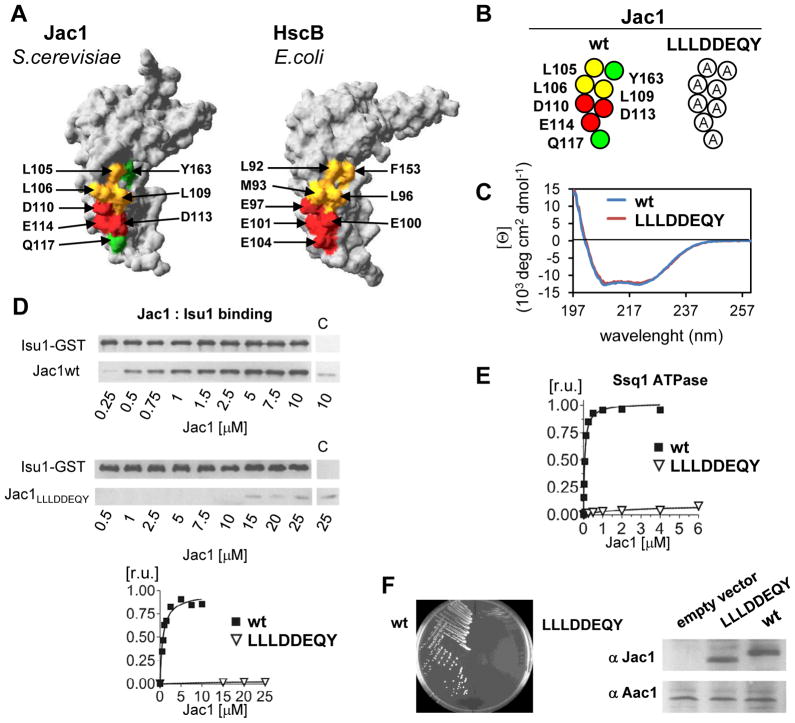
Fig. 2. Alanine replacements of conserved charged and hydrophobic residues on the surface of the C-terminal domain of Jac1 result in defective Isu binding and inability to support cell growth.
A. Corresponding residues are highlighted on the surface of protein crystal structures: (left) Jac1 (this report) and (right) HscB (PDB:1FPO) in the region implicated in interaction with Isu/IscU (see text). Yellow, hydrophobic residues; green, polar residues; red, negatively charged residues.
B. A map of Jac1 surface residues and introduced substitutions to alanine in Jac1LLLDDEQY variant.
C. CD spectra measured for purified wild-type (wt) Jac1 and Jac1LLLDDEQY as described in Materials and Methods.
D. (top panel) Isu1-GST (2.5μM) and Jac1 wt or LLLDDEQY variant at the indicated concentrations were mixed to allow complex formation. Glutathione resin was added to pull-down the complex. Isu1-GST and Jac1 proteins were separated by SDS-PAGE and visualized by immunoblot analysis using antibodies specific for Isu1 and Jac1. As a control (c) Isu1-GST was omitted for 10 μM of Jac1 or Jac1LLLDDEQY as indicated. (bottom panel) Bound Jac1 was quantitated by densitometry. Values were plotted in Prism using 1:1 binding hyperbola to fit data for wt Jac1 (Kd = 0.69 ± 0.12 μM). Bmax was set to 1.
E. Stimulation of Ssq1 ATPase activity by wt Jac1 or Jac1LLLDDEQY variant was measured in the presence of 0.5 μM Ssq1, 10 μM Isu1, 0.5 μM Mge1 and indicated concentrations of Jac1 proteins. Values were plotted in Prism using Michaelis-Menten hyperbolic equation to fit the data. The ATPase activity corresponding to maximal stimulation (MS) of wt Jac1 was set to 1. The concentration giving half-maximal stimulation was C05 = 0.065 ± 0.002 μM.
F. (top) jac1-Δ cells harboring plasmid-borne copies of both wt JAC1(URA3 marked) and wt JAC1(HIS3 marked) or mutant jac1LLLDDEQY(TRP1 marked), as indicated, were plated on glucose-minimal medium containing 5-FOA, which selects for cells having lost the plasmid containing the wt copy of JAC1(URA3 marked). The plate was incubated at 30°C for 3 days. (bottom) Immunoblots of 0.1 optical density of cells lysates from indicated strains probed with antibodies specific to Jac1 and Aac1, a loading control. Empty vector- lysate of GAL-JAC1 strain, harbouring chromosomal copy of JAC1 under control of glucose repressible GAL-10 promoter, prepared following 64 hours of growth in glucose containing media. LLDDEQY- lysate of GAL-JAC1 strain transformed with plasmid harbouring copy of jac1LLDDEQY mutant under control of the native JAC1 promoter prepared after 64 hours of growth in glucose containing media. wt- lysate of wt yeast strain.
