Abstract
The cornea, the most densely innervated tissue on the surface of the body, becomes innervated in a series of highly coordinated developmental events. During cornea development, chick trigeminal nerve growth cones reach the cornea margin at embryonic day (E)5, where they are initially repelled for days from E5-8, instead encircling the corneal periphery in a nerve ring prior to entering on E9. The molecular events coordinating growth cone guidance during cornea development are poorly understood. Here we evaluated a potential role for the Robo-Slit nerve guidance family. We found that Slit 1, 2 and 3 expression in the cornea and lens persisted during all stages of cornea innervation examined. Robo1 expression was developmentally regulated in trigeminal cell bodies, expressed robustly during nerve ring formation (E5-8), then later declining concurrent with projection of growth cones into the cornea. In this study we provide in vivo and in vitro evidence that Robo-Slit signaling guides trigeminal nerves during cornea innervation. Transient, localized inhibition of Robo-Slit signaling, by means of beads loaded with inhibitory Robo-Fc protein implanted into the developing eyefield in vivo, led to disorganized nerve ring formation and premature cornea innervation. Additionally, when trigeminal explants (source of neurons) were oriented adjacent to lens vesicles or corneas (source of repellant molecules) in organotypic tissue culture both lens and cornea tissues strongly repelled E7 trigeminal neurites, except in the presence of inhibitory Robo-Fc protein. In contrast, E10 trigeminal neurites were not as strongly repelled by cornea, and presence of Robo-Slit inhibitory protein had no effect. In full, these findings suggest that nerve repulsion from the lens and cornea during nerve ring formation is mediated by Robo-Slit signaling. Later, a shift in nerve guidance behavior occurs, in part due to molecular changes in trigeminal neurons, including Robo1 downregulation, thus allowing nerves to find the Slit-expressing cornea permissive for growth cones.
Keywords: cornea, lens, Robo, Slit, nerves, axon guidance
Introduction
The cornea, one of the most densely innervated tissues on the surface of the body, becomes populated by sensory and autonomic nerve fibers during embryonic development. The vast majority of corneal nerves are sensory (Marfurt, et al. 1989; Muller, et al. 2003), derived from the neural crest component of the ophthalmic branch (OpV) of the trigeminal ganglion (Tg) (Lwigale. 2001). These nerves are critical for the protection of the eye as they are involved in transmission of painful or irritating stimuli encountered from the external environment (Belmonte, et al. 2004), while also maintaining a healthy cornea by secreting trophic factors that stimulate growth and survival of corneal epithelial cells (Beuerman and Schimmelpfennig. 1980; Baker, et al. 1993; Garcia-Hirschfeld, et al. 1994). Understanding the mechanisms that regulate cornea innervation is becoming increasingly important due to the observation that corneal nerves are often damaged during modern corrective surgical procedures, such as LASIK and cornea transplantation, or following corneal trauma and disease (Benitez-del-Castillo, et al. 2001; Davis and Dohlman. 2001; Wilson and Ambrosio. 2001). Corneal nerve damage is associated with degenerative conditions ranging from dry eye to transient or chronic neurotrophic keratitis, characterized by abnormal epithelial cell metabolism, impaired corneal sensitivity and desiccation of the corneal surface (Davis and Dohlman. 2001; Muller, et al. 2003).
During chicken eye development, neuronal growth cones of trigeminal axons approach the corneal periphery at embryonic day (E)4–5, yet are repelled from entering the cornea for days, instead forming a nerve ring around the corneal periphery (Bee. 1982; Lwigale and Bronner-Fraser. 2007; Conrad, et al. 2008). Observations of early nerve exclusion from the cornea suggest the presence of nerve growth cone-repulsive signals released from both the embryonic cornea and underlying lens vesicle (Lwigale and Bronner-Fraser. 2007; Kubilus and Linsenmayer. 2010). Nerve growth cone inhibition by the cornea and lens are at least partly mediated by the chemorepellant guidance molecule Semaphorin3a (Sema3a), since disrupting Sema3a binding with its nerve growth cone receptor Neuropilin 1 (Npn1) during cornea development leads to trigeminal nerves invading the cornea in a premature and disorganized fashion (Lwigale and Bronner-Fraser. 2007). Currently, it is not known if Sema3a-Npn1 acts alone to control nerve growth cone exclusion from the early developing cornea, or whether other molecular signals act in parallel with the receptor-ligand pair. Later on during cornea development, at a critical time between E9–E10, a subset of the trigeminal growth cones within the pericorneal nerve ring find the cornea stroma permissive, and nerve ingrowth to the cornea commences along all radii (Riley, et al. 2001; Lwigale and Bronner-Fraser. 2007; Conrad, et al. 2008). The change in nerve growth cone behavior at this stage may be due to dynamic, temporal modulations to the presence, concentrations, or identity of nerve guidance molecules in the cornea itself, to the receptors on the growth cones of axons in the pericorneal ring, or both. To date, the identities of the molecular signals which regulate nerve growth into the cornea, as well as the nature of their regulation within the cornea or on the surface of the trigeminal growth cones, are unknown.
In this study, a role for the Roundabout (Robo)-Slit family of nerve guidance family molecules in cornea innervation was examined. The Slit family of nerve guidance molecules represent a membrane-bound or secreted signaling factor which is capable of guiding nerve growth through interactions with their cognate Robo receptors located on nerve growth cones (reviewed in Dickson and Gilestro. 2006; Chedotal. 2007; Ypsilanti, et al. 2010). Slit proteins are generally thought of as chemorepellants to sensory nerve growth and branching (Zallen, et al. 1998; Brose, et al. 1999; Kidd, et al. 1999; Li, et al. 1999; Fricke, et al. 2001; Hao, et al. 2001; Plump, et al. 2002; Long, et al. 2004); however, Robo-Slit interactions can also serve as positive regulators for peripheral sensory axon elongation and branching, especially by the trigeminal axons of the embryonic head (Ozdinler and Erzurumlu. 2002; Ma and Tessier-Lavigne. 2007; Kubilus and Linsenmayer. 2010). In mammals and birds, three Slit genes (1–3) have been identified (Itoh, et al. 1998; Holmes and Niswander. 2001; Vargesson, et al. 2001) and each found to govern nerve guidance decisions in various organ systems during embryogenesis (reviewed in Chedotal. 2007). Slit2 gene expression has been observed in the lens and corneal tissues of multiple model organisms during embryonic development, including chick (Conrad, et al. 2009; Kubilus and Linsenmayer. 2010) and mouse (Niclou, et al. 2000; Thompson, et al. 2006), and Robo expression has been observed in rat trigeminal neuron cell bodies at concurrent stages of development (Ozdinler and Erzurumlu. 2002; Ma and Tessier-Lavigne. 2007).
Herein we show that Slit 1–3 are expressed in the embryonic lens and cornea during all stages of cornea innervation examined. Robo1 expression is broad in trigeminal neuronal cell bodies during early cornea development when pathfinding nerves encircle the cornea in a nerve ring. By studying trigeminal neuronal growth cone behavior in organotypic co-culture and in vivo, we show that repulsion of trigeminal nerves by the embryonic lens and cornea could be abrogated by inhibiting Robo-Slit interactions. These results provide new insight into the mechanism(s) of nerve exclusion from the cornea during the earliest stages of cornea innervation. Moreover, we reveal that the nerve guidance behavior switch, from cornea-prohibitory to cornea-permissive, is a result of age-related molecular changes occurring within both the cornea and trigeminal neurons. With regard to molecular changes occurring in trigeminal neurons over time, Robo1 gene expression weakened in trigeminal neurons from the OpV by E10, concurrent with nerve growth into the cornea. Likewise, Robo receptor activity was reduced, or absent, as a consequence of age since older, E10, neurons became desensitized to Robo-Slit inhibitors in organotypic co-culture studies. These findings collectively suggest that developmental regulation to Robo receptor expression and activity in trigeminal neurons represent a key molecular event mediating the nerve guidance behavior shift occurring during cornea development. In full, this study increases our understanding of the complex and multi-faceted regulatory events that govern cornea innervation.
Material and Methods
Embryo Husbandry and Tissue Isolation
Fertile White Leghorn chicken eggs (Nelson’s Hatchery, Manhattan, KS) were stored at 15°C for up to a week before being transferred to a 38°C humidified poultry incubator on E0 and incubated at 45% humidity for up to 10 days. Corneas, lens vesicles or Tgs from embryos of the desired age were dissected into sterile Howard Ringer’s saline solution (7.2 g NaCl, 0.17 g CaCl2-2H2O, 0.37 g KCl in 1 liter distilled water, pH 7.3). Saline was treated with DEPC (Sigma, St. Louis, MO) to remove RNases when appropriate. For RNA isolation, tissues were immediately quick frozen in liquid nitrogen and stored at minus 70°C until used. For RNA or protein staining, tissues were immediately fixed in modified Carnoy’s fixative (3 parts 100% ethanol, 1 part 32% paraformaldehdye, 1 part glacial acetic acid) overnight at 4°C, followed by multiple washes in phosphate buffered saline (PBS) and transferred serially to 100% methanol and stored at minus 20°C until used.
RNA Extraction and RT-PCR
cDNA was synthesized (SuperScript III RT First-Strand Synthesis Systems, Invitrogen, Carlsbad, CA) using RNA isolated and purified (RNeasy Mini Kit, Qiagen, Valencia, CA) from dissected corneas or lens vesicles snap-frozen in liquid nitrogen. The primers used for RT-PCR are shown in Table 1. Primers were used to amplify products by PCR.
Table 1.
RT-PCR primers used in this study.
| Gene | Basepairs | F’ primer | R’ primer |
|---|---|---|---|
| Slit1 | 2147–2403 | 5’ GTCAGGAGGAGACGAGTTGC 3’ | 5’ TGAGCTGATCCTGTTGTTGC 3’ |
| Slit2 | 3678–3904 | 5’ ATGGTTTTACCTCGCAGGAG 3’ | 5’ TCGTCTGTGGCAATCTGAAG 3’ |
| Slit3 | 3731–3921 | 5’ CTCTGAACCTGGTGGTGGAT 3’ | 5’ ATTGATGCGAACATCGTGAA 3’ |
| Gapdh | 120–319 | 5’ CCTCTCTGGCAAAGTCCAAG 3’ | 5’ CATCTGCCCATTTGATGTTG 3’ |
In situ hybridization
Whole-mount in situ hybridization was performed on lens vesicles or Tgs dissected from E5–E10 embryos as previously described (Thisse, et al. 1993). Following staining, the tissues were washed in PBS, dehydrated through an ethanol series, rinsed twice in xylenes, and embedded in paraffin. Paraffin block serial sections of 12 µm were mounted on slides (SuperFrost Plus, Fisher, Pittsburgh, PA), dried overnight at 40°C, dewaxed in xylenes and mounted in EMS Shield Mount (Electron Microscopy Sciences, Hatfield, PA) for imaging. In situ hybridization on 12 µm eyefront sections were performed essentially as described previously (Etchevers, et al. 2001) with minor modifications previously reported (Conrad, et al. 2009). Antisense and sense digoxigenin labeled probes were generated for Robo1 and Slit1-3 (Vargesson, et al. 2001). No signal was observed using sense probes. Stained tissues were visualized using a Leica MZ16F microscope equipped with a Leica DFC 320 digital camera.
