Abstract
Background
The Differentiator Model predicts that individuals with a positive family history of alcoholism or heavy alcohol consumers will feel more sensitive to the effects of alcohol on the ascending phase of the blood alcohol content while feeling less sedated on the descending phase. This study tested if subjective perceptions are sensitive to the slope of breath alcohol concentration and if that sensitivity is associated with a family history of alcoholism (FHA) and/or recent drinking history (RDH).
Methods
Family history positive (FHP, N=27) and family history negative (FHN, N=27) young adult non-dependent drinkers were infused intravenously with alcohol in 2 sessions separated by one week. After 20 minutes, one session had an ascending BrAC (+3.0mg% per min) while the other session had a descending BrAC (−1 mg% per min). The BrAC for both sessions at this point was approximately 60 mg%, referred to as the crossover point. Subjective perceptions of intoxication, high, stimulated, and sedation were sampled frequently, then interpolated to the crossover point. Within-subject differences between ascending and descending responses were examined for associations with FHA and/or RDH.
Results
Recent moderate drinkers reported increased perceptions of feeling intoxicated (p<0.023) and high (p<0.023) on the ascending slope compared to the descending slope. In contrast, recent light drinkers felt more intoxicated and high on the descending slope.
Conclusion
Subjective perceptions in young adult social drinkers depend on the slope of the BrAC when examined in association with RDH. These results support the Differentiator Model hypothesis concerning the ascending slope and suggest that moderate alcohol consumers could be at risk for increased alcohol consumption because they feel more intoxicated and high on the ascending slope. Subjects did not feel less sedated on the descending slope, contrary to the Differentiator Model but replicating several previous studies.
Keywords: subjective perceptions, slope of brain exposure to alcohol, family history of alcoholism (FHA), recent drinking history (RDH)
Introduction
Alcohol abuse is the second most common lifetime disorder, with a prevalence of about 13% (Hasin et al., 2007; Kessler, 2005), and alcohol dependence affects 4–5% of the United States population at any given time (Li et al., 2007). Family, twin, and adoption studies have shown a substantial genetic component to the risk of alcoholism (Bornovalova et al., 2010; Heath et al., 1997; Kendler et al., 1994; McGue, 1999), with heritability estimates of 50–60% (Bienvenu et al., 2011; Reed et al., 1996). While genes have been identified which increase the risk for alcohol dependence, they individually predict only a small fraction of the risk for this disorder. Therefore, when evaluating those at greatest risk for alcohol dependence, it is still most efficient to identify those with a family history of alcoholism and heavy recent drinking rather than screen on a particular genetic variant.
Family history of alcoholism (FHA) has been used as a proxy for genetic variability, under the assumption that individuals with a positive family history (FHP) carry more of the genes predisposing to an increased risk for developing alcohol dependence as compared to those who are family history negative (FHN) (Morean and Corbin, 2010). FHP status increases the likelihood for future alcoholism in young adults, and sensitivity to the rate of change of exposure (ascending vs descending) could compound that risk by reinforcing increased alcohol consumption. One hypothesis posited by Newlin & Thomson (Newlin and Thomson, 1990) as the Differentiator Model, proposed that individuals who were FHP would be more sensitive to the effects of alcohol on the ascending phase of the blood alcohol content (BAC), while the sedating effects of alcohol during the descending phase of the BAC would be attenuated, increasing the potential for alcohol abuse. A recent review of this literature has shown that most studies support the notion that individuals with a family history of alcohol dependence or heavy recent drinkers demonstrate increased sensitivity to alcohol on the ascending limb but not attenuated sedation on the descending limb (Morean and Corbin, 2010), which only partially supports the prediction of the Differentiator Model.
More recently, studies have extended the Differentiator Model to address the postulate that patterns of personal recent drinking history (RDH), without regard to FHA, also contribute to the risk of alcohol abuse. For example, individuals who need to consume more alcohol in order to feel the same subjective effects as their lightly-consuming counterparts may do so because of tolerance to the effects of alcohol (Morean and Corbin, 2010; Schuckit et al., 2000; Trim et al., 2009). These findings indicate that moderate to heavy non-dependent drinkers experience greater stimulant-like effects during the ascending limb as compared with individuals who have lower recent alcohol consumption. In addition, the moderate and heavy drinkers also have fewer sedative-like effects on the descending limb (Holdstock et al., 2000; King et al., 2002). In a study of a different type of drinking pattern, binge drinkers were found to exhibit greater intoxication on the ascending limb as compared with non-binge drinkers. The binge drinkers also demonstrated tolerance to the subjective effect of intoxication on the descending limb (Marczinski and Fillmore, 2009). In a recent prospective study, binge drinkers experienced greater stimulant and rewarding (liking and wanting) responses and lower sedative responses compared to light drinkers, most marked on the ascending limb, (King et al., 2011). A recent meta-analysis demonstrated that while heavy drinkers respond with slightly less sedation on the descending limb of the BrAC, they experience more stimulation on the ascending limb, relative to light drinkers (Quinn and Fromme, 2011).
In this study, we examined the role of FHA and RDH on the subjective perceptions of the effects of alcohol. Unlike previous studies that explored the effect of either FHA or RDH and used a traditional oral alcohol challenge, we have utilized the intravenous alcohol infusion method. There are several advantages to alcohol administration by infusion, including the ability to minimize variance in the time course of the breath alcohol concentration (BrAC) across subjects (Marczinski and Fillmore, 2009; O’Connor et al., 1998; Ramchandani et al., 1999) The method has been shown to be useful in examining subjective perceptions (Morzorati et al., 2002) as well as eye movements (Blekher et al., 2002) and alcohol elimination rate (Ramchandani and O’Connor, 2006).
