Abstract
Increased consumption of cruciferous vegetables is associated with decreased risk in prostate cancer (PCa). The active compound in cruciferous vegetables appears to be the self dimerized product [3,3’-diindolylmethane (DIM)] of indole-3-carbinol (I3C). Nutritional grade B-DIM (absorption-enhanced) has proven safe in a Phase I trial in PCa. We investigated the anti-cancer activity of B-DIM as a new biological approach to improve the effects of radiotherapy for hormone refractory prostate cancer cells, which were either positive or negative for androgen receptor (AR) expression. B-DIM inhibited cell growth in a dose-dependent manner in both PC-3 (AR−) and C4-2B (AR+) cell lines. B-DIM was effective at increasing radiation-induced cell killing in both cell lines, independently of AR expression. B-DIM inhibited NF-κB and HIF-1α DNA activities and blocked radiation-induced activation of these transcription factors in both PC-3 and C4-2B cells. In C4-2B (AR+) cells, AR expression and nuclear localization were significantly increased by radiation. However, B-DIM abrogated the radiation–induced AR increased expression and trafficking to the nucleus, which was consistent with decreased PSA secretion. In vivo, treatment of PC-3 prostate tumors in nude mice with B-DIM and radiation resulted in significant primary tumor growth inhibition and control of metastasis to para-aortic lymph nodes. These studies demonstrate that B-DIM augments radiation-induced cell killing and tumor growth inhibition. B-DIM impairs critical survival signaling pathways activated by radiation, leading to enhanced cell killing. These novel observations suggest that B-DIM could be used as a safe compound to enhance the efficacy of radiotherapy for castrate-resistant PCa.
Keywords: radiation, B-DIM, prostate cancer, androgen receptor
1. Introduction
Several nutritional compounds, including soy isoflavones (genistein), indole-3-carbinol (I3C), 3,3'-diindolylmethane (DIM), curcumin, resveratrol, (–)-epigallocatechin-3-gallate, and others, have been recognized as cancer chemopreventive agents because of their anti-carcinogenic activity [1,2]. We found that soy isoflavones enhance the therapeutic efficacy of radiotherapy for prostate cancer (PCa) in pre-clinical studies in vitro and in vivo [3].
In addition to the beneficial chemopreventive effects of soy in Asian countries resulting in decreased incidence of prostate and breast cancers [4], an increased consumption of cruciferous vegetables has also been found to be associated with decreased risk in prostate cancer [5]. The major bio-active compounds I3C and its in vivo dimeric derivative DIM are found in cruciferous vegetables [2]. I3C is chemically unstable in aqueous and gastric acidic environment, and is rapidly converted to DIM. DIM has demonstrated pleiotropic anti-cancer effects on PCa cells and various malignant cell lines, leading to cancer cell apoptosis [2,6]. For clinical application, a formulation of nutritional grade B-DIM (or BR-DIM) with higher bioavailability was manufactured by BioResponse (LLC, Boulder, CO), which contains pharmaceutically pure DIM microdispersed in spray-dried starch particles [7]. Treatment of castrate-resistant PCa patients with B-DIM capsules has proven safe in a recent Phase I trial with modest therapeutic efficacy [7]. B-DIM was previously found to enhance the activity of chemotherapeutic agents in pre-clinical studies in vitro and in vivo [2]. Since radiotherapy is commonly used for early stage and locally advanced PCa, we investigated whether B-DIM could enhance the effects of radiation in a pre-clinical setting in vitro and in vivo using PCa cells.
PCa is the most common cancer diagnosed in men in the United States and is the second leading cause of cancer death in men. It has been estimated that 217,730 new cases of PCa were diagnosed in 2010 and 32,050 men were expected to die [8]. Localized PCa is sensitive to conventional radiotherapy, yet this treatment was reported to be insufficient to eradicate PCa in a significant proportion of patients resulting in clinical recurrence [9,10]. Local failure after radiotherapy for PCa predisposes to distant metastases.
Resistant and progressive PCa tumors could still express a functional androgen receptor (AR) even though they become non-responsive to androgen ablation therapy, defined as castrate-resistant stage. AR is a nuclear transcription factor, which upon activation by androgen binding, interacts with androgen response elements (ARE) in the promoter of target genes, including the prostate-specific antigen (PSA) [11,12]. Binding of androgen to AR plays a critical role in cancer cell proliferation, and in the development and progression of PCa [11,12]. It has been reported that Akt and the nuclear transcription factor κB (NF-κB) regulate the AR signaling by phosphorylation of AR or transcriptional regulation of AR [13]. Interestingly, B-DIM affected these AR-associated pathways by inhibiting Akt activation, NF-κB DNA binding activity, AR phosphorylation, AR expression and PSA expression, contributing to apoptosis of PCa cells [6]. To dissect further the role of AR in the molecular response of PCa cells to B-DIM, two cell lines PC-3 (AR−) cells and C4-2B (AR+) (LNCaP derived cell line), which are androgen independent and mimic castrate-resistant phenotypes, were compared. We investigated whether B-DIM could target the signaling pathways essential for the progression of PCa including AR, NF-κB and hypoxia-inducible factor (HIF-1α), and thereby impair the radioresistance of PCa cells. The combined B-DIM and radiation therapeutic approach was tested in vitro and in vivo using an orthotopic pre-clinical PCa tumor model.