Alterations in C-domain of Jac1 that abolish Isu1 binding in vitro result in a null phenotype in vivo
To better understand the interaction between Jac1 and Isu, we constructed a mutant JAC1 gene encoding a variant having an alanine in place of each of the eight surface exposed residues (Fig. 2B), encoding Jac1LLLDDEQY. We purified Jac1LLLDDEQY and compared its CD spectra to that of wild-type (wt) protein (Fig. 2C). The two CD spectra were indistinguishable, indicating that the extensive alteration of surface-exposed residues did not substantially affect overall structural properties. To test whether Jac1LLLDDEQY was able to bind Isu1 we developed a pull-down assay utilizing a fusion between Isu1 and glutathione-S-transferase (GST) and thus taking advantage of the ability of GST to bind glutathione. Different concentrations of purified Jac1 were incubated with a fixed concentration of Isu1-GST to allow complex formation. Glutathione resin was then used to pull down Isu1-GST and any Jac1 bound to it. Binding of wt Jac1 was saturable with an apparent Kd of ~0.7 μM (Fig. 2D). Jac1LLLDDEQY did not detectably bind Isu1-GST. Even at 10 fold molar excess, the amount of mutant protein pulled down by Isu1-GST was similar to the background level observed for Jac1 incubated with glutathione resin alone. We also determined the ability of Jac1 to stimulate the ATPase activity of Ssq1 in the presence of saturable amounts of Isu1 (Fig. 2E). In contrast to wt Jac1, which stimulated the ATPase activity of Ssq1 very efficiently with concentration at which half-maximal stimulation was observed (C0.5) at ~0.07 μM, Jac1LLLDDEQY was unable to stimulate Ssq1’s ATPase above the background level (Fig. 2E).
To determine whether the drastic diminution in the ability of Jac1LLLDDEQY to interact with Isu1 affects the in vivo function of Jac1 we transformed a jac1-Δ strain harboring a wt copy of the JAC1 gene and the URA3 marker on a centromeric plasmid with a second plasmid carrying a different selectable marker and the jac1LLLDDEQY gene. Cells were then plated on media containing 5-fluororotic acid (5-FOA). Only those cells having lost the plasmid containing the URA3 gene, and therefore the wt copy of JAC1, can grow on such media, thus allowing the growth phenotype of cells harboring only a mutant copy of JAC1 to be scored (Fig. 2F). No 5-FOA resistant cells carrying jac1LLLDDEQY were recovered. To ensure that the null phenotype was caused by altered protein function rather than low expression, the level of Jac1LLDDEQY was measured. We took advantage of the repression of transcription of the GAL-10 promoter by glucose. Cells expressing wt JAC1 under control of the GAL-10 promoter were transformed with a plasmid carrying jac1LLDDEQY under control of the native JAC1 promoter or a control vector containing no JAC1 gene (Fig. 2F). After growth in glucose containing media cellular extracts were prepared and Jac1 detected using immunoblot analysis. Wt Jac1 was depleted below the level of immunodetection whereas the level of Jac1LLDDEQY was similar to that of Jac1 in a wt strain. Therefore, we conclude that Jac1LLDDEQYcan not support cell growth, indicating that the Jac1:Isu1 interaction is essential.
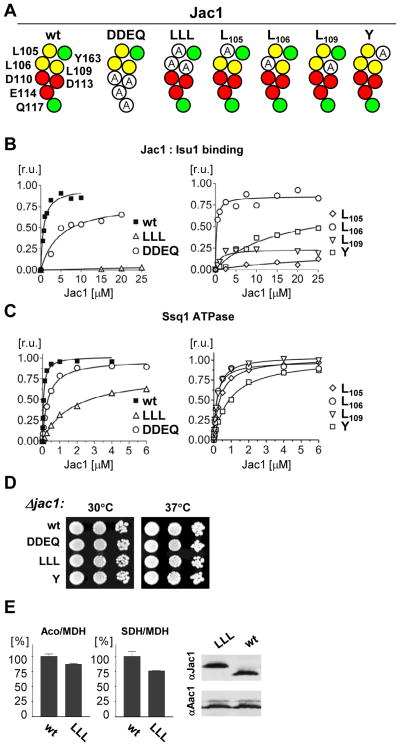
Three residues contribute most significantly to the Jac1:Isu interaction
To continue our systematic analysis of the contribution of surface-exposed residues to the Jac1:Isu interaction we created the JAC1 mutant, jac1DDEQ, which encodes alanines in place of the four residues in the conserved charged patch described above: D110, D113, D114 and Q117 (Fig. 3A). The ability of the Jac1DDEQ to bind Isu1 was impaired in the Isu1-GST pull-down assay. Its apparent Kd value was approximately six fold higher then that of wt Jac1, 4.7 μM versus 0.8 μM (Fig. 3B). When tested for ability to stimulate the ATPase activity of Ssq1 in the presence of saturable concentrations of Isu1, only a minor decrease in stimulatory activity was detected (Fig. 3C, Suppl. Tab. 3). No growth defect of cells harboring jac1DDEQ, as the only copy of JAC1 was observed (Fig. 3D). Thus, we conclude that although the charged region of Jac1 plays a role in Isu1 interaction, its contribution to Isu1 binding is not critical for in vivo function and, consistent with previous results 17, that the affinity of Jac1DDEQ and Isu is higher than that required in vivo under typical laboratory conditions.
Fig. 3. Importance of hydrophobic region for Isu binding in vitro.
A. Maps of Jac1 surface residues and introduced substitutions to alanine in Jac1 variants.
B. Isu1-GST (2.5μM) and Jac1 wt or Jac1 variants as indicated were mixed to allow complex formation. Glutathione resin was added to pull -down the complex and the samples were treated as described in Fig. 1. Bound Jac1 was quantitated by densitometry. Values were plotted in Prism using single binding hyperbola to fit data for wtJac1 (Bmax was set to 1; Kd =0.69 ± 0.12 μM), for Jac1DDEQ (Bmax = 0.81± 0.10 μM; Kd = 4.73 ±1.79 μM) and for Jac1L106(Bmax = 0.85±0.02 μM; Kd = 0.25±0.07 μM).
