Abstract
Chitosan/tripolyphosphate/chondroitin sulfate (Chi/TPP/CS) nanoparticles were prepared by an ionic gelation method to obtain a controlled release of proteins. Using Nel-like molecule-1 (Nell-1), a novel osteogenic protein, as a model protein, it was demonstrated that adjusting the composition of the particles modulated the protein association and release kinetics of incorporated proteins. Increasing the amounts of chitosan crosslinking agents, TPP and CS, in the particles achieved sustained protein release. An increase in crosslinking density decreased degradation rates of the particles. Furthermore, the bioactivity of the protein was preserved during the encapsulating procedure into the particles. To demonstrate the feasibility of Chi/TPP/CS nanoparticles as sustained release carriers for tissue engineering scaffold applications, protein-loaded nanoparticles were successfully incorporated into collagen hydrogels or prefabricated porous poly (lactide-co-glycolide) (PLGA) scaffolds without obstructing the integrity of the hydrogels or porous structure of the scaffolds. Thus, we expect that these particles have a potential for efficient protein carriers in tissue engineering applications, and will be further evaluated in vivo.
Keywords: Chitosan, Nanoparticle, Protein, Controlled release, Tissue engineering
INTRODUCTION
Numerous growth factors are released to control cellular functions and promote tissue regeneration during natural tissue healing. However, their therapeutic applications pose a clinical challenge due to the short half-life and rapid diffusion of the agents from the defective area,1 leading to adverse side effects and inefficient tissue formation. Thus, significant research efforts have been made to develop appropriate carriers/delivery systems that prolong the biological activity of protein and release bioactive factors over time in a controlled manner at the defect site.
Various approaches have been investigated to incorporate protein into polymeric release carriers or tissue engineering scaffolds, including chemical conjugation and physical encapsulation.2–5 However, the formulation methods with a polymer matrix are often not suitable for the incorporation of proteins due to their denaturation and loss of bioactivity under the harsh conditions used, such as high temperatures, organic solvents, and sonication.6,7 Furthermore, post-processing steps such as porogen leaching or solvent evaporation can cause the premature release or deactivation of incorporated proteins.
Chitosan (Chi) is a naturally-derived polysaccharide and has been extensively used in pharmaceutical and medical applications due to its favorable biological properties, such as biocompatibility, biodegradability, and nontoxicity.8, 9 In addition, its cationic nature allows the formation of stable ionic complexes with multivalent anionic ions or polymers in various forms, such as gels, sponges, beads, and micro/nanoparticles.10–17 Ionic crosslinking process is attractive in preparation of protein delivery devices because of its simple and mild procedure without the application of harmful organic solvents, minimizing protein denaturation during formulation preparation. The first Chi nanoparticles obtained by an ionic gelation method were reported in the late 1990s using tripolyphosphate (TPP) where the nanoparticles are formed via electrostatic interactions of the positively charged Chi molecules with TPP anions used as crosslinkers.18 Since then, because of its hydrophilic and mucoadhesive features, and its capacity for the association with therapeutic nucleic acids, Chi nanoparticles have received considerable attention to enhance the transport of peptides and proteins across mucosal barriers and deliver genetic molecules.19–21 Although the Chi nanoparticles have been extensively explored as mucosal delivery systems and as non-viral gene delivery vectors, very few studies investigated their use for growth factor delivery systems in tissue engineering applications.
In this study, we sought to create Chi nanoparticles designed to support controlled release of growth factors in tissue engineering strategies. Chondroitin sulfate (CS), one of the major glycosaminoglycans found in tissue extracellular matrices, and TPP were used as chitosan crosslinkers. Because crosslinking density can influence important properties of ionically crosslinked particles, such as drug association, release, and particle degradation,22, 23 we investigated the effects of crosslinker concentrations on protein association efficiency, release kinetics of incorporated protein, and in vitro enzymatic degradation of the particles. Nel-like molecule-1 (Nell-1), a novel osteoinductive molecule,24, 25 was used as a model protein. The bioactivity of protein released from nanoparticles was evaluated. To examine further the potential of Chi/TPP/CS nanoparticles as sustained release carriers applicable to tissue engineering scaffolds, protein-loaded nanoparticles were incorporated into hydrogels or prefabricated porous scaffolds. Uniform distribution of particles in the hydrogels or throughout the pores of scaffolds, and sustained release kinetics of loaded proteins, were determined using fluorescently-labeled bovine serum albumin (BSA) as a model protein.
