Abstract
Objective
To estimate the impact of the mandatory National Collegiate Athletic Association (NCAA) sickle cell trait (SCT) screening policy on the identification of sickle cell carriers and prevention of sudden death.
Data Source
We used NCAA reports, population-based SCT prevalence estimates, and published risks for exercise-related sudden death attributable to SCT.
Study Design
We estimated the number of sickle cell carriers identified and the number of potentially preventable sudden deaths with mandatory SCT screening of NCAA Division I athletes. We calculated the number of student-athletes with SCT using a conditional probability based upon SCT prevalence data and self-identified race/ethnicity status. We estimated sudden deaths over 10 years based on published attributable risk of exercise-related sudden death due to SCT.
Principal Findings
We estimate that over 2,000 NCAA Division I student-athletes with SCT will be identified under this screening policy and that, without intervention, about seven NCAA Division I student-athletes would die suddenly as a complication of SCT over a 10-year period.
Conclusion
Universal sickle cell screening of NCAA Division I student-athletes will identify a substantial number of sickle cell carriers. A successful intervention could prevent about seven deaths over a decade.
Keywords: Sickle cell trait, National Collegiate Athletic Association, screening, sudden death, athletes
On August 1, 2010, the National Collegiate Athletic Association (NCAA) implemented one of the largest mandated genetic screening programs in the United States: universal sickle cell trait (SCT) screening of all Division I student-athletes (NCAA 2010b). The policy resulted from a legal settlement with the family of Dale Lloyd II, a Rice University football player, who collapsed during football practice and later died from acute exertional rhabdomyolysis attributed to SCT (Figure 1).
Figure 1.
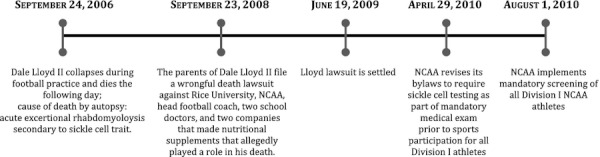
Timeline of Significant Events in the Implementation of NCAA Division I Sickle Cell Screening Program
The morbidity associated with SCT has long been debated (Sears 1978). Complications of SCT are rare and include infarction associated with hypoxia from high altitudes and renal medullary carcinoma (Tsaras et al. 2009). However, there is also concern that athletes with SCT have an elevated risk of exercise-related sudden death secondary to exertional heat illness or acute exertional rhabdomyolysis (Eichner 2010).
The NCAA's mandatory, universal SCT screening program (NCAA Proposal No. 2009-75-B) has sparked significant debate. Endorsement of this policy from professional organizations has been mixed (College of American Pathologists 2007; National Athletic Trainers’ Association 2007; Secretary's Advisory Committee on Heritable Disorders and Genetic Diseases in Newborns and Children's 2010). This is due to fear that screening may lead to discrimination and confusion (Bonham, Dover, and Brody 2010), as occurred with previous community and public health sickle cell screening programs (Whitten 1973; Rutkow and Lipton 1974), as well as a paucity of evidence that screening improves health outcomes for student-athletes.
Given the scope and controversy of the screening program, we conducted a policy impact analysis (Porell and Adams 1995) to estimate the number of athletes with SCT identified and the anticipated number of exercise-related sudden deaths attributable to SCT among NCAA student-athletes.
Methods
Study Population: Number of Student-Athletes
We used the most recently published, publicly available NCAA participation rates (academic year 2007–2008) to estimate the number of Division I student-athletes in a 4-year cohort (e.g., all participating student-athletes in an academic year) (NCAA 2009). These data are self-reported by the 335 NCAA Division I member institutions (National Collegiate Athletic Association 2011).
The NCAA defines student-athletes as individuals who meet at least one of the following criteria by the first scheduled competition: (1) are listed as a team member, (2) practice with the varsity team and receive coaching from one or more varsity coaches, or (3) receive athletically related student aid. If a student participates in more than one sport, the NCAA counts him/her as a student-athlete participant for each sport. However, we are unaware of any publicly available NCAA statistics on the number of multisport student-athletes. To decrease the likelihood of double-counting athletes, we assumed as follows: (1) athletes who competed in cross-country also competed in track and (2) athletes who competed in indoor track also competed in outdoor track and so only included participants in outdoor track (i.e., did not include participants listed for cross country and indoor track) in our analyses.
