Abstract
The axonal connections between the retina and its midbrain target, the superior colliculus (SC), is mapped topographically, such that the spatial relationships of cell bodies in the retina are maintained when terminating in the SC. Topographic map development uses a Cartesian mapping system such that each axis of the retina is mapped independently. Along the nasal-temporal mapping axis, EphAs and ephrin-As, are graded molecular cues required for topographic mapping while the dorsal-ventral axis is mapped in part via EphB and ephrin-Bs. Because both Ephs and ephrins are cell surface molecules they can signal in the forward and reverse directions. Eph/ephrin signaling leads to changes in cytoskeletal dynamics that lead to actin depolymerization and endocytosis guiding an axons via attraction and repulsion.
Keywords: Eph, ephrin, Topographic mapping, retina, Superior colliculus, vision
1. Introduction
Owing to its accessibility and amenability to experimental perturbation, the retina’s projection to the superior colliculus (SC, or its lower vertebrate equivalent, the optic tectum, OT) has served as a model for investigating guidance mechanisms that lead to ordered connectivity. The retinocollicular projection, like many projections in the brain, is topographically organized, such that neighbor-neighbor relationships of cell bodies in the retina are maintained in their terminations in the SC. In this manner spatial relationships within an image are preserved as it is relayed between visual processing centers. In the retinocollicular projection, the dorsal-ventral (D-V) axis of the retina maps topographically onto the medial-lateral (M-L) axis of the SC/OT, and the nasal-temporal (N-T) axis of the retina maps topographically onto the anterior-posterior (A-P) axis (Figure 1) [1,2]. While many molecular and activity-dependent cues have been implicated in mapping the retinocollicular projection (see [3] for review), here, we focus on the roles and mechanisms of action of the Eph and ephrin families of cell surface proteins in retinocollicular mapping.
Figure 1. Expression patterns of Ephs and ephrins in the retinocollicular system.
A) The temporal-nasal (T-N) axis of the retina maps along the anterior-posterior (A-P) axis of the SC/OT. Graded expression of EphA and ephrin-A expression patterns in each structure in the mouse are indicated by blue and red bars, respectively. EphAs are expressed high temporally and anteriorly, while ephrin-As are expressed high nasally and posteriorly in complentary gradients. B) The dorsal-ventral (D-V) axis of the retina maps along the lateral-medial (L-M) axis of the SC/OT. Graded EphB and ephrin-B expression patterns in each structure are indicated by blue and red bars, respectively. In the retina, EphBs are expressed in a V > D gradient, while ephrin-Bs are expressed in a complementary D > V gradient. In the SC, EphBs are expressed uniformly across the L-M axis, and ephrin-B1 expression is high at the midline, but is steeply reduced laterally.
2. The Eph and ephrin families of cell surface signaling proteins
Ephs represent the largest family of receptor tyrosine kinases (RTKs) and have been shown to play important roles in a wide variety of cellular activities, including cell proliferation, survival, migration, and axon guidance [4]. There are 14 distinct Eph receptors in mammals [5], characterized by an extracellular region with a unique cysteine rich motif extending over the amino-terminal half, followed by two fibronectin repeats. The intracellular domain of Ephs contains a juxtamembrane tyrosine-containing motif, a tyrosine kinase domain, and a C-terminal SAM domain [6]. Based on sequence similarities, Eph receptors are subdivided into A and B classes.
Ligands for these receptors, called ephrins, are also divided into A and B classes. There are 8 ephrins in mammals, which share a conserved extracellular sequence that includes 4 invariant cysteine residues. All ephrins are membrane anchored: ephrin-A’s are glycosylphosphatidylinositiol (GPI) linked to the membrane, and ephrin B ligands have a single transmembrane domain with C-terminal PDZ binding motifs. In vitro binding assays suggest promiscuity in receptor-ligand interactions within each class. That is, each EphA receptor can bind with high affinity to all ephrin-A’s, and each EphB can bind to each ephrin-B, with very little cross talk between A and B families [7,8].
Among the major RTK/ligand families, Eph/ephrin signaling is distinctive in that both ephrin “ligands” and Eph “receptors” are membrane bound, resulting in signals that are localized to sites of cell-cell contact. Additionally, Eph/ephrin binding induces signaling in both Eph- and ephrin-expressing cells [9]. Thus, Ephs and ephrins signal bidirectionally, with signaling in the Eph-bearing cell termed “forward” and signaling in the ephrin-bearing cell termed “reverse” signaling (Figure 3). Eph/ephrin signaling is dependent upon receptor/ligand clustering, such that pre-clustering of ephrin ligands is required to induce Eph phosphorylation in vitro, while unclustered ligands can act as functional antagonists to Eph signaling [10].
Figure 3. Signaling pathways downstream of Eph/ephrin binding and termination of cell-cell contact.
A) Ephrin binding to Eph receptors initiates intracellular signaling events in both directions, termed “forward” in the Eph containing cells and “reverse” in the ephrin-bearing cells. Binding to EphA receptors (blue) results in activation of the RhoA GTPase via ephexin, a guanine nucleotide exchange factor (GEF), ultimately resulting in growth cone collapse. In addition, regulation of Tsc2, a GEF, and its downstream target, Rheb, results in decreased translation, contributing to growth cone collapse. Reverse signaling through the GPI-linked ephrin-As (red) is mediated by co-receptors, TrkB and p75NTR, which results in axonal branching and axon repulsion, respectively. Forward signaling through EphB receptors (cyan) results in growth cone attraction or repulsion, depending on cellular context. Reverse signaling through ephrin-Bs (orange) is important for synapse maturation in the SC/OT. B & C) Ephrin signaling and cell-cell contact is terminated in two possible ways. Endocytosis of EphA receptors occurs in a Vav/Rac dependent manner (B). Termination of EphB/ephrin-B receptors can occur in either cell, each dependent on Rac signaling (B). Cleavage of ephrin-As by ADAM10 protease also results in signal termination and EphA receptor endocytosis (C). ADAM10 can associate with either ephrin-As or EphAs.
3. Chemoaffinity: implication of molecular cues in retinocollicular mapping
Prior to the identification of Ephs and ephrins, a role for molecular cues in topographic map formation was established using both in vivo manipulations and in vitro assays. In many lower vertebrates such as frogs and fish, retinotectal axons were shown to regenerate after optic nerve severing and maintain the same patterns of connections as they did during development..If the eyes are rotated 180 degrees, axons regenerate and grow back to their original destinations rather than reorienting to the rotated position, even though this led to maladaptive behaviors in response to visual stimuli [11,12]. This work led to the idea that each RGC contains specific “physio-chemical” properties that direct them to a particular location in the tectum. These conclusions were expanded into the chemoaffinity hypothesis, in which Sperry postulated that the presence of graded cues in both the retina and OT gave each RGC axon a unique positional identity that could be used to establish topography [13].