MATERIALS AND METHODS
Materials
Chitosan (Mw 400,000, 85% deacetylated), pentasodium tripolyphosphate (TPP), chondroitin-4-sulfate (CS) from bovine trachea, and lysozyme were purchased from Sigma-Aldrich (St. Louis, MO). Poly(D,L-lactic-co-glycolic) acid (PLGA, latide:glycolide ratio 85:15, intrinsic viscosity 0.61 dL/g; 50:50, intrinsic viscosity 0.67 dL/g) were purchased from Birmingham Polymers (Birmingham, AL). Fluorescein isothiocyanate conjugated bovine serum albumin (FITC-BSA) was obtained from Invitrogen (Carlsbad, CA). Type I collagen solution purified from bovine skin was obtained from Vitrogen (Palo Alto, CA). Bioactive recombinant human Nell-1 protein was produced and purified from Chinese hamster ovary cells (Aragen Bioscience, Morgan Hill, CA). The murine chondrogenic cell line ATDC5 cells were obtained from the RIKEN cell bank (Tsukuba, Japan). All chemicals were analytical grade and used as received.
Preparation of nanoparticles
Chi/TPP/CS nanoparticles were prepared by ionic gelation of Chi solution with polyanion TPP and CS solution (Figure 1). Polyanion solutions were prepared by dissolving TPP (0.1% w/v) or CS (0.1% w/v) in deionized water. Chi solution (0.1% w/v) was prepared by dissolving Chi in 0.05% aqueous acetic acid. All solutions were sterile filtered through a 0.22 μm polyethersulfone (PES) membrane (Nalgene, NY). Nanoparticles were formed by dropping TPP/CS solution (0.1%, w/v) into Chi solution (0.1% w/v) with various ratio Chi:TPP:CS of 10:1:0, 10:2:0, 10:2:1, 10:2:2 (w/w/w) under magnetic stirring. Nell-1 protein was loaded onto the nanoparticles by dissolving protein in the TPP/CS solution. Nanoparticles were collected by centrifugation at 14,000 g for 30 min and were washed twice with distilled water.
Figure 1.
Schematic diagrams depicting preparation of nanoparticles.
Characterization of nanoparticles
The morphology of nanoparticles was analyzed with scanning electron microscopy (SEM; FEI/NOVA 230). Prior to SEM analysis, the samples were mounted on aluminum stubs and gold-coated with a sputter coater at 20 mA under 70 mTorr for 90 sec. The particles sizes and zeta potential of nanoparticles were examined using Zetasizer Nano (Malvern Instruments, Westborough, MA).
Protein loading and in vitro release
The amount of Nell-1 protein associated with the nanoparticles was determined indirectly by measuring the difference between the initial amount of protein dissolved in the TPP/CS solution and the amount of protein remaining in the supernatant after centrifugation. Nell-1 content was quantified using the 3-(4-carboxybenzoyl) quinoline-2-carboxaldehyde (CBQCA) protein assay (Invitrogen, Carlsbad, CA). Protein release was determined by incubating protein-loaded nanoparticles in 1 ml of 10 mM phosphate-buffered saline (PBS, pH 7.4) at 37 C with gentle shaking. Nanoparticles were centrifuged and the incubating solution was replaced with fresh solution at various time points up to 14 days. The released Nell-1 protein in the supernatant was measured using the CBQCA protein assay. The experiment was performed in triplicate and the amount of protein released was expressed as a percentage of the initial amount of protein loaded.
Bioactivity of released protein
The bioactivity of the Nell-1 released from nanoparticles was determined by measuring its ability to increase the expression of alkaline phosphatase (ALP) in the ATDC5 cells. Protein-loaded particles were incubated in PBS at 37 C for 24 h, and the supernatant was collected for the assay. Supernatant from nanoparticles not containing protein served as a control. ATDC5 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F-12 medium (50:50) supplemented with 5% fetal bovine serum (FBS), 100U/mL penicillin, and 100 mg/mL streptomycin at 37 C in 5% CO2 humidified incubators. At the beginning of the assay, cultures were washed once with PBS, and the media was supplemented with 50 μg/ml ascorbic acid and 50 μg/ml bone morphogenetic protein-2 (BMP-2). After 24h of culture, the culture medium was removed and the culture medium containing Nell-1 or control supernatant was added. After incubation for 72h, cells were solubilized in lysis buffer (0.2% NP-40, 1 mM magnesium chloride) at 4 C for 30 min. ALP activity was determined colorimetrically using p-nitrophenol phosphate (Sigma, St. Louis, MO) as a substrate and measuring at an absorbance at 405 nm. Measurements were performed in triplicate and normalized to total protein content determined by the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA).
In vitro Degradation
The nanoparticles with various ratios Chi:TPP:CS were prepared as described above. The prepared particles were incubated in 5 mL of PBS containing lysozyme (0.1 mg/ml) at 37 C with gentle shaking. The incubating media was replaced once per week. At varying time points, nanoparticles were centrifuged, the incubating solution was removed, and the samples were dialyzed against 200 ml water in dialysis tubing with cutoff molecular weight of 50,000 Da. The purified particles were then freeze-dried and weighed. Degradation ratios were expressed as the ratio of the remaining weight to the initial weight of particles. Nanoparticles incubated in PBS were used as a control. All the experiments were performed in triplicate.
