Abstract
Background
Chronic ethanol consumption increases the risk of hepatic cirrhosis and hepatocellular carcinoma (HCC). While sex differences exist in susceptibility to ethanol-induced liver damage-HCC development, little is known about the effects of ethanol on tumor progression.
Methods
Neonatal male and female mice were initiated with a single dose of diethylnitrosamine (DEN). 16 or 40 weeks later animals were placed on a 10/20% (v/v) ethanol-drinking water (EtOH-DW; alternate days) regime for 8 weeks. At study end liver tissue and serum were analyzed for liver pathology/function and cytokine expression.
Results
DEN reproducibly induced hepatic foci/tumors in male and female mice. Ethanol diminished hepatic function and increased liver damage, but ethanol alone did not induce hepatic foci-HCC formation. In DEN-initiated EtOH-DW animals, ethanol significantly increased tumor incidence and burden, but only in male mice. Male and female mice (±DEN) demonstrated comparable blood-alcohol content at necropsy, yet increased hepatic damage and diminished hepatic function/anti-oxidant capacity was significantly greater in males. Analysis of liver mRNA for Th1, Th2 or T-regulatory factors demonstrated significantly elevated SMAD3 in male compared to female mice in response to EtOH, DEN-initiation and DEN+EtOH-DW.
Conclusions
These data demonstrate male mice are more susceptible to HCC incidence and progression in the setting of chronic ethanol feeding than females. Differences in markers of hepatic immune response in male mice suggest increased TGFβ-SMAD3 signaling may enhance promotion in this model of HCC progression, effects modulated by chronic ethanol feeding.
Keywords: Ethanol, liver, hepatocarcinogenesis, hepatocellular carcinoma, diethylnitrosamine, sex
INTRODUCTION
On a global scale hepatocellular carcinoma (HCC) is the most common primary liver cancer diagnosed, the fifth most common cancer diagnosed overall, and the third leading cause of cancer-related mortality (Altekruse, 2009; El-Serag, 2007). Unlike many other common cancers in which hereditary risk factors have been identified, similar patterns of incidence are not evidenced for HCC. Rather, incidence of HCC is directly linked to exposure to known risk factors, of which viral hepatitis B and C, aflatoxin, and chronic, heavy ethanol consumption are the most common (Altekruse, 2009; El-Serag, 2007; McKillop, 2006). Despite the varied nature of these insults, exposure is most commonly linked to progressive liver disease toward hepatic cirrhosis, the most common precursor to HCC development (El-Serag, 2007; McKillop, 2006). Similarly, while each factor alone represents a significant risk for progression to cirrhosis and HCC, these agents act synergistically to enhance the probability of eventual HCC. (McKillop, 2006).
The increasing prevalence of viral hepatitis represents a burgeoning health problem globally. In developed countries chronic ethanol intake, in the absence or presence of underlying viral hepatitis, remains an important risk factor for hepatic cirrhosis and progression to HCC (El-Serag, 2007; McKillop, 2006; McKillop and Schrum, 2009). Following ingestion, ≈80% of ethanol is metabolized in the liver. Ethanol metabolism occurs (predominantly) in hepatocytes via three pathways; alcohol dehydrogenase (ADH), cytochrome P4502E1 (CYP2E1) and catalase (Crabb and Liangpunsakul, 2007; Lieber, 1997; McKillop and Schrum, 2009). In the setting of moderate or infrequent ethanol consumption, the majority of ethanol is oxidized by ADH to acetaldehyde that is rapidly metabolized by acetaldehyde dehydrogenase (ALDH) to acetate. Acetaldehyde represents a highly reactive compound capable of binding to cell constituents, including DNA, to form adducts (Albano et al., 1999; Crabb and Liangpunsakul, 2007; McKillop and Schrum, 2009). While direct DNA damage is a significant factor in ethanol toxicity, acetaldehyde also depletes glutathione, the main cellular antioxidant involved in detoxification (Lu, 2009). Chronic, heavy ethanol ingestion leads to microsomal CYP2E1 induction (Crabb and Liangpunsakul, 2007; Lieber, 1997; McKillop and Schrum, 2009). CYP2E1-mediated metabolism induces direct cellular damage due to production of reactive oxygen species (ROS) /oxidative stress, as well as elevated acetaldehyde production and acetaldehyde-dependent cellular damage. Additionally, CYP2E1 induction is reported to alter cell cycle progression, pro-carcinogen activation, and immune responses (Crabb and Liangpunsakul, 2007; Ekstrom and Ingelman-Sundberg, 1989; McKillop and Schrum, 2005).
Ethanol metabolism profoundly affects hepatocyte integrity, as well as that of other hepatic cells (McKillop and Schrum, 2005). However, increasing evidence indicates that the effects of ethanol (metabolism) on hepatic cells are further augmented by other, systemic events. Prolonged ethanol intake causes a hepatic inflammatory response due to increased GI-permeability to LPS (Enomoto et al., 2001; Jirillo et al., 2002). Elevated intrahepatic LPS promotes pro-inflammatory cytokine release from Kupffer cells (KCs) and neutrophil/macrophage infiltration, leading to increased cytokine release and apoptosis (Jirillo et al., 2002; Mathurin et al., 2000). Stimulation of stress-activated cytokine cascades also increases ROS formation which, given ethanol-related GSH depletion, further enhances stress responses, incidence of DNA damage, apoptosis, and compensatory regeneration (Enomoto et al., 2001; Jirillo et al., 2002; Mathurin et al., 2000).
Several studies in humans and animal models of HCC identified sexual dimorphism during ALD development and progression. Differences in gene expression encoding for ethanol metabolizing enzymes influences predisposition to ethanol sensitivity, and ALD development/progression (Gramenzi et al., 2006). Differences in ADH activity exist between males and females, activity being lower in males than females, resulting in less acetaldehyde accumulation (Harada et al., 1998). Studies also report estrogens positively influence both ADH and CYP2E1, suggesting ethanol should be more rapidly metabolized in females vs. males (Harada et al., 1998). Yet this is not the case, and BACs following ethanol ingestion are not lower in females. Nonetheless, sex differences in ALD are well documented; women are more susceptible to the deleterious effects of ethanol than men, and are thus more likely to develop liver disease earlier (Muller, 2006). Several mechanisms have been proposed to explain this phenomena including different ADH activities in the stomach and liver, higher concentrations of toxic byproducts (from ethanol metabolism), and increases in estrogen (E2)-induced inflammation due to ethanol (Muller, 2006). Despite higher susceptibility of women to ethanol-induced liver damage, development of cirrhosis is more common in men than women (Altekruse, 2009; El-Serag, 2007; McKillop, 2006).
A considerable body of research has addressed the role of ethanol in ALD development and hepatocyte transformation(McKillop and Schrum, 2009). In comparison, relatively few studies have addressed potential mechanisms whereby ethanol may affect progression of hepatic tumorigenesis. In this study we sought to determine the effects of chronic ethanol intake on hepatocarcinogenesis in a well-described mouse model of HCC development generated via neonatal initiation with diethylnitrosamine (DEN). Additionally, we employed male and female mice for these studies in an attempt to identify potential sexual dimorphism in HCC progression in response to chronic ethanol feeding.
MATERIALS AND METHODS
Animal Assurances
Male and female B6C3 mice (21-25 days old, Jackson Laboratories (Bar Harbor, ME)) were used for these studies. All experiments were approved by the Institutional Animal Care and Use Committee and conformed to NIH Guidelines for the Care and Use of Animals.