Immunostaining
Nerves were visualized in whole heads, dissected eyefronts or Tg explants in culture with anti-neuronal β-tubulin-specific antibody (Tuj1, R & D systems, Minneapolis, MN) used at a 1:200 dilution in antibody blocking solution (PBS, pH 7.2), containing 10% goat serum, 1% bovine serum albumin, 0.1% Triton-X 100), overnight at 4°C with mild rocking. Following three washes in PBS-T (PBS with 0.1% Triton-X 100), tissue was incubated in Alexa Fluor 488 goat anti-mouse IgG2a antibody (Molecular Probes, Carlsbad, CA) used at a 1:200 dilution in antibody block. Fluorescent images were captured using a Leica MZ16F epifluorescent microscope equipped with a Leica DFC 320 digital camera.
Tissue Culture
Tg tissues were dissected from E7–E10 chick embryos and the proximal region of each ganglion was cut into tissue explants using a tungsten needle (Fine Science Tools, Foster City, CA). Neuron explants were cultured alone or co-cultured with E5 lens vesicles or E5–E9 corneas in collagen matrix as described (Keynes, et al. 1997). Briefly, explants were cultured in Opti-MEM media (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum, antibiotics (100 units penicillin, 0.1 mg/ml streptomycin; Sigma, St. Louis, MO) and 25 ng/ml nerve growth factor (NGF; Sigma, St. Louis, MO) to support neurogenesis of the neural crest-derived cells of Tg explants (Dillon, et al. 2004; Lwigale and Bronner-Fraser. 2007). Inhibitory recombinant protein (Recombinant Rat Robo1-Fc Chimera; R & D Systems, Minneapolis, MN) or control protein (Recombinant Human IgG1-Fc Chimera; R & D Systems, Minneapolis, MN) were used at 1–12 nanomolar and some controls were cultured in media with NGF alone. Tissues were oriented in a collagen matrix solution, comprised of 3 ng/ml rat tail collagen (BD Biosciences, San Jose, CA) and Opti-MEM which is allowed to solidify at 37 °C under sterile conditions prior to addition of media. Co-cultures were prepared in 8 well Lab Tek II chamber slides and incubated at 37°C in a humidified CO2 incubator for 42–48 hours. After incubation, co-cultures were fixed in 4% paraformaldehyde and neurites were visualized by immunostaining as described above.
Neurite guidance assays
For trigeminal explant and lens co-cultures, a ‘neurite guidance’ index was used to quantify the behavior of neurites, which has been previously described (Keynes, et al. 1997; Lwigale and Bronner-Fraser. 2007). One to three explants were arranged in close proximity to a single lens in the presence or absence of recombinant proteins: 5, all neurite growth occurs in the distal quadrant away from the lens; 4, a small minority of neurites grow in the lateral quadrants from the distal quadrant; 3, neurites grow in the quadrant that is just lateral to the proximal quadrant, but no neurites enter the promixal quadrant; 2, neurites grow in the proximal quadrant, but do not touch the lens tissue; 1, neurites grow in the proximal quadrant and touch the lens tissue; 0, neurites are not impeded from extending within the lens tissues. Statistical analyses are given for the differences between the mean of the control and each treatment group analyzed by a two-tailed Student’s t-test (*p < 0.05). For trigeminal explants and cornea co-cultures, one or two explants were arranged in close proximity to the cornea. Neurite growth from a single explant was scored as either “repelled” or “not repelled” by the cornea in the presence or absence of recombinant proteins. Explants whose neurites failed to extend within or over the cornea were scored as “repelled.” Explants whose neurites grew into, over or transversed the cornea were scored as “not repelled.” Upon scoring all explants for a given culture condition, a repulsion frequency was assigned for that culture condition which was determined by dividing the number of explants given a “repelled” scored by the total number of explants analyzed.
In vivo bead implantation
Localized inhibition of Robo-Slit signaling was performed in vivo using beads soaked in Robo1-Fc inhibitor. Chick eggs incubated for 4 to 5 days were windowed and the embryonic membranes dissected away to expose the right eye. Affi-gel beads (Bio-Rad Laboratories, 153-7302) were rinsed several times in Howard Ringer’s saline solution and then incubated overnight at 4°C in 25 µM Robo1-Fc recombinant protein or control IgG-Fc recombinant protein. Using a fine tungsten needle, 1–2 beads were implanted into the anterior region of the eye at E4-5. Beads were delivered to many different locations throughout the anterior eye to determine the optimal location to influence nerve growth cones. Optimal bead locations for triggering nerve growth cone response from the Robo-Slit inhibitor were adjacent to the lens under the limbus epithelium at E4 or in the anterior chamber, between the lens and cornea, at E5. Beads loaded with control solutions at these locations, or other sites in the eye field, never elicited differences in nerve guidance. Implantation of beads soaked in Alexa Fluor 546-Albumin protein (Molecular Probes, Carlsbad, CA), which is similar in charge and molecular weight to Robo1-Fc, was also conducted to monitor protein diffusion from implanted beads and protein stability in the developing eye in vivo. Following bead implantation, eggs were moistened with a few drops of Ringer’s saline spiked with antibiotics (100 units penicillin, 0.1 mg/ml streptomycin; Sigma, St. Louis, MO), resealed and incubated for 1–1.5 days before being collected at E5–E6. Anterior halves of the eyeballs were dissected and processed by immunostaining as described above.
Results
Stages of Corneal Innervation
To provide observations of cornea innervation in developing chick, nerves were visualized in whole heads or dissected eyefronts through staining with the anti-neuronal β-tubulin-specific antibody (Tuj1) antibody. During E4–E5, nerves extended from the OpV branch of the Tg to the eyelid and the anterior surface of the eye (Fig. 1A), reaching the corneal periphery by E5 (arrows, Fig. 1A,B). Next, rather than extending into the cornea, nerve growth cones remained in the limbal mesenchyme and extended dorsally and ventrally around the cornea (Fig. 1C). From E6–E8, a pericorneal nerve ring formed as the main bundles of nerves (trunks) grew circumferentially around the cornea (Fig. 1C–E). During these stages, the cornea appeared repulsive to trigeminal nerves, as the growth cones of individual fibers branching from the trunks of the nerve ring bent away from the cornea. At E9, a striking change in nerve growth cone behavior was visualized, as the cornea first became permissive to nerve fibers that extended from the bundles of nerves in the pericorneal nerve ring and invaded the corneal stroma (Fig. 1F,G). As development proceeded, the growth cones continued to extend from the nerve ring along all radii toward the center of the cornea, advancing one-third of the way toward the center by E10 (Fig. 1H) and reaching the center by E15 (not shown), consistent with previous reports (Bee. 1982; Lwigale and Bronner-Fraser. 2007).
Figure 1.
Cornea innervation by trigeminal sensory nerves during development. (A–E) Sensory nerve growth cones from the OpV of the Tg seem attracted to the cornea (arrows in A,B), but find it non-permissive from E5–E8, instead forming a pericorneal nerve ring around the corneal periphery. (F,G) Beginning at E9, growth cones find the corneal stroma to be permissive and begin to project into the stroma. (G) E9 cornea at higher magnification. (H) By E10, nerve growth cones continue to extend to the corneal center, with individual nerve fibers extending from the nerve ring along all radii. Asterisk denotes choroid fissure. Abbreviations: Co, cornea; E, embryonic day; EL, eyelid; MmV, maxillary/mandibular branches; OpV, ophthalmic branch. Scale bar: 1 mm.
These results confirm previous observations (Bee. 1982; Lwigale and Bronner-Fraser. 2007; Conrad, et al. 2008; Kubilus and Linsenmayer. 2010) showing that cornea innervation during development is highly regulated, and begs the following questions: (1) What molecules in the anterior eyefield mediate the repulsion of trigeminal nerves, inhibiting them from populating corneal tissues during E5-8?, and (2) what molecular changes occur in the cornea or on the trigeminal nerve growth cones at later stages (E9–E10) to transition the corneal tissue from growth cone-prohibitory to -permissive?