The NCAA assigns sports to one of three categories: championship (men and women, e.g., basketball), nonchampionship (men only, e.g., rowing), and emerging (women only, e.g., rugby). The NCAA policy does not explicitly exempt student-athletes in nonchampionship and emerging sports from screening, so we included these athletes in our overall calculation of “screen-able” student-athletes. Moreover, the NCAA requires that “the [medical] examination or evaluation [which includes a sickle cell solubility test] must have been administered within six months prior to participation in any weight-training or conditioning activity” (NCAA 2010b). As all athletic activities involve weight-training or conditioning activity, student-athletes in all three NCAA sport categories meet criteria for SCT screening.
Study Population: Race/Ethnicity of Student-Athletes
Because the prevalence of SCT is increased in Black and Hispanic/Latino individuals compared to Whites (Blacks: 1/14, Hispanic/Latino: 1/183, Whites: 1/625) (Lorey, Arnopp, and Cunningham 1996), we gathered data on the race/ethnicity distribution among all NCAA Division I student-athetes for the years 2007–2008 (NCAA 2010a). These data are self-reported by athletes. The NCAA categorizes race/ethnicity as follows: American Indian/Alaskan Native, Asian, Black/African American, Hispanic/Latino, Native Hawaiian/Pacific Islander, Other, Two or More Races, White/Non-Hispanic. “Black, Non-Hispanic” was defined as “A person having origins in any of the black racial groups of Africa (except those of Hispanic origin)”; “Hispanic/Latino” was defined as “a person of Mexican, Puerto Rican, Cuban, Central or South American or other Spanish culture or origin, regardless of race” (NCAA 2010a). We collapsed these categories as follows (Lorey, Arnopp, and Cunningham 1996): Black, Non-Hispanic; Hispanic; All Other.
Prevalence of SCT
Literature-derived estimates for the prevalence of SCT vary by ethnicity. Classically, discussion of the prevalence of SCT has focused exclusively on individuals of African ancestry. However, studies report an increased prevalence in Hispanics/Latinos compared to individuals of non-Hispanic/non-African ancestry. We used the following published point estimates for prevalence: 7 percent of blacks, 0.5 percent of Hispanics/Latinos, and 0.2 percent of whites (Lorey, Arnopp, and Cunningham 1996). We applied the 0.2 percent prevalence for whites to our “Other” race/ethnicity category.
Rates of Death for Exercise-Related Sudden Death from SCT
We identified only one study that systematically quantifies the risk of exercise-related sudden death associated with SCT. It was conducted in U.S. military recruits and categorized reasons for death into three categories: sudden unexplained death, sudden nonunexplained death (e.g., silent structural heart disease, asthma), nonsudden death (Kark et al. 1987). “Sudden death” was defined as “death due to an illness producing an irreversible critical condition within 1 hour of onset.” The authors categorized exertional heat stress, heat stroke, rhabdomyolysis, and unknown mechanism as “sudden unexplained death.” We used the death rate for “sudden unexplained death” in our analyses because it included hypothesized mechanisms (e.g., exertional heat stress, rhabdomyolysis) of exercise-related sudden death among individuals with SCT (Eichner 2010). For the risk of sudden death due to SCT in our analysis, we used the attributable death rates for sudden death among black recruits (31 deaths per 100,000 individuals) with SCT, calculated by subtracting the death rate among black recruits with SCT (32.2 per 100,000) from the death rate among black recruits without SCT (1.2 per 100,000).
Analysis
To estimate the number of student-athletes who would suffer exercise-related sudden death due to SCT, we calculated the conditional probability of an exercise-related sudden death for student-athletes based upon the likelihood of an athlete having SCT. We assumed full compliance of all Division I member institutions with this mandatory screening policy.
Number of Athletes with SCT
First, we calculated the conditional probability of having SCT based upon ethnic group status, stratified by sport and by gender:
We multiplied this probability by the number of Division I student-athletes in a 4-year cohort to estimate the number of individuals who have SCT among all Division I student-athletes. We multiplied this estimate by the sensitivity of the sickle cell solubility test (98.9 percent) to determine the number of athletes with SCT identified by testing (Hicks and Hughes 1975). We then divided our total student body estimate by 4 to determine the number of athletes with SCT identified in each academic class (i.e., those identified annually).