Materials
Diethylnitrosamine (DEN), β-nitrophenol, and ethanol (99% (v/v)) were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies against glutathione S-transferase placental isoform (GSTpi), proliferating cell nuclear antigen (PCNA), ADH, and ALDH, and an avidin-biotin complex (ABC) detection kit were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rodent Decloaker, Rodent Block M, goat probe and goat polymer kit with Betazoid DAB, were purchased from BioCare Medical (Concord, CA). An anti-CYP2E1 antibody was purchased from Millipore (Temecula, CA). Antibodies against caspase 3 and cyclin D1 were purchased from Cell Signaling Technologies (Beverly, MA). An in situ terminal dUTP nick end label (TUNEL) kit was purchased from Roche (Indianapolis, IN). The EnzyChrom ethanol detection assay was purchased from BioAssay Systems (Hayward, CA). The RNeasy mini kit was purchased from Qiagen (Valencia, CA). cDNA synthesis was carried out using RQ1 DNase and ImpromII RT-PCR followed by amplification using GoTaq green master mix (Promega, Madison, WI) or IQTMSYBR Green Supermix (BioRad, Hercules, CA). An assay kit to measure serum alanine-aminotransferase (ALT) was purchased from Thermo-Scientific (Rockford, IL). Assay kits to measure thiobarbituric acid reactive species (TBARS) and glutathione (GSH) were purchased from Cayman Chemical (Ann Arbor, MI).
In vivo model of hepatocarcinogenesis
B6C3 mice (21-25 days old) were randomized, weighed, and injected with a single dose of DEN (1mg/Kg body weight, (i.p.)) dissolved in sterile olive oil, or vehicle (olive oil (i.p.). Upon recovery animals were randomized to receive control or ethanol feeding regimes for early (16-24-weeks) or late (40-48-weeks) hepatocarcinogenesis studies. Animals were allowed free access to AIN-93M rodent chow diet throughout (Dyets Inc., Bethlehem, PA).
Ethanol feeding
Animals assigned to ethanol-feeding groups were weaned on to an ethanol-drinking water (EtOH-DW) regime by replacing DW with 5% (v/v) EtOH in DW (3 days), followed by 10% (v/v) EtOH in DW (3 days). At the end of this period animals were maintained on 10/20% (v/v) EtOH-DW (alternate days) for a further 8-weeks. For animals assigned to the early (16-24-weeks) hepatocarcinogenesis studies initiation of EtOH-DW began at 15 -weeks and ceased at 24-weeks. For animals assigned to the late (40-48-weeks) hepatocarcinogenesis studies initiation of EtOH -DW began at 39-weeks and ceased at 48-weeks. All animals were weighed twice weekly.
Necropsy
At 24 or 48-weeks (depending on pre-assigned groups) mice were weighed, anesthetized (isofluorane by inhalation), examined grossly and sacrificed by exsanguination. Livers were excised, weighed, examined for visible lesions, and representative liver sections (4-6mm) taken from the left, right, median, and anterior lobes. Sections were either snap frozen in liquid nitrogen and stored prior to analysis (−80°C), or fixed in 10% neutral buffered formalin (24 hours) prior to transfer to 70% (v/v) EtOH for histological processing/analysis.
Histology
Multiple liver lobes (minimum of 2 lobes per animal) were sectioned (4-6μm) and stained with Mayer’s hematoxylin and eosin (H & E) or Picrosirius red. Representative sections (5 random fields/lobe) were examined microscopically (200x magnification) and blind-scored for steatosis (0-4), necrosis (0-4), inflammation (0-4), and/or fibrosis (0-4) using modified scoring scales reported by others (Morgan et al., 2002) (Supplemental Data; Table 1). Degree of fibrosis (mean % Picrosirius red staining) was performed using ImageJ software (NIH, Bethesda, MD). The four independent scores were combined to generate a total liver injury score (TLIS).
Table 1.
Animal/liver weights and liver-body weight ratios at necropsy.
| Group | n | Weight (g) | Liver (g) | Liver:BW ratio |
Visible Lesions |
|
|---|---|---|---|---|---|---|
| MALE (24 Wks) |
Control (C) | 9 | 35.6±0.5 | 1.81±0.05 | 0.050±0.001 | 0 |
| EtOH (E) | 9 | 35.1±0.7 | 2.14±0.09 | 0.056±0.002 | 0 | |
| DEN (D) | 9 | 36.9±2.4 | 2.10±0.17 | 0.057±0.002 | 0 | |
| DEN+EtOH (D+E) | 14 | 34.8±0.7 | 2.08±0.12 | 0.059±0.002 | 0 | |
| FEMALE (24 Wks) |
Control (C) | 10 | 25.7±0.4 | 1.30±0.03 | 0.051±0.001 | 0 |
| EtOH (E) | 10 | 27.5±0.4 | 1.48±0.03 | 0.054±0.015 | 0 | |
| DEN (D) | 9 | 27.9±0.6 | 1.64±0.09* | 0.059±0.003 | 0 | |
| DEN+EtOH (D+E) | 14 | 25.4±0.4 | 1.35±0.03 | 0.053±0.006 | 0 | |
| Group | n | Weight (g) | Liver (g) |
Liver:BW
ratio |
Visible
Lesions |
|
| MALE (48 Wks) |
Control (C) | 9 | 41.2±1.0 | 1.93±0.05 | 0.047±0.001 | 0 |
| EtOH (E) | 9 | 42.1±0.8 | 2.19±0.06 | 0.054±0.001 | 0 | |
| DEN (D) | 8 | 36.3±2.3# | 2.59±0.35# | 0.070±0.007# | 87.5% (7/8) |
|
| DEN+EtOH (D+E) | 14 | 34.7±0.7# | 4.44±0.16* | 0.129±0.006* | 93.7% (13/14) |
|
| FEMALE (48 Wks) |
Control (C) | 10 | 27.9±0.6 | 1.37±0.05 | 0.049±0.001 | 0 |
| EtOH (E) | 10 | 28.6±0.5 | 1.41±0.04 | 0.049±0.001 | 0 | |
| DEN (D) | 9 | 28.2±0.8 | 1.78±0.14* | 0.063±0.005* | 44.4% (4/9) |
|
| DEN+EtOH (D+E) | 14 | 25.7±1.1* | 1.31±0.07 | 0.050±0.001 | 35.7% (5/14) |
|
p<0.05 versus all other treatments groups within male or females assayed at matching time points (24 or 48 weeks).
p<0.05 versus C or E within male or female groups assayed at matching time points.
Immunohistochemistry (IHC)
Sections (4-6μm) of fixed liver were immuno-stained as previously reported (Kitano, 1998). Briefly, following deparaffinization, rehydration and antigen retrieval, sections were placed in a humidified chamber and blocked with goat serum. Sections were incubated with anti-glutathione S-transferase placental isoform (GSTpi) (1:100 dilution) or anti-proliferating cell nuclear antigen (PCNA; 1:1000 dilution). Detection was performed using an ABC kit counterstained with Mayer’s hematoxylin (for GSTpi IHC) or goat IgG probe/goat polymer kit with Betazoid DAB, counterstained with methyl green (for PCNA IHC). Five random fields/lobe (minimum of 2 lobes) were examined microscopically (200x magnification), photographed, and analyzed for number of altered hepatic foci (AHF) and AHF area (mm2), or number of PCNA positive nuclei (dark brown staining) using ImageJ software. To measure apoptosis, an in situ TUNEL assay was preformed as per the manufacturer’s instructions and sections counterstained with 4′-6-Diamidino-2-phenylindole (DAPI) for nuclear visualization. To confirm antibody specificity mouse spleen and embryonic tissue were used as positive controls (PCNA and TUNEL respectively). Negative control for TUNEL employed enzyme free probe.
