Abstract
The aim of this study was to relate altered corpus callosum (CC) integrity in 106 very preterm (VPT) infants (<30 weeks’ gestational age or <1250 g birth weight) at term equivalent to perinatal predictors and neurodevelopmental outcomes at two years. T1 and diffusion magnetic resonance images were obtained. The CC was traced, and divided into six sub-regions for cross-sectional area and shape analyses. Fractional anisotropy, mean, axial and radial diffusivity was sampled within the CC, and probabilistic tractography performed. Perinatal predictors were explored. The Bayley Scales of Infant Development (BSID-II) was administered at two years. Intraventricular hemorrhage was associated with a smaller genu and altered diffusion values within the anterior and posterior CC of VPT infants. White matter injury was associated with widespread alterations to callosal diffusion values, especially posteriorly, and radial diffusivity was particularly elevated, indicating altered myelination. Reduced CC tract volume related to lower gestational age, particularly posteriorly. Reduced posterior callosal skew was associated with postnatal corticosteroid exposure. This more circular CC was associated with delayed cognitive development. Higher diffusivity, particularly in splenium tracts, was associated with impaired motor development. This study elucidates perinatal predictors and adverse neurodevelopmental outcomes associated with altered callosal integrity in VPT infants.
Keywords: Brain, Prematurity, Neonate, Magnetic resonance imaging, Diffusion tensor imaging, Tractography
Introduction
Very preterm (VPT) infants born <30 weeks’ gestational age (GA) or weighing <1250 g at birth are at risk for adverse neurodevelopmental outcome, which is mediated by alterations in cerebral development in the neonatal period. Perinatal variables related to alterations in cerebral development are not fully understood but may include the following: respiratory disease such as bronchopulmonary dysplasia (BPD) (Anjari et al., 2009); exposure to postnatal corticosteroids (PCS), which are used to treat BPD (Halliday et al., 2009a); infection, such as necrotizing enterocolitis and sepsis (Shah et al., 2008); immaturity at birth; low birth weight (BW); and cerebral injury such as white matter injury (WMI) and intraventricular hemorrhage (IVH).
VPT infants are born during a sensitive time of brain development, and are vulnerable to hypoxia-ischemia and infection. Periventricular leukomalacia (PVL) is the most common form of brain injury in the VPT infant, and pre-oligodendrocytes are particularly vulnerable (Volpe, 2009). Importantly, VPT infants have high rates of adverse long term neurodevelopmental outcomes with up to 15% of VPT infants being diagnosed with cerebral palsy (CP) and a further 50% having significant deficits including visual-motor problems, attention difficulties, impaired memory, delayed language skills and executive dysfunction (Holsti et al., 2002). Such neurodevelopmental delays lead to considerable educational burden, with both economic and social implications (Doyle, 2004).
The corpus callosum (CC) is the major inter-hemispheric commissure that connects the majority of the neocortical areas (Schmahmann and Pandya, 2006) and is the largest WM fibre bundle in the human brain. The CC is important for inter-hemispheric communication of sensory, motor and higher-order information. Deficits in the CC have been previously implicated in delayed motor functioning (Rademaker et al., 2004), and reduced intelligence quotient (Caldu et al., 2006), which are problems VPT infants often display. Therefore, alterations to the CC in VPT infants may contribute to later adverse neurodevelopmental outcomes.
Magnetic resonance imaging (MRI) is a safe and non-invasive tool to study the development of the VPT brain in vivo. MR image analysis techniques can detect and quantify the size and morphological characteristics of WM structures within VPT infants. In conjunction with structural MRI, diffusion tensor imaging (DTI) provides insight into the micro-structure and connectivity of WM tracts (Pierpaoli et al., 1996). The literature is consistent in confirming that WM anisotropy increases, and overall diffusivity decreases with increasing age and maturation (Huppi et al., 1998; Schneider et al., 2004). Furthermore, both axial diffusivity (λ||) and radial diffusivity (λ⊥) decrease with age and maturation as water content decreases and myelination increases (Partridge et al., 2004). Thus, higher λ|| and λ⊥ in infants likely represents immaturity of the WM.
It has previously been shown in children, adolescents and adults that prematurity is associated with a smaller CC size (Caldu et al., 2006; Lawrence et al., 2010; Narberhaus et al., 2007; Nosarti et al., 2004; Peterson et al., 2000), and altered diffusion characteristics (Andrews et al., 2009; Constable et al., 2008; Kontis et al., 2009; Nagy et al., 2009; Nagy et al., 2003). A few studies have reported altered CC diffusion in VPT infants (Anjari et al., 2007; Rose et al., 2008; Skiold et al., 2010). One study found VPT males had delayed splenium development on diffusion imaging (Rose et al., 2009). We recently reported that VPT infants had smaller corpora callosa, particularly for the mid-body and posterior sub-regions, and the shape was more circular compared with full-term infants. Further, we found that fractional anisotropy (FA) was lower, mean diffusivity (MD), λ||, and λ⊥ were higher, particularly posteriorly, and CC connectivity was reduced (Thompson et al., 2011). However no study to date has assessed associations between altered callosal measures obtained from structural MRI and DTI at term-equivalent, perinatal variables and neurodevelopmental outcomes. Thus, the aims of this study were to determine the perinatal correlates for changes to VPT CC development, and to associate alterations in CC development at term with functional outcomes at 2 years of age. It was hypothesized that CC size reductions and shape alterations would be associated with younger GA and WMI, and that males would have smaller posterior CC sub-regions. It was also hypothesized that reduced FA values, increased MD, λ||, and λ⊥ within the VPT CC and callosal tracts, and reduced interhemispheric tract volume would relate to WMI. Regarding neurodevelopmental outcomes, the hypotheses were that altered CC area, shape, diffusion and tractography measures would relate to adverse cognitive and motor functioning at 2 years, where anterior regions of the CC would relate to cognitive measures, while posterior regions would relate to motor scores.
Materials and methods
Subjects and scanning
A prospective observational cohort study was conducted at the Royal Women’s Hospitals in Melbourne, Australia between July 2001 and December 2003. Of 348 eligible VPT infants (<30 weeks’ GA and/or <1250 g at birth), 227 VPT infants (65% of those eligible) were recruited, as described previously (Thompson et al., 2011). Infants with congenital anomalies were excluded (3%). There were no significant differences between infants recruited or not included for sex, GA at birth, BPD (defined as requirement for oxygen at 36 weeks’ GA), grade III or IV IVH, or cystic PVL. Informed parental consent was obtained and the study was approved by the Research and Ethics Committee at the Royal Women’s Hospital, Melbourne. A total of 106 stable VPT infants were able to be analyzed for the current study (47% of those recruited). The reasons for non-inclusion were that DTI was not attempted (34%), DTI was unsuccessful or of insufficient quality for further analysis (17%), or structural MRI was unsuccessful or of insufficient quality (2%) largely due to movement or imaging artifact. There were no significant differences between infants included or not included for sex, GA at birth, BPD, grade III or IV IVH, or cystic PVL.
MRI acquisition and pre-processing
All infants were scanned at term equivalent (median 40, range 38–42 weeks’ GA) in a 1.5 T General Electric MRI scanner. Infants were fed and tightly swaddled, immobilized in a vacuum fixation bean-bag and scanned during natural sleep, without sedation. Whole brain structural 3D T1 spoiled gradient recalled images (0.8–1.6 mm coronal slices; flip angle 45°; repetition time (TR) 35 ms; echo time (TE) 9 ms; field of view (FOV) 210 × 157 mm; matrix 256 × 192; in plane voxel dimensions 0.82 mm2), T2 dual echo fast spin echo images with interleaved acquisition (1.7 – 3 mm coronal slices; TR 4000 msec; TE 60 / 160 msec; FOV 220 × 165 mm; matrix 256 × 192, interpolated 512 × 512; in plane voxel dimensions 0.43 mm2), and linescan diffusion images (4 – 6 mm axial slices; 2 baselines, b = 5 s/mm2; six non-collinear gradient directions, b = 700 s/mm2; in plane voxel dimensions 0.86 mm2) were acquired.
Post-acquisition MRI analyses were undertaken on sun Microsystems workstations (Palo Alto, CA). T1-weighted and diffusion images were pre-processed and aligned as previously described (Thompson et al., 2011). In short, T1 images were aligned to the anterior commissure to posterior commissure line (AC-PC) (Talairach and Tournoux, 1988) and brain extraction was achieved by creating an intracranial cavity mask (Kikinis et al., 1992). Diffusion weighted images were preprocessed using the Oxford centre for functional magnetic resonance imaging of the brain software library (FSL), correcting for eddy current distortions (Jenkinson and Smith, 2001), creating a binary brain mask (Smith, 2002), fitting the diffusion tensor model (Behrens et al., 2003), and estimating probabilistic diffusion orientation and diffusion parameters (Behrens et al., 2003). The diffusion images were co-registered with the AC-PC aligned T1 structural images using FSL’s linear registration tool (Jenkinson and Smith, 2001).
Corpus callosum structural measures
Corpora callosa were manually delineated on the mid-sagittal slice of the AC-PC aligned T1-weighted image using 3D slicer software (www.slicer.org). The operator (D.K.T) was blind to all perinatal data, and neurodevelopmental test results. Each CC was delineated twice, and the overlap of the two delineations was obtained and used as the final mask. Reliability, as measured by intraclass correlation coefficient, was 0.84 (95% CI 0.45, 0.95; p=0.003), and the Dice overlap score (Pfefferbaum et al., 2006) was 0.89 (range 0.82 to 0.96, SD 0.04). The CC mask was sub-divided at intervals of one sixth, one third, one half, two thirds, and four fifths the distance from the most anterior to most posterior points, creating regions representing the genu, rostral body, anterior mid-body, posterior mid-body, isthmus and splenium, based on well established schemes (Hofer and Frahm, 2006; Witelson, 1989) as previously described (Thompson et al., 2011). The cross-sectional area of the whole CC and all sub-regions were corrected for the cross-sectional area of the intracranial cavity measured on the mid-sagittal slice from which the CC was delineated, as previously described (Thompson et al., 2011).