Number of Sudden Deaths Attributable to SCT
We estimated the probability of exercise-related death from SCT conditioned upon the likelihood of having SCT, using published risk estimates (Kark et al. 1987):
To estimate the number of expected sudden deaths in an academic year, we multiplied this probability by the number of Division I athletes participating in each sport during an academic year (i.e., 4-year cohort). We used this statistic because each year every participating athlete (newly identified SCT or already known) is at risk of sudden death from SCT. We multiplied the number of deaths by 10 to estimate deaths over a 10-year time period, which allowed us to benchmark our model against estimates of sudden death attributable to SCT based on case reports.
Although female athletes anecdotally constitute a lower proportion of exercise-related death cases related to SCT than male athletes, we are unaware of any published data confirming this and so did not apply a gender-based differential probability of death.
Number Needed to Screen and Cost of Testing
Finally, we calculated the number needed to screen (NNS) to prevent one student-athlete death from SCT (assuming a 100 percent effective intervention). Because our data do not provide the athlete's eligibility year, we calculated the NNS over a 4-year period by assuming that ¼ of participants represented a freshman class that would only be screened once during this 4-year period.
The NCAA policy recommends that athletes be screened with a sickle cell solubility test (National Collegiate Athletic Association 2010). Positive sickle cell solubility tests must be confirmed with hemoglobin (Hb) electrophoresis. We calculated the number of student-athletes with positive sickle cell solubility tests that would undergo Hb electrophoresis by multiplying the published sensitivity estimate of 98.9 percent for sickle cell solubility testing by the number of athletes with SCT in the total athletic student body (Table 1). We did not examine the costs for false-positive results since the published specificity estimate for sickle cell solubility testing is 100 percent (Hicks and Hughes 1975). To calculate the cost of screening and confirmatory testing alone (excluding education and counseling costs), we used a range of costs for the sickle cell solubility testing ($10–$20) provided by different collegiate institutions (Secretary's Advisory Committee on Heritable Disorders in Newborns Meeting 2010; University of California-Berkeley 2010; Wesleyan University 2010). We used $50 for the cost of hemoglobin electrophoresis (Chasen, Loeb-Zeitlin, and Landsberger 1999; January 22, 2010).
Table 1.
Estimated Number of Division I NCAA Student-Athletes with Sickle Cell Trait
| Black | Hispanic | Other | Total | |
|---|---|---|---|---|
| Archery | 0 | 0 | 0 | 0 |
| Badminton | 0 | 0 | 0 | 0 |
| Baseball | 43 | 3 | 18 | 64 |
| Basketball | 384 | 1 | 9 | 393 |
| Bowling | 9 | 0 | 0 | 9 |
| Equestrian | 0 | 0 | 1 | 2 |
| Fencing | 3 | 0 | 1 | 4 |
| Field hockey | 2 | 0 | 3 | 6 |
| Football | 833 | 3 | 26 | 863 |
| Golf | 13 | 1 | 9 | 23 |
| Gymnastics | 5 | 0 | 3 | 8 |
| Ice hockey | 1 | 0 | 5 | 6 |
| Lacrosse | 6 | 0 | 9 | 16 |
| Rifle | 0 | 0 | 1 | 1 |
| Rowing | 11 | 1 | 12 | 24 |
| Rugby | 0 | 0 | 0 | 0 |
| Sailing | 0 | 0 | 0 | 0 |
| Skiing | 0 | 0 | 1 | 1 |
| Soccer | 68 | 4 | 23 | 95 |
| Softball | 28 | 2 | 9 | 39 |
| Squash | 0 | 0 | 1 | 1 |
| Swimming/diving | 8 | 1 | 17 | 26 |
| Sync. swimming | 0 | 0 | 0 | 0 |
| Team handball | 0 | 0 | 0 | 0 |
| Tennis | 22 | 2 | 10 | 34 |
| Track, outdoor | 430 | 4 | 29 | 463 |
| Volleyball | 40 | 1 | 9 | 50 |
| Water polo | 1 | 0 | 2 | 4 |
| Wrestling | 11 | 1 | 5 | 16 |
| All sports | 1,918 | 25 | 204 | 2,147* |
This number is multiplied by 0.989 to calculate the number of athletes identified by sickle cell solubility testing = 2,123 athletes.
