Abstract
Mammalian cells have the ability to recognize virus infection and mount a powerful antiviral response. Pattern recognition receptor proteins detect molecular signatures of virus infection and activate antiviral signaling cascades. The RIG-I-like receptors are cytoplasmic DExD/H box proteins that can specifically recognize virus-derived RNA species as a molecular feature discriminating the pathogen from the host. The RIG-I-like receptor family is composed of three homologous proteins, RIG-I, MDA5, and LGP2. All of these proteins can bind double-stranded RNA species with varying affinities via their conserved DExD/H box RNA helicase domains and C-terminal regulatory domains. The recognition of foreign RNA by the RLRs activates enzymatic functions and initiates signal transduction pathways resulting in the production of antiviral cytokines and the establishment of a broadly effective cellular antiviral state that protects neighboring cells from infection and triggers innate and adaptive immune systems. The propagation of this signal via the interferon antiviral system has been studied extensively, while the precise roles for enzymatic activities of the RNA helicase domain in antiviral responses are only beginning to be elucidated. Here, current models for RLR ligand recognition and signaling are reviewed.
Introduction
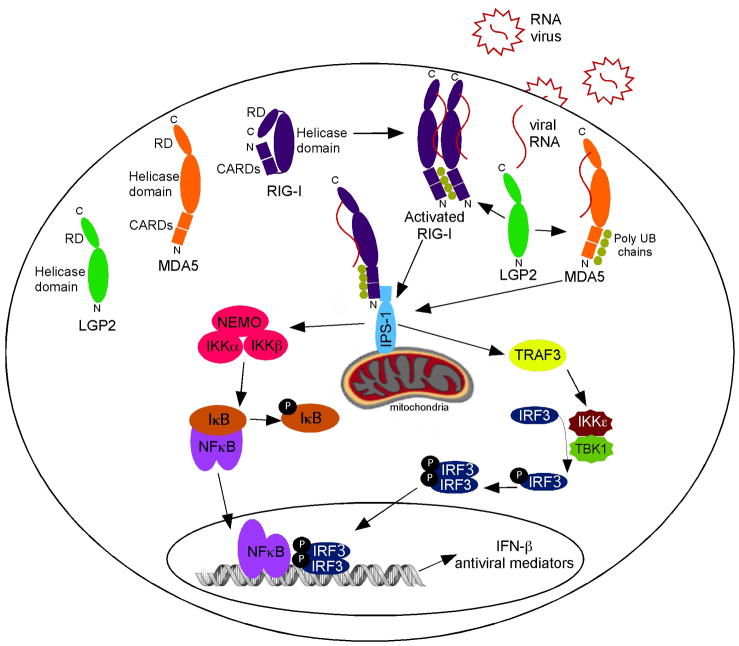
The innate immune system serves to protect the host from pathogen infection and disease. The first step for an efficient antimicrobial response is pathogen recognition at the site of primary infection by pattern-recognition receptor (PRR) proteins. These PRRs detect a specific molecular signature of the pathogen, referred to as a pathogen-associated molecular pattern (PAMP). Recognition of PAMPs allows the host to distinguish self from non-self and mount the appropriate immune response. In the case of RNA virus infections, induction of type I interferon (IFN) and other antiviral effectors drives immediate antiviral protection and maturation of innate immunity, inflammatory responses, and adaptive immunity for the control of infection. During RNA virus infection, the viral genomic RNA and replication intermediates serve as PAMPs that can be recognized by various classes of PRRs. A subset of the transmembrane Toll-like receptors (TLRs 3, 7, 8, and 9) expressed in immune cells can detect these foreign nucleic acids in topologically extracellular compartments (Takeuchi and Akira, 2010). Recognition of cytosolic pathogen-derived RNA is mediated in most cell types by a family of intracellular PRRs defined by three DExD/H box proteins collectively referred to as RIG-I like receptor (RLR) proteins. These proteins, RIG-I, MDA5, and LGP2 have the capacity to distinguish between self and non-self RNA in the cytoplasm and initiate host defenses against invading RNA viruses (Yoneyama and Fujita, 2009). In the current model, RNA virus infection produces cytoplasmic PAMP RNAs that are detected by the RLRs, initiating a serine-kinase signaling cascade resulting in the production of type I IFN and other antiviral effectors that together create a broadly effective antiviral state (Figure 1).
Figure 1. Signal Transduction by the RIG-I-like receptors.
The RIG-I-like receptors (RIG-I: blue, MDA5: orange, and LGP2: green) are activated upon recognition of virus-derived RNA (red stars/lines). RIG-I is present in the cytoplasm in an autoinhibited state, and upon activation a conformational change exposes the CARD domains for interaction with IPS-1. Activated RIG-I and MDA5 both signal through IPS-1 and their signaling activity may be positively regulated by LGP2. IPS-1 transmits the signal through protein kinase complexes leading to the activation of NFκB and IRF3 transcription factors, resulting in the production of IFN-β and other cytokines that initiate antiviral immunity. See text for details.
A color version of this figure is available online.
Identification and characterization of the RIG-I-like receptors
The RLR genes were originally identified in distinct biological contexts by transcriptional profiling. RIG-I was identified as a retinoic acid-inducible mRNA in promyelocytic leukemia cells (Sun, 1997). MDA5, together with other melanoma differentiation-associated genes, was first identified in human melanoma cells after induction of terminal differentiation by treatment with IFNβ and a protein kinase C agonist (Kang et al., 2002). LGP2 was described as a gene neighboring the Stat3 and Stat5 loci on mouse chromosome 11, conducted at the Laboratory of Genetics and Physiology (Cui et al., 2001).
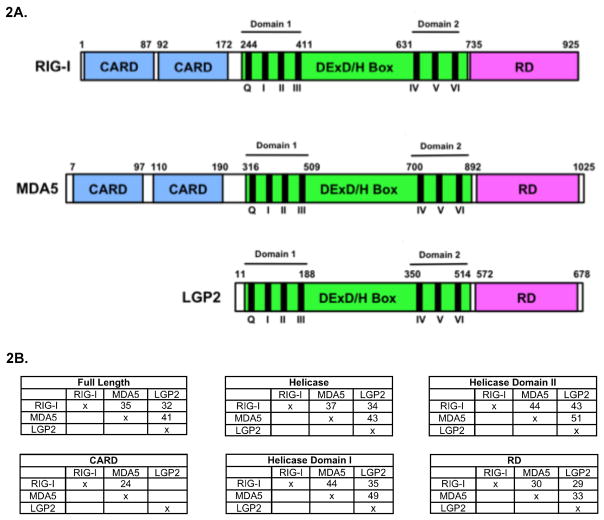
The biological functions of the RLR proteins were better understood when RIG-I was identified in a function-based cDNA library screen designed to discover proteins that could induce the expression of an IFNβ promoter reporter gene. This screen isolated an N-terminal fragment of RIG-I that encompassed key signaling domains. The C-terminal fragment of RIG-I was found to act as a dominant inhibitor of IFNβ induction (Yoneyama et al., 2004). Sequence alignment studies revealed MDA5 and LGP2 as close homologs to RIG-I, with an overall amino acid sequence identity of approximately 30%. The sequence identity was even higher within key domains, suggesting potential functional similarity (Yoneyama et al., 2005) (see figure 2B). The evolutionary conservation of the RLRs among vertebrates, and their relationships to other RNA processing enzymes has been noted (Zou et al., 2009). It is of interest to point out that the next closest homologs outside of the RLR family are the RNA interference components Dicer and Dicer-related helicase (DRH). These proteins share 30% sequence identity within their conserved helicase domains, but lack significant homology in surrounding regions, and the importance of this similarity, if any, is yet to be appreciated (Bamming and Horvath, 2009). MDA5 was found to have structural and functional similarity with RIG-I when it was characterized as an activator of IFN expression in response to RNA virus infection or transfection with the double-stranded RNA analog polyinosinic:polycytidilic acid (poly(I:C)) (Yoneyama et al., 2005). No functional insight was obvious for LGP2 other than its ability to bind dsRNA and clear structural similarity to RIG-I and MDA5. Under different experimental conditions LGP2 has been characterized as both a feedback inhibitor of RLR signaling (Komuro and Horvath, 2006, Rothenfusser et al., 2005, Yoneyama et al., 2005) and an activator of RLR signaling (Satoh et al., 2010, Venkataraman et al., 2007).
Figure 2. Diagram of the structural protein domains of RIG-I, MDA5, and LGP2.
A.) The RLRs are composed of a shared central DExD/H box helicase domain (green) containing conserved helicase motifs (roman numerals) which function to coordinate RNA binding and ATP hydrolysis. The C-terminal regulatory domain (CTD or RD, pink) implicated in RNA binding and inhibition of basal RIG-I signaling. RIG-I and MDA5 contain two tandem caspase activation and recruitment (CARD) domains (blue) that are critical for downstream signaling activity. The paramyxovirus V protein targets MDA5 and LGP2 at a minimal region coinciding with the boundaries of helicase domain 2 (motifs IV, V, and VI).
B.) Tables comparing the percent amino acid identity of the full length RLR proteins and defined protein domains.
A color version of this figure is available online.
