Abstract
Objective
Intravenous glucose tolerance tests (IVGTT) have demonstrated lower whole-body insulin sensitivity (SI) among African Americans (AA) compared to European Americans (EA). Whole-body SI represents both insulin-stimulated glucose disposal, primarily by skeletal muscle, and insulin's suppression of endogenous glucose production (EGP) by liver. A mathematical model was recently introduced that allows for distinction between disposal and hepatic insulin sensitivity. The purpose of this study was to examine specific indexes of insulin sensitivity among AA and EA women to determine whether lower whole-body insulin sensitivity in AA may be attributed to insulin action at muscle, liver, or both.
Methods
Participants were 53 non-diabetic, premenopausal AA and EA women. Profiles of EGP and indexes of Disposal SI and Hepatic SI were calculated by mathematical modeling and incorporation of a stable isotope tracer (6,6-2H2glucose) into the IVGTT. Body composition was assessed by dual energy X-ray absorptiometry.
Results
After adjustment for percent fat, both Disposal SI and Hepatic SI were lower among AA (p=0.009 for both). Time profiles for serum insulin and EGP revealed higher peak insulin response and corresponding lower EGP among AA women compared to EA.
Conclusions
Indexes from a recently-introduced mathematical model suggest that lower whole-body insulin sensitivity among non-diabetic AA women is due to both hepatic and peripheral components. Despite lower Hepatic SI, AA displayed lower EGP, resulting from higher post-challenge insulin levels. Future research is needed to determine the physiological basis of lower insulin senstivity among AA and its implications for type 2 diabetes risk.
Keywords: mathematical modeling, intravenous glucose tolerance test, hepatic glucose production
Introduction
Compared to European Americans (EA), African Americans (AA) are twice as likely to be diagnosed with type 2 diabetes (T2D) [1]. In particular, AA women have higher prevalence rates of T2D than any other gender-ethnic group [2]. The reasons for this disparity are not clear, but ethnic differences in tissue sensitivity to insulin may play a role.
Indexes of whole-body insulin sensitivity (SI) and glucose effectiveness (Sg) can be estimated by mathematical modeling of glucose and insulin values from an intravenous glucose tolerance test (IVGTT) [3,4]. Previous studies that have estimated insulin sensitivity by the traditional one-compartment minimal model have consistently shown lower whole-body insulin sensitivity among healthy AA compared to EA, independent of body composition [5-8]. However, the one-compartment model is not ideal for comparing groups such as EA and AA that differ regarding the magnitude of the post-challenge insulin response [9] due to overestimation of the effects of glucose itself (Sg) in subjects with a higher insulin response [10,11]. Further, the one-compartment minimal model assesses insulin sensitivity only at the whole-body level. Whole-body insulin sensitivity comprises both insulin action to promote glucose uptake as well as to suppress endogenous glucose production by the liver [12]. Whether lower insulin sensitivity among AA represents impairment in glucose uptake or hepatic glucose production is not known. Discerning whether lower insulin sensitivity among AA relates to hepatic or peripheral glucose regulation could help elucidate the etiology behind racial/ethnic differences in T2D.
When a stable isotope tracer is included in the IVGTT, a two-compartment model can be used to provide an index of disposal-specific insulin sensitivity and a more reliable index of glucose effectiveness [13,14]. In 2005, researchers expanded on the traditional two-compartment minimal model by describing endogenous glucose production (EGP) from tracer-labeled IVGTT data [15]. In 2009, Tokuyama and colleagues further developed the two-compartment model framework by introducing model-derived insulin sensitivity indexes specific to hepatic glucose regulation [16]. Thus, these latest advances in two-compartment modeling offer the opportunity to provide novel data comparing disposal-specific insulin sensitivity, hepatic insulin sensitivity, and hepatic glucose production in AA and EA.
The aim of this study was to describe insulin action and hepatic glucose metabolism among non-diabetic, premenopausal women of AA and EA ancestry using a recently-introduced integrated model [16] with the goal of determining whether lower insulin sensitivity among AA may be attributed to either hepatic or peripheral sensitivity to insulin.
Methods
Participants
Fifty-three non-diabetic, premenopausal women were categorized by ethnicity as African American (AA) or European American (EA) based on self-report and assertion that both parents shared the same ethnicity as the participant. Normal glucose tolerance was verified by 2-hour oral glucose tolerance testing, and premenopausal status was confirmed by serum follicle stimulating hormone concentration ≤ 35 IU/mL in addition to self-report of regular menstrual cycles. Exclusion criteria included history of polycystic ovary disease, hypoglycemia, and any medication known to influence glucose metabolism (including oral contraceptives). All participants provided oral and written consent, and the study was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham (UAB).
Protocol
For three days prior to testing, participants were instructed to consume ∼250 g carbohydrates. The evening before testing, they reported to the General Clinical Research Center (GCRC). After a 12-hour overnight fast, metabolic parameters were determined by insulin-modified intravenous glucose tolerance test (IVGTT). Body composition was assessed by dual energy X-ray absorptiometery (DXA; Lunar Prodigy, GE Healthcare, Madison, WI), and scans were analyzed with software version 1.5.