2. Materials and Methods
2.1. Tissue culture
PC-3 human PCa cell line (AR− and non responsive to androgen) was cultured in F-12K culture medium (CM) containing 7% heat-inactivated fetal bovine serum (FBS) with supplements [14]. The C4-2B human PCa cell line (AR+ and non responsive to androgen) was cultured in RPMI 1640 CM containing 10% FBS [14].
2.2. Cell growth assays and clonogenic analysis of cell survival
C4-2B or PC-3 cells were plated at 105 cells per well in duplicate wells, in 6-well plates. A day later, cells were treated with doses of 5–25 μM B-DIM alone or combined with 3Gy irradiation. B-DIM was generously provided by Dr. Michael Zeligs (BioResponse, Boulder, CO). B-DIM was dissolved in DMSO to 50 mmol/L stock solution and further diluted in CM. Radiation treatment was done with an X-ray generating Pantak HF 320 instrument (settings at 320kV, 10 mA, @ ~ 0.9 Gy per minute). After 72h incubation, viable cells were counted using trypan blue exclusion dye [14]. For clonogenic assays, cells were pre-treated with B-DIM for 24 h and then irradiated. After radiation of PC-3 cells, cells re-plated in triplicate wells in 6-well plates at: 500 cells per well for control; 1,000 cells for B-DIM; 2,000 for 3 Gy and 3,000 cells for combined B-DIM + radiation treatment. On day 10 of culture, colonies were fixed and stained using 2% crystal violet and counted [14]. For C4-2B cells, cells were seeded, in triplicates, on matrigel coated 35mm plates (BD Biosciences, MA) in 2 mL CM as follows: 5,000 cells for control; 10,000 cells for B-DIM; 10,000 cells for 3 Gy radiation and 20,000 cells for radiation + B-DIM. Colony plates were supplemented with B-DIM. On day 15, colonies were stained with para-iodonitrotetrazolium violet overnight at 37°C and counted using a Nikon Eclipse TS 100 inverted microscope. The surviving fraction (SF) was calculated as previously detailed [14].
2.3. Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) was performed on nuclear extracts using IRDye-700 labeled NF-κB oligonucleotide or IRDye-700 labeled HIF-1α oligonucleotide (LI-COR Biosciences, Lincoln, NE) as previously detailed [14].
2.4. Western blot analysis of androgen receptor expression
C4-2B cells were pre-treated with B-DIM for 48 h, stimulated with 1nM Dihydrotestosterone (DHT) overnight [6], and then irradiated with 3 Gy. At 5h after radiation, cytosolic and nuclear extracts proteins were prepared using Sigma CelLytic™ NuCLEAR™ Extraction Kit (Sigma-Aldrich, St. Louis, MO) [14]. Proteins were loaded and separated on 9% SDS-PAGE gel and transferred to Trans-Blot membranes (Bio-Rad, Hercules, CA). Membranes were immuno-blotted with primary antibodies (Ab) directed against androgen receptor (Santa Cruz, CA) and re-probed with β-Actin or retinoblastoma (Rb) antibodies (Santa Cruz, CA) as internal controls for the cytosolic and nuclear fractions, respectively. Membranes were incubated in IgG-HRP secondary Abs and signals detection and analysis was done as previously described [14].
2.5. Immunofluorescence staining of androgen receptor
C4-2B cells were cultured onto cover slips in six-well plates and treated with 25 μM B-DIM for 24 hrs, then stimulated with 1 nM DHT overnight. Cells were irradiated with 3 Gy and five hours later, cells were fixed with 4% formaldehyde, permeabilized with 0.5% Triton X100 and incubated with anti-AR antibody for 2 h. Cells were stained for 2 h with Alexa Fluor 488-conjugated secondary goat anti-mouse IgG (Invitrogen, Carlsbad, CA) prepared in DAPI. Stained cells were analyzed using NIKON E-800 UV microscope for detection of DAPI and Alexa Fluor 488 staining [6,14].
2.6. PSA determination in conditioned media
C4-2B cells were plated at 2 × 105 in triplicate wells of 6-well plates for 24 h. The cells were washed with serum-free medium and cultured with B-DIM for 24h in CM with 1% FBS + 1nM DHT, then irradiated at 3Gy. At 24h after radiation, conditioned medium was collected and PSA concentration was quantified using Human PSA ELISA Kit (Anogen, Mississauga, Ontario, Canada) [6].