Structure and function of the RLR proteins
The amino acid sequence conservation among RIG-I, MDA5, and LGP2 clearly defines these proteins as members of the same family, sharing conserved structural and functional domains (Figure 2). The most prominent feature is the central DExD/H-box region that is homologous to RNA helicase domains. This domain is capable of ATP hydrolysis activity in vitro, and is involved in dsRNA interactions. At their extreme C-terminus, the RLRs share a regulatory domain (RD) that has been implicated in regulation of RIG-I signaling activity (Saito et al., 2007) and is crucial for the specific recognition of RNA substrate features (Cui et al., 2008, Takahasi et al., 2008). In addition to these domains shared by all the RLRs, RIG-I and MDA5 have two N-terminal caspase activation and recruitment domain (CARD) regions that are absent in LGP2. The CARDs are protein interaction modules required for mediating antiviral signal transduction downstream of RNA recognition (Saito et al., 2007, Saito et al., 2008, Takahasi et al., 2008). Due to the absence of CARD regions in LGP2, positive antiviral effects are not observed upon expression of LGP2 in cells (Yoneyama et al., 2005). Each of these RLR domains is considered in greater detail below.
Helicase domain
RIG-I, MDA5, and LGP2 are members of the large helicase superfamily II (SF2). In general, SF2 enzymes participate in nearly all cellular pathways involving nucleic acids and can be described as a ubiquitous group of energy-dependent, nucleic acid remodeling proteins. RNA-specific SF2 proteins are found in processes including transcription, pre mRNA splicing, tRNA maturation, nucleocytoplasmic transport, translation, RNA decay and ribosome biogenesis (Abdelhaleem, 2005). Their role in RNA metabolism typically requires ATP hydrolysis-driven modulation of RNA or RNA-protein complexes, including RNA folding, RNA unwinding, and displacement of RNA-binding proteins (de la Cruz et al., 1999).
Like other SF2 members, the RLRs share a catalytic core composed of two RecA-like domains that encompass eight conserved helicase domain motifs (Cordin et al., 2006). The most highly conserved and prominent helicase domain sequence motifs, motif I-VI, cluster into either of the two RecA subdomains and function to coordinate RNA binding and ATP hydrolysis. These motifs are identifiable in the three RLRs, but shared variations clearly distinguish these motifs as related versions of the consensus sequences (Bamming and Horvath, 2009). The name DExD/H box refers to the signature sequence in motif II, also called the Walker B motif, which is “DECH” in the three RLR proteins (Walker et al., 1982). The presence of this signature motif contributed to the RLRs being defined as “helicase” proteins. Although the term “helicase” is used to indicate unwinding of double stranded DNA and RNA, DExD/H box proteins are now known to have many diverse functions, including translocation along single-stranded and double-stranded nucleic acids, unwinding of double-stranded nucleic acids, annealing of complementary strands, and displacement of proteins from ribonucleoprotein complexes (Pyle, 2008). Comparison of the RLR sequences with structurally characterized helicase proteins indicates that the RLRs may also contain a third domain inserted between the two core helicase domains, similar to the organization of the helicase domain of the Hef helicase protein of Pyrococcus furiosus (Nishino et al., 2005). As the Hef inter-domain plays a role in the recognition of specific nucleic acid structures, it has been speculated that Hef and the RLR helicase domains may have acquired the insertion domains to increase substrate specificity (Plumet et al., 2007).
Several studies indicate that ATP hydrolysis may be critical for proper RLR function and antiviral signaling. Initial experiments demonstrated that a point mutation in RIG-I motif I, the ATP binding Walker A motif, (Walker et al., 1982) yielded an ATPase-deficient protein that was also described as defective in antiviral signaling (Yoneyama et al., 2004), despite retention of RNA-binding ability (Plumet et al., 2007). The ATPase activity of RIG-I and MDA5 were found to strictly require RNA ligand stimulation, while LGP2 exhibited a low level of basal ATP hydrolysis in the absence of RNA (Bamming and Horvath, 2009). A more comprehensive mutational analysis was undertaken to evaluate six core helicase domain motifs of RIG-I, MDA5, and LGP2 in parallel. For all RLR proteins, mutation of any of the motifs resulted in defective ATP hydrolysis activity. For RIG-I, the mutated proteins acted as dominant negative inhibitors of endogenous cellular RIG-I signal transduction in trans. For MDA5, no trans inhibition was observed for any of the mutated proteins. These findings suggest fundamental differences in the stoichiometry of RIG-I or MDA5 signaling complexes. Despite their defective helicase domains, an unexpected consequence of the mutations was observed with MDA5: mutation to helicase motif I or motif III produced MDA5 proteins with constitutive RNA-independent hyperactive signal transduction activity. Closer examination revealed hyperactive signaling for the RIG-I motif III mutant as well, but only in cell lines that lacked active endogenous RIG-I pathways. These mutations clearly demonstrated the uncoupling of RLR signal transduction from RLR enzymatic activity by creating ATPase deficient hyperactive signaling proteins. A complementary phenotype was observed for another RIG-I mutant with a point mutation to motif V, producing a signaling-deficient protein that retained the ability to hydrolyze ATP. Together, these studies point to a regulatory role for ATP hydrolysis in RLR signal transduction that may be idiosyncratic to the individual receptor and its signaling context (Bamming and Horvath, 2009).
These studies also raise a question regarding the role of helicase enzymatic activity in RLR signaling. All three RLR proteins are able to hydrolyze ATP, but to date, ATP dependent RNA processing has only been reported for RIG-I. Experiments using purified truncated RIG-I containing only the helicase domain and short blunt-end dsRNA molecules as substrates showed weak activity interpreted as dsRNA strand displacement or unwinding activity (Marques et al., 2006). RIG-I-mediated unwinding of RNA/RNA duplexes and RNA/DNA heteroduplexes with at least 5 nucleotide-long 3′ overhangs and 5′ monophosphate ends in vitro was also reported (Takahasi et al., 2008). Intriguingly, an inverse correlation was observed between RNA substrate unwinding and the antiviral signaling stimulation capacity of that substrate. When transfected into mammalian cells, dsRNA substrates that could be unwound by RIG-I were poor inducers of IFNβ. Conversely, poor helicase substrates were found to be more potent IFNβ inducers (Takahasi et al., 2008). Despite these reports, no strand displacement was observed in single molecule FRET assays used to monitor RNA unwinding activity by RIG-I (Myong et al., 2009), and there are no reports of MDA5 or LGP2 mediated helicase activity. This uncertainty raises questions regarding the ability of RLRs in general to function as true RNA helicase proteins, and, if so, what are the biological consequences of dsRNA unwinding by RLRs.
Single molecule fluorescence microscopy techniques used to evaluate RIG-I protein-RNA interactions have yielded valuable insights into the enzymatic functions of RIG-I. Taking advantage of protein-induced fluorescence enhancement induced by RIG-I binding to immobilized dsRNA substrates, experiments demonstrated that in the presence of ATP, purified RIG-I protein is able to translocate along dsRNA substrates (Myong et al., 2009). While a double-stranded substrate is required for translocation activity, RIG-I was also able to translocate on RNA/DNA heteroduplex substrates, suggesting that RIG-I might track along a single RNA strand within the duplex. The rate of RIG-I translocation on dsRNA was found to be dependent on the concentration of ATP, and a truncated RIG-I protein lacking the CARD domains was able to translocate more rapidly than the full-length protein, suggesting the CARD domains may inhibit RIG-I translocation. However, when the dsRNA substrate contained a 5′-triphosphate (5′-PPP) modification, the presence of the CARD domains no longer had an inhibitory effect on the rate of RIG-I translocation. Based on these studies, a model was proposed that integrates more than one PAMP for RIG-I activity. Activation of the RIG-I ATPase and subsequent dsRNA translocation require both a 5′-PPP and double stranded regions of RNA (Myong et al., 2009). The ability of RIG-I to translocate along dsRNA without unwinding the duplex has been suggested to be an exclusive function of RLR proteins (Myong and Ha, 2010), however the universality of dsRNA translocation as a property of the RLRs, and the important question of its biological significance in RLR-mediated antiviral signaling will require further investigation.
The C-terminal regulatory domain (RD)
The regulatory domain (RD) at the C-terminus of RIG-I, MDA5, and LGP2 was identified as an autoinhibitory domain for RIG-I. Originally termed the repressor domain, expression of the RD of either RIG-I or LGP2 can inhibit RIG-I-mediated signaling, and deletion of the RIG-I RD region increases basal signaling activity (Cui et al., 2008, Saito et al., 2007, Takahasi et al., 2008). This form of autoregulation is a key feature of RIG-I, which is generally found to be inactive in the absence of activating ligand even when highly expressed (Saito et al., 2007). Autoregulation by the RD does not appear to be conserved in MDA5, as expression of MDA5 in cells results in high basal signaling activity in the absence of RNA, though including RNA ligand can increase this signaling (Bamming and Horvath, 2009). Analysis of the RLR RDs, both alone and in complex with various RNA ligands demonstrated that the RD regions of LGP2 and MDA5 have an affinity for the termini of blunt-ended dsRNA (Cui et al., 2008, Li et al., 2009a, Li et al., 2009b, Takahasi et al., 2009), while the RIG-I RD was implicated in the specific recognition of RNA 5′ end modifications (Cui et al., 2008, Takahasi et al., 2008, Wang et al., 2010). The discovery of the dual role of the RD in the inhibition of basal RIG-I signaling and the detection of specific RNA substrates led to the current model of RIG-I-mediated signaling (Figure 1). In this model, RIG-I is present in the cytoplasm in an auto-inhibited latent conformation in which the CARD domains are not available for downstream signaling. When the RD recognizes a foreign RNA molecule, a conformational change occurs exposing the CARDs for interaction with downstream signaling partners.
