Abstract
One unique physiological characteristic of frogs is that their main route for intake of water is across the skin. In these animals, the skin acts in concert with the kidney and urinary bladder to maintain electrolyte homeostasis. Water absorption across the skin is driven by the osmotic gradient that develops as a consequence of solute transport. Our recent study demonstrated that chytridiomycosis, an infection of amphibian skin by the fungal pathogen, Batrachochytrium dendrobatidis, inhibits epithelial Na+ channels, attenuating Na+ absorption through the skin. In frogs that become severely affected by this fungus, systemic depletion of Na+, K+ and Cl− is thought to cause deterioration of cardiac electrical function, leading to cardiac arrest. Here we review the ion transport mechanisms of frog skin, and discuss the effect of chytridiomycosis on these mechanisms.
Keywords: Epithelial Na+ Channel, chytridiomycosis, Batrachochytrium dendrobatidis, frog skin, hyponatremia, hypokalemia
1. Introduction
Fluid and electrolyte homeostasis in amphibians is maintained by fine balance of the activity of the kidneys, urinary bladder and skin. In these animals, the kidneys produce copious volumes of dilute urine, and the bladder serves mostly as a reservoir of water during terrestrial activity (Uchiyama and Konno, 2006). The unique properties of amphibian skin of having high permeability to water and electrolytes, therefore, allow this tissue to contribute to osmoregulation and electrolyte and fluid homeostasis. The outermost layer of frog skin, the stratum corneum, is composed of a thin layer of keratinized cells, offering very little resistance to movement of water between internal and external environments (Lillywhite, 2006). Consequently, terrestrial and semiterrestrial frogs are subject to water loss via evaporative dehydration (Lillywhite, 2006). Frogs do not exhibit primary drinking behavior for the purposes of relieving thirst or for rehydration. Instead, the main route for water intake is across the ventral skin, especially the highly-vascularized pelvic patch (Parsons and Mobin, 1991).
Water intake is under tight control by various physiological factors, in particular the neurohypophyseal hormone, vasotocin (Uchiyama and Konno, 2006). At least two types of amphibian-specific vasotocin-sensitive aquaporin water channels, AQP-h2 and AQP-h3, have been identified in cells of the pelvic patch (Hasegawa et al., 2003). Water absorption across the skin occurs more quickly when frogs are in solutions containing NaCl, than when bathed with deionised water (Hillyard and Larsen, 2001). The rate of water absorption is linearly related to the rate of Na+ influx, and is dependent upon the activity of the Na+/K+ ATPase (Larsen et al., 2009). Absorption of water, therefore, is coupled to Na+ transport and energized by the activity of the pump (Larsen et al., 2009). Recent studies suggest that reduced Na+ transport in frog skin is a key feature of the pathophysiology of chytridiomycosis (Voyles et al., 2009). This amphibian disease is caused by the lethal fungal pathogen, Batrachochytrium dendrobatidis (Bd), and has been implicated in global declines in amphibian populations (Fisher et al., 2009b).
2. Cellular Origin
The epidermal surface of frog skin is derived from ectoderm (Jones and Woodland, 1986). The basal layer of the epidermis, the stratum germinativum, is composed of columnar or cuboidal cells (Farquhar and Palade, 1965). These cells migrate superficially through the stratum spinosum and stratum granulosum layers as they mature, ultimately becoming keratinized in the stratum corneum (Farquhar and Palade, 1965). Principal cells comprise 90% of the stratum granulosum, the layer most prominently involved in active electrolyte transport (Larsen, 1991). Interspersed throughout, and comprising up to 10% of the epithelial volume of the stratum granulosum, are flask-shaped mitochondria-rich (MR) cells (Larsen, 1991).