Incorporation of nanoparticles into hydrogels
FITC-BSA was loaded onto nanoparticles with a final ratio Chi:TPP:CS of 10:2:1 (w/w/w). Type I collagen solution (3 mg/ml) was neutralized with 0.1 N NaOH and mixed with BSA or BSA-loaded nanoparticles at 4°C. The mixture was incubated for 1 h at 37°C for gelation. Distribution of nanoparticles in the gels was visualized using fluorescent microscope (Leica Microsystems Inc., Bannockburn, IL). Collagen gels containing BSA or BSA-loaded nanoparticles were immersed in 1 ml of 10 mM PBS at 37 °C with gentle shaking. At various time points, the whole incubating solution was removed and replaced with 1 mL of fresh solution. The amount of released protein in the supernatant was determined by measuring the fluorescence of the sample.
Incorporation of nanoparticles into porous 3D scaffolds
Polymer scaffolds were fabricated by the solvent casting and particulate leaching technique.26, 27 Briefly, PLGA (15%, w/w) was dissolved in a mixture of chloroform and methanol (67/33, w/w). Sucrose particles were sieved to range 200~300 μm in diameter. Polymer solution mixed with sucrose (polymer/sucrose ratio 5/95, w/w) was cast into Teflon molds (inner diameter: 8 mm, height: 2 mm). Scaffolds were dried in a fume hood and solvents were removed by freeze-drying at 100 mTorr and 110°C (SP Industries, Inc., Warminster, PA) overnight. Sucrose was removed by immersing scaffolds in deionized water. BSA-loaded Chi:TPP:CS nanoparticles were dispersed in neutralized 0.025% collagen solution at 4 °C, and the mixture was dropped onto PLGA scaffolds at room temperature for 30 min. Distribution of nanoparticles in the scaffolds was visualized using fluorescent microscope. The internal morphology of scaffolds was observed using SEM.
Statistical analysis
Statistical analysis was performed using student’s t test to compare differences between two groups. A value of p<0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Preparation and characterization of nanoparticles
Chi nanoparticles processed by an ionic gelation method have attracted much attention as delivery carriers of therapeutic molecules because of its simple and mild procedure.9, 11, 15 In the present study, we created Chi nanoparticles using TPP and CS as chitosan crosslinkers and evaluated their feasibility as a protein delivery platform that can be readily used in common tissue graft materials such as injectable hydrogels or 3D porous scaffolds. The particles with submicron size would be desirable for the uniform incorporation into the graft materials without lowering the structural integrity of hydrogels or obstructing the pores of prefabricated scaffolds.
The prepared particles showed a particle size in the range of 550–850 nm and incorporation of increasing amounts of crosslinkers led to an increase of the particle size (Table 1). The similar size of the nanoparticles was observed when the CS crosslinker was incorporated either before or after the incorporation of TPP crosslinker.
Table 1.
Mean particle size and zeta potential of chitosan nanoparticles
| Formulation | Chi:TPP:CS ratio (w/w/w) | Mean diameter (nm) | Zeta potential (mV) |
|---|---|---|---|
| Chi/TPP | 10:1:0 | 554 ± 16 | +43.5 ± 0.6 |
| Chi/2TPP | 10:2:0 | 659 ± 36 | +36.8 ± 0.9 |
| Chi/2TPP/CS | 10:2:1 | 717 ± 19 | +31.1 ± 1.7 |
| Chi/2TPP/2CS | 10:2:2 | 852 ± 29 | +26.9 ± 1.1 |
All of the prepared Chi nanoparticles showed a positive zeta potential of +28–42 mV, which is a typical characteristic of Chi nanoparticles mediated by an ionic gelling process.15, 18–20 Their positive nature is from residual amino groups of Chi after particle formation. Increasing amounts of crosslinkers led to a decrease on their surface charge, indicating the incorporation of TPP and CS in the Chi nanoparticles where the electro-positive amine groups of Chi are neutralized by their interaction with the electro-negative groups in crosslinker molecules. Since the surface charge is an important parameter affecting the particle stability/aggregation and adhesion of particles onto biological surface,28, 29 crosslinker concentration needs to be optimized to form particulate carrier systems that can be readily integrated with tissue engineering constructs. Higher crosslinker concentrations over a Chi:TPP:CS ratio of 5:1:1 (w/w/w) resulted in nanoparticle aggregation after several centrifugation/resuspension cycles due to the strong intermolecular hydrogen bonding.