Evidence that these cues were cell surface proteins was demonstrated by Bonhoeffer and colleagues using an in vitro “stripe” assay that recapitulated some key aspects of retinal tectal mapping along the nasotemporal retinal mapping axis. In this assay, temporal or nasal retinal explants were presented with alternating lanes, or stripes, of membrane derived from the anterior and posterior tectum [14]. They found that temporal axons avoided membranes form the posterior OT, while nasal axons showed no preference for either substrate [15]. It was later found that treatment of posterior membranes with either a protease or PI-phospholipase C (PI-PLC) abolished the preference of temporal axons for anterior membranes, while nasal axons still showed no preference [16]. This suggested that a GPI-linked cell surface repellent molecule enriched in posterior OT was responsible for the choice of temporal axons in this assay. Taken together, these data set parameters for potential molecular candidates hypothesized to mediate topographic mapping.
3.1 Identification of EphAs and ephrin-As in topographic mapping of the retinocollicular projection
The first evidence of Eph/ephrin signaling being used in topographic mapping came from experiments using two distinct approaches. In a molecular approach, Cheng and Flanagan cloned the gene for ephrin-A2 (then called Eph Ligand Family-1 Elf-1) in their search for ligands of orphan RTKs [17]. They used soluble, enzyme tagged, extracellular domains of Mek4 and Sek (now, EphA3 and EphA4) as a probe to screen for cDNAs that allowed binding of these receptors to tissue culture cells after transfection [17]. Subsequently, they showed that the expression patterns of EphA3 and ephrin-A2 implicated them as being candidates for the chemospecifity labels proposed by Sperry [18]. They found that ephrin-A2 was expressed in a high posterior to low anterior gradient across the tectum while EphA3 was expressed in a high temporal to low nasal gradient in the ganglion cells of the retina. These complementary patterns of graded expression fit well into the theoretical framework established by Sperry in the chemoaffinity hypothesis [13,18].
In a separate biochemical approach, Drescher, Bonhoeffer and colleagues purified and cloned a GPI-linked protein enriched in posterior tectum that had homology to Elf-1. This protein, Repulsive Axon Guidance Signal (RAGS) (now called ephrin-A5) [19] was also expressed in a high posterior to low anterior gradient in the OT, consistent with a role in topographic mapping. Further, the membrane stripe assay confirmed that RAGS was capable of repelling temporal RGC axons and inducing growth cone collapse in isolated RGCs [19].
3.2 Expression patterns of EphAs and ephrin-As in the developing retinocollicular projection
Over the following years, it was found that of the vertebrates examined, all have multiple EphA and ephrin-A family members expressed in complementary gradients along the nasal-temporal axis of the retina and anterior-posterior axis in the SC/OT, though with slight differences in the specific members expressed in each species (frog: [20], [21]; chick: [18], [19], [22]; mouse: [23], [24], [25]; human: [26]). For example, in the chick, EphA3, -A5 and -A6 are expressed in a high temporal to low nasal gradient in the retina, while EphA4 is expressed uniformly across the T-N axis. In addition, gradients of ephrin-A2, -A5 and -A6 are found in a high nasal to low temporal counter-gradient. Correspondingly, in the OT, ephrin-A2 and -A5 are expressed in a high posterior to low anterior gradient, while EphA3, -A4, -A5, -A6 and -A7 are found in a high anterior to low posterior gradient. In the mouse, a pattern of complementary counter-gradients exists, but with EphA5 and EphA6 expressed in a high temporal to low nasal gradient in the retina, and ephrin-A3 expressed uniformly along the A-P axis of the SC (Figure 1).
4. Mapping the anterior-posterior axis of the SC
Based on the graded expression patterns of EphAs in the retina, ephrin-As in the SC, and the in vitro repellent activity of ephrin-As, a “mass action” model for topographic mapping was developed in which axons would project to the SC based on their sensitivity to posteriorly derived ephrin-As [18]. Thus, all axons end up with an equivalent amount of negative signal, which counterbalances their tendency to grow toward posterior OT [27]. Temporal axons, with high levels of receptor, terminate in anterior SC/tectum where ephrin-A levels are low, and nasal axons, with low receptor levels, will terminate in posterior tectum where ephrin-A levels are high.
4.1 EphAs and ephrin-As are required for topographic mapping of the temporal-nasal axis of the visual field
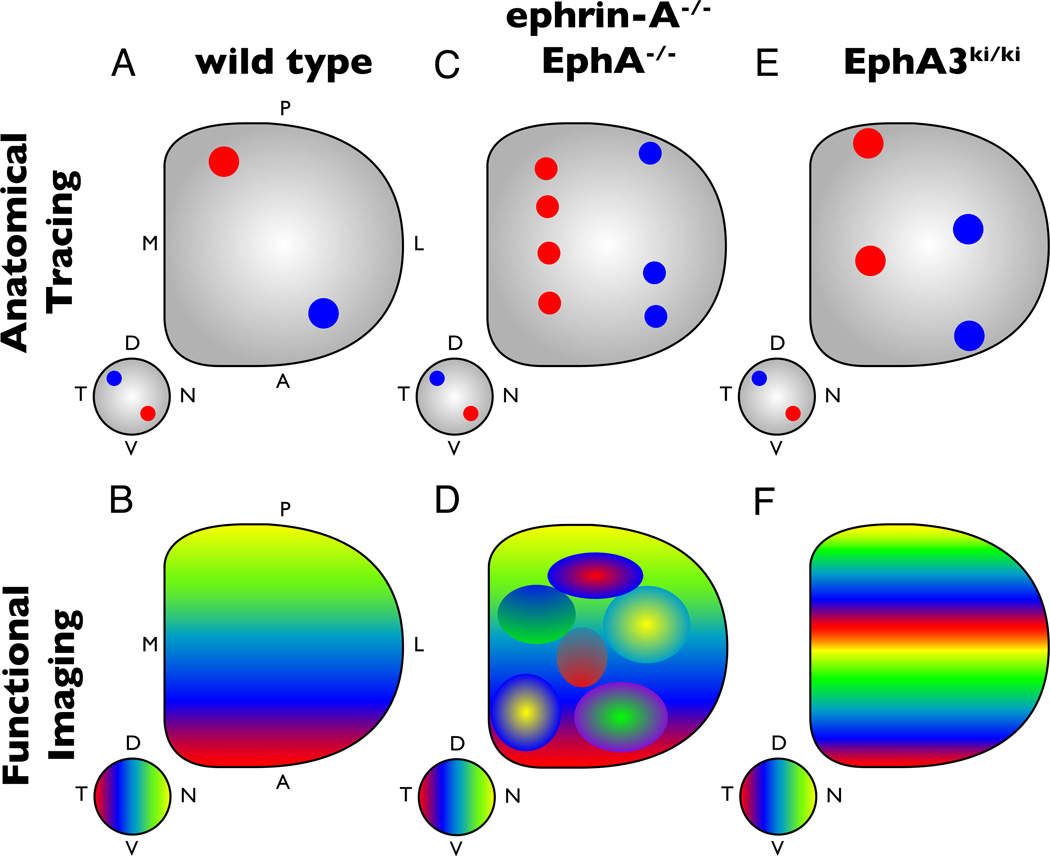
The mass action model was tested in both loss and gain of function experiments that manipulated EphA or ephrin-A expression and asked how topographic mapping was affected. Ectopic expression of ephrin-A2 in the chick tectum results in the avoidance of these areas by temporal but not nasal axons [27], showing ephrin-A2 can act as a repellent molecule in vivo. Loss of ephrin-A5 in the mouse results in topographic mapping errors of temporal and nasal axons in the SC [23,28]; these errors are enhanced in ephrin-A2/A5 double knockout and ephrin-A2/A3/A5 triple knockout mice [28,29], suggesting some redundancy in the roles of these molecules in topographic mapping. Importantly, these anatomic effects lead to deficits in the functional topography in the SC, showing that ephrin-As play a critical role in establishing the retinocollicular map [30](Figure 2).
