Abstract
Purpose
To assess whether in addition to sparing parotid glands (PGs), xerostomia after chemo-IMRT of head and neck cancer is affected by reducing doses to other salivary glands.
Methods
Prospective study: 78 patients with stages III/IV oropharynx/nasopharynx cancers received chemo-IMRT aiming to spare the parts outside the targets of bilateral PGs, oral cavity (OC) containing the minor salivary glands, and contralateral submandibular gland (SMG) (when contralateral level I was not a target). Pretherapy and periodically through 24 months, validated patient-reported xerostomia questionnaires (XQ) scores and observer-graded xerostomia were recorded, and stimulated and unstimulated saliva measured selectively from each of the PGs and SMGs. Mean OC doses served as surrogates of minor salivary glands dysfunction. Regression models assessed XQ and observer-graded xerostomia predictors.
Results
Statistically significant predictors of the XQ score in univariate analysis included OC, PG, and SMG mean doses, as well as baseline XQ score, time since RT, and both stimulated and unstimulated PG saliva flow rates. Similar factors were statistically significant predictors of observer-graded xerostomia. OC, PG and SMG mean doses were moderately inter-correlated (r=0.47–0.55). In multivariate analyses, after adjusting for PG and SMG doses, OC mean dose (p < 0.0001), time from RT (p < 0.0001), and stimulated PG saliva (p < 0.0025) were significant predictors for XQ scores, and OC mean dose and time for observer-graded xerostomia. While scatter plots showed no thresholds, OC mean doses <40 Gy and contralateral SMG mean <50 Gy were each associated with low patient-reported and observer-rated xerostomia at almost all post-therapy time points.
Conclusion
PG, SMG and OC mean doses were significant predictors of both patient-reported and observer-rated xerostomia after chemo-IMRT, with OC doses remaining significant after adjusting for PG and SMG doses. These results support efforts to spare all salivary glands by IMRT, beyond the PGs alone.
Keywords: head neck cancer, xerostomia, IMRT, submandibular glands
INTRODUCTION
Reducing xerostomia by sparing parotid glands (PGs) has been the main rationale of IMRT for head and neck cancer (HNC), improving xerostomia compared with conventional radiotherapy in randomized studies (1–3), with continuous improvement over time (4). However, these achievements are relatively modest. While salivary output and observer-rated xerostomia such as RTOG scales have consistently been significantly better using IMRT, a rate of post-IMRT xerostomia grade ≥2 as high as 40% at 12 months, reported in one of the randomized studies (3), is typical. It has been even harder to demonstrate significant improvements in patient-reported xerostomia. Kam et al reported no advantage of IMRT over 2D radiotherapy (RT) in patient-reported xerostomia (1), and Nutting et al reported that the advantage through months post-therapy was smaller than 10 points on a 0–100 scale, regarded as less than clinically relevant difference (3). Thus, IMRT aiming to spare only the PGs achieves partial gains in observer-rated, and even smaller gains in patient-reported xersotomia.
We have previously hypothesized that in addition to sparing the PGs, whose secretions are serous and constitute the majority of the saliva during eating, there is potential benefit in sparing the minor salivary glands and the submandibular/sublingual glands (SMGs), which, in addition to their important mucinous secretions, are the dominant non-stimulated saliva producers (4). Our previous analysis of the predictors of xerostomia, in a very heterogeneous patient cohort receiving IMRT or 3D RT, showed that the mean doses delivered to the PGs and the SMGs, as well as the doses delivered to the oral cavity (OC),where the minor glands are dispersed, were all statistically significant predictors of patient-reported xerostomia (4). Following these findings we have routinely included sparing of the non-involved OC in the IMRT plans. In addition, after assessing the relationships between the doses to the SMGs and their post-therapy salivary output, we have used these relationships to set IMRT cost functions for sparing these glands when neck level I was not considered at risk (5).
We have recently assessed prospectively the predictors of xerostomia in patients with stage III/IV oropharyngeal/nasopharyngeal cancers treated with chemo-IMRT in whom the planning goals included sparing the parts of all the salivary glands outside the targets. We sought to learn how did xerostomia predictors in these patients differ from those found in our previous study, whose goal was the sparing of the contralateral PGs only. Also, while ample data exists regarding the relationships between PG doses and patient-reported or observer-rated xerostomia (6), very little is known about such dose-effect relationships for the other salivary glands. These potential relationships are presented in this paper.