Expression of Slit and Robo genes during stages of cornea innervation
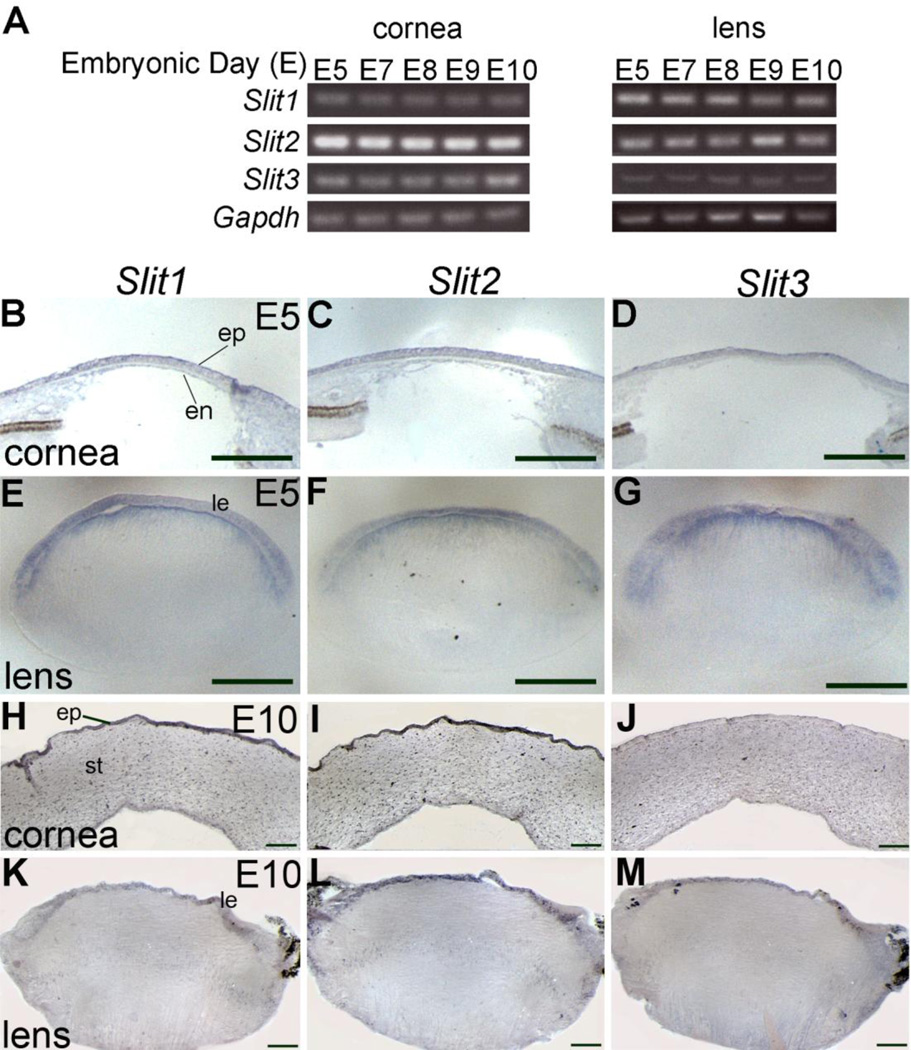
To determine if repellant Slit nerve guidance molecules represented good candidate molecules for regulating cornea innervation, we examined the expression profiles of Slit 1, 2, and 3 mRNAs by gene expression analyses from E5, when trigeminal nerves approach the cornea, up to E10, when the cornea is actively being innervated. Expression of Slit2 in chick tissues that are relevant to cornea innervation has been described previously (Conrad, et al. 2009; Kubilus and Linsenmayer. 2010). By performing semi-quantitative RT-PCR analyses on both whole lens vesicles and corneas, we found that Slit 1, 2 and 3 mRNAs were present consistently in both tissues throughout stages of cornea innervation (Fig. 2A). Next, we performed in situ hybridization to characterize the expression patterns for each Slit mRNA in the developing anterior eyefield. At E5, Slit1, 2 and 3 showed a similar pattern of expression in the cornea (Fig. 2B,C,D) and the lens epithelium (Fig. 2E,F,G). Likewise, Slit1, 2 and 3 could be detected in the corneal cells at E10. In E10 corneal sections, Slit 1 and 2 appeared to be more robustly expressed in the cornea epithelium than Slit3 (Fig. 2H–J), while Slit1-3 each displayed uniform expression throughout the cornea stroma (Fig. 2H–J). In the lens at E10, Slit1, 2 and 3 were each present in the epithelium, whereas Slit2 expression appeared to be uniform throughout the epithelium, while Slit1 and 3 mRNA expression was localized primarily to epithelial cells in the center of lens which directly underlie the cornea (Fig. 2K–M).
Figure 2.
Slit expression in anterior eye tissues during stages of cornea innervation. (A) Semi-quantitative RT-PCR for Slit1, 2 and 3 in lens vesicles and corneas harvested at daily intervals from E5–E10. Gapdh is included as a loading control. (B–G) Transverse sections through E5 anterior eye revealed that Slit1, 2 and 3 are expressed in epithelium (ep) and endothelium (en) of the cornea (B–D) and the lens epithelium (le) (E–G). (H–J) Transverse corneal sections at E10 reveal robust Slit1 and 2 expression in corneal epithelium and stroma (st) (H,I) and strong Slit3 expression in the stroma, but weak expression in the epithelium (J). (K–M) Transverse sections through E10 lens show robust expression of Slit1, 2 and 3 in lens epithelium. Positive reaction product in sectioned material is the blue-staining region. Abbreviations: E, embryonic day; le, lens epithelium; en, corneal endothelium; ep, corneal epithelium; st, stroma. Scale bar: 0.2 mm.
We next investigated whether Robo1, the cognate receptor for Slit 1, 2, and 3, was expressed in trigeminal neurons during concurrent timepoints associated with cornea innervation. We found that during stages of pericorneal nerve ring formation, when nerve growth cones seem actively repelled from entering the cornea (E5–E8), Robo1 was expressed uniformly throughout neuronal cell bodies in the proximal half of the Tg within both the OpV and maxillary/mandibular (MmV) branches (Fig. 3A,B). Later, at E10 when nerves from the OpV branch are actively innervating the cornea, the expression pattern for Robo1 varied from earlier stages, with expression becoming primarily localized within the MmV branch, with weaker expression in the OpV branch (Fig. 3C). In some cases (2/6 ganglia), Robo1 expression could only be detected in neuronal cell bodies within the MmV branch (Fig. 3D), although the former expression pattern was more prevalent (4/6 ganglia).
Figure 3.
Robo1 receptor expression in trigeminal neurons during stages of cornea innervation. (A–D) Robo1 expression in transverse sections of E5 (A) and E8 (B) Tg was prominent throughout OpV and MmV branches, while expression became reduced (C) or absent (D) in the OpV of E10 Tg. Positive reaction product in sectioned material is the blue-staining region. Abbreviations: E, embryonic day; MmV, maxillary/mandibular branches; OpV, ophthalmic branch. Scale bar: 0.5 mm.
Overall, evidence obtained from investigating Robo and Slit gene expression patterns raised the possibility for Robo-Slit receptor ligand interactions to be involved in nerve exclusion from early corneas resulting in pericorneal nerve ring formation during E5–E8. Moreover, downregulation of Robo1 in older E10 OpV neurons suggested that older growth cones may express a different receptor repertoire than earlier neurons, giving them a different potential for sensing repellant nerve guidance cues at developmental stages when their growth cones are actively migrating into the cornea. These dual hypotheses provided the rationale for the following functional studies that we chose to perform in this study.
Robo-Slit signaling is involved in trigeminal nerve repulsion by the embryonic lens
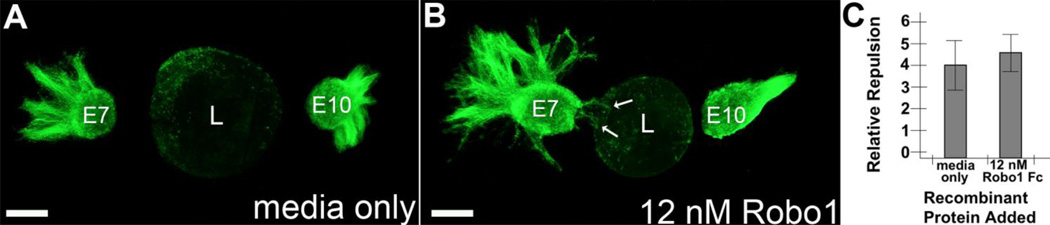
The lens, which directly underlies the cornea, is a source of repellant nerve guidance cues that at least in part mediates nerve growth cone exclusion from the cornea and pericorneal nerve ring formation prior to E9 (Lwigale and Bronner-Fraser. 2007). To explore the possibility that Robo-Slit interactions between the trigeminal nerves and embryonic lens may be involved in cornea innervation, trigeminal explants derived from the proximal half of the OpV of E7 Tg were co-cultured in a collagen matrix adjacent to lens vesicles in the presence of an inhibitory Robo recombinant protein (Robo1-Fc) to inhibit Robo-Slit interactions and nerve growth factor (NGF) to stimulate neurite outgrowth. Robo1-Fc recombinant protein, which was provided in varying concentrations in the culture medium, can abrogate Robo-Slit interactions by binding to lens-secreted or membrane-bound Slit molecules on the lens epithelium, thereby preventing them from interacting with available endogenous Robo receptors on the transmembrane surface of trigeminal nerve growth cones. First, to show that culture conditions were conducive for neurite outgrowth, trigeminal neuron explants that were cultured for two days in collagen displayed neurites extending from around the explant in every direction (Fig. 4A). In contrast, when trigeminal explants were co-cultured in the collagen matrix adjacent to an E5 lens vesicle the neurites were strongly repelled and outgrowth occurred only from the distal edge of the explant (Fig. 4B). Strikingly, when Robo1-Fc protein was added to the culture medium in varying concentrations, the repulsive effects of the lens were greatly diminished in a dose-dependent fashion (Fig. 4D–F).
Figure 4.
Neurite repulsion by embryonic lens vesicles is mediated by Robo-Slit signaling. (A) Neurites from E7 trigeminal ganglion explants (Tg) cultured alone in collagen with medium containing NGF extended in a halo around the explant in every direction. (B–F) Neurites from E7 trigeminal explants co-cultured in collagen adjacent to an E5 lens vesicle were strongly repelled by lens vesicles (B); however, the lens-mediated neurite repulsion diminished with increasing concentrations (1–12 nM) of an inhibitory Robo1-Fc protein (D–F), but not by 12 nM control IgG-Fc protein solution (C). (G) Schematic showing how repulsion of neurite outgrowth by the lens was quantified (neurite guidance index, see Material and Methods for further details) in this study. (H) Quantification of the effects of Robo1 inhibitor and controls on neurite guidance. Error bar in each graph represents standard deviation. Abbreviations: L, lens; Tg, Trigeminal ganglion explant. Scale bar: 0.5 mm.
To quantitate the effects of neurite outgrowth from trigeminal explants co-cultured adjacent to lens vesicles, we used a relative repulsion scheme (Fig. 4G), which has been previously described (Lwigale and Bronner-Fraser. 2007). A score of 5 was given when neurite outgrowth from the explant was fully repelled by the lens, whereas a score of 2 or less indicated that outgrowth was not inhibited by the lens. Full details of the scoring criteria used in this scheme are described in the methods section. When trigeminal explants were cultured in the presence of 12 nM control IgG-Fc protein solution (Fig. 4C), neurites were strongly repelled by adjacent lens vesicles. However, in the presence of low concentrations of Robo1-Fc protein solution (1 nm; Fig. 4D), neurite repulsion by the adjacent lens was slightly reduced compared to controls. At intermediate concentrations of Robo1-Fc (6 nm; Fig. 4E), neurite repulsion was significantly abrogated, with many neurites extending from the explant towards the adjacent lens vesicle. Most significantly, at even higher concentrations of Robo1-Fc (12 nM; Fig. 4F), neurite repulsion was completely blocked, with neurites extending toward, or growing over, the adjacent lens vesicle. Overall, the relative repulsion scores ascribed to the explants cultured next to an embryonic lens for each culture condition is summarized in Fig. 4H. These data reveal that the embryonic lens produces repellant Slit molecules, and that blocking Robo-Slit interactions with inhibitory protein can overcome the repulsive effects of embryonic lens vesicles on trigeminal nerves.