Intravenous Glucose Tolerance Test (IVGTT)
For the IVGTT, flexible intravenous catheters were placed in the antecubital spaces of both arms. Blood was sampled 3 times over 15 minutes, and averages of these three fasting samples were used to determine fasting glucose, insulin, and C-peptide concentrations. At time zero, a bolus of glucose (50% dextrose, 270 mg/kg, plus [6,6-2H2]glucose, 30 mg/kg) was infused intravenously. Insulin (0.02 U/kg) was administered intravenously over a 5-minute period from 20-25 minutes after the glucose injection. Blood was sampled at the following times (minutes) following glucose administration: 2, 3, 4, 5, 6, 8, 10, 12, 15, 19, 20, 21, 22, 24, 26, 28, 30, 35, 40, 45, 50, 55, 60, 70, 80, 100, 120, 140, 180, 210, 240, for a total of 34 samples). Sera were stored at −85°C until laboratory analysis of glucose and insulin.
AIRg, the integrated incremental area under the curve for insulin during the first 10 min of the IVGTT, was calculated using the trapezoidal method. An integrated two-compartment mathematical model [16] was used to estimate the time course of endogenous glucose production (EGP) in mg/kg/min. EGP primarily reflects hepatic glucose production [17,18]. Indexes of insulin sensitivity specific to glucose disposal (Disposal Si) and hepatic insulin action (Hepatic Si) were calculated [16]. The same model was used to derive indexes of disposal and hepatic glucose effectiveness (Disposal Sg and Hepatic Sg) as estimates of the ability of glucose itself to promote disposal and suppress EGP, respectively. Details of the integrated two-compartment minimal model have been previously described by Tokuyama et al[16], and indexes from the model are summarized by the following equations:
IC50 is representative of insulin action required for 50% inhibition of EGP. H2 represents a function of insulin's inhibitory effect on EGP; H2 (= x(t)/[IC50 + x(t)]), and GL signifies available glucose in the liver.
V1 is the volume of the accessible compartment, sk is a parameter of insulin action, and kp, k02, k12, and k21 are rate constant parameters.
Basal glucose is quantified by Gb, while kin and kout are rate constants of hepatic glucose production and hepatic glucose loss, respectively.
Data were modeled using SCIENTIST software (version 2.01; St. Louis, MO). For each participant, Disposal SI and Hepatic SI from the 2-compartment model were summed to yield a value for Total SI. A surrogate index for fasting hepatic insulin resistance was calculated by multiplying basal EGP × fasting insulin concentration as previously described [19].
Laboratory Analyses
All analyses were performed in the Core Laboratories of the General Clinical Research Center (GCRC), Nutrition Obesity Research Center (NORC), and Diabetes Research and Training Center (DRTC). Glucose was measured in 10 μL sera using the Ektachem DT II analyzer (Johnson and Johnson Clinical Diagnostics, Rochester, NY). This analysis had a mean intra-assay coefficient of variation (CV) of 0.61%, and a mean inter-assay CV of 1.45%. Insulin was measured by radioimmunoassay (Linco Research Inc., St. Charles, MO; now Millipore Corporation, Billerica, MA). This assay has a sensitivity of 3.35 μIU/ml, mean intra-assay CV of 3.49%, and mean interassay CV of 5.57%. C-peptide was measured by radioimmunoassay (Siemens Healthcare Diagnostics, Los Angeles, CA) in duplicate 25 μL aliquots. Sensitivity for this assay is 0.318 ng/mL, mean intra-assay CV is 3.57%, and mean interassay CV is 5.59%. Serum concentrations of free fatty acids (FFA) were measured with “NEFA-C” assays (Wako Diagnostics, Richmond, VA [20]. This assay has an intra-assay CV was 3.89%, and the inter-assay CV is 5.87%. Minimum assay sensitivity was 0.0014mEq/L. [6,6-2H2]glucose enrichment for each of the 34 blood samples listed above were analyzed by gas chromatography mass as previously described [21]. Briefly, serum samples were deproteinized, evaporated, and prepared with N,O-bis[Trimethylsilyl]trifluoroacetamide (BSTFA) and 1% trimethylchlorosilane (TMSC). Derivatives were analyzed on an Agilent 6890 gas chromatograph coupled to a 5973 mass spectrometer in Electron Impact mode. This analysis uses a standard curve prepared with in-house control serum samples and monitoring of M+0 and M+2 ions. Mole fractions were calculated from total area counts. CV of the [6,6-2H2]glucose among the fasting samples was 1.75%.
Statistical Analysis
Ethnic group differences for age, body mass index (BMI), kin, kout, and IC50 were compared by nonparametric Mann Whitney-U tests, percentages of overweight subjects in each group were compared by the Fisher exact test, and independent t-tests were used to determine between-group differences for all other variables of interest. Variables were Log10 transformed for normality when appropriate. ANCOVA analyses were performed to examine group differences in SI and Sg indexes with adjustment for % body fat.
Mann Whitney-U tests and independent t-tests were used to identify ethnic differences in glucose, insulin, and EGP at individual time points over the duration of the IVGTT. Composite scores for glucose, insulin, and EGP time courses were calculated as incremental area under the curve (AUC) by the trapezoidal method.