Figure 2. Structure of the retinocollicular map in EphA/ephrin-A knockout and transgenic mice.
A–B) In wild type mice, the retina is mapped topographically onto the SC. Temporal RGCs (blue dots) map to anterior SC, while nasal RGCs (red dots) project to posterior SC.. Intrinsic optical imaging reveals topography by measuring the visual responses in the SC to a drifting bar along the N-T axis of the retina. Here depicted as a change in coloration as one moves across the retina and SC (B). C–D) Genetic deletion of ephrin-As or EphAs results in abberant topographic map formation. Anatomical tracing reveals multiple termination zones along the A-P axis of the SC for both temporal and nasal RGCs (C). Functionally, this results in areas of the SC with topographically inappropriate responses, with the general polarity of the map intact (D). E–F) Relative levels of EphA expressed are used to map along the A-P axis. In Islet2-EphA3 knock-in mice, half of retinal ganglion cells express exogenous EphA3, resulting in neighboring cells with drastically different EphA expression levels. This results in a duplicated retinocollicular map as assesed by anatomical tracing (E) and functional imaging studies (F).
Consistent with these experiments, manipulation of Eph receptor expression also causes topographic mapping defects. Expression of a dominant negative version of EphA3 in temporal axons of the chick retina or deletion of EphA5 in mice reduces RGC sensitivity to ephrin-As in vitro and results in temporal axons terminating posterior to their topographically appropriate location in vivo [31]. Alternatively, ectopic expression of EphA6 in the mouse retina causes RGCs to become more sensitive to ephrin-As and map to more anterior regions of the SC [32].
4.2 Relative not absolute levels of EphA receptors are used to sort RGC axons topographically
An important insight into how EphA/ephrin-A gradients direct topographic mapping comes from experiments by Lemke and colleagues that demonstrated that topography is established through relative, as opposed to absolute levels of EphA signaling [33]. Taking advantage of the fact that EphA3 is not expressed in the mouse retina, Brown et al. introduced EphA3 into the 3’ untranslated region of the Islet-2 locus. Since Islet-2 is expressed in approximately half of all RGCs distributed relatively evenly across the retina, two subpopulations of RGCs existed: 1) Islet-2− cells, which express endogenous levels of EphA4, EphA5 and EphA6 based on their position along the T-N axis and 2) Islet2+ cells, which express endogenous EphAs and exogenous EphA3 in high amounts. As a result, RGCs expressing EphA3 are compressed into the anterior half of the SC, while the Islet-2− RGCs, which have wild type levels of EphA, are restricted to the posterior half of the SC, which is reflected in the functional responses [34](Figure 2). Thus, the mapping of RGCs is not determined by the absolute level of EphAs, but by the relative difference in EphA signaling [33,35].
5. Models of nasal axon mapping
A number of models for topographic mapping have been derived that are consistent with the observed topographic mapping phenotypes exhibited by RGCs in the in vivo gain and loss of function experiments. Each model incorporates experimental evidence demonstrating that a posterior > anterior gradient of ephrin-A repellent activity is differentially detected by RGC axons. EphA receptors are expressed by RGCs in a low-nasal to high-temporal gradient, which results in temporal axons being more easily repelled by ephrin-As than nasal axons, thereby terminating in the anterior SC [24,27,36]. Where these models differ is in the explanation of why nasal axons, which express low levels of EphA, terminate in the posterior SC.
5.1 Reverse signaling through ephrin-As
In the ephrin-A reverse signaling model, nasal RGCs (that express high levels of ephrin-A) are repelled by EphA receptors and project to the posterior SC because of the high anterior to low posterior gradient of EphA receptors in the SC. Several lines of evidence support this hypothesis. First, Rashid et al. examined the retinocollicular projection in mice lacking EphA7, which is expressed in a high anterior to low posterior gradient in the SC but not expressed in the retina. In these mice, nasal RGCs have ectopic termination zones in the anterior SC, consistent with the idea that SC-derived EphA normally repels nasal axons to the posterior SC [37]. In addition, axons from nasal RGCs avoid EphA7 when presented on surfaces in vitro [37]. Recent studies using in vivo retinal electroporation shed more light on this subject. By co-expressing constitutively active EphA6 (EphA6EE) and GFP in central retina, Carreras et al. found that the electroporated RGC axons terminated in the anterior SC. When ephrin-A5 was co-expressed with EphA6EE the termination zones of central RGCs were pushed back to the central and posterior SC, suggesting that ephrin-A5 mediates signaling that counter-balances those of active EphA6 [32].
Because ephrin-A acts as a receptor, even though it has no intracellular domain, a co-receptor is required to transduce the signal across the membrane. Two different neurotrophin receptors, p75 and TrkB, have been proposed to play this role. p75 is expressed uniformly in the retina, co-immunoprecipitates with ephrin-A5, and is required for EphA7-Fc-induced tyrosine phosphorylation in stably transfected cells [38]. Furthermore, retinal axons derived from p75 mutant mice are no longer repelled by EphA7 in vitro, suggesting that p75 is an essential component of the reverse signaling repellent response [38]. To test the role of p75 in retinocollicular mapping, Lim et al. assayed retinocollicular topography in mice lacking p75 in the retina. They found that nasal RGC termination zones shifted anteriorly, consistent with a role for p75 acting as an ephrin-A5 coreceptor expressed on nasal axons that is required to mediate EphA-induced repulsive signals.