Results
In the 2007–2008 season, 81,073 male and 63,108 female student-athletes participated in NCAA Division I sports. Football (n = 25,658) had the most participants for men's sports and track for women's sports (n = 11,230) (Appendix SA2, Supporting Information). Overall, the majority of Division I student-athletes self-reported a non-Black, non-Hispanic/Latino race/ethnicity (male 71.1 percent, female 80.4 percent, Appendix SA3).
Among the estimated 2,147 athletes with SCT, we estimate that 2,123 NCAA Division I student-athletes in one 4-year cohort of athletes (i.e., total athletic student body) would be identified as having SCT under this new screening policy (about 530 new students annually) (Table 1). Of these, 89 percent will self-report as Black race/ethnicity. Most student-athletes with SCT will compete in football (n = 863), track (n = 463), and basketball (n = 393).
In a 4-year cohort of screened NCAA student-athletes (e.g., a 4-year period with each athlete screened only once), there would be one death. Based on our model, we estimated that without intervention, about seven student-athletes with SCT would suffer exercise-related sudden death by year 10 of the screening program (Table 2).
Table 2.
Estimated Exercise-Related Deaths from Sickle Cell Trait in NCAA Division I Student-Athletes That Would Be Prevented over 10 Years
| Deaths | |
|---|---|
| Archery | 0.0 |
| Badminton | 0.0 |
| Baseball | 0.2 |
| Basketball | 1.2 |
| Bowling | 0.0 |
| Equestrian | 0.0 |
| Fencing | 0.0 |
| Field hockey | 0.0 |
| Football | 2.7 |
| Golf | 0.1 |
| Gymnastics | 0.0 |
| Ice hockey | 0.0 |
| Lacrosse | 0.0 |
| Rifle | 0.0 |
| Rowing | 0.1 |
| Rugby | 0.0 |
| Sailing | 0.0 |
| Skiing | 0.0 |
| Soccer | 0.2 |
| Softball | 0.1 |
| Squash | 0.0 |
| Swimming/diving | 0.1 |
| Sync. swimming | 0.0 |
| Team handball | 0.0 |
| Tennis | 0.1 |
| Track, outdoor | 1.4 |
| Volleyball | 0.2 |
| Water polo | 0.0 |
| Wrestling | 0.1 |
| All sports | 6.5 |
Assuming a 100 percent effective intervention, this screening program requires that 144,181 student-athletes (e.g., a 4-year cohort of athletes) be screened to prevent one death. For each death prevented, we estimate that the cost of testing alone (i.e., without education or counseling) would range from $1,441,810 to $2,883,620 for sickle cell solubility testing and be about $106,150 for hemoglobin electrophoresis confirmatory testing for those with positive sickle cell solubility tests.
Discussion
The NCAA recently implemented mandatory SCT screening of all Division I student-athletes. Using publicly available data, we estimated that this policy would identify over 2,000 athletes with SCT among a 4-year cohort of student-athletes. Alongside a 100 percent effective intervention, screening could prevent the deaths of seven student athletes over a 10-year period. Our estimate approximates the number of case reports of collegiate student-athletes that have suffered exercise-related death attributed to SCT over a similar historical time interval (Eichner 2010, 2011).
A powerful motivating force behind the mandatory NCAA SCT screening policy was a wrongful death suit. As part of the settlement, the NCAA agreed to require that all Division I athletes be screened for SCT before sports participation (Lanier Law Firm 2009). Previously, the NCAA had recommended, but not required, that member institutions screen athletes for SCT (NCAA 2008b); compliance from member institutions had been variable (Clarke et al. 2006).