Perturbing Robo-Slit signaling in vivo alters nerve behavior during cornea innervation
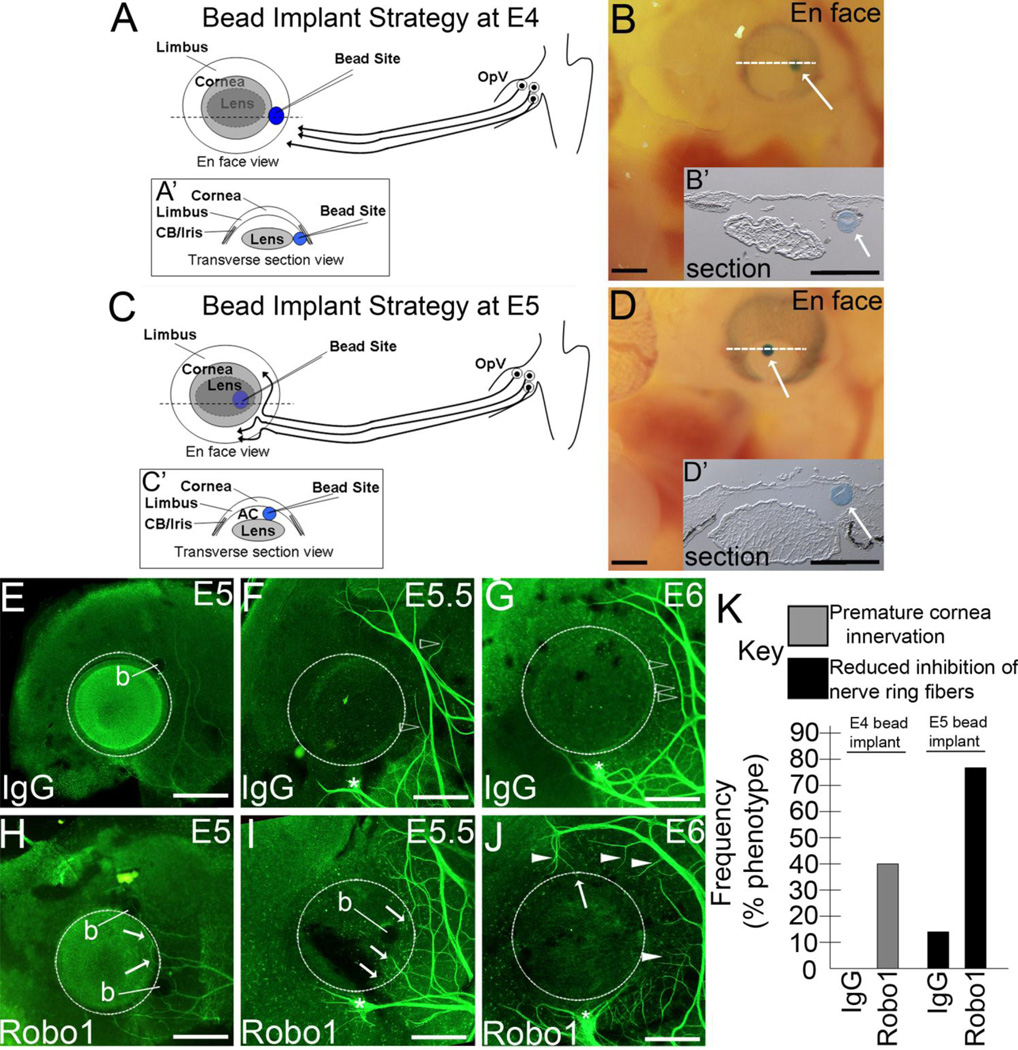
To determine if Robo1-Fc inhibitory protein could influence nerve behavior in vivo, Robo1-Fc or control IgG-Fc-coated beads were transplanted into the developing eye of E4-5 chick embryos. At E4, beads were implanted at a ventrotemporal position in the eye, next to the lens/cornea and under the limbal epithelium, directly in the path of approaching trigeminal nerve growth cones projecting from the OpV (Fig. 5A,B). To deliver Robo1-Fc inhibitory protein at E5, a time wherein the nerve ring is forming and nerve trunks have advanced both dorsally and ventrally around the cornea’s periphery, beads were implanted to the anterior chamber between the lens and cornea (Fig. 5C,D). This site represents a central location in the eyefield most likely to influence growth cones in both nerve trunks. The exact locations of the beads following implantation, at the limbal mesenchyme near the lens and corneal periphery at E4, or to the anterior chamber at E5, was confirmed in transverse cryostat sections of the anterior eye following implantation (Fig. 5A’,B’,C’,D’).
Figure 5.
Robo1-Fc coated beads implanted in the developing eyefield leads to a disrupted nerve ring and premature cornea innervation. (A–D) Schematics (A,C) and images of eyes receiving beads (B,D) represent bead implantation strategy at E4 (A,B) and E5 (C,D). (A,A’) At E4, protein coated beads (blue circle) were inserted under the limbal epithelia on the ventrotemporal side of the eye, directly in the path of nerves advancing from the OpV. Schematic of en face (A) or transverse section (A’, dotted white line in A delineates location of section) views show the bead lying adjacent to the lens/cornea as nerve growth cones (black arrows) approach. (B,B’) En face (B) view showing bead (white arrow) in eye of live E4 chick embryo and transverse section (B’, dotted white line in B delineates location of section) view showing bead (white arrow) in fixed eye following bead implantation at E4. (C,C’) At E5, protein coated beads (blue circle) were inserted into anterior chamber, in between the nerve trunks (black arrows) migrating dorsal and ventral to the cornea. Schematic of en face (C) or transverse section (C’, dotted white line in C delineates location of section) views show the bead lying in anterior chamber in between lens and cornea. (D,D’) En face (D) view showing bead (white arrow) in eye of live E5 chick embryo and transverse section (D’, dotted white line in D delineates location of section) view showing bead (white arrow) in fixed eye following bead implantation at E5. (E–G) In control eyes implanted with IgG-Fc coated beads, sensory trigeminal nerve growth cones have advanced to the anterior eye but are inhibited from entering cornea, instead forming a nerve ring. Open arrowheads in F and G highlight individual nerve fibers bifurcating from main nerve bundles of nerve ring which display repulsion by bending away, or actively growing away, from the cornea. An IgG-Fc coated bead (b) remained attached to fixed tissue in E, but in eyefronts shown in F and G the beads were removed when lens and other posterior tissues were freed from cornea prior to nerve staining. (H,I) In E5–E5.5 eyes implanted with Robo1-Fc coated beads, sensory trigeminal nerve growth cones show aberrant and premature growth into the cornea (arrows). (J) In E6 eyes receiving Robo1-Fc coated beads, nerve fibers branching away from the main nerve bundles of the nerve ring fail to show inhibition by the cornea, displaying active growth toward the cornea periphery (white arrowheads) or beginning premature innervation (arrow). Robo1-Fc coated bead(s) remained in H and I. In E–J the E-age accompanying the image denotes the timepoint when the eyefronts were fixed and nerve growth examined, in each case being 1 to 1.5 days following implantation. (K) Percentages of embryos receiving Robo1-Fc or control IgG-Fc beads at different embryonic ages which displayed premature cornea innervation (gray) or defects associated with a loss of inhibition of nerves bifurcating from nerve ring (black). Embryos not represented by these percentages in each group were characterized as having normal nerve growth patterns during innervation. n values: IgG-Fc coated bead implanted at E4, 9 embryos, or E5, 8 embryos. Robo1-Fc coated beads implanted at E4, 10 embryos, or E5, 8 embryos. Abbreviations: b, bead; cb, ciliary body; E, embryonic day; OpV, ophthalmic branch. Asterisk denotes choroid fissure. Scale bar: 1 mm.
To track protein diffusion from beads and protein stability in the developing eyefield, some beads were loaded with a fluorescent molecule of similar molecular weight and charge to the Robo1-Fc protein and fluorescence was monitored in ovo in live embryos over a 24 hour time-period. Protein diffusion from the beads occurred within twenty minutes post-implantation, generating a diffusion gradient of fluorescence emanating from the bead and detected near the lens, cornea and limbal tissues for 24 hours (Supplementary Fig. 1). Fluorescence could be detected in these regions of the eye regardless of the embryonic age at which the implantation occurred, indicating that protein solutions delivered by the bead as a diffusion gradient should remain stable in the anterior eye for at least 1 day.
When embryos were fixed at E5 and nerve growth examined, 1 day following E4 bead implantation, nerves in embryos receiving one or two beads with the control IgG-Fc protein avoid the cornea (Fig. 5E), advancing around its periphery similar to unbeaded controls. In contrast, a different pattern of nerve growth was seen in E5 embryos that received one or two beads loaded with Robo1-Fc at E4. Nerve bundles in these embryos advanced prematurely into the cornea instead of projecting around its periphery (Fig. 5H, arrows). When embryos were allowed to develop to E5.5 prior to being examined following E4 bead implantation, nerves in control embryos grew in tight bundles, advancing in a highly organized fashion around the cornea (Fig. 5F). However, nerves in E5.5 embryos implanted with Robo1-Fc beads grew in a disorganized fashion, with many nerves bifurcating randomly from the main nerve trunks and growing towards the cornea, some advancing prematurely into the cornea (Fig. 5I, arrows). Finally, upon examining E6 control embryos that received IgG-Fc beads a day earlier at E5, we found that nerve trunks continued to grow around the cornea in the nerve ring (Fig. 5G). In these control embryos, individual nerve fibers that bifurcated from the main nerve trunks were seen actively growing away from the cornea in the majority of embryos examined (Fig. 5G, open arrowheads). In contrast, nerves in E6 embryos which had been implanted with Robo1-Fc beads at E5 displayed a different pattern. The majority of these embryos displayed many, random bifurcations branching away from the main nerve trunks and these individual nerve fibers advanced directly towards the cornea and into its periphery, not appearing to be inhibited like those nerve fibers in controls (Fig. 5J, arrowheads). The pattern of nerves in these embryos differed from those receiving Robo1-Fc coated beads at E4, in that nerve trunks did not grow directly into the cornea, likely due to the fact that beads were implanted at a timepoint (E5) after the pioneer nerves had begun their advancement around the corneal periphery. Nevertheless, the normal pattern of innervation was disrupted in embryos receiving Robo1-Fc beads, regardless of E-age when implantation occurred. Overall, the frequency of aberrant nerve guidance phenotypes described in these studies following implantation of Robo1-Fc or IgG-Fc loaded beads is summarized in Fig. 5K. Combined, these data further suggest a requirement for Robo-Slit signaling in repelling pioneer nerves from entering the cornea. Moreover, data obtained herein also suggest that Robo-Slit signaling may be required to maintain the nerve ring, keeping individual nerve fibers from bifurcating early, prior to the closing of the nerve ring at E9, at which time nerves from all along the nerve ring radii begin to advance into the corneal stroma.