PCR and quantitative RT-PCR
Total RNA was extracted from liver tissue. cDNA was synthesized from random hexamers, and used to perform PCR CYP2E1 mRNA expression performed using mouse CYP2E1 primer pairs (forward: 5′AGG CTG TCA AGG AGG TGC TA 3′, and reverse: 5′GGA AGT GTG CCT CTC TTT GG 3′). Final product was semi-quantified using NIH-ImageJ software and corrected to β-2 microgoblulin (housekeeping gene) levels. qRT-PCR was preformed using 50ng of cDNA followed by SYBR Green Supermix using mouse specific primer pairs for T-bet (forward: 5′ CCT GGA CCC AAC TGT CAA CT 3′, reverse: 5′ AAC TGT GTT CCC GAG GTG TC 3′); GATA3 (forward: 5′ GCA AAA AGG AGG GTT TAG GG 3′, reverse: 5′ GTG GTC ACA CTC GGA TTC CT 3′) or SMAD3 (forward: 5′ GGG CCA ACA AGT CAA CAA GT 3′ , reverse: 5′ CTG GCT GGC TAA GGA GTG AC 3′) in an iCycler IQ™ real time PCR detection system (BioRad) as previously described (Mantel et al., 2007; Yu et al., 2006). Samples were assayed in duplicate, data analyzed according to the comparative CT method using an internal housekeeping control (GAPDH), and normalized to pair-matched controls.
Immunoblot protein detection
Liver tissue (≈100mg) was homogenized in radioimmuno-precipitation assay (RIPA) buffer, the resulting homogenate sonicated (4°C), protein levels measured, and normalized using RIPA buffer. Protein detection was performed by immunoblot using antibodies specific against ADH, ALDH, CYP2E1 or cyclin D1 as previously reported (Brandon-Warner et al., 2010). Signal intensity was determined using NIH-ImageJ software and equal protein loading confirmed by stripping/re-probing membranes with an anti-β-actin (housekeeping protein) antibody.
Liver function and oxidative stress status
Serum alanine-aminotransferase (ALT) was measured using an ALT/GPT 2-part reagent. Hepatic lipid peroxidation was measured using a TBARS kit on liver tissue homogenates. Hepatic GSH levels were determined by colorimetric analysis of liver tissue lysates collected in 2-(N-morpholino) ethanesulfonic acid (MES) buffer using a GHS assay kit.
Ethanol assay
Aliquots (50μl) of serum were collected and stored at −80°C prior to measurement of ethanol concentration (Brandon-Warner et al., 2010).
Cytochrome P4502E1 activity
CYP2E1 activity was measured as the rate of oxidation of β-nitrophenol to 4-nitrocatechol in the presence of NADPH and O2 (Brandon-Warner et al., 2010).
Changes in serum cytokine levels
To evaluate and compare cytokine expression patterns a custom (7) Plex murine cytokine array (BioRad, Hercules, CA) was performed. Cytokines were selected to reflect changes in T-effector responses (Th1; IFNγ, IL1β, IL12p70, and Th2; IL13, T-reg-IL10) and inflammatory liver disease (TNFα and IL-6). Pooled serum (12.5μL) from each group was diluted 1:4 in proprietary serum sample diluents. Premixed standards were used to generate a standard curve. The assay was performed in a 96-well filtration plate supplied with the assay kit as per the manufacturer’s instructions, before being read on the Bio-Plex suspension array system and analyzed using Bio-Plex manager software.
Statistical Analysis
Comparisons of pathological scoring, foci size and number in each group were made by Fishers exact test. Comparisons of ALT/AST, TBARS and GSH levels were made by either one-way ANOVA followed by Tukey’s or Dunnet’s post-hoc test, as appropriate or two-way ANOVA followed by Bonferonni correction. p<0.05 was considered significant.
RESULTS
Mortality, body-liver weight, and necropsy
Male and female mice tolerated the EtOH-DW regime well either without, or with, prior DEN initiation. Male mice were significantly heavier than female counterparts at all time points (Table 1, and Figure 1; Supplemental Data). Overall no significant difference in body weight was measured within male or female groups maintained on control (C) versus EtOH-DW regimes (E), nor between these groups and pair matched animals initiated with DEN alone (D), or DEN concomitant with EtOH-DW (D+E) (Table 1, and Figure 1; Supplemental Data). Of note, male mice in the D and D+E group (48 -weeks) exhibited significantly lower body weight compared to pair-matched male C or E groups at the end of the 48-week experimental period (Figure 1A; Supplemental Data, and Table 1). No mortality occurred in any of the experimental groups in the 24-week group. Within the 48-week group, 1 male DEN and 1 female DEN+EtOH animal died prior to time course completion (47-weeks and 13-weeks respectively).
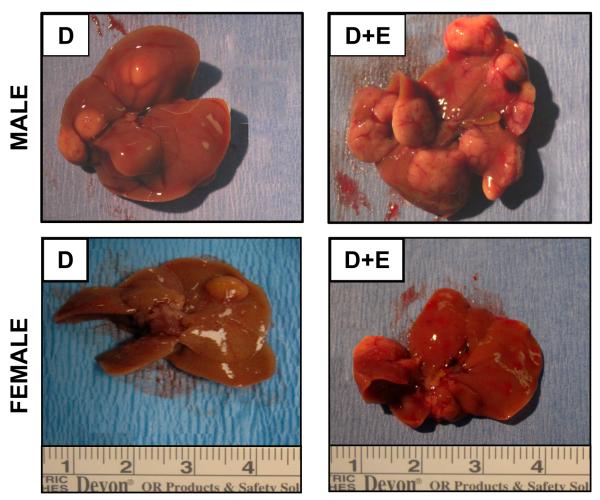
Figure 1. Ethanol feeding enhances DEN-induced hepatocarcinogenesis in male mice.
Representative images of livers resected from male and female mice (48 weeks) initiated with DEN (D) or DEN with ethanol feeding (D+E).
At necropsy, livers were resected, photographed, and weights recorded. Upon gross examination no visible lesions were observed in any of the groups at 24-weeks (Table 1). By 48-weeks visible lesions were observed in both male and female mice initiated with DEN, a significantly greater number being visible in males (87.5% versus 44.4%, male versus female, p<0.05, Figure 1, Table 1). In male mice (48-weeks) the effect of DEN was exacerbated by chronic ethanol feeding (93.7% (D+E) versus 87.5% (D)), whereas fewer visible lesions were observed in DEN-initiated females maintained on the EtOH-DW regime than DEN-initiated alone (35.7% (D+E) versus 44.4% (D); Figure 1 and Table 1).
Calculation of liver-body weight ratios (L:BW) demonstrated no significant difference between male and female mice in pair-matched groups. However, by 48-weeks, male D and D+E mice exhibited a significantly higher L:BW ratio as compared to C and E groups, and D+E versus DEN-initiated alone (p<0.01, Table 1). Conversely only DEN-initiated female mice in the 48-week groups, demonstrated significantly higher L:BW ratios. In comparing these data, differences in liver weight and L:BW ratios were attributed to increased size/number of tumors in the male (D and DE groups) and the female (D group) compared to control and ethanol only groups (Figure 1, Table 1).