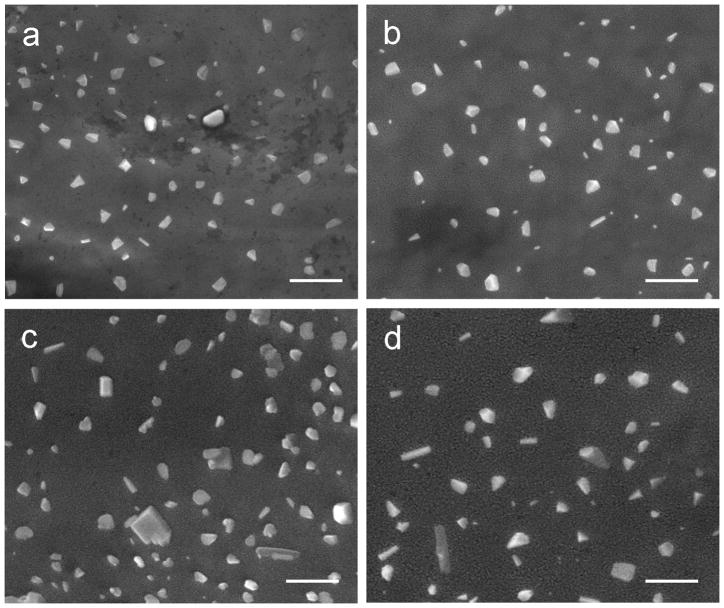
SEM was used to observe the surface morphology of Chi nanoparticles (Figure 2). Most Chi nanoparticles were rounded with some irregularly-shapes particles. The addition of CS crosslinker did not alter the morphology of particles.
Figure 2.
SEM micrographs of the nanoparticles prepared with various ratio chitosan:TPP:CS of 10:1:0 (a), 10:2:0 (b), 10:2:1 (c), 10:2:2 (d). Scale bar = 1 μm.
Association efficiency and protein release
Because crosslinking density can influence protein association and release from ionically crosslinked Chi particles, we investigated the effects of crosslinker concentrations on protein association efficiency and release kinetics using Nell-1 as a model protein. The incorporation of protein in the Chi nanoparticles was achieved by dissolving protein in crosslinker solutions. The protein loading did not significantly changed the size and zeta potential of the nanoparticles. The association efficiency was in the range of 40–90% depending on the TPP and CS concentration, as determined by CBQCA protein assay (Figure 3). An increase in the concentration of CS or TPP resulted in higher association capacity, indicating that crosslinking density affected the entrapment of proteins within the Chi molecules.
Figure 3.
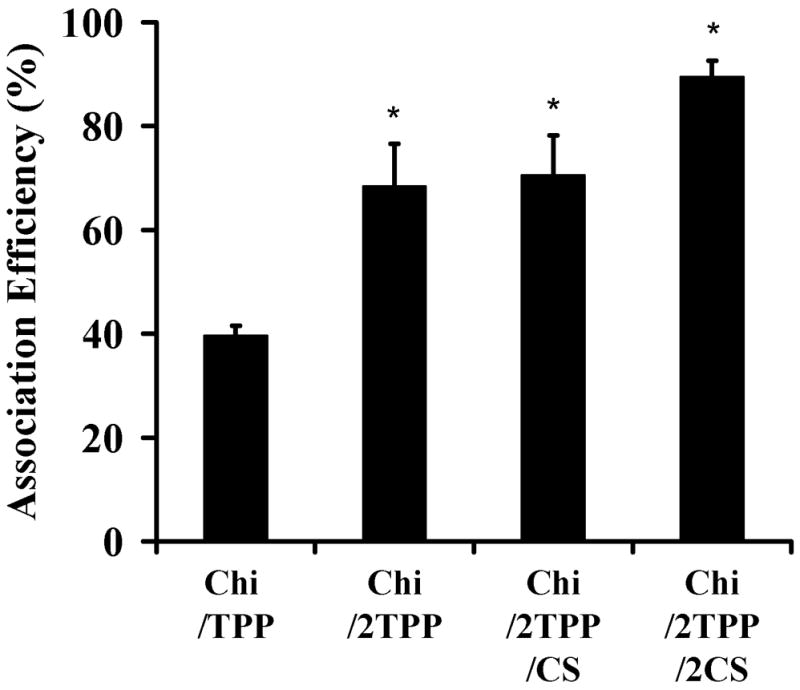
Nell-1 association efficiency of the nanoparticles prepared with various ratio chitosan:TPP:CS of 10:1:0 (Chi/TPP), 10:2:0 (Chi/2TPP), 10:2:1 (Chi/2TPP/CS), 10:2:2 (Chi/2TPP/2CS) (n=3, mean ± standard deviation [SD]) (*p < 0.01).
To investigate the protein delivery capacity of Chi nanoparticles, we examined the release kinetics of Nell-1 protein from the nanoparticles by incubating the particles in PBS (Figure 4). At a low TPP concentration (Chi/TPP, Chi:TPP ration of 10:1), approximately 50% of the initially loaded protein was released from the Chi/TPP particles during the first day, followed by a gradual release of ~1.5% per day up to 14 days. This may be due to a dissociation of proteins located at the surface of the particles, followed by diffusion of more tightly bound proteins through the network of the particles. Higher TPP concentration (Chi/2TPP, Chi:TPP ration of 5:1) reduced the initial burst of protein to 30% with a subsequent slow release at ~1% per day. The burst release of protein was reduced further by the addition of CS crosslinker. Approximately 20% of loaded protein was released from the Chi/2TPP/2CS particles during the first day with a slow gradual release at ~0.7% per day during the subsequent incubation time. An increase in crosslinking density induces the formation of the compact network in Chi chains and improves the network stability, resulting in reduced protein release. Thus, these results suggest that adjusting the composition of the particles can modulate the rate of protein release.