The NCAA SCT screening policy yields interesting comparisons to the controversy surrounding cardiovascular screening of college athletes to prevent sudden death. The NCAA does not currently recommend universal ECG screening for student athletes despite the fact that cardiovascular conditions underlie more than half of medically related sudden deaths among college athletes (Harmon et al. 2011) and data exist to support the notion that ECG screening may reduce the incidence of cardiovascular sudden death (Corrado et al. 1998). Admittedly, issues remain to be resolved (e.g., nontrivial incidence and consequence of false-positive test results, uncertainty that screening would lead to a reduction in the number of deaths from cardiovascular causes). However, a reasonable argument could be made that the evidence base supporting an NCAA recommendation for universal ECG screening appears more robust than that for universal SCT screening.
The relationship between exercise-related death and SCT has long been debated. While ample evidence suggests that SCT has not limited the competitiveness of professional football players (Murphy 1973), national track champions (Pearson 1989; Bile et al. 1998), or elite sprinters (Marlin et al. 2005), case reports of exercise-related sudden death in athletes with SCT at all levels of sports (e.g., high school through professional) suggest a compelling pattern. Exercise-related sudden deaths attributed to SCT tend to occur in male athletes, early in training (often on the first day), and during conditioning workouts. They tend to be associated with high levels of exertion, such as short, maximum-effort drills/testing, such as those frequently used in football, a sport in which numerous sudden deaths have been attributed to SCT (Eichner 2010, 2011). Environmental conditions, such as the heat and hydration status of the player, also play an important role. These data suggest that a gene–environment interaction promotes the initiation of acidosis and rhabdomyolysis that triggers sickling and creates a rapid downward spiral from which recovery is difficult (Eichner 2010).
The “treatment” for the athlete with SCT is proactive prevention that focuses on appropriate preseason and in-season conditioning. To this end, the NCAA recommends a number of precautionary strategies for athletes with SCT (Figure 2). There is evidence that preventive hydration and temperature-monitoring strategies are effective. A 1970s study of U.S. military recruits found an increase in unexplained sudden death among individuals with SCT, compared to those without (Kark et al. 1987). However, unlike the NCAA, the U.S. military implemented universal intervention rather than screening. In an experimental study of universal precautionary measures (e.g., aggressive hydration, temperature monitoring) without a priori knowledge of recruits’ SCT status, the military was able to prevent all subsequent sudden death in recruits with SCT (Kark et al. 1999). This universal intervention program may yield positive spillover effects to other life-threatening conditions unrelated to SCT (e.g., exertional heat stroke) that benefit all athletes, not just those with SCT.
Figure 2.

NCAA Recommended Precautions for Student-Athletes with Sickle Cell Trait (NCAA 2010b)
Of course, successful screening programs depend upon effective and accessible interventions. It is unclear how the NCAA enforces compliance with its “safe conditioning” recommendation and there does not seem to be a strong mandate for intervention. The NCAA informs athletes and member institutions that “Student-athletes with SCT should be knowledgeable of these precautions and institutions should provide an environment in which these precautions may be activated” (2008a). However, the ongoing challenge of compliance with sports-related concussion guidelines highlights the power of the “pressure to play” after an individual is identified as being at risk for sports-related injury. Such pressure to perform and dismiss early symptoms is likely no different for student-athletes with SCT. For example, in 2008 a collegiate football player died during a football-conditioning workout despite the fact that he and the athletic staff were aware that he had SCT. He had reportedly tested positive for SCT twice in 2007 (Fainaru-Wada 2008)—highlighting that screening can lead to a false sense of security if an effective intervention is not enforced.
Although the NCAA policy may be based more litigation based, rather than evidence based, it has real-life implications for the athletes and schools forced to comply with it. First, there are economic costs to the member institutions that must carry out the education and testing. Second, there is the potential for discrimination toward athletes both during and after their collegiate athletic careers. While precautions should be taken, athletic programs should not exclude these athletes or discourage them from being competitive, but rather, according to the NCAA “enable them to thrive in their sport” (2008a). Similarly, when some of these student-athletes transition to professional athletics, coaches and athletic directors should ensure that athletes with SCT do not suffer employment discrimination due to their genetic status. The history of screening for SCT is littered with stories of misinformation, stigma, and unjustified exclusion from activities and professions (Levin 1958; Whitten 1973).