Statistical tests were performed with SPSS software (version 19.0; Chicago, IL) and GraphPad Prism (version 5.0; La Jolla, CA). All tests were two-sided with a Type I error rate of 0.05.
Results
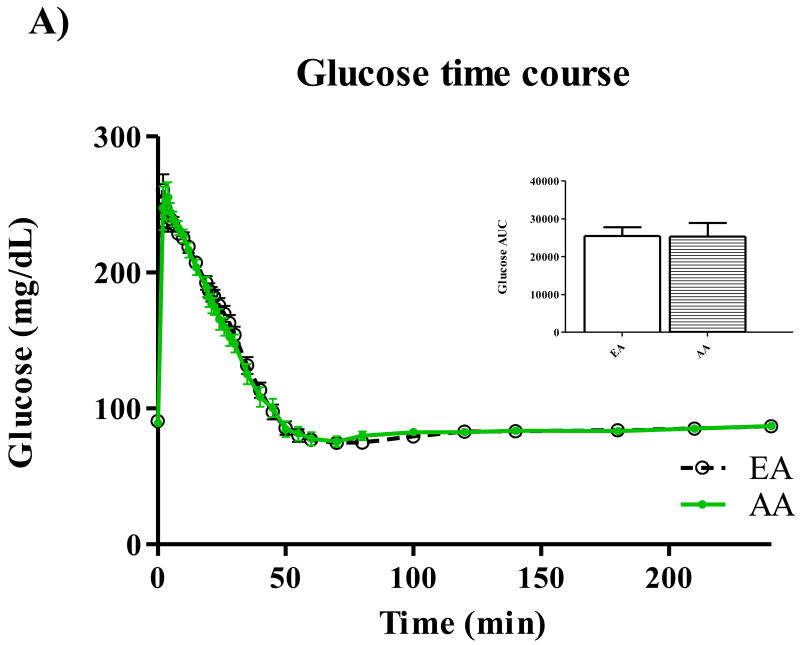
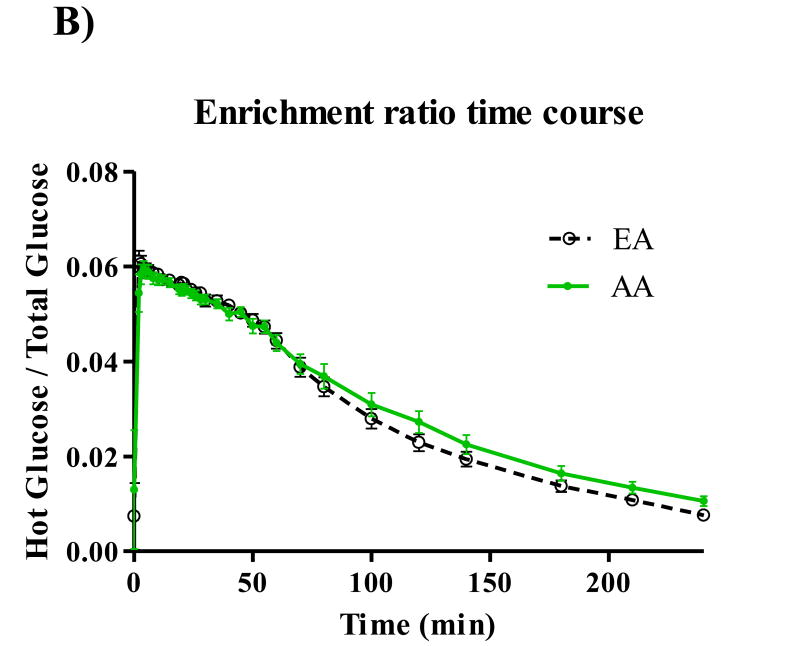
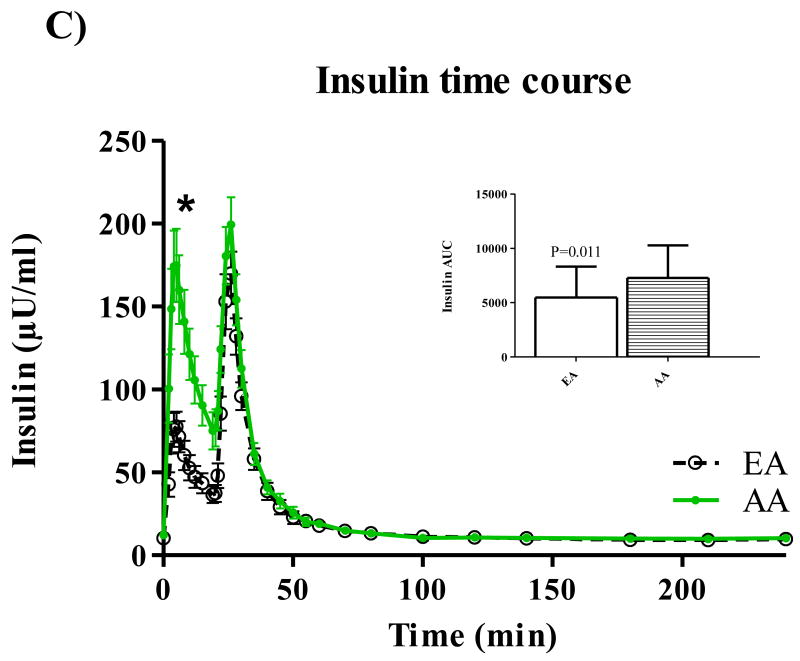
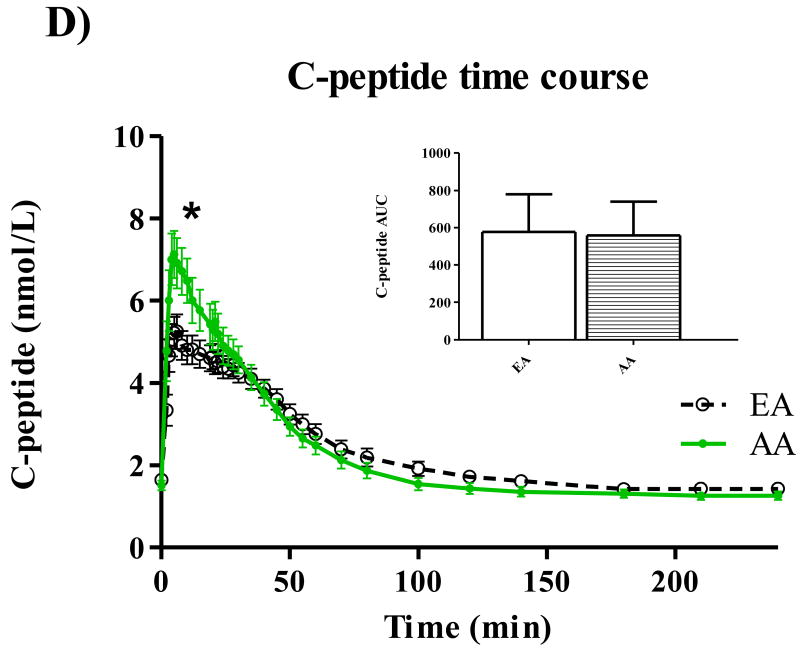
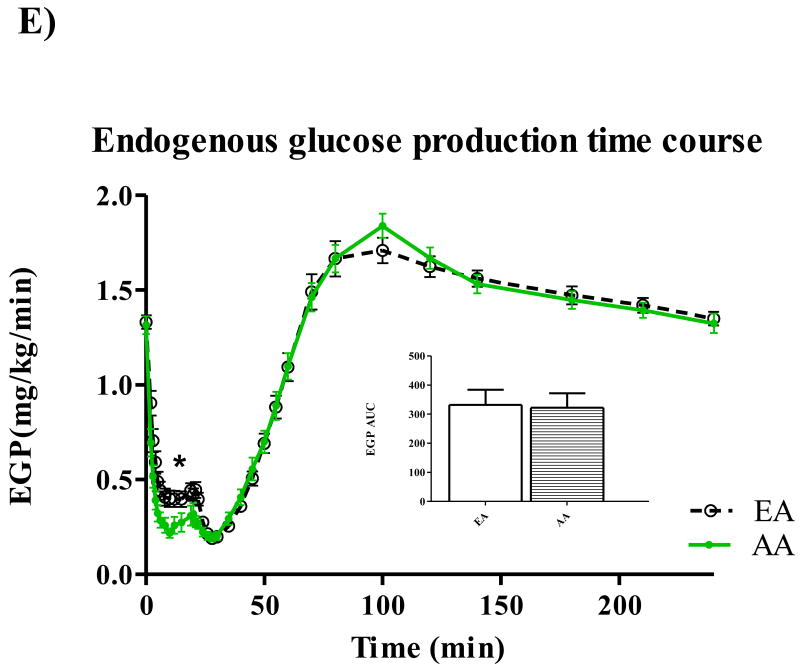
Participant characteristics and metabolic parameters are displayed as mean ± SD by ethnic group in Table 1. Groups were similar in age, body weight, and body composition. Both Hepatic SI and Disposal SI were lower for AA (p=0.009 for both), and these ethnic differences intensified with adjustment for body composition. On average, Hepatic SI accounted for approximately 30% Total SI in both EA (range: 24.5 – 39.6) and AA (range 22.2 – 42.2). Hepatic Sg did not differ between ethnic groups, but Disposal Sg was significantly lower among AA independent of body composition (p=0.020). AIRg was more than two-fold higher among AA (p<0.001), and after adjustment for percent body fat, the surrogate index of fasting hepatic insulin resistance [19] was higher for AA (p=0.048). Time profiles for serum total glucose, glucose enrichment, insulin, C-peptide, and EGP are shown in Figure 1. Total glucose concentrations did not differ between groups at any point during the test (panel A), but AA demonstrated higher peak insulin levels (panel C) (p<0.05 for minutes 3-22 following the glucose challenge). AUC for insulin was also greater for AA compared to EA (p=0.01). C-peptide measurements were available for 50 of the 53 participants. Panel D displays higher post-challenge C-peptide concentrations among AA (p<0.05 for minutes 4-10). EGP among AA was significantly lower (p<0.05) from minutes 2 to 22 of testing (Panel E). Although total AUC of EGP did not differ between ethnic groups, AUC for the first 30 minutes of testing was significantly lower for AA p=0.016). Serum concentrations of FFA were similar between groups at baseline, and circulating FFA did not differ between groups at any point of blood sampling (data not shown).