Measures of CC shape for this cohort have been previously described in detail (Thompson et al., 2011). In brief, CC eccentricity was measured by fitting an ellipse to each CC mask and calculating the ratio between the semi and major axes. CC skew was calculated by dividing the CC midway between two end points and determining the area under the curve of the most anterior and most posterior segments.
Diffusion measures and tractography within the corpus callosum
The CC region of interest (ROI) was overlaid onto the diffusion map that had been registered to the AC-PC aligned T1 image. MD, FA, λ||, and λ⊥ (calculated as the average of λ2 and λ3: (λ2+λ3)/2) were quantified within the CC ROI, genu, rostral body, anterior mid-body, posterior mid-body, isthmus and splenium.
Probabilistic tractography was carried out using FSL’s Diffusion Toolbox (Behrens et al., 2003), with a curvature threshold of 0.2, 5000 samples, with 1800 steps of 0.4 mm. The CC ROIs were used as seed masks. Measures of connectivity were made by taking the volume of tracts that had been normalized by the number of voxels in the CC ROI, and thresholded as previously described (Thompson et al., 2011). Diffusivity measures (FA, MD, λ⊥, and λ||) within the tracts were obtained.
Perinatal data and neurodevelopmental outcomes
Perinatal data were established by chart review. Data were recorded on GA at birth, sex, birth weight (BW), birth weight standard deviations score (BWSDS), infection, BPD, PCS, grade III or IV IVH and WMI. BWSDS was computed relative to the British Growth Reference data (Cole et al., 1998). Intrauterine growth restriction was defined as a BWSDS more than 2 SD below the mean weight for GA. Neonatal infection was defined as one or more episodes of proven necrotizing enterocolitis and/or one or more episodes of proven sepsis. IVH was recorded as the highest grade from serial ultrasound scans throughout the neonatal intensive care course. WMI was graded qualitatively from MRIs by two blinded independent readers, including a qualified neonatal neurologist (T.E.I) and a trained neonatologist (R.W.H). WM was scored between 1 and 3 for five variables: WM signal abnormality (shortening of T1-weighted images), reduction in WM volume, presence of cystic abnormality, lateral ventricular size, and CC/myelin maturation, as previously reported and validated (Inder et al., 2003; Woodward et al., 2006). As there was overlap with the CC measures in the current study, the CC/myelin maturation variable was disregarded to create a WMI scale ranging from scores of 4 to 12. The median score was 6, and therefore WMI was dichotomized into two categories: lower WMI score (≤6, n=57), or higher WMI score (>6, n=46).
At two years’ corrected age, infants were assessed for developmental delay using the Bayley Scales of Infant Development – 2nd Edition (BSID-II) (Bayley, 1993). Assessors were blinded to all perinatal data. The BSID-II has two indices: the Mental Developmental Index (MDI) and the Psychomotor Developmental Index (PDI). Cognitive development, including language, reasoning ability, memory and learning was assessed by the MDI. Motor development, including gross and fine motor skills was evaluated by the PDI. Children who scored below the lower limit of published normative data were assigned a standardized score of 45, consistent with previous procedures (Woodward et al., 2006). Those too impaired to complete the test were assigned a standardized score of 40, four standard deviations less than the mean. A diagnosis of definite or probable CP was also made at two years’ corrected age according to standardized neurological examination.
Statistical analyses
Data were analyzed using SPSS version 15.0 (SPSS, Chicago, IL), SAS version 8 (SAS Institute Inc., Cary, NC) and Matlab Release 2008b (http://www.mathworks.com). Exploratory analyses were performed on all data to assess normality, homogeneity of variance, linearity, and multi-collinearity as appropriate. Correction for multiple comparisons was achieved by using an alpha level of p<0.01 as the cut-off for statistical significance.
The relationships between CC measures and perinatal variables were initially determined by stepwise linear regression where clinically important variables were provided, but only those that were statistically significant were included in the model. Variables included GA, sex, BWSDS, BPD, PCS therapy, infection, IVH, and WMI (excluding CC aspect). Furthermore, an adjusted standard multivariate regression was performed where GA at MRI was included in the model, as well as all perinatal variables in order to adjust for their effect. The regression coefficients (β) were used to describe mean difference change in the dependent variables per unit change in the independent variables.
The relationships between neurodevelopmental outcomes (MDI, PDI or CP) and VPT infants CC structural and diffusion measures were carried out initially using univariate linear regression. Perinatal variables that were related to the MDI, PDI, or CP were determined using stepwise linear regression. Male sex, PCS, and WMI were related to lower MDI, while PCS was related to lower PDI. No perinatal predictors related to CP. The perinatal variables that were related to the outcome measure of interest were then included in the final multivariate model, along with GA at MRI, to adjust for their effects while examining the relationships between neurodevelopmental outcomes and CC measures.
Results
Subjects (Table 1)
Table 1.
Perinatal and demographic characteristics of the very preterm infant cohort.
| Characteristic | Preterm, n=106 |
|---|---|
| Antenatal corticosteroid administration, n (%) | 94 (89) |
| Gestational age at birth (wk), mean (SD) | 27.6 (1.7) |
| Male gender, n (%) | 55 (52) |
| Multiple births, n (%) | 43 (41) |
| Birth weight (g), mean (SD) | 996 (216) |
| Birth weight SD score, mean (SD) | −0.5 (0.9) |
| Intrauterine growth restriction a, n (%) | 8 (7) |
| Positive pressure ventilation (hr), median (IQR) | 56 (2.3,255) |
| Inotropic Support, n (%) | 46 (43) |
| Patent ductus arteriosus (indomethacin therapy), n (%) | 53 (50) |
| Parenteral nutrition (days), median (IQR) | 11 (6.8,17) |
| Bronchopulmonary dysplasia b, n (%) | 35 (33) |
| Postnatal corticosteroid therapy c, n (%) | 9 (9) |
| Proven necrotizing enterocolitis, n (%) | 3 (3) |
| Proven sepsis, n (%) | 41 (39) |
| Intraventricular hemorrhage (any grade), n (%) | 14 (13) |
| Intraventricular hemorrhage (grade III/IV), n (%) | 5 (5) |
| WM injury (any grade), n (%) | 72 (68) |
| WM injury (grade III/IV), n (%) | 16 (15) |
| Gestational age at MRI (wk), mean (SD) | 40.1 (1.1) |
| Mental Developmental Index at 2 years, mean (SD) | 86 (18) |
| Psychomotor Developmental Index at 2 years, mean (SD) | 89 (17) |
| Definite or probable cerebral palsy at 2 years, n (%) | 8 (8) |
Birth weight SD score >2 SD below mean weight for gestational age,
Required oxygen at 36 weeks gestational age,
Postnatal dexamethasone, usual dose 0.15 mg/kg per day for 3 days, reducing over 10 days: total dose 0.89 mg/kg. MRI = magnetic resonance imaging, SD = standard deviation, IQR = inter-quartile range
Data were analyzed on 106 VPT infants. Characteristics of the cohort studied are reported in Table 1.
Perinatal correlates
Corpus callosum structure (Table 2)
Table 2.
Comparison of structural corpus callosum measures between very preterm infants with or without grade III or IV intraventricular hemorrhage (IVH), females and males, and those exposed to postnatal corticosteroid (PCS) or not.
| Perinatal variable | Mean (SD) | Unadjusted β (95% CI)a | p | Adjusted β (95% CI)b | p | |
|---|---|---|---|---|---|---|
| Whole CC (mm2)* | IVH < III | 78.66 (13.80) | −16.35 (−28.9, −3.8) | 0.01 | −14.48 (−28.0, −0.96) | 0.04 |
| IVH III/IV | 62.31 (13.90) | |||||
| Female | 78.60 (12.70) | −1.36 (−6.8, 4.1) | 0.6 | −1.47 (−7.0, 4.1) | 0.7 | |
| Male | 77.24 (15.50) | |||||
| No PCS | 77.97 (13.54) | −0.61 (−10.5, 9.3) | 0.9 | 0.85 (−10.0, 11.7) | 0.9 | |
| PCS | 77.36 (21.39) | |||||
| Genu (mm2) | IVH < III | 24.41 (5.44) | −9.14 (−14.2, −4.1) | 0.001 | −9.08 (−14.7, −3.5) | 0.002 |
| IVH III/IV | 15.27 (8.09) | |||||
| Female | 23.68 (5.32) | 0.56 (−1.7, 2.8) | 0.6 | 0.45 (−1.9, 2.8) | 0.7 | |
| Male | 24.24 (6.38) | |||||
| No PCS | 23.77 (5.59) | 2.40 (−1.7, 6.5) | 0.2 | 3.03 (−1.5, 7.5) | 0.2 | |