PATIENTS AND METHODS
This was a prospective longitudinal study of IMRT concurrent with chemotherapy for HNC, approved by the Institutional Review Board of the University of Michigan. All patients signed study-specific informed consent. Eligibility included stages III/IV squamous cell carcinoma of the oropharynx or nasopharynx requiring bilateral neck treatment, no prior therapy, Karnofsky performance status ≥ 60, and primary therapy with chemoradiotherapy. The study assessed sparing the swallowing structures, detailed elsewhere (7), as well as sparing the salivary glands. Details of therapy have been published elsewhere (7). In brief, all patients required treatment of the bilateral neck. IMRT planning objectives included the dosimetric sparing of the parts of the bilateral PGs, contralateral SMGs, and OC which were outside the targets. The OC was defined schematically as the surfaces of the inner lips, buccal mucosa, tongue, base of tongue, floor of mouth, and palate, representing sites of the minor salivary glands, as detailed elsewhere (4). Early in the study, contralateral SMGs in cases where contralateral level Ib was outside the targets were assigned low weights in the optimization cost function striving to reduce their mean doses as much as possible (ipsilateral level Ib was a target in all patients). After establishing dose-response relationships for SMG saliva output, the cost function for these glands was changed to achieve mean dose <39 Gy, and its weight increased, as previously detailed (5). Similar cost functions were set to achieve mean PG dose ≤26 Gy bilaterally and mean OC dose as low as possible. The cost functions for sparing the salivary glands had lower weights than target doses.
PG mean dose was calculated as a volume-weighted average of both parotid glands. Only contralateral SMG was considered in the analysis because in all patients the ipsilateral SMGs resided within the targets, received high doses, and were not expected to produce any saliva. Mean doses were calculated for the whole organs, including the parts overlapping with the targets, while optimization was aimed only at the parts of the organs outside the targets.
The clinical target volumes were each expanded uniformly by 3 mm to yield planning target volumes (PTVs). 70 Gy were prescribed to PTV1 and 63 - 56 Gy to PTV2/PTV3, all in 35 fractions. Achieving adequate target doses superseded sparing of any organ except the spinal cord. Online imaging and correction before each treatment were done to assure correct set-up.
Concurrent carboplatin (AUC 1) and paclitaxel 30 mg/m2 once weekly were delivered to oropharyngeal cancer patients, and cisplatin 100 mg/m2 every 3 weeks to nasopharyngeal patients. No salivary protectors or stimulants were allowed during therapy or the 2-year study follow-up.
Assessment of Xerostomia
Patients completed previously-validated, xerostomia-specific questionnaire (XQ), detailed elsewhere (4). In brief, the questionnaire consisted of four items asking about dryness while eating/speaking and four items about dryness while not eating. Subjects rated each symptom on an 11-point ordinal Likert scale from 0 to 10, with higher scores indicating greater xerostomia. Each item score was added, and the sum was linearly transformed to produce a summary score from 0 to 100. Observer-graded xerostomia was assessed according to the Common Toxicity Criteria Adverse Effects (CTCAE v2) (8). Both toxicity assessments were completed before RT started and at 1, 3, 6, 12, 18, and 24 months after completion of therapy during clinic visits.
Salivary Flow Measurements
Selective stimulated and unstimulated flow rates from each of the major salivary glands were measured as previously detailed (4,5) at the same time intervals as the xerostomia assessments. PG saliva was collected separately from each PG duct orifice. SMG and sublingual glands output are referred to as SMG, although they represent secretions from both, as they frequently share common ducts. Due to the proximity of the orifices of Wharton’s ducts, flow measurements for the SMGs were made from both orifices and represent combined output of the bilateral SMGs in each patient.
Statistical Analysis
Regression models were used to evaluate the effect of covariates on XQ score and CTCAE grade. XQ scores were modeled as a continuous variable and CTCAE grades as binary (0–1 vs ≥2). Models for XQ included subject level random intercept and slope terms to account for correlation with subject over time. Similarly, logistic regression models for CTCAE grades used generalized estimating equations with an autoregressive covariance structure (9). The effect of each of the dose terms on XQ score and CTCAE grades was found to be nearly constant over months 3 through 24, thus the final model included 3–24 months data and did not include a dose-time interaction term. Each univariate model contained the listed predictor as well as time from therapy. Multivariate models were fit to assess impact on XQ scores and CTCAE grades while accounting for mean PG dose and other covariates. All tests were 2-sided. The software package SAS V9.2 was used for analysis.
RESULTS
Patient Characteristics
Seventy-eight patients were enrolled between 2003–2008: 72 with oropharyngeal and 6 with nasopharyngeal cancers, all with American Joint Committee on Cancer (AJCC) stages III/IV. Patient and tumor characteristics are detailed in Table S1 (on-line supplement). The median (range) of the mean doses to the OC, combined PGs, and contralateral SMG, was 46 Gy (17 – 66), 36 Gy (22 – 61), and 62 Gy (29–75). The median (range) of the mean dose to the contralateral PGs was 25 Gy (14 – 61). OC mean doses were moderately correlated with both the PG mean dose (r = 0.55, p < 0.001) and SMG mean dose (r = 0.56, p < 0.001), and the latter two were correlated with each other (r = 0.47, p < 0.001).