The alcohol infusion method has the unique ability to control the ascending and descending slopes of the BrAC as an experimental variable. The current study employed this advantage by using a within-subject design. Individuals were infused such that in one session, their BrAC was increasing and after 20 minutes was approximately 60mg%, while in the alternate session their BrAC was decreasing, and after 20 minutes was also approximately 60mg%. This point at which the BrAC is the same in both sessions, after the same amount of time, yet is ascending in one session and descending in the alternate session, is termed the “crossover point.” More broadly, this protocol allows us to investigate if the effects of alcohol are associated with FHA and/or RDH by assessing subjective perceptions at the same target BrAC, after the same interval of exposure, on both the ascending and descending limbs. It should be noted that this protocol can only test if there is association between these factors and subjective feelings, and that no conclusions can be made regarding a causal relationship between either FHA or RDH and subjective perceptions. We hypothesized that subjects who are FHP or have a moderate to heavy RDH will experience increased subjective perceptions of intoxication on the ascending limb and less sedation on the descending limb compared to FHN or light drinkers. Changes in subjective perceptions can be attributed to the direction of the slope, since the data were assessed at the same target BrAC during both phases, and at the same period of time after the infusion begins.
Materials and methods
Subjects
All 263 people who responded to local advertisement (flyers, newspapers, and a community website) were screened by telephone for general eligibility. Of these, 85 completed a face-to-face interview after obtaining informed consent approved by the Indiana University School of Medicine IRB. Sixty-one healthy, young-adult (21–30 years), social drinkers were selected, balanced by gender and representing positive (FHP n=27) or negative (FHN n=31) FHA. FHA status was assessed using the family history assessment module of the Semi-Structured Assessment of the Genetics of Alcoholism (SSAGA) (Bucholz et al 1994) (Bucholz et al., 1994). FHP subjects had at least one 1st degree, and at least one or more 1st or 2nd degree relative with a lifetime diagnosis of DSM-IV alcohol dependence; FHN subjects had none.
Exclusion criteria were a biological mother affected with alcoholism prior to delivery of the subject, a clinically significant personal history of renal, hepatic, cardiovascular, pulmonary, or gastrointestinal disease, any current DSM-IV Axis I illness including alcohol dependence, a history of seizures or loss of consciousness, mental illness requiring hospitalization, or current use or abuse of psychoactive medications besides alcohol, nicotine or caffeine (such as cocaine, amphetamines, opiates, sedatives, benzodiazepines or hallucinogens). Past personal drinking history and drug use was assessed with the SSAGA alcohol and drug use module. RDH was assessed by using a computerized questionnaire (Time Line Follow-Back, (Sobell et al., 1988)) which estimates daily consumption using the European-standard, 12 g ethanol drinks.
Each subject participated in two experimental sessions beginning at the same time of day and on the same day of the week, at least one week apart, in randomized order. Subjects were informed only that they would receive alcohol intravenously in both experimental sessions and that their BrAC would not exceed the legal limit for driving a car in Indiana. Subjects were not informed about the time courses of BrAC or the intent of the study to examine the rate of change of alcohol exposure. Subjects received partial payment after the first infusion session, and the remainder at the end of the second session.
Experimental design and alcohol infusion method
The study was conducted using a 2-session, single-blind within-subject crossover design. In each session, a subject was exposed to one of two predetermined time courses of BrAC. In one session, the BrAC rose at twice the rate of the alternate session, until BrAC reached 68 mg%, and then began to descend such that after 20 minutes, the BrAC was descending and was at 60 mg%. The BrAC of the alternate session rose at a slower rate such that after 20 minutes, the BrAC was still rising, and was at 60 mg%. At this point (60 mg%), the elapsed time was the same in both sessions, the BrAC was the same in both sessions, but the BrAC was ascending in one session and descending in the other. This point is called the crossover point, depicted in Figure 1. For purposes of potential replication, the technical description of the protocol is as follows. In one session, the BrAC rose at a steady rate of +3.0 mg%/min. In the other session, the BrAC rose at twice that rate, +6.0 mg%/min, until it reached a value of 68 mg%, nominally 11.8 min later, then descended steadily at −1.0 mg%/min. These two time courses of BrAC, when plotted as a function of time beginning at the start of alcohol infusion, intersect at a point, nominally at 60 mg%, 20 min after the infusions begin. At that point, called “the crossover point”, the BrAC and the time of exposure are identical, and only the slopes differ (+3.0 mg%/min vs. −1.0 mg%/min). Projected and actual BrAC curves, and the crossover point, are shown in Figure 1.
Figure 1.