A second proposed co-receptor for ephrin-A5 in RGCs is the high affinity neurotrophin receptor TrkB. In co-expression studies, TrkB was shown to form a complex with ephrin-A5 via its CC2 domain [39]. Using in vitro branching assays, it was shown that an ephrin-A5/TrkB complex promotes axon branching in a BDNF-dependent manner by activating the PI3 kinase pathway [39]. Upon EphA binding, PI3 kinase activity decreases and branching is decreased. Thus, in this model, the role of ephrin-A5 reverse signaling is to regulate branching via a BDNF/TrkB pathway [39]. Interestingly, the same group also examined the effects of the p75 ligand, proBDNF, on axon branching and ephrin-A reverse signaling. Treatment of cells co-expressing ephrin-A5 and p75 with proBDNF results in increased interaction between these proteins, reduced sensitivity to EphA7 in the stripe assay and inhibiting BDNF-induced branching [40]. Taken together, the authors propose a model in which regulated cleavage of proBDNF to BDNF ensures that RGC branching is localized to the topographically appropriate location.
5.2 Ephrin-As are bifunctional, acting as both repellents and attractants
Another model to explain nasal axons mapping to the posterior SC incorporates in vitro experiments that showed that low to moderate levels of ephrin-A2 or ephrin-A5 can increase outgrowth from nasal retinal explants [41]. The idea here is that ephrin-As expressed in the SC are bifunctional, such that each RGC axon terminates where the ephrin-A attraction and repellent activities balance out.
5.3 Competition can counterbalance the ephrin-A repellent gradient
A third model that explains nasal RGC mapping behavior hypothesizes that axon-axon competition for a limited target-derived cue is used to counterbalance the ephrin-A repellent signal. Since temporal axons express high levels of EphA they are restricted to the anterior SC because of the repellent activity of SC-derived ephrin-As.. This forces nasal axons to terminate more posteriorly in the SC where synaptic targets are unoccupied. In support of this, ablation studies performed in amphibian, fish, and rodents have shown that retinal axons fill the available target space after partial ablation of the retina or tectum/SC while maintaining topographic order. This result is taken to demonstrate that axons normally compete for target space and sort based on their relative rather than absolute sensitivities to guidance molecules. Regenerating axons in fish and frogs seem to create more ordered maps after ablation than do those of rodents, suggesting that there may be differences in the degree or range of competition used between cold and warm blooded species [42–45]. This model is strengthened by the findings of Brown et. al that demonstrated that there is not a strict gradient matching mechanism for topography [33]. On the other hand, a study in zebrafish has challenged a requirement for competition in topographic mapping for some features of the retinotectal projection [46]. In this study, RGC transplantation into a mutant lacking RGCs was done to create a zebrafish with a single RGC that could innervate the tectum. They found that distal tips of solitary axons project to the topographically correct position in the absence of competition from other RGCs, suggesting a cell autonomous, strict matching mechanism for establishing topography [46].
One mechanism by which nasal axons could tolerate the repulsive environment in the posterior SC is through modulation of sensitivity to ephrin-As via cis interactions between EphAs and ephrin-As on the axon [47]. In co-transfection studies, ephrin-A5 and EphA3 were shown to interact within the same cell, which reduced ephrin-A-induced EphA3 tyrosine phosphorylation [48]. Additionally, over-expression of ephrin-A5 in temporal retinal explants reduced their sensitivity to ephrin-As in the membrane stripe assay [49], while removal of ephrin-As via PI-PLC treatment or gene knockout increased the sensitivity of nasal explants to ephrin-As [28,49]. These experiments suggest that ephrin-As can bind or block EphA function when expressed in cis, which may be useful in modulating or smoothening the EphA gradient in RGC axons.
6. Signaling events downstream of EphA activation
The above experiments show that the EphAs and ephrin-As are required for topographic mapping, but do not shed light on the signaling mechanisms used to execute the EphA repellent response. It is now known that EphA/ephrin-A interactions can trigger a number of pathways that are important for the execution of axon repulsion: endocytosis of EphA/ephrin-A complexes used to disassociate axons from a target cell, activation of small GTP binding proteins that lead to destabilization of the actin cytoskeleton, and regulation of local translation in the growth cone.
6.1 EphA forward signaling
EphA forward signaling seems to follow principles similar to those of other RTKs but rather than acting in the nucleus, the responses are activated on and near the cell surface. Ephrin-A binding leads to receptor dimerization; this in turn activates the kinase domain, which autophosphoylates on specific tyrosine residues, generating SH2-binding sites for adaptor proteins and kinases. One response to ephrin-A binding is growth cone collapse induced by the activation of Rho family GTPases that trigger depolymerization of the actin cytoskeleton [50]. A number of guanine nucleotide exchange factors (GEFs) are activated by EphA forward signaling; these then regulate Rho family GTP binding proteins to change cytoskeletal and membrane dynamics in growth cones. In retinal neurons, ephexin is a GEF that, when bound to unoccupied EphAs, causes activation of Rho, Cdc42 and Rac1, which lead to net growth cone extension. However, when EphAs are bound to ephrin-As, ephexin changes its activity such that Rho activation is higher, while Cdc42 and Rac1 activation is lowered to cause growth cone collapse [51](Figure 3). This is due to tyrosine phosphorylation of ephexin-1 in response to EphA activation, which enhances its GEF activity only towards RhoA [52]. Consistent with this model, ephexin-1 mutant RGCs do not display ephrin-A-dependent repulsion in vitro; however, no topographic mapping errors were observed in ephexin-1 knockout mice, suggesting redundancy among the five ephexin family members [52].
6.2 Regulation of translation
EphA forward signaling also interacts with the Tsc2-Rheb signaling pathway, which is involved in regulating the translation of proteins in the growth cone. Protein synthesis in the growth cone is needed for axon extension, while inhibition of protein synthesis promotes growth cone collapse. In this pathway, EphA activation leads to inhibition of ERK activity, which activates Tsc2, which in turn inactivates the GTP binding protein Rheb. mTOR, the downstream target of Rheb, becomes less active and leads to a reduction in protein synthesis in axons, thus leading to growth cone collapse [53] (Figure 3). Importantly, mutations in Tsc2 cause RGC mapping defects in mice that are similar to those found in ephrin-A mutants. This provides in vivo evidence for the Tsc-Rheb signaling pathway being involved in ephrin-mediated axon guidance [53].
6.3 Terminating cell-cell contact to mediate repulsion
A paradox in the ephrin-A/EphA repulsion model arises when considering that cell-cell contact is required for signaling to occur, but that contact must be terminated in order for the EphA-bearing cell to be repelled from the ephrin-A bearing cell. Two mechanisms have been shown to achieve this: cell-cell contacts can be terminated by cleavage of the extracellular domain of either protein, and receptor-ligand complexes can be endocytosed into either cell. Experiments in transfected cells showed that the metalloproteinase Kuzbanian/ADAM10 forms a complex with ephrin-A2 and cleaves it in a juxtamembrane domain in response to treatment with clustered EphA3. If Kuzbanian activation is prevented, growth cones become stuck on the ephrin-A bearing cell [54] (Figure 3). Other studies have shown that ADAM10 forms a complex with EphA3 upon ligand binding and cleaves the ephrin-A from the axon, resulting in internalization of ephrin-A into the EphA-bearing cell [55].