Saliva Flow Rates
PG saliva flow rates are listed in Tables S2–S3 (on-line supplement). At 12 months, stimulated and unstimulated flow rates from individual glands were >25% of baseline in 78% and 47% of the patients, respectively. For the SMGs, flow rates at 12 months > 25% of baseline (representing flow rates from both glands in each patient) were observed in 14% and 17% of the patients.
Patient-reported Xerostomia
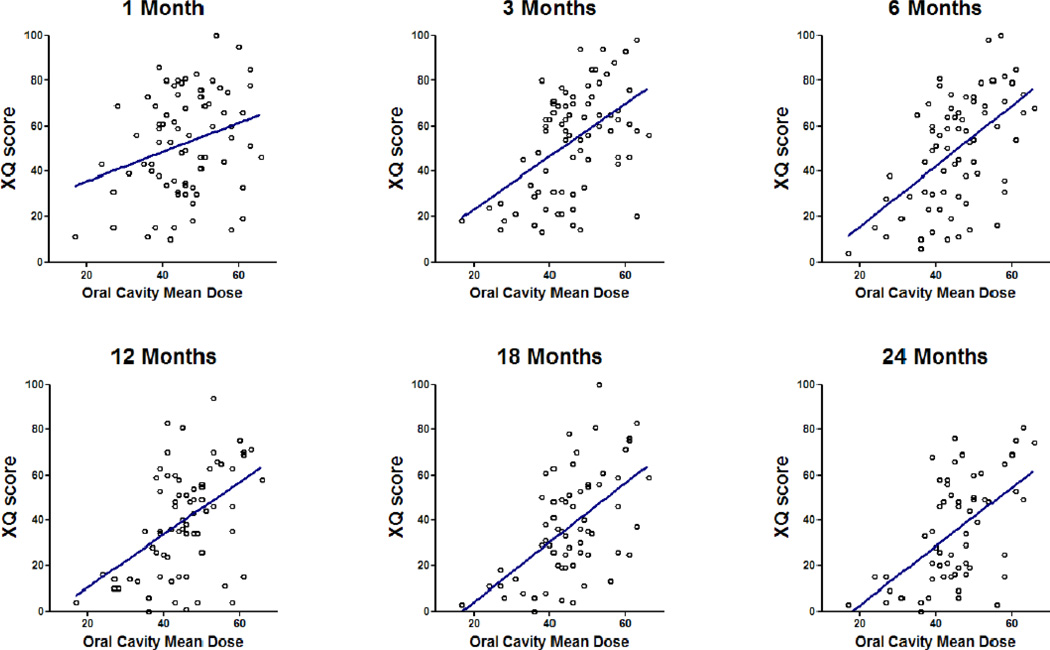
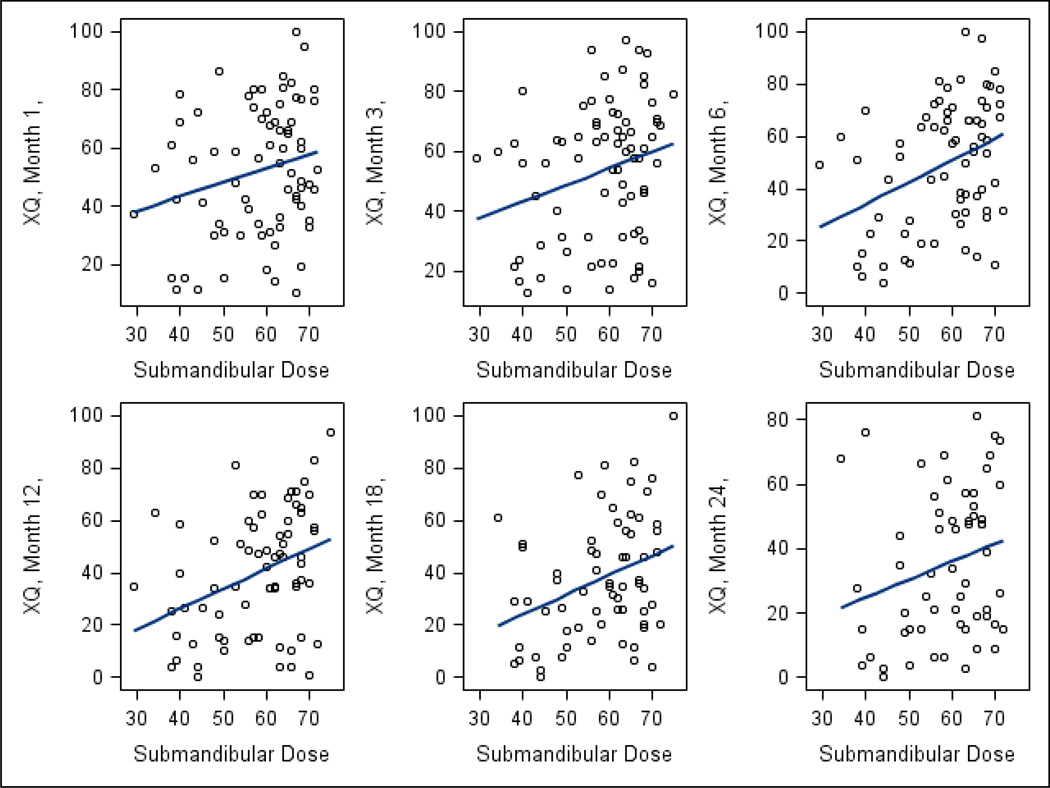
XQ scores were available from 100%, 93%, 97%, 90%, 90%, 84%, and 75% of patients at baseline, 1, 3, 6, 12, 18, and 24 months, respectively. The scores are detailed in Table S4 (online supplement). Significant worsening of scores was noted from pre-therapy to early post-therapy, followed by improvement over time through 2 years (p < 0.001). Factors correlated with XQ scores on univariate analysis are summarized in Table 1. OC mean dose, PG mean dose, contralateral SMG mean dose, baseline XQ score, and both stimulated and unstimulated PG saliva output were each statistically significant correlates. SMG saliva output, stage, sex, and age were not significant. Figures 1–2 demonstrate that the effects of OC or SMG mean doses on XQ scores was each similar across all time points. Figs S1–S2 (Supplement) summarize these relationships by plotting the XQ scores associated with the highest through the lowest quartiles of mean OC (Fig S1) or contralateral SMG doses (Fig S2) at each time point. The lowest dose quartiles (mean SMG <53 Gy or mean OC< 41 Gy) were associated with significantly better XQ scores compared with the other quartiles (p<0.001).
Table 1.
Predictors of patient-reported xerostomia (XQ) scores
| Univariate analysis | Multivariate Model | |||||
|---|---|---|---|---|---|---|