Table 1. Participant characteristics and metabolic parameters.
| EA (n = 30) | AA (n = 23) | P-value | P-value adjusted for %FAT | |
|---|---|---|---|---|
| Age | 26.02 ± 3.45 (19.3 − 32.9) |
24.27 ± 4.13 (18.0 − 29.9) |
0.141 | |
| Weight (kg) | 69.86 ± 12.82 (52.6 − 103.0) |
73.27 ± 16.05 (51.9 − 111.8) |
0.442 | |
| BMI (kg/m2) | 25.22 ± 4.33 (18.7 − 35.2) |
26.62 ± 5.84 (18.5 − 38.6) |
0.440 | |
| BMI > 25 (%) | (43.3%) | (43.5%) | 1.000 | |
| FFM (kg) | 41.14 ± 5.09 (32.6 − 49.7) |
42.72 ± 4.32 (36.1 − 50.9) |
0.212 | |
| FM (kg) | 25.56 ± 9.79 (9.8 − 50.9) |
27.06 ± 13.36 (10.9 − 57.8) |
0.954 | |
| Percent body fat (%) | 35.92 ± 7.91 (16.4 − 49.9) |
35.31 ± 10.34 (21.0 − 52.0) |
0.814 | |
| kin (mg/kg/min) | 2.66 ± 0.40 (2.23 − 3.82) |
2.63 ± 0.45 (1.47 − 3.73) |
0.872 | |
| kout (min−1) | 0.027 ± 0.004 (0.020 − 0.038) |
0.027 ± 0.005 (0.021 − 0.043) |
0.693 | |
| IC50 (min−1) | 0.013 ± 0.002 (0.011 − 0.018) |
0.016 ± 0.012 (0.010 − 0.071) |
0.844 | |
| Basal FFA (mEq/L) | 0.50 ± 0.16 (0.13 − 0.79) |
0.56 ± 0.15 (0.38 − 0.96) |
0.143 | 0.141 |
| Basal glucose (mg/dL) | 90.71 ± 6.32 (77.0 − 105.0) |
88.86 ± 7.97 (79.0 − 112.6) |
0.311 | 0.326 |
| Basal insulin (μU/mL) | 10.29 ± 4.56 (4.0 − 26.0) |
12.18 ± 4.26 (7.0 − 23.2) |
0.062 | 0.027 |
| Basal C-peptide (nmol/L) | 1.64 ± 0.0.48 (0.64 − 2.66) |
1.52 ± 0.52 (0.66 − 2.72) |
0.359 | 0.328 |
|
AIRg (μU/mL × 10 min) |
481.45 ± 384.28 (66.8 − 1958.5) |
1178.03 ± 799.80 (262.0 − 3080.7) |
<0.001 | <0.001 |
| Basal EGP (mg/kg/min) | 1.33 ± 0.20 (1.12 − 1.91) |
1.32 ± 0.23 (0.73 − 1.86) |
0.702 | 0.737 |
| Basal EGP × basal insulin | 13.59 ± 6.02 (5.47 − 35.17) |
16.10 ± 6.64 (7.68 − 35.87) |
0.112 | 0.048 |
|
Disposal Sg (×102/min) |
0.736 ± 0.235 (0.337 − 1.342) |
0.586 ± 0.189 (0.249 − 1.214) |
0.020 | 0.008 |
|
Hepatic Sg (×102/min) |
0.57 ± 0.09 (0.460 − 0.871) |
0.538 ± 0.109 (0.315 − 0.838) |
0.156 | 0.152 |
|
Disposal SI (×104/min/μU/ml) |
10.51 ± 4.54 (3.49 − 20.53) |
7.46 ± 3.81 (1.66 − 20.31) |
0.009 | 0.002 |
|
Hepatic SI (×104/min/μU/ml) |
4.46 ± 1.71 (2.29 − 10.22) |
3.42 ± 1.34 (0.94 − 6.52) |
0.009 | 0.005 |
|
Total Si (×104/min/μU/ml) |
14.97 ± 6.13 (5.77 − 30.75) |
10.88 ± 4.97 (2.60 − 26.13) |
0.009 | 0.002 |
(Mean ± SD and Range); BMI = body mass index, FFM = fat-free mass, FM = fat mass, FFA = free fatty acids, AIRg = acute insulin response to glucose, EGP = endogenous glucose production, kin = rate constant of hepatic glucose production, kout = rate constant of hepatic glucose loss, IC50 = insulin action required to suppress EGP by 50%, Sg = glucose effectiveness index, SI = insulin sensitivity index, Total SI = (Disposal SI + Hepatic SI)
Figure 1.
Time courses for glucose (A), isotope enrichment (B), insulin (C), C-peptide (D), and EGP (E). *P < 0.05.