An alternative cell-cell contact termination mechanism involves the endocytosis of the receptor-ligand complex without cleavage. Recent studies have implicated this process as necessary for topographic mapping. The fact that Eph/ephrin complexes can be endocytosed first came from studies that demonstrated in tissue culture cells, EphB/ephrin-B complexes can be endocytosed in both cis and trans, i.e. into the EphB or ephrin-B bearing cell, respectively [56,57]. Endocytosis leads to cell-cell retraction in tissue culture cells and is mediated via the small GTPase Rac, which also regulates cytoskeletal dynamics [56]. This result is consistent with data demonstrating a requirement for the Rac GEF, Vav, in EphA and EphB endocytosis [58] (Figure 3). Interestingly, Vav-deficient mice show errors in eye-specific segregation in the dorsal lateral geniculate nucleus, suggesting an in vivo role for endocytosis in axon guidance [58]. Clear evidence for endocytosis in topographic mapping comes from recent work examining BAC transgenic mice expressing an endocytosis-deficient version of EphA8, which prevents axon repulsion of RGC axons[59,60]. In these mice, focal DiI tracing in nasal retina shows that preventing endocytosis results in ectopic termination zones anterior to their topographically appropriate location, similar to the phenotype observed in ephrin-A and EphA mutant mice [60]. A similar mapping defect was seen in mice expressing a dominant-negative form of Rac, showing that Rac is needed to execute endocytosis and retraction.
7. Mapping the medial-lateral axis of the SC
An important result from the gain and loss of function experiments that manipulate EphA and ephrin-As is that the topographic mapping defects are predominantly restricted to the N-T mapping axis, leaving the D-V mapping axis intact; this strongly indicates that each axis uses independent mechanisms for mapping. Although details are not well understood, a combination of pre-target sorting and graded signaling by EphB/ephrin-Bs and Wnts are used to establish topography along the M-L axis.
7.1 Pre-target sorting
As RGC axons exit the optic disc, they fasciculate to form the optic nerve and defasiculate prior to entering the SC. Experiments in which dorsal or ventral retina is labeled with DiI reveal that D-V order is already established prior to axons entering the SC [61,62]. Mutations that affect D-V patterning in the retina also affect D-V sorting and D-V mapping in the SC, suggesting that optic tract sorting is important for topographic mapping [63]. While the molecular mechanisms regulating RGC axon sorting are unknown, similar tract organization in the olfactory system has recently been shown to rely on Sema3a and its receptor, neuropilin-1 [64]. Interestingly, neuropilin-1 is expressed by RGCs, and RGC growth cones are responsive to Sema3a in in vitro assays [65–67].
7.2 EphB/ephrin-B signaling in topographic mapping
Though RGCs enter the SC with some degree of topographic order along the M-L axis, it is clear that RGC axons also refine their D-V position within the SC by interstitial branching off of their primary axon toward their appropriate D-V position. These branches can form either medial or lateral from the primary axon, suggesting that the exact entrance location of the axon into the SC is not important. Based on expression patterns and functional analysis, ephrin-B/EphB molecules are candidates for D-V mapping labels.
Like EphAs and ephrin-As, EphBs and ephrin-Bs are expressed in counter-gradients along the D-V axis of the retina. Ephrin-Bs are expressed in a high dorsal to low ventral gradient, while EphBs are expressed in the opposite,high ventral to low dorsal gradient [25,68–70]. However, the expression patterns of EphBs and ephrin-Bs are not as obviously graded in the medial-lateral axis of the SC. In the mouse SC, mRNA in situ hybridization reveals ephrin-B1 in a high medial to low lateral gradient restricted to the ventricular zone of the SC [71], far from the superficial retinorecipient layers and in different cells than those that express ephrin-As. A similar pattern of expression exists in the chick OT, where ephrin-B1 protein expression is associated with radial glia that span the depth of the OT [70], suggesting expression on these cells could guide incoming retinal axons. However, recent experiments in frog did not detect significant ephrin-B expression in the OT, suggesting that the role of ephrin-Bs in the SC in not conserved in vertebrates [20,21]. EphBs are widely expressed in the vertebrate tectum, but not in an obvious medial-lateral gradient [20].
Evidence supporting a requirement for ephrin-B/EphB signaling in D-V mapping comes from in vivo studies in which ephrin-Bs are over-expressed or EphBs have been removed. In EphB2/B3 double knockout mice, DiI tracing of ventral RGCs reveals ectopic termination zones in addition to a largely correct termination [71]. Interestingly, the ectopic termination zones were always lateral to the topographically appropriate location, supporting a model in which ephrin-Bs expressed high medially act as attractants for ventral RGCs that express EphB receptors. Because a kinase dead version of EphB2 made similar topographic mapping errors [71], it seems that forward rather than reverse signaling is used for D-V mapping. On the other hand, experiments done in frogs have led to models whereby attractive ephrin-B reverse signaling is used in D-V mapping. This is supported by data that show that dorsal RGC axons prefer EphB1 coated surfaces in vitro, and ectopic expression of a dominant-negative ephrin-B2 in dorsal retina causes D-V mapping errors [68].
Like ephrin-As, ephrin-Bs can also act as both attractants and repellents. When ephrin-B1 is ectopically expressed in the chick OT, RGC axons fail to form termination zones in these patches, suggesting that ephrin-B1 acts as a repellent. However, interstitial branches formed within an ephrin-B1 patch were consistently oriented laterally, regardless of the position of the axon branch relative to the future termination zone [72]. These data were interpreted to indicate that ephrin-B1 acts bifunctionally to direct interstitial branches depending on the relative concentration level. However, the molecular mechanisms by which this bifunctionality is manifested remain to be determined.
8. Ephrin-B/EphB signaling in synaptic development and plasticity
Another role for ephrin-B/EphB signaling in the developing nervous system is in the maturation of synapes [73]. Recently, a role for ephrin-B reverse signaling in retinotectal synaptic maturation was established. In these experiments, the authors infused clustered EphB2-Fc fusion proteins into the ventricle and found increased synaptobrevin-positive puncta, as well as increased mEPSP frequency and amplitude in the OT [74]. These data suggest an important role specific to reverse signaling, as infusion of ephrin-B1-Fc had no effect on any of these synaptic parameters [74]. This group went on to show an important role for ephrin-B signaling in regulating visual receptive field development and plasticity [75]. Using a conditioning stimulus known to result in receptive field shifts, the authors found that plasticity was enhanced in the dorsal and ventral OT compared to cells in the central regions. Expression of mutated ephrin-B1 in RGCs inhibited this enhanced plasticity, while overexpression of wild type ephrin-B1 increased receptive field shifts [75]. The authors also implicate Wnt signaling in this process, a molecular pathway previously implicated in map development along the M-L axis [76]. Together, these data suggest that a primary role for ephrin-Bs in retinocollicular map development may be to ensure precise synaptic connectivity by modulating activity-dependent plasticity.