C. Ssq1 ATPase stimulation was measured as described in Fig. 1. Values were plotted in Prism using Michaelis-Menten hyperbolic equation to fit the data. The ATPase activity corresponding to maximal stimulation (MS) of the wt Jac1 was set to 1. Kinetic parameters are listed in supplementary Table 3.
D. jac1-Δ cells harboring plasmid-borne copies of wt JAC1 or mutant jac1, as indicated, were plated as 10-fold serial dilutions on glucose-rich medium and incubated at 30°C and 37°C for 3 days.
E. (top) Aconitase activity (left) and succinate dehydrogenase activity (right) were measured in lysates of mitochondria isolated from jac1-Δ cells harboring plasmid -borne copies of wt JAC1or jac1LLL grown in glucose-minimal medium. As a standard, the non-FeS cluster-containing protein malate dehydrogenase (MDH) was measured. The ratio of activities of aconitase and MDH or SDH and MDH was calculated and expressed as percent of the ratio in wt mitochondrial extracts. Bars represents average values for three repeated measurements with presented error bars as S.D. (bottom) Protein concentrations of Jac1 and Aac1, a loading control, were determined in mitochondrial extracts from cells described in top panel. Mitochondrial extracts were separated by SDS-PAGE and proteins were visualized by immunoblot analysis using polyclonal antibodies as indicated.
Next, we turned our attention to the hydrophobic patch. We created a JAC1 mutant that encoded alanine in place of leucine codons at positions 105, 106 and 109, generating jac1LLL (Fig. 3A), to test how significantly these closely positioned hydrophobic residues contribute to Isu binding. The ability of Jac1LLL to bind Isu1 was very strongly reduced, as no binding signal above the background level was detected in the pull-down assay (Fig. 3B). Jac1LLL’s ability to stimulate Ssq1’s ATPase activity was also reduced, but easily detectable. Maximal stimulation was decreased by ~20% and the concentration (C0.5) at which 50% the maximal stimulation was attained, was increased ~24 fold compared to the wt control (Fig. 3C, Suppl. Tab. 3). Next, we assessed the in vivo functionality of the Jac1LLL variant, testing growth and the activity of two Fe-S cluster containing enzymes, aconitase and succinate dehydrogenase. Cells expressing Jac1LLL grew normally at both 30 and 37°C (Fig. 3D). To determine enzyme activity, extracts were prepared from mitochondria of cells expressing wt Jac1 or Jac1LLL, The activities were standardized in relation to that of malate dehydrogenase (MDH), which does not contain an Fe-S cluster. Little difference in activity between the strains was observed; aconitase or succinate dehydrogenase activity was 15–25% lower in jac1LLL compared to wt mitochondria (Fig. 3E). That only a slight defect in Fe-S cluster enzyme activity was observed is consistent with robust growth of the mutant cells and is consistent with our previous observation17 that cells can tolerate significant reduction in the affinity of Jac1 for Isu.
However, because the interaction between Jac1 and Isu1 was significantly reduced upon alteration of leucines 105, 106 and 109 and our overall goal was to identify the most critical residues for Jac1 interaction with Isu, we decided to assess the contribution of individual Leu residues. We created three JAC1 mutants, each encoding replacement of a single Leu by an Ala (Fig. 3B). Jac1L106 bound to Isu1 almost as efficiently as wt Jac1 in the pull-down assay (Fig. 3B). However, both Jac1L105 and Jac1L109 bound poorly, but detectably (Fig. 3B), while having only a slightly reduced ability to stimulate Ssq1’sATPase activity (Fig. 3C, Suppl. Tab. 3). From this analysis we concluded that two Leu residues, 105 and 109, are candidates for playing an important role in Jac1:Isu interaction in vivo.
Finally, we also created a mutant, in which the codon for Tyr163 was replaced by an Ala codon, yielding Jac1Y. Jac1Y had obviously reduced, but detectable, interaction in both the pull-down and the ATPase assays (Fig. 3A,B). The ATPase defect was the strongest we observed for a single amino acid substitution, with a ~15 fold increase of the C0.5 value (Fig. 3C, Suppl. Tab. 3). Based on these results we concluded that Tyr163 also plays a significant role in the Isu1 interaction. Next, we assessed the in vivo functionality of variants Jac1LLL and Jac1Y. As expected, based on the results described above, cells expressing Jac1LLL or Jac1Y grew normally (Fig. 3D).