3. Electrolyte Transport in Frog Skin
Most of our knowledge about active Na+ absorption in epithelial cells originated from the seminal studies by Hans Ussing on the isolated skin of Rana temporaria. Prior to Ussing, it had been established that frog skin can absorb electrolytes from pond water against a large chemical gradient (Krogh, 1937). The detail of the mechanism underlying this process was elucidated only later when Ussing developed a technique to quantitatively evaluate active transepithelial Na+ absorption. Ussing’s system allowed each side of the isolated frog skin to be exposed to different bathing solutions. He then used two different approaches to measure active Na+ absorption. Firstly, he bathed both sides of the skin with symmetrical solutions and introduced the radioisotope, 24Na+, into the solution bathing the pond-side membrane to measure net Na+ flux (Ussing, 1949). He then used an electrophysiological method to determine the transepithelial potential difference, observing that the pond side of the skin was negative relative to the blood side (Ussing, 1949). Subsequently, Ussing applied a novel technique to determine the current required to drive the potential difference across the skin to 0 mV, which he then named the “short-circuit current”. Since both sides of epithelium were exposed to symmetrical solutions, all passive transepithelial ion movement was eliminated. The short-circuit current measured under these conditions, therefore, is equal to net flux of ions transported via active mechanisms. Since the current predicted from the transepithelial 24Na+ flux, was almost equivalent to the short-circuit current (Ussing and Zerahn, 1951), he concluded that frog skin actively transports Na+ from pond water to blood plasma with negligible retrograde movement.
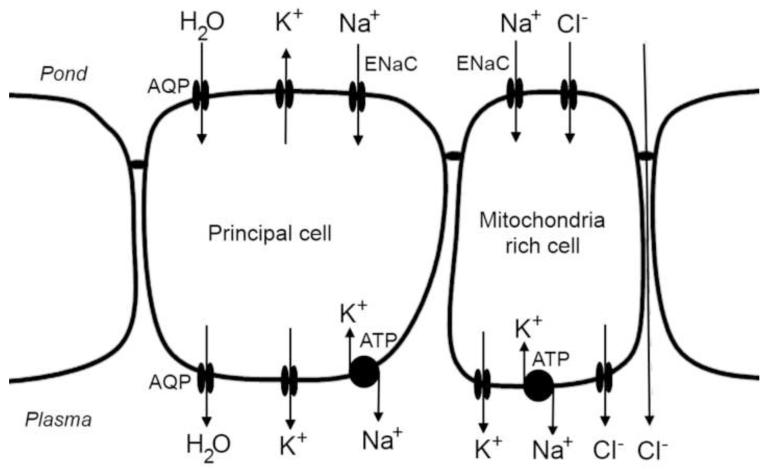
In subsequent studies, Ussing removed Cl− from the solution on both sides to eliminate the contribution of the major anions to the short-circuit current. This approach allowed assessment of ion permeability of the membrane on either side of the epithelium. He observed that the pond side membrane of frog skin is primarily Na+ selective, while the blood side was highly selective for K+ (Koefoed-Johnsen and Ussing, 1958). These observations led to the formulation of the ‘Two-Membrane Model of Epithelial Transport’, one of the most important paradigms in epithelial physiology. In this model, activity of the Na+/K+ ATPase in the basolateral membrane of the principal cells exchanges cytosolic Na+ for plasma K+ (Figure 1). By maintaining a low intracellular Na+ concentration, Na+ ions in the pond water passively moved into the cell via Na+-selective channels. Excess intracellular K+ generated by activity of the Na+/K+ ATPase is returned to the plasma across the basolateral membrane via K+-selective channels (Nagel and Hirschmann, 1980, Larsen, 2011). The combination of a Na+-selective apical membrane and K+-selective basolateral membrane allows absorption of Na+ to generate a large transepithelial potential difference (Koefoed-Johnsen and Ussing, 1958). In turn, this potential drives parallel uptake of Cl− from pond water through Cl− channels in mitochondria-rich cells (Voûte and Meier, 1978, Larsen, 2011) and paracellular pathways (Ussing and Windhager, 1964) (Figure 1). The electroneutral, absorption of NaCl generates an osmotic driving force that facilitates uptake of water across the skin. The molecular identity of these Na+-selective channels was not confirmed for almost 40 years after Ussing’s studies (Canessa et al., 1994, Takada et al., 2006).
Figure 1.