Liver pathology - tumor burden
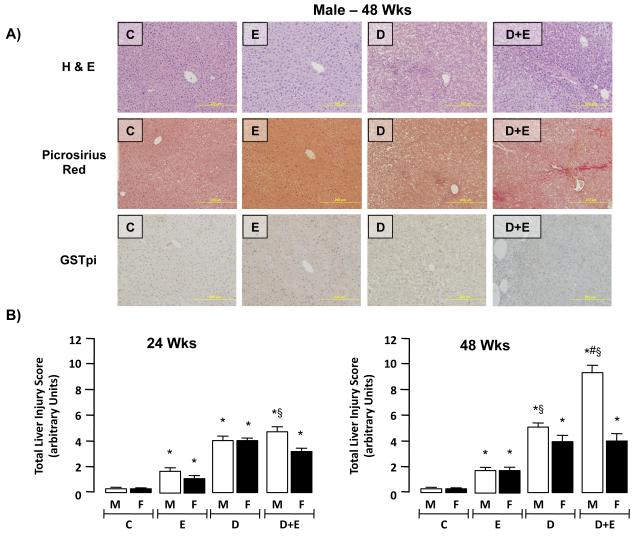
Total liver injury score (TLIS) were generated following blind scoring for total fat, necrosis, inflammatory cell infiltration, and collagen deposition (Figure 2A; male 48-week data shown, and Table 2; Supplementary data). Using this approach, increased injury was identified in male EtOH, DEN and DEN+EtOH mice in all lobes at both 24 and 48-weeks (Figure 2B). Conversely, pair-matched female mice were observed to exhibit more localized liver injury that was typically identified in only one or two lobes. Moderate, though significant, liver injury was measured in both male and female mice maintained on EtOH alone (E) compared to controls (C) at both 24 and 48-week time points (Figure 2B). Additional analysis demonstrated DEN-initiation alone (D) significantly increased the TLIS in both male and female mice at 24 and 48-week time points compared to C and E groups (Figure 2B, p<0.05). Within the 24-week group, maintenance on the EtOH-DW regime led to significantly greater TLIS in male mice compared to female (Figure 2B, p<0.05). This effect was further magnified in the 48-week study groups in which EtOH-DW significantly increased TLIS in male mice versus pair-matched female animals and male DEN-only initiated animals (Figure 2B, p<0.05). When comparing 24 to 48-week groups, no significant difference in TLIS was measured between pair-matched groups in female mice (Figure 2B). Conversely, the effects of D or D+E had significantly greater impact on TLIS in male mice at 48 -weeks compared to 24 -weeks (Figure 2B, p<0.05).
Figure 2. Ethanol feeding promotes hepatic injury in a DEN model of hepatocarcinogenesis in male mice.
A) Representative images (x200 magnification) of sections from control (C) male mice (48 weeks) and pair-matched male mice maintained on ethanol feeding (E), initiated with DEN (D), or initiated with DEN and ethanol fed (D+E). Sections were stained with H&E, Picrosirius Red or an antibody against Glutathione S-transferase placental isoform (GSTpi). B) Cumulative total liver injury score (TLIS) was blind scored from representative sections (2 lobes/mouse, 5 fields/lobe) from each experimental group (male and female, 24- and 48-weeks; C, E, D, D+E). *p<0.05 versus respective male or female controls (C), #p<0.05 D+E (male or female) versus D mice, §p<0.05 male versus female within same treatment group/time points. Minimum n = 8 independent mice/group.
Table 2.
Blood alcohol content (BAC), alanine aminotransferase (ALT), maliondyadehyde (MDA), and glutathione (GSH) levels at necropsy.
| Group | n | BAC mmol/L |
ALT IU |
MDA nmol/mg |
GSH μmol/mg |
|
|---|---|---|---|---|---|---|
| MALE (24 Wks) |
Control (C) | 9 | 1.3±0.1* | 11.3±0.9* | 1.64±0.16* | 5.74±0.16* |
| EtOH (E) | 9 | 14.3±1.2 | 29.1±3.9 | 7.70±0.75 | 4.58±0.24 | |
| DEN (D) | 9 | 1.2±0.1* | 45.5±4.4* | 6.87±0.67§ | 4.02±0.04§ | |
| DEN+EtOH (D+E) | 14 | 13.3±1.1# | 60.7±7.2*# | 8.81±0.49*# | 2.98±0.11*#§ | |
| FEMAL E (24 Wks) |
Control (C) | 10 | 1.3±0.1* | 11.2±1.1* | 2.10±0.27* | 6.06±0.15* |
| EtOH (E) | 10 | 10.4±1.0 | 35.3±3.2 | 8.12±0.72 | 5.15±0.13 | |
| DEN (D) | 9 | 1.3±0.1* | 37.3±4.0 | 5.43±0.83* | 4.99±0.08 | |
| DEN+EtOH (D+E) | 14 | 12.2±1.0# | 57.0±5.2*# | 8.51±0.61# | 3.87±0.18*# | |
| Group | n | BAC mg/dL |
ALT IU |
MDA nmol/mg |
GSH μmol/mg |
|
| MALE (48 Wks) |
Control (C) | 9 | 1.5±0.1* | 14.7±0. 9* | 2.77±0.09* | 5.86±0.14* |
| EtOH (E) | 9 | 11.5±0.9 | 53.5±7.5 | 6.85±0.92§ | 4.34±0.36 | |
| DEN (D) | 8 | 3.0±0.6* | 116.1±16.1*§ | 8.16±0.53* | 4.09±0.14§ | |
| DEN+EtOH (D+E) | 14 | 14.2±0.8# | 131.3±17.3* | 14.35±0.76*#§ | 2.43±0.11*#§ | |
| FEMAL E (48 Wks) |
Control (C) | 10 | 1.3±0.2* | 15.3±2.2* | 2.19±0.31* | 5.97±0.14* |
| EtOH (E) | 10 | 12.5±0.9 | 45.2±6.8§ | 5.26±0.79 | 4.91±0.08 | |
| DEN (D) | 9 | 2.5±0.2* | 124.6±19.6* | 7.79±0.81* | 4.90±0.08 | |
| DEN+EtOH (D+E) | 14 | 13.1±0.8# | 107.0±13.9*# | 11.52±1.14*# | 3.06±0.15*# | |
p<0.05 versus respective male or female E mice,
p<0.05 D+E mice versus respective male or female D mice,
p<0.05 male versus female within same treatment group/time points.
Analysis of individual components of the TLIS revealed that the primary mode of hepatic injury was lipid accumulation in DEN-initiated groups at both 24 and 48 - weeks, and lipid scoring was further increased by EtOH exposure, this effect being more pronounced in male compared to female mice (Table 2; Supplemental Data).
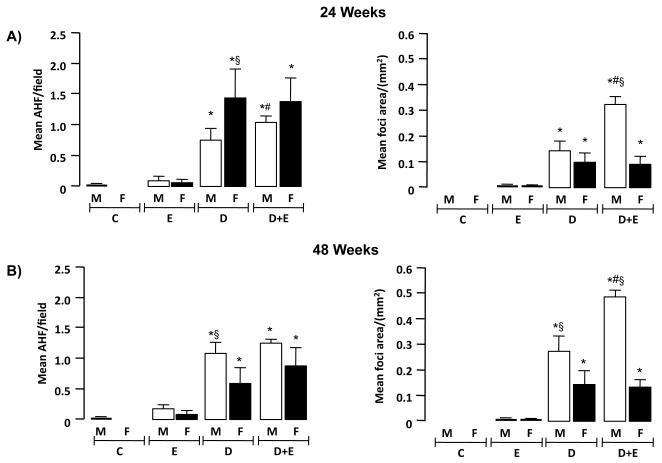
Immunohistochemistry (IHC) was initially performed using an antibody against GSTpi to identify altered hepatic foci (AHF). Using this approach number of AHF/field were significantly greater in female D versus male groups at 24-weeks (p<0.05) (Figure 3A). Conversely, by 48-weeks, number of AHF/field were significantly greater in male D mice versus female counterparts (Figure 3B, p<0.05). Area of AHF were next calculated and expressed as mean foci area (MFA; mm2). These data demonstrated significantly greater MFA in D and D+E groups compared to E and C in males and females at 24 and 48 -weeks (Figure 3A and B, p<0.05). In analyzing male versus female DEN-initiated animals, greater MFA were measured in males compared to females in D+E mice (24-weeks) and in D and D+E groups (48-weeks) (Figure 3A and B, p<0.05). Finally, EtOH-DW significantly increased MFA in male D+E mice versus all other groups at both 24- and 48-weeks (Figure 3B, p<0.05).
Figure 3. Ethanol feeding promotes hepatic tumor incidence and size preferentially in male mice.