CARDS
Originally identified in a subset of adaptor molecules that are required for caspase processing and activation during apoptosis (Hofmann et al., 1997), the caspase activation and recruitment domain (CARD) is characterized as a protein-protein interaction domain involved in the assembly of protein complexes involved in diverse cellular functions including innate immune and inflammatory response pathways (Bouchier-Hayes and Martin, 2002). The tandem CARDs located at the N-terminus of RIG-I and MDA5 are the primary effector domains that transduce an RNA detection signal downstream. Expression of the RIG-I or MDA5 CARDs alone is sufficient to induce unregulated signal transduction (Yoneyama et al., 2005, Yoneyama et al., 2004). LGP2 lacks CARD sequences and is therefore unable to propagate an antiviral signal like RIG-I or MDA5, suggesting a distinct role for LGP2 despite its sequence conservation with RIG-I and MDA5.
Due to the critical function of the CARDs in antiviral signaling, it is not surprising to find these domains are highly regulated. The ubiquitin system has been shown to play diverse roles in the modification and regulation of the RLR CARDs. Certain ubiquitin modifications of RIG-I are critical for the creation of an active RIG-I signaling complex, while others serve to target RIG-I for proteasome-mediated degradation. The E3 ubiquitin ligase, TRIM25, adds ubiquitin to RIG-I on lysine 172 within the second CARD. This modification is essential for maximal RIG-I signaling (Gack et al., 2007). The tumor suppressor, CYLD, acts as an antagonist of TRIM25, removing the ubiquitin modifications within the RIG-I CARD, and possibly functioning to prevent inappropriate RIG-I signaling (Friedman et al., 2008, Zhang et al., 2008). Some advances have been made towards the mechanism that controls TRIM25-mediated ubiquitination and CYLD-mediated deubiquitination. Two ubiquitin ligases HOIP and HOIL-1L, which polymerize linear ubiquitin chains were shown to target both RIG-I and TRIM25, preventing the activating RIG-I ubiquitination by TRIM25 and also leading to TRIM25 degradation (Inn et al., 2011).
Another mechanism of ubiquitin-mediated regulation of RIG-I signaling was recently uncovered. Employing an in vitro reconstitution system, a non-covalent interaction between the RIG-I CARDs and linear ubiquitin chains was observed to activate RIG-I signaling (Zeng et al., 2010). The interaction is both RNA and ATP dependent, suggesting linear ubiquitin may be an additional endogenous ligand that cooperates in signaling. Other ubiquitin ligases have been shown to modify RIG-I, including RNF135/REUL/Riplet and RNF125, which ubiquitin modify the C-terminal domain of RIG-I or mark RIG-I for proteasomal degradation, respectively (Arimoto et al., 2007, Gao et al., 2009, Oshiumi et al., 2009, Oshiumi et al., 2010). Both MDA5 and its major downstream interaction partner were shown to be ubiquitination targets of RNF125, indicating it may be part of a general inhibitory mechanism (Arimoto et al., 2007).
RLRs in antiviral signaling
The RLRs are inducible by antiviral stimuli (Komuro et al., 2008) and are expressed widely in the cytoplasm of virtually all mammalian cells (Kato et al., 2005). RIG-I is essential for the induction of type I interferons after RNA virus infection in conventional dendritic cells (DCs) and fibroblasts, however plasmacytoid DCs use the TLR system rather than RIG-I for viral detection (Kato et al., 2005). Many studies of the RLRs have focused on RIG-I, and the accumulated evidence supports a well-characterized model of RIG-I-mediated signal transduction (Figure 1). A central protein within the RLR signaling pathway is the mitochondrial resident called IPS-1, MAVS, VISA, or CARDIFF (Kawai et al., 2005, Meylan et al., 2005, Seth et al., 2005, Xu et al., 2005), which exhibits CARD-CARD domain interactions with RIG-I and MDA5. Although the mitochondrial localization is essential for IPS-1 signaling, the requirement for mitochondria-specific functions is not fully understood. It has been suggested recently that in the presence of K63 ubiquitin chains, RIG-I may catalyze the conversion of MAVS on the mitochondrial membrane into functional prion-like aggregates (Hou et al., 2011). IPS-1 serves as a scaffold that mediates the assembly and activation of a serine-kinase cascade that includes canonical and non-canonical IκB kinases (Clemént et al., 2008). The signaling cascade between IPS-1 and the IKKs is not completely understood, but it requires E3 ubiquitin ligases of the TNF (tumor necrosis factor) receptor associated factor family (TRAF3 and TRAF6) (Kawai et al., 2005, Oganesyan et al., 2006, Saha et al., 2006, Xu et al., 2005). Association of RLR CARDs with the CARD of IPS-1 activates IKKα and IKKβ that derepress the NFκB transcription factor, allowing it to translocate to the nucleus and assemble on target gene promoters. In addition, the Tank-binding kinase, TBK1, and the inducible IKK, IKKε are activated to phosphorylate the IRF3 transcription factor, which then moves into the nucleus activating transcription of antiviral genes (Fitzgerald et al., 2003, Hiscott, 2007). The activated NFκB and IRF3 transcription factors are essential components for transcriptional regulation of the IFNβ gene, the central antiviral cytokine, and several other antiviral mediators and inflammatory effector genes (Figure 1). Recognition of foreign RNA by MDA5 also functions to activate this antiviral signaling cascade.
The exact role of LGP2 in RNA recognition and antiviral signaling remains unresolved, in part due to apparently opposing actions of LGP2 depending on the experimental approaches used. LGP2 has been demonstrated to both negatively and positively regulate RIG-I and MDA5-mediated antiviral signaling. Expression of LGP2 protein in cells effectively inhibits RLR-mediated IFN induction (Komuro and Horvath, 2006, Rothenfusser et al., 2005, Yoneyama et al., 2005). LGP2 is thought to be in low abundance at steady state, but is strongly inducible by antiviral stimulation, including poly(I:C) transfection, IFNα treatment, or virus infection. Based on these findings, and the absence of CARD signaling motifs, LGP2 was described as a feedback inhibitor for RLR signaling (Komuro et al., 2008, Rothenfusser et al., 2005, Saito et al., 2007).
Several mechanisms for LGP2 feedback inhibition were proposed (Vitour and Meurs, 2007, Komuro et al., 2008). Due to its strong RNA binding ability, LGP2 was originally suggested to sequester viral RNA from detection by RIG-I (Yoneyama et al., 2005). However, LGP2 proteins that are defective for RNA binding can still inhibit RLR signaling, indicating that RNA competition could not explain the effect of LGP2 expression (Bamming and Horvath, 2009). Based on titration experiments, it was suggested that CARD-independent LGP2 interaction with IPS-1 could compete with kinase recruitment to IPS-1 that is required for antiviral signaling. This model may account for the ability of LGP2 to inhibit antiviral signaling triggered in the absence of RNA through tandem CARD or IPS-1 expression (Komuro and Horvath, 2006). Further experimentation is needed to fully appreciate the biological role, if any, for LGP2 feedback inhibition.
In contrast to these examples of negative regulation by LGP2, targeted gene disruption studies have indicated a positive role for LGP2 in IFNβ expression and antiviral signaling (Moresco and Beutler, 2010, Pollpeter et al., 2011, Satoh et al., 2010). Mice lacking LGP2 exhibited decreased IFN-β production in response to various RNA viruses, but overexpression of the CARD domains of RIG-I and MDA5 in LGP2−/− cells rescued IFN-β promoter activity, suggesting LGP2 may act upstream of MDA5 and RIG-I (Satoh et al., 2010). The absence of LGP2 also reveals a role in the recognition of Listeria monocytogenes, DNA viruses, and cytosolic DNA (Pollpeter et al., 2011), suggesting a diverse positive effect of LGP2 in anti-microbial signaling.
Protein overexpression studies have suggested the RLRs exhibit various forms of signaling crosstalk. RIG-I homo-oligomerization has been shown to be important for the formation of an active signaling complex (Saito et al., 2007, Takahasi et al., 2008), and LGP2 was suggested to inhibit RIG-I by RD-mediated hetero-oligomerization (Saito et al., 2007). Other proteins and posttranslational modifications have also been suggested to positively or negatively regulate RIG-I and MDA5. For example, the IFN-inducible ZAPS protein may associate with RIG-I promoting oligomerization and ATP hydrolysis (Hayakawa et al., 2011), and dihydroxyacetone kinase (DAK) has been suggested to inhibit MDA5 but not RIG-I-mediated signaling (Diao et al., 2007). Phosphorylation of RIG-I by casein kinase II may function to maintain the RD autoregulation in the absence of ligand (Sun et al., 2011). Lysine acetylation of RIG-I has also been observed, but the biological significance of this modification remains to be determined (Choudhary et al., 2009). Due to the importance of RLR signaling in response to virus infection, it is likely that further experimentation will reveal additional layers of regulation and mechanisms of crosstalk among the RLRs under different biological contexts.
Nucleic acid recognition by RLRs
Many studies have contributed to an understanding of molecular features recognized by RIG-I (Schlee and Hartmann, 2010), but the precise RNA structures recognized by MDA5 and LGP2 in vivo remain largely undefined. The preexisting knowledge that virus-derived double-stranded RNA can induce IFN in a TLR-independent manner (Diebold et al., 2003), and the observation that the synthetic dsRNA analogue poly(I:C), a known inducer of antiviral signaling responses, binds to RIG-I (Yoneyama et al., 2004), suggested dsRNA as an important signature for RIG-I activation (Yoneyama et al., 2005). However, it was subsequently discovered that a more specific feature of pathogen RNA, the presence of 5′-end triphosphorylation, was optimal for RIG-I-specific detection (Hornung et al., 2006). While cellular mRNAs are processed to add a 5′ 7-methylguanosine cap, uncapped RNA molecules with 5′-triphosphate (5′-PPP) are produced by virus-encoded polymerases throughout the course of infection, and 5′-PPP is often present in native RNA genomes. The ability of RIG-I to recognize the single-stranded influenza A virus RNA segments required at least a monophosphate group at the 5′-end, but long dsRNA stretches were dispensable for RIG-I activation (Pichlmair et al., 2006). Evaluation of a variety of modified RNA species revealed that RNA molecules with a 5′-PPP, such as T7 phage polymerase transcripts, potently induce IFNβ gene expression in a RIG-I-dependent manner (Hornung et al., 2006). Enzymatic removal of the 5′-PPP greatly diminished the potency of IFN induction. These findings explain the previously reported observation that phage polymerase transcribed RNA is a more potent type I IFN inducer than synthetic RNA (Kim et al., 2004).