| PCS | 26.17 (8.66) | |||||
| RB (mm2) | IVH < III | 10.27 (1.90) | −1.66 (−3.4, 0.08) | 0.06 | −1.34 (−3.2, 0.54) | 0.2 |
| IVH III/IV | 8.61 (2.41) | |||||
| Female | 10.06 (1.81) | 0.26 (−0.49, 1.0) | 0.5 | 0.36 (−0.41, 1.1) | 0.4 | |
| Male | 10.32 (2.07) | |||||
| No PCS | 10.25 (1.88) | −1.00 (−2.3, 0.33) | 0.1 | −0.63 (−2.1, 0.87) | 0.4 | |
| PCS | 9.25 (2.46) | |||||
| AMB (mm2) | IVH < III | 8.80 (2.85) | −1.81 (−4.4, 0.76) | 0.2 | −1.51 (−4.2, 1.2) | 0.3 |
| IVH III/IV | 6.99 (2.47) | |||||
| Female | 8.98 (2.93) | −0.51 (−1.6, 0.59) | 0.4 | −0.60 (−1.7, 0.51) | 0.3 | |
| Male | 8.47 (2.78) | |||||
| No PCS | 8.72 (2.79) | 0.20 (−1.8, 2.2) | 0.8 | 0.69 (−1.5, 2.8) | 0.5 | |
| PCS | 8.92 (3.66) | |||||
| PMB (mm2) | IVH < III | 6.72 (2.28) | −1.50 (−3.5, 0.54) | 0.1 | −1.28 (−3.4, 0.88) | 0.2 |
| IVH III/IV | 5.22 (1.16) | |||||
| Female | 7.18 (2.44) | −1.02 (−1.9, −0.16) | 0.02 | −1.11 (−2.0, −0.21) | 0.02 | |
| Male | 6.16 (1.98) | |||||
| No PCS | 6.55 (2.29) | 1.35 (−0.21, 2.9) | 0.09 | 1.13 (−0.6, 12.9) | 0.2 | |
| PCS | 7.89 (1.67) | |||||
| Isthmus (mm2) | IVH < III | 6.47 (1.84) | −0.52 (−2.2, 1.1) | 0.5 | −0.17 (−2.0, 1.7) | 0.9 |
| IVH III/IV | 5.95 (1.14) | |||||
| Female | 6.42 (1.79) | 0.05 (−0.66, 0.75) | 0.9 | 0.005 (−0.75, 0.76) | 0.9 | |
| Male | 6.47 (1.86) | |||||
| No PCS | 6.42 (1.75) | 0.45 (−0.81, 1.7) | 0.5 | 0.43 (−1.0, 1.9) | 0.6 | |
| PCS | 6.87 (2.57) | |||||
| Splenium (mm2) | IVH < III | 21.99 (5.42) | −2.77 (−7.7, 2.1) | 0.3 | −2.72 (−7.9, 2.4) | 0.3 |
| IVH III/IV | 19.22 (5.27) | |||||
| Female | 21.94 (5.10) | −0.15 (−2.2, 1.9) | 0.9 | 0.02 (−2.1, 2.1) | 0.9 | |
| Male | 21.79 (5.74) | |||||
| No PCS | 22.21 (5.28) | −4.02 (−7.7, −0.33) | 0.03 | −3.73 (−7.8, 0.39) | 0.08 | |
| PCS | 18.18 (6.07) | |||||
| Eccentricity | IVH < III | 0.62 (0.14) | 0.03 (−0.10, 0.16) | 0.7 | 0.003 (−0.14, 0.15) | 0.9 |
| IVH III/IV | 0.65 (0.21) | |||||
| Female | 0.64 (0.14) | −0.04 (−0.09, 0.02) | 0.2 | −0.03 (−0.09, 0.03) | 0.3 | |
| Male | 0.60 (0.14) | |||||
| No PCS | 0.62 (0.15) | 0.03 (−0.07, 0.13) | 0.5 | 0.05 (−0.07, 0.16) | 0.4 | |
| PCS | 0.65 (0.12) | |||||
| Skew (posterior) | IVH < III | 0.52 (0.07) | 0.02 (−0.05, 0.08) | 0.6 | 0.02 (−0.06, 0.09) | 0.7 |
| IVH III/IV | 0.54 (0.12) | |||||
| Female | 0.52 (0.07) | 0.004 (−0.02, 0.03) | 0.8 | 0.006 (−0.02, 0.04) | 0.7 | |
| Male | 0.52 (0.08) | |||||
| No PCS | 0.53 (0.07) | −0.07 (−0.12, −0.02) | 0.008 | −0.08 (−0.14, −0.02) | 0.009 | |
| PCS | 0.46 (0.06) |
Only other significant variables included in the model,
Adjusted for gestational age at MRI as well as other clinically relevant perinatal variables: gestational age at birth, gender, birth weight standard deviation score, bronchopulmonary dysplasia, PCS, infection, IVH, and white matter injury.
corrected for cross-sectional area of the intracranial cavity. SD = Standard deviation, CI = Confidence interval, RB = rostral body, AMB = Anterior mid-body, PMB = Posterior mid-body, β = regression coefficient, which indicates the amount of change in the dependent variable for each unit change in the independent variable. Bolded = significant at p <0.01 level (corrected for multiple comparisons).
After adjustment for multiple comparisons, GA at MRI and other clinically relevant perinatal variables, grade III or IV IVH was associated with smaller cross-sectional callosal areas for the genu of VPT infants. PCS was related to reduced posterior skew of the CC (Table 2). There was a trend toward an association between male sex and a smaller posterior mid-body cross-sectional area (Table 2).
Apart from the significant associations with perinatal predictors and CC structure before adjustment reported in Table 2, increased rostral body area was also associated with increased GA [β (95% CI): 0.33 (0.09, 0.57); p = 0.008], but the association was no longer statistically significant once adjusting for GA at MRI and other perinatal variables (p = 0.2). There were no clear associations between CC structural measures and either BPD, infection, BWSDS, or WMI.
Corpus callosum diffusion (Table 3)
Table 3.
Comparison of diffusion measures within the corpus callosum and sub-regions between very preterm infants with high or low white matter injury (WMI) scores, grade III or IV intraventricular hemorrhage (IVH), or infection.
| WMI (CC score excluded) | IVH | Infection | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD), mm2 | Unadjusteda | Adjustedb | Mean (SD), mm2 | Unadjusteda | Adjustedb | Mean (SD), mm2 | Unadjusteda | Adjustedb | |||||||||||
| Diffusion measure c |
Low | High | β (95% CI) |
p | β (95% CI) |
p | < grade III |
grade III/IV |
β (95% CI) |
p | β (95% CI) |
P | No | Yes | β (95% CI) |
p | β (95% CI) |
p | |
| Whole CC | FA | 0.32 (0.06) |
0.28 (0.06) |
−0.03 (−0.05, −0.01) |
0.004 | −0.03 (−0.05, −0.009) |
0.006 | 0.30 (0.06) |
0.20 (0.04) |
−0.09 (−0.14, −0.04) |
<0.0005 | −0.08 (−0.14, −0.03) |
0.004 | 0.31 (0.06) |
0.28 (0.06) |
−0.02 (−0.04, −0.002) |
0.04 | −0.02 (−0.04, 0.008) |
0.2 |
| λ⊥ (×10−3mm2/s) | 1.33 (0.15) |
1.38 (0.15) |
N/A | N/A | N/A | N/A | 1.35 (0.15) |
1.48 (0.10) |
0.14 (0.001, 0.27) |
0.05 | 0.13 (−0.01, 0.28) |
0.07 | 1.33 (0.13) |
1.38 (0.18) |
N/A | N/A | N/A | N/A | |
| Genu | FA | 0.34 (0.08) |
0.29 (0.06) |
−0.05 (−0.07, −0.02) |
0.001 | −0.04 (−0.07, −0.01) |
0.004 | 0.32 (0.07) |
0.21 (0.03) |
−0.09 (−0.15, −0.03) |
0.005 | −0.09 (−0.16, −0.02) |
0.01 | 0.32 (0.07) |
0.30 (0.07) |
N/A | N/A | N/A | N/A |
| λ⊥ (×10−3mm2/s) | 1.31 (0.18) |
1.39 (0.15) |
0.07 (0.003, 0.13) |
0.04 | 0.07 (−0.001, 0.13) |
0.06 | 1.34 (0.16) |
1.53 (0.26) |
0.16 (0.01, 0.31) |
0.03 | 0.16 (−0.007, 0.32) |
0.06 | 1.35 (0.17) |
1.35 (0.16) |
N/A | N/A | N/A | N/A | |
| RB | FA | 0.21 (0.04) |
0.19 (0.07) |
−0.03 (−0.05, −0.002) |
0.03 | −0.02 (−0.05, 0.001) |
0.06 | 0.20 (0.06) |
0.16 (0.04) |
N/A | N/A | N/A | N/A | 0.20 (0.07) |
0.20 (0.05) |
N/A | N/A | N/A | N/A |
| AMB | FA | 0.25 (0.04) |
0.21 (0.07) |
−0.04 (−0.06, −0.02) |
<0.0005 | −0.04 (−0.06, −0.02) |
0.001 | 0.23 (0.06) |
0.20 (0.04) |
N/A | N/A | N/A | N/A | 0.23 (0.06) |
0.22 (0.05) |
N/A | N/A | N/A | N/A |
| λ⊥ (×10−3mm2/s) | 1.37 (0.15) |
1.46 (0.19) |
0.09 (0.02, 0.16) |
0.01 | 0.08 (0.01, 0.16) |
0.02 | 1.41 (0.17) |
1.46 (0.17) |
N/A | N/A | N/A | N/A | 1.40 (0.16) |
1.43 (0.02) |
N/A | N/A | N/A | N/A | |
| PMB | FA | 0.23 (0.05) |
0.20 (0.05) |
−0.03 (−0.05, −0.008) |
0.007 | −0.02 (−0.05, −0.002) |
0.04 | 0.22 (0.06) |
0.18 (0.05) |
N/A | N/A | N/A | N/A | 0.21 (0.05) |
0.21 (0.06) |
N/A | N/A | N/A | N/A |
| λ⊥ (×10−3mm2/s) | 1.40 (0.14) |
1.48 (0.18) |
0.08 (0.02, 0.14) |
0.01 | 0.07 (0.003, 0.13) |
0.04 | 1.44 (0.16) |
1.47 (0.11) |
N/A | N/A | N/A | N/A | 1.44 (0.14) |
1.44 (0.18) |
N/A | N/A | N/A | N/A | |
| Splenium | FA | 0.42 (0.10) |
0.39 (0.10) |
N/A | N/A | N/A | N/A | 0.41 (0.09) |
0.24 (0.10) |
−0.18 (−0.26, −0.09) |
<0.0005 | −0.15 (−0.24, −0.05) |
0.002 | 0.42 (0.09) |
0.37 (0.11) |
−0.05 (−0.09, −0.01) |
0.009 | −0.04 (−0.08, 0.001) |
0.05 |
| MD (×10−3mm2/s) |
1.52 (0.21) |
1.53 (0.25) |
N/A | N/A | N/A | N/A | 1.52 (0.23) |
1.66 (0.18) |
N/A | N/A | N/A | N/A | 1.48 (0.14) |
1.61 (0.30) |
0.14 (0.05, 0.23) |
0.003 | 0.12 (0.03, 0.22) |
0.01 | |
| λ⊥
(×10−3mm2/s) |
2.27 (0.20) |
2.21 (0.25) |
N/A | N/A | N/A | N/A | 2.25 (0.22) |
2.09 (0.19) |
0.29 (0.06, 0.52) |
0.01 | 0.31 (0.06, 0.56) |
0.02 | 2.21 (0.17) |
2.28 (0.29) |
0.16 (0.06, 0.27) |
0.002 | 0.15 (0.03, 0.26) |
0.01 | |
Only other significant variables included in the model,
Adjusted for gestational age at MRI as well as other clinically relevant perinatal variables: gestational age at birth, gender, birth weight standard deviation score, bronchopulmonary dysplasia, postnatal corticosteroids, infection, IVH, and WMI,
Only diffusion values which had significant correlations with perinatal variables are shown. CC = corpus callosum, RB = rostral body, PMB = posterior mid-body, FA = fractional anisotropy, MD = mean diffusivity, λ|| = axial diffusivity, λ⊥ = radial diffusivity, SD = standard deviation, CI = confidence interval, N/A = not applicable (no significant perinatal predictors), β = regression coefficient, which indicates the amount of change in the dependent variable for each unit change in the independent variable. Bolded = significant at p <0.01 level (corrected for multiple comparisons).