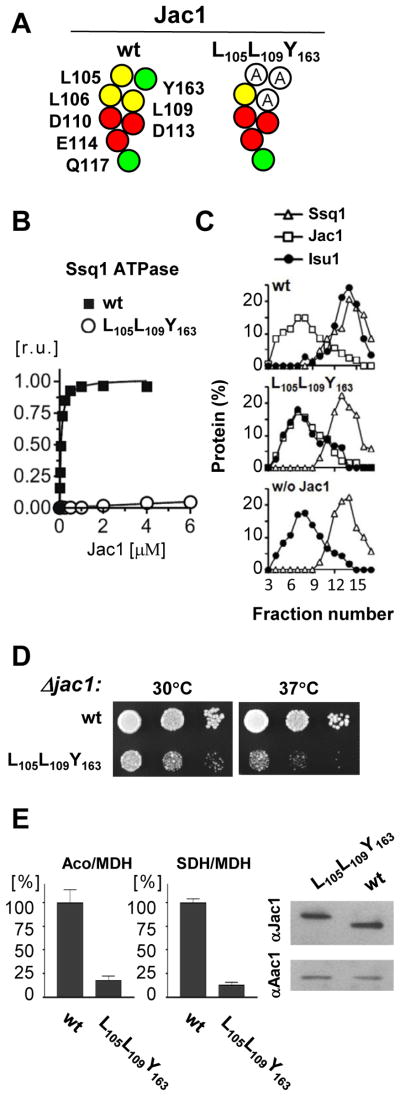
jac1L105L109Y163 cells grow slowly and have reduced Fe-S cluster enzyme activity
Alteration of three residues, L105, L109 and Y163, had the largest effects when changed individually. Therefore we tested the effect of combining the three (Fig. 4A), with a goal of finding a variant that was defective in the in vitro assays, but, unlike the original Jac1LLLDDEQY mutant, was sufficiently active in vivo to support viability. Indeed, Jac1L105L109Y163 was strongly defective in ATPase stimulation (Fig. 4B). However, cells expressing this variant as the only copy of Jac1 grew significantly slower than wt cells at 30°C and extremely poorly at 37°C (Fig. 4D). To ensure that the phenotypic defect of jac1L105L109Y163 was not due to low levels of expression, we compared Jac1 levels in mitochondrial extracts prepared from cells expressing wt Jac1 or jac1L105L109Y163. Wt Jac1 and Jac1L105L109Y163 were expressed at comparable levels (Fig. 4E).
Fig. 4. Alanine replacement of L105, L109 and Y163 results in defective Fe-S cluster biogenesis in vivo.
A. Map of Jac1 surface residues and introduced substitutions to alanine in Jac1L105L109Y163
B. Ssq1 ATPase stimulation was measured as described in Fig. 1.
C. Isu1 binding to Ssq1 analyzed using glycerol gradient centrifugation. Purified proteins: Isu1 (2.5 μM), Jac1 (5 μM), Ssq1 (5 μM) were incubated, as indicated, in the presence of ATP (2 mM) in 70 μl reaction mixture prior to loading onto a gradient. Plots representing quantification of protein content obtained by densitometry after analysis of protein content of fractions by SDS-PAGE and silver staining. w/o Jac1- a negative control with no Jac1 in the reaction mixture.
D. jac1-Δ cells harboring plasmid -borne copies of wt or mutant jac1, as indicated, were plated as 10-fold serial dilutions on glucose-rich medium and incubated at 30°C and 37°C for 3 days.
E. (top) Aconitase (left) and succinate dehydrogenase (right) activities were measured in lysates of mitochondria isolated from jac1-Δ cells harboring plasmid -borne copies of wt JAC1or jac1L105L109Y163 grown in glucose-minimal medium. As a standard, the non-FeS cluster-containing protein malate dehydrogenase (MDH) was measured. The ratio of activities of aconitase and MDH or SDH and MDH was calculated and expressed as percent of the ratio in wt mitochondrial extracts. Bars represents average values for three repeated measurements with presented error bars as S.D. (bottom) Levels of Jac1 and Aac1, a loading control, in the mitochondrial extracts described in top panel were determined. Extracts were subjected to SDS-PAGE and proteins detected by immunoblot analysis using polyclonal antibodies as indicated.
As expected, because of the severe interaction defect caused by alteration of individual residues, no interaction of Jac1L105L109Y163 was detected in the GST-Isu pull-down assay (data not shown). To further characterize the biochemical properties of Jac1L105L109Y163, we also used a previously developed biochemical assay based on glycerol gradient centrifugation of a mixture of Jac1, Isu1, Ssq1 and ATP 15, which serves as a test of targeting of Isu to Hsp70. In a control experiment we observed that greater than 80% of Isu1 migrated deep into the gradient, co-localizing with Ssq1, indicating formation of Isu1:Ssq1 complex (Fig. 4C). In contrast, when Jac1 was omitted from the reaction mixture, less than 10% of Isu1 co-localized with Ssq1, indicating that formation of a stable Ssq1:Isu1 complex requires Jac1. However, when we replaced wt Jac1 by Jac1L105L109Y163, less then 10% of Isu1 was found in complex with Ssq1. From these results we concluded that the Jac1 variant defective in Isu1 binding was unable to target Isu1 to its partner Hsp70, Ssq1.
To test whether the in vitro and in vivo defects of Jac1L105L109Y163 are consistent with an effect on Fe-S biogenesis, we compared the activity of two Fe-S cluster containing enzymes, aconitase and succinate dehydrogenase in mitochondrial extracts prepared from cells expressing wt Jac1 and Jac1L105L109Y163 (Fig. 4E). Activities were ~80% lower in Jac1L105L109Y163 extracts than in the wt extracts. As both enzymes require Fe-S cluster for their activity, the slow growth phenotype of mutant defective in Jac1:Isu1 interaction can be explained by a defective Fe-S biogenesis pathway.