Transport model of the principal and mitochondria-rich cells of the frog skin epithelium. Na+ is moved from the pond solution via epithelial Na+ channels (ENaC) in the apical membrane and extruded via the Na+/K+ ATPase in the basolateral membrane. Excess cytosolic K+ generated by activity of the Na+/K+ ATPase is recycled to the plasma via K+ channels in the basolateral membrane. The transepithelial potential difference generated by this mechanism drives absorption of Cl− through mitochondria-rich cells and through paracellular pathways. NaCl absorption generates an osmotic gradient that drives absorption of water via aquaporin channels (AQP).
These epithelial Na+ channels (ENaC) play an important role in active Na+ absorption in a variety of epithelia. In mammals, the function of ENaC is important for the regulation of body fluid volume, blood pressure and maintenance of the depth of alveolar fluid (Garty and Palmer, 1997). Dysfunction of ENaC has been associated with disorders of Na+ and fluid homeostasis, blood pressure, and lung fluid balance (Schild, 2004). It has also been reported that activity of ENaC in the lung is adversely affected by respiratory pathogens (Kunzelmann et al., 2004, Hee et al., 2011). Recent studies suggest that activity of ENaC in frog skin is inhibited by the fungus, Batrachochytrium dendrobatidis (Bd), leading to the fatal pathogenesis of chytridiomycosis (Voyles et al., 2009).
4. Associated pathologies: Pathogenesis of chytridiomycosis
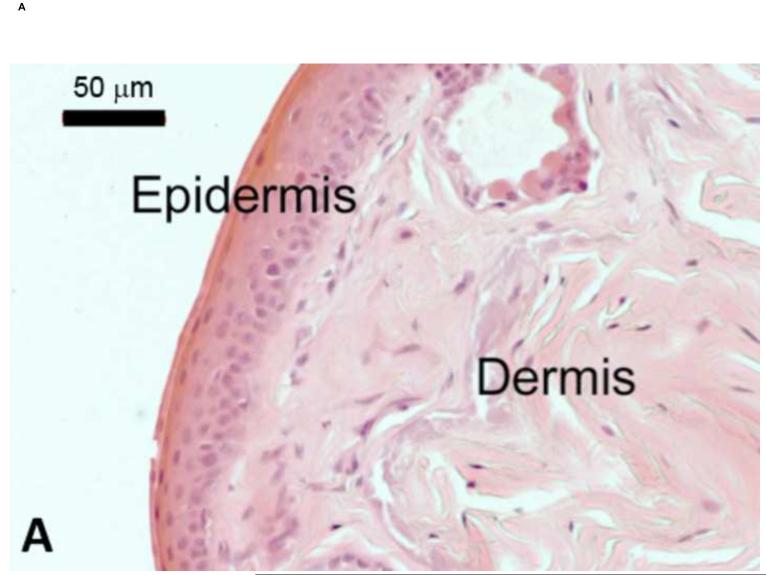
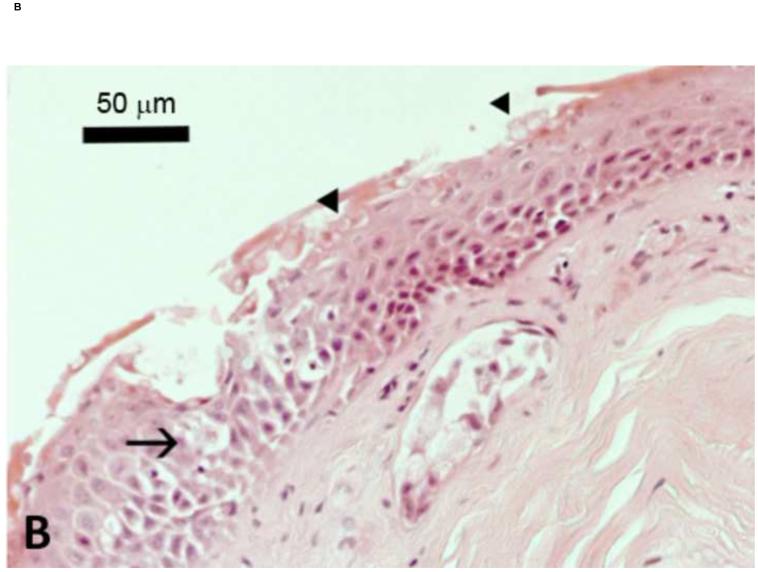
The global decline and, in some cases, extinction, of amphibian species has been attributed to the chytridiomycosis pandemic (Fisher et al., 2009b). Identified in 1998 (Berger et al., 1998), Bd belongs to the phylum Chytridiomycota, and is the only member of this fungal family known to parasitize vertebrate hosts with fatal effect (Longcore et al., 1999). Bd zoospores predominantly colonize the skin of the ventral abdomen and toes, but are rarely found on the dorsal skin of infected frogs (Berger et al., 2005b). Infection is contained within the superficial layers of the epidermis, and causes hyperkeratosis with cytoplasmic degeneration and vacuolation (Berger et al., 1998) (Figure 2). Visible lesions of the skin, however, are not common and no histologically detectable changes in internal organs have been observed (Berger et al., 1998, Voyles et al., 2009).