Discussion
It is well-established that AA are at higher risk than EA for developing T2D. Previous studies quantifying insulin sensitivity by IVGTT and the traditional one-compartment minimal model [22] have repeatedly reported lower whole-body SI among AA participants, independent of body composition [5-8]. However, T2D is a disease involving multiple organs and tissues, and whole-body SI represents a composite of both peripheral and hepatic components. Whether lower SI among AA relates to lower Disposal SI (insulin-mediated glucose uptake), lower Hepatic Si (suppression of EGP in response to insulin concentration), or both has not previously been investigated. The results presented here indicate that both Disposal SI and Hepatic SI were lower among a cohort of healthy, premenopausal AA vs EA women. Additionally, a surrogate index of basal hepatic insulin resistance was higher among AA. However, despite the lower Hepatic SI, AA demonstrated lower model-derived EGP following a glucose challenge. Lower EGP among AA was concomitant with higher insulin and C-peptide concentrations. The physiological basis for lower insulin sensitivity in AA remains to be determined.
This study provides a novel contribution to the literature by comparing specific indexes of SI between EA and AA. Previous studies describing lower whole-body SI among AA by one-compartment modeling [5-8] were unable to tease apart specific effects of insulin on glucose disposal and glucose production, and inherent limitations of the one-compartment model warrant caution about its estimation of glucose effectiveness (Sg) [9-11]. Incorporation of a stable isotope tracer of glucose into the IVGTT in combination with two-compartment modeling provides a more accurate estimate of Sg as well as discrimination of disposal-specific SI and Sg [12-14]. Based on a description of endogenous glucose kinetics by Krudys et al [15], Tokuyama et al recently expanded modeling of the glucose system to provide new indexes of Hepatic S I and Hepatic Sg that correspond to the effects of insulin and glucose on EGP specifically [16]. Using this new integrated mathematical model, we report for the first time that both Disposal SI and Hepatic SI appear lower in AA compared to EA. Moreover, although the ability of glucose per se to suppress EGP (Hepatic Sg) did not differ between groups, the action of glucose to facilitate its own disposal (Disposal Sg) appeared lower among AA.
Lower Disposal SI and Disposal Sg among AA women may relate to inherent differences in aspects of skeletal muscle function. A previous study among premenopausal women reported lower muscle oxidative capacity among AA vs. EA, as well as an independent correlation between in vivo mitochondrial function and whole-body SI [23]. Our group also recently demonstrated that disposal-specific SI was independently associated with systemic markers of oxidative stress in AA but not EA [24]. In contrast, infiltration of skeletal muscle by lipid was related to decreased insulin sensitivity among EA but not AA [25-27]. Taken together, these observations suggest that mitochondrial dysfunction and consequent oxidative stress may compromise muscle function and consequent glucose disposal in AA women.
The source of lower Hepatic SI among AA is not clear. Previous studies in animals [28] and humans [29,30] have related compromised hepatic insulin resistance to accumulation of visceral adipose tissue, with increased free fatty acids into portal circulation as a proposed mechanism [30,31]. However, AA have been shown to have lower hepatic triglyceride content [32,33], and it is well-established that AA women tend to deposit less fat as visceral adipose [5,34-37]. Among our cohort, neither fasting nor post-challenge FFA concentrations differed between AA and EA, and ethnic differences in Hepatic SI intensified with adjustment for percent body fat. Although it is possible that hepatic triglycerides or portal FFA may have contributed to differences between groups, these analyses were beyond the scope of this study. Thus, while it seems likely that lower Hepatic SI among AA is attributable to factors other than body composition, the source of this difference awaits further study.
Despite significantly lower Hepatic SI, time course comparisons revealed lower EGP among AA during the early period of testing. Little data are available regarding ethnic differences in EGP, but one study examining basal hepatic glucose production among obese adolescents similarly reported lower EGP in AA compared to EA [38]. Although the physiological mechanism for lower EGP in AA is not known, higher insulin is likely influential. The model-derived estimate of EGP involves both IC50 and insulin response. IC50 did not significantly differ between the two groups, suggesting that greater suppression of EGP resulted from the relatively robust early insulin response of AA. Fasting insulin concentration was higher in AA vs EA in this sample, and average AIRg was approximately two-fold higher in AA, as previously reported [6,8,39]. First-phase insulin secretion has been identified as a determinant of EGP [40], and higher C-peptide concentrations in AA concurrent with higher AIRg suggests that early insulin secretion was higher in AA vs EA [41]. Further research is indicated to explain the inter-relationships of fasting and post-challenge insulin and EGP as well as the physiological significance of these relationships for diabetes risk within healthy, non-diabetic individuals.
Major strengths of this study were incorporation of a stable isotope tracer and application of recent advances in mathematical modeling to differentiate peripheral and hepatic insulin sensitivity. However, this mathematical model has yet to be validated against model-independent measures of insulin sensitivity, so our analysis may be subject to limitations inherent to model-derived indexes of insulin sensitivity [9,12]. Additional limitations included modest sample size and cross-sectional design. Although we adjusted for percent body fat and confirmed equal distribution of normal weight and overweight women in each group, we lacked statistical power for further subgroup analysis of normal weight vs overweight participants.
In conclusion, present results indicate that lower whole-body insulin sensitivity previously reported among healthy AA may be due to both disposal and hepatic components. Further research is indicated to determine why AA women exhibit lower insulin sensitivity and higher risk for T2DM despite their lower post-challenge EGP, and future studies using model-independent methods are needed to determine the physiological basis of lower insulin sensitivity among AA.