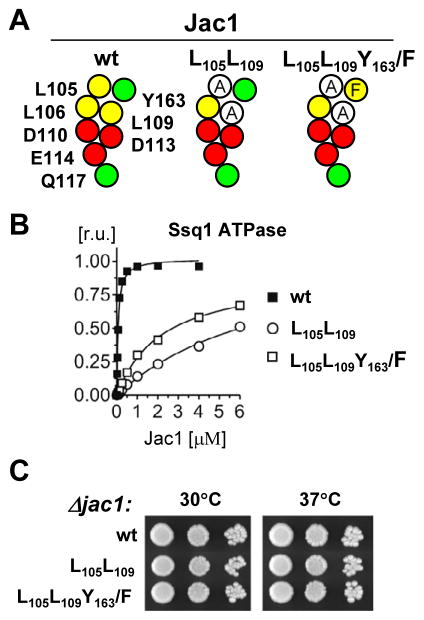
Phenylalanine can replace Tyrosine-163 without functional consequences
Our observation that the three residues, L105, L109 and Y163, play a critical role in the Jac1:Isu interaction is consistent with previously published data 19 showing that the three homologous residues, L92, L96 and F153, are important for the HscB:IscU interaction in E. coli. Whereas leucine residues are invariant between Jac1 and HscB, phenylalanine is at the position of Tyr163 in HscB. While, Phe is predominantly involved in nonpolar interactions, Tyr, with its mixed hydrophobic and polar character could form hydrogen bounds, as well as participate in nonpolar interactions. To test whether the presence of Tyr at position 163 is critical, or whether Phe would suffice in S. cerevisiae, we prepared a Jac1 variant in which Tyr163 was replaced by Phe. To allow scoring of the effects of the Y/F replacement in vivo, as well as in vitro, we placed it in the context of Jac1 having the L105A and L109A substitutions (Fig. 5A). The ability of Jac1L105A,L109A,Y163F to stimulate the ATPase activity of Ssq1 were comparable to that of the control Jac1L105A, L109A variant (Fig. 5B), indicating that the Y/F replacement had no obvious detrimental effects on biochemical properties of Jac1. Also, no growth defect was visible for cells expressing jac1L105A,L109A,Y163F (Fig. 5C). Thus, these residues can be exchanged for one another without obvious functional consequences pointing to the conclusion that it is the similar size and hydrophobic character shared by Tyr and Phe that are critical for the Jac1:Isu interaction.
Fig. 5. Tyrosine 163 of Jac1 can be functionally replaced by Phenylalanine.
A. Maps of Jac1 surface residues and introduced substitutions to alanine in Jac1 variants.
B. jac1-Δ cells harboring plasmid-borne copies of wtJAC1 or jac1 mutant, as indicated, were plated as 10-fold serial dilutions on glucose-rich medium and incubated at 30°C and 37°Cfor 3 days.
C. Ssq1 ATPase stimulation was measured as described in Fig. 1.
Perspectives
The results presented here point to two key conclusions: (i) the “adhesive surface” of Jac1’s C-domain, consisting of hydrophobic and charged regions and responsible for the Jac1:Isu interaction, is evolutionary conserved and (ii) the Jac1:Isu interaction is indispensible for in vivo biological functions. Hydrophobic residues consisting of leucines 105 and 109 on helix 6 and tyrosine 163 on helix 8 play a critical role in the Isu interaction both in vitro and in vivo. Yet, jac1L105L109Y163 cells are viable. Only when replacements of the hydrophobic residues were combined with replacements of charged residues was a null phenotype observed. Thus, the charged region does play a role, though not a critical one under laboratory conditions. We note that throughout this work measurements of Jac1 interaction with Isu1 were performed under aerobic conditions, thus Isu1 was in the apo-form, that is no Fe-S cluster was present. However, we reported previously that the biochemical defects measured for apo-Isu1 interaction with both Jac1 and Ssq1 correlated well with in vivo Fe-S biogenesis defects determined for cells expressing protein variants defective in apo-Isu1 interactions9; 17; 23; 24 Moreover, these findings are consistent with those of a previously published biochemical and biophysical analysis of the HscB:IscU interaction 18; 19, which shows that the three evolutionary conserved hydrophobic residues of HscB have the strongest contribution to IscU binding, with the charged residues contributing to a lesser extent to binding stability. Such a contribution of a number of residues toward the strength of protein:protein interaction across a binding interface has been observed previously for variety of interacting proteins 25. It is often the case that hydrophobic residues provide stability to the interaction, with the charged region providing specificity and directing the precise orientation of interacting partners18. The evolutionary conservation of the spatial orientation of both hydrophobic and charged patches across the binding interface of Jac1 and its bacterial and eukaryotic orthologs is consistent with such a mechanism.
How does our finding that the Jac1:Isu interaction is indispensible in vivo fit into the current picture of the role of Jac1 in Fe-S cluster biogenesis? Biochemical data, including those presented here, are consistent with the hypothesis that Jac1, by direct interaction, can target Isu for Ssq1 binding, thus orchestrating the Isu binding and ATPase stimulation events. In the larger picture, a similar mechanism of client protein targeting has been proposed for other J-protein co-chaperones, including bacterial DnaJ and its partner DnaK, as well as their homologues from eukaryotic cells 8; 11. It is also possible that the Jac1:Isu interaction plays roles in addition to the targeting of Isu to Ssq1, as the process of Fe-S cluster assembly on and transfer from Isu to recipient apo-proteins is a complex interplay of protein:protein interactions, involving other proteins required for cluster biogenesis 1. Recent data 26; 27 suggests two possibilities: facilitating dissociation of holo-Isu from the desulfurase complex involved in formation of the cluster and stabilization of the ordered conformation of cluster loaded Isu prior to interaction with Ssq1 and cluster transfer. Further experimentation is under way to test these attractive hypotheses.