2.7. B-DIM and radiation treatments in PC-3 orthotopic tumor model in vivo
PC-3/PI prostate tumor cell lines were generated by passaging PC-3 cells in the prostate of nude mice [14]. PC-3/PI cells were injected, at a concentration of 5×105 cells in 20 μl HBSS, into the prostate of 5–6-week-old male Balb/c nu/nu nude mice (Harlan Sprague Dawley, Indianapolis, IN). By day 10 after cell injection, mice had established prostate tumors of about 0.4 cm compared to 0.2 cm normal prostate [14]. On day 10–13, mice were pretreated for 4 days with oral B-DIM. B-DIM was dissolved 1:9 in DMSO / sesame seed oil and administered by gavage at the dose of 5 mg/day (250 mg/kg body weight / day). Control and radiation-only treated groups received vehicle only. On day 14, mice received 5 Gy photon radiation to the tumor-bearing prostate, as previously detailed [14]. One day after radiation, B-DIM treatments were resumed and administered daily for the duration of the experiment. Five mice were used per experimental group. Mice were killed on day 34 after PC-3/PI cell injection, necropsied and examined for gross tumors in the prostate. The tumor-bearing prostates and para-aortic lymph nodes were resected and weighed [14]. Mice were housed and handled under sterile conditions in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care. The animal protocol was approved by the Institutional Animal Care and Use Committee of Wayne State University.
2.8. Statistical analysis
Comparisons between means of various treatment groups in all in vitro assays and tumor weights in vivo were analyzed by two-tailed unpaired Student's t test [14].
3. RESULTS
3.1. B-DIM Enhances Radiation-Induced Cell Killing of PC-3 and C4-2B Cells
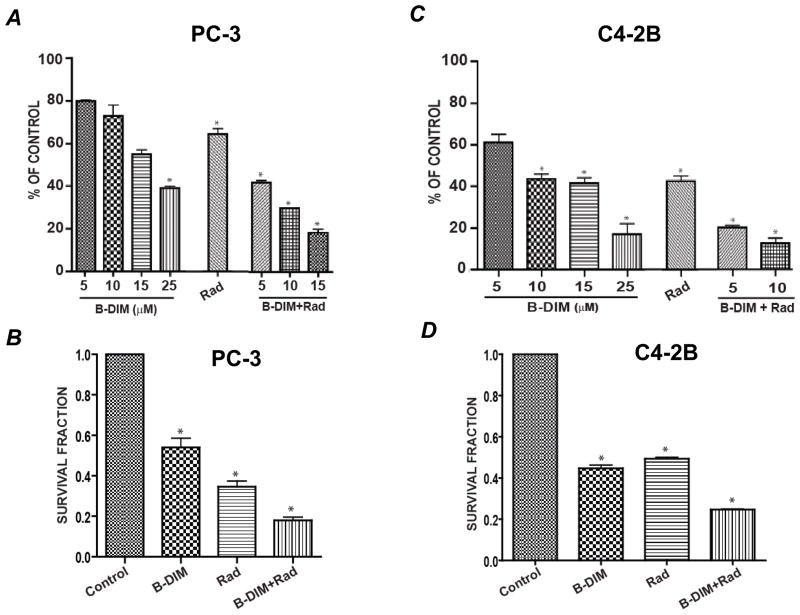
The short-term effect of doses of 5–25 μM of B-DIM on the growth of PC-3 cells (AR−) was assessed after 72 h treatment in vitro. B-DIM inhibited PC-3 cell growth in a dose-dependent manner with 45–60% inhibition at doses of 15 and 25 μM compared to control cells (p<0.005) (Fig. 1A). This effect was enhanced by 3 Gy radiation resulting in greater inhibition of 70–82% at low doses of 10 and 15 μM compared to radiation alone (36%, p<0.005), or 25–45% effect caused by 10–15 μM B-DIM (p<0.05) (Fig. 1A). The long-term effect of B-DIM and/or radiation on cell killing was assessed using clonogenic assays. A low dose of only 5 μM B-DIM significantly enhanced cell killing induced by radiation leading to only 18% cell survival (p<0.001 relative to control), compared to 54% survival with B-DIM alone (p<0.01) and 34% survival with radiation alone (p<0.01) (Fig. 1B).
Figure 1. PC-3 and C4 -2B cell growth inhibition by B-DIM and radiation.
(A) PC-3 short-term cell growth. PC-3 cells were treated with B-DIM at 5, 10, 15 and 25 μM for 72 hrs, and viable cells were counted. For combination of B-DIM and radiation, PC-3 cells were pretreated with 5, 10 and 15 μM B-DIM for 24 h, then irradiated with 3Gy photons and incubated for 48 h prior to viable cell counting. Bars represent the mean number of cells from duplicate wells calculated as the percentage of control cells ± SE. (B) PC-3 long-term survival. PC-3 cells were pre-treated with 5 μM of B-DIM for 24 h, then irradiated with 3Gy photons and plated in a clonogenic assay in the presence of B-DIM (5 μM). Bars represent the mean survival fraction of triplicate wells ± SE. (C) C4-2B short-term cell growth. C4-2B cells were treated with B-DIM, radiation or both as described in (A) and viable cells were counted. Bars represent the mean number of cells from duplicate wells calculated as the percentage of control cells ± SE. (D) C4-2B long-term survival. C4-2B cells were pre-treated with 5μM of B-DIM for 48 hrs, then irradiated with 3 Gy photons and plated in matrigel coated plates for clonogenic assay in the presence of B-DIM (5 μM). Bars represent the mean survival fraction of triplicate wells ± SE. In panels A, B, C and D, *p < 0.005 relative to control group.