Acknowledgments
This work was supported by R01DK58278, M01-RR-00032, UL 1RR025777, P30-DK56336, and P60DK079626. Doctoral training support for Amy Ellis was provided by Kraft Foods Global, Inc. and F31 AT005384-01. Maryellen Williams and Cindy Zeng conducted laboratory analyses; Tena Hilario, Crystal Douglas, and Jeannine Lawrence supported this study with project coordination.
Funding: R01DK58278, R01DK067426, M01RR00032, P30DK56336, P60DK079626, F31AT005384-01
Abbreviations
- AA
African Americans
- EA
European Americans
- IVGTT
intravenous glucose tolerance tests
- EGP
endogenous glucose production
- T2D
type 2 diabetes
- SI
insulin sensitivity
- Sg
glucose effectiveness
- AIRg
acute insulin response to glucose
- BMI
body mass index
- FFM
fat-free mass
- FM
at mass
- FFA
free fatty acids
Footnotes
Disclosure Statement: The authors have no conflicts of interest
Author Contributions: BAG designed the study and supervised all aspects of the project. ACE analyzed the data and prepared the manuscript. JAA performed data analyses and provided input into manuscript preparation. WMG performed mathematical modeling of the data. FO served as study physician and provided conceptual advice. All authors contributed to interpretation and implications of these results as well as review of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. Table 54. Accessed at http://www.cdc.gov/nchs/data/hus/hus08.pdf on February 1, 2011.
- 2.Centers for Disease Control and Prevention. Table 8. Accessed at http://www.cdc.gov/nchs/data/series/sr10/sr10240.pdf on February 1, 2011.
- 3.Bergman R, Ider Y, Bowden C, et al. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236:E667–677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 4.Bergman R. The minimal model of glucose regulation: A biography. Adv Exp Med Biol. 2003;537:1–19. doi: 10.1007/978-1-4419-9019-8_1. [DOI] [PubMed] [Google Scholar]
- 5.Lovejoy J, de la Bretonne J, Klemperer M, et al. Abdominal fat distribution and metabolic risk factors: Effects of race. Metabolism. 1996;45:1119–1124. doi: 10.1016/s0026-0495(96)90011-6. [DOI] [PubMed] [Google Scholar]
- 6.Haffner S, D'Agostino R, Saad M, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-hispanic whites. The insulin resistance atherosclerosis study. Diabetes. 1996;45:742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 7.Osei K, Schuster D. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white americans. Diabet Med. 1994;11:755–762. doi: 10.1111/j.1464-5491.1994.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 8.Gower B, Ard J, Hunter G, Fernandez J, et al. Elements of the metabolic syndrome: Association with insulin sensitivity and effects of ethnicity. Metab Syndr Relat Disord. 2007;5:77–86. doi: 10.1089/met.2006.0027. [DOI] [PubMed] [Google Scholar]
- 9.Prigeon R, Røder M, Porte DJ, et al. The effect of insulin dose on the measurement of insulin sensitivity by the minimal model technique. Evidence for saturable insulin transport in humans. J Clin Invest. 1996;97:501–507. doi: 10.1172/JCI118441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finegood D, Tzur D. Reduced glucose effectiveness associated with reduced insulin release: An artifact of the minimal-model method. Am J Physiol. 1996;271:E485–495. doi: 10.1152/ajpendo.1996.271.3.E485. [DOI] [PubMed] [Google Scholar]
- 11.Cobelli C, Bettini F, Caumo A, et al. Overestimation of minimal model glucose effectiveness in presence of insulin response is due to undermodeling. Am J Physiol. 1998;275:E1031–1036. doi: 10.1152/ajpendo.1998.275.6.E1031. [DOI] [PubMed] [Google Scholar]
- 12.Caumo A, Vicini P, Zachwieja J, et al. Undermodeling affects minimal model indexes: Insights from a two-compartment model. Am J Physiol. 1999;276:E1171–1193. doi: 10.1152/ajpendo.1999.276.6.E1171. [DOI] [PubMed] [Google Scholar]
- 13.Toffolo G, Cobelli C. The hot ivgtt two-compartment minimal model: An improved version. Am J Physiol Endocrinol Metab. 2003;284:E317–321. doi: 10.1152/ajpendo.00499.2001. [DOI] [PubMed] [Google Scholar]
- 14.Vicini P, Caumo A, Cobelli C. The hot ivgtt two-compartment minimal model: Indexes of glucose effectiveness and insulin sensitivity. Am J Physiol. 1997;273:E1024–1032. doi: 10.1152/ajpendo.1997.273.5.E1024. [DOI] [PubMed] [Google Scholar]
- 15.Krudys K, Dodds M, Nissen S, et al. Integrated model of hepatic and peripheral glucose regulation for estimation of endogenous glucose production during the hot ivgtt. Am J Physiol Endocrinol Metab. 2005;288:E1038–1046. doi: 10.1152/ajpendo.00058.2004. [DOI] [PubMed] [Google Scholar]
- 16.Tokuyama K, Nagasaka S, Mori S, et al. Hepatic insulin sensitivity assessed by integrated model of hepatic and peripheral glucose regulation. Diabetes Technol Ther. 2009;11:487–492. doi: 10.1089/dia.2009.0011. [DOI] [PubMed] [Google Scholar]
- 17.Cherrington AD. Banting lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999;48:1198–1214. doi: 10.2337/diabetes.48.5.1198. [DOI] [PubMed] [Google Scholar]