After covarying for GA at MRI and other perinatal variables, WMI was significantly related to lower FA values within the whole CC, genu, and anterior mid-body of VPT infants at the alpha level p < 0.01 (Table 3).
IVH was significantly related to reduced FA within the whole CC and the splenium of VPT infants, with a trend toward reduced FA within the genu and higher λ⊥ within the splenium after correcting for GA at MRI and other perinatal variables (Table 3).
After correcting for GA at MRI and other potential perinatal confounders, infection trended toward a positive relationship with MD and λ⊥ values within the splenium (Table 3).
Diffusion values within the CC and sub-regions that were not significantly related to any perinatal predictors are not reported. There were no clear statistically significant relationships between diffusion values within the CC and GA, sex, BWSDS, BPD, or PCS. No perinatal variables were related to isthmus diffusion values. Neither were there any clear statistically significant associations between perinatal variables and λ|| values.
Inter-hemispheric fibre bundles (Tables 4 and 5)
Table 4.
Relationships between gestational age at birth and callosal tract volumes: whole corpus callosum (CC) and sub-regions.
| Unadjusted β (95% CI)a | p | Adjusted β (95% CI)b | p | |
|---|---|---|---|---|
| Whole CC | 1.09 (0.65, 1.5) | <0.0005 | 0.98 (0.28, 1.7) | 0.007 |
| Genu | 0.58 (0.28, 0.87) | <0.0005 | 0.54 (0.07, 1.0) | 0.02 |
| RB | 0.41 (0.18, 0.64) | 0.001 | 0.43 (0.07, 0.78) | 0.02 |
| AMB | 0.23 (0.03, 0.44) | 0.03 | 0.30 (−0.03, 0.63) | 0.07 |
| PMB | N/A | N/A | N/A | N/A |
| Isthmus | N/A | N/A | N/A | N/A |
| Splenium | 1.33 (0.85, 1.8) | <0.0005 | 1.30 (0.54, 2.1) | 0.001 |
Only other significant variables included in the model.
Adjusted for gestational age at MRI as well as other clinically relevant perinatal variables: gestational age at birth, gender, birth weight standard deviation score, bronchopulmonary dysplasia, postnatal corticosteroids, infection, intraventricular hemorrhage, and white matter injury. CI = Confidence interval, RB = rostral body, AMB = Anterior mid-body, PMB = Posterior mid-body, N/A = not applicable (no significant perinatal predictors), β = regression coefficient, which indicates the amount of change in the dependent variable for each unit change in the independent variable. Bolded = significant at p <0.01 level (corrected for multiple comparisons).
Table 5.
Correlations between diffusion measures within the corpus callosum (CC) and callosal sub-regional tracts of very preterm infants with high or low white matter injury (WMI) scores, or grade III or IV intraventricular hemorrhage (IVH).
| WMI (CC score excluded) | IVH | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| low | high | Unadjusted β (95% CI)a | p | Adjusted β (95% CI)b | p | < grade III | grade III/IV | Unadjusted β (95% CI)a | p | Adjusted β (95% CI)b | p | ||
| Whole CC (×10−3 mm2/s) | FA | 0.21 (0.02) | 0.19 (0.03) | −0.02 (−0.03, −0.01) | <0.0005 | −0.02 (−0.03, −0.01) | <0.0005 | 0.20 (0.03) | 0.18 (0.02) | N/A | N/A | N/A | N/A |
| MD | 1.62 (0.07) | 1.70 (0.11) | 0.07 (0.03, 0.10) | <0.0005 | 0.07 (0.03, 0.10) | <0.0005 | 1.65 (0.09) | 1.76 (0.13) | 0.08 (0.003, 0.16) | 0.04 | 0.10 (0.009, 0.18) | 0.03 | |
| λ|| | 1.98 (0.09) | 2.04 (0.13) | 0.05 (0.009, 0.10) | 0.02 | 0.05 (0.002, 0.09) | 0.04 | 2.00 (0.11) | 2.08 (0.18) | N/A | N/A | N/A | N/A | |
| λ⊥ | 1.44 (0.07) | 1.53 (0.10) | 0.08 (0.05, 0.11) | <0.0005 | 0.08 (0.04, 0.11) | <0.0005 | 1.48 (0.09) | 1.60 (0.12) | 0.09 (0.02, 0.17) | 0.02 | 0.11 (0.03, 0.19) | 0.01 | |
| Genu | FA | 0.18 (0.02) | 0.18 (0.04) | N/A | N/A | N/A | N/A | 0.18 (0.03) | 0.18 (0.03) | N/A | N/A | N/A | N/A |
| MD | 1.59 (0.08) | 1.66 (0.15) | 0.06 (0.01, 0.10) | 0.01 | 0.06 (0.02, 0.10) | 0.006 | 1.62 (0.11) | 1.79 (0.20) | 0.16 (0.06, 0.26) | 0.002 | 0.17 (0.07, 0.27) | 0.001 | |
| λ|| | 1.90 (0.10) | 1.97 (0.24) | N/A | N/A | N/A | N/A | 1.92 (0.17) | 2.13 (0.26) | 0.21 (0.05, 0.37) | 0.009 | 0.21 (0.05, 0.38) | 0.01 | |
| λ⊥ | 1.44 (0.07) | 1.51 (0.12) | 0.06 (0.02, 0.09) | 0.002 | 0.06 (0.03, 0.10) | <0.0005 | 1.46 (0.09) | 1.62 (0.17) | 0.14 (0.06, 0.23) | 0.002 | 0.15 (0.07, 0.23) | 0.001 | |
| RB | FA | 0.20 (0.02) | 0.18 (0.04) | −0.02 (−0.03, −0.005) | 0.008 | −0.02 (−0.03, −0.003) | 0.02 | 0.19 (0.03) | 0.18 (0.03) | N/A | N/A | N/A | N/A |
| MD | 1.67 (0.11) | 1.75 (0.20) | N/A | N/A | N/A | N/A | 1.69 (0.14) | 1.91 (0.31) | 0.23 (0.09, 0.37) | 0.002 | 0.23 (0.08, 0.38) | 0.003 | |
| λ|| | 2.01 (0.16) | 2.07 (0.30) | N/A | N/A | N/A | N/A | 2.02 (0.20) | 2.28 (0.41) | 0.26 (0.06, 0.46) | 0.01 | 0.28 (0.07, 0.50) | 0.01 | |
| λ⊥ | 1.50 (0.10) | 1.58 (0.16) | 0.06 (0.01, 0.12) | 0.02 | 0.08 (0.02, 0.13) | 0.009 | 1.52 (0.12) | 1.73 (0.26) | 0.18 (0.07, 0.30) | 0.002 | 0.21 (0.08, 0.33) | 0.002 | |
| AMB | FA | 0.20 (0.02) | 0.19 (0.03) | −0.02 (−0.03, −0.006) | 0.004 | −0.01 (−0.03, 0.0001) | 0.05 | 0.20 (0.03) | 0.18 (0.03) | N/A | N/A | N/A | N/A |
| MD | 1.61 (0.10) | 1.70 (0.16) | 0.08 (0.02, 0.14) | 0.008 | 0.08 (0.02, 0.14) | 0.01 | 1.65 (0.12) | 1.76 (0.29) | N/A | N/A | N/A | N/A | |
| λ|| | 1.96 (0.13) | 2.03 (0.23) | N/A | N/A | N/A | N/A | 1.98 (0.16) | 2.10 (0.40) | N/A | N/A | N/A | N/A | |
| λ⊥ | 1.44 (0.09) | 1.54 (0.13) | 0.08 (0.04, 0.13) | 0.001 | 0.08 (0.03, 0.13) | 0.002 | 1.48 (0.10) | 1.60 (0.24) | N/A | N/A | N/A | N/A | |
| PMB | FA | 0.21 (0.02) | 0.19 (0.03) | −0.02 (−0.03, −0.006) | 0.002 | −0.02 (−0.03, −0.003) | 0.01 | 0.20 (0.03) | 0.19 (0.01) | N/A | N/A | N/A | N/A |
| MD | 1.60 (0.09) | 1.71 (0.14) | 0.11 (0.06, 0.16) | <0.0005 | 0.10 (0.05, 0.16) | <0.0005 | 1.65 (0.12) | 1.79 (0.10) | N/A | N/A | N/A | N/A | |
| λ|| | 1.95 (0.12) | 2.06 (0.19) | 0.11 (0.04, 0.17) | 0.001 | 0.09 (0.02, 0.16) | 0.009 | 1.99 (0.16) | 2.14 (0.12) | N/A | N/A | N/A | N/A | |
| λ⊥ | 1.43 (0.08) | 1.54 (0.12) | 0.12 (0.08, 0.16) | <0.0005 | 0.10 (0.06, 0.15) | <0.0005 | 1.47 (0.11) | 1.61 (0.09) | N/A | N/A | N/A | N/A | |
| Isthmus | FA | 0.21 (0.02) | 0.19 (0.02) | −0.02 (−0.03, −0.007) | 0.001 | −0.02 (−0.03, −0.004) | 0.008 | 0.20 (0.03) | 0.17 (0.01) | N/A | N/A | N/A | N/A |
| MD | 1.64 (0.09) | 1.77 (0.14) | −0.13 (−0.08, 0.17) | <0.0005 | 0.13 (0.08, 0.18) | <0.0005 | 1.69 (0.13) | 1.73 (0.05) | N/A | N/A | N/A | N/A | |
| λ|| | 1.99 (0.13) | 2.12 (0.17) | 0.12 (0.06, 0.19) | <0.0005 | 0.13 (0.06, 0.20) | <0.0005 | 2.05 (0.16) | 2.04 (0.05) | N/A | N/A | N/A | N/A | |
| λ⊥ | 1.46 (0.08) | 1.59 (0.12) | 0.13 (0.09, 0.17) | <0.0005 | 0.13 (0.08, 0.17) | <0.0005 | 1.52 (0.12) | 1.57 (0.06) | N/A | N/A | N/A | N/A | |
| Splenium | FA | 0.20 (0.02) | 0.19 (0.03) | −0.02 (−0.03, −0.01) | <0.0005 | −0.02 (−0.03, −0.009) | <0.0005 | 0.20 (0.03) | 0.17 (0.03) | N/A | N/A | N/A | N/A |
| MD | 1.63 (0.08) | 1.72 (0.15) | 0.08 (0.04, 0.13) | <0.0005 | 0.08 (0.03, 0.12) | 0.001 | 1.66 (0.10) | 1.88 (0.28) | 0.20 (0.10, 0.30) | <0.0005 | 0.21 (0.10, 0.33) | <0.0005 | |
| λ|| | 1.99 (0.11) | 2.06 (0.17) | 0.06 (0.001, 0.11) | 0.05 | 0.06 (0.003, 0.12) | 0.04 | 2.01 (0.12) | 2.22 (0.31) | 0.18 (0.06, 0.30) | 0.005 | 0.20 (0.06, 0.33) | 0.006 | |
| λ⊥ | 1.45 (0.07) | 1.55 (0.15) | 0.09 (0.05, 0.13) | <0.0005 | 0.09 (0.04, 0.13) | <0.0005 | 1.48 (0.10) | 1.72 (0.28) | 0.21 (0.11, 0.30) | <0.0005 | 0.22 (0.12, 0.33) | <0.0005 | |
Only other significant variables included in the model,
Adjusted for gestational age at MRI as well as other clinically relevant perinatal variables: gestational age at birth, gender, birth weight standard deviation score, bronchopulmonary dysplasia, postnatal corticosteroids, infection, IVH, and WMI. RB = rostral body, AMB = anterior mid-body, PMB = posterior mid-body, FA = fractional anisotropy, MD = mean diffusivity(×10−3 mm2/s), λ|| = axial diffusivity (×10−3 mm2/s), λ⊥ = radial diffusivity (×10−3 mm2/s), CI = confidence interval, N/A = not applicable, β = regression coefficient, which indicates the amount of change in the dependent variable for each unit change in the independent variable. Bolded = significant at p <0.01 level (corrected for multiple comparisons).