Figure 2.
Skin of Litoria caerulea. A) Histological section through the epithelium of frog skin from a healthy animal. The epidermis consists of multiple layers of cells overlying the dermis. In uninfected frogs, the epidermal surface is smooth with regular epithelial cell layers. B) Histological section of epithelium collected from an experimentally infected frog showing hyperkeratosis, characteristic of the pathology of chytridiomycosis. Sporangia of Batrachochytrium dendrobatidis are indicated by arrowheads. The arrow shows cellular vacuolation. Scale bars represent 50 μm. Figure modified from Voyles et al. (2009).
Concomitant with the pathological changes in the superficial epidermis, frogs develop inappetance, lethargy, loss of righting reflex, skin sloughing (Voyles et al., 2009), and slower rehydration (Carver et al., 2010), all becoming more noticeable at the terminal stages of disease. At this point, diseased frogs appear to maintain body mass and have stable hematocrit, plasma albumin, urea and total protein levels. However, significant reductions in plasma osmolarity, Na+, K+ and Cl− concentrations (Voyles et al., 2009, Marcum et al., 2010) suggest loss of plasma electrolytes without dramatic changes in plasma volume. Although sample sizes were low in preliminary studies, analyses of urine samples from these animals did not reveal significant changes in renal function or indicate an increase in wasting of electrolytes in the urine (Voyles et al., 2009). This loss of electrolytes coincides with deterioration of cardiac electrical function preceding death by asystolic arrest (Voyles et al., 2009). It appears that there are no detectable changes in plasma CO2 levels, therefore changes in respiratory gases seem an unlikely cause of cardiac arrest (Voyles et al., 2007). While not able to overcome the infection, electrolyte supplementation prolongs survival and allows frogs to regain physical activity (Voyles et al., 2009), supporting the conclusion that depletion of plasma electrolytes underpins the ultimate cause of death in chytridiomycosis.
Analysis of electrophysiological parameters measured across the isolated pelvic patch epithelium of Bd-infected green tree frogs (Litoria caerulea) indicate that frogs with advanced clinical signs exhibit a significant reduction in transepithelial potential, resistance and amiloride-sensitive short-circuit current, consistent with a reduction in the activity of ENaC (Voyles et al., 2009). Given that there is no concurrent reduction in the activity of the Na+/K+ ATPase (Campbell, unpublished findings), it is likely that Bd directly inhibits activity of ENaC in the skin. In the absence of evidence for excess secretion of Na+ in urine and stool, reduced absorption of Na+ across the skin via ENaC remains the most likely cause of hyponatremia in chytridiomycosis.