Figure 4.
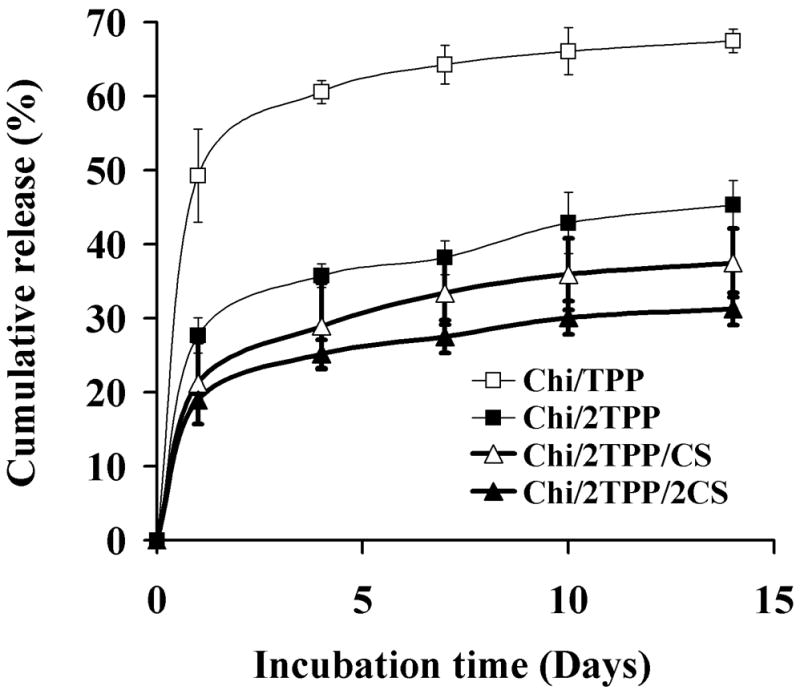
In vitro release of Nell-1 from nanoparticles prepared with various ratio chitosan:TPP:CS in phosphate-buffered saline (PBS).
Degradation of nanoparticles
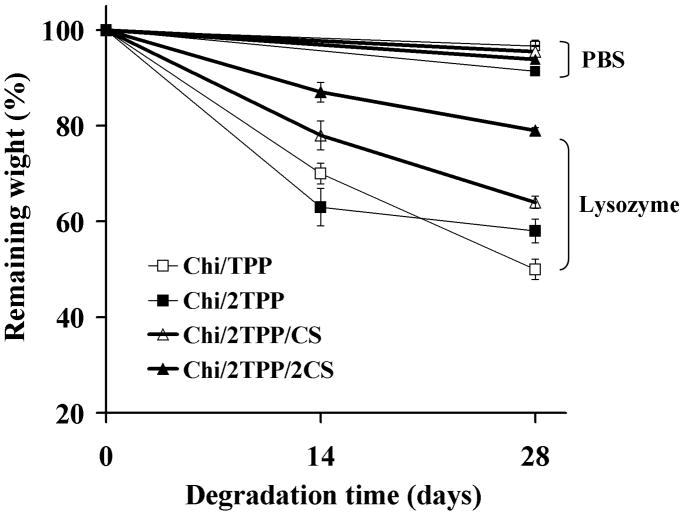
In vivo, enzymes, such as lysozyme or chitosanase, degrade Chi through binding onto the six sugar chains present in the Chi backbone.30, 31 The release kinetics of protein from the chitosan nanoparticles are thus controlled by a combination of diffusion and carrier degradation after implantation. Carrier degradation is also preferable for the complete tissue replacement of the graft, as well as the complete release of protein. The degradation of Chi nanoparticles was evaluated in PBS containing lysozyme (Figure 5). The lysozyme concentration of 100 μg/ml was chosen to better mimic the in vivo physiological conditions, as the lysozyme level in the extracellular matrix of human tissues can increase up to 1,000-fold greater than that in serum (0.95~2.45 μg/ml).32, 33 The rate of nanoparticle mass loss significantly increased in the presence of lysozyme after 4 weeks. Approximately 50% of particle weight remained for the Chi/TPP nanoparticles prepared at a low TPP concentration (Chi/TPP) in the presence of lysozyme, whereas only 4% of particle mass loss was observed in the absence of lysozyme during the incubation. The increase of crosslinker concentrations slowed the rate of gel mass loss and 80% of particle weight remained for the Chi/2TPP/2CS particles after 4-week incubation with lysozyme. These results indicate that a higher crosslinking density reduces penetration and accessibility of the lysozyme to the particle network, and subsequently slows degradation.
Figure 5.
In vitro degradation of nanoparticles prepared with various ratio chitosan:TPP:CS in PBS containing 0 or 0.1 mg/ml lysozyme.