Materials and Methods
Yeast Strains, Plasmids, Media, and Chemicals
PJ53 jac1-Δ strain of S. cerevisiae used in this study is isogenic to W303 and was described previously by Andrew et al.,17. GAL-JAC1 strain harbouring a chromosomal copy of JAC1 under control of the glucose repressible GAL-10 promoter was a kind gift of Dr. Roland Lill (Philipps-Universität Marburg, Germany) and was described by Muhlenhoff et al.,28. Full length mature Jac1 was cloned into pET11a starting at amino acid 10 to give Jac-C1. JAC1 mutants were constructed by changing selected codons to encode alanine by site-directed mutagenesis (QuikChange protocol, Stratagene) using wtJAC1 (−350 to +824) cloned into pRS314 or pRS313 29 as a template. An expression vector harboring Isu1-GST was prepared by amplifying the Isu1-GSTfusion from p416 - Isu1-GST, a kind gift of Dr. Roland Lill (Philipps-Universität Marburg, Germany) described by Gerber etal., 3, using primers which encode a NdeI site at the mature start of Isu1 and a BamHI site downstream of the stop codon for GST. The resulting NdeI-BamHI fragment was cloned into pET3A (Novagen, Gibbstown, NJ). Yeast were grown on YPD (1% yeast extract, 2% peptone, and 2% glucose) or synthetic media as described by Sherman,30. All chemicals, unless stated otherwise, were purchased from Sigma.
Protein purification
Expression of Isu1-GST was induced in the E. coli strain C41(DE3) carrying the pET3aISU1-GST plasmid by addition of 1 mM isopropyl-1-thio-D-galactopyranoside at A600=0.6. After 2.5 hours, cells were harvested and lysed in a French Press in buffer L (25mM Tris-HCl pH 8.0; 200 mM NaCl; 1 mM phenylmethylsulfonyl fluoride (PMSF); 1 mM dithiothreitol; 10% glycerol; 0.05% Triton X-100). After a clarifying spin, the supernatant was loaded on a 1 ml glutathione agarose column (Fluka) equilibrated with 10 volumes of buffer L. Next the column was washed with 100 ml of buffer L without PMSF and with 10 volumes of buffer L with 0.5M NaCl and with 10 volumes of buffer L with 10mM MgCl2 and 1 mM ATP. After a final wash with 10 volumes of buffer L, proteins were eluted with buffer E (25 mM Tris-HCl pH 8.0; 200mM NaCl; 1 mM dithiothreitol; 10% glycerol; 0.05% Triton X-100, 50mM reduced glutathione). Fractions containing Isu1-GST were pooled, dialyzed against buffer CM (20 mM MOPS pH 7.0; 25 mM NaCl; 1 mM dithiothreitol; 0.05% Triton X-100) and loaded onto a 2 ml CM-Sepharose column (Amersham Biosciences) equilibrated with buffer CM containing 25 mM NaCl. The CM-Sepharose column was washed with 10 volumes of buffer CM containing 25 mM NaCl. Isu1-GST was eluted with a 50 ml linear NaCl gradient (25 mM – 600 mM) in buffer CM. Fractions containing Isu1-GST were pooled, dialyzed against buffer Q (20 mM Tris pH 8.0, 10% glycerol, 5 mM β-mercaptoethanol, 0.05% Triton X-100, 50 mM NaCl) and loaded onto a 1 ml Q-Sepharose column (GE Healthcare). After washing with 10 volumes of buffer Q containing 50 mM NaCl, Isu1-GST was step eluted with buffer Q containing 600 mM NaCl. Fractions containing Isu1-GST were pooled, dialyzed against buffer F (20 mM Tris-HCl pH 8.0; 10% glycerol; 5 mM β-mercaptoethanol; 0.05% Triton X-100; 200 mM KCl) and stored at −70°C.
Recombinant Mge1His, Isu1His, Ssq1His were purified as described previously by Dutkiewicz et al.,15. Jac1His mutant proteins were purified according to Dutkiewiczet al.,15 but with use of E.coli strain C41(DE3) for expression. In all cases, protein concentrations, determined by using the Bradford (Bio-Rad) assay system with bovine serum albumin as a standard, are expressed as the concentration of monomers.
Pulldown Assay
Titration pulldown experiments were performed by incubating indicated concentrations of Jac1Hiswith 2. 5 μM of Isu1 -GST in 150 μl of buffer PD (40 mM HEPES-KOH, pH 7.5, 5% (v/v) glycerol, 100 mM KCl, 0.001 mM dithiothreitol, 0.01 mM MgCl 2, 0,002 mM ATP)for 30 min at 25°C to allow complex formation. Glutathione immobilized agarose beads were incubated with 0.1% of BSA and 10% (v/v) glycerolin buffer PD. 20 μl of beads were added to each reaction and incubated at 4°C for 1h with rotation. The beads were washed once with 500 μl and then three times with 200 μl of buffer PD. After the final wash, four times concentrated Laemmli sample buffer (20 μl) was added to the reaction mixtures and samples were incubated for 10 min at 90°C. 3 μl aliquots were separated by SDS-PAGE and visualized by immunoblot analysis using polyclonal antibodies specific for Isu1 and Jac1.
ATPase activity of Ssq1
ATPase activity was measured as described by Dutkiewicz et al.,15 with 0.5 μM Ssq1, 10 μM Isu1, 0.5 μM Mge1 and Jac1 protein at the indicated concentrations in buffer A (40 mM HEPES-KOH, pH 7.5, 100 mM KCl, 1 mM dithiothreitol, 10 mM MgCl2, 10% (v/v) glycerol). Reactions (15 μl) were initiated by the addition of ATP (2 μCi, DuPont NEG-003H, 3000 Ci/mmol) to a final concentration of 120 μM. Incubation was carried out at 25°C and the reaction was terminated after 15 min by the addition of 100 μl of 1 M perchloric acid and 1 mM sodium phosphate After addition of 400 μl of 20 mM ammonium molybdate and 400 μl of isopropyl acetate, samples were mixed and the phases separated by a short centrifugation. An aliquot of the organic phase (150 μl), containing the radioactive orthophosphate-molybdate complex, was removed and radioactivity was determined by liquid scintillation counting. Control reactions lacking protein were included in all experiments.