C4-2B cells, which are AR+, were more sensitive to B-DIM mediated-cell killing than PC-3 cells. In short-term cell growth assays, C4-2B cells were significantly inhibited by 40% at a dose as low as 5 μM (p<0.01) (Fig. 1C). This effect was enhanced by 3 Gy radiation resulting in 80–85% inhibition at low doses of 5 and 10 μM compared to radiation alone (55%, p<0.01), or 40–55% effect caused by 5–10 μM B-DIM (p<0.01) (Fig. 1C). A long-term clonogenic assay, also showed significantly greater cell killing by B-DIM leading to only 24% cell survival, compared to about 50% survival with B-DIM or radiation alone (p<0.001) (Fig. 1D). In these cell growth assays, C4-2B cells were not cultured in the presence of DHT because these cells do not depend on androgen stimulation for proliferation, despite having basal levels of AR.
3.2. Radiation-induced DNA Binding Activity of Transcription Factors is Inhibited by B-DIM in both PC-3 and C4-2B cells
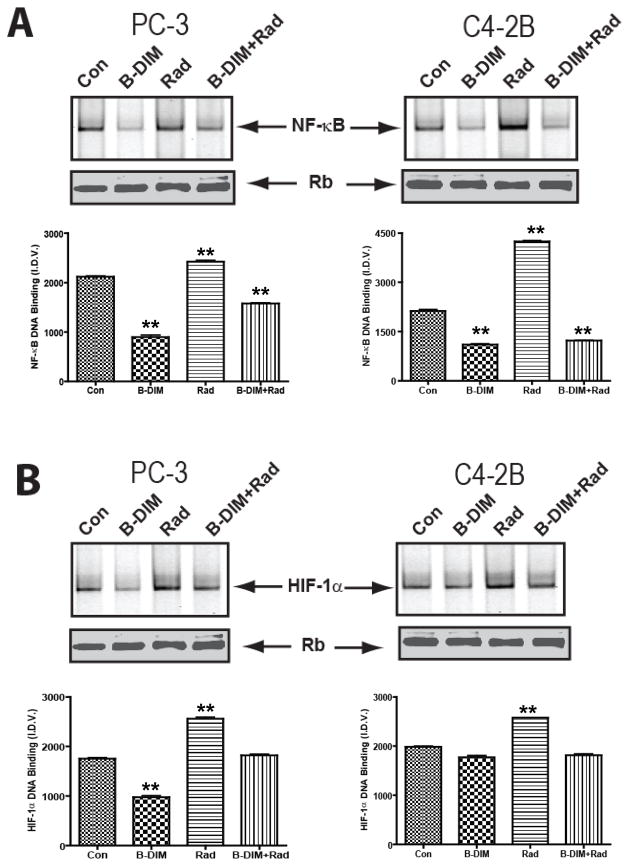
To assess the effect of B-DIM on molecular survival pathways activated by radiation, cells were pre-treated with B-DIM (25 μM) for 48 h. Then, cells were irradiated (3Gy), and 5h later, were tested for the DNA binding activities of NF-κB and HIF-1α transcription factors. We have previously shown that NF-κB and HIF-1α DNA binding activities are upregulated in response to radiation in both PC-3 and C4-2B cells [14]. This effect was reproduced in the current studies (Fig. 2A,B). However, B-DIM inhibited the DNA binding activity of NF-κB and blocked NF-κB-induced activation by radiation in both PC-3 and C4-2B cells (p<0.001) (Fig. 2A). Likewise, the DNA binding activity of HIF-1α and its radiation-induced activity were decreased by B-DIM in PC-3 cells (Fig. 2B). In C4-2B cells, HIF-1α DNA binding activity was significantly enhanced by radiation (p<0.001). This radiation-induced increase in HIF-1α DNA binding activity in C4-2B cells was reduced to baseline levels following treatment with B-DIM and radiation compared to control and B-DIM treated cells (p<0.001) (Fig. 2B).
Figure 2. Pre-treatment with B-DIM inhibits NF-κB and HIF-1α DNA-binding activity induced by radiation in PC-3 and C4-2B cells.
(A) Effect of treatment on NF-κB DNA binding activity. Cells were pre-treated with 25μM B-DIM for 48h. Then, cells were irradiated (3Gy) and 5h later, nuclear extracts were subjected to EMSA to test for NF-κB DNA binding activity. Data from untreated control (Con), B-DIM, radiation (Rad) and both combined (B-DIM+Rad) are shown. (B) Effect of treatment on HIF-1α DNA binding activity. Nuclear extracts were subjected to EMSA to test for HIF-1α DNA binding activity. Data are also presented as the mean I.D.V. of the band per μg protein loaded (±S.E.). **p < 0.001 relative to control. Western blot for Rb protein in the nuclear extract was performed as an internal loading control.