Bioactivity of released protein
One of the major problems associated with the fabrication of protein release carriers is the loss of bioactivity of incorporated proteins during the encapsulation process. This is due to a short half-life and rapid degradation of proteins once they are exposed to organic solvents or the method of chemical conjugation used. Chi can be easily manipulated into various forms such as beads, micro-, and nanoparticles based on an ionic gelation process under very mild conditions. In this study, Chi nanoparticles consisted solely of hydrophilic TPP and CS crosslinkers that are soluble in aqueous solution.
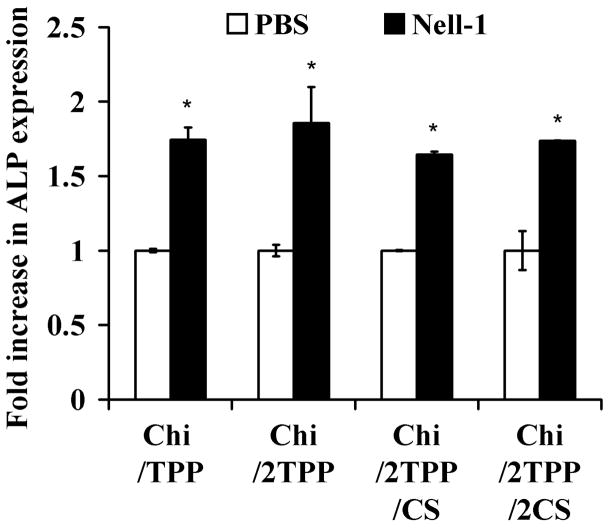
The bioactivity of Nell-1 protein released from Chi nanoparticles was investigated by assessing its ability to activate alkaline phosphatase (ALP) expression in ATDC5 cells (Figure 6). The supernatant from Nell-1/Chi particles crosslinked with TPP stimulated a 1.74 (low TPP)-or 1.82 (high TPP)-fold increase in ALP activity of ATDC5 cells compared with negative controls (supernatant from PBS-loaded chitosan particles). ALP activity was significantly higher than that of control particles, indicating that the bioactivity of protein was maintained during the incorporation procedure into chitosan particles. Nell-1/Chi particles crosslinked with CS at low and high concentrations had a similar increase in ALP activity of 1.66 and 1.71, respectively, to that of Nell-1/chitosan particles crosslinked with TPP.
Figure 6.
Bioactivity of Nell-1 released from nanoparticles prepared with various ratio chitosan:TPP:CS. Supernatant collected from Nell-1 loaded nanoparticles (solid bars) increased ALP activity of ATDC5 cells, compared with supernatant collected from PBS-loaded nanoparticles (*p < 0.01).
Incorporation of chitosan nanoparticles into hydrogels
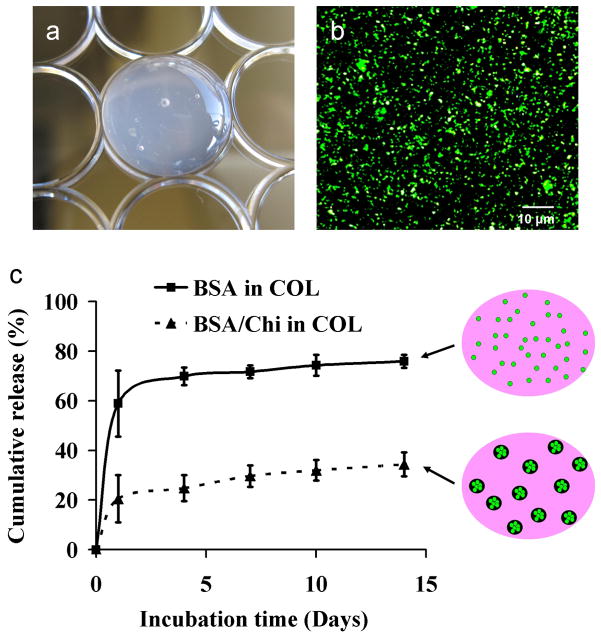
To investigate the feasibility of Chi nanoparticles as sustained protein release carriers in common tissue graft materials, we encapsulated BSA into Chi/TPP/CS nanoparticles and subsequently embedded them into a collagen hydrogel (Figure 7A). Collagen is a major component of extracellular matrix in bone tissue and is widely used for bone grafts. Chi/TPP/CS nanoparticles encapsulating fluorescently-tagged BSA were uniformly distributed within the collagen hydrogels (Figure 7B).
Figure 7.
Incorporation of nanoparticles into collagen gels. (a) Gross morphology of collagen composite hydrogels. (b) Fluorescent image of composite hydrogels showing uniform distribution of BSA-loaded particles in collagen gels. (c) In vitro release of BSA from collagen gels (■) or nanoparticles embedded in collagen gels (▲).