However, 5′-PPP ends are not sufficient to account for all of the RNA recognition properties of the RLRs. Studies revealed that single-stranded RNA, even with 5′-PPP ends, was insufficient for RIG-I activation (Schlee et al., 2009, Schmidt et al., 2009). Instead, base pairing near the 5′ end was required for RIG-I binding and subsequent signal transduction. Experiments also suggested a minimal requirement of 19 base paired nucleotides at the 5′-PPP end, but small bulge loops, such as the ones found in panhandle structures of several negative-sense RNA viruses were tolerated (Schlee and Hartmann, 2010, Schmidt et al., 2010). Current models suggest that the helicase domain contacts the dsRNA region, while the RD of RIG-I discriminates the 5′ end modifications (Cui et al., 2008, Takahasi et al., 2008, Wang et al., 2010).
Gel filtration chromatography experiments have demonstrated that the RD of RIG-I has the highest affinity for 5′-PPP dsRNA, but also binds appreciably to 5′-PPP ssRNA and blunt-ended dsRNA (Lu et al., 2010). Protein-RNA contact sites have been revealed in the crystal structure of the RIG-I RD bound to a 5′-PPP dsRNA (Lu et al., 2010). Comparison to the structure of the RIG-I RD bound to blunt ended dsRNA revealed distinct but overlapping amino acids are involved in substrate recognition, and that the orientation of two RNAs relative to the protein is dramatically different (Lu et al., 2011). These studies demonstrate that the RD of RIG-I has versatile RNA-binding properties allowing it to recognize different forms of pathogen derived RNA that may be present in the cytoplasm during virus infection. Comparing the crystal structures of RIG-I and LGP2 demonstrates that the two proteins use similar binding surfaces to interact with RNA, but that the RNA is shifted in orientation (Lu et al., 2011). A similar RD binding surface is also likely to be involved in MDA5 recognition of dsRNA (Li et al., 2009a, Takahasi et al., 2009), but it is notable that critical residues involved in detecting the 5′-PPP present in the RIG-I RD are lacking in LGP2 and MDA5, possibly indicating a structural basis for differences in RLR ligand specificity (Li et al., 2009a, Li et al., 2009b, Lu et al., 2010).
In addition to the study of synthetic RNAs, more natural virus-derived RNA ligands for RIG-I have also been characterized. Immunoprecipitation of RIG-I:RNA complexes from influenza A virus or Sendai virus -infected cells demonstrated that full-length virus genomes can act as physiological RIG-I agonists. The production of these ligands was found to be dependent on virus genome replication but not mRNA transcription (Rehwinkel et al., 2010). In contrast, sequencing of RNAs immunoprecipitated from Sendai virus and influenza A virus-infected cells demonstrated a preference for shorter 5′-PPP containing RNAs (Baum et al., 2010). For Sendai virus, these RNA molecules originated primarily from the 5′ ends of defective interfering (DI) genomes that contaminate many Sendai virus stocks, and are the main RNA species present in infected cells (Johnston, 1981). In addition to terminal 5′-end modifications, internal RNA sequence preferences of RIG-I have been investigated in the context of hepatitis C virus (HCV). Scanning the HCV RNA genome for RIG-I interactions revealed preferential RIG-I activation by a conserved polyuridine tract present in the 3′ untranslated region of the HCV genome (Saito et al., 2008, Uzri, 2009).
While much is known about RNA ligands for RIG-I, the potential ligands for LGP2 and MDA5 remain largely enigmatic. LGP2 binds strongly to dsRNA, irrespective of 5′ triphosphate (Bamming and Horvath, 2009), but the ligand specificity of LGP2 has yet to be revealed. The lack of detailed information regarding MDA5 substrates is in part due to the apparently weak RNA binding activity of MDA5 for the RNAs tested. However, a few studies have elucidated potential RNA features recognized by MDA5. Using an RNA substrate that had been enzymatically digested or sheared to create populations of discrete lengths, MDA5 was found to be most effectively activated by RNA molecules longer than 2kbp, whereas RIG-I was efficiently activated by shorter molecules (Kato et al., 2008). Testing reovirus genomic dsRNA segments of distinct lengths for the ability to activate RIG-I or MDA5 signaling supported this observation. The largest class of reovirus dsRNA segments (3.9 kbp) is detected by both proteins, while the small (1.2–1.4 kbp) and medium (2.2–2.3 kbp) subgenomic segments appear to signal solely through RIG-I (Kato et al., 2008). In addition to RNA substrate length, secondary structure may play a role in RNA recognition by MDA5. High molecular weight RNAs extracted from virus-infected cells were shown to preferentially activate MDA5-mediated signaling (Pichlmair et al., 2009). Interestingly, purely dsRNA species, even of significant length (>2 kb), that were extracted from the infected cells were not sufficient for MDA5 activation. Instead, it was proposed that structural features, such as RNA branches found in RNAs with both single-stranded and double-stranded regions might be required for recognition by MDA5. This is consistent with the notion that poly(I:C), the synthetic dsRNA analogue, which forms branches due to annealing of poly I and poly C strands of non-defined length, is primarily detected by MDA5 in vitro and in vivo (Gitlin et al., 2006, Kato et al., 2006). MDA5 may also be able to discriminate features specific to virus-derived mRNAs. The 5′ cap structures of eukaryotic mRNAs are methylated at the 2′ hydroxyl position of the ribose, and many viruses have evolved methyltransferases to modify their own mRNA to mimic the 2′-O-methylation of cellular mRNAs. Mutant coronaviruses with defective 2′-O-methyltransferase enzymes were found to induce much stronger antiviral responses than wild type coronaviruses. This differential response was found to be dependent on MDA5, as macrophages from MDA5-deficient mice did not hyperactivate IFN secretion when infected with methyltransferase-defective virus (Züst et al., 2011). The connection between MDA5 and 2′-O-methylation is an intriguing result, and studies confirming direct molecular interactions and MDA5 signaling activation will strengthen the evidence for mRNAs lacking 2′-O-methylation as natural substrates for MDA5.
Virus specificity of RLR signaling
RLR functions in RNA virus infections have also been investigated in vivo using mice with targeted gene disruptions (Barral et al., 2010). Increased susceptibility to virus infection and measurement of antiviral responses, such as IFN production or virus replication, allowed the assignment of specific viruses to individual RLR responses (Table 1). RIG-I-deficient mice were found to be more susceptible to infection with several negative strand RNA genome viruses including Newcastle disease virus, vesicular stomatitis virus, influenza A virus, and Sendai virus. Cells derived from these mice failed to initiate antiviral signaling programs, resulting in reduced production of IFNβ (Kato et al., 2006). Comparison of RIG-I-deficiency with MDA5-deficiency confirmed that several negative-strand RNA viruses elicited a lower IFN and cytokine induction and greater replicative capacity in the absence of RIG-I. However, no defects in IFN production were observed in response to the same viruses in the absence of MDA5. In contrast, MDA5 deficiency created a greater susceptibility to certain positive-sense single-stranded RNA genome viruses tested, specifically the picornaviruses, poliovirus and encephalomyocarditis virus (EMCV) (Gitlin et al., 2006, Kato et al., 2006). Infection with the positive-sense norovirus also induced less IFN and grew to higher titers in the absence of MDA5 (McCartney et al., 2008). However, the simplistic distinction between negative-sense and positive-sense single stranded RNA viruses as being recognized by RIG-I and MDA5, respectively, cannot be generalized (Table 1). For example, the positive-sense flaviviruses including HCV, West Nile virus, and dengue fever virus are more potent activators of IFN responses in wild type than RIG-I-deficient mice, suggesting RIG-I is involved in their detection (Kato et al., 2006, Sumpter et al., 2005). Similarly, dengue virus and West Nile virus, as well as dsRNA genome reovirus are thought to be detected by both RIG-I and MDA5, because cytokine responses were only abrogated in RIG-I deficient cells that were also subjected to siRNA-mediated MDA5 depletion (Fredericksen et al., 2008, Kato et al., 2008, Loo et al., 2008). MDA5 has also recently been shown to play a role in sustaining expression of IFN in response to negative-sense ssRNA viruses including the paramyxovirus Sendai virus (Gitlin et al., 2010). Comparing wild-type mice to MDA5 deficient mice, antiviral responses were comparable during early Sendai virus infection, but at later times IFN levels were significantly decreased in the absence of MDA5. This study suggests that MDA5 is critical for sustained IFN expression during Sendai virus infection and provides the first evidence of a role for MDA5 during infections in vivo caused by negative sense RNA viruses (Gitlin et al., 2010).
Table 1.