3.3. B-DIM inhibits the nuclear AR expression and PSA secretion in C4-2B (AR+) cells in vitro
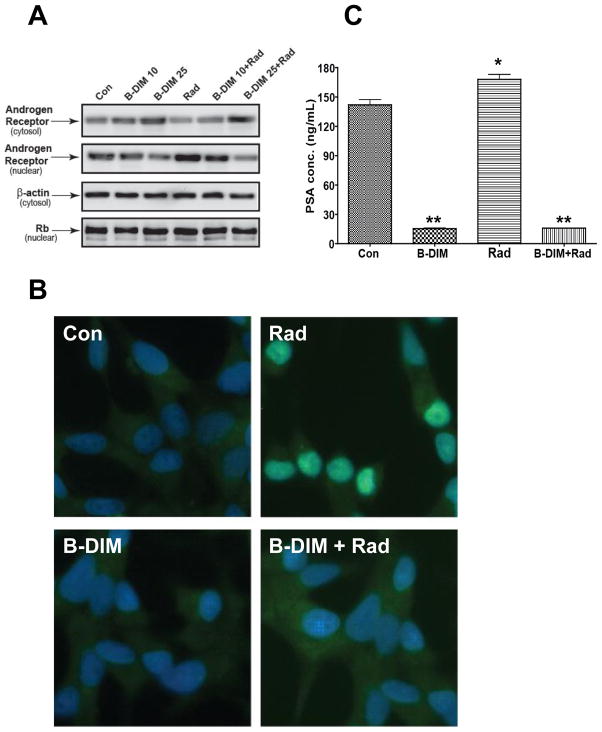
To activate AR in C4-2B (AR+) cells and study its expression following treatment with B-DIM and radiation, cells were stimulated with DHT prior to radiation [6]. C4-2B cells were treated with two different doses of 10 and 25 μM B-DIM for 48 h. Cells were stimulated with 1nM DHT for 18h and then irradiated with 3 Gy. At 5h after radiation, cells were processed to separate cytosolic and nuclear cell protein fractions which were tested for AR expression in Western blot analysis (Fig. 3A). Control cells showed expression of AR at a low level in the cytosolic and nuclear fractions. With increasing doses of B-DIM, AR expression increased in the cytosolic fraction and decreased in the nuclear fraction (Fig. 3A). In contrast, cells treated with radiation showed increased nuclear localization of AR and decreased cytosolic expression (Fig. 3A). Cells treated with B-DIM combined with radiation showed inhibition of radiation-increased nuclear expression concomitant with increased cytosolic expression which was B-DIM dose dependent (Fig. 3A). Immunostaining experiments using 25 μM B-DIM confirmed the findings observed by Western blots. Cytoplasmic staining of AR was dominant with B-DIM treatment whereas a dramatic increase in AR nuclear staining was observed with radiation (Fig. 3B). Pre-treatment of cells with B-DIM prior to radiation significantly decreased AR nuclear localization, compared to cells treated with radiation alone (Fig. 3B). PC-3 cells tested negative for AR expression by Western blots confirming their AR− phenotype. Furthermore, radiation did not induce AR expression (data not shown).
Figure 3. B-DIM inhibits the nuclear androgen receptor expression and PSA secretion in C4-2B cells in vitro.
(A) Effect of B-DIM treatment on AR in C4-2B cells. Cells were either untreated (Con) or treated with 10 and 25 μM B-DIM (B-DIM 10, B-DIM 25) or radiation (Rad) or combined with radiation (B-DIM 10 + Rad or B-DIM 25 + Rad ). Cells were pre-treated for 48 h with B-DIM, stimulated with 1nM DHT for 18h and then irradiated with 3 Gy. At 5h after radiation, nuclear and cytosolic fractions were obtained from cells and analyzed for AR expression by Western blot. β-Actin in the cytosolic extracts and Rb protein in the nuclear extracts were used as internal loading controls. (B) B-DIM decreases nuclear AR expression in C4-2B cells. C4-2B cells were pre-treated with 25 μM B-DIM for 48 h, stimulated with 1nM DHT for 18h and treated with 3Gy radiation. At 5 h after radiation, cells were processed for AR immuno-staining. Control cells show AR cytoplasmic staining and minimal nuclear staining. Radiation-treated cells exhibit intense nuclear AR staining. Cells treated with B-DIM show cytoplasmic staining but minimal nuclear AR staining. Cells treated with B-DIM combined with radiation have a significant decrease in nuclear AR staining compared to radiation-treated cells. All magnifications X40. (C) B-DIM inhibits PSA secretion. C4-2B cells were pre-treated for 24 h with 25 μM of B-DIM in CM containing 1% FBS + 1nM DHT, then treated with 3Gy radiation. At 24 h after radiation, conditioned media were harvested and tested for PSA by ELISA assay. Bars represent the mean PSA concentration of triplicate wells ± SE. *p<0.05, **p <0.001 relative to control.