9. Concluding remarks
The retina’s projection to the SC/OT has served as a model to understand the mechanisms used to establish ordered neuronal connections. The Eph family of RTKs and their binding partners, ephrins, has been shown to play a crucial role in topographic mapping of this and many other brain projections [77–79][80]. Despite this, several aspects of both retinotopic mapping and the function of Eph/ephrins in this context remain unknown. For instance, determining the mechanisms by which ephrin-A reverse signaling is transmitted across the membrane to cytoskeletal machinery is a necessary step towards understanding how the posterior portion of the SC/OT is organized. In addition, the molecular cues that regulate pre-target sorting along the D-V/M-L axis within the optic tract are completely unknown. Finally, how a molecular gradient is detected with such detailed precision remains a mystery. Future studies taking advantage of the power of transgenic animals, single axon tracing, high-resolution microscopy and in vitro techniques will answer these questions.
Highlights.
Topographic maps are used in the visual system to relay spatial information of the visual world.
Eph and ephrins are cell surface signaling proteins expressed in gradients along each axis of the visual field
EphAs and ephrin-As are required for mapping of the nasal-temporal visual field
The dorsal-ventral axis of the visual field is mapped using pre-target sorting and EphB/ephrin-B signaling
Eph/ephrin signaling leads to changes in actin stabilization in the axon and endocytosis of receptor/ligand complexes
Acknowledgements
We would like to thank Jena Yamada for editing the manuscript and the NIH (R01-EYO14689 to DAF and F32-EY18531 to JWT) for funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lewin B. On neuronal specificity and the molecular basis of perception. Cell. 1994;79:935–943. doi: 10.1016/0092-8674(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 2.Udin SB, Fawcett JW. Formation of topographic maps. Annu Rev Neurosci. 1988;11:289–327. doi: 10.1146/annurev.ne.11.030188.001445. [DOI] [PubMed] [Google Scholar]
- 3.Feldheim DA, O'Leary DDM. Visual map development: bidirectional signaling, bifunctional guidance molecules, and competition. Cold Spring Harbor Persp Biol. 2010 doi: 10.1101/cshperspect.a001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasquale E. Eph-Ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Mellott DO, Burke RD. The molecular phylogeny of eph receptors and ephrin ligands. BMC Cell Biol. 2008;9:27. doi: 10.1186/1471-2121-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Himanen J-P, Nikolov DB. Eph signaling: a structural view. Trends Neurosci. 2003;26:46–51. doi: 10.1016/s0166-2236(02)00005-x. [DOI] [PubMed] [Google Scholar]
- 7.Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, Pawson T, Davis S, Yancopoulos GD. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- 8.Himanen J-P, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- 9.Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17:230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- 11.Sperry RW. Effect of 180 degree rotation of the retinal field on visuomotor coordination. J Exp Zool. 1943;92:263–279. [Google Scholar]
- 12.Attardi DG, Sperry RW. Preferential selection of central pathways by regenerating optic fibers. Exp Neurol. 1963;7:46–64. doi: 10.1016/0014-4886(63)90093-1. [DOI] [PubMed] [Google Scholar]
- 13.Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns. Proc Natl Acad Sci USA. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter J, Kern-Veits B, Huf J, Stolze B, Bonhoeffer F. Recognition of position-specific properties of tectal cell membranes by retinal axons in vitro. Development. 1987;101:685–696. doi: 10.1242/dev.101.4.685. [DOI] [PubMed] [Google Scholar]
- 15.Walter J, Henke-Fahle S, Bonhoeffer F. Avoidance of posterior tectal membranes by temporal retinal axons. Development. 1987;101:909–913. doi: 10.1242/dev.101.4.909. [DOI] [PubMed] [Google Scholar]
- 16.Walter J, Müller B, Bonhoeffer F. Axonal guidance by an avoidance mechanism. J Physiol (Paris) 1990;84:104–110. [PubMed] [Google Scholar]
- 17.Cheng HJ, Flanagan JG. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell. 1994;79:157–168. doi: 10.1016/0092-8674(94)90408-1. [DOI] [PubMed] [Google Scholar]
- 18.Cheng HJ, Nakamoto M, Bergemann AD, Flanagan JG. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell. 1995;82:371–381. doi: 10.1016/0092-8674(95)90426-3. [DOI] [PubMed] [Google Scholar]
- 19.Drescher U, Kremoser C, Handwerker C, Löschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- 20.Scalia F, Currie JR, Feldheim DA. Eph/ephrin gradients in the retinotectal system of Rana pipiens: developmental and adult expression patterns. J. Comp. Neurol. 2009;514:30–48. doi: 10.1002/cne.21968. [DOI] [PubMed] [Google Scholar]
- 21.Higenell V, Han SM, Feldheim DA, Scalia F, Ruthazer ES. Expression patterns of Ephs and ephrins throughout retinotectal development in Xenopus laevis. Devel Neurobio. 2011 doi: 10.1002/dneu.20930. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connor RJ, Menzel P, Pasquale EB. Expression and tyrosine phosphorylation of Eph receptors suggest multiple mechanisms in patterning of the visual system. Dev Biol. 1998;193:21–35. doi: 10.1006/dbio.1997.8786. [DOI] [PubMed] [Google Scholar]
- 23.Frisén J, Yates PA, McLaughlin T, Friedman GC, O'Leary DD, Barbacid M. Ephrin-A5 (AL-1/RAGS) is essential for proper retinal axon guidance and topographic mapping in the mammalian visual system. Neuron. 1998;20:235–243. doi: 10.1016/s0896-6273(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 24.Feldheim DA, Vanderhaeghen P, Hansen MJ, Frisén J, Lu Q, Barbacid M, Flanagan JG. Topographic guidance labels in a sensory projection to the forebrain. Neuron. 1998;21:1303–1313. doi: 10.1016/s0896-6273(00)80650-9. [DOI] [PubMed] [Google Scholar]
- 25.Marcus RC, Gale NW, Morrison ME, Mason CA, Yancopoulos GD. Eph family receptors and their ligands distribute in opposing gradients in the developing mouse retina. Dev Biol. 1996;180:786–789. doi: 10.1006/dbio.1996.0347. [DOI] [PubMed] [Google Scholar]
- 26.Lambot M-A, Depasse F, Noel J-C, Vanderhaeghen P. Mapping labels in the human developing visual system and the evolution of binocular vision. J Neurosci. 2005;25:7232–7237. doi: 10.1523/JNEUROSCI.0802-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamoto M, Cheng HJ, Friedman GC, Mclaughlin T, Hansen MJ, Yoon CH, O'Leary DD, Flanagan JG. Topographically specific effects of ELF-1 on retinal axon guidance in vitro and retinal axon mapping in vivo. Cell. 1996;86:755–766. doi: 10.1016/s0092-8674(00)80150-6. [DOI] [PubMed] [Google Scholar]