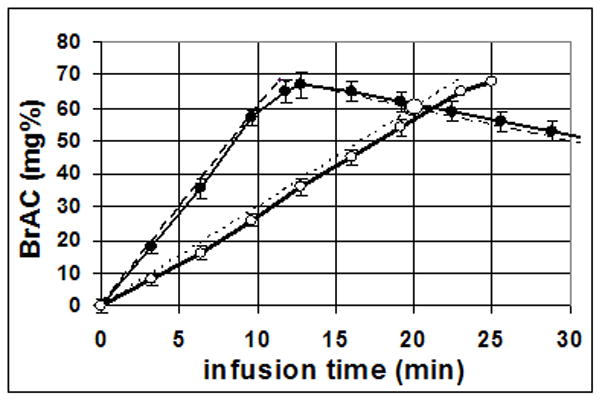
The mean ± standard deviation of BrAC measurements obtained during 108 sessions of the experiment from the 54 subjects included in subsequent analyses. Solid lines with: open circles represent actual average BrAC for time course 1 (+3.0 mg%/min); closed circles represent actual average BrAC for time course 2 (rose at +6.0 mg%/min). Dashed lines represent average predicted BrAC for the two time courses. The large open circle depicts the average interpolation of the crossover point.
Measurement of Subjective Perceptions
Subjective perceptions of the effects of alcohol exposure were assessed by six items presented in the same order, in brief blocks that were repeated approximately every 3.5 minutes throughout each experimental session. Questions about perceptions were displayed on a computer monitor. Monopolar visual analog scales were used to rate feelings of being intoxicated, high, stimulated and sedated, attributable to the current effect of alcohol. Subjects responded by placing a mouse pointer at any one of 100 positions along a horizontal scale. Upon clicking the mouse, the subjects’ responses were automatically time-stamped and logged, then the program advanced to the next question. The extremes of the scales were anchored with “not at all” and “most ever”. The words “a little”, “somewhat”, “fairly”, and “very” were evenly spaced along the scale. When a new question was displayed on the screen, an arrow indicated the position the subject had chosen on that question in the immediately preceding block. Subjects were told that high was synonymous with “a pleasant feeling of being buzzed”, intoxicated with “impaired and drunk”, sedated with “an unpleasant experience of feeling “sluggish, inactive or heavy”, and stimulated with “a pleasant experience of being energized, excited and vigorous”.
In addition, a bipolar scale asked How much do I like or dislike the effect of alcohol right now?, with “dislike a lot!” and “like a lot!” as anchors with “somewhat” and “quite a bit” progressing outward from a “neutral” center position along the horizontal scale. A final screen asked the subject to indicate how many drinks it usually takes to feel the way they did at that moment, with up and down buttons to adjust the integer number between 0 and 10. Subjects averaged 24 seconds to answer all 6 subjective perception questions each time the block was presented.
Protocol
A subject was admitted to the General Clinical Research Center at University Hospital in Indianapolis, at the same time for both sessions, beginning at either 7:30 am or 12:30 pm. Subjects were instructed to abstain from alcohol for at least 24 hours and from food and drink (except water) after midnight or 8:00 am, respectively. Subjects were not tested if they had a non-zero BrAC on arrival. The subject ate a standardized ~350 calorie meal and an indwelling catheter was inserted into a vein in the antecubital fossa of one arm. Two hours after admission, the subject was seated in a soft reclining chair in a 4′×7′ electrically-shielded, acoustically quiet chamber (Industrial Acoustics Co, New York) and prepared for testing. An elastic cap with 64 evenly spaced scalp electrodes (Easy Cap, Herrsching, Germany), was positioned on the head for measuring electroencephalographic (EEG) activity. Use of the Draeger, Model 7410, hand-held breath alcohol meter (Irving, TX) was demonstrated, and the test battery of dependent measures of brain function was practiced. At the end of each 40-min experimental session, the catheter was removed and a lunch or dinner was provided. The subject was paid and discharged when the BrAC decreased to less than 20 mg%, typically 5 hours after arrival.
All procedures from a subject’s vantage were identical for both experimental sessions and subjects were never aware of the result of BrAC readings. Since the crossover point for an individual could not be determined until both sessions were completed, dependent measures were sampled frequently to allow estimation of the values at that moment. The test battery comprised identical 3.0 min blocks of measurements that were initiated every 3.5 min, beginning 7.0 min before the infusion started, for a total of 15 acquisitions. For each session, linear interpolation of the subjective perceptions scores bracketing each individual’s crossover point was employed to obtain one single measure at the crossover point for each session. This one measure for each slope, representing the subjective perception for the same time and same BrAC was used in all analyses. In addition to evaluation of subjective perceptions, the battery included BrAC, EEG, heart rate and gaze fixation (results presented elsewhere).
Statistical Analyses
A dichotomous categorization of personal RDH was constructed based on the number of drinks consumed per drinking day using NIAAA standards for safe drinking levels: 2 drinks/drinking day for men; 1 drink/drinking day for women. Thus, subjects that exceeded this limit (based on total drinks in the last 7 days divided by the number of drinking days) were classified as moderate consumers; remaining subjects were classified as light consumers.
Differences in demographic data between FHA and RDH subjects were examined using t-tests for the quantitative variables: age, education, height, weight, age when regular drinking began and the number of drinking days in the preceding week. Chi-squared tests were used to test for differences attributable to gender, race, FHA, or RDH. For each of the subjective perceptions, the difference between the rating at crossover and baseline was computed for each individual on both ascending and descending limbs separately. A paired t-test was employed for each limb separately to verify that the average difference on each limb was significantly different from zero (p<0.05), signifying an effect of alcohol. The average of the baseline subjective perception from each of the two sessions was used in additional t-tests to test for differences between FHA and RDH at baseline for each subjective measure.