Enhanced virus replication in the absence of RIG-I and MDA5.
| Absence of RIG-I | Absence of MDA5 | Absence of MDA5 and RIG-I |
|---|---|---|
|
Rhabdoviridae: Vesticular stomatitis virus (VSV) 1, 2 Rabies virus 12 |
Picornaviridae: Encephalomyocarditis virus (EMCV) 5, 2 Theiler’s disease virus 2 Mengo virus 2 |
Flaviviridae: Dengue virus 3 West Nile virus 7 |
|
Orthomyxoviridae: Influenza A virus 2, 3 Influenza B virus 3 |
Calciviridae: Norovirus 6 |
Reoviridae: Orthoreovirus 3,8 |
|
Paramyxoviridae: Newcastle disease virus (NDV) 1, 2, 3 Respiratory syncytial virus (RSV) 3 Measles virus 11 |
Paramyxoviridae: Sendai virus (SeV)10 |
Paramyxoviridae: Sendai virus (SeV) 2, 9 |
|
Flaviviridae: Hepatitis C virus (HCV) 4 Japanese encephalitis virus (JEV) 2 |
Viruses listed here have been tested in mice or cells with targeted gene disruption in RIG-I, MDA5, or both.
(Yount et al., 2008)
The precise role for LGP2 in virus recognition was not completely resolved by analysis of mice with LGP2 gene disruption. The first report found disparate functions for LGP2 in virus recognition, and the LGP2-deficient mice were more resistant to VSV and less resistant to EMCV (Venkataraman et al., 2007). The second strain of LGP2-deficient mice produce less IFN following infection with Sendai virus, Japanese encephalitis virus, reovirus, EMCV and Mengo virus (Satoh et al., 2010). An exception was influenza A virus, for which LGP2-deficiency had no effect on IFN levels. In contrast to the previous LGP2 knockout, VSV also elicited a weaker antiviral response in the absence of LGP2. Together with the report of impaired responses to dsDNA and DNA pathogens, these studies indicate a wide ranging positive regulatory role of LGP2 on antiviral responses (Pollpeter et al., 2011, Satoh et al., 2010). The exact mechanism of LGP2 in mediating RNA detection is not clear, but it was proposed that LGP2 might function upstream of both MDA5 and RIG-I, based on the observation that expression of isolated RIG-I or MDA5 CARD fragments induces IFN synthesis irrespective of LGP2 presence (Satoh et al., 2010). Interestingly, mice harboring a point mutation in LGP2 that inactivates ATP hydrolysis displayed a phenotype similar to LGP2 deficient mice (Satoh et al., 2010), indicating that LGP2 enzymatic activity is entirely essential for its function in the antiviral system (Moresco and Beutler, 2010).
The observed differential effects of MDA5 or RIG-I deficiencies revealed that recognition of viruses by RIG-I and MDA5 can be explained in large part by the presence or absence of identified RIG-I-specific molecular RNA signatures, serving as PAMPs present throughout the course of virus infection. For example, some negative-strand viruses have complementary 5′ and 3′ ends that can form panhandle structures and have 5′-PPP ends, as documented for the genomic RNAs of measles virus, Sendai virus and VSV (Schlee and Hartmann, 2010). Similarly, each of the 8 ssRNA segments that comprise the genome of influenza A virus contains complementary 5′ and 3′ ends that form double-stranded panhandles (Hsu et al., 1987) required for transcription (Fodor et al., 1994). In addition to genomic RNA, products of viral transcription can also potently activate RIG-I. One example is the measles virus leader sequence, a small RNA that accumulates in infected cells, which is non-capped, non-polyadenylated, and carries 5′-PPP ends (Plumet et al., 2007). As a result of these features, all of these RNA viruses signal antiviral responses in a RIG-I dependent manner.
In contrast, the absence of specific RNA features prevents RIG-I recognition. Lack of 5′-PPP in genomic RNA can also explain the apparent lack of phenotype in RIG-I-deficient cells and mice after infection with viruses such as EMCV, Hantaan virus, Crimean Congo hemorrhagic fever virus, and borna disease virus (Habjan et al., 2008). All of these viruses are known for permanent genomic RNA processing, either by viral nuclease cleavage of 5′-PPP ends (Schneider et al., 2005) or by linkage of a viral protein, such as VPg protein of EMCV (Vartapetian et al., 1980). Such modifications can be viewed as an active viral immune evasion strategy (Habjan et al., 2008).
Viral evasion of RLR-mediated signaling
One complication that arises when attempting to assign specific viruses to individual RLR proteins is the evolution of virus-encoded immune evasion mechanisms that prevent normal recognition and response. As such, a lack of phenotype in virus infected RLR-deficient cells does not necessarily correlate with a complete lack of involvement of the RLR in recognition of that virus. The importance of RLR-mediated initiation of the IFN antiviral response is underscored by the fact that many viruses have evolved mechanisms to disrupt antiviral signaling, targeting key proteins in the RLR signaling pathway, regulators of RLR signaling, or the RLRs directly. For example Hepatitis C virus (HCV) can disrupt the RLR-mediated signaling cascade by targeting the signaling adaptor molecule IPS-1 (Foy et al., 2003, Li et al., 2005, Meylan et al., 2005). The HCV protease called NS3/4A cleaves IPS-1 at the transmembrane domain interface, removing it from the mitochondria and preventing downstream signaling (Breiman et al., 2005, Li et al., 2005, Meylan et al., 2005). Other viruses have evolved mechanisms to target critical regulators of RLR-mediated signaling. The nonstructural protein (NS1) of influenza A virus is a well-characterized antagonist of antiviral host responses including dsRNA signaling (Krug et al., 2003). It has been demonstrated that NS1 suppresses RIG-I by targeting the activating ubiquitin ligase, TRIM25, an essential positive regulator of RIG-I (Gack et al., 2009).
Several other viral inhibitory mechanisms have been described, including some that directly target the RLR proteins (Komuro et al., 2008). The paramyxovirus V protein is a well described IFN evasion protein that specifically disrupts MDA5 mediated signal transduction (Randall and Goodbourn, 2008). A highly conserved zinc-binding domain of the paramyxovirus V protein binds to MDA5 preventing MDA5-dependent stimulation of the IFN-β promoter (Andrejeva et al., 2004). The V protein does not interact with or inhibit the activity of RIG-I (Childs et al., 2007), but does associate with LGP2, suggesting LGP2 may play a positive role in antiviral defense (Parisien et al., 2009). The V protein binds to the helicase domain of MDA5, targeting approximately 130 amino acids that exactly coincide with the boundaries of helicase domain 2, the C-terminal RecA domain (Figure 2). The minimal V protein binding region of MDA5 has higher amino acid sequence identity with LGP2 than with the analogous region of RIG-I, correlating with the targeting specificity of the V proteins for MDA5 and LGP2, but not RIG-I (Parisien et al., 2009) (see Table 1-helicase domain II). Viruses have evolved many strategies to target the RLR-signaling pathway both directly and indirectly, illustrating the importance of this pathway in detection of virus infection.
Perspectives
The RLRs have been studied extensively since their discovery as a class of intracellular pattern recognition receptors. While research has yielded many insights into the signaling cascades initiated by activated RLRs, the RNA features they recognize, their mechanisms of activation, their enzymatic activities, and many aspects of their diverse biological roles in pathogen recognition and innate immunity remain unresolved.
One area of future investigation will seek to define the specific RNA ligands that regulate the activity of LGP2 and MDA5. Although some studies have suggested MDA5 may preferentially bind RNA with higher order structures (Pichlmair et al., 2009), or viral mRNA lacking 2′-O-methylation (Züst et al., 2011), no direct molecular interaction has been shown, most likely due to the low RNA binding affinity of MDA5. More sensitive techniques to assay MDA5 RNA binding will likely help resolve this question. Little is known about the ligand specificity of LGP2, although it binds strongly to dsRNA, irrespective of 5′ triphosphate (Bamming and Horvath, 2009). It is also possible that LGP2 does not detect a specific molecular feature of foreign RNA, but is rather working in conjunction with or upstream of MDA5 and RIG-I (Moresco and Beutler, 2010), sampling various foreign RNA molecules and modifying their conformation to be more readily detectable by RIG-I or MDA5 (Figure 3A).
Figure 3. Potential model of LGP2 function.
A.) LGP2 may have enzymatic activity capable of remodeling RNA substrates, making them more readily detectable by RIG-I and MDA5, thus serving a positive role in antiviral signaling.
B.) During steady state and initially during virus infection the concentrations of LGP2 may be low, allowing LGP2 to function independently or together with RIG-I and MDA5 to positively regulate signaling, perhaps by remodeling RNA substrates (see 3A). LGP2 is inducible by antiviral stimuli, and accumulation of LGP2 to higher levels would allow it to interact with other proteins (blue box, red oval) to form complexes capable of inhibiting RIG-I- and MDA5-mediated antiviral signaling.
A color version of this figure is available online.
Like the RNA ligand specificity of LGP2, the general role of LGP2 in RLR-mediated antiviral signaling is undetermined. The demonstrated positive and negative regulatory roles for LGP2 suggest multiple functions for this protein manifested under specific biological contexts. One could speculate that if the steady state concentration of LGP2 is low, it may work alone or together with RIG-I and MDA5 to positively regulate signaling pathways, perhaps by utilizing its enzymatic activity to remodel RNA substrates (Figure 3B). Antiviral signaling responses cause increased accumulation of LGP2 sufficient for trans inhibition of pre-existing protein or RNA complexes, acting as a negative regulator (Figure 3B). As the concentration of LGP2 continues to rise, these complexes would become saturated, allowing LGP2 to function independently or form homo-oligomers with distinct functional consequences. Determination of the protein interaction partners and oligomeric states of LGP2 throughout infectious cycles will help clarify these potential mechanisms. It is likely that the inhibitory RD interactions, the RNA binding properties, and ATP hydrolysis activity of LGP2 all contribute to its diverse functional roles, many of which will undoubtedly become clear with further research.