AR is a nuclear transcription factor, which upon activation by androgen, binds to androgen response elements (ARE) in the promoter of target genes including PSA, therefore the effects of B-DIM and radiation on PSA secretion were tested [6]. C4-2B cells were pre-treated with 25 μM of B-DIM, stimulated with 1nM DHT, and then, irradiated with 3Gy. At 24h after radiation, conditioned media were tested for PSA secretion. PSA secretion in culture media was high in C4-2B (AR+) cells, but was drastically decreased by B-DIM treatment (p<0.001) [Fig. 3C]. Consistent with increased expression of AR by radiation, PSA secretion was also increased by radiation (p<0.05) and corroborated the increased AR nuclear localization observed in Fig 3A,B. B-DIM pre-treatment of C4-2B cells followed by radiation caused almost complete inhibition of PSA secretion (p<0.001) [Fig. 3C]. No secretion of PSA was observed in PC-3 cells (AR−) (data not shown).
3.4. Response of PC-3 orthotopic prostate tumors to B-DIM and radiation
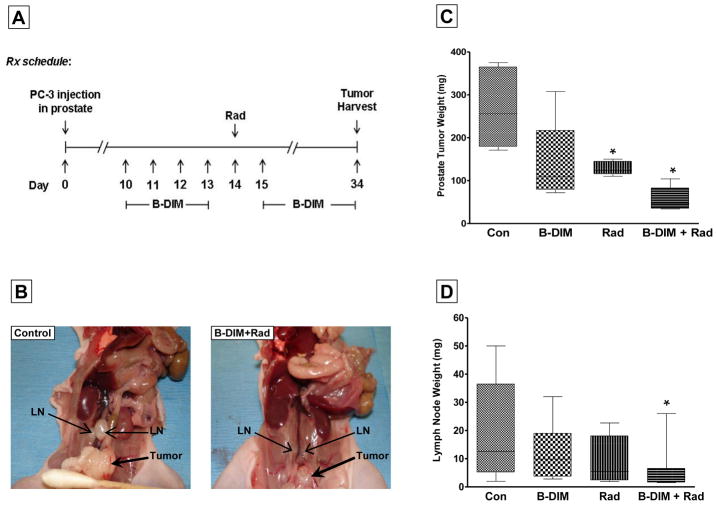
We have shown that B-DIM enhanced radiation-induced cell killing in both PC-3 (AR−) and C4-2B (AR+) androgen-independent PCa cells regardless of AR expression status (Fig. 1). B-DIM also inhibited transcription factors activated by radiation (Fig. 2). As a proof of concept, we tested the effect of the combined therapy in orthotopic PC-3 prostate tumors. Enhanced cell killing by B-DIM combined with radiation was observed in cells treated with B-DIM prior to radiation and continuously exposed to B-DIM after radiation, as shown in cell growth and clonogenic assays in vitro. We tested a similar sequence of pre-treatment with B-DIM followed by radiation and continuous exposure to B-DIM for the treatment of PC-3 prostate tumors in nude mice (Fig. 4A). The PC-3 orthotopic xenograft model, established in our laboratory, leads to spontaneous metastasis from primary tumors to regional para-aortic lymph nodes located along the spine [14]. Mice bearing established PC-3 prostate tumors were first treated with oral B-DIM at 5mg/day for 4 days followed by radiation administered selectively to the prostate area at 5Gy. B-DIM was resumed on a daily basis (Fig. 4A). Mice were killed on day 34 after cell injection to assess the sizes and weights of the prostate tumors, and those of the para-aortic lymph nodes. As shown in Figure 4B, the size of the prostate tumors treated with B-DIM and radiation was much smaller (0.3–0.4 cm) than that of control mice (1–1.2 cm). Whereas the para-aortic lymph nodes in control mice were markedly enlarged (0.4–0.7 cm), these lymph-nodes were much smaller in mice treated with B-DIM and radiation (0.2–0.3cm) (Fig. 4B). These observations were confirmed by the weight measurements of prostate tumors showing a greater 80% tumor inhibition mediated by B-DIM and radiation compared to B-DIM alone (48%, p≤0.05), radiation alone (52%, p<0.01) and to control mice (p<0.005) (Fig. 4C). The distribution of weights of para-aortic lymph nodes showed a similar trend with the majority of the lymph nodes much smaller in mice treated with B-DIM and radiation compared to control mice (p<0.05) (Fig. 4D).
Figure 4. Treatment of PC-3 prostate tumors with B-DIM and tumor irradiation.
(A) Treatment schedule diagram. On day 10–13 after PC-3 cell injection in prostate, mice bearing established prostate tumors were pre-treated daily with oral B-DIM (5mg/mouse/day). Tumor-bearing-prostates were irradiated with 5Gy photons on day 14. One day later, B-DIM treatment was resumed and given every day. On day 34, mice were killed and the prostates, including the tumors, were resected and weighed. (B) Picture of mice showing primary prostate tumors and para-aortic lymph node metastasis in control mice and mice treated with B-DIM and radiation. Note smaller prostate tumor and smaller lymph nodes following combined treatments. (C) Response of primary prostate tumors following B-DIM and radiation. The weights of the tumor-bearing prostates and their median are reported for 5 mice per treatment group treated with vehicle (Con for control), B-DIM or radiation (Rad) or both combined (B-DIM+Rad). (D) Response of para-aortic lymph node metastases. At least 2 lymph nodes were obtained from each mouse and weighed. The distribution of lymph nodes weights and their median is shown for 5 mice per treatment group. *p<0.05 relative to control.