- 28.Feldheim DA, Kim YI, Bergemann AD, Frisén J, Barbacid M, Flanagan JG. Genetic analysis of ephrin-A2 and ephrin-A5 shows their requirement in multiple aspects of retinocollicular mapping. Neuron. 2000;25:563–574. doi: 10.1016/s0896-6273(00)81060-0. [DOI] [PubMed] [Google Scholar]
- 29.Pfeiffenberger C, Yamada J, Feldheim DA. Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system. J Neurosci. 2006;26:12873–12884. doi: 10.1523/JNEUROSCI.3595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cang J, Wang L, Stryker MP, Feldheim DA. Roles of ephrin-as and structured activity in the development of functional maps in the superior colliculus. J Neurosci. 2008;28:11015–11023. doi: 10.1523/JNEUROSCI.2478-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldheim DA, Nakamoto M, Osterfield M, Gale NW, DeChiara TM, Rohatgi R, Yancopoulos GD, Flanagan JG. Loss-of-function analysis of EphA receptors in retinotectal mapping. J Neurosci. 2004;24:2542–2550. doi: 10.1523/JNEUROSCI.0239-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carreres MI, Escalante A, Murillo B, Chauvin G, Gaspar P, Vegar C, Herrera E. Transcription factor foxd1 is required for the specification of the temporal retina in mammals. J Neurosci. 2011;31:5673–5681. doi: 10.1523/JNEUROSCI.0394-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown A, Yates PA, Burrola P, Ortuño D, Vaidya A, Jessell TM, Pfaff SL, O'Leary DD, Lemke G. Topographic mapping from the retina to the midbrain is controlled by relative but not absolute levels of EphA receptor signaling. Cell. 2000;102:77–88. doi: 10.1016/s0092-8674(00)00012-x. [DOI] [PubMed] [Google Scholar]
- 34.Triplett JW, Owens MT, Yamada J, Lemke G, Cang J, Stryker MP, Feldheim DA. Retinal input instructs alignment of visual topographic maps. Cell. 2009;139:175–185. doi: 10.1016/j.cell.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reber M, Burrola P, Lemke G. A relative signalling model for the formation of a topographic neural map. Nature. 2004;431:847–853. doi: 10.1038/nature02957. [DOI] [PubMed] [Google Scholar]
- 36.Monschau B, Kremoser C, Ohta K, Tanaka H, Kaneko T, Yamada T, Handwerker C, Hornberger MR, Löschinger J, Pasquale EB, Siever DA, Verderame MF, Müller BK, Bonhoeffer F, Drescher U. Shared and distinct functions of RAGS and ELF-1 in guiding retinal axons. EMBO J. 1997;16:1258–1267. doi: 10.1093/emboj/16.6.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rashid T, Upton AL, Blentic A, Ciossek T, Knöll B, Thompson ID, Drescher U. Opposing gradients of ephrin-As and EphA7 in the superior colliculus are essential for topographic mapping in the mammalian visual system. Neuron. 2005;47:57–69. doi: 10.1016/j.neuron.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 38.Lim Y-S, Mclaughlin T, Sung T-C, Santiago A, Lee K-F, O'Leary DDM. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59:746–758. doi: 10.1016/j.neuron.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marler KJM, Becker-Barroso E, Martinez A, Llovera M, Wentzel C, Poopalasundaram S, Hindges R, Soriano E, Comella J, Drescher U. A TrkB/EphrinA interaction controls retinal axon branching and synaptogenesis. J Neurosci. 2008;28:12700–12712. doi: 10.1523/JNEUROSCI.1915-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marler KJM, Poopalasundaram S, Broom ER, Wentzel C, Drescher U. Pro-neurotrophins secreted from retinal ganglion cell axons are necessary for ephrinA-p75NTR-mediated axon guidance. Neural Dev. 2010;5:30. doi: 10.1186/1749-8104-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen MJ, Dallal GE, Flanagan JG. Retinal axon response to ephrin-as shows a graded, concentration-dependent transition from growth promotion to inhibition. Neuron. 2004;42:717–730. doi: 10.1016/j.neuron.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Finlay BL, Schneps SE, Schneider GE. Orderly compression of the retinotectal projection following partial tectal ablation in the newborn hamster. Nature. 1979;280:153–155. doi: 10.1038/280153a0. [DOI] [PubMed] [Google Scholar]
- 43.Fraser SE, Hunt RK. Retinotectal specificity: models and experiments in search of a mapping function. Annu Rev Neurosci. 1980;3:319–352. doi: 10.1146/annurev.ne.03.030180.001535. [DOI] [PubMed] [Google Scholar]
- 44.Fraser SE, Perkel DH. Competitive and positional cues in the patterning of nerve connections. J Neurobiol. 1990;21:51–72. doi: 10.1002/neu.480210105. [DOI] [PubMed] [Google Scholar]
- 45.Simon DK, Roskies AL, O'Leary DD. Plasticity in the development of topographic order in the mammalian retinocollicular projection. Dev Biol. 1994;162:384–393. doi: 10.1006/dbio.1994.1095. [DOI] [PubMed] [Google Scholar]
- 46.Gosse N, Nevin L, Baier H. Retinotopic order in the absence of axon competition. Nature. 2008 doi: 10.1038/nature06816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin Y, Yamashita Y, Noda H, Okafuji T, Go MJ, Tanaka H. EphA receptor tyrosine kinases interact with co-expressed ephrin-A ligands in cis. Neurosci Res. 2004;48:285–296. doi: 10.1016/j.neures.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 48.Carvalho RF, Beutler M, Marler KJM, Knöll B, Becker-Barroso E, Heintzmann R, Ng T, Drescher U. Silencing of EphA3 through a cis interaction with ephrinA5. Nat Neurosci. 2006;9:322–330. doi: 10.1038/nn1655. [DOI] [PubMed] [Google Scholar]
- 49.Hornberger MR, Dütting D, Ciossek T, Yamada T, Handwerker C, Lang S, Weth F, Huf J, Wessel R, Logan C, Tanaka H, Drescher U. Modulation of EphA receptor function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron. 1999;22:731–742. doi: 10.1016/s0896-6273(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 50.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 51.Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, Bazalakova M, Neve RL, Corfas G, Debant A, Greenberg ME. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- 52.Sahin M, Greer PL, Lin MZ, Poucher H, Eberhart J, Schmidt S, Wright TM, Shamah SM, O'connell S, Cowan CW, Hu L, Goldberg JL, Debant A, Corfas G, Krull CE, Greenberg ME. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46:191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 53.Nie D, Nardo AD, Han JM, Baharanyi H, Kramvis I, Huynh T, Dabora S, Codeluppi S, Pandolfi PP, Pasquale EB, Sahin M. Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nat Neurosci. 2010;13:163–172. doi: 10.1038/nn.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hattori M, Osterfield M, Flanagan JG. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289:1360–1365. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- 55.Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, Nievergall E, Blobel CP, Himanen J-P, Lackmann M, Nikolov DB. Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell. 2005;123:291–304. doi: 10.1016/j.cell.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 56.Marston DJ, Dickinson S, Nobes CD. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat Cell Biol. 2003;5:879–888. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- 57.Zimmer M, Palmer A, Köhler J, Klein R. EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol. 2003;5:869–878. doi: 10.1038/ncb1045. [DOI] [PubMed] [Google Scholar]