A) Mean number of altered hepatic foci (AHF)/field and mean foci area (MFA) were calculated from glutathione S-transferase placental isoform (GSTpi) positive cells in microscopic fields (x200 magnification, 2 lobes/mouse, 5 fields/lobe) from control (C) male and female mice, and mice undergoing ethanol feeding (E), DEN-initiation (D) or DEN initiation followed by 8 weeks of ethanol feeding (D+E). *p<0.05 versus respective male or female E, #p<0.05 D+E versus respective male or female D mice, §p<0.05 male versus female within same treatment group/time points. Minimum n = 8 independent mice/group. B) Mean AHF and mean area occupied by foci were calculated from GSTpi+ cells from microscopic fields (x200 magnification, 2 lobes/mouse, 5 fields/lobe) from control (C) male and female mice, and mice undergoing E, D, or D+E. *p<0.05 versus respective male or female E, #p<0.05 D+E versus respective male or female D mice, §p<0.05 male versus female within same treatment group/time points. Minimum n = 8 independent mice/group.
Proliferation
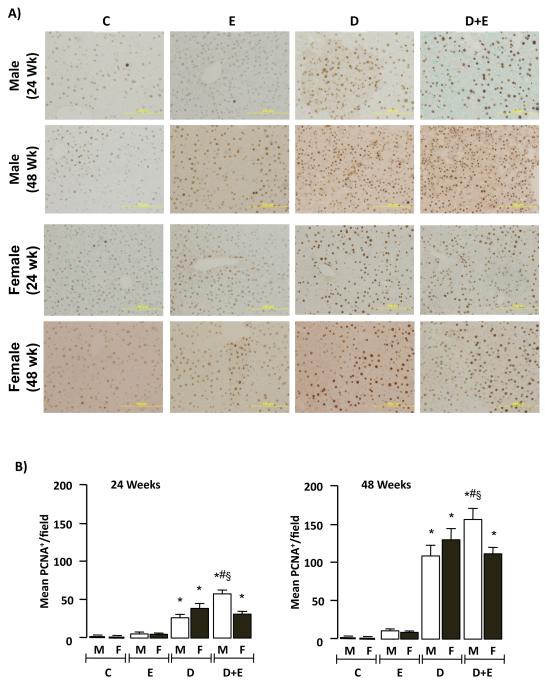
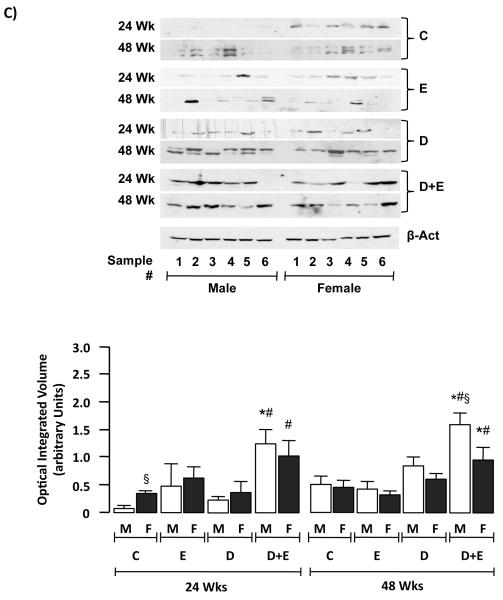
To assess mitogenesis a second series of IHC was performed using an antibody specific against proliferating cell nuclear antigen (PCNA; Figure 4A). Quantification of these results demonstrated significantly increased PCNA staining in male and female mice initiated with DEN (D) or DEN+EtOH (D+E) compared to control (C), or EtOH-only (E) groups at 24 and 48-weeks (Figure 4B, p<0.05). Furthermore, significantly increased PCNA staining was measured in male D+E mice versus D in both 24 and 48-week groups (Figure 4B, p<0.05). Finally, PCNA staining in D and D+E was significantly higher in male and female mice at 48-week compared to 24-week groups (Figure 4B, p<0.05). Since increased PCNA staining is also associated with DNA repair (Essers, 2005), we performed immunoblot analysis of cyclin D1 in hepatic tissue lysates. These data showed significantly increased cyclin D1 expression in D+E male and female mice compared to other groups (Figure 4C, p<0.05). Furthermore, significantly increased cyclin D1 expression was detected in male D+E mice at 48-weeks compared to pair-matched females (Figure 4C, p<0.05).
Figure 4. Ethanol feeding promotes hepatic tumorigenesis preferentially in male mice.
A) Representative proliferating cell nuclear antigen (PCNA) immunohistochmistry of hepatic sections (x200 magnification) from control (C) mice, or mice maintained on ethanol (E), DEN-initiation (D), or DEN and ethanol (D+E) at 24 or 48 weeks. B) Number of PCNA positive cells per microscopic field (x200 magnification) were measured in representative sections (2 lobes/mouse, 5 fields/lobe) from individual male and female mice on different treatment regimes (24 and 48 weeks) and mean values (± SEM) were calculated. *p<0.05 versus respective male or female E, #p<0.05 D+E versus respective male or female D mice, §p<0.05 male versus female within same treatment group/time points. Minimum n = 8 independent mice/group. C) Representative immunoblots of cyclin D1 expression in hepatic tissue lysates from male and female mice on different treatment regimes at 24 and 48 weeks (upper panel). Optical integrated volume was calculated, corrected for sample loading (stripping-re-probing membranes with anti-β-actin (β-Act)) and expressed as mean values (arbitrary units) ± SEM (lower panel). *p<0.05 versus respective male or female E, #p<0.05 D+E versus respective male or female D mice, §p<0.05 male versus female within same treatment group/time points. Minimum n = 6 independent samples per group.
Apoptosis
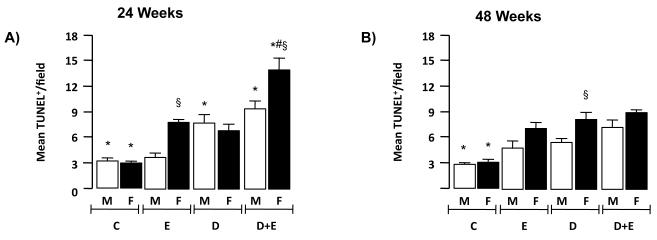
An in situ TUNEL assay was performed and TUNEL positive nuclei/field (TUNEL+) calculated. These data demonstrated a significant increase in TUNEL+ nuclei in male D+E versus D only at 24 and 48-weeks (Figure 5A and B, p<0.05). Of interest, the number of TUNEL+ nuclei was significantly greater in female D+E mice at 24 -weeks compared to all other groups (Figure 5A), and in female D-only mice compared to male counterparts in the 48-week group (Figure 5B, p<0.05).
Figure 5. Ethanol feeding promotes cell death in hepatic tumorigenesis preferentially in female mice.
Immunohistochemistry was performed on representative sections of control male and female mice (C) and mice maintained on ethanol (E), DEN-initiated (D), or DEN-initiated followed by ethanol feeding (D+E) at A) 24 and B) 48 weeks. Number of TUNEL positive cells per microscopic field (x200 magnification) were measured in representative sections (minimum of 2 lobes/mouse, 5 fields/lobe) and mean values (± SEM) calculated. *p<0.05 versus respective male or female E, #p<0.05 D+E versus respective male or female D mice, §p<0.05 male versus female within same treatment group/time points. Minimum n = 8 independent mice/group.
Blood-alcohol content (BAC), liver function, and REDOX status
As anticipated, blood alcohol content (BAC) was elevated in animals maintained on the EtOH-DW regime, a range of 10.4 ± 1.0 to 14.3 ± 1.2mmol/L being detected (Table 2). No significant differences were measured between male and female DEN-initiated animals maintained on ethanol (D+E) compared to ethanol alone (E), and no significant differences were measured between respective groups at 24 versus 48-weeks (Table 2).
As an indicator of liver injury/function serum alanine transferase (ALT) levels were measured. Within the 24-week groups ALT levels were significantly elevated in all three treatment groups (E, D and E+D) compared to control, although these levels remained within the normal range expected for ALT levels in mice (11-70U/L) (Yan and Stanley, 2001). Of note, ALT levels in both male and female mice initiated with DEN and maintained on EtOH-DW (D+E) were significantly higher than either D, or E alone at 24-weeks (Table 2, p<0.05). In the 48-week groups ALT was again significantly greater in E, D and D+E groups compared to control (Table 2, p<0.05). Furthermore, in male mice, D+E and D alone caused significant increases in ALT levels compared to E alone at 24- and 48-weeks, while in female animals this effect was only evident in the 48-week mice (Table 2, p<0.05). In both instances, ALT levels were significantly higher than in corresponding groups at 24-weeks (Table 2, p<0.05).