4. DISCUSSION
We have explored the beneficial role of B-DIM as a new biological approach which could favorably improve the clinical response of prostate cancer to radiotherapy. We found that B-DIM inhibited cell growth in a dose-dependent manner in both PC-3 (AR−) and the C4-2B (AR+) cell lines, although the C4-2B cell line was more sensitive, in agreement with previous studies [16]. B-DIM was effective at increasing radiation-induced cell killing of both PC-3 (AR−) and C4-2B (AR+) cell lines. This effect was demonstrated in short-term cell growth assays and long-term clonogenic assays. Potentiation of radiation cell killing by B-DIM occurred independently of AR expression, suggesting that the combination of B-DIM with radiation could be applied to castrate-resistant patients regardless of AR expression.
We previously demonstrated that radiation caused up-regulation of the transcription factors NF-κB and HIF-1α in vitro both in PC3 and C4-2B cells [14,15] and in vivo in PC-3 orthotopic prostate tumors [17]. B-DIM inhibited the radiation-induced increase DNA binding activity of both NF-κB and HIF-1α and this effect was independent of AR expression because it was observed both in PC-3 (AR−) and C4-2B (AR+) cells. In our studies AR was not detectable in the cytosol or nucleus of PC-3 cells and was not upregulated by radiation. Previous studies showed an interesting cross-talk between NF-κB and the AR pathway in the AR positive cell lines LNCaP and C4-2B, contributing to B-DIM induced apoptosis [6]. Our findings suggest that such a cross-talk is not necessarily involved in B-DIM mediated alterations of the survival pathways that are activated by radiation, which leads to greater cell killing. B-DIM could directly impair the activity of NF-κB and HIF-1α, independently of AR pathway, as shown in AR− cells.
In early stage PCa, which is hormone responsive, androgen controls AR signaling and this interaction can be inhibited by androgen ablation therapy. However, when PCa converts to castrate-resistant stage, AR expression can be still maintained and overexpressed, albeit dysregulated [11,12]. In AR+ and androgen independent C4-2B cells, radiation caused an increase in AR expression and induced its nuclear localization that was concomitant with a decreased cytosolic AR expression. Whether AR activation is implicated in radioresistance is an intriguing question. Previous studies reported that radiation enhances AR expression using Western blot analysis of whole cell lysates [18,19]. By Western blots of nuclear and cytosolic fractions and AR immunostaining cell localization, we further show that radiation not only increases AR expression but augments its trafficking to the nucleus where it could potentially act as a transcription factor. This hypothesis is supported by the increased in PSA secretion observed in irradiated cells as PSA is a downstream gene transcribed under the control of AR transcription factor. In contrast to the radiation effect, B-DIM decreased AR nuclear expression and increased cytosolic expression, thereby altering nuclear localization of AR, as previously reported [6]. We have now shown that B-DIM also blocked the radiation-induced upregulation of AR expression and AR nuclear localization, thus affecting its trafficking to the nucleus. The treatment of C4-2B cells with B-DIM alone and combined with radiation completely inhibited PSA secretion. These novel findings indicate that B-DIM could function as an AR antagonist by impairing the AR cell survival pathway activated by radiation and its downstream PSA gene in AR+ cells which are androgen-independent and representative of castrate-resistant PCa.
As a proof of concept, our pre-clinical studies in orthotopic prostate tumors demonstrate the feasibility and enhanced therapeutic efficacy of B-DIM combined with radiotherapy. Treatment of mice-bearing prostate tumors with B-DIM and radiation controlled tumor growth in the prostate and spontaneous metastasis to regional lymph nodes.
These studies demonstrate that both in vitro and in vivo, B-DIM could augment radiation-induced cell killing and tumor growth inhibition. B-DIM impairs critical survival signaling pathways activated by radiation including HIF-1α and NF-κB, leading to enhanced cell killing. These molecular alterations occurred in AR+ and AR− cell lines. Thereby, this strategy could be applied to both AR+ and AR− androgen-independent PCa. In AR+ PCa, B-DIM also alters the AR survival pathway activated by radiation, making it a potential biological approach to improve radiotherapy in patients presenting with progressive AR+ PCa tumors. These novel observations suggest that B-DIM, a clinically-proven safe compound, could be useful as a complementary approach to enhance the efficacy of radiotherapy for castrate-resistant PCa.
Acknowledgments
Grant Support: The Fund for Cancer Research (GGH), American Institute for Cancer Research #10A108 (GGH), Funds from the Department of Radiation Oncology at Wayne State University (GGH) and National Cancer Institute, NIH # R01CA108535-06 (FHS).