To determine if Chi/TPP/CS nanoparticles can sustain the release of protein from collagen gels, we incorporated BSA or BSA-loaded nanoparticles into collagen gels and determined the release profile of protein from the gels by incubating in PBS. The release of protein directly from the gels exhibited a rapid burst release of ~60 % over the first day and the cumulative amount of protein released reached a maximum of ~75% over 14 days. This may be due to weak non-specific binding and electrostatic repulsion between BSA and collagen, as both are negatively charged at physiological pH. In contrast, the release of protein from the Chi/TPP/CS nanoparticles in collagen gel showed a burst release of ~20% over the first day, followed by a gradual release of ~0.5% per day up to 14 days (Figure 7C).
Incorporation of chitosan nanoparticles into porous 3D scaffolds
Adsorption of proteins onto tissue engineering scaffolds or surgical implants provides a local delivery of bioactive signaling molecules in a defect area, but the capability to control the release is minimal. The controlled release of proteins from scaffolds can be achieved by incorporating proteins into various scaffolding biomaterial during fabrication. However, the process used to fabricate scaffolds often is not suitable for the encapsulation of bioactive agents due to harsh conditions employed such as high temperatures and toxic organic solvents.
In this study, we proposed a practical method of protein incorporation by coupling protein-loaded Chi/TPP/CS nanoparticles onto prefabricated PLGA scaffolds instead of entrapping the proteins into scaffolds during fabrication. Model protein BSA was encapsulated into Chi/TPP/CS nanoparticles and subsequently incorporated into collagen for coating the porous PLGA scaffolds. Fluorescent BSA-containing nanoparticles were uniformly incorporated over the porous scaffolds (Figure 8A). The internal microstructure of nanoparticle/collagen-coated scaffolds demonstrated uniform distribution of nanoparticles on the wall of pores without obstructing the porous structure of the scaffolds (Figures 8B and C).
Figure 8.
Incorporation of nanoparticles into PLGA scaffolds. (a) Gross morphology of PLGA composite scaffolds. (b) Fluorescent image of composite scaffolds showing uniform coating of BSA-loaded particles. SEM micrographs of composite scaffolds showing open pore structure (c) and uniform distribution of nanoparticles on the wall of the pores (d).
CONCLUSIONS
We report Chi/TPP/CS nanoparticles for sustained release of growth factors, which can be used for tissue engineering. This study demonstrated that modification of particle composition modulated protein association, release kinetics, and particle degradation. Furthermore, material process, performed under very mild conditions, did not reduce the biological activity of the released growth factors. In addition, the Chi/TPP/CS nanoparticles could be incorporated into tissue engineering scaffolds to provide sustained protein delivery. This particulate delivery system appears to be a suitable carrier for controlled growth factor release in tissue engineering applications.
Acknowledgments
This work was supported by the UC Discovery grant Bio 07-10677, the National Institutes of Health grant R01 AR060213, the Jonsson Cancer Center Foundation, the International Association for Dental Research, the Academy of Osseointegration, and UCLA School of Dentistry Dean’s start-up funds.
References
- 1.Manning MC, Patel K, Borchardt RT. Stability of protein pharmaceuticals. Pharm Res. 1989;6:903–18. doi: 10.1023/a:1015929109894. [DOI] [PubMed] [Google Scholar]
- 2.Mooney DJ, Lee K, Silva EA. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gopferich AM, Tessmar JK. Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Deliver Rev. 2007;59:274–291. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Mikos AG, Kretlow JD, Klouda L. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliver Rev. 2007;59:263–273. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Shin H, Lee SH. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliver Rev. 2007;59:339–359. doi: 10.1016/j.addr.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Mikos AG, Bao Y, Cima LG, Ingber DE, Vacanti JP, Langer R. Preparation of poly(glycolic acid) bonded fiber structures for cell attachment and transplantation. J Biomed Mater Res. 1993;27:183–9. doi: 10.1002/jbm.820270207. [DOI] [PubMed] [Google Scholar]
- 7.Zhu G, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly (lactide- co-glycolide) Nat Biotechnol. 2000;18:52–7. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 8.Di Martino A, Sittinger M, Risbud MV. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Dodane V, Vilivalam VD. Pharmaceutical applications of chitosan. Pharm Sci Technol To. 1998;1:246–253. [Google Scholar]