GA at birth was related to callosal tract volume. Larger volumes of fibre tracts radiating from the whole CC were significantly associated with a later GA (Fig. 1A,B), even after adjustment for GA at MRI and other perinatal variables (Table 4). In particular, larger splenium tract volumes were related to a higher GA, even after adjustment (Table 4). There was also a trend toward larger genu and rostral body tract volumes with higher GA (Table 4). There were no clear associations between perinatal variables and tract volume within the posterior mid-body or isthmus.
Fig 1.
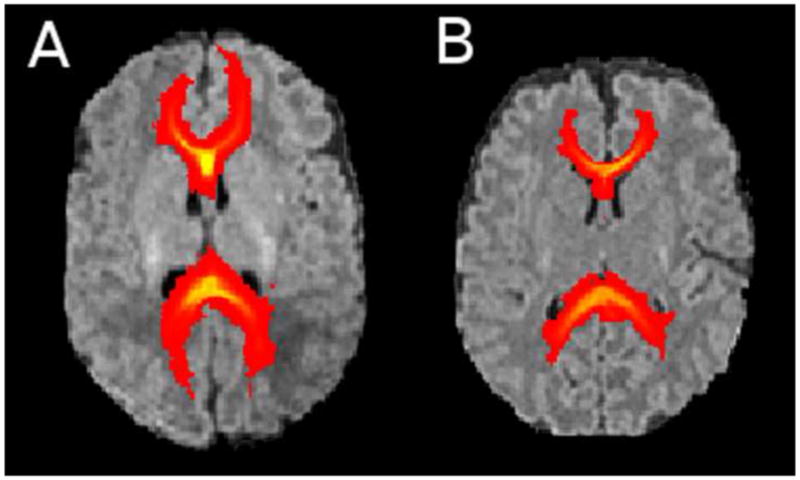
Probabilistic fibre tracts of the corpus callosum overlaid on the T1 image for very preterm infants of 29 weeks’ gestational age (A) and 25 weeks’ gestational age (B).
WMI was significantly associated with lower FA within the VPT infant tracts originating from the whole CC, isthmus and splenium with a trend for the rostral body, and posterior mid-body, once GA at MRI and other perinatal variables were covaried (Table 5). WMI was associated with higher MD in all callosal tracts, except those of the rostral body (and a trend for the anterior mid-body), after adjustment (Table 5). Higher λ|| values within the tracts of the posterior mid-body and isthmus were associated with WMI, and these associations remained significant after adjustment (Table 5). WMI was also related to increased λ⊥ values within the tracts of the whole CC, including all sub-regions (excepting a trend for rostral body), after covarying for GA at MRI and other perinatal variables (Table 5).
IVH was related to higher MD and λ⊥ values within the tracts of the VPT infants genu, rostral body, and splenium, and the association remained significant after correction for GA at MRI and the effect of other perinatal variables (Table 5). There was a trend for higher λ⊥ in the whole CC associated with IVH. IVH also significantly correlated with higher λ|| values within the splenium, and this association remained significant after adjustment (Table 5). There was a trend toward higher λ|| values in the genu and rostral body of infants with grade III or IV IVH after adjusting for GA at MRI and other perinatal variables (Table 5).
There was some evidence that BPD was related to higher diffusivity values within the posterior mid-body of VPT infants. Specifically, BPD was related to higher MD values within the posterior mid-body tracts [mean (SD): no BPD 1.63 (0.11), BPD 1.70 (0.14) ×10−3 mm2/s; β (95%CI): 0.06 (0.006, 0.11); p = 0.004]. However this association did not remain statistically significant once GA at MRI and other perinatal variables were included in the model.
Neurodevelopmental outcomes
From the perinatal predictors tested, male sex, PCS, and WMI were related to lower MDI, while PCS was related to lower PDI. However no perinatal variables related to CP. Therefore the relevant perinatal variables were adjusted for in the following analyses, and GA at MRI was also included in the final model.
Corpus callosum structure
There were no substantial relationships between neurodevelopmental outcomes (MDI or PDI) and the cross-sectional area of the whole CC or any of the relevant sub-regions within VPT infants once correcting for GA at MRI and other relevant perinatal variables. There was a significant positive correlation between VPT infants’ skew measures and the MDI. The more the infant’s CC was skewed toward the posterior, the higher the MDI scores [β (95% CI): 71.4 (25.0, 118); p=0.003]. After adjustment for GA at MRI, sex, PCS and WMI, there was still a trend toward posterior skew being associated with MDI scores [β (95% CI): 51.7 (8.7, 94.7); p=0.02]. There was a trend to greater eccentricity of the CC being related to CP [β (95% CI): 0.43 (0.07, 0.78); p=0.02], and this trend remained after adjustment for GA at MRI [β (95% CI): 0.40 (0.05, 0.75); p=0.03].
Corpus callosum diffusivity
There were no clear significant relationships between MDI, PDI or CP for any diffusivity values within the whole CC or sub-regions of VPT infants after adjustment for GA at MRI and relevant perinatal variables.
Inter-hemispheric fibre bundles (Table 6)
Table 6.
(A)Relationships between mental developmental index (MDI) and diffusion measures within the callosal tracts: whole corpus callosum (CC) and sub-regions. (B) Relationships between psychomotor developmental index (PDI) and diffusion measures within the callosal tracts: whole CC and sub-regions.
| (A) MDI | |||||
|---|---|---|---|---|---|
| Diffusion value | Unadjusted β (95% CI) | p | Adjusted β (95% CI)a | p | |
| Whole CC | FA | 213.9 (83.1, 344.8) | 0.002 | 157.7 (25.3, 290.2) | 0.02 |
| λ⊥ | −41.1 (−81.0, −1.21) | 0.04 | −19.3 (−61.0, 22.4) | 0.4 | |
| Genu | FA | 138.7 (6.08, 271.2) | 0.04 | 98.6 (−26.2, 223.4) | 0.1 |
| AMB | FA | 228.4 (102.1, 354.7) | 0.001 | 152.9 (25.8, 280.1) | 0.02 |
| PMB | FA | 174.1 (23.8, 324.4) | 0.02 | 157.5 (11.5, 303.4) | 0.04 |
| Isthmus | FA | 184.0 (44.2, 323.8) | 0.01 | 124.7 (−12.4, 261.7) | 0.07 |
| Splenium | FA | 173.9 (48.0, 299.9) | 0.007 | 125.3 (−0.97, 251.6) | 0.05 |
| MD | −31.2 (−59.8, −2.60) | 0.03 | −10.5 (−38.2, 17.1) | 0.5 | |
| λ⊥ | −37.8 (−66.7, −8.9) | 0.01 | −16.5 (−45.5, 12.5 | 0.3 | |
| (B) PDI | |||||
| Whole CC | MD | −38.6 (−75.5, −1.70) | 0.04 | −19.4 (−30.6, −8.25) | 0.001 |
| λ⊥ | −45.0 (−82.1, −7.96) | 0.02 | −44.4 (−79.8, −9.04) | 0.01 | |
| Splenium | MD | −44.1 (−70.1, −18.2) | 0.001 | −35.4 (−60.0, −10.7) | 0.005 |
| λ|| | −32.6 (−55.5, −9.68) | 0.006 | −24.9 (−46.6, −3.13) | 0.03 | |
| λ⊥ | −46.5 (−72.9, −20.1) | 0.001 | −38.2 (−63.3, −13.1) | 0.003 | |
Adjusted for gestational age at MRI as well as other perinatal variables related to MDI (gender, postnatal corticosteroids and white matter injury) or PDI (postnatal corticosteroids). CI = Confidence interval, AMB = Anterior mid-body, PMB = Posterior mid-body, FA = fractional anisotropy, MD = mean diffusivity(×10−3 mm2/s), λ|| = axial diffusivity(×10−3 mm2/s), λ⊥ = radial diffusivity (×10−3 mm2/s), β = regression coefficient, which indicates the amount of change in the dependent variable for each unit change in the independent variable
There was a trend toward higher MDI scores being related to higher FA values within the tracts of the whole CC, and particularly the anterior mid-body after adjustment for GA at MRI, sex, PCS and WMI (Table 6). Before adjustment, there was an additional significant positive relationship between MDI and splenium FA, as well as a trend for posterior mid-body and isthmus FA. Furthermore, before adjustment, there was a trend toward negative relationships between MDI and λ⊥ within the splenium tracts (Table 6A).