To measure hepatic REDOX status tissue lipid peroxidation (maliondyadehyde; MDA) and glutathione levels were measured. These data demonstrated significantly increased MDA levels in E, D and D+E groups at both 24 and 48-weeks compared to control (Table 2, p<0.05). Within the 24-week treatment groups no significant difference was measured between MDA levels between the different treatment regimes, or between male and female mice. Conversely, in the 48-week groups, MDA levels were significantly higher in the D+E groups compared to all other groups, and MDA levels were significantly higher in male D+E animals compared to females (Table 2, p<0.05). Analysis of GSH levels revealed an inverse correlation to MDA data; all three treatment groups (E, D and D+E) exhibiting significantly less GSH compared to controls, the most significant differences being measured in the D+E groups at both 24 and 48 -weeks. Additionally, the most pronounced effect on GSH depletion was measured in the male D+E group at 48-weeks (Table 2, p<0.05).
Ethanol metabolizing enzyme expression and activity
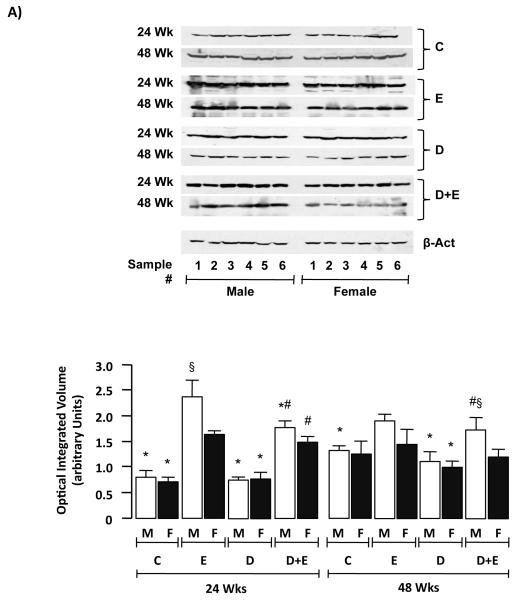
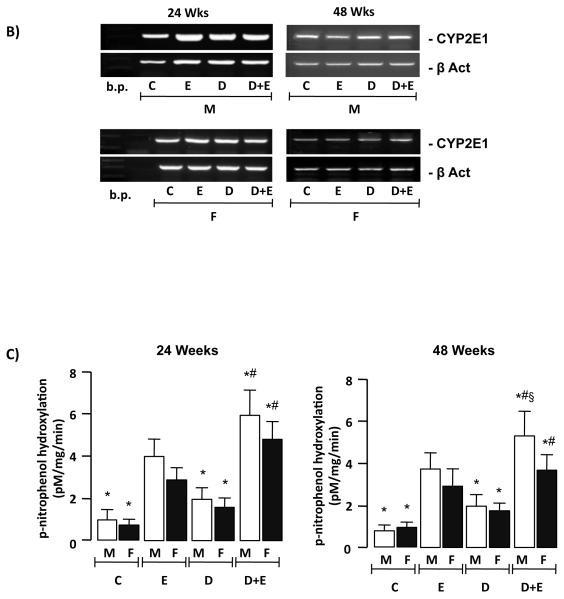
Expression of ADH, ALDH and CYP2E1 was measured by immunoblot using liver tissue lysates. These data demonstrated no significant differences in ADH or ALDH expression within the groups analyzed at 24 or 48-week time points (Supplemental Data, Figure 2). Conversely, analysis of CYP2E1 protein demonstrated increased CYP2E1 expression in E and D+E versus C and D mice at 24 weeks. In contrast CYP2E1 expression was only significantly increased in male E and D+E mice versus C and D counterparts (Figure 6A). Additionally, there was no significant difference in CYP2E1 expression between DEN-initiated animals (D) compared to C, nor between male and female mice within the C and D groups at either 24 or 48 weeks time points (Figure 6A). To further understand these differences, CYP2E1 mRNA expression was measured by RT-PCR. Using this approach no differences in CYP2E1 mRNA were detected in male or female animals in any of the groups at either 24- or 48-weeks (Figure 6B). To determine whether biochemical differences in CYP2E1 activity existed p-nitrophenol hydroxylation was measured. This approach demonstrated β-nitrophenol hydroxylation was significantly higher in E male and female mice compared to C and D at 24 and 48-weeks (Figure 6C, p<0.05). Furthermore, CYP2E1 activity was significantly higher in D+E versus E mice at 24-weeks (male and female) and 48-weeks (male only), by 48-weeks CYP2E1 activity being significantly higher in male versus female D+E mice (Figure 6C, p<0.05).
Figure 6. Ethanol induces cytochrome P4502E1 expression and activity preferentially in male mice.
A) Representative immunoblots of cytochrome P4502E1 (CYP2E1) expression in hepatic tissue lysates from male and female mice from control (C) and pair-matched animals maintained on ethanol (E), DEN-initiated (D), or DEN-initiated followed by ethanol feeding (D+E) at 24 and 48 weeks (upper panels). Optical integrated volume was calculated, corrected for sample loading (stripping-re-probing membranes with anti-β-actin (β-Act)) and expressed as mean values (arbitrary units) ± SEM (lower panel). *p<0.05 versus respective male or female E, #p<0.05 D+E versus respective male or female D mice, §p<0.05 male versus female within same treatment group/time points. Minimum n = 6 independent samples per group. B) RT-PCR analysis of CYP2E1 mRNA expression from pooled total mRNA samples from male and female mice on the different treatment regimes indicated at 24 and 48 weeks. C) p-nitrophenol hydroxylation was measured as a marker of CYP2E1 activity in hepatic lysates from male and female mice on different treatment regimes at 24 and 48 weeks. *p<0.05 versus respective male or female E, #p<0.05 D+E versus respective male or female D mice, §p<0.05 male versus female within same treatment group/time points.
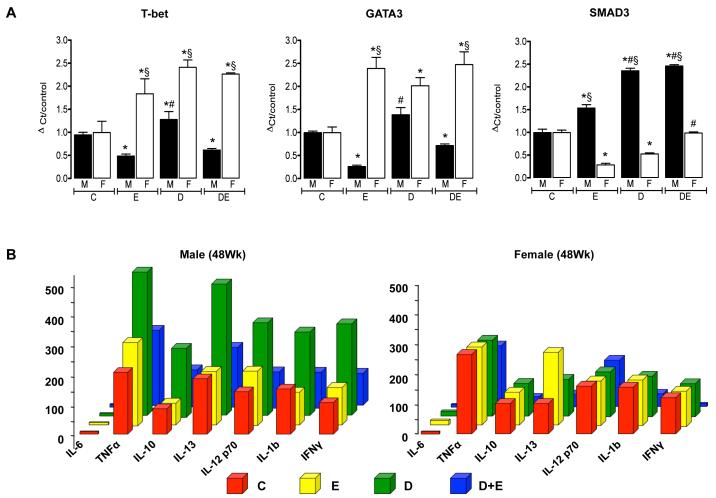
Immunological status
To identify changes in hepatic immune responses, we performed qRT-PCR analysis of hepatic T-bet, GATA3, and SMAD3 mRNA expression (Sundrud and Nolan, 2010). These data demonstrated significantly increased T-bet and GATA3 mRNA expression in female experimental groups (E, D and D+E) compared to control (Figure 7A, p<0.05 all groups versus C) and compared to male counterparts, with the exception of GATA3 expression in DEN-only initiated animals (Figure 7A). Furthermore, T-bet and GATA3 mRNA expression were significantly decreased in male E and D+E mice versus control and DEN-only male counterparts (Figure 7A, p<0.05). Concomitant with decreased T-bet/GATA3 mRNA expression, male mice demonstrated significantly increased SMAD3 mRNA in all experimental groups (E, D, and D+E) compared to control (C) (Figure 7A, p<0.05 all groups versus C). Hepatic SMAD3 mRNA expression was in turn significantly increased in D and D+E male mice compared to E-only counterparts (Figure 7A, p<0.05). Conversely SMAD3 mRNA was significantly decreased in female mice treatment groups as compared to male counterparts and female controls (Figure 7A, p<0.05).