Depletion of plasma K+ preceding abnormal cardiac electrical activity (Voyles et al., 2009), suggests that hypokalemia may be the direct cause of cardiac arrest and death in chytridiomycosis. So far, the mechanism underlying the development of hypokalemia in Bd-infected frogs remains elusive. Low plasma K+ often results from K+-wasting by the kidney, however, in chytridiomycosis, there is no evidence for excess K+ secretion in the urine (Voyles et al., 2009). Although difficult to determine, because frogs are maintained in an aquatic environment, there have been no indications of diarrhea, that might suggest K+-wasting in the stool. With the lack of evidence that Bd has any direct effect on other tissues, with the exception of inhibiting Na+ transport across the skin, could hypokalemia be caused by Bd increasing skin K+ secretion? Although the apical membrane of the frog skin is considered to be almost exclusively permeable to Na+ (Koefoed-Johnsen et al., 1952), K+ permeability (Nagel and Hirschmann, 1980) and K+ secretion (Huf and Wills, 1953) of this tissue has been reported. In Rana temporaria, the apical membrane became permeable to K+ only after absorption of Na+ was inhibited (Nagel and Hirschmann, 1980), suggesting that K+ secretion is augmented by the reduction in the cytosolic concentration of Na+. This prompts us to speculate that, in cases of severe chytridiomycosis, K+ may be wasted by the skin as a consequence of inhibition of ENaC.
5. Conclusion
To date, the strongest evidence available suggests that the pathophysiology of chytridiomycosis involves abnormal electrolyte homeostasis. Most notably, this fungal infection causes severe reduction in Na+ absorption across the skin, and loss of Na+, K+ and Cl− from the plasma (Voyles et al., 2009), leading to asystolic cardiac arrest and death. It is possible that hypokalemia in Bd-infected frogs is due to K+ loss through the skin, caused by low cytosolic Na+ concentration following inhibition of ENaC.
Interestingly, most frog species that become infected do not appear to be adversely affected by the development of Bd infection, or alternatively, they may be susceptible only at certain times of the year (Carver et al., 2010). The reasons for interspecific and seasonal differences in susceptibility to this fungal infection are unresolved, but may include environmental variables (Rödder et al., 2008), host immunological (Rollins-Smith et al., 2011), behavioral and ecological characteristics (Woodhams et al., 2007, Woodhams et al., 2008), virulence of different Bd strains (Berger et al., 2005a, James et al., 2006, Retallick and Miera, 2007, Fisher et al., 2009a), or a combination of all of these factors. The ion transport characteristics, especially that of K+ secretion, of frog skin vary between species, and with physical, seasonal, and environmental conditions (Nagel and Hirschmann, 1980). It is, therefore, possible that properties inherent to amphibian epidermis render some species of frogs more vulnerable to fatal chytridiomycosis than others.
Cell Facts.
Frog skin is an electrically tight epithelium, comprised primarily of principal cells, with a minority of mitochondria-rich cells interspersed.
Principal cells of frog skin can absorb Na+ against its concentration gradient.
Superficial infection by Batrachochytrium dendrobatidis inhibits absorption of Na+ across the skin, leading to depletion of plasma electrolytes and death.
Acknowledgements
The authors’ laboratories are supported by funding from the Australian Research Council (DP1096313), the National Health and Medical Research Council of Australia (1007447 and 1011356), NIH/NCRR (P20RR16448) and NSF (EF-0723871). We thank L. Berger and R. Webb for assistance with histology.
References
- Berger L, Marantelli G, Skerratt LF, Speare R. Virulence of the amphibian chytrid fungus Batrachochytium dendrobatidis varies with the strain. Dis Aquat Organ. 2005a;68:47–50. doi: 10.3354/dao068047. [DOI] [PubMed] [Google Scholar]
- Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci U S A. 1998;95:9031–6. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger L, Speare R, Skerratt LF. Distribution of Batrachochytrium dendrobatidis and pathology in the skin of green tree frogs Litoria caerulea with severe chytridiomycosis. Dis Aquat Organ. 2005b;68:65–70. doi: 10.3354/dao068065. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, et al. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–7. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Carver S, Bell BD, Waldman B. Does chytridiomycosis disrupt amphibian skin function? Copeia. 2010;2010:487–95. [Google Scholar]
- Farquhar MG, Palade GE. Cell junctions in amphibian skin. J Cell Biol. 1965;26:263–91. doi: 10.1083/jcb.26.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MC, Bosch J, Yin Z, Stead DA, Walker J, Selway L, et al. Proteomic and phenotypic profiling of the amphibian pathogen Batrachochytrium dendrobatidis shows that genotype is linked to virulence. Mol Ecol. 2009a;18:415–29. doi: 10.1111/j.1365-294X.2008.04041.x. [DOI] [PubMed] [Google Scholar]
- Fisher MC, Garner TWJ, Walker SF. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu Rev Microbiol. 2009b;63:291–310. doi: 10.1146/annurev.micro.091208.073435. [DOI] [PubMed] [Google Scholar]
- Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359–96. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Tanii H, Suzuki M, Tanaka S. Regulation of water absorption in the frog skins by two vasotocin-dependent water-channel aquaporins, AQP-h2 and AQP-h3. Endocrinology. 2003;144:4087–96. doi: 10.1210/en.2003-0418. [DOI] [PubMed] [Google Scholar]
- Hee L, Dinudom A, Mitchell AJ, Grau GE, Cook DI, Hunt NH, et al. Reduced activity of the epithelial sodium channel in malaria-induced pulmonary oedema in mice. Int J Parasitol. 2011;41:81–8. doi: 10.1016/j.ijpara.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SD, Larsen EH. Lymph osmolality and rehydration from NaCl solutions by toads, Bufo marinus. J Comp Physiol B. 2001;171:283–92. doi: 10.1007/s003600100175. [DOI] [PubMed] [Google Scholar]
- Huf EG, Wills J. The relationship of sodium uptake, potassium rejection, and skin potential in isolated frog skin. J Gen Physiol. 1953;36:473–87. doi: 10.1085/jgp.36.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TY, Letcher PM, Longcore JE, Mozley-Standridge SE, Porter D, Powell MJ, et al. A molecular phylogeny of the flagellated fungi (Chytridiomycota) and description of a new phylum (Blastocladiomycota) Mycologia. 2006;98:860–71. doi: 10.3852/mycologia.98.6.860. [DOI] [PubMed] [Google Scholar]
- Jones EA, Woodland HR. Development of the ectoderm in Xenopus: Tissue specification and the role of cell association and division. Cell. 1986;44:345–55. doi: 10.1016/0092-8674(86)90769-5. [DOI] [PubMed] [Google Scholar]
- Koefoed-Johnsen V, Levi H, Ussing HH. The mode of passage of chloride ions through the isolated frog skin. Acta Physiol Scand. 1952;25:150–63. doi: 10.1111/j.1748-1716.1952.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Koefoed-Johnsen V, Ussing HH. The nature of the frog skin potential. Acta Physiol Scand. 1958;42:298–308. doi: 10.1111/j.1748-1716.1958.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Krogh A. Osmotic regulation in the frog (R. esculenta) by active absorption of chloride ion. Skand Arch Physiol. 1937;76:60–74. [Google Scholar]
- Kunzelmann K, Konig J, Sun J, Markovich D, King NJ, Karupiah G, et al. Acute effects of parainfluenza virus on epithelial electrolyte transport. J Biol Chem. 2004;279:48760–6. doi: 10.1074/jbc.M409747200. [DOI] [PubMed] [Google Scholar]
- Larsen EH. Chloride transport by high-resistance heterocellular epithelia. Physiol Rev. 1991;71:235–83. doi: 10.1152/physrev.1991.71.1.235. [DOI] [PubMed] [Google Scholar]
- Larsen EH. Reconciling the Krogh and Ussing interpretations of epithelial chloride transport - presenting a novel hypothesis for the physiological significance of the passive cellular chloride uptake. Acta Physiol. 2011;202:435–64. doi: 10.1111/j.1748-1716.2010.02239.x. [DOI] [PubMed] [Google Scholar]