| Variable | Estimate | Standard error |
p value | Estimate | Standard error |
p value |
| Baseline XQ score | 0.37 | 0.17 | 0.03 | 0.11 | 0.14 | 0.44 |
| Oral cavity mean dose | 1.3 | 0.20 | < 0.001 | 1.2 | 0.26 | < 0.0001 |
| PG mean dose | 0.77 | 0.29 | 0.008 | −0.27 | 0.29 | 0.36 |
| SMG mean dose | 0.72 | 0.21 | < 0.001 | 0.082 | 0.22 | 0.71 |
| Stimulated parotid saliva rate | −18 | 4.7 | < 0.001 | −16 | 4.6 | 0.0007 |
| Unstimulated parotid saliva rate | −37 | 18 | 0.04 | NS | ||
| Stimulated SMG saliva rate | −11 | 21 | 0.6 | |||
| Unstimulated SMG saliva rate | −27 | 40 | 0.5 | |||
| AJCC Stage III vs. IV | ||||||
| Gender | 5.8 | 6.9 | 0.4 | |||
| Age | 0.20 | 0.28 | 0.5 | |||
| Time (categorical) | - | - | - | - | - | <0.0001 |
Fig 1.
Correlations between oral cavity mean doses and patient-reported xerostomia (XQ) scores at various times post-therapy: Individual data points and linear regression fits.
Fig 2.
Correlations between contralateral submandibular gland mean doses and patient-reported xerostomia (XQ) scores at various times post-therapy: Individual data points and linear regression fits.
Mean OC dose and stimulated parotid saliva flow rate were significant predictors of XQ after adjusting for PG and SMG mean doses (Table 1). In addition, time since completion of therapy was predictive of XQ score, with increasing time (during 3–24 months) predicting a better score (p < 0.001). We have further explored the relationship between PG and OC mean doses and XQ scores. Plotting OC mean dose by residuals from regression of PG mean dose on the XQ score at 12 months, we found significant relationship (r=0.4, p<0.01), confirming the finding of the multivariate model that the OC mean dose was an important predictor of patient-reported xerostomia even after accounting for the effect of PG mean doses.