To test for associations of the slope of BrAC with FHA and RDH, the rating of subjective perception at the target BrAC on the descending slope at crossover was subtracted from the corresponding rating for the ascending slope. The difference was used as the dependent variable in a two-way analysis of covariance (ANCOVA), covarying for the individual’s average subjective perceptions at baseline for the two sessions. Gender and race were included in each model but were not significant (all p>0.11); therefore these covariates were not included in the final analyses. Separate analysis for each subjective perception included FHA and RDH as separate independent variables, along with the FHA*RDH interaction. Post-hoc t-tests were used to assess differences between subgroups.
Results
Of the 61 subjects originally enrolled in the study, 54 completed both alcohol sessions with adequate infusion performance (Figure 1). Seven subjects were eliminated because of inadequate infusion performance at crossover or complaints of nausea in one of the sessions. There were no complaints of nausea in the remaining 27 FHP (15 male) and 27 FHN (14 male) subjects. The majority of the subjects were Caucasian (n=46, 85%) in addition to 6 Black or African American (11%), and 2 Asian (4%) subjects. The distribution of Caucasians, African Americans, and Asians was similar among FHA (p=1.0) and RDH (p=0.21) groups. Both Asian subjects were included in the moderate drinking category and did not experience adverse effects due to alcohol consumption. The demographic characteristics of both family history groups and drinking groups are summarized in Table 1. There were no significant (p<0.05) differences in gender, age, education, race, height, weight, age at which the subject began to drink regularly or drinking history for the preceding week between FHA or RDH groups. There was no difference in the distribution of drinkers between the FHP (18 moderate drinkers, 9 light drinkers) and FHN (14 moderate drinkers, 13 light drinkers) groups (χ2(1)=1.23, p=0.27).
Table 1.
Demographics of the study population.
| Family History of Alcoholism | Family History of Alcoholism | Family History of Alcoholism | Recent Drinking History | Recent Drinking History | Recent Drinking History | |
|---|---|---|---|---|---|---|
| FHP | FHN | p-value | Moderate | Light | p-value | |
| Gender: # males (%) | 12 (44%) | 13 (48%) | 0.78 | 16 (50%) | 9 (41%) | 0.51 |
| Race: # Caucasian/African American/Asian | 23/3/1 | 23/3/1 | 1.00 | 28/2/2 | 18/4/0 | 0.21 |
| Age: mean years (SEM) | 24.7 (0.5) | 24.3 (0.6) | 0.64 | 24.7 (0.6) | 24.2 (0.5) | 0.53 |
| Education: mean years (SEM) | 16.0 (0.4) | 15.4 (0.4) | 0.34 | 15.9 (0.4) | 15.4 (0.5) | 0.44 |
| Height: mean cm (SEM) | 177.3 (4.3) | 174.5 (2.5) | 0.57 | 176.8 (3.9) | 174.7 (2.2) | 0.65 |
| Weight: mean kg (SEM) | 81.3 (3.7) | 76.9 (3.9) | 0.42 | 79.6 (3.7) | 78.3 (3.8) | 0.83 |
| Age at regular drinking: mean years (SEM) | 19.1 (0.4) | 19.5 (0.3) | 0.40 | 19.0 (0.3) | 19.9 (0.3) | 0.054 |
| # drinking days in past 7 days: mean days (SEM) | 2.0 (0.2) | 2.5 (0.3) | 0.22 | 2.4 (0.2) | 1.9 (0.4) | 0.30 |
SEM = standard error of the mean, kg = kilograms.
There was a significant effect of alcohol at the crossover point compared to baseline for all subjective perceptions for both trajectories (all p≤0.0003). In general, subjects liked the effects of alcohol and reported feeling intoxicated, high, stimulated and sedated on both ascending and descending BrAC slopes. In addition, subjects reported consuming an average of 2.8 drinks to feel the way they did at crossover; a reasonably accurate estimate of the number of 12 g ethanol standard drinks required to achieve a mean BrAC of 60mg%, if those drinks had been ingested quickly in a fasting state. No baseline differences attributable to FHA or RDH were detected (all p>0.60).
All means for ascending and descending BrAC slopes for FHA and RDH groups are provided in Table 2. The difference between the ascending and descending limbs was not significantly different between FHP and FHN subjects (all p>0.16), for any of the subjective perceptions. However, there were significant main effects for RDH and for interactions of FHA*RDH, described below.
Table 2.
Mean subjective perceptions for family history positive (FHP) and family history negative (FHN) subjects, and for subjects with moderate recent drinking historys (Mod RDH) and light recent drinking histories (light RDH) at 60 mg% BrAC after 20 minutes on ascending (asc) and descending (desc) BrAC slopes.
| FHP | FHP | FHN | FHN | Mod RDH | Mod RDH | Light RDH | Light RDH | |
|---|---|---|---|---|---|---|---|---|
| Asc | Desc | Asc | Desc | Asc | Desc | Asc | Desc | |
| Intoxicated | 25.1 (4.9) | 29.7 (5.9) | 35.3 (5.8) | 35.3 (5.1) | 29.1 (4.5) | 27.5 (4.7) | 31.7 (6.9) | 39.6 (6.5) |
| High | 31.6 (4.8) | 32.7 (5.9) | 32.6 (5.2) | 34.7 (5.5) | 32.2 (4.2) | 27.6 (4.4) | 31.9 (6.2) | 42.5 (7.1) |
| Stimulated | 22.1 (3.8) | 22.6 (4.6) | 25.3 (5.1) | 24.4 (4.7) | 26.3 (4.3) | 22.3 (4.0) | 19.9 (4.6) | 24.4 (5.6) |
| Sedated | 17.0 (4.3) | 16.3 (4.3) | 25.0 (5.20) | 21.5 (4.2) | 20.3 (3.8) | 14.9 (3.1) | 22.1 (6.2) | 24.8 (5.6) |
Table entries are mean values, numbers in parentheses are standard errors of the mean.