- Larsen EH, Willumsen NJ, Mobjerg N, Sorensen JN. The lateral intercellular space as osmotic coupling compartment in isotonic transport. Acta Physiol. 2009;195:171–86. doi: 10.1111/j.1748-1716.2008.01930.x. [DOI] [PubMed] [Google Scholar]
- Lillywhite HB. Water relations of tetrapod integument. J Exp Biol. 2006;209:202–26. doi: 10.1242/jeb.02007. [DOI] [PubMed] [Google Scholar]
- Longcore JE, Pessier AP, Nichols DK. Batrachochytrium Dendrobatidis gen. et sp. nov., a Chytrid pathogenic to amphibians. Mycologia. 1999;91:219–27. [Google Scholar]
- Marcum RD, St-Hilaire S, Murphy PJ, Rodnick KJ. Effects of Batrachochytrium dendrobatidis infection on ion concentrations in the boreal toad Anaxyrus (Bufo) boreas boreas. Dis Aquat Organ. 2010;91:17–21. doi: 10.3354/dao02235. [DOI] [PubMed] [Google Scholar]
- Nagel W, Hirschmann W. K+-permeability of the outer border of the frog skin (R. temporaria) J Membr Biol. 1980;52:107–13. doi: 10.1007/BF01869115. [DOI] [PubMed] [Google Scholar]
- Parsons RH, Mobin F. Water flow across the pectoral and ventral pelvic patch in Rana catesbeiana. Physiol Zool. 1991;235:812–22. [Google Scholar]
- Retallick RWR, Miera V. Strain differences in the amphibian chytrid Batrachochytrium dendrobatidis and non-permanent, sub-lethal effects of infection. Dis Aquat Organ. 2007;75:201–7. doi: 10.3354/dao075201. [DOI] [PubMed] [Google Scholar]
- Rödder D, Veith M, Lötters S. Environmental gradients explaining the prevalence and intensity of infection with the amphibian chytrid fungus: the host’s perspective. Animal Conservation. 2008;11:513–7. [Google Scholar]
- Rollins-Smith LA, Ramsey JP, Pask JD, Reinert LK, Woodhams DC. Amphibian immune defenses against chytridiomycosis: Impacts of changing environments. Integrative and Comparative Biology. 2011;51:552–62. doi: 10.1093/icb/icr095. [DOI] [PubMed] [Google Scholar]
- Schild L. The epithelial sodium channel: from molecule to disease. Rev Physiol Biochem Pharmacol. 2004;151:93–107. doi: 10.1007/s10254-004-0023-7. [DOI] [PubMed] [Google Scholar]
- Takada M, Shimomura T, Hokari S, Jensik P, Cox T. Larval bullfrog skin expresses ENaC despite having no amiloride-blockable transepithelial Na+ transport. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2006;176:287–93. doi: 10.1007/s00360-005-0050-y. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Konno N. Hormonal regulation of ion and water transport in anuran amphibians. Gen Comp Endocrinol. 2006;147:54–61. doi: 10.1016/j.ygcen.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Ussing HH. The active ion transport through the isolated frog skin in the light of tracer studies. Acta Physiol Scand. 1949;17:1–37. doi: 10.1111/j.1748-1716.1949.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Ussing HH, Windhager EE. Nature of shunt path and active sodium transport path through frog skin epithelium. Acta Physiol Scand. 1964;61:484–504. [PubMed] [Google Scholar]
- Ussing HH, Zerahn K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951;23:110–27. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Voûte CL, Meier W. The mitochondria-rich cell of frog skin as hormone-sensitive “shunt-path”. J Membr Biol. 1978;40:151–65. doi: 10.1007/BF02026003. Spec No. [DOI] [PubMed] [Google Scholar]
- Voyles J, Berger L, Young S, Speare R, Webb R, Warner J, et al. Electrolyte depletion and osmotic imbalance in amphibians with chytridiomycosis. Dis Aquat Organ. 2007;77:113–8. doi: 10.3354/dao01838. [DOI] [PubMed] [Google Scholar]
- Voyles J, Young S, Berger L, Campbell CR, Voyles WF, Dinudom A, et al. Pathogenesis of chytridiomycosis, a cause of amphibian declines. Science. 2009;326:582–5. doi: 10.1126/science.1176765. [DOI] [PubMed] [Google Scholar]
- Woodhams DC, Alford RA, Briggs CJ, Johnson M, Rollins-Smith LA. Life-history trade-offs influence disease in changing climates: Strategies of an amphibian pathogen. Ecology. 2008;89:1627–39. doi: 10.1890/06-1842.1. [DOI] [PubMed] [Google Scholar]
- Woodhams DC, Ardipradja K, Alford RA, Marantelli G, Reinert LK, Rollins-Smith LA. Resistance to chytridiomycosis varies among amphibian species and is correlated with skin peptide defenses. Animal Conservation. 2007;10:409–17. [Google Scholar]