Observer-graded Xerostomia
Observer-rated CTCAE grades were available from 100%, 91%, 95%, 92%, 88%, 76%, and 77% of patients at baseline, 1, 3, 6, 12, 18, and 24 months, respectively. The incidence of CTCAE xerostomia grade ≥2 at baseline, 1, 3, 6, 12, 18, and 24 months, was 0, 40%, 55%, 38%, 24%, 16%, and 14%, respectively. Statistically significant predictors of CTCAE grade ≥2 xerostomia on univariate analysis included OC mean dose, PG mean dose, and contralateral SMG mean dose (Table 2). PG saliva flow rates tended but did not reach statistical significance, while stage, age, and gender were not predictive. Figures 3–4 demonstrate the relationships between OC or SMG mean doses and observer-rated xerostomia grades at each time point. Table 2 summarizes also multivariate analysis for the observer-graded xerostomia, where significant dosimetric predictors, after accounting for PG and SMG mean doses, were OC mean doses and time since completion of radiotherapy.
Table. 2.
Predictors of observer-rated toxicity (CTCAE xerostomia grade ≥2)
| Univariate Analysis | Multivariate model | |||
|---|---|---|---|---|
| Variable | Odds ratio estimate (CI) |
p value | Odds ratio estimate (CI) |
p value |
| Oral cavity mean dose | 1.06 (1.02, 1.10) | 0.002 | 1.04 (1.00, 1.08) | 0.05 |
| PG mean dose | 1.05 (1.00, 1.11) | 0.04 | 1.01 (0.96, 1.07) | 0.6 |
| SMG mean dose | 1.05 (1.01, 1.08) | 0.009 | 1.02 (0.98, 1.06) | 0.3 |
| Baseline XQ score | 1.02 (0.99, 1.04) | 0.06 | ||
| Stimulated parotid saliva rate | 0.18 (0.026, 1.26) | 0.08 | ||
| Unstimulated parotid saliva rate | 0.00 (0.00, 3.85) | 0.09 | ||
| Stimulated SMG saliva rate | 1.13 (0.001, 2.35) | 0.9 | ||
| Unstimulated SMG saliva rate | 0.00 (0.00, 3.88) | 0.2 | ||
| AJCC Stage III vs. IV | 0.93 (0.42, 1.49) | 0.8 | ||
| Gender | 1.23 (0.47, 3.2) | 0.7 | ||
| Age | 1.03 (0.99, 1.07) | 0.1 | ||
| Time (categorical) | - | - | - | <0.0001 |
Fig 3.
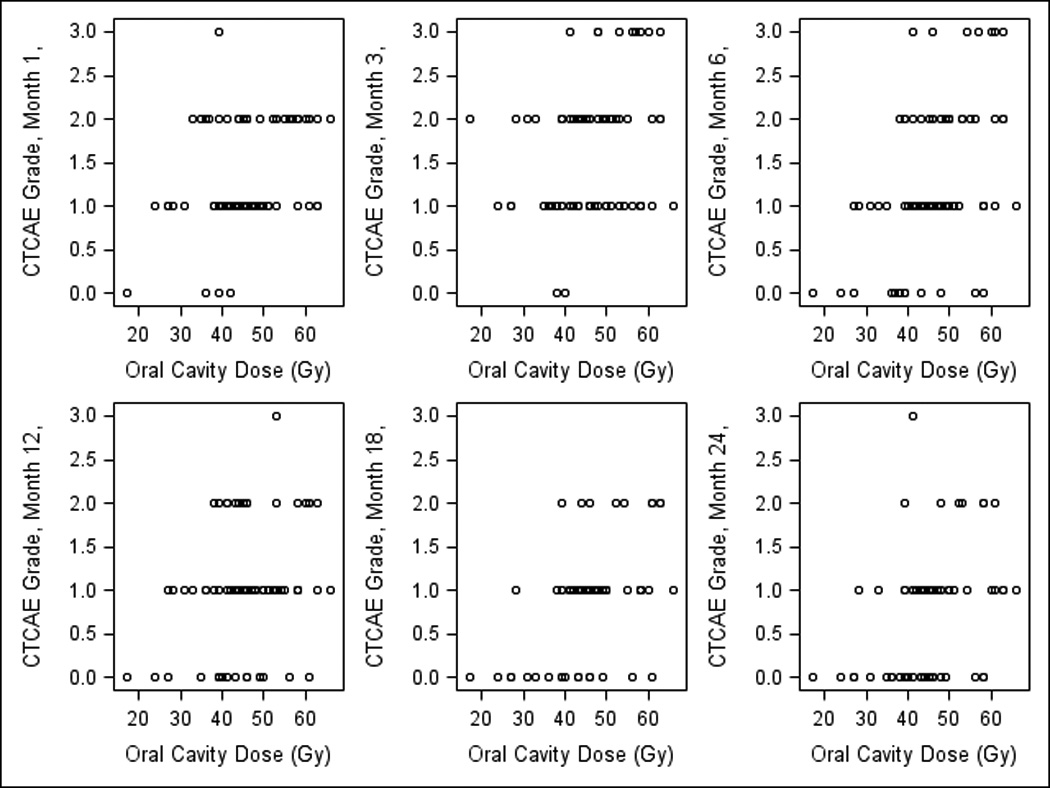
Correlations between observer-rated (CTCAE v2) xerostomia grades at various times post therapy and oral cavity (OC) mean doses.