Glycerol gradient centrifugation
Glycerol gradient centrifugation was carried out as described by Dutkiewicz et al.,15. Purified proteins: Isu (2.5 mM), Jac1 (5 mM), Ssq1 (5 mM) were incubated in the presence of ATP (2 mM) in buffer G (40 mM HEPES-KOH, pH 7.5, 100 mM KCl, 1 mM dithiothreitol, 10 mM MgCl2, 5% (v/v) glycerol) in a total volume of 80μl for 10 min at 25°C. Then, 70 μl of this mixture was loaded onto a 3-ml linear 15–35% (v/v) glycerol gradient prepared in buffer G, and centrifuged at 4°C in a Beckman SW60 rotor for 28 h at 46,000 rpm. Fractions (130 μl each) were collected from the top of the tube and their protein contents were analyzed by SDS-PAGE followed by silver staining.
Circular Dichroism (CD)
Measurements were performed on a Jasco J-815 CD Spectrometer from 197 to 260 nm with 3-s averaging times and 1-nm step size at 25°C. The protein concentration was 9.8 μM in 20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 5 mM β-mercaptoethanol, 10% glycerol) in a quartz cuvette with 1-mm path length. Spectra were measured in milidegrees, corrected for buffer effects, and converted to mean residue ellipticity (Θ)
Mitochondrial enzymes activities
Activities of the respiratory enzymes were measured in mitochondria isolated as described previously by Gambill et al.,31. Succinate dehydrogenase activity was measured by using succinate as a substrate as described Stehling et al.,32. Aconitase activity was measured by monitoring the decrease in absorbance of the substrate isocitrate at 235 nm as described by Stehling et al.,32 Malate dehydrogenase activity was measured using oxaloacetate as a substrate and monitoring the decrease in absorbance of NADH at 340 nm as described by Stehling et al.,32. Data were normalized to the protein content of the mitochondrial samples.
Supplementary Material
Acknowledgments
This work was supported by the Polish Ministry of Science and Higher Education Grant N N301 314137 (R.D.), National Institutes of Health grant GM278709 (E.A.C) and GM074942 and the U.S. Department of Energy, Office of Biological and Environmental Research, under contract DE-AC02-06CH11357 (A.J.). We thank all members of the Structural Biology Center at Argonne National Laboratory for their help in conducting these experiments. Also, we thank Gyorgy Babnigg and Brian Feldman for statistical analysis and cloning of truncation mutants, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lill R, Muhlenhoff U. Maturation of iron-sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu Rev Biochem. 2008;77:669–700. doi: 10.1146/annurev.biochem.76.052705.162653. [DOI] [PubMed] [Google Scholar]
- 2.Garland SA, Hoff K, Vickery LE, Culotta VC. Saccharomyces cerevisiae ISU1 and ISU2: members of a well-conserved gene family for iron-sulfur cluster assembly. J Mol Biol. 1999;294:897–907. doi: 10.1006/jmbi.1999.3294. [DOI] [PubMed] [Google Scholar]
- 3.Gerber J, Muhlenhoff U, Lill R. An interaction between frataxin and Isu1/Nfs1 that is crucial for Fe/S cluster synthesis on Isu1. EMBO Rep. 2003;4:906–11. doi: 10.1038/sj.embor.embor918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai CL, Barondeau DP. Human frataxin is an allosteric switch that activates the FeS cluster biosynthetic complex. Biochemistry. 2010;49:9132–9. doi: 10.1021/bi1013062. [DOI] [PubMed] [Google Scholar]
- 5.Craig EA, Marszalek J. A specialized mitochondrial molecular chaperone system: a role in formation of Fe/S centers. Cell Mol Life Sci. 2002;59:1658–65. doi: 10.1007/PL00012493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vickery LE, Cupp-Vickery JR. Molecular chaperones HscA/Ssq1 and HscB/Jac1 and their roles in iron-sulfur protein maturation. Crit Rev Biochem Mol Biol. 2007;42:95–111. doi: 10.1080/10409230701322298. [DOI] [PubMed] [Google Scholar]
- 7.Hennessy F, Nicoll WS, Zimmermann R, Cheetham ME, Blatch GL. Not all J domains are created equal: implications for the specificity of Hsp40-Hsp70 interactions. Protein Sci. 2005;14:1697–709. doi: 10.1110/ps.051406805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579–92. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knieszner H, Schilke B, Dutkiewicz R, D’Silva P, Cheng S, Ohlson M, Craig EA, Marszalek J. Compensation for a defective interaction of the hsp70 ssq1 with the mitochondrial Fe-S cluster scaffold isu. J Biol Chem. 2005;280:28966–72. doi: 10.1074/jbc.M503031200. [DOI] [PubMed] [Google Scholar]
- 10.Voisine C, Cheng YC, Ohlson M, Schilke B, Hoff K, Beinert H, Marszalek J, Craig EA. Jac1, a mitochondrial J-type chaperone, is involved in the biogenesis of Fe/S clusters in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2001;98:1483–8. doi: 10.1073/pnas.98.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–84. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wawrzynow A, Banecki B, Wall D, Liberek K, Georgopoulos C, Zylicz M. ATP hydrolysis is required for the DnaJ-dependent activation of DnaK chaperone for binding to both native and denatured protein substrates. J Biol Chem. 1995;270:19307–11. doi: 10.1074/jbc.270.33.19307. [DOI] [PubMed] [Google Scholar]