Figure 7. Ethanol feeding differentially affects immune responses in male versus female mice in the setting of hepatocarcinogenesis.
A) Quantitative real time PCR (qRT-PCR) analysis of T-bet, GATA3, and SMAD3 mRNA expression in total hepatic mRNA isolated from control (C) and pair-matched animals maintained on ethanol (E), DEN-initiated (D), or DEN-initiated followed by ethanol feeding (D+E) at 48-weeks. *p<0.05 versus C, #p<0.05 versus respective male or female E mice, §p<0.05 male versus female within same treatment group/time points. B) Custom BioPlex assay was used to determine cytokine expression in pooled serum samples from control male (left) and female (right) mice (C), and pair matched animals maintained on ethanol (E), DEN-initiated (D), or DEN-initiated followed by ethanol feeding (D+E) at 48 weeks. Minimum n = 8 independent samples per group.
In an attempt to further understand changes in hepatic markers of immune status, a custom mouse bioplex assay for 7 immunological cytokines (IL-6, TNFα, IL-10, IL-13, IL12p70, IL1b, and IFNγ) was performed in serum pooled from C, E, D, and D+E, 48-week animals. IL-6 was not detectable in serum from any of the experimental groups (Figure 7B). In male animals, chronic ethanol feeding (E) did not alter cytokine levels compared to control (C). However, serum levels of the 6 detectable cytokines increased by 1.6-2.8 fold in DEN-initiated (D) males compared to pair-matched C and E mice. Conversely, levels of all 6 cytokines decreased in D+E mice to levels equal, or lower, than those measured in C and E mice (Figure 7B). Analysis of serum from female mice demonstrated ethanol alone led to a 1.65 fold increase in IL-13 compared to C, no notable differences being measured for the other detectable cytokines between E and C groups (Figure 7B). Unlike their male counterparts DEN-initiation (D) did not lead to any notable changes in detectable cytokine levels compared to C (Figure 7B). However, female D+E mice did demonstrate a substantial decrease in IL-10, IL-13, IL-1b, and IFNγ as compared to C, E and D-only mice. Of particular note, IL-10 was ≈5.0 fold lower in female D+E mice compared to C, E or D mice, as well as pair-matched males, without any notable change in IL-12 p70 or TNFα (D+E versus C, E, and D, Figure 7B).
DISCUSSION
Chronic ethanol abuse, in the absence or presence of other factors, is a significant risk factor for developing HCC (El-Serag, 2007; McKillop and Schrum, 2009; Yang and Roberts, 2010). Considerable evidence points to many of the deleterious effects of ethanol arising from metabolic byproducts and ROS produced during ethanol metabolism (Crabb and Liangpunsakul, 2007; Hoek, 2002; McKillop and Schrum, 2009). Similarly, epidemiological evidence points to sexual dimorphism for HCC development (Altekruse, 2009; El-Serag, 2007). To date though, relatively few studies have addressed the effects of ethanol on promotion of hepatocarcinogenesis, and the potential role that gender plays in these disparities.
Epidemiological data report chronic, heavy ethanol consumption is a significant risk factor for the development of hepatic cirrhosis, the leading precursor to HCC development (Altekruse, 2009; El-Serag, 2007; McKillop and Schrum, 2005). However, experimental approaches to better understand these mechanisms report disparate results depending on the model employed. Using HCC cells in vitro, ethanol is reported to either increase (Brandon-Warner et al., 2010) or inhibit (Castañeda and Kinne, 2000) proliferation depending on the cell line employed. Studies aimed at evaluating the promotional effects of ethanol on hepatocarcinogenesis in vivo report similarly diverse results, whereby no effect, co-carcinogenesis, or promotional effects occur (Mandl, 1989; Schwarz, 1983; Takada, 1986). However, these studies were limited to male animals such that the role of gender was not examined. Additionally, these models commonly utilize nutritional deficits (low methionine diet) or partial hepatectomy as a means to promote hepatocyte proliferation concomitant with ethanol feeding (Mandl, 1989; Schwarz, 1983; Takada, 1986). In doing so, it is possible that differences in timing, duration of ethanol exposure (relative to DEN initiation), and the effects of partial hepatectomy/altered nutritional status significantly affects outcome.
In our study DEN was administered to neonatal mice and dysplastic foci were allowed to develop prior to ethanol administration in the absence of nutritional deficits or partial hepatectomy. Following administration, DEN like ethanol, induces hepatic CYP2E1 resulting in hepatocyte transformation. As the liver develops, hepatic DNA damage is magnified and hepatic tumors form that exhibit a high proliferative index, a subtype genetically similar to that of human HCC, and correspondingly poor prognostic outcomes (Fausto and Campbell, 2010; Kang, 2007). To ensure the effects of DEN did not interfere with those of ethanol feeding on CYP2E1 induction, DEN-initiation was performed in neonatal mice (21-24 days) 13- or 37-weeks prior to commencing ethanol feeding. In this way ethanol feeding was timed to coincide with hepatic foci (16-weeks) or HCC (40-week) formation (following DEN injection) (Fausto and Campbell, 2010; Goldfarb et al., 1983; Goldsworthy, 2002; Kang, 2007).
In our study, rate of HCC for males to females was ≈2:1 in DEN-only mice and 2.6:1 in DEN+EtOH mice at 48-weeks. Additionally increased incidence and size of tumors was measured in DEN-initiated male mice maintained on EtOH-DW (E+D) compared to DEN-initiation alone (D). In contrast, female mice maintenance on the EtOH-DW regime exhibited a decrease in both tumor size and burden. Of note, PCNA was higher in DEN-only female mice, while there was no apparent difference in cyclin D1 expression (compared to pair-matched males) in this group. This disparity may indicate changes in PCNA expression in female mice occurs, in part, due to activation of DNA repair mechanisms. That is, chemical carcinogens induce DNA damage that can in turn activate endogenous repair pathways, PCNA having been reported to accumulate at sites of DNA damage as a requirement for DNA repair (Essers, 2005). Given the importance of oxyradicals and changes in cellular REDOX balance during ethanol metabolism (Albano et al., 1999; Hoek, 2002), the differences in DNA repair mechanisms between male and female mice may thus be of significance in hepatic tumorigenesis in the setting of chronic ethanol feeding in a model pre-disposed to hepatic tumor formation. No differences in PCNA were detected between male and female E-only mice, but female D+E mice had significantly lower PCNA staining compared to pair-matched males with significant increases in TUNEL positive cells. This may indicate that females eliminate cells with ethanol-induced DNA-damage, and that these differences in apoptosis are more efficient during early hepatocarcinogenesis than late, although apoptosis (as indicated by TUNEL+ cells) remains higher in females than males in E and D+E groups, even at 48 wks.
In addition to differences in timing of DEN administration and/or nutritional or surgical promotion, our model employed an ethanol-drinking water (EtOH-DW) feeding regime. Several models of chronic ethanol feeding have been described, including the Leiber DeCarli (LDC) liquid diet, the Tsukamoto-French intra-gastric model, daily gavage, and exposure to ethanol vapor (Tabakoff and Hoffman, 2000). Each of these models has advantages and potential limitations depending on study design and duration. The choice of the EtOH-DW model for our studies was based on practical and experimental considerations. While the LDC and intra-gastric approaches are most commonly reported for studying alcoholic liver disease (Nanji and French, 2003; Tabakoff and Hoffman, 2000), the number of animals in our study group, the use of calorically pair-matched control animals, and the feeding period (8-weeks) made this approach unfeasible. Rather preliminary data from pilot studies, and data generated from our experimental series, demonstrated no significant differences in body weight in animals maintained on EtOH-DW compared to pair-matched DW-only animals, with BACs of ≈10-14mmol/L.