Fig 4.
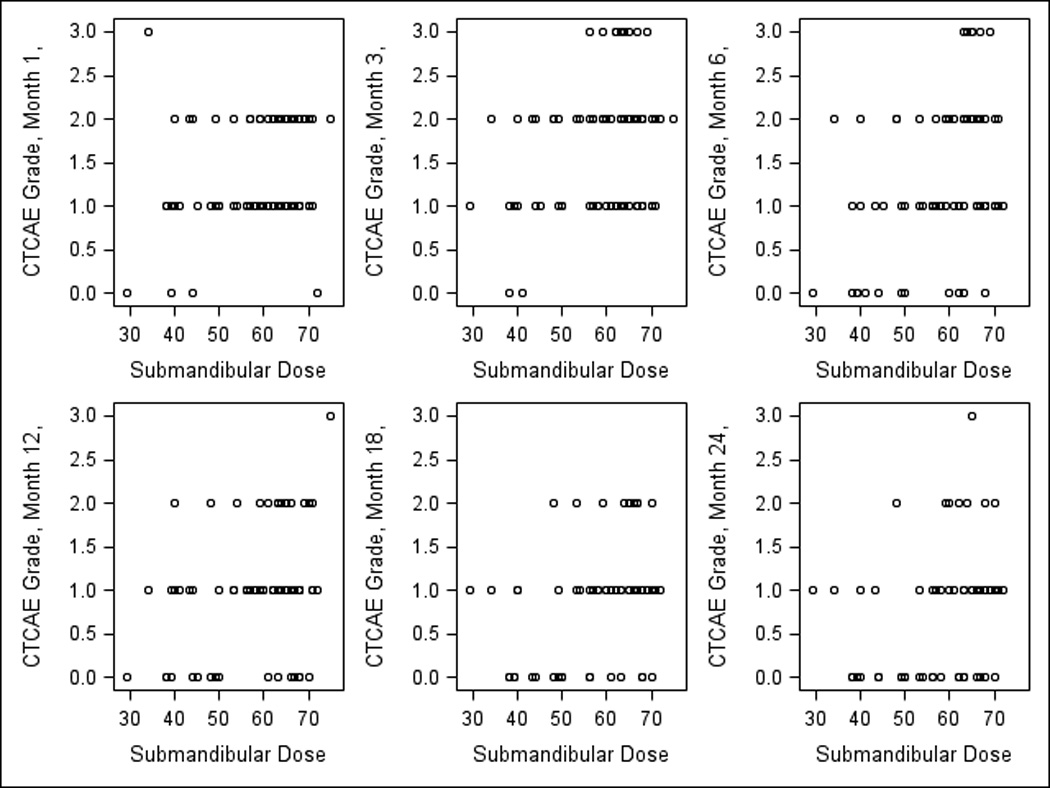
Correlations between observer-rated (CTCAE v2) xerostomia grades at various times post-therapy and contralateral submandibular (SMG) mean doses.
Individual dose-response scatter plots for XQ vs. OC mean dose (Fig 1) suggest no threshold relationships. However, it demonstrates that if the mean OC dose is <40 Gy, almost all XQ scores are lower (better) than 50 on the 0–100 scale at all time points after one month post-therapy, while higher mean doses cause higher (worse) scores in many patients. Similarly, scatter plots for XQ vs. contralateral SMG mean doses (Fig 2) demonstrate no thresholds, however, mean doses <50 Gy were associated with low XQ scores in the large majority of the patients, at multiple post-therapy time points.
Scatter plots for the observer-graded xerostomia (Figs 3–4) showed dose –effect relationships for the OC or the SMG mean doses that were similar to their respective patient-reported scatter plots: if mean OC dose was <40 Gy, no patient had grade 3 xerostomia at 1–3 months, and no grade ≥2 xerostomia at 6–24 months post-therapy (Fig 3). Also, if mean contralateral SMG dose was <50 Gy, no xerostomia grade ≥2 was observed in any patient at any time except for one month (Fig 4).