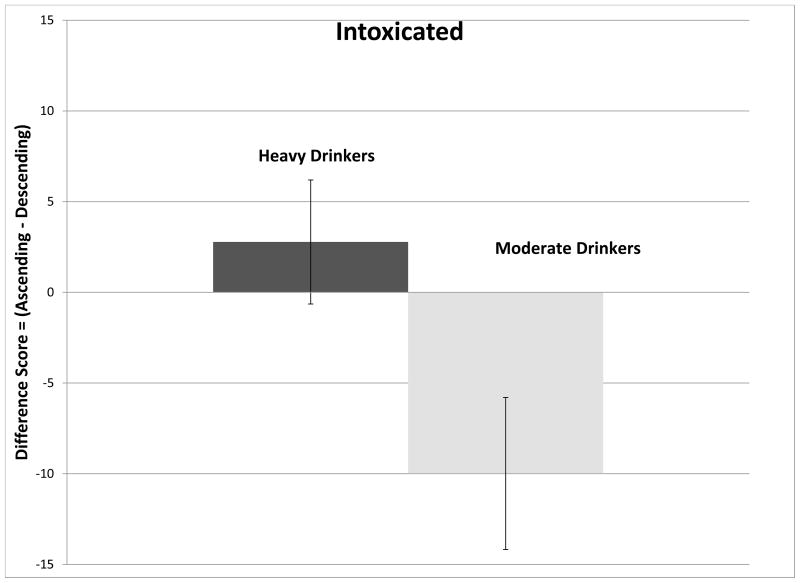
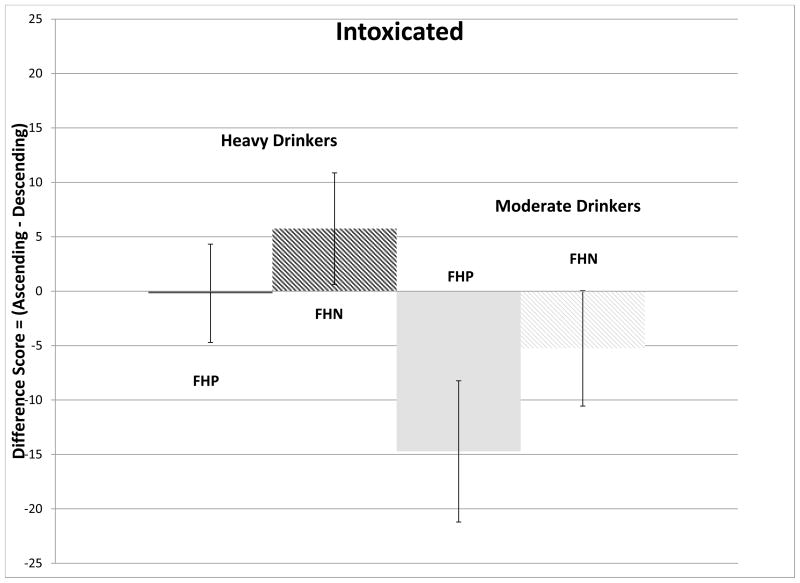
The ANCOVA model for testing differences between ratings at crossover yielded a significant overall F-ratio for the subjective perception of intoxicated (F(4,49)=2.64, p=0.04), primarily evidenced by a main effect of RDH (F(1,49)=5.49, p=0.023). Figure 2A demonstrates that moderate drinkers felt more intoxicated on the ascending slope while light drinkers felt more intoxicated on the descending slope. A post-hoc t-test revealed that within FHP subjects, individuals who were light drinkers felt somewhat more intoxicated on the descending slope compared to moderate drinkers (p=0.07, Figure 2B).