The cornea repels trigeminal neurites in vitro, dependent on Robo-Slit signaling and both cornea and neuron age
In addition to their expression in the lens, mRNAs for repellant Slits were found in the corneal stroma and epithelium throughout all stages of cornea innervation we examined. Thus, we next chose to examine if trigeminal neurite outgrowth could be repelled or spatially regulated by the embryonic cornea itself in organotypic collagen co-cultures and what role, if any, Robo-Slit signaling played in the process. To do this, the front half of eyes were harvested from developing embryos and the cornea and pericorneal tissues were freed from the lens, iris and retina, which was then cultured endothelium side-up with one or two trigeminal explants oriented near the corneal periphery (circular dotted line in Fig. 6 B–G delineates cornea), resting on the surface of the pericorneal tissue (culture set-up is schematized in Fig. 6G). Following two days in culture, neurites could be visualized extending from explants toward the E6 cornea; however, neurites that approached the corneal periphery were repelled and either stopped or made sharp turns to avoid extending over or through the cornea tissue (Fig. 6A,B). Strikingly, many neurite growth cones that were repelled at the corneal periphery continued to migrate over or within the surrounding pericorneal tissue, invariably extending near the corneal periphery (Fig. 6B), reminiscent of pericorneal nerve ring behavior observed in developing chick embryos (see Fig. 1). Similar to lens and trigeminal explant organotypic co-culture experiments, adding Robo1-Fc protein to the culture medium at 12 nM concentration considerably abrogated the repulsive effect of the E6 cornea on E7 trigeminal neurites. Rather, a portion of the neurites in these cultures that grew toward the cornea extended over or through the cornea tissue, while the remaining neurite growth cones migrated along the cornea margin as in controls (Fig. 6C). Importantly, explants cultured in a similar fashion in the presence of IgG-Fc failed to abolish the repulsive effects of the cornea on trigeminal neurites (data not shown). These data reveal that, like the lens, the E6 cornea appears to release chemorepellants that strongly repel E7 trigeminal neurites and that blocking Robo-Slit interactions interferes with that repulsion.
Figure 6.
Neurite repulsion by the embryonic cornea is dependent on Robo-Slit signaling and neuron age. (A–B) Neurites from E7 trigeminal explants co-cultured with E6 corneas in culture medium containing no recombinant proteins (A,B) sent out neurites preferentially to the cornea, yet failed to migrate into, or over, the cornea tissue, instead remaining in the pericorneal mesenchyme (B). Brightfield image is shown in (A) revealing that cornea (Co) cells appear darker than surrounding mesenchyme allowing detection of it post-staining. (C) In the presence of inhibitory Robo1-Fc recombinant protein solution, a higher percentage of E7 trigeminal neurites grow directly into, or over, the E6 cornea, than controls. (D–F) Co-culture of trigeminal explants of varying ages, E7 or E10, with corneas and surrounding pericorneal mesenchyme of varying ages, E6 or E9, in different combinations in culture medium containing no recombinant proteins, revealed that the potential of the cornea to repel trigeminal neurites declines with both cornea and neuron age. (G) Schematic showing the organotypic co-culture set-up used in these analyses. (H) Percentages of trigeminal (Tg) explants being repelled by corneas under different culture conditions. n values: E7 Tg, E6 cornea media only, n = 21; IgG-Fc treated, n = 18; Robo1-Fc treated, n = 21; E7 Tg, E9 cornea media only, n =16 ; E10 Tg, E6 cornea media only, n = 20; E10 Tg, E9 cornea media only, n = 11. Abbreviations: Co, cornea; E, embryonic day; Tg, Trigeminal explant. The white, dotted circles in B–G demarcate the periphery of the cornea. Scale bar: 0.5 mm.
These results demonstrate that the E6 cornea is a source of diffusible repellant molecules that can influence trigeminal nerve behavior. This leaves open the question of how trigeminal nerve growth cones are capable of migrating into cornea stroma at later E9–10 stages (see Fig. 1), timepoints when the cornea continues to contain mRNAs for repellant Slit molecules. We reasoned that the stage-specific change in nerve behavior is likely due to dynamic, temporal modulations to the presence or identity of nerve guidance molecule(s) in the cornea itself, to the receptors on the growth cones of nerves in the pericorneal ring, or both. To explore these possibilities, corneas and trigeminal explants were prepared for organotypic culture assays from different aged chick embryos, with ages corresponding to stages of corneal non-permissiveness (E6–E7) or permissiveness (E9–E10) in response to approaching trigeminal growth cones.
First, when older, E9, corneas were used (Fig. 6D), the repulsive effect on E7 trigeminal neurites was diminished compared to when they were cultured adjacent to younger corneas (Fig. 6B), i.e., a higher percentage of E7 Tg explants sent out neurites that extended into, or over, the E9 cornea tissue (Fig. 6D), than when confronted with an E6 cornea (Fig. 6B). Conversely, when older, E10, trigeminal neuronal explants were placed next to young E6 corneas, the repulsive capacity of the corneas was significantly negated, as the majority of trigeminal explants sent out neurites capable of extending into, or over, the E6 cornea (Fig. 6E). These data indicated that the age of both the cornea and neurons were important in promoting the transition of the cornea from growth cone non-permissive, prior to E9, to permissive at E9–10. Consistent with this, when older, E10, trigeminal neuronal explants were co-cultured adjacent to older, E9, corneas in collagen, neurite growth cones from the explants invariably extended into, or over, the corneas (Fig. 6F). The growth cone behavior in collagen gel assays under the various culture conditions described herein is summarized in Fig. 6H.
Inhibitory Robo1-Fc recombinant protein has no effect on lens repulsion of older trigeminal neurites
Different nerve repulsion behaviors between younger (E7) and older (E10) trigeminal neurons in organotypic co-cultures with corneas of a single age suggested that changes in nerve guidance receptor expression normally occur during development. Thus, older trigeminal growth cones are more often permitted to enter developing corneas, whereas younger trigeminal growth cones are prohibited. Consistent with that change in trigeminal growth cone behavior, Robo1 gene expression within the cell bodies of trigeminal neurons in the OpV branch becomes reduced or absent by E10, compared with its robust expression at earlier embryonic ages (Fig. 3), suggesting that age-specific changes may occur in the expression of Robo receptors at the trigeminal growth cones, thus altering their ability to detect and respond to Slit molecules diffusing from the cornea.
To examine this possibility, older E10 OpV trigeminal explants were co-cultured next to E5 repellant lens vesicles. We provided evidence earlier (Fig. 4) that neurite repulsion from E7 trigeminal explants by the lens was mediated by Robo-Slit interactions; thus, we reasoned that the embryonic lens would provide a robust source of repellant Slit molecules for challenging E10 trigeminal neuron outgrowth. Unlike the cornea, the lens strongly repelled neurites, regardless of the trigeminal E-age, when the tissues were co-cultured adjacently in control solution (Fig. 7A). Once again, the repulsive capacity of the lens on E7 neurites could be abrogated by adding 12 nM Robo1-Fc protein; however, no significant difference in neurite repulsion by the lens could be observed in older E10 neurons, even when the different aged neurons were cultured on opposite sides of the same lens (Fig. 7B). Neurite repulsion by the lens on older, E10 neurons in the different culture conditions are summarized in a bar graph in Figure 7C.
Figure 7.
Abrogation of the repulsive nature of the lens on trigeminal neurites by Robo1-Fc recombinant protein is dependent on the age of trigeminal neurons. (A,B) Trigeminal explants of different E-ages (E7 or E10) juxtaposed on either side of a single E5 lens vesicle. (A) The E5 lens repelled neurites, regardless of neuron E-age, in culture medium with no recombinant proteins, yet (B) the addition of inhibitory Robo1-Fc protein neutralized lens-mediated repulsion on younger E7 neurites (arrows), but did not influence older E10 neurites. (C) Quantification of the effects of Robo1-Fc inhibitor and controls on neurite guidance of older, target-innervating E10 trigeminal neurons. Error bar indicates standard deviation. Abbreviations: E7, embryonic day 7 trigeminal ganglion explant; E10, embryonic day 10 trigeminal ganglion explant; L, lens. Scale bar: 0.5 mm.
The results of these lens and trigeminal explant co-culture experiments and all others performed in this study are summarized in Table 2. These data indicate that while both younger and older trigeminal neurons express some combination of nerve guidance receptors which mediate neurite repulsion by the lens, repulsion by the lens is influenced by Robo-Slit interactions only on younger neurons, while older neurons are no longer sensitive to perturbations induced by the Robo1-Fc inhibitory protein. Assuming that the levels and forms of Slit molecules diffusing from the lens in these experiments remained constant, older trigeminal neurons likely possess quantities or identities of Robo receptor molecules different from younger trigeminal neurons.
Table 2.
Effects of inhibitory Robo1-Fc recombinant protein on lens repulsion of different aged trigeminal neurons.
| Culture Conditions | Relative Repulsion | Relative Repulsion |
|---|---|---|
| (N= explants analyzed) Tg age = E7 |
(N= explants analyzed) Tg age = E10 |
|
| Control (media only) | 4.02 ± 1.13 (47)* | 4.00 ± 1.29 (25) |
| Media + 12 nM IgG-Fc | 4.10 ± 1.10 (25) | -- |
| Media + 1 nM Robo1-Fc | 3.67 ± 1.54 (15) | -- |
| Media + 6 nM Robo1-Fc | 2.41 ± 1.23 (34) | -- |
| Media + 12 nM Robo1-Fc | 1.97 ± 1.09 (47)* | 4.33 ± 1.06 (30) |
Discussion
In this study we show that between E4-5, trigeminal nerves extend from the OpV branch of the Tg toward the developing cornea. Upon arriving at the cornea periphery, Robo expressing nerves are repelled by Slit molecules diffusing from both the presumptive cornea epithelium and underlying lens vesicle. Interactions between Robo receptors on the nerve growth cones and Slit molecules in and adjacent to the cornea cause the nerve growth cones to remain in the pericorneal limbus tissue for several days, at which time they form a pericorneal nerve ring (Fig. 8A). At later stages of development, around E9–10, Robo1 expression decreases in the OpV branch, likely resulting in fewer Robo1 receptor molecules on the growth cones of nerves at the cornea periphery. At this time, nerve growth cones become insensitive to repulsive Slit molecules, which continue to be present in the lens and cornea epithelium and stroma, and for the first time during embryonic development these growth cones are able to transverse the cornea periphery and project extensively into the stroma (Fig. 8B), subsequently innervating the epithelium at later stages.