At median follow-up 38 months, no local-regional recurrences were observed in contralateral level I or in the OC outside the targets.
Discussion
These results imply that in patients whose bilateral parotid glands and contralateral SMGs are spared as much as possible, mean OC doses, serving as surrogates to the RT-induced damage of the minor salivary gland function, are statistically significant predictors of both patient-reported and observer-rated xerostomia, even after the doses to the PGs and SMGs, as well as PG salivary flow rates, are taken into account. Both patient-reported and observer-graded xerostomia were favorable in multiple post-therapy time points if oral cavity mean dose was <40 Gy and contralateral SMG mean dose was <50 Gy. Similar factors and doses predicted both observer-rated and patient-reported low xerostomia. This is reassuring, taking into account the generally low correlation between patients and observers in grading xerostomia (10).
This study had relatively homogeneous patient population, all receiving concurrent chemotherapy and treated with similar IMRT optimization goals. It validates the xerostomia predictors found in our previous study from 2001, which included very heterogeneous treatment techniques and patient population (4). These results are therefore relevant to current IMRT use, and support a change in the prevalent practice: in addition to sparing the PGs, efforts should be made to spare also the SMGs and the OC outside the targets.
The results of the multivariate analysis imply that once controlled for PG and SMG doses, the variation in OC mean doses is an important predictor of xerostomia. The mean doses to each of the major salivary glands, PGs and contralateral SMGs, were significantly correlated with xerostomia in the univariate analysis but were not independent predictors in the multivariate model. Moderate correlations existed between the major salivary gland doses and OC mean doses, and the lack of statistical significance in the multivariate model should not be interpreted to imply lack of a causal relationship, given the difficulties in interpreting such results in a regression model with inter-correlated predictors (11).
The clinical rationale for assessing OC doses as potential predictors of xerostomia has been the minor salivary glands. They are dispersed in the oral cavity, produce less than 10% of the whole saliva volume, but contribute the majority of the total mucins (12). As mucins bind water molecules, their presence on the mucous membranes help maintain a hydrated state and contribute to the patient’s sense of hydration (12). Mean doses <40 Gy to the whole OC were associated in this study with favorable patient-reported and observer-rated xerostomia. It currently serves as our dosimetric goal for IMRT optimization, to reduce xerostomia as well as acute mucositis.
Jellema et al assessed patient-reported xerostomia and its relationships to OC as well as the major salivary gland mean doses (13). They found highly statistically significant relationships between OC doses and xerostomia, however, as correlations between OC and the major salivary gland doses were very high in this 2D RT study, OC doses were not significant in multivariate models, likely due to multicollinearity (10).
We were unable to measure the output of the minor salivary glands, relying instead on the mean dose to the OC as surrogate. Outlining the OC was schematic. Efforts for a better delineation of the minor glands have been made, however, they still lack accuracy, for example, inability to outline the parts of the tongue bearing minor glands (14). Novel imaging of minor salivary gland activity may improve our understanding of RT effects on these glands.
Preserving SMGs function to reduce xerostomia has been evaluated by few additional investigators. SMG doses were significantly correlated with either observer-rated or patient-reported xerostomia in few studies (4, 13, 15–16). Surgical transfer of the contralateral SMG to the submental space, off the RT fields, resulted in preservation of their salivary flows and gains in xerostomia (17). Very few previous IMRT studies sparing the contralateral SMGs also found significant benefit (15–16). Dose-effect relationships for the SMGs, determined by selective measurements of their function, include Tc-pertechnetate scintigraphy showing steep reduction at 50 Gy (18), and our group’s study of selective salivary flow measurements demonstrated exponential reduction in SMG flow rates through mean dose of 39 Gy, beyond which very little saliva was noted (5). Reducing mean contralateral SMG dose to <40 Gy in patients receiving bilateral neck RT, without compromising contralateral level II PTV doses, has been challenging, but feasible in our experience (5) and others(19). Efforts to achieve such dose reductions is our current practice in patients at low risk at contralateral level Ib (no oral cavity involvement by tumor or contralateral level II nodal involvement). Wang et al reported significantly lower xerostomia in patients receiving contralateral mean SMG doses near 20 Gy (16). We could not reproduce such dose reduction without compromising contralateral level II doses, specifically the doses to the contralateral jugulodigastric (subdigastric) nodes. The post-therapy total SMG salivary output compared to pre-therapy was on average relatively low in the current study because the ipsilateral SMG was within the targets in all patients, and the contralateral one was within target in many. This limitation cannot be overcome by IMRT. However, the findings in the current study, of mild xerostomia in almost all patients receiving mean contralateral SMG doses <50 Gy, support efforts to limit SMG doses to lower levels whenever possible.