It is evident that an enzymatically active helicase domain is required for proper RLR-signaling (Bamming and Horvath, 2009), but the functions of the helicase domain and ATP hydrolysis are only beginning to be understood. The most recent study regarding potential enzymatic activity of the RLRs demonstrated that RIG-I has the ability to translocate on dsRNA substrates in an ATP-dependent manner (Myong et al., 2009). The translocation activity of RIG-I may serve to integrate two PAMPs by scanning dsRNA molecules searching for a 5′-PPP modification to activate downstream antiviral signaling. The RLRs are members of the DExH family of SF2 helicases, many of which have evolved to function as protein displacement enzymes, remodeling ribonucleoprotein (RNP) complexes (Jankowsky and Bowers, 2006, Pyle et al., 2007), or assisting the catalysis of tertiary RNA structure formation (Pyle et al., 2007). It is possible that the potential translocation activity of the RLRs could displace proteins from viral RNPs, or perhaps catalyze the breakdown of RNA tertiary structure, in either case allowing the RNA to be recognized as an activating ligand for downstream signaling. The biological significance of this translocation activity in antiviral signaling and the potential conservation as a property of the RLR protein family remain to be determined. The field of RLR biology has grown rapidly since their discovery in antiviral responses to RNA viruses less than ten years ago. Future directions will synthesize research from animal knockout models and the fields of molecular and cell biology, biophysics, and biochemistry, revealing new layers of signaling regulation, RNA recognition, and enzymatic activities, leading to a more complete understanding of RLR biology.
Acknowledgments
We are grateful to members of the Horvath laboratory for helpful discussions and to Darja Pollpeter for her significant intellectual and experimental contributions to the RLR research in the group.
Footnotes
Declaration of Interest:
The authors report no declarations of interest. Research on the RLRs and antiviral responses in the Horvath lab are funded by NIH grants AI073919, AI050707, IMVC Pilot Project Translocation Activity of RLR Family Members AI083005 to CMH. A.M.B. is funded by the NIH Cellular and Molecular Basis of Disease Training Grant GM08061.
References
- ABDELHALEEM M. RNA helicases: regulators of differentiation. Clin Biochem. 2005;38:499–503. doi: 10.1016/j.clinbiochem.2005.01.010. [DOI] [PubMed] [Google Scholar]
- ANDREJEVA J, CHILDS KS, YOUNG DF, CARLOS TS, STOCK N, GOODBOURN S, RANDALL RE. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci USA. 2004;101:17264–9. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARIMOTO K, TAKAHASHI H, HISHIKI T, KONISHI H, FUJITA T, SHIMOTOHNO K. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc Natl Acad Sci U S A. 2007;104:7500–5. doi: 10.1073/pnas.0611551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAMMING D, HORVATH CM. Regulation of signal transduction by enzymatically inactive antiviral RNA helicase proteins MDA5, RIG-I, and LGP2. J Biol Chem. 2009;284:9700–12. doi: 10.1074/jbc.M807365200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRAL P, SARKAR D, SU Z-Z, BARBER GN, DESALLE R, RACANIELLO V, FISHER PB. Functions of the cytoplasmic RNA sensors RIG-I and MDA-5- Key regulators of innate immunity. Pharmacology and Therapeutics. 2010:1–16. doi: 10.1016/j.pharmthera.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUM A, SACHIDANANDAM R, GARCIA-SASTRE A. Preference of RIG-I for short viral RNA molecules in infected cells revealed by next-generation sequencing. Proceedings of the National Academy of Sciences. 2010;107:16303–16308. doi: 10.1073/pnas.1005077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUCHIER-HAYES L, MARTIN SJ. CARD games in apoptosis and immunity. EMBO Rep. 2002;3:616–21. doi: 10.1093/embo-reports/kvf139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREIMAN A, GRANDVAUX N, LIN R, OTTONE C, AKIRA S, YONEYAMA M, FUJITA T, HISCOTT J, MEURS EF. Inhibition of RIG-I-dependent signaling to the interferon pathway during hepatitis C virus expression and restoration of signaling by IKKepsilon. J Virol. 2005;79:3969–78. doi: 10.1128/JVI.79.7.3969-3978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHILDS K, STOCK N, ROSS C, ANDREJEVA J, HILTON L, SKINNER M, RANDALL R, GOODBOURN S. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology. 2007;359:190–200. doi: 10.1016/j.virol.2006.09.023. [DOI] [PubMed] [Google Scholar]
- CHOUDHARY C, KUMAR C, GNAD F, NIELSEN ML, REHMAN M, WALTHER TC, OLSEN JV, MANN M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–40. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- CLÉMENT JF, MELOCHE S, SERVANT MJ. The IKK-related kinases: from innate immunity to oncogenesis. Cell Research. 2008;18:889–899. doi: 10.1038/cr.2008.273. [DOI] [PubMed] [Google Scholar]
- CORDIN O, BANROQUES J, TANNER NK, LINDER P. The DEAD-box protein family of RNA helicases. Gene. 2006;367:17–37. doi: 10.1016/j.gene.2005.10.019. [DOI] [PubMed] [Google Scholar]
- CUI S, EISENÄCHER K, KIRCHHOFER A, BRZÓZKA K, LAMMENS A, LAMMENS K, FUJITA T, CONZELMANN KK, KRUG A, HOPFNER KP. The C-terminal regulatory domain is the RNA 5′-triphosphate sensor of RIG-I. Molecular Cell. 2008;29:169–79. doi: 10.1016/j.molcel.2007.10.032. [DOI] [PubMed] [Google Scholar]
- CUI Y, LI M, WALTON KD, SUN K, HANOVER JA, FURTH PA, HENNIGHAUSEN L. The Stat3/5 locus encodes novel endoplasmic reticulum and helicase-like proteins that are preferentially expressed in normal and neoplastic mammary tissue. Genomics. 2001;78:129–34. doi: 10.1006/geno.2001.6661. [DOI] [PubMed] [Google Scholar]
- DE LA CRUZ J, KRESSLER D, LINDER P. Unwinding RNA in Saccharomyces cerevisiae: DEAD-box proteins and related families. Trends Biochem Sci. 1999;24:192–8. doi: 10.1016/s0968-0004(99)01376-6. [DOI] [PubMed] [Google Scholar]
- DIAO F, LI S, TIAN Y, ZHANG M, XU LG, ZHANG Y, WANG RP, CHEN D, ZHAI Z, ZHONG B, TIEN P, SHU HB. Negative regulation of MDA5-but not RIG-I-mediated innate antiviral signaling by the dihydroxyacetone kinase. Proc Natl Acad Sci U S A. 2007;104:11706–11. doi: 10.1073/pnas.0700544104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIEBOLD SS, MONTOYA M, UNGER H, ALEXOPOULOU L, ROY P, HASWELL LE, AL-SHAMKHANI A, FLAVELL R, BORROW P, REIS E SOUSA C. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424:324–8. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- FITZGERALD KA, MCWHIRTER SM, FAIA KL, ROWE DC, LATZ E, GOLENBOCK DT, COYLE AJ, LIAO SM, MANIATIS T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–6. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- FODOR E, PRITLOVE DC, BROWNLEE GG. The influenza virus panhandle is involved in the initiation of transcription. J Virol. 1994;68:4092–6. doi: 10.1128/jvi.68.6.4092-4096.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOY E, LI K, WANG C, SUMPTER R, JR, IKEDA M, LEMON SM, GALE M., JR Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science. 2003;300:1145–8. doi: 10.1126/science.1082604. [DOI] [PubMed] [Google Scholar]
- FREDERICKSEN BL, KELLER BC, FORNEK J, KATZE MG, GALE M., JR Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1. J Virol. 2008;82:609–16. doi: 10.1128/JVI.01305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN CS, O’DONNELL MA, LEGARDA-ADDISON D, NG A, CARDENAS WB, YOUNT JS, MORAN TM, BASLER CF, KOMURO A, HORVATH CM, XAVIER R, TING AT. The tumour suppressor CYLD is a negative regulator of RIG-I-mediated antiviral response. EMBO Reports. 2008;9:930–936. doi: 10.1038/embor.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GACK MU, ALBRECHT RA, URANO T, INN KS, HUANG IC, CARNERO E, FARZAN M, INOUE S, JUNG JU, GARCÍA-SASTRE A. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host & Microbe. 2009;5:439–49. doi: 10.1016/j.chom.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GACK MU, SHIN YC, JOO CH, URANO T, LIANG C, SUN L, TAKEUCHI O, AKIRA S, CHEN Z, INOUE S, JUNG JU. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- GAO D, YANG YK, WANG RP, ZHOU X, DIAO FC, LI MD, ZHAI ZH, JIANG ZF, CHEN DY. REUL is a novel E3 ubiquitin ligase and stimulator of retinoic-acid-inducible gene-I. PLoS ONE. 2009;4:e5760. doi: 10.1371/journal.pone.0005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GITLIN L, BARCHET W, GILFILLAN S, CELLA M, BEUTLER B, FLAVELL R, DIAMOND MS, COLONNA M. Essential role of mda-5 in type I IFN responses to polyriboinosinic-polyribocytidylic acid and encephalomyocarditis picornavirus. PNAS. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GITLIN L, BENOIT L, SONG C, CELLA M, GILFILLAN S, HOLTZMAN MJ, COLONNA M. Melanoma differentiation-associated gene 5 (MDA5) is involved in the innate immune response to Paramyxoviridae infection in vivo. PLoS Pathog. 2010;6:e1000734. doi: 10.1371/journal.ppat.1000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABJAN M, ANDERSSON I, KLINGSTRÜM J, SCHÜMANN M, MARTIN A, ZIMMERMANN P, WAGNER V, PICHLMAIR A, SCHNEIDER U, MÜHLBERGER E, MIRAZIMI A, WEBER F. Processing of genome 5′ termini as a strategy of negative-strand RNA viruses to avoid RIG-I-dependent interferon induction. PLoS ONE. 2008;3:e2032. doi: 10.1371/journal.pone.0002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYAKAWA S, SHIRATORI S, YAMATO H, KAMEYAMA T, KITATSUJI C, KASHIGI F, GOTO S, KAMEOKA S, FUJIKURA D, YAMADA T, MIZUTANI T, KAZUMATA M, SATO M, TANAKA J, ASAKA M, OHBA Y, MIYAZAKI T, IMAMURA M, TAKAOKA A. ZAPS is a potent stimulator of signaling mediated by the RNA helicase RIG-I during antiviral responses. Nat Immunol. 2011;12:37–44. doi: 10.1038/ni.1963. [DOI] [PubMed] [Google Scholar]