- 18.Tonelli J, Kishore P, Lee DE, et al. The regulation of glucose effectiveness: How glucose modulates its own production. Curr Opin Clin Nutr Metab Care. 2005;8:450–456. doi: 10.1097/01.mco.0000172588.47811.63. [DOI] [PubMed] [Google Scholar]
- 19.Vangipurapu J, Stanèáková A, Kuulasmaa T, et al. A novel surrogate index for hepatic insulin resistance. Diabetologia. 2011;54:540–543. doi: 10.1007/s00125-010-1966-7. [DOI] [PubMed] [Google Scholar]
- 20.Gower BA, Herd SL, Goran MI. Anti-lipolytic effects of insulin in African American and white prepubertal boys. Obes Res. 2001;9:224–228. doi: 10.1038/oby.2001.25. [DOI] [PubMed] [Google Scholar]
- 21.Bier D, Leake R, Haymond M, et al. Measurement of “True” Glucose production rates in infancy and childhood with 6,6-di-deuteroglucose. Diabetes. 1977;26:1016–1023. doi: 10.2337/diab.26.11.1016. [DOI] [PubMed] [Google Scholar]
- 22.Bergman R, Cobelli C. Minimal modeling, partition analysis, and the estimation of insulin sensitivity. Fed Proc. 1980;39:110–115. [PubMed] [Google Scholar]
- 23.Sirikul B, Gower BA, Hunter GR, et al. Relationship between insulin sensitivity and in vivo mitochondrial function in skeletal muscle. Am J Physiol Endocrinol Metab. 2006;291:E724–728. doi: 10.1152/ajpendo.00364.2005. [DOI] [PubMed] [Google Scholar]
- 24.Fisher G, Alvarez JA, Ellis AC, et al. Independent association of oxidative stress and insulin sensitivity in African American but not European American women. Abstract to be published in the Proceedings of the Obesity Society 29th Annual Scientific Meeting; October 1-5; Orlando, Florida. 2011. abstract. [Google Scholar]
- 25.Ingram KH, Lara-Castro C, Gower BA, et al. Intramyocellular lipid and insulin resistance: Differential relationships in European and African Americans. Obesity. 2011;19(7):1469–75. doi: 10.1038/oby.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrence JC, Newcomer BR, Buchthal SD, et al. Relationship of intramyocellular lipid to insulin sensitivity may differ with ethnicity in healthy girls and women. Obesity. 2011;19:43–48. doi: 10.1038/oby.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith LM, Yao-Borengasser A, Starks T, et al. Insulin resistance in African-American and Caucasian women: Differences in lipotoxicity, adipokines, and gene expression in adipose tissue and muscle. J Clin Endocrinol Metab. 2010;95:4441–4448. doi: 10.1210/jc.2010-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barzilai N, She L, Liu B, et al. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes. 1999;48:94–98. doi: 10.2337/diabetes.48.1.94. [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki Y, Glass L, Triplitt C, et al. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2002;283:E1135–1143. doi: 10.1152/ajpendo.0327.2001. [DOI] [PubMed] [Google Scholar]
- 30.Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insuli resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 31.Jensen M. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;(93):S57–63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerrero R, Vega GL, Grundy SM, et al. Ethnic differences in hepatic steatosis: An insulin resistance paradox? Hepatology. 2009;49:791–801. doi: 10.1002/hep.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liska D, Dufour S, Zern TL, et al. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS One. 2007;2:e569. doi: 10.1371/journal.pone.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lara-Castro C, Weinsier R, Hunter G, et al. Visceral adipose tissue in women: Longitudinal study of the effects of fat gain, time, and race. Obes Res. 2002;10:868–874. doi: 10.1038/oby.2002.119. [DOI] [PubMed] [Google Scholar]
- 35.Albu J, Murphy L, Frager D, et al. Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes. 1997;46:456–462. doi: 10.2337/diab.46.3.456. [DOI] [PubMed] [Google Scholar]
- 36.Ryan A, Nicklas B, Berman D. Racial differences in insulin resistance and mid-thigh fat deposition in postmenopausal women. Obes Res. 2002;10:336–344. doi: 10.1038/oby.2002.47. [DOI] [PubMed] [Google Scholar]
- 37.Katzmarzyk P, Bray G, Greenway F, et al. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91:7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bacha F, Saad R, Gungor N, et al. Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: Race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab. 2003;88:2534–2540. doi: 10.1210/jc.2002-021267. [DOI] [PubMed] [Google Scholar]
- 39.Albu J, Kovera A, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82:1210–1217. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luzi L, DeFronzo R. Effect of loss of first-phase insulin secretion on hepatic glucose production and tissue glucose disposal in humans. Am J Physiol. 1989;257:E241–246. doi: 10.1152/ajpendo.1989.257.2.E241. [DOI] [PubMed] [Google Scholar]
- 41.Polonsky KS, Rubenstein AH. C-peptide as a measure of the secretion and hepatic extraction of insulin. Pitfalls and limitations. Diabetes. 1984;33:486–494. doi: 10.2337/diab.33.5.486. [DOI] [PubMed] [Google Scholar]