Figure 8.
Proposed model of the nature of Robo-Slit signaling regulation on cornea innervation. (A) Schematic represents early stages of cornea innervation, E5–E8. At early stages, some unidentified guidance factor(s) initially attract the trigeminal nerves from the OpV to the limbus and cornea margin. Upon arriving at the cornea margin, the trigeminal neurons initially express Robo1/2 (+ Robo expression, indicated by red coloring in neuronal cell body, axon and growth cone) and are repelled by Slit expressing ocular tissues. Slit mRNA expression is indicated by blue shading in lens and cornea. (B) At later stages, E9–E10, trigeminal neurons have downregulated Robo1 (− Robo1 expression, indicated by black shading in neuronal cell body, axon and growth cone) and show reduced sensitivity to repellant Slit molecules in ocular tissues (blue shading). This proposed molecular change to the Robo-Slit interaction would lead to loss of a negative guidance signal between the cornea and trigeminal growth cones. This molecular event may act in part with unidentified positive guidance signals from the cornea to the trigeminal growth cones, to instruct the growth cones to enter the cornea for the first time during development. Abbreviation: OpV, ophthalmic branch.
Spatiotemporal expression patterns of Robo1 and Slits during stages of cornea innervation
The hypothesis described above provides the first explanation of how nerves within the pericorneal nerve ring may undergo a precise temporal transition from cornea-prohibitory to cornea-permissive during development. The finding that nerves become less sensitive over time to repulsive molecules secreted by the lens and cornea, namely repellant Slit ligands for Robo receptors, helps to explain gene expression evidence reported herein and in preceding reports, that the lens and cornea continue to express repellant nerve guidance cues during embryonic stages where nerves are beginning to enter the cornea and then later migrating extensively through the anterior stroma and epithelium (Lwigale and Bronner-Fraser. 2007; Conrad, et al. 2008; Conrad, et al. 2009; Kubilus and Linsenmayer. 2010). During chick development, innervation of the cornea occurs in a series of discrete stages, each seemingly involving a high degree of spatiotemporal molecular regulation. Stages of cornea innervation involve, first, a period of nerve repulsion, characterized by pericorneal nerve ring formation from E5 to E8, followed by a period of nerve ingrowth to the corneal stroma, beginning at E9. The switch in nerve behavior from nerve repulsion to ingrowth between these two stages likely reflects changes in the expression patterns of regulatory factors in the cornea, the nerve growth cones, or both, occurring over time. Previous reports have indicated that the repellant nerve guidance molecule Slit2 is expressed in the developing cornea and lens during timepoints corresponding to both repulsion and ingrowth stages (Conrad, et al. 2009; Kubilus and Linsenmayer. 2010). Herein we extended these studies to include other Slit family members involved in nerve repulsion and we showed that both Slit1 and Slit3 are expressed in synonymous cornea and lens tissues as Slit2 throughout all developmental stages of cornea innervation examined. These mRNA expression data suggests that temporal regulation of Slit molecules cannot explain the change in nerve behavior during development, however further work will be required to determine if Slit protein levels in the anterior eye are altered during different stages of cornea innervation.
The response of nerves to available Slit signals requires its binding to Robo receptors on the surface of growth cones, with interactions occurring between the second leucine-rich repeat (LRR) of Slit and the first two Ig-like (Ig) domains of Robo (Howitt, et al. 2004; Liu, et al. 2004). We report here that Robo1 is robustly expressed in trigeminal neurons during nerve ring formation, while another recent study reported that Robo2 is expressed at concurrent stages, albeit at low levels, and Robo2 expression is expressed consistently between nerve ring formation stages and later timepoints when nerve growth cones project into the cornea (Kubilus and Linsenmayer. 2010). Unlike Slit expression in ocular tissues and Robo2 in trigeminal neurons during different stages of cornea innervation, Robo1 expression in trigeminal neurons appears to be spatiotemporally regulated during cornea innervation. At E5 and E8, when the pericorneal nerve ring is forming, Robo1 is expressed throughout trigeminal neurons in both the OpV and MmV branches; however, at E10, Robo1 expression in the OpV branch became reduced, or in some cases, absent. Thus, temporal downregulation of Robo1 in the Tg coincided with developmental behavioral changes to their nerve growth cones at the cornea, which suggested a potential correlation between trigeminal neuron Robo1 dynamic gene expression with modulations in nerve pathfinding. Consistent with this, temporal regulation of Robo-Slit signaling is involved in nerve guidance in other vertebrate organs during development, as removal of Robo receptors from the growth cones membranes has been shown to contribute to the precise control of nerve guidance during spinal cord/midline development (Keleman, et al. 2002; Keleman, et al. 2005).
Patterns of corneal innervations during development are regulated by Robo-Slit signaling
Multiple lines of evidence from this study suggest that Robo-Slit signaling regulates the patterning of corneal nerves during cornea development. First, the concurrent mRNA expression patterns of Slit ligands in ocular tissues and Robo1 receptors in trigeminal neurons provided indirect evidence for their involvement in nerve guidance. Next, by using an organotypic co-culture assay and employing a soluble recombinant Robo1-Fc protein encoding the Ig-like domains that confer binding to the LRR of Slit molecules to reduce the quantity of lens-derived Slit molecules for binding to native Robo receptors on nerve growth cones, we found that perturbing Robo-Slit interactions in vitro inhibited the repulsive effects of the lens in a dose-dependent fashion. Moreover, when trigeminal explants were cultured on pericorneal tissue, adjacent to repulsive embryonic corneas, the majority of neurite growth cones avoided the cornea except in the presence of the recombinant Robo1-Fc protein. Finally, when Robo-Slit interactions were perturbed in the eyefield in vivo, pioneer nerves grew prematurely into the cornea. Combined, these findings indicate that Robo receptors on trigeminal nerve growth cones are involved in proper nerve pathfinding during cornea development.
Robo 1–2 receptors bind identical regions of Slit 1–3 proteins and this interaction occurs with similar affinity (Brose, et al. 1999), thus the Robo1-Fc recombinant protein inhibitor employed in this study is likely promiscuous in nature, binding to the LRR of any available Slit to prevent their interaction with any endogenous Robo receptors. Slit ligands and Robo receptors often function redundantly in the outgrowth and guidance of neurons (Plump, et al. 2002), thus it remains to be determined which Slit molecules produced by the embryonic ocular tissues bind which Robo receptors on trigeminal nerve growth cones during nerve ring formation to induce repulsion.
Robo1 is a likely candidate receptor for mediating nerve repulsion by ocular tissues, as its expression in neurons diminishes concurrent with growth cones no longer being repelled by the cornea. On the other hand, Robo2 expression is low in neurons during repulsion stages (data not shown), and its expression does not change until later stages, when it increases at E14 concurrent with nerve branching in the cornea epithelium (Kubilus and Linsenmayer. 2010). Slit2 represents a strong candidate molecule as it has been shown to repel nerves growing from ventral spinal cord, olfactory bulb, retinal ganglion and dentate gyrus explants and also induces growth cone collapse in olfactory bulb and hippocampal neurons (Brose, et al. 1999; Li, et al. 1999; Niclou, et al. 2000; Nguyen Ba-Charvet, et al. 2001). Moreover, a recent report found that when chick OpV explants were cultured near embryonic corneas or lens vesicles in the presence of function-blocking Slit2 antibodies, neurite length increased compared to control levels (Kubilus and Linsenmayer. 2010), revealing that Slit2 may influence trigeminal neuron outgrowth, although outcomes to nerve guidance were not examined in this study. Certainly, the identity and combination of Slit ligands binding to Robos can influence the outcome on nerve growth and guidance (Ypsilanti, et al. 2010). Currently, the molecular regulators secreted by the lens and cornea which interact with Robo to influence nerve behavior remain in question and provide an important topic for future experimentation.
In the rat, Robo1 and Robo2, but not Robo3, are expressed in sensory nuclei of the Tg during different stages of cornea development (Ozdinler and Erzurumlu. 2002; Ma and Tessier-Lavigne. 2007), while Slits 1–3 are expressed in the lens at concurrent stages, with Slit3 showing the highest expression levels in the epithelium of lens vesicles (Niclou, et al. 2000). These expression data indirectly suggest a requirement for Robo-Slit signaling in trigeminal nerve guidance in other model organisms. Consistent with this suggestion, nerves within Slit1:Slit2 knock-out mice show multiple deficits related to trigeminal nerve guidance (Ma and Tessier-Lavigne. 2007), although cornea innervation per se was not directly assessed in that study. The recent work of McKenna and Lwigale showing how the mouse cornea becomes innervated during normal embryonic development will help to provide further insight into the mechanisms of cornea innervation (McKenna and Lwigale. 2011), as many mouse mutants currently exist and have been described with Robo-Slit function deficit, as well as other knock-outs of nerve guidance receptors and ligands.
Temporal changes within the cornea and trigeminal neurons, but not within the lens, influences cornea innervation
The eye contains a variety of tissues that each may influence nerve growth behavior. In this study we focused on two of those tissues, the lens and the cornea. Inhibitory factors secreted by the lens are likely required at early embryonic stages to promote pericorneal nerve ring formation. Lensectomy prior to the arrival of trigeminal nerve growth cones to the presumptive cornea results in premature innervation of the cornea by E5 (Lwigale and Bronner-Fraser. 2007). Moreover, negative guidance factors are secreted by the lens as early as E3, since trigeminal explants co-cultured with lens of this age are highly repelled by the lens vesicle (Lwigale and Bronner-Fraser. 2007). At E3–E5, the lens epithelium directly underlies the presumptive cornea (Hay. 1980) likely allowing lens-secreted factors to diffuse into the cornea at these early stages since the cornea has not yet formed tight junctions between corneal endothelial cells (Hay and Revel. 1969). The repulsive capacity of the lens persists to late developmental stages (Lwigale and Bronner-Fraser. 2007), at timepoints beyond when nerves have entered the cornea (E9–E15), potentially due to continued developmental expression of inhibitory Slits (this study) and Sema3a (Kubilus and Linsenmayer. 2010) nerve guidance molecules. Therefore, changes in the repertoire of secreted factors released by the lens that may diffuse anteriorly to the cornea likely does not account for the developmental change from nerve repulsion to cornea in-growth.