- HISCOTT J. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem. 2007;282:15325–9. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- HOFMANN K, BUCHER P, TSCHOPP J. The CARD domain: a new apoptotic signalling motif. Trends Biochem Sci. 1997;22:155–6. doi: 10.1016/s0968-0004(97)01043-8. [DOI] [PubMed] [Google Scholar]
- HORNUNG V, ELLEGAST J, KIM S, BRZÓZKA K, JUNG A, KATO H, POECK H, AKIRA S, CONZELMANN KK, SCHLEE M, ENDRES S, HARTMANN G. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–7. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- HOU F, SUN L, ZHENG H, SKAUG B, JIANG QX, CHEN ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–61. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSU MT, PARVIN JD, GUPTA S, KRYSTAL M, PALESE P. Genomic RNAs of influenza viruses are held in a circular conformation in virions and in infected cells by a terminal panhandle. Proc Natl Acad Sci U S A. 1987;84:8140–4. doi: 10.1073/pnas.84.22.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INN KS, GACK MU, TOKUNAGA F, SHI M, WONG LY, IWAI K, JUNG JU. Linear ubiquitin assembly complex negatively regulates RIG-I- and TRIM25-mediated type I interferon induction. Mol Cell. 2011;41:354–65. doi: 10.1016/j.molcel.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANKOWSKY E, BOWERS H. Remodeling of ribonucleoprotein complexes with DExH/D RNA helicases. Nucleic Acids Res. 2006;34:4181–8. doi: 10.1093/nar/gkl410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON MD. The characteristics required for a Sendai virus preparation to induce high levels of interferon in human lymphoblastoid cells. J Gen Virol. 1981;56:175–84. doi: 10.1099/0022-1317-56-1-175. [DOI] [PubMed] [Google Scholar]
- KANG DC, GOPALKRISHNAN RV, WU Q, JANKOWSKY E, PYLE AM, FISHER PB. mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci USA. 2002;99:637–42. doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATO H, SATO S, YONEYAMA M, YAMAMOTO M, UEMATSU S, MATSUI K, TSUJIMURA T, TAKEDA K, FUJITA T, TAKEUCHI O, AKIRA S. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- KATO H, TAKEUCHI O, MIKAMO-SATOH E, HIRAI R, KAWAI T, MATSUSHITA K, HIIRAGI A, DERMODY TS, FUJITA T, AKIRA S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–10. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATO H, TAKEUCHI O, SATO S, YONEYAMA M, YAMAMOTO M, MATSUI K, UEMATSU S, JUNG A, KAWAI T, ISHII KJ, YAMAGUCHI O, OTSU K, TSUJIMURA T, KOH CS, REIS E SOUSA C, MATSUURA Y, FUJITA T, AKIRA S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–5. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- KAWAI T, TAKAHASHI K, SATO S, COBAN C, KUMAR H, KATO H, ISHII KJ, TAKEUCHI O, AKIRA S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nature Immunology. 2005;6:981–8. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- KIM DH, LONGO M, HAN Y, LUNDBERG P, CANTIN E, ROSSI JJ. Interferon induction by siRNAs and ssRNAs synthesized by phage polymerase. Nat Biotechnol. 2004;22:321–5. doi: 10.1038/nbt940. [DOI] [PubMed] [Google Scholar]
- KOMURO A, BAMMING D, HORVATH CM. Negative regulation of cytoplasmic RNA-mediated antiviral signaling. Cytokine. 2008;43:350–8. doi: 10.1016/j.cyto.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMURO A, HORVATH CM. RNA-and virus-independent inhibition of antiviral signaling by RNA helicase LGP2. J Virol. 2006;80:12332–42. doi: 10.1128/JVI.01325-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRUG RM, YUAN W, NOAH DL, LATHAM AG. Intracellular warfare between human influenza viruses and human cells: the roles of the viral NS1 protein. Virology. 2003;309:181–9. doi: 10.1016/s0042-6822(03)00119-3. [DOI] [PubMed] [Google Scholar]
- LI X, LU C, STEWART M, XU H, STRONG RK, IGUMENOVA T, LI P. Structural basis of double-stranded RNA recognition by the RIG-I like receptor MDA5. Archives of Biochemistry and Biophysics. 2009a;488:23–33. doi: 10.1016/j.abb.2009.06.008. [DOI] [PubMed] [Google Scholar]
- LI X, RANJITH-KUMAR CT, BROOKS MT, DHARMAIAH S, HERR AB, KAO C, LI P. The RIG-I-like receptor LGP2 recognizes the termini of double-stranded RNA. J Biol Chem. 2009b;284:13881–91. doi: 10.1074/jbc.M900818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI XD, SUN L, SETH RB, PINEDA G, CHEN ZJ. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc Natl Acad Sci USA. 2005;102:17717–22. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOO YM, FORNEK J, CROCHET N, BAJWA G, PERWITASARI O, MARTINEZ-SOBRIDO L, AKIRA S, GILL MA, GARCÍA-SASTRE A, KATZE MG, GALE M. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82:335–45. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LU C, RANJITH-KUMAR CT, HAO L, KAO CC, LI P. Crystal structure of RIG-I C-terminal domain bound to blunt-ended double-strand RNA without 5′ triphosphate. Nucleic Acids Res. 2011;39:1565–75. doi: 10.1093/nar/gkq974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LU C, XU H, RANJITH-KUMAR CT, BROOKS MT, HOU TY, HU F, HERR AB, STRONG RK, KAO CC, LI P. The Structural Basis of 52 Triphosphate Double-Stranded RNA Recognition by RIG-I C-Terminal Domain. Structure. 2010:1–12. doi: 10.1016/j.str.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARQUES JT, DEVOSSE T, WANG D, ZAMANIAN-DARYOUSH M, SERBINOWSKI P, HARTMANN R, FUJITA T, BEHLKE MA, WILLIAMS BR. A structural basis for discriminating between self and nonself double-stranded RNAs in mammalian cells. Nat Biotechnol. 2006;24:559–65. doi: 10.1038/nbt1205. [DOI] [PubMed] [Google Scholar]
- MCCARTNEY SA, THACKRAY LB, GITLIN L, GILFILLAN S, VIRGIN HW, COLONNA M. MDA-5 recognition of a murine norovirus. PLoS Pathog. 2008;4:e1000108. doi: 10.1371/journal.ppat.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYLAN E, CURRAN J, HOFMANN K, MORADPOUR D, BINDER M, BARTENSCHLAGER R, TSCHOPP J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–72. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- MORESCO EMY, BEUTLER B. LGP2: Positive about viral sensing. Proceedings of the National Academy of Sciences. 2010;107:1261–1262. doi: 10.1073/pnas.0914011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYONG S, CUI S, CORNISH PV, KIRCHHOFER A, GACK MU, JUNG JU, HOPFNER KP, HA T. Cytosolic Viral Sensor RIG-I Is a 5′-Triphosphate-Dependent Translocase on Double-Stranded RNA. Science. 2009;323:1070–1074. doi: 10.1126/science.1168352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYONG S, HA T. Stepwise translocation of nucleic acid motors. Curr Opin Struct Biol. 2010;20:121–7. doi: 10.1016/j.sbi.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHINO T, KOMORI K, TSUCHIYA D, ISHINO Y, MORIKAWA K. Crystal structure and functional implications of Pyrococcus furiosus hef helicase domain involved in branched DNA processing. Structure. 2005;13:143–53. doi: 10.1016/j.str.2004.11.008. [DOI] [PubMed] [Google Scholar]
- OGANESYAN G, SAHA SK, GUO B, HE JQ, SHAHANGIAN A, ZARNEGAR B, PERRY A, CHENG G. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–11. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- OSHIUMI H, MATSUMOTO M, HATAKEYAMA S, SEYA T. Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I to promote interferon-beta induction during the early phase of viral infection. J Biol Chem. 2009;284:807–17. doi: 10.1074/jbc.M804259200. [DOI] [PubMed] [Google Scholar]
- OSHIUMI H, MIYASHITA M, INOUE N, OKABE M, MATSUMOTO M, SEYA T. The Ubiquitin Ligase Riplet Is Essential for RIG-I-Dependent Innate Immune Responses to RNA Virus Infection. Cell Host & Microbe. 2010;8:496–509. doi: 10.1016/j.chom.2010.11.008. [DOI] [PubMed] [Google Scholar]