In conclusion, after chemo-IMRT aiming to spare all salivary tissues outside the targets, mean doses to all the salivary glands: PG, SMGs, and the minor glands (represented by mean OC doses), were significant predictors of both patient-reported and observer-rated xerostomia, and OC doses remained significant even after adjusting to PG and SMG doses. These results justify efforts to spare all the salivary glands, beyond the PGs alone.
Highlights.
Xerostomia after chemo-IMRT of head and neck cancer improves if in addition to the parotid glands, submandibular glands and oral cavity are spared.
Supplementary Material
Acknowledgments
Supported by NIH grant PO1 CA59827 and the Newman Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 52nd Annual Meeting of ASTRO, San Diego, CA, Oct 31 2010.
Conflicts of Interest : none
Financial disclosure: none
REFERENCES
- 1.Kam MK, LSF, Zee B, Chau RM. Prospective randomized study of IMRT on salivary gland function in early-stage nasopharyngeal carcinoma patients. Journal of Clinical Oncology. 2007;25(31):4873–4879. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 2.Pow EHN, Kwong DLW, McMillan AS, et al. Xerostomia and quality of life after IMRT vs conventional radiotherapy for early nasopharyngeal carcinoma. Int J Rad Onc Biol Phys. 2006;66:981–991. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated vs conventional radiotherapy in head neck cancer (PARSPORT) Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisbruch A, Kim HM, Terrell JE, et al. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50(3):695–704. doi: 10.1016/s0360-3016(01)01512-7. [DOI] [PubMed] [Google Scholar]
- 5.Murdoch-Kinch CA, Kim HM, Vineberg KA, et al. Dose-effect relationships for the submandibular salivary glands and implications for their sparing by intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(2):373–382. doi: 10.1016/j.ijrobp.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deasy JO, Moiseenko V, Marks L, et al. Radiotherapy dose-volume effects on salivary gland function. Int J Rad Onc Biol Phys. 2010;76(3 Suppl):S58–S63. doi: 10.1016/j.ijrobp.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol. 2010;28(16):2732–2738. doi: 10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbuck SG, ISP, Setser A. The Revised Common Toxicity Criteria: Version 2.0. CTEP. [Website] : http://ctep.info.nih.gov.
- 9.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: A generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 10.Meirovitz A, Murdoch-Kinch CA, Schipper M, et al. Grading xerostomia by physicians or by patients after IMRT. Int J Rad Onc Biol Phys. 2006;66:445–453. doi: 10.1016/j.ijrobp.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Motulski H. Intuitive Biostatistics. Oxford: Oxford University Press; 1995. [Google Scholar]
- 12.Tabak LA. In defense of the oral cavity: salivary mucins. Annu Rev Physiol. 1995;57:547–564. doi: 10.1146/annurev.ph.57.030195.002555. [DOI] [PubMed] [Google Scholar]
- 13.Jellema AP, Doornaert P, Slotman B, et al. Does radiation dose to salivary glands and oral cavity predict patient-rated xersotomia? Radiother Oncol. 2005;77:164–171. doi: 10.1016/j.radonc.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 14.van de Water TA, Bijl HP, Westerlaan HE, et al. Delineation guidelines for organs at risk involved in radiation-induced salivary dysfunction. Radiother Oncol. 2009;93:545–552. doi: 10.1016/j.radonc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Saarilahti K, Kouri M, Collan J, et al. Sparing submandibular glands by IMRT. Radiother Oncol. 2006;78:270–275. doi: 10.1016/j.radonc.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Wang ZH, Yan C, Zhang ZY, et al. Impact of salivary gland dosimetry on post-IMRT recovery of saliva and xerostomia. Int J Rad Onc Biol Phys. doi: 10.1016/j.ijrobp.2010.07.1990. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 17.Seikaly H, Jha N, Harris J, et al. Outcomes of submandibular gland transfer for prevention of xerostomia. Arch Otolaryngol Head Neck Surg. 2004;130:956–961. doi: 10.1001/archotol.130.8.956. [DOI] [PubMed] [Google Scholar]
- 18.Tsujii H. Quantitative dose-response analysis of salivary function using RI-sialography. Int J Rad Onc Biol Phys. 1985;11:1603–1612. doi: 10.1016/0360-3016(85)90212-3. [DOI] [PubMed] [Google Scholar]
- 19.Houweling AC, Dijkema T, Roesink JM, et al. Sparing contralateral submandibular gland in oropharyngeal cancer: planning study. Radiother Oncol. 20008;69:64–70. doi: 10.1016/j.radonc.2008.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.