Our in vitro studies suggest that by E5-6, the cornea alone, independent of the lens, can influence the behavior of trigeminal neurites to grow to its periphery then extend around its periphery, rather than crossing over its surface, reminiscent of pericorneal nerve ring formation in the developing eyefield. Like the lens, the repulsive molecules in the cornea are sufficient to repel neuronal growth cones in culture for up to two days. This reveals that, in addition to the lens, the cornea is a source of inhibitory factors which keep trigeminal nerves from prematurely entering its stroma. In support of this hypothesis, the cornea produces and secretes inhibitory Slits (this study) and Sema3a (Lwigale and Bronner-Fraser. 2007; Kubilus and Linsenmayer. 2010) as nerves first approach the cornea and form the pericorneal nerve ring. Moreover, the repulsive nature of the cornea is likely enhanced by lens-derived inhibitory molecules that may diffuse into the cornea during early embryonic development, due to the close proximity of the tissues.
Unlike the lens, the cornea undergoes a change from nerve-repulsive to nerve-permissiveness as a consequence of age. By harvesting corneas at different stages of development (E5–E9) and culturing them next to similar aged trigeminal neuron explants to examine the consequence on neurite guidance, we found that neurites were much less repelled by corneas at later stages of development. This begs the question: what changes occur in the cornea or the eye over time to make the cornea more amenable for nerve ingrowth? One explanation may be developmental expression of positive or negative guidance cue molecules in the cornea. Quantitative comparison of Sema3a levels in the cornea stroma during cornea innervation stages showed that both mRNA and protein levels become significantly reduced during nerve in-growth stages compared to earlier timepoints when the pericorneal nerve ring is forming (Kubilus and Linsenmayer. 2010). On the other hand, positive guidance cues Netrin 1 and 4 and their positive guidance receptor Neo1 are present in high levels in the developing cornea as nerves first invade the stroma (Conrad, et al. 2009), as well as at later stages. The presence of both negative and positive cues in the cornea likely reflects a balance between nerve guidance signals, dependent on concentration. Thus, the switch in nerve behavior at E9 may represent a diminution of negative signals or receptors in the cornea or trigeminal nerves, an increase in positive signals or receptors, or simply a change in the balance between the two signals and receptor expression profiles.
In addition, the switch from nerve repulsion to cornea nerve ingrowth may also be a result of physiological changes occurring in the developing eyefield. Concurrent with the arrival of nerves is the formation of the fluid-filled anterior chamber (Bard and Abbott. 1979) and the consequent appearance of intraocular pressure (Coulombre and Coulombre. 1957; Coulombre. 1957) which leads to a spatial separation between the cornea and lens, potentially making it more difficult for molecules to diffuse from the lens anteriorly to the cornea. Additionally, the formation of tight junctions between corneal endothelial cells (Hay and Revel. 1969) at approximately the same developmental timepoints may further impede secreted molecules from diffusing from the lens/aqueous humor into the cornea stroma (Hay. 1980; Tuft and Coster. 1990). Combined action of these developmental events could effectively lead to a barrier for diffusible molecules to pass from the lens into the corneal stroma. However, this would not preclude the possibility that by E6, or older, the cornea contains residual inhibitory molecules secreted by the lens during timepoints prior to the formation of physical barriers between the two tissues. In this case, lens-derived protein content in the cornea would eventually degrade or diffuse away over a matter of days, diminishing over time with fewer inhibitory proteins present in corneas as they age, consistent with the findings in this study.
Similar to nerve growth being influenced by changes in guidance cues in the target tissue, the levels of receptors within a nerve growth cone are highly regulated in time and space and these changes have been suggested to modify the response of a nerve to signaling molecules (Tessier-Lavigne and Goodman. 1996; Dickson. 2002). In the present study we have obtained evidence revealing that molecular changes occur in trigeminal nerves as a consequence of time (age), since increasing the age of trigeminal neurons in organotypic culture negatively impacted repulsion by an adjacent cornea whose age was unchanged. Transcriptional regulation of Robo1 receptor expression may help to explain the different responses to the cornea, since Robo1 expression diminished in a sub-population (OpV) of trigeminal neuronal cell bodies as development progressed. Likewise, transcriptional regulation of Robo receptor expression at the Drosophila midline modulates central and peripheral nerve guidance decisions (Stennard and Harvey. 2005; Liu, et al. 2009). Changes in the levels of receptor molecules on a pathfinding growth cone allows precise guidance decisions to be made in a target field whose expression of nerve guidance cues is more stable. In this regard, we show that Slit expression remained constant in the eyefield during stages of cornea innervation. Therefore we hypothesize that molecular changes at the level of the nerve growth cone plays a critical role in the transition from nerve repulsion to permissive during cornea innervation (Figure 8). This reveals that much information concerning cornea innervation can be gleaned from studying the expression and identities of receptor molecules on trigeminal neurons during cornea development. Such molecular changes remain to be characterized, and will represent a major focus of future research.
While many medical advances have been made in healing corneal trauma and in corrective surgeries, little is known concerning the mechanisms of corneal innervation or how to activate regeneration of the cut ends of corneal nerves once they become damaged. Results from this study suggest that the Robo-Slit family of nerve guidance molecules function during cornea innervation and should be considered when determining mechanisms to temporarily stimulate nerve growth in post-transplantation cornea stroma and post-LASIK nerve regeneration.
Highlights.
-
>
Robo-Slit nerve guidance family as mediators for trigeminal nerves during cornea innervation.
-
>
Slit1-3 expressed consistently in cornea and lens throughout cornea innervation.
-
>
Robo1 expression in trigeminal neurons was developmentally regulated, declining with neuron age.
-
>
Young trigeminal nerves are repelled by lens and cornea, in vivo and in vitro, except in the presence of Robo-Slit inhibitor.
-
>
Age-dependent molecular changes, including Robo1 reduction, allow nerve in-growth to cornea.
Supplementary Material
Supplementary Figure 1. Diffusion of fluorescent protein from beads implanted into E4-5 eyefield. (A–H) Fluorescence was monitored in live embryos in ovo following implantation at E4 under the limbal epithelia (A–H) or E5 in the anterior chamber (I–P) of beads (arrows) loaded with red fluorescent Alexa Fluor 546-albumin protein. Fluorescence could be detected near the lens and cornea as soon as thirty minutes following implantation and fluorescence persisted for 24 hours. Scale bar: 1 mm.
Acknowledgements
We kindly thank Abigail H. Conrad for insightful discussion and technical advice at the onset of this work. The authors would also like to acknowledge members of Dr. Gary W. Conrad’s laboratory for support and advice during the duration of this work. We are indebted to Dr. Edward Laufer (Columbia University) for providing plasmids used in this manuscript. This work was supported through funding by an individual Ruth L. Kirschstein postdoctoral NRSA award (F32 EY021708) from the National Eye Institute to T.S. as well as NIH grants R00 EY018050 to P.Y.L and R01 EY000952 to G.W.C and funding from the Research Career Development Core (Brychta) in the Division of Biology at Kansas State University GOBO000657 to G.W.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Eye Institute or the National Institutes of Health.