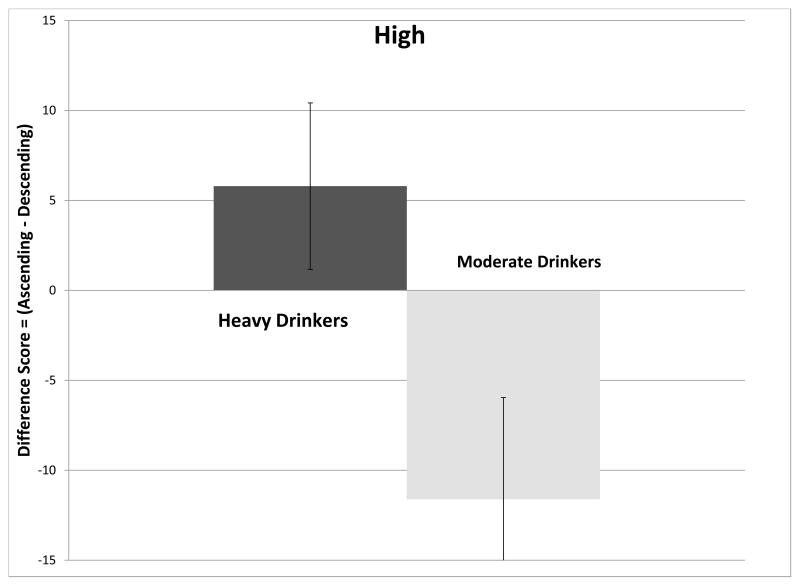
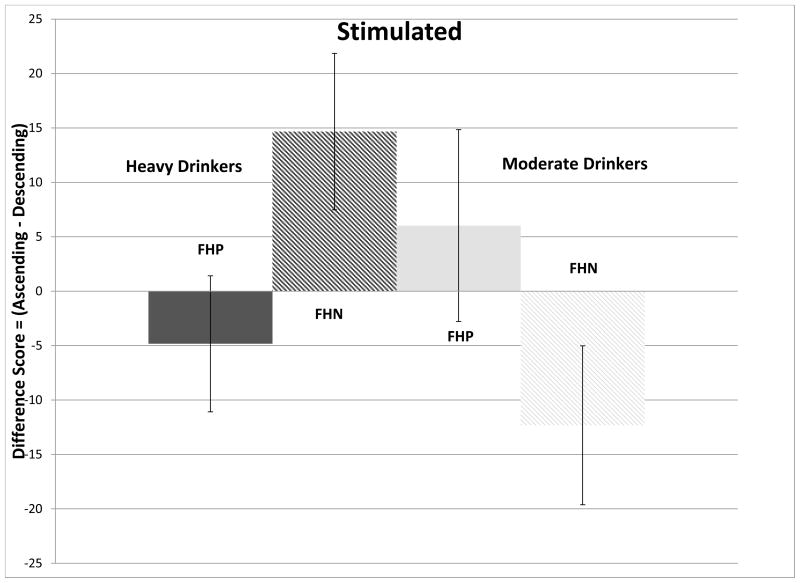
Figure 2.
(Ascending-Descending) slope differences (mean ± standard error) in scoring of subjective perceptions attributed to the effects of alcohol at the crossover point. Figure 2A compare changes in Intoxicated for moderate (men>2 drinks per drinking day; women>1) and light drinkers. Figure 2B compares changes in Intoxicated for moderate and light drinkers, divided into family history of alcoholism subgroups. Figure 2C compares changes in High, for moderate and light drinkers. Figure 2D compares changes in Stimulated for moderate and light drinkers divided into family history of alcoholism subgroups.
There was no overall effect for feeling high (F(4,49)=1.62, p=0.18), although there was a main effect of RDH (F(1,49)=5.50, p=0.023). Figure 2C shows that moderate drinkers felt more high on the ascending slope while light drinkers felt more high on the descending slope. A post-hoc t-test revealed that FHN individuals who were light drinkers felt somewhat more high on the descending slope (p=0.07).
Although there was minimal evidence for an overall effect for feeling stimulated (F(4,49)=2.08, p<0.10), there was a significant FHA*RDH interaction (F(1,49)=6.53, p=0.014), which is depicted in Figure 2D. FHN moderate drinkers were more stimulated on the ascending limb compared to FHN light drinkers who were more stimulated on the descending limb (p=0.011).
There were no significant overall effects (all p>0.43), main effects of FHA (all p>0.47), main effects of RDH (all p>0.12), or significant FHA*RDH interactions (all p>0.65) for the subjective perceptions of sedation, like/dislike the effects of alcohol and the number of drinks it takes to feel the way the subject did at that moment.
Discussion
The brain’s acute responses to alcohol can be considered as a function of extracellular brain alcohol concentration, the duration of alcohol exposure, and the slope of blood alcohol exposure (whether rising or falling). The rate of intravenous infusion of alcohol that is required to achieve the same trajectory of BrAC in all subjects depends on physiologic parameters that are computed from each subject’s gender, age, height and weight. The use of this physiologically-based pharmacokinetic model of alcohol distribution and elimination enabled the precise control of the rise and fall of the BrAC throughout each session, thus regulating the brain’s exposure to alcohol (O’Connor et al., 1998; O’Connor et al., 2000; Ramchandani et al., 1999). To our knowledge, this is the first study to isolate the slope of alcohol concentration as an independent variable and examine the association of the ascending and descending slopes with both FHA and RDH using subjective perceptions of the effects of alcohol.
The primary finding of this study was that subjective perceptions measuring the rewarding effects of alcohol consumption were increased during the ascending limb of BrAC compared to the descending limb in more moderately-consuming young adult social drinkers when BrAC is approximately 60 mg%, and 20 minutes have elapsed since the exposure began. This was true for both FHP and FHN individuals, emphasizing the conclusion made by Schuckit (Schuckit, 2011) that studies should balance ascertainment groups such as FHA by individuals’ recent drinking histories. The pattern was remarkably similar across all subjective perceptions tested. That is, moderate non-dependent drinkers (men who consumed more than 2 drinks per drinking day and females who consumed more than 1 drink per drinking day) perceived greater subjective effects (especially intoxicated and high) on the ascending BrAC slope at crossover than on the descending slope at crossover. The opposite effect was observed for light drinkers (those who recently consumed less alcohol per drinking day), who felt more intoxicated and high on the descending BrAC slope. For both intoxicated and high, the difference between the ascending and descending limbs was much larger for the light drinkers (negative difference) than for the moderate drinkers (positive difference), indicating that perhaps light drinkers are more sensitive to the effect of a rising or falling BrAC. This supports the findings of Ramchandani and colleagues, who showed that individuals with a higher RDH demonstrated a lower initial response to alcohol for subjective measures during an extended intravenous infusion of alcohol (Ramchandani et al., 2002). More specifically, these results support the conclusion drawn by the meta-analysis of Quinn and Fromme (Quinn and Fromme, 2011), that subjective perceptions of stimulation are increased on the ascending limb of the BAC in individuals with a heavy recent drinking history.