- PARISIEN JP, BAMMING D, KOMURO A, RAMACHANDRAN A, RODRIGUEZ JJ, BARBER G, WOJAHN RD, HORVATH CM. A Shared Interface Mediates Paramyxovirus Interference with Antiviral RNA Helicases MDA5 and LGP2. J Virol. 2009;83:7252–7260. doi: 10.1128/JVI.00153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICHLMAIR A, SCHULZ O, TAN CP, REHWINKEL J, KATO H, TAKEUCHI O, AKIRA S, WAY M, SCHIAVO G, REIS E SOUSA C. Activation of MDA5 Requires Higher-Order RNA Structures Generated during Virus Infection. J Virol. 2009;83:10761–10769. doi: 10.1128/JVI.00770-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICHLMAIR A, SCHULZ O, TAN CP, NÄSLUND TI, LILJESTRÖM P, WEBER F, REIS E SOUSA C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- PLUMET S, HERSCHKE F, BOURHIS JM, VALENTIN H, LONGHI S, GERLIER D. Cytosolic 5′-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS ONE. 2007;2:e279. doi: 10.1371/journal.pone.0000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLPETER D, KOMURO A, BARBER GN, HORVATH CM. Impaired cellular responses to cytosolic DNA or infection with Listeria monocytogenes and vaccinia virus in the absence of the murine LGP2 protein. PLoS ONE. 2011;6:e18842. doi: 10.1371/journal.pone.0018842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PYLE AM. Translocation and unwinding mechanisms of RNA and DNA helicases. Annu Rev Biophys. 2008;37:317–36. doi: 10.1146/annurev.biophys.37.032807.125908. [DOI] [PubMed] [Google Scholar]
- PYLE AM, FEDOROVA O, WALDSICH C. Folding of group II introns: a model system for large, multidomain RNAs? Trends Biochem Sci. 2007;32:138–45. doi: 10.1016/j.tibs.2007.01.005. [DOI] [PubMed] [Google Scholar]
- RANDALL RE, GOODBOURN S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- REHWINKEL J, TAN CP, GOUBAU D, SCHULZ O, PICHLMAIR A, BIER K, ROBB N, VREEDE F, BARCLAY W, FODOR E. RIG-I Detects Viral Genomic RNA during Negative-Strand RNA Virus Infection. Cell. 2010;140:397–408. doi: 10.1016/j.cell.2010.01.020. [DOI] [PubMed] [Google Scholar]
- ROTHENFUSSER S, GOUTAGNY N, DIPERNA G, GONG M, MONKS BG, SCHOENEMEYER A, YAMAMOTO M, AKIRA S, FITZGERALD KA. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J Immunol. 2005;175:5260–8. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- SAHA SK, PIETRAS EM, HE JQ, KANG JR, LIU SY, OGANESYAN G, SHAHANGIAN A, ZARNEGAR B, SHIBA TL, WANG Y, CHENG G. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 2006;25:3257–63. doi: 10.1038/sj.emboj.7601220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO T, HIRAI R, LOO YM, OWEN D, JOHNSON CL, SINHA SC, AKIRA S, FUJITA T, GALE M. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc Natl Acad Sci USA. 2007;104:582–7. doi: 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO T, OWEN DM, JIANG F, MARCOTRIGIANO J, GALE M. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523–7. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATOH T, KATO H, KUMAGAI Y, YONEYAMA M, SATO S, MATSUSHITA K, TSUJIMURA T, FUJITA T, AKIRA S, TAKEUCHI O. From the Cover: LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proceedings of the National Academy of Sciences. 2010;107:1512–1517. doi: 10.1073/pnas.0912986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLEE M, HARTMANN G. The Chase for the RIG-I Ligand-Recent Advances. Molecular therapy: the journal of the American Society of Gene Therapy. 2010 doi: 10.1038/mt.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLEE M, ROTH A, HORNUNG V, HAGMANN CA, WIMMENAUER V, BARCHET W, COCH C, JANKE M, MIHAILOVIC A, WARDLE G. Recognition of 52 Triphosphate by RIG-I Helicase Requires Short Blunt Double-Stranded RNA as Contained in Panhandle of Negative-Strand Virus. Immunity. 2009;31:25–34. doi: 10.1016/j.immuni.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT A, ENDRES S, ROTHENFUSSER S. Pattern recognition of viral nucleic acids by RIG-I-like helicases. J Mol Med. 2010:1–8. doi: 10.1007/s00109-010-0672-8. [DOI] [PubMed] [Google Scholar]
- SCHMIDT A, SCHWERD T, HAMM W, HELLMUTH JC, CUI S, WENZEL M, HOFFMANN FS, MICHALLET MC, BESCH R, HOPFNER KP, ENDRES S, ROTHENFUSSER S. 5′-triphosphate RNA requires base-paired structures to activate antiviral signaling via RIG-I. Proc Natl Acad Sci USA. 2009;106:12067–72. doi: 10.1073/pnas.0900971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER U, SCHWEMMLE M, STAEHELI P. Genome trimming: a unique strategy for replication control employed by Borna disease virus. Proc Natl Acad Sci USA. 2005;102:3441–6. doi: 10.1073/pnas.0405965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETH RB, SUN L, EA CK, CHEN ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–82. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- SUMPTER R, LOO YM, FOY E, LI K, YONEYAMA M, FUJITA T, LEMON SM, GALE M. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol. 2005;79:2689–99. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN YW. Thesis. Shanghai Second Medical University; 1997. RIG-I, a human homolog gene of RNA helicase, is induced by retinoic acid during the differentiation of acute promyelocytic leukemia cell. [Google Scholar]
- SUN Z, REN H, LIU Y, TEELING JL, GU J. Phosphorylation of RIG-I by casein kinase II inhibits its antiviral response. J Virol. 2011;85:1036–47. doi: 10.1128/JVI.01734-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASI K, KUMETA H, TSUDUKI N, NARITA R, SHIGEMOTO T, HIRAI R, YONEYAMA M, HORIUCHI M, OGURA K, FUJITA T, INAGAKI F. Solution Structures of Cytosolic RNA Sensor MDA5 and LGP2 C-terminal Domains: IDENTIFICATION OF THE RNA RECOGNITION LOOP IN RIG-I-LIKE RECEPTORS. Journal of Biological Chemistry. 2009;284:17465–17474. doi: 10.1074/jbc.M109.007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASI K, YONEYAMA M, NISHIHORI T, HIRAI R, KUMETA H, NARITA R, GALE M, INAGAKI F, FUJITA T. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Molecular Cell. 2008;29:428–40. doi: 10.1016/j.molcel.2007.11.028. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI O, AKIRA S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- UZRI D, GEHRKE L. Nucleotide sequences and modifications that determine RIG-I/RNA binding and signaling activities. J Virol. 2009;83:4174–84. doi: 10.1128/JVI.02449-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARTAPETIAN AB, DRYGIN YF, CHUMAKOV KM, BOGDANOV AA. The structure of the covalent linkage between proteins and RNA in encephalomyocarditis virus. Nucleic Acids Res. 1980;8:3729–42. doi: 10.1093/nar/8.16.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENKATARAMAN T, VALDES M, ELSBY R, KAKUTA S, CACERES G, SAIJO S, IWAKURA Y, BARBER GN. Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J Immunol. 2007;178:6444–55. doi: 10.4049/jimmunol.178.10.6444. [DOI] [PubMed] [Google Scholar]
- VITOUR D, MEURS EF. Regulation of interferon production by RIG-I and LGP2: a lesson in self-control. Sci STKE. 2007;2007:pe20. doi: 10.1126/stke.3842007pe20. [DOI] [PubMed] [Google Scholar]
- WALKER JE, SARASTE M, RUNSWICK MJ, GAY NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–51. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y, LUDWIG J, SCHUBERTH C, GOLDECK M, SCHLEE M, LI H, JURANEK S, SHENG G, MICURA R, TUSCHL T, HARTMANN G, PATEL DJ. Structural and functional insights into 5′-ppp RNA pattern recognition by the innate immune receptor RIG-I. Nature Structural & Molecular Biology. 2010;17:781–7. doi: 10.1038/nsmb.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU LG, WANG YY, HAN KJ, LI LY, ZHAI Z, SHU HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Molecular Cell. 2005;19:727–40. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- YONEYAMA M, FUJITA T. RNA recognition and signal transduction by RIG-I-like receptors. Immunological Reviews. 2009;227:54–65. doi: 10.1111/j.1600-065X.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- YONEYAMA M, KIKUCHI M, MATSUMOTO K, IMAIZUMI T, MIYAGISHI M, TAIRA K, FOY E, LOO YM, GALE M, AKIRA S, YONEHARA S, KATO A, FUJITA T. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–8. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- YONEYAMA M, KIKUCHI M, NATSUKAWA T, SHINOBU N, IMAIZUMI T, MIYAGISHI M, TAIRA K, AKIRA S, FUJITA T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature Immunology. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- ZENG W, SUN L, JIANG X, CHEN X, HOU F, ADHIKARI A, XU M, CHEN ZJ. Reconstitution of the RIG-I Pathway Reveals a Signaling Role of Unanchored Polyubiquitin Chains in Innate Immunity. Cell. 2010;141:315–330. doi: 10.1016/j.cell.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG M, WU X, LEE AJ, JIN W, CHANG M, WRIGHT A, IMAIZUMI T, SUN SC. Regulation of IkappaB kinase-related kinases and antiviral responses by tumor suppressor CYLD. J BiolChem. 2008;283:18621–6. doi: 10.1074/jbc.M801451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOU J, CHANG M, NIE P, SECOMBES CJ. Origin and evolution of the RIG-I like RNA helicase gene family. BMC Evol Biol. 2009;9:85. doi: 10.1186/1471-2148-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZÜST R, CERVANTES-BARRAGAN L, HABJAN M, MAIER R, NEUMAN BW, ZIEBUHR J, SZRETTER KJ, BAKER SC, BARCHET W, DIAMOND MS, SIDDELL SG, LUDEWIG B, THIEL V. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nature Immunology. 2011;12:137–43. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]