References
- Baker KS, Anderson SC, Romanowski EG, Thoft RA, SundarRaj N. Trigeminal Ganglion Neurons Affect Corneal Epithelial Phenotype. Influence on Type VII Collagen Expression in Vitro. Invest Ophthalmol Vis Sci. 1993;34:137–144. [PubMed] [Google Scholar]
- Bard JB, Abbott AS. Matrices Containing Glycosaminoglycans in the Developing Anterior Chambers of Chick and Xenopus Embryonic Eyes. Dev Biol. 1979;68:472–486. doi: 10.1016/0012-1606(79)90219-7. [DOI] [PubMed] [Google Scholar]
- Bee JA. The Development and Pattern of Innervation of the Avian Cornea. Dev Biol. 1982;92:5–15. doi: 10.1016/0012-1606(82)90145-2. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Aracil A, Acosta MC, Luna C, Gallar J. Nerves and Sensations from the Eye Surface. Ocul Surf. 2004;2:248–253. doi: 10.1016/s1542-0124(12)70112-x. [DOI] [PubMed] [Google Scholar]
- Benitez-del-Castillo JM, del Rio T, Iradier T, Hernandez JL, Castillo A, Garcia-Sanchez J. Decrease in Tear Secretion and Corneal Sensitivity After Laser in Situ Keratomileusis. Cornea. 2001;20:30–32. doi: 10.1097/00003226-200101000-00005. [DOI] [PubMed] [Google Scholar]
- Beuerman RW, Schimmelpfennig B. Sensory Denervation of the Rabbit Cornea Affects Epithelial Properties. Exp Neurol. 1980;69:196–201. doi: 10.1016/0014-4886(80)90154-5. [DOI] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit Proteins Bind Robo Receptors and have an Evolutionarily Conserved Role in Repulsive Axon Guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Chedotal A. Slits and their Receptors. Adv Exp Med Biol. 2007;621:65–80. doi: 10.1007/978-0-387-76715-4_5. [DOI] [PubMed] [Google Scholar]
- Conrad AH, Albrecht M, Pettit-Scott M, Conrad GW. Embryonic Corneal Schwann Cells Express some Schwann Cell Marker mRNAs, but no Mature Schwann Cell Marker Proteins. Invest Ophthalmol Vis Sci. 2009;50:4173–4184. doi: 10.1167/iovs.08-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad AH, Strafuss JM, Wittman MD, Conway S, Conrad GW. Thyroxine Increases the Rate but does Not Alter the Pattern of Innervation during Embryonic Chick Corneal Development. Invest Ophthalmol Vis Sci. 2008;49:139–153. doi: 10.1167/iovs.07-0800. [DOI] [PubMed] [Google Scholar]
- Coulombre AJ. The Role of Intraocular Pressure in the Development of the Chick Eye. II. Control of Corneal Size. AMA Arch Ophthalmol. 1957;57:250–253. doi: 10.1001/archopht.1957.00930050260015. [DOI] [PubMed] [Google Scholar]
- Coulombre AJ, Coulombre JL. The Role of Intraocular Pressure in the Development of the Chick Eye: III. Ciliary Body. Am J Ophthalmol. 1957;44:85–93. doi: 10.1016/0002-9394(57)90435-x. [DOI] [PubMed] [Google Scholar]
- Davis EA, Dohlman CH. Neurotrophic Keratitis. Int Ophthalmol Clin. 2001;41:1–11. doi: 10.1097/00004397-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Dickson BJ, Gilestro GF. Regulation of Commissural Axon Pathfinding by Slit and its Robo Receptors. Annu Rev Cell Dev Biol. 2006;22:651–675. doi: 10.1146/annurev.cellbio.21.090704.151234. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular Mechanisms of Axon Guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Dillon TE, Saldanha J, Giger R, Verhaagen J, Rochlin MW. Sema3A Regulates the Timing of Target Contact by Cranial Sensory Axons. J Comp Neurol. 2004;470:13–24. doi: 10.1002/cne.11029. [DOI] [PubMed] [Google Scholar]
- Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The Cephalic Neural Crest Provides Pericytes and Smooth Muscle Cells to all Blood Vessels of the Face and Forebrain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- Fricke C, Lee JS, Geiger-Rudolph S, Bonhoeffer F, Chien CB. Astray, a Zebrafish Roundabout Homolog Required for Retinal Axon Guidance. Science. 2001;292:507–510. doi: 10.1126/science.1059496. [DOI] [PubMed] [Google Scholar]
- Garcia-Hirschfeld J, Lopez-Briones LG, Belmonte C. Neurotrophic Influences on Corneal Epithelial Cells. Exp Eye Res. 1994;59:597–605. doi: 10.1006/exer.1994.1145. [DOI] [PubMed] [Google Scholar]
- Hao JC, Yu TW, Fujisawa K, Culotti JG, Gengyo-Ando K, Mitani S, Moulder G, Barstead R, Tessier-Lavigne M, Bargmann CI. C. Elegans Slit Acts in Midline, Dorsal-Ventral, and Anterior-Posterior Guidance Via the SAX-3/Robo Receptor. Neuron. 2001;32:25–38. doi: 10.1016/s0896-6273(01)00448-2. [DOI] [PubMed] [Google Scholar]
- Hay ED. Development of the Vertebrate Cornea. Int Rev Cytol. 1980;63:263–322. doi: 10.1016/s0074-7696(08)61760-x. [DOI] [PubMed] [Google Scholar]
- Hay ED, Revel JP. Fine Structure of the Developing Avian Cornea. Monogr Dev Biol. 1969;1:1–144. [PubMed] [Google Scholar]
- Holmes G, Niswander L. Expression of Slit-2 and Slit-3 during Chick Development. Dev Dyn. 2001;222:301–307. doi: 10.1002/dvdy.1182. [DOI] [PubMed] [Google Scholar]
- Howitt JA, Clout NJ, Hohenester E. Binding Site for Robo Receptors Revealed by Dissection of the Leucine-Rich Repeat Region of Slit. EMBO J. 2004;23:4406–4412. doi: 10.1038/sj.emboj.7600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh A, Miyabayashi T, Ohno M, Sakano S. Cloning and Expressions of Three Mammalian Homologues of Drosophila Slit Suggest Possible Roles for Slit in the Formation and Maintenance of the Nervous System. Brain Res Mol Brain Res. 1998;62:175–186. doi: 10.1016/s0169-328x(98)00224-1. [DOI] [PubMed] [Google Scholar]
- Keleman K, Ribeiro C, Dickson BJ. Comm Function in Commissural Axon Guidance: Cell-Autonomous Sorting of Robo in Vivo. Nat Neurosci. 2005;8:156–163. doi: 10.1038/nn1388. [DOI] [PubMed] [Google Scholar]
- Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, Huber LA, Technau GM, Dickson BJ. Comm Sorts Robo to Control Axon Guidance at the Drosophila Midline. Cell. 2002;110:415–427. doi: 10.1016/s0092-8674(02)00901-7. [DOI] [PubMed] [Google Scholar]
- Keynes R, Tannahill D, Morgenstern DA, Johnson AR, Cook GM, Pini A. Surround Repulsion of Spinal Sensory Axons in Higher Vertebrate Embryos. Neuron. 1997;18:889–897. doi: 10.1016/s0896-6273(00)80329-3. [DOI] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS. Slit is the Midline Repellent for the Robo Receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Kubilus JK, Linsenmayer TF. Developmental Guidance of Embryonic Corneal Innervation: Roles of Semaphorin3A and Slit2. Dev Biol. 2010;344:172–184. doi: 10.1016/j.ydbio.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS, Chen JH, Wu W, Fagaly T, Zhou L, Yuan W, Dupuis S, Jiang ZH, Nash W, Gick C, Ornitz DM, Wu JY, Rao Y. Vertebrate Slit, a Secreted Ligand for the Transmembrane Protein Roundabout, is a Repellent for Olfactory Bulb Axons. Cell. 1999;96:807–818. doi: 10.1016/s0092-8674(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Liu QX, Hiramoto M, Ueda H, Gojobori T, Hiromi Y, Hirose S. Midline Governs Axon Pathfinding by Coordinating Expression of Two Major Guidance Systems. Genes Dev. 2009;23:1165–1170. doi: 10.1101/gad.1774209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Patel K, Schmidt H, Andrews W, Pini A, Sundaresan V. Extracellular Ig Domains 1 and 2 of Robo are Important for Ligand (Slit) Binding. Mol Cell Neurosci. 2004;26:232–240. doi: 10.1016/j.mcn.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved Roles for Slit and Robo Proteins in Midline Commissural Axon Guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- Lwigale PY, Bronner-Fraser M. Lens-Derived Semaphorin3A Regulates Sensory Innervation of the Cornea. Dev Biol. 2007;306:750–759. doi: 10.1016/j.ydbio.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Lwigale PY. Embryonic Origin of Avian Corneal Sensory Nerves. Dev Biol. 2001;239:323–337. doi: 10.1006/dbio.2001.0450. [DOI] [PubMed] [Google Scholar]
- Ma L, Tessier-Lavigne M. Dual Branch-Promoting and Branch-Repelling Actions of Slit/Robo Signaling on Peripheral and Central Branches of Developing Sensory Axons. J Neurosci. 2007;27:6843–6851. doi: 10.1523/JNEUROSCI.1479-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfurt CF, Kingsley RE, Echtenkamp SE. Sensory and Sympathetic Innervation of the Mammalian Cornea. A Retrograde Tracing Study. Invest Ophthalmol Vis Sci. 1989;30:461–472. [PubMed] [Google Scholar]
- McKenna CC, Lwigale PY. Innervation of the Mouse Cornea during Development. Invest Ophthalmol Vis Sci. 2011;52:30–35. doi: 10.1167/iovs.10-5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal Nerves: Structure, Contents and Function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet KT, Brose K, Ma L, Wang KH, Marillat V, Sotelo C, Tessier-Lavigne M, Chedotal A. Diversity and Specificity of Actions of Slit2 Proteolytic Fragments in Axon Guidance. J Neurosci. 2001;21:4281–4289. doi: 10.1523/JNEUROSCI.21-12-04281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niclou SP, Jia L, Raper JA. Slit2 is a Repellent for Retinal Ganglion Cell Axons. J Neurosci. 2000;20:4962–4974. doi: 10.1523/JNEUROSCI.20-13-04962.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdinler PH, Erzurumlu RS. Slit2, a Branching-Arborization Factor for Sensory Axons in the Mammalian CNS. J Neurosci. 2002;22:4540–4549. doi: 10.1523/JNEUROSCI.22-11-04540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump AS, Erskine L, Sabatier C, Brose K, Epstein CJ, Goodman CS, Mason CA, Tessier-Lavigne M. Slit1 and Slit2 Cooperate to Prevent Premature Midline Crossing of Retinal Axons in the Mouse Visual System. Neuron. 2002;33:219–232. doi: 10.1016/s0896-6273(01)00586-4. [DOI] [PubMed] [Google Scholar]
- Riley NC, Lwigale PY, Conrad GW. Specificity of Corneal Nerve Positions during Embryogenesis. Mol Vis. 2001;7:297–304. [PubMed] [Google Scholar]
- Stennard FA, Harvey RP. T-Box Transcription Factors and their Roles in Regulatory Hierarchies in the Developing Heart. Development. 2005;132:4897–4910. doi: 10.1242/dev.02099. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The Molecular Biology of Axon Guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the Zebrafish snail1 Gene and its Expression in Wild-Type, Spadetail and no Tail Mutant Embryos. Development. 1993;119:1203–1215. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- Thompson H, Camand O, Barker D, Erskine L. Slit Proteins Regulate Distinct Aspects of Retinal Ganglion Cell Axon Guidance within Dorsal and Ventral Retina. J Neurosci. 2006;26:8082–8091. doi: 10.1523/JNEUROSCI.1342-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuft SJ, Coster DJ. The Corneal Endothelium. Eye (Lond) 1990;4(Pt 3):389–424. doi: 10.1038/eye.1990.53. [DOI] [PubMed] [Google Scholar]
- Vargesson N, Luria V, Messina I, Erskine L, Laufer E. Expression Patterns of Slit and Robo Family Members during Vertebrate Limb Development. Mech Dev. 2001;106:175–180. doi: 10.1016/s0925-4773(01)00430-0. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Ambrosio R. Laser in Situ Keratomileusis-Induced Neurotrophic Epitheliopathy. Am J Ophthalmol. 2001;132:405–406. doi: 10.1016/s0002-9394(01)00995-3. [DOI] [PubMed] [Google Scholar]
- Ypsilanti AR, Zagar Y, Chedotal A. Moving Away from the Midline: New Developments for Slit and Robo. Development. 2010;137:1939–1952. doi: 10.1242/dev.044511. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Yi BA, Bargmann CI. The Conserved Immunoglobulin Superfamily Member SAX-3/Robo Directs Multiple Aspects of Axon Guidance in C. Elegans. Cell. 1998;92:217–227. doi: 10.1016/s0092-8674(00)80916-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Diffusion of fluorescent protein from beads implanted into E4-5 eyefield. (A–H) Fluorescence was monitored in live embryos in ovo following implantation at E4 under the limbal epithelia (A–H) or E5 in the anterior chamber (I–P) of beads (arrows) loaded with red fluorescent Alexa Fluor 546-albumin protein. Fluorescence could be detected near the lens and cornea as soon as thirty minutes following implantation and fluorescence persisted for 24 hours. Scale bar: 1 mm.