Our policy analysis has limitations. First, NCAA Division II and III athletes are also at risk for exercise-related sudden death due to SCT. While they comprise a significant proportion (61 percent) of collegiate athletes, we did not include them in our formal analyses because the current NCAA SCT screening policy is limited to Division I athletes. Using identical methods, we calculated that 1,210 Division II and 675 Division III athletes would be identified as having SCT (Appendices SA4 and SA5). Assuming an effective intervention, it will take about 3 years to prevent a death in Division II and over 4 years in Division III (data not shown). Second, student-athletes that participate in multiple Division I sports could have been double-counted, leading to an overestimate of individuals with SCT. We have attempted to account for this by only including athletes who participated in outdoor track (i.e., not indoor track or cross country). Although some multisport athletes probably remain in our model, their effect on our results should be small and lead to only a slight overestimation of sudden deaths. Third, testing costs are based on publicly available costs provided to student-athletes by institutions and probably vary across institutions. However, we feel that the testing cost estimates used in our analysis are reasonable and illustrate that while the costs of individual tests are inexpensive, the cost of testing across all student-athletes is not trivial. Finally, we assumed that Division I college student-athletes do not have a greater or lesser proportion of individuals with SCT compared to the general population. Based on previous studies of elite athletes, we have no reason to believe that we have overestimated the prevalence of SCT in this population (Murphy 1973; Bile et al. 1998). In fact, a study of elite sprinters found an overrepresentation of individuals with SCT among title winners (Marlin et al. 2005).
Despite these limitations, we feel that our analysis has important implications for policy makers. First, it provides data about the scope and impact of a policy whose implementation was driven by litigation rather than evidence. Additional studies could explore the scope of screening-related harms, the costs of screening, and the effectiveness of and compliance with interventions. A successful screening program consists of both identification and action—in this case, rigorous compliance with conditioning precautions. Evidence of this intervention's effectiveness is sorely needed, because interventions, not screening tests, save lives. For this screening policy to be successful, testing must be only the first step in the NCAA's overall strategy. However, the question still remains whether SCT screening is a necessary step to prevent exercise-related sudden death in student-athletes.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: The content herein is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development, the National Institutes of Health, Robert Wood Johnson Foundation, Johns Hopkins University, or the University of Michigan. Dr. Tarini is supported by a K23 Mentored Patient-Oriented Research Career Development Award from the National Institute for Child Health and Human Development (K23HD057994). Dr. Bundy is supported by the Robert Wood Johnson Foundation Physician Faculty Scholars Program and the Johns Hopkins University Clinician Scientist Award. Dr. Brooks is supported by Award Number K12 HD055894 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The authors would like to acknowledge Ugochukwu Agbakwuru for his work in gathering background information for this manuscript.
Disclosures: None.
Disclaimers: None.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
Appendix SA2: Number of NCAA Division I Athletes in 2007–2008, by Sport and Gender.
Appendix SA3: Race/Ethnicity Distribution for NCAA Division I Athletes, 2007–2008, by Sport and Gender (%).
Appendix SA4: Estimated Number of Division II NCAA Student-Athletes Who Have Sickle Cell Trait.
Appendix SA5: Estimated Number of Division III NCAA Student-Athletes Who Have Sickle Cell Trait.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Bile A, Le Gallais D, Mercier J, Bogui P, Prefaut C. “Sickle Cell Trait in Ivory Coast Athletic Throw and Jump Champions, 1956-1995”. International Journal of Sports Medicine. 1998;19(3):215–9. doi: 10.1055/s-2007-971907. [DOI] [PubMed] [Google Scholar]
- Bonham VL, Dover GJ, Brody LC. “Screening Student Athletes for Sickle Cell Trait—A Social and Clinical Experiment”. New England Journal of Medicine. 2010;363(11):997–9. doi: 10.1056/NEJMp1007639. [DOI] [PubMed] [Google Scholar]
- Chasen ST, Loeb-Zeitlin S, Landsberger EJ. “Hemoglobinopathy Screening in Pregnancy: Comparison of Two Protocols”. American Journal of Perinatology. 1999;16(4):175–80. doi: 10.1055/s-2007-993853. [DOI] [PubMed] [Google Scholar]
- Clarke C, Paul S, Stilson M, Senf J. “Sickle Cell Trait Preparticipation Screening Practices of Collegiate Physicians”. Clinical Journal of Sport Medicine. 2006;16:440a. [Google Scholar]
- College of American Pathologists. 2007. “Sickle Cell Trait and the Athlete” [accessed on 2007]. Available at http://www.cap.org/apps/cap.portal?_nfpb= true&cntvwrPtlt_actionOverride=%2Fportlets%2FcontentViewer%2Fshow&_windowLabel=cntvwrPtlt&cntvwrPtlt{actionForm.contentReference}=statements%2Fsickle_cell_and_the_athlete_statement.html&_state=maximized&_pageLabel=cntvwr.