When moderate and light drinkers were further categorized by FHA, we found that FHN subjects who were considered moderate drinkers were more intoxicated and stimulated on the ascending BrAC slope while FHN subjects considered light drinkers were more intoxicated and stimulated as BrAC was descending. While this appears to be an interaction between FHA and RDH, this is most likely driven by the moderate vs light drinkers, since there was no main effect of FHA. These results support previous findings (Holdstock et al., 2000; King et al., 2011; King et al., 2002; Marczinski and Fillmore, 2009) which showed that following alcohol ingestion, heavy social drinkers with a binge style of alcohol consumption were more stimulated or intoxicated while BrAC was rising than light social drinkers. There was no evidence that FHP or moderate non-dependent drinkers felt less sedated on the descending slope, confirming that individuals with a high RDH demonstrate acute tolerance to alcohol (Ramchandani et al., 2002), and lending credibility to the summary statement of the review by (Morean and Corbin, 2010) that most studies “fail to show reduced sedation among high-risk groups”. This lack of a significantly reduced sedation among the high-risk heavy drinkers was corroborated by the Quinn and Fromme meta-analysis (Quinn and Fromme, 2011).
This study had several limitations. First, this protocol could only test the association between the slope and subjective perceptions; it could not determine if RDH predisposed individuals to feel more intoxicated and high, or if these subjective feelings were the impetus for a heavier drinking pattern. Second, the techniques employed did not control for the cumulative exposure to alcohol and any effect it might have on subjective perceptions of alcohol’s effects. In fact, the cumulative exposure (represented in Figure 1 by the area under the curve up to the crossover point) differed in the two sessions. It is also possible that sensitivity to BrAC slope varies as a non-linear function of alcohol exposure or changes as a function of time. Third, the study was conducted in a laboratory setting. Doty and de Wit (Doty and de Wit, 1995) reported that subjects who were tested with a choice paradigm in a social setting drank more and perceived greater stimulant effects than those who were tested in isolation, as our subjects were. Yet the laboratory setting is necessary for measurement of parameters assessing the brain’s response to alcohol (e.g., EEG and gaze fixation to be reported separately). Although the moderate level of alcohol exposure (60 mg%) was similar to previously published studies, Holdstock and de Wit (Holdstock and de Wit, 1998) have suggested that a sharper resolution of subjective responses that are sensitive to the slope of BrAC may emerge at higher levels. An unfortunate limitation of this study was that the conditions of this protocol did not allow for interpretation of results in the context of the Low Response (LR) to alcohol(Schuckit, 2011). Most data demonstrating a LR are obtained on the descending limb after oral ingestion, an hour or more after exposure begins, while the time point of interest in this experiment was 20 minutes after the exposure began. Finally, there was no placebo session because the focus of this within-subject design was to directly compare the ascending vs descending slope of the BrAC, within the same amount of time and at the same BrAC. Thus, we acknowledge that whatever placebo, learning, or fatigue effects that may have influenced the results were the same for each subject, in both alcohol sessions.
Finally, due to the time-intensive nature of the study, only a minimal number of subjects could be used. This reduced power could potentially account for the lack of a significant association of FHA and sensitivity to the slope of alcohol exposure, with regard to subjective perceptions, as well as the marginally significant p-values for RDH. FHA is a traditional proxy for the segregation of multiple genes that, as an ensemble, influence the risk for alcoholism, but certainly also reflect environmental influences. The sample population for this study was restricted to be 21 years or older, as required by current law in Indiana. Thus, it is possible that any association of subjective perceptions with FHA that would be present in a younger sample has been blunted in the current sample.
The primary advantage of this study is the use of intravenous administration of alcohol, which minimized variability in the BrAC time course by controlling for alcohol absorption kinetics to achieve a reliable crossover point. Thus the slope of BrAC could be investigated at the same BrAC level and after the same amount of time of exposure, controlling for those factors which are quite variable in studies using oral alcohol administration. The within-subject design allowed for direct comparison of the ascending vs descending slope of the BrAC. Although the concept of intoxication could have a positive effect (e.g., stimulating) for moderate alcohol consumers while having a negative effect (e.g., sedating) for light consumers (Morean and Corbin, 2010; Reich et al., 2009), there was evidence that light drinkers felt more intoxicated, high, and stimulated on the descending slope. The result of moderate alcohol consumers feeling more intoxicated, high, and stimulated on the ascending slope is consistent with the Differentiator Model and suggests that these heavy drinkers could beat a higher risk for abusing alcohol.
Acknowledgments
This study was supported by Public Health Service Grants P60 AA 07611 and MO1 RR 750. The authors wish to acknowledge Brian McCammon, Kerri McCullough, Julie Piper, and Victor Vitvitskiy, as well as the General Clinical Research Center nursing staff, for their excellent support. We also appreciate a consultation by Dr. Harriet de Wit early in the implementation phase of the NIAAA grant to the Indiana Alcohol Research Center that funded this research. The results were presented, in part, in a symposium at the 2007 RSA conference in Chicago and in a poster at the same conference.
This study was supported by Public Health Service Grants P60 AA 07611 and MO1 RR 750.