- 13.Chandramouli K, Johnson MK. HscA and HscB stimulate [2Fe-2S] cluster transfer from IscU to apoferredoxin in an ATP-dependent reaction. Biochemistry. 2006;45:11087–95. doi: 10.1021/bi061237w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silberg JJ, Hoff KG, Vickery LE. The Hsc66-Hsc20 chaperone system in Escherichia coli: chaperone activity and interactions with the DnaK-DnaJ-grpE system. J Bacteriol. 1998;180:6617–24. doi: 10.1128/jb.180.24.6617-6624.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutkiewicz R, Schilke B, Knieszner H, Walter W, Craig EA, Marszalek J. Ssq1, a mitochondrial Hsp70 involved in iron-sulfur (Fe/S) center biogenesis. Similarities to and differences from its bacterial counterpart. J Biol Chem. 2003;278:29719–27. doi: 10.1074/jbc.M303527200. [DOI] [PubMed] [Google Scholar]
- 16.Schilke B, Voisine C, Beinert H, Craig E. Evidence for a conserved system for iron metabolism in the mitochondria of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1999;96:10206–11. doi: 10.1073/pnas.96.18.10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrew AJ, Dutkiewicz R, Knieszner H, Craig EA, Marszalek J. Characterization of the interaction between the J-protein Jac1p and the scaffold for Fe-S cluster biogenesis, Isu1p. J Biol Chem. 2006;281:14580–7. doi: 10.1074/jbc.M600842200. [DOI] [PubMed] [Google Scholar]
- 18.Fuzery AK, Tonelli M, Ta DT, Cornilescu G, Vickery LE, Markley JL. Solution structure of the iron-sulfur cluster cochaperone HscB and its binding surface for the iron-sulfur assembly scaffold protein IscU. Biochemistry. 2008;47:9394–404. doi: 10.1021/bi800502r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuzery AK, Oh JJ, Ta DT, Vickery LE, Markley JL. Three hydrophobic amino acids in Escherichia coli HscB make the greatest contribution to the stability of the HscB-IscU complex. BMC Biochem. 2011;12:3. doi: 10.1186/1471-2091-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson DC, Dean DR, Smith AD, Johnson MK. Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem. 2005;74:247–81. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- 21.Cupp-Vickery JR, Vickery LE. Crystal structure of Hsc20, a J-type Co-chaperone from Escherichia coli. J Mol Biol. 2000;304:835–45. doi: 10.1006/jmbi.2000.4252. [DOI] [PubMed] [Google Scholar]
- 22.Bitto E, Bingman CA, Bittova L, Kondrashov DA, Bannen RM, Fox BG, Markley JL, Phillips GN., Jr Structure of human J-type co-chaperone HscB reveals a tetracysteine metal-binding domain. J Biol Chem. 2008;283:30184–92. doi: 10.1074/jbc.M804746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pukszta S, Schilke B, Dutkiewicz R, Kominek J, Moczulska K, Stepien B, Reitenga KG, Bujnicki JM, Williams B, Craig EA, Marszalek J. Co-evolution-driven switch of J-protein specificity towards an Hsp70 partner. EMBO Rep. 2010;11:360–5. doi: 10.1038/embor.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schilke B, Williams B, Knieszner H, Pukszta S, D’Silva P, Craig EA, Marszalek J. Evolution of mitochondrial chaperones utilized in Fe-S cluster biogenesis. Curr Biol. 2006;16:1660–5. doi: 10.1016/j.cub.2006.06.069. [DOI] [PubMed] [Google Scholar]
- 25.Bogan AA, Thorn KS. Anatomy of hot spots in protein interfaces. J Mol Biol. 1998;280:1–9. doi: 10.1006/jmbi.1998.1843. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Fuzery AK, Tonelli M, Ta DT, Westler WM, Vickery LE, Markley JL. Structure and dynamics of the iron-sulfur cluster assembly scaffold protein IscU and its interaction with the cochaperone HscB. Biochemistry. 2009;48:6062–71. doi: 10.1021/bi9002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi R, Proteau A, Villarroya M, Moukadiri I, Zhang L, Trempe JF, Matte A, Armengod ME, Cygler M. Structural basis for Fe-S cluster assembly and tRNA thiolation mediated by IscS protein-protein interactions. PLoS Biol. 2010;8:e1000354. doi: 10.1371/journal.pbio.1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhlenhoff U, Gerber J, Richhardt N, Lill R. Components involved in assembly and dislocation of iron-sulfur clusters on the scaffold protein Isu1p. EMBO J. 2003;22:4815–25. doi: 10.1093/emboj/cdg446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 31.Gambill BD, Voos W, Kang PJ, Miao B, Langer T, Craig EA, Pfanner N. A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J Cell Biol. 1993;123:109–17. doi: 10.1083/jcb.123.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stehling O, Smith PM, Biederbick A, Balk J, Lill R, Muhlenhoff U. Investigation of iron-sulfur protein maturation in eukaryotes. Methods Mol Biol. 2007;372:325–42. doi: 10.1007/978-1-59745-365-3_24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.