- Corrado D, Basso C, Schiavon M, Thiene G. “Screening for Hypertrophic Cardiomyopathy in Young Athletes”. New England Journal of Medicine. 1998;339(6):364–9. doi: 10.1056/NEJM199808063390602. [DOI] [PubMed] [Google Scholar]
- Eichner ER. “Sickle Cell Trait in Sports”. Curr Sports Medicine Reports. 2010;9(6):347–51. doi: 10.1249/JSR.0b013e3181fc73d7. [DOI] [PubMed] [Google Scholar]
- Eichner ER. “Sickle Cell Considerations in Athletes”. Clinics in Sports Medicine. 2011;30(3):537–49. doi: 10.1016/j.csm.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Fainaru-Wada M. 2008. “Autopsy Reveals UCF's Plancher Had Gene Trait Tied to 10 Similar Deaths.” Available at http://www.ESPN.com.
- Harmon KG, Asif IM, Klossner D, Drezner JA. “Incidence of Sudden Cardiac Death in National Collegiate Athletic Association Athletes”. Circulation. 2011;123(15):1594–600. doi: 10.1161/CIRCULATIONAHA.110.004622. [DOI] [PubMed] [Google Scholar]
- Hicks EJ, Hughes BJ. “Comparison of Electrophoresis on Citrate Agar, Cellulose Acetate, or Starch for Hemoglobin Identification”. Clinical Chemistry. 1975;21(8):1072–6. [PubMed] [Google Scholar]
- Kark JA, Posey DM, Schumacher HR, Ruehle CJ. “Sickle-Cell Trait as a Risk Factor for Sudden Death in Physical Training”. New England Journal of Medicine. 1987;317(13):781–7. doi: 10.1056/NEJM198709243171301. [DOI] [PubMed] [Google Scholar]
- Kark JA, Garder JW, Ward FT, Virmani R. 1999. “Prevention of Exertional Heat Illness Protects Recruits with Sickle Cell Trait from Exercise-Related Death(abstract).” SRGL, Sickle Cell Disease Program, National Institute of Health [accessed on November 27, 2011]. Available at http://www.dtic.mil/cgibin/GetTRDoc?Location=U2&doc=GetTRDoc.pdf&AD=ADA500648.
- Lanier Law Firm. 2009. “NCAA Sickle Cell Settlement” [accessed on December 2009]. Available at http://www.lanierlawfirm.com/law_firm_news/ncaa_sickle_cell_settlement.htm.
- Levin WC. “Asymptomatic Sickle Cell Trait”. Blood. 1958;13(9):904–7. [PubMed] [Google Scholar]
- Lorey FW, Arnopp J, Cunningham GC. “Distribution of Hemoglobinopathy Variants by Ethnicity in a Multiethnic State”. Genetic Epidemiology. 1996;13(5):501–12. doi: 10.1002/(SICI)1098-2272(1996)13:5<501::AID-GEPI6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Marlin L, Etienne-Julan M, Le Gallais D, Hue O. “Sickle Cell Trait in French West Indian Elite Sprint Athletes”. International Journal of Sports Medicine. 2005;26(8):622–5. doi: 10.1055/s-2004-830377. [DOI] [PubMed] [Google Scholar]
- Murphy JR. “Sickle Cell Hemoglobin (Hb AS) in Black Football Players”. Journal of the American Medical Association. 1973;225(8):981–2. [PubMed] [Google Scholar]
- National Athletic Trainers’ Association. Consensus Statement: Sickle Cell and the Athlete. Dallas, TX: National Athletic Trainers’ Association; 2007. [Google Scholar]
- National Collegiate Athletic Association. 2010. “NCAA Division I Proposal No. 2009-75-B-1. Question and Answer Document” [accessed on June 24, 2011, 2010]. Available at http://www.ncaa.org/wps/wcm/connect/e833fc8042c462ce92bdfbffd1ce0240/NCAA+Division+I+Sickle+Cell+Trait+QA+for+institutions+5+25+2010.pdf?MOD=AJPERES&CACHEID=e833fc8042c462ce92bdfbffd1ce0240.