Higher PDI scores, and hence better motor outcomes, were significantly related to lower MD and λ⊥ within the tracts originating from the splenium. These associations remained statistically significant after adjustment. Furthermore, higher PDI scores were related to lower λ|| within the splenium tracts (with only a trend after adjustment), and there was a trend toward an association between higher PDI scores and lower λ⊥ values within the whole CC tracts (Table 6B).
Discussion
Summary of results
Alterations to callosal morphology in VPT infants were related to IVH and PCS, and to a lesser extent male sex. Abnormal microstructure of the CC was associated mainly with WMI and IVH, and to a lesser extent infection, while inter-hemispheric connectivity was reduced in VPT infants born at a younger GA. There was evidence that CC shape alterations were associated with later cognitive impairment, and that microstructural alterations within the callosal tracts were associated with motor impairment at 2 years of age.
Perinatal predictors
Intraventricular hemorrhage
Grade III or IV IVH within VPT infants was associated with a smaller cross-sectional area, and the genu appeared particularly vulnerable. IVH has previously been associated with CC structural abnormalities in VPT infants (de Vries et al., 1998). De Vries et al. showed that thinning of the CC was evident in the vast majority of VPT infants with a large IVH (de Vries et al., 1998). A related study showed that VPT adolescents who had experienced periventricular hemorrhage had a greatly reduced CC area (Nosarti et al., 2004). Anisotropy was lower within the CC ROI in association with grade III or IV IVH in VPT infants, particularly at the splenium, and to a lesser extent the genu. IVH was also related to increased MD, λ|| and λ⊥ values in VPT infant’s callosal tracts, indicating abnormal microstructure. IVH was mainly associated with alterations to the fibres running through the ends of the CC, namely the genu, rostral body and splenium. These results indicate that microstructure and organization of tracts connecting left and right prefrontal, premotor, sensorimotor, occipital and inferior temporal cortices are compromised by IVH in VPT infants. Myelination of the occipital and inferior temporal inter-hemispheric tracts also appears to be disrupted or delayed in VPT infants with a grade III or IV IVH. Severe IVH is known to affect both local and distant cerebral regions (Chua et al., 2009; Luciano et al., 2007; Tam et al., 2009). Considering the CC forms the roof of the anterior horns, body and part of the posterior horns of the lateral ventricles, it follows that the CC is vulnerable to IVH, particularly in relation to post-hemorrhagic ventriculomegaly (Juliet et al., 2009; Luciano et al., 2007). The vulnerability of the anterior and posterior-most ends of the CC is possibly a result of active organization and pre-myelination of these regions during perinatal development (Gilmore et al., 2007; Hofer and Frahm, 2006; Huppi and Dubois, 2006; Partridge et al., 2004). Another possible explanation is that IVH selectively affects the small diameter fibres of the CC, common at the ‘extremities’ (Aboitiz et al., 1992; LaMantia and Rakic, 1990; Yazgan et al., 1995). Until now, CC microstructure had not been examined in relation to IVH in VPT infants.
White matter injury
WMI was uniquely associated with abnormal microstructure of the CC ROI, specifically reduced anisotropy within the genu and anterior mid-body. Within the VPT infants’ callosal tracts, WMI was related to a reduced FA and increased λ|| in the whole CC, particularly the posterior half (isthmus and splenium, with a trend for posterior mid-body), as well as a widespread increase in MD and particularly λ⊥ values. This indicates that WMI interferes with the maturation of the CC, including its microstructural organization and myelination. The mechanisms by which this injury may occur include hypoxic-ischemia, infection and inflammation, which lead to excitotoxicity, and potentially necrosis, apoptosis, astrogliosis and microgliosis as well as damage to and reduction of pre-myelinating oligodendrocytes (Volpe, 2009).
Although all sub-regions of the CC appear affected in some way, the greatest association with WMI was in the posterior regions connecting inter-hemispheric somatosensory, temporal, parietal and occipital cortices. Posterior callosal regions are first to myelinate during development (Bloom and Hynd, 2005; van der Knaap and Valk, 1995), which may explain why microstructural alterations as a result of WMI during the preterm period are particularly evident in these regions at term equivalent. WMI has not been previously studied in relation to callosal diffusion characteristics in VPT infants, however altered λ⊥ in the splenium of VPT infants has been associated with WM signal abnormality (Counsell et al., 2006). Furthermore, Benjak et al. found that PVL caused by hypoxia-ischemia in VPT infants damaged the CC, being topographically close to the lateral ventricles (Benjak et al., 2008). No studies have reported the effects of WMI on CC tractography findings in VPT infants. However, studies specifically examining PVL have found that the commissural fibres are frequently injured (Davatzikos et al., 2003; Nagae et al., 2007). Related studies have shown that PVL in children is associated with adverse CC tractography measures in the genu (Fan et al., 2006) and splenium (Davatzikos et al., 2003; Fan et al., 2006; Nagae et al., 2007). Furthermore, higher MD values are evident in the callosal tracts of children with mild ventriculomegaly (Gilmore et al., 2008).
Postnatal corticosteroids
PCS exposure was uniquely associated with reduced posterior skew of the CC. The CC grows from anterior to posterior, so the later developing posterior region is likely more vulnerable during the preterm period, when PCS are administered. A possible explanation for reduced posterior skew is that fewer interhemispheric fibres extend into the posterior section of the CC during development. Shape measures may be a sensitive marker for morphological changes to the CC, reflecting underlying regional alterations to fibre organization or myelination. Reduced posterior skew has previously been reported in VPT compared with full-term (FT) infants, making the CC appear more circular (Thompson et al., 2011), and the current study suggests this alteration may be mediated by PCS exposure. Early PCS exposure (Halliday et al., 2009a), and to some extent late exposure (Halliday et al., 2009b), is thought to negatively affect brain development. However, very little literature exists relating PCS exposure to CC alterations. Before adjustment for other perinatal variables, BPD was related to microstructural alterations of the posterior mid-body tracts. Together these results would indicate an adverse affect of BPD, or the PCS used to treat this condition, on the posterior sub-regions of the CC. In contrast, Anjari and colleagues associated respiratory disease with reduced FA in the anterior portion (genu) of the CC in VPT infants (Anjari et al., 2009).
Sex
Male sex was associated with a smaller posterior mid-body in VPT infants, remaining a trend after adjustment for other perinatal variables. The posterior mid-body connects somatosensory and posterior parietal regions of the brain, involved in sensory integration. The only previous study comparing sex effects within VPT corpora callosa found that VPT males had delayed splenium development on diffusion imaging, which was associated with a higher rate of neurodevelopmental abnormalities (Rose et al., 2009). Previous studies of sex differences in the CC have also suggested that females and males generally differ in the posterior sub-regions (Allen et al., 1991; DeLacoste-Utamsing and Holloway, 1982; Dubb et al., 2003). Such sex specific alterations to the CC may partially relate to the vulnerability of VPT males to adverse neurodevelopmental outcomes. The neurobiological basis for this remains unknown.
Infection
There was some evidence that infection was related to increased MD and λ⊥ values within the splenium of the CC, suggesting there may be increased water content and delayed or disrupted myelination of the CC region connecting occipital and inferior temporal inter-hemispheric areas. Although not specific to the CC, the only study to examine sepsis and necrotizing enterocolitis in VPT infants associated infection with a greater risk of motor impairment at 2 years of age; however this relationship appeared to be mediated by WM abnormality (Shah et al., 2008).
Gestational age
A younger GA was associated with significantly fewer inter-hemispheric tracts, particularly those of the splenium and to a lesser extent the genu and rostral body at the ‘extremities’ of the CC, indicating that inter-hemispheric connectivity is more disrupted the earlier the VPT infant is born. These earlier organizing, small diameter fibres common at the ‘extremities’ of the CC appear more vulnerable the earlier the VPT infant is born. Although no studies have examined the effects of GA on CC tracts per se, younger GAs have been associated with a slower rate of callosal growth, and hence smaller callosal size in VPT infants (Anderson et al., 2005; Anjari et al., 2007), adolescents (Caldu et al., 2006; Narberhaus et al., 2007), and adults (Lawrence et al., 2010). In particular, Narberhaus et al., reported a smaller genu, anterior mid-body, posterior mid-body, isthmus, and splenium in association with lower GA in VPT adolescents (Narberhaus et al., 2007). Thus it is possible that CC reduction in VPT infants who are born earlier is not compensated for during childhood.
Neurodevelopmental outcomes
This study did not find any associations between CC size and neurodevelopmental functioning at two years. In contrast, previous studies have found associations between the size of the CC and motor performance in 2 year old (Anderson et al., 2006) and 7–8 year old VPT children (Rademaker et al., 2004), general cognition in 8 year old VPT children (Peterson et al., 2000), IQ and everyday memory performance in VPT adolescents (Caldu et al., 2006), and language functions in adolescent boys born VPT (Nosarti et al., 2004).
Reduced posterior skew in VPT corpora callosa was associated with impaired cognitive development. Reduced posterior skew has been reported in VPT compared with FT infants (Thompson et al., 2011), and thus it appears there are functional consequences to altered callosal curvature. A possible explanation for the association between skew and cognition is that reduced posterior skew equates to reduced interhemispheric connectivity between the posterior parietal, temporal and occipital cortical regions associated with sensory processing, language, memory, hearing and vision. Although no studies have examined callosal shape in relation to prematurity, thinning of the CC has been previously associated with CP (Iai et al., 1994), altered CC morphology in children with PVL has been associated with motor and cognitive impairment (Davatzikos et al., 2003), and more circular CC morphology has been associated with impairment on phonological tests in adult males with developmental dyslexia (Robichon and Habib, 1998).