References
- Bienvenu OJ, Davydow DS, Kendler KS. Psychiatric ‘diseases’ versus behavioral disorders and degree of genetic influence. Psychol Med. 2011;41(1):33–40. doi: 10.1017/S003329171000084X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blekher T, Ramchandani VA, Flury L, Foroud T, Kareken D, Yee RD, Li TK, O’Connor S. Saccadic eye movements are associated with a family history of alcoholism at baseline and after exposure to alcohol. Alcohol Clin Exp Res. 2002;26(10):1568–73. doi: 10.1097/01.ALC.0000033121.05006.EF. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Hicks BM, Iacono WG, McGue M. Familial transmission and heritability of childhood disruptive disorders. Am J Psychiatry. 2010;167(9):1066–74. doi: 10.1176/appi.ajp.2010.09091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Doty P, de Wit H. Effect of setting on the reinforcing and subjective effects of ethanol in social drinkers. Psychopharmacology (Berl) 1995;118(1):19–27. doi: 10.1007/BF02245245. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27(6):1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Holdstock L, de Wit H. Individual differences in the biphasic effects of ethanol. Alcohol Clin Exp Res. 1998;22(9):1903–11. [PubMed] [Google Scholar]
- Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res. 2000;24(6):789–94. [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Heath AC, Kessler RC, Eaves LJ. A twin-family study of alcoholism in women. Am J Psychiatry. 1994;151(5):707–715. doi: 10.1176/ajp.151.5.707. [DOI] [PubMed] [Google Scholar]
- Kessler DA. Alcohol marketing and youth: the challenge for public health. J Public Health Policy. 2005;26(3):292–5. doi: 10.1057/palgrave.jphp.3200041. [DOI] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68(4):389–99. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26(6):827–35. [PubMed] [Google Scholar]
- Li TK, Hewitt BG, Grant BF. The Alcohol Dependence Syndrome, 30 years later: a commentary. The 2006 H. David Archibald lecture. Addiction. 2007;102(10):1522–1530. doi: 10.1111/j.1360-0443.2007.01911.x. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Acute alcohol tolerance on subjective intoxication and simulated driving performance in binge drinkers. Psychol Addict Behav. 2009;23(2):238–47. doi: 10.1037/a0014633. [DOI] [PubMed] [Google Scholar]
- McGue M. Phenotyping alcoholism. Alcohol Clin Exp Res. 1999;23(5):757–758. doi: 10.1111/j.1530-0277.1999.tb04180.x. [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective response to alcohol: a critical review of the literature. Alcohol Clin Exp Res. 2010;34(3):385–95. doi: 10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Morzorati SL, Ramchandani VA, Flury L, Li TK, O’Connor S. Self-reported subjective perception of intoxication reflects family history of alcoholism when breath alcohol levels are constant. Alcohol Clin Exp Res. 2002;26(8):1299–306. doi: 10.1097/01.ALC.0000025886.41927.83. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108(3):383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res. 1998;22(1):202–10. [PubMed] [Google Scholar]
- O’Connor S, Ramchandani VA, Li TK. PBPK modeling as a basis for achieving a steady BrAC of 60 +/− 5 mg% within ten minutes. Alcohol Clin Exp Res. 2000;24(4):426–7. [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Subjective response to alcohol challenge: A quantitative review. Alcoholism: Clinical and Experimental Research. 2011 doi: 10.1111/j.1530-0277.2011.01521.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Flury L, Morzorati SL, Kareken D, Blekher T, Foroud T, Li TK, O’Connor S. Recent drinking history: association with family history of alcoholism and the acute response to alcohol during a 60 mg% clamp. J Stud Alcohol. 2002;63(6):734–44. doi: 10.15288/jsa.2002.63.734. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, O’Connor S. Studying alcohol elimination using the alcohol clamp method. Alcohol Res Health. 2006;29(4):286–90. [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, O’Connor S, Blekher T, Kareken D, Morzorati S, Nurnberger J, Jr, Li TK. A preliminary study of acute responses to clamped alcohol concentration and family history of alcoholism. Alcohol Clin Exp Res. 1999;23(8):1320–30. [PubMed] [Google Scholar]
- Reed T, Page WF, Viken RJ, Christian JC. Genetic predisposition to organ- specific endpoints of alcoholism. Alcohol Clin Exp Res. 1996;20(9):1528–33. doi: 10.1111/j.1530-0277.1996.tb01695.x. [DOI] [PubMed] [Google Scholar]
- Reich RR, Darkes J, Goldman MS. What do you mean ‘drunk’? Ambiguity and similarly among alcohol expectancies as a function of drinker type. Alcoholism: Clinical and Experimental Research. 2009;33(Supplement s1):11A–265A. [Google Scholar]
- Schuckit MA. Comment on the Paper by Quinn and Fromme Entitled Subjective Response to Alcohol Challenge: A Quantitative Review. Alcohol Clin Exp Res. 2011;35(12) doi: 10.1111/j.1530–0277.2011.01561.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Tsuang J, Hesselbrock V, Bucholz K. Response to alcohol in daughters of alcoholics: a pilot study and a comparison with sons of alcoholics. Alcohol Alcohol. 2000;35(3):242–8. doi: 10.1093/alcalc/35.3.242. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83(4):393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Trim RS, Schuckit MA, Smith TL. The relationships of the level of response to alcohol and additional characteristics to alcohol use disorders across adulthood: a discrete-time survival analysis. Alcohol Clin Exp Res. 2009;33(9):1562–70. doi: 10.1111/j.1530-0277.2009.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]