- National Collegiate Athletic Association. 2011. “Differences among the Three Divisions: Division I” [accessed on June 23, 2011]. Available at http://www.ncaa.org/wps/wcm/connect/public/ncaa/about+the+ncaa/who+we+are/differences+among+the+divisions/division+i/about+division+i.
- NCAA. 2008-2009 NCAA Sports Medicine Handbook. Indianapolis, IN: NCAA; 2008a. [Google Scholar]
- NCAA. Guideline 3C: The Student-Athlete with Sickle Cell Trait. Indianapolis, IN: NCAA; 2008b. [Google Scholar]
- NCAA. 1981-82–2007-08 NCAA Sports Sponsorship and Participation Rates. Indianapolis, IN: NCAA; 2009. [Google Scholar]
- NCAA. 2008-09 NCAA Student-Athlete Race/Ethnicity Report. Indianapolis, IN: NCAA; 2010a. [Google Scholar]
- NCAA. 2010-11 NCAA Division I Manual. Indianapolis, IN: NCAA; 2010b. [Google Scholar]
- Pearson HA. “Sickle Cell Trait and Competitive Athletics: Is There a Risk?”. Pediatrics. 1989;83(4):613–4. [PubMed] [Google Scholar]
- Porell FW, Adams EK. “Hospital Choice Models: A Review and Assessment of Their Utility for Policy Impact Analysis”. Medical Care Research and Review. 1995;52(2):158–95. doi: 10.1177/107755879505200202. [DOI] [PubMed] [Google Scholar]
- Rutkow IM, Lipton JM. “Some Negative Aspects of State Health Departments’ Policies Related to Screening for Sickle Cell Anemia”. American Journal of Public Health. 1974;64(3):217–21. doi: 10.2105/ajph.64.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears DA. “The Morbidity of Sickle Cell Trait: A Review of the Literature”. American Journal of Medicine. 1978;64(6):1021–36. doi: 10.1016/0002-9343(78)90458-8. [DOI] [PubMed] [Google Scholar]
- Secretary's Advisory Committee on Heritable Disorders and Genetic Diseases in Newborns and Children. Screening U.S. College Athletes for Their Sickle Cell Disease Carrier Status. Rockville, MD: Health Resources and Services Administration; 2010. Briefing paper. [Google Scholar]
- Secretary's Advisory Committee on Heritable Disorders in Newborns Meeting. Carrier Screening for Sickle Cell Disease: Report from the Sickle Cell Disease Association of America Workshop on Carrier Screening. Briefing paper. Rockville, MD: Health Resources and Services Administration; 2010. January 22. [Google Scholar]
- Tsaras G, Owusu-Ansah A, Boateng FO, Amoateng-Adjepong Y. “Complications Associated with Sickle Cell Trait: A Brief Narrative Review”. American Journal of Medicine. 2009;122(6):507–12. doi: 10.1016/j.amjmed.2008.12.020. [DOI] [PubMed] [Google Scholar]
- University of California-Berkeley. 2010. “Letter to Parents of Cal Student-Athlete” [accessed in 2010]. Available at http://uhs.berkeley.edu/students/athletics/pdf/Sickle Cell Parent Letter, Final Winter 2011.pdf.
- Wesleyan University. 2010. “Wesleyan University Sports Medicine—Sickle Cell Trait Testing” [accessed in 2010]. Available at http://www.wesleyan.edu/athletics/injurycare/Wesleyan Sickle Cell Trait Testing Waiver.pdf.
- Whitten CF. “Sickle-Cell Programming—An Imperiled Promise”. New England Journal of Medicine. 1973;288(6):318–9. doi: 10.1056/NEJM197302082880612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