- 10.Zhang MQ, Bhattarai N, Gunn J. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliver Rev. 2010;62:83–99. doi: 10.1016/j.addr.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Aminabhavi TM, Agnihotri SA, Mallikarjuna NN. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release. 2004;100:5–28. doi: 10.1016/j.jconrel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Zhang MQ, Li ZS, Ramay HR, Hauch KD, Xiao DM. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 2005;26:3919–3928. doi: 10.1016/j.biomaterials.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 13.Chandy T, Sharma CP. Chitosan Beads and Granules for Oral Sustained Delivery of Nifedipine - Invitro Studies. Biomaterials. 1992;13:949–952. doi: 10.1016/0142-9612(92)90119-9. [DOI] [PubMed] [Google Scholar]
- 14.Bakos D, Vodna L, Bubenikova S. Chitosan based hydrogel microspheres as drug carriers. Macromol Biosci. 2007;7:629–634. doi: 10.1002/mabi.200600290. [DOI] [PubMed] [Google Scholar]
- 15.Alonso MJ, Janes KA, Calvo P. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv Drug Deliver Rev. 2001;47:83–97. doi: 10.1016/s0169-409x(00)00123-x. [DOI] [PubMed] [Google Scholar]
- 16.Brack HP, Tirmizi SA, Risen WM. A spectroscopic and viscometric study of the metal ion-induced gelation of the biopolymer chitosan. Polymer. 1997;38:2351–2362. [Google Scholar]
- 17.Draget KI, Varum KM, Moen E, Gynnild H, Smidsrod O. Chitosan Cross-Linked with Mo(Vi) Polyoxyanions - a New Gelling System. Biomaterials. 1992;13:635–638. doi: 10.1016/0142-9612(92)90032-j. [DOI] [PubMed] [Google Scholar]
- 18.Calvo P, RemunanLopez C, VilaJato JL, Alonso MJ. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci. 1997;63:125–132. [Google Scholar]
- 19.Alonso MJ, Vila A, Sanchez A, Janes K, Behrens I, Kissel T, Jato JLV. Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur J Pharm Biopharm. 2004;57:123–131. doi: 10.1016/j.ejpb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Urrusuno R, Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharmaceut Res. 1999;16:1576–1581. doi: 10.1023/a:1018908705446. [DOI] [PubMed] [Google Scholar]
- 21.Selvamurugan N, Saranya N, Moorthi A, Saravanan S, Devi MP. Chitosan and its derivatives for gene delivery. Int J Biol Macromol. 2011;48:234–238. doi: 10.1016/j.ijbiomac.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 22.RemunanLopez C, Bodmeier R. Mechanical, water uptake and permeability properties of crosslinked chitosan glutamate and alginate films. J Control Release. 1997;44:215–225. [Google Scholar]
- 23.Zhu KJ, Shu XZ. Controlled drug release properties of ionically cross-linked chitosan beads: the influence of anion structure. Int J Pharm. 2002;233:217–225. doi: 10.1016/s0378-5173(01)00943-7. [DOI] [PubMed] [Google Scholar]
- 24.Ting K, Vastardis H, Mulliken JB, Soo C, Tieu A, Do H, Kwong E, Bertolami CN, Kawamoto H, Kuroda S, Longaker MT. Human NELL-1 expressed in unilateral coronal synostosis. J Bone Miner Res. 1999;14:80–9. doi: 10.1359/jbmr.1999.14.1.80. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Kuroda S, Carpenter D, Nishimura I, Soo C, Moats R, Iida K, Wisner E, Hu FY, Miao S, Beanes S, Dang C, Vastardis H, Longaker M, Tanizawa K, Kanayama N, Saito N, Ting K. Craniosynostosis in transgenic mice overexpressing Nell-1. J Clin Invest. 2002;110:861–70. doi: 10.1172/JCI15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee M, Chen TT, Iruela-Arispe ML, Wu BM, Dunn JC. Modulation of protein delivery from modular polymer scaffolds. Biomaterials. 2007;28:1862–70. doi: 10.1016/j.biomaterials.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Mikos AG, Sarakinos G, Leite SM, Vacanti JP, Langer R. Laminated 3-Dimensional Biodegradable Foams for Use in Tissue Engineering. Biomaterials. 1993;14:323–330. doi: 10.1016/0142-9612(93)90049-8. [DOI] [PubMed] [Google Scholar]
- 28.van der Lubben IM, Verhoef JC, van Aelst AC, Borchard G, Junginger HE. Chitosan microparticles for oral vaccination: preparation, characterization and preliminary in vivo uptake studies in murine Peyer's patches. Biomaterials. 2001;22:687–694. doi: 10.1016/s0142-9612(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 29.Lee DW, Powers K, Baney R. Physicochemical properties and blood compatibility of acylated chitosan nanoparticles. Carbohyd Polym. 2004;58:371–377. [Google Scholar]
- 30.Tomihata K, Ikada Y. In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials. 1997;18:567–575. doi: 10.1016/s0142-9612(96)00167-6. [DOI] [PubMed] [Google Scholar]
- 31.Lee KY, Ha WS, Park WH. Blood Compatibility and Biodegradability of Partially N-Acylated Chitosan Derivatives. Biomaterials. 1995;16:1211–1216. doi: 10.1016/0142-9612(95)98126-y. [DOI] [PubMed] [Google Scholar]
- 32.Brouwer J, Vanleeuwenherberts T, Ottingvanderuit M. Determination of Lysozyme in Serum, Urrine, Cerebrospinal-Fluid and Feces by Enzyme-Immunoassay. Clin Chim Acta. 1984;142:21–30. doi: 10.1016/0009-8981(84)90097-4. [DOI] [PubMed] [Google Scholar]
- 33.Greenwald R, Josephso A, Diamond H, Tsang A. Human Cartilage Lysozyme. Journal of Clinical Investigation. 1972;51:2264–2270. doi: 10.1172/JCI107035. [DOI] [PMC free article] [PubMed] [Google Scholar]