Higher diffusivity values in the splenium tracts were associated with lower PDI scores, and hence altered motor development. Previously we have shown that VPT infants’ callosal tract microstructure was altered compared with FT infants, particularly for posterior connections (Thompson et al., 2011) and the present results suggest there are functional consequences to these alterations. The splenium connects the occipital lobe and inferior temporal cortices, and is traditionally thought to transfer visual information (Gazzaniga and Freedman, 1973). There is a large component of motor coordination that relies upon visual processing, and it appears that motor functions in VPT infants rely on the integrity of callosal tracts involved in lateralization of vision. Similarly to the current results, Rose et al. associated reduced splenium anisotropy in VPT males with a higher incidence of abnormal neurodevelopment at 1-½ years of age (Rose et al., 2009). Studies examining healthy subjects have associated lower anisotropy within various regions of the CC with motor skills and bimanual co-ordination (Johansen-Berg et al., 2007), as well as visually guided motor task performance (Wahl et al., 2007).
Adverse functioning as a result of altered connectivity and structural organization of the CC likely relates to reduced inter-hemispheric transfer times. Westerhausen et al. found negative correlations between MD and inter-hemispheric transfer time, concluding that stronger callosal connectivity allows for better or faster inter-hemispheric transfer time (Westerhausen et al., 2006).
Limitations
Infants of this cohort were scanned over a long time frame, during which upgrades in scanning protocols were made. This resulted in a range of slice thicknesses within our cohort which possibly led to inconsistencies and bias in volume measurements. However, approximately equal numbers of infants in each group were scanned with the different slice thicknesses, minimizing the likelihood of confounded results. Furthermore, the CC was traced on the sagittal plane of coronally acquired T1 images, and considering voxels were not isotropic, partial volume artifacts are likely.
There are important technological limitations to the diffusion acquisition utilized in this study. DTI was acquired in only 6 non-collinear directions, which is reportedly sub-optimal for robust tractography and rotationally invariant diffusivity measures (Jones, 2004). While multi-tensor models are suggested for resolving crossing fibres, tractography for dominant fibre pathways such as the CC, with few crossing fibres, are unlikely to gain from such a technique (Behrens et al., 2007). Regardless, the DTI results presented in this study require confirmation by other studies using more technologically advanced DTI acquisition protocols, higher field strength, increased image resolution, more gradient directions, and improved signal to noise ratio, as well as more advanced analysis algorithms and techniques.
There is rapid maturation and a dramatic decrease in brain water content with increasing GA during the perinatal time period (Huppi and Dubois, 2006; Mukherjee et al., 2002; Neil et al., 1998; Partridge et al., 2004). As a result, there may be changes in diffusivity measures over the 4 week GA range at which the infants were scanned, which was a possible confounder. Therefore, GA at MRI was statistically corrected for in this study.
The relatively generalized PDI and MDI tests at only 2 years of age are insufficient to pick up the specific functional outcomes related to each CC sub-region. The infants in the current study are being reassessed at 7 years of age with MRI, DTI and comprehensive neurodevelopmental tests.
Conclusion
IVH in VPT infants was related to smaller CC size, and abnormal diffusivity values within the CC ROIs and the callosal tracts, particularly at the anterior and posterior ends. As hypothesized, WMI was also a major perinatal predictor associated with widespread alterations to CC microstructural development in VPT infants. VPT infants who were male, born at an earlier GA, or exposed to PCS or infection also had more vulnerable corpora callosa.
This study has provided evidence for poorer cognitive and motor outcomes associated with alterations to callosal morphology and microstructure in VPT infants, which likely result from poorer inter-hemispheric transfer time and slower information processing.
Previously we have shown how the CC is affected by prematurity, with a smaller overall size, less posterior skew, and altered diffusion properties, particularly within posterior sub-regions (Thompson et al., 2011). The current study reveals possible perinatal causes and functional consequences for these alterations.
Research Highlights.
We associated corpus callosum measures in very preterm infants with perinatal predictors
Intraventricular hemorrhage and white matter injury associate with corpora collosa
This indicates altered development, microstructural organization and myelination
We also associated infant corpus callosum measures with 2 year neurodevelopment
Altered callosal shape and microstructure associated with cognitive and motor delay
Acknowledgments
We gratefully thank Merilyn Bear for recruitment, Kelly Howard and Karli Treyvaud for neurobehavioural assessments, Hong X Wang for image processing, the VIBeS and Developmental imaging teams at the Murdoch Childrens Research Institute for their ideas and support, as well as the families and children who participated in this study.
Funding
This work was supported by the National Health and Medical Research Council of Australia [237117 to L.W.D., 400317 to G.F.E., 628371 to P.J.A., the Australian Postgraduate Award to D.K.T.); the National Institute of Health [R01 RR021885, R01 GM074068, R01 EB008015, P30 HD018655 to S.K.W.]; the United Cerebral Palsy Foundation (USA) to T.E.I.; the Leila Y. Mathers Charitable Foundation (USA) to G.F.E.; the Brown Foundation (USA) to G.F.E.; and the Victorian Government’s Operational Infrastructure Support Program to D.K.T., and P.J.A.
Abbreviations
- AC-PC
anterior commissure to posterior commissure line
- BPD
bronchopulmonary dysplasia
- CC
corpus callosum
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FOV
field of view
- FSL
Oxford centre for functional magnetic resonance imaging of the brain software library
- FT
full-term
- GA
gestational age
- IVH
intraventricular hemorrhage
- MD
mean diffusivity
- MDI
mental developmental index
- PCS
postnatal corticosteroids
- PDI
psychomotor developmental index
- VPT
preterm
- PVL
periventricular leukomalacia
- ROI
region of interest
- TE
echo time
- TR
repetition time
- WM
white matter
- WMI
white matter injury
- λ||
axial diffusivity
- λ⊥
radial diffusivity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Allen LS, Richey MF, Chai YM, Gorski RA. Sex differences in the corpus callosum of the living human being. J Neurosci. 1991;11:933–942. doi: 10.1523/JNEUROSCI.11-04-00933.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson NG, Laurent I, Cook N, Woodward L, Inder TE. Growth rate of corpus callosum in very premature infants. Am J Neuroradiol. 2005;26:2685–2690. [PMC free article] [PubMed] [Google Scholar]
- Anderson NG, Laurent I, Woodward LJ, Inder TE. Detection of impaired growth of the corpus callosum in premature infants. Pediatrics. 2006;118:951–960. doi: 10.1542/peds.2006-0553. [DOI] [PubMed] [Google Scholar]
- Andrews JS, Ben-Shachar M, Yeatman JD, Flom LL, Luna B, Feldman HM. Reading performance correlates with white-matter properties in preterm and term children. Dev Med Child Neurol. 2009 doi: 10.1111/j.1469-8749.2009.03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjari M, Counsell SJ, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD. The association of lung disease with cerebral white matter abnormalities in preterm infants. Pediatrics. 2009;124:268–276. doi: 10.1542/peds.2008-1294. [DOI] [PubMed] [Google Scholar]
- Anjari M, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD, Counsell SJ. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. NeuroImage. 2007;35:1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley scales of infant development. 2. The Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Benjak V, Culjat M, Pavlovic M, Kostovic-Srzentic M. Changes of the corpus callosum in children who suffered perinatal injury of the periventricular crossroads of pathways. Coll Antropol. 2008;32(Suppl 1):25–29. [PubMed] [Google Scholar]
- Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol Rev. 2005;15:59–71. doi: 10.1007/s11065-005-6252-y. [DOI] [PubMed] [Google Scholar]
- Caldu X, Narberhaus A, Junque C, Gimenez M, Vendrell P, Bargallo N, Segarra D, Botet F. Corpus callosum size and neuropsychologic impairment in adolescents who were born preterm. J Child Neurol. 2006;21:406–410. doi: 10.1177/08830738060210050801. [DOI] [PubMed] [Google Scholar]
- Chua CO, Chahboune H, Braun A, Dummula K, Chua CE, Yu J, Ungvari Z, Sherbany AA, Hyder F, Ballabh P. Consequences of intraventricular hemorrhage in a rabbit pup model. Stroke. 2009;40:3369–3377. doi: 10.1161/STROKEAHA.109.549212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17:407–429. [PubMed] [Google Scholar]
- Constable RT, Ment LR, Vohr BR, Kesler SR, Fulbright RK, Lacadie C, Delancy S, Katz KH, Schneider KC, Schafer RJ, Makuch RW, Reiss AR. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121:306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- Counsell SJ, Shen Y, Boardman JP, Larkman DJ, Kapellou O, Ward P, Allsop JM, Cowan FM, Hajnal JV, Edwards AD, Rutherford MA. Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at term-equivalent age. Pediatrics. 2006;117:376–386. doi: 10.1542/peds.2005-0820. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Barzi A, Lawrie T, Hoon AH, Jr, Melhem ER. Correlation of corpus callosal morphometry with cognitive and motor function in periventricular leukomalacia. Neuropediatrics. 2003;34:247–252. doi: 10.1055/s-2003-43259. [DOI] [PubMed] [Google Scholar]
- de Vries LS, Rademaker KJ, Groenendaal F, Eken P, van Haastert IC, Vandertop WP, Gooskens R, Meiners LC. Correlation between neonatal cranial ultrasound, MRI in infancy and neurodevelopmental outcome in infants with a large intraventricular haemorrhage with or without unilateral parenchymal involvement. Neuropediatrics. 1998;29:180–188. doi: 10.1055/s-2007-973558. [DOI] [PubMed] [Google Scholar]