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarkar FH, Li Y. Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Res. 2006;66:3347–3350. doi: 10.1158/0008-5472.CAN-05-4526. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee S, Kong D, Wang Z, Bao B, Hillman GG, Sarkar FH. Attenuation of multi-targeted proliferation-linked signaling by 3,3'-diindolylmethane (DIM): From bench to clinic. Mutat Res. 2011;728:47–66. doi: 10.1016/j.mrrev.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hillman GG, Singh-Gupta V. Soy isoflavones sensitize cancer cells to radiotherapy. Free Radic Biol Med. 2011;51:289–298. doi: 10.1016/j.freeradbiomed.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 4.Hebert JR, Hurley TG, Olendzki BC, Teas J, Ma Y, Hampl JS. Nutritional and socioeconomic factors in relation to prostate cancer mortality: a cross-national study. J Natl Cancer Inst. 1998;90:1637–1647. doi: 10.1093/jnci/90.21.1637. [DOI] [PubMed] [Google Scholar]
- 5.Kristal AR, Lampe JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2002;42:1–9. doi: 10.1207/S15327914NC421_1. [DOI] [PubMed] [Google Scholar]
- 6.Bhuiyan MM, Li Y, Banerjee S, Ahmed F, Wang Z, Ali S, Sarkar FH. Down-regulation of androgen receptor by 3,3'-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in both hormone-sensitive LNCaP and insensitive C4-2B prostate cancer cells. Cancer Res. 2006;66:10064–10072. doi: 10.1158/0008-5472.CAN-06-2011. [DOI] [PubMed] [Google Scholar]
- 7.Heath EI, Heilbrun LK, Li J, Vaishampayan U, Harper F, Pemberton P, Sarkar FH. A phase I dose-escalation study of oral BR-DIM (BioResponse 3,3'-Diindolylmethane) in castrate-resistant, non-metastatic prostate cancer. Am J Transl Res. 2010;2:402–411. [PMC free article] [PubMed] [Google Scholar]
- 8.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 9.Swanson GP, Hussey MA, Tangen CM, Chin J, Messing E, Canby-Hagino E, Forman JD, Thompson IM, Crawford ED. Predominant treatment failure in postprostatectomy patients is local: analysis of patterns of treatment failure in SWOG 8794. J Clin Oncol. 2007;25:2225–2229. doi: 10.1200/JCO.2006.09.6495. [DOI] [PubMed] [Google Scholar]
- 10.Morgan PB, Hanlon AL, Horwitz EM, Buyyounouski MK, Uzzo RG, Pollack A. Radiation dose and late failures in prostate cancer. Int J Radiat Oncol Biol Phys. 2007;67:1074–1081. doi: 10.1016/j.ijrobp.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 12.Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. J Cell Biochem. 2006;99:333–344. doi: 10.1002/jcb.20794. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Charron M, Wright WW, Chatterjee B, Song CS, Roy AK, Brown TR. Nuclear factor-kappaB activates transcription of the androgen receptor gene in Sertoli cells isolated from testes of adult rats. Endocrinology. 2004;145:781–789. doi: 10.1210/en.2003-0987. [DOI] [PubMed] [Google Scholar]
- 14.Singh-Gupta V, Zhang H, Yunker CK, Ahmad Z, Zwier D, Sarkar FH, Hillman GG. Daidzein effect on hormone refractory prostate cancer in vitro and in vivo compared to genistein and soy extract: potentiation of radiotherapy. Pharm Res. 2010;27:1115–1127. doi: 10.1007/s11095-010-0107-9. [DOI] [PubMed] [Google Scholar]
- 15.Singh-Gupta V, Zhang H, Banerjee S, Kong D, Raffoul JJ, Sarkar FH, Hillman GG. Radiation-induced HIF-1alpha cell survival pathway is inhibited by soy isoflavones in prostate cancer cells. Int J Cancer. 2009;124:1675–1684. doi: 10.1002/ijc.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nachshon-Kedmi M, Yannai S, Haj A, Fares FA. Indole-3-carbinol and 3,3'-diindolylmethane induce apoptosis in human prostate cancer cells. Food Chem Toxicol. 2003;41:745–752. doi: 10.1016/s0278-6915(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 17.Raffoul JJ, Banerjee S, Singh-Gupta V, Knoll ZE, Fite A, Zhang H, Abrams J, Sarkar FH, Hillman GG. Down-regulation of apurinic/apyrimidinic endonuclease 1/redox factor-1 expression by soy isoflavones enhances prostate cancer radiotherapy in vitro and in vivo. Cancer Res. 2007;67:2141–2149. doi: 10.1158/0008-5472.CAN-06-2147. [DOI] [PubMed] [Google Scholar]
- 18.Harashima K, Akimoto T, Nonaka T, Tsuzuki K, Mitsuhashi N, Nakano T. Heat shock protein 90 (Hsp90) chaperone complex inhibitor, radicicol, potentiated radiation-induced cell killing in a hormone-sensitive prostate cancer cell line through degradation of the androgen receptor. Int J Radiat Biol. 2005;81:63–76. doi: 10.1080/09553000400029460. [DOI] [PubMed] [Google Scholar]
- 19.Udayakumar TS, Hachem P, Ahmed MM, Agrawal S, Pollack A. Antisense MDM2 enhances E2F1-induced apoptosis and the combination sensitizes androgen-sensitive and androgen-insensitive prostate cancer cells to radiation. Mol Cancer Res. 2008;6:1742–1754. doi: 10.1158/1541-7786.MCR-08-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]