- 58.Cowan CW, Shao YR, Sahin M, Shamah SM, Lin MZ, Greer PL, Gao S, Griffith EC, Brugge JS, Greenberg ME. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron. 2005;46:205–217. doi: 10.1016/j.neuron.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 59.Yoo S, Shin J, Park S. EphA8-ephrinA5 signaling and clathrin-mediated endocytosis is regulated by Tiam-1, a Rac-specific guanine nucleotide exchange factor. Mol Cells. 2010;29:603–609. doi: 10.1007/s10059-010-0075-2. [DOI] [PubMed] [Google Scholar]
- 60.Yoo S, Kim Y, Noh H, Lee H, Park E, Park S. Endocytosis of EphA receptors is essential for the proper development of the retinocollicular topographic map. EMBO J. 2011;30:1593–1607. doi: 10.1038/emboj.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simon DK, O'Leary DD. Relationship of retinotopic ordering of axons in the optic pathway to the formation of visual maps in central targets. J Comp Neurol. 1991;307:393–404. doi: 10.1002/cne.903070305. [DOI] [PubMed] [Google Scholar]
- 62.Plas DT, Lopez JE, Crair MC. Pretarget sorting of retinocollicular axons in the mouse. J Comp Neurol. 2005;491:305–319. doi: 10.1002/cne.20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plas DT, Dhande OS, Lopez JE, Murali D, Thaller C, Henkemeyer M, Furuta Y, Overbeek P, Crair MC. Bone morphogenetic proteins, eye patterning, and retinocollicular map formation in the mouse. J Neurosci. 2008;28:7057–7067. doi: 10.1523/JNEUROSCI.3598-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Imai T, Yamazaki T, Kobayakawa R, Kobayakawa K, Abe T, Suzuki M, Sakano H. Pre-target axon sorting establishes the neural map topography. Science. 2009;325:585–590. doi: 10.1126/science.1173596. [DOI] [PubMed] [Google Scholar]
- 65.Kawakami A, Kitsukawa T, Takagi S, Fujisawa H. Developmentally regulated expression of a cell surface protein, neuropilin, in the mouse nervous system. J Neurobiol. 1996;29:1–17. doi: 10.1002/(SICI)1097-4695(199601)29:1<1::AID-NEU1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 66.Claudepierre T, Koncina E, Pfrieger F, Bagnard D, Aunis D, Reber M. Implication of neuropilin 2/semaphorin 3F in retinocollicular map formation. Dev Dyn. 2008;237:3394–3403. doi: 10.1002/dvdy.21759. [DOI] [PubMed] [Google Scholar]
- 67.Campbell DS, Regan AG, Lopez JS, Tannahill D, Harris WA, Holt CE. Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones. J Neurosci. 2001;21:8538–8547. doi: 10.1523/JNEUROSCI.21-21-08538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mann F, Ray S, Harris W, Holt C. Topographic mapping in dorsoventral axis of the Xenopus retinotectal system depends on signaling through ephrin-B ligands. Neuron. 2002;35:461–473. doi: 10.1016/s0896-6273(02)00786-9. [DOI] [PubMed] [Google Scholar]
- 69.Holash JA, Soans C, Chong LD, Shao H, Dixit VM, Pasquale EB. Reciprocal expression of the Eph receptor Cek5 and its ligand(s) in the early retina. Dev Biol. 1997;182:256–269. doi: 10.1006/dbio.1996.8496. [DOI] [PubMed] [Google Scholar]
- 70.Braisted JE, McLaughlin T, Wang HU, Friedman GC, Anderson DJ, O'Leary DD. Graded and lamina-specific distributions of ligands of EphB receptor tyrosine kinases in the developing retinotectal system. Dev Biol. 1997;191:14–28. doi: 10.1006/dbio.1997.8706. [DOI] [PubMed] [Google Scholar]
- 71.Hindges R, Mclaughlin T, Genoud N, Henkemeyer M, O'Leary DDM. EphB forward signaling controls directional branch extension and arborization required for dorsal-ventral retinotopic mapping. Neuron. 2002;35:475–487. doi: 10.1016/s0896-6273(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 72.Mclaughlin T, Hindges R, Yates PA, O'Leary DDM. Bifunctional action of ephrin-B1 as a repellent and attractant to control bidirectional branch extension in dorsal-ventral retinotopic mapping. Development. 2003;130:2407–2418. doi: 10.1242/dev.00467. [DOI] [PubMed] [Google Scholar]
- 73.Lai K, Ip N. Synapse development and plasticity: roles of ephrin/Eph receptor signaling. Curr Op Neurobiol. 2009 doi: 10.1016/j.conb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 74.Lim BK, Matsuda N, Poo M-M. Ephrin-B reverse signaling promotes structural and functional synaptic maturation in vivo. Nat Neurosci. 2008;11:160–169. doi: 10.1038/nn2033. [DOI] [PubMed] [Google Scholar]
- 75.Lim BK, Cho S-J, Sumbre G, Poo M-M. Region-specific contribution of ephrin-B and Wnt signaling to receptive field plasticity in developing optic tectum. Neuron. 2010;65:899–911. doi: 10.1016/j.neuron.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 76.Schmitt AM, Shi J, Wolf AM, Lu C-C, King LA, Zou Y. Wnt–Ryk signalling mediates medial–lateral retinotectal topographic mapping. Nature. 2005:7. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- 77.Vanderhaeghen P, Lu Q, Prakash N, Frisén J, Walsh CA, Frostig RD, Flanagan JG. A mapping label required for normal scale of body representation in the cortex. Nat Neurosci. 2000;3:358–365. doi: 10.1038/73929. [DOI] [PubMed] [Google Scholar]
- 78.Torii M, Levitt P. Dissociation of corticothalamic and thalamocortical axon targeting by an EphA7-mediated mechanism. Neuron. 2005;48:563–575. doi: 10.1016/j.neuron.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 79.Galimberti I, Bednarek E, Donato F, Caroni P. EphA4 signaling in juveniles establishes topographic specificity of structural plasticity in the hippocampus. Neuron. 2010;65:627–642. doi: 10.1016/j.neuron.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 80.Cang J, Kaneko M, Yamada J, Woods G, Stryker MP, Feldheim DA. Ephrin-as guide the formation of functional maps in the visual cortex. Neuron. 2005;48:577–589. doi: 10.1016/j.neuron.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]