- DeLacoste-Utamsing C, Holloway RL. Sexual dimorphism in the human corpus callosum. Science. 1982;216:1431–1432. doi: 10.1126/science.7089533. [DOI] [PubMed] [Google Scholar]
- Doyle LW. Neonatal intensive care at borderline viability--is it worth it? Early Hum Dev. 2004;80:103–113. doi: 10.1016/j.earlhumdev.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Dubb A, Gur R, Avants B, Gee J. Characterization of sexual dimorphism in the human corpus callosum. NeuroImage. 2003;20:512–519. doi: 10.1016/s1053-8119(03)00313-6. [DOI] [PubMed] [Google Scholar]
- Fan GG, Yu B, Quan SM, Sun BH, Guo QY. Potential of diffusion tensor MRI in the assessment of periventricular leukomalacia. Clin Radiol. 2006;61:358–364. doi: 10.1016/j.crad.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, Freedman H. Observations on visual processes after posterior callosal section. Neurology. 1973;23:1126–1130. doi: 10.1212/wnl.23.10.1126. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Corouge I, Vetsa YS, Smith JK, Kang C, Gu H, Hamer RM, Lieberman JA, Gerig G. Early postnatal development of corpus callosum and corticospinal white matter assessed with quantitative tractography. AJNR Am J Neuroradiol. 2007;28:1789–1795. doi: 10.3174/ajnr.A0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Smith LC, Wolfe HM, Hertzberg BS, Smith JK, Chescheir NC, Evans DD, Kang C, Hamer RM, Lin W, Gerig G. Prenatal mild ventriculomegaly predicts abnormal development of the neonatal brain. Biol Psychiatry. 2008;64:1069–1076. doi: 10.1016/j.biopsych.2008.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday HL, Ehrenkranz RA, Doyle LW. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2009a:CD001146. doi: 10.1002/14651858.CD001146.pub2. [DOI] [PubMed] [Google Scholar]
- Halliday HL, Ehrenkranz RA, Doyle LW. Late (>7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2009b:CD001145. doi: 10.1002/14651858.CD001145.pub2. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited--Comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Holsti L, Grunau RV, Whitfield MF. Developmental coordination disorder in extremely low birth weight children at nine years. J Dev Behav Pediatr. 2002;23:9–15. doi: 10.1097/00004703-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med. 2006;11:489–497. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998;44:584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- Iai M, Tanabe Y, Goto M, Sugita K, Niimi H. A comparative magnetic resonance imaging study of the corpus callosum in neurologically normal children and children with spastic diplegia. Acta Paediatr. 1994;83:1086–1090. doi: 10.1111/j.1651-2227.1994.tb12991.x. [DOI] [PubMed] [Google Scholar]
- Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003;143:171–179. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Della-Maggiore V, Behrens TE, Smith SM, Paus T. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. NeuroImage. 2007;36(Suppl 2):T16–21. doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn Reson Med. 2004;51:807–815. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- Juliet PA, Frost EE, Balasubramaniam J, Del Bigio MR. Toxic effect of blood components on perinatal rat subventricular zone cells and oligodendrocyte precursor cell proliferation, differentiation and migration in culture. J Neurochem. 2009;109:1285–1299. doi: 10.1111/j.1471-4159.2009.06060.x. [DOI] [PubMed] [Google Scholar]
- Kikinis R, Shenton ME, Gerig G, Martin J, Anderson M, Metcalf D, Guttmann CR, McCarley RW, Lorensen W, Cline H, et al. Routine quantitative analysis of brain and cerebrospinal fluid spaces with MR imaging. J Magn Reson Imaging. 1992;2:619–629. doi: 10.1002/jmri.1880020603. [DOI] [PubMed] [Google Scholar]
- Kontis D, Catani M, Cuddy M, Walshe M, Nosarti C, Jones D, Wyatt J, Rifkin L, Murray R, Allin M. Diffusion tensor MRI of the corpus callosum and cognitive function in adults born preterm. Neuroreport. 2009;20:424–428. doi: 10.1097/WNR.0b013e328325a8f9. [DOI] [PubMed] [Google Scholar]
- LaMantia AS, Rakic P. Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. J Neurosci. 1990;10:2156–2175. doi: 10.1523/JNEUROSCI.10-07-02156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence EJ, Allen GM, Walshe M, Allin M, Murray R, Rifkin L, McGuire PK, Nosarti C. The corpus callosum and empathy in adults with a history of preterm birth. J Int Neuropsychol Soc. 2010;16:716–720. doi: 10.1017/S1355617710000500. [DOI] [PubMed] [Google Scholar]
- Luciano R, Baranello G, Masini L, Ricci D, Gallini F, Ciotti S, Leone D, Serrao F, De Santis M, Zecca E, Zuppa A, Romagnoli C, Di Rocco C, Guzzetta F, Mercuri E. Antenatal post-hemorrhagic ventriculomegaly: a prospective follow-up study. Neuropediatrics. 2007;38:137–142. doi: 10.1055/s-2007-985366. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Philip JV, Nehra D, Snyder AZ, Conturo TE, Neil JJ, McKinstry RC. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol. 2002;23:1445–1456. [PMC free article] [PubMed] [Google Scholar]
- Nagae LM, Hoon AH, Jr, Stashinko E, Lin D, Zhang W, Levey E, Wakana S, Jiang H, Leite CC, Lucato LT, van Zijl PC, Johnston MV, Mori S. Diffusion tensor imaging in children with periventricular leukomalacia: variability of injuries to white matter tracts. AJNR Am J Neuroradiol. 2007;28:1213–1222. doi: 10.3174/ajnr.A0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Ashburner J, Andersson J, Jbabdi S, Draganski B, Skare S, Bohm B, Smedler AC, Forssberg H, Lagercrantz H. Structural correlates of preterm birth in the adolescent brain. Pediatrics. 2009;124:e964–972. doi: 10.1542/peds.2008-3801. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Skare S, Andersson JL, Lilja A, Flodmark O, Fernell E, Holmberg K, Bohm B, Forssberg H, Lagercrantz H, Klingberg T. Preterm children have disturbances of white matter at 11 years of age as shown by diffusion tensor imaging. Pediatric Research. 2003;54:672–679. doi: 10.1203/01.PDR.0000084083.71422.16. [DOI] [PubMed] [Google Scholar]
- Narberhaus A, Segarra D, Caldu X, Gimenez M, Junque C, Pueyo R, Botet F. Gestational age at preterm birth in relation to corpus callosum and general cognitive outcome in adolescents. J Child Neurol. 2007;22:761–765. doi: 10.1177/0883073807304006. [DOI] [PubMed] [Google Scholar]
- Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli CR, Akbudak E, Aronovitz JA, Miller JP, Lee BC, Conturo TE. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology. 1998;209:57–66. doi: 10.1148/radiology.209.1.9769812. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Rushe TM, Woodruff PW, Stewart AL, Rifkin L, Murray RM. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain. 2004;127:2080–2089. doi: 10.1093/brain/awh230. [DOI] [PubMed] [Google Scholar]
- Partridge SC, Mukherjee P, Henry RG, Miller SP, Berman JI, Jin H, Lu Y, Glenn OA, Ferriero DM, Barkovich AJ, Vigneron DB. Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. NeuroImage. 2004;22:1302–1314. doi: 10.1016/j.neuroimage.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Adalsteinsson E, Kemper CA, Deresinski S, Sullivan EV. Contribution of alcoholism to brain dysmorphology in HIV infection: effects on the ventricles and corpus callosum. NeuroImage. 2006;33:239–251. doi: 10.1016/j.neuroimage.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Rademaker KJ, Lam JN, Van Haastert IC, Uiterwaal CS, Lieftink AF, Groenendaal F, Grobbee DE, de Vries LS. Larger corpus callosum size with better motor performance in prematurely born children. Semin Perinatol. 2004;28:279–287. doi: 10.1053/j.semperi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Robichon F, Habib M. Abnormal callosal morphology in male adult dyslexics: relationships to handedness and phonological abilities. Brain Lang. 1998;62:127–146. doi: 10.1006/brln.1997.1891. [DOI] [PubMed] [Google Scholar]
- Rose J, Butler EE, Lamont LE, Barnes PD, Atlas SW, Stevenson DK. Neonatal brain structure on MRI and diffusion tensor imaging, sex, and neurodevelopment in very-low-birthweight preterm children. Dev Med Child Neurol. 2009;51:526–535. doi: 10.1111/j.1469-8749.2008.03231.x. [DOI] [PubMed] [Google Scholar]
- Rose SE, Hatzigeorgiou X, Strudwick MW, Durbridge G, Davies PS, Colditz PB. Altered white matter diffusion anisotropy in normal and preterm infants at term-equivalent age. Magn Reson Med. 2008;60:761–767. doi: 10.1002/mrm.21689. [DOI] [PubMed] [Google Scholar]
- Schmahmann J, Pandya D. Fiber pathways of the brain. Oxford University Press; New York: 2006. [Google Scholar]
- Schneider JF, Il’yasov KA, Hennig J, Martin E. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46:258–266. doi: 10.1007/s00234-003-1154-2. [DOI] [PubMed] [Google Scholar]
- Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, Inder TE. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153:170–175. 175, e171. doi: 10.1016/j.jpeds.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Skiold B, Horsch S, Hallberg B, Engstrom M, Nagy Z, Mosskin M, Blennow M, Aden U. White matter changes in extremely preterm infants, a population-based diffusion tensor imaging study. Acta Paediatr. 2010;99:842–849. doi: 10.1111/j.1651-2227.2009.01634.x. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Tam EW, Ferriero DM, Xu D, Berman JI, Vigneron DB, Barkovich AJ, Miller SP. Cerebellar development in the preterm neonate: effect of supratentorial brain injury. Pediatr Res. 2009;66:102–106. doi: 10.1203/PDR.0b013e3181a1fb3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Inder TE, Faggian N, Johnston L, Warfield SK, Anderson PJ, Doyle LW, Egan GF. Characterization of the corpus callosum in very preterm and full-term infants utilizing MRI. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap MS, Valk J. Magnetic resonance of myelination and myelin disorders. 3. Springer-Verlag; Berlin: 1995. [Google Scholar]
- Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27:12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhausen R, Kreuder F, Woerner W, Huster RJ, Smit CM, Schweiger E, Wittling W. Interhemispheric transfer time and structural properties of the corpus callosum. Neurosci Lett. 2006;409:140–145. doi: 10.1016/j.neulet.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- Yazgan MY, Wexler BE, Kinsbourne M, Peterson B, Leckman JF. Functional significance of individual variations in callosal area. Neuropsychologia. 1995;33:769–779. doi: 10.1016/0028-3932(95)00018-x. [DOI] [PubMed] [Google Scholar]