Measures of morbidity (ALT, pathology scores: steatosis, necro-inflammatory changes) corresponded to tumor burden and incidence in male and females and was affected by ethanol. Serum ALT, was elevated in animals maintained on EtOH-DW, without or in addition to DEN-initiation, at both 24 and 48-weeks. Additionally, ALT levels were several fold higher in late (48-week) stage models while only modest increases over normal levels (11-70U/L) were identified at early time points (24-week). To directly compare degrees of hepatic injury, we presented our data as total liver injury score (TLIS). Using this approach, data demonstrate TLIS are (generally) commensurate with ALT data. However, it is of interest to note that both male and female D and D+E mice had significantly elevated ALT values (compared to C or E counterparts), yet TLIS were significantly lower in female D+E mice versus male D+E mice. The most likely explanation for this disparity is the difference in tumor burden between the two groups; in females, HCC formation was most commonly restricted to 1 or 2 lobes, whereas in males tumors formed throughout all hepatic lobes. As such histological examination using 2 randomly assigned lobes (5 fields/lobe scored) is more likely to encounter/score non-tumor tissue in females compared to males resulting in a lower TLIS. Additionally, when considering the individual components used to compile TLIS (Supplemental Table), lipid deposition is observed as the most pronounced individual factor influencing TLIS at both early and late time points. Of further note, in the 24-week animals lipid scoring (male and female) was comprised primarily of microvessicular lipid droplets indicative of active lipid loading. However, by the 48-week time point lipid deposition was primarily large globules indicative of steady state steatosis. Additionally, male mice had high levels of foamy degeneration of cells with large areas of necrosis, effects exacerbated by ethanol exposure in DEN-initiated animals. Overall, scores for individual criteria were generally lower in female mice, the only exception being the presence of more infiltrating immune cells in female DEN-initiated mice at 24 and 48-weeks compared to pair matched males.
The differences we detected in hepatic injury are most likely to be related to changes in cell REDOX state due to ethanol metabolism, generation of ROS, and changes in cellular GSH. Induction of CYP2E1 accentuates the imbalance in acetaldehyde/ALDH and contributes to the generation of ROS with subsequent depletion of GSH and oxidative injury. Male and female mice maintained on the EtOH-DW regime had consistently higher levels of MDA compared to control or DEN-initiated only mice at 24-weeks. Conversely, GSH levels were decreased in both male and female animals. However, female mice exhibited consistently higher GSH levels than males in both 24 and 48-week groups. These data suggest that, despite the increased potential for injury in females, greater GSH levels may protect against hepatic ROS/oxidative injury.
Hepatic inflammatory responses to ethanol, and their influence on the tumor microenvironment, have emerged as important factors during hepatocarcinogenesis (Mandrekar and Szabo, 2009). mRNA analysis of transcription factors for Th1 (T-bet) and Th2 (GATA3) T-effector responses demonstrated female mice maintain higher expression of both T-bet and GATA3 compared to pair-matched males in E, D, and D+E groups. Furthermore, EtOH-DW alone, or EtOH-DW in DEN-initiated male mice acted to suppress T-bet and GATA3 expression below that of C levels. These events also occurred in the presence of increased SMAD3 mRNA expression in male experimental mice compared to their female counterparts. Collectively these data suggest females maintain a more robust inflamation response, a property reflected by lower tumor incidence and burden as compared to male counterparts. Indeed, as indicated by TUNEL analysis, increased apoptosis was detected in female E and D+E groups compared to male counterparts (24 weeks), an event that may be indicative of more efficient immuno-surveillance and/or clearance of transformed cells in females; factors that will directly contribute to subsequent decreases in hepatic tumor size and burden in females at the later time point (48-weeks). Of further note, we also detected increased SMAD3 mRNA in E, D and D+E male mice, a factor that may indicate increased activation of a T-regulatory response through TGFβ signaling and immunosuppression in male versus female mice.
Cytokine profiles within the tumor milieu can have critical effects on tumor promotion and progression. Data presented herein demonstrated that DEN-initiation led to increased serum levels of all measurable cytokines in male mice compared to control. In contrast, similar changes in serum cytokines were not measured in DEN-initiated female mice, an effect that is likely attributable to the increased incidence and tumor burden in male DEN mice compared to female. Ethanol alone did not noticeably alter cytokine expression in male mice, yet serum IL-13 was increased by a factor of 1.65 in female E-only mice compared to control and did correlate to increased GATA3 identified in this group. Serum cytokine levels were not completely reflective of hepatic gene expression, but indicate systemic immune responses. As such, they can be influenced by many factors outside the liver, including sex-hormone levels, blood LPS, and overall oxidative stress. It was also noted that male D+E mice exhibited an increased ratio of IL-10 to IL12p70 expression relative to C that was not detected in female mice. This is significant as high IL-10/low IL-12p70 has been reported to influence alternative activation of macrophages which can in-turn impair dendritic cell (DC) maturation (Corinti et al., 2001; Mantovani et al., 2002). This is associated with conversion of macrophages from an inflammatory (M1) phenotype, to a tumor-associated (M2) phenotype that promotes tumor progression.
Multiple factors influence cytokine expression, and this is particularly true in the setting of chronic ethanol consumption. For example, a significant component of hepatic immune responses are attributed to oxidative stress arising from ethanol metabolism (Crabb and Liangpunsakul, 2007; Hoek, 2002). Conversely, indirect effects of ethanol on intestinal permeability, as well as composition of the gut flora, is equally important in defining Kupffer cell (KC) activation and intrahepatic immune signaling (Enomoto et al., 2001; Mandrekar and Szabo, 2009). As such, changes in serum cytokine levels we report may not accurately reflect specific immune responses within the milieu of tumor per se, or for that matter the environment around the tumor. Similarly, this type of cytokine panel does not allow us to address the potential role of the multiple cell types likely to be contributing to change in systemic cytokine levels (e.g. KCs, stellate cells, endothelial cells, monocytes and/or T/B-cell populations). However, gender differences are reported in cytokine signaling as well as recruitment of neutrophils and macrophages to the site of injury (Bird, 2008; Naugler, 2007). The potential to differentially affect DC activation, immunosuppression and modulation of T-reg cells thus raises intriguing questions regarding the potential role of cytokines/immune responses in mediating inherent differences in male and female responses during EtOH-dependent changes in tumor progression (Hoek, 2002; Lin and Karin, 2007).
While differences in immune system responsiveness are likely to be important mediators of the gender specific effects of ethanol on tumor promotion, other factors must also be considered. Most notable amongst these are the likely effects of ethanol on sex hormone signaling. While our study did not measure androgen or estrogen levels, or signaling pathways regulated by these sex hormones, a considerable body of data indicates ethanol plays a significant role in regulating the synthesis and action of sex hormones in the liver and other organs. Gender specific differences have been identified in a number of transcription factors, NF B signaling, p38, AP-1 that may influence HCC progression and immune response (Karin, 2008; Seki et al., 2011). Additionally, small molecule mediators such as adiponectin have been demonstrated to possess hepatoprotective and anti-tumor qualities and exhibit sex-dependent differences in expression (Shen et al., 2010; You and Rogers, 2009). Indeed, it seems increasingly likely that future studies of the dynamic between sex hormone synthesis, metabolism and signaling, and the intra and extra-hepatic immune system responses will prove central to fully understanding the dramatic gender differences that occur during hepatic tumorigenesis in the setting of chronic ethanol intake.
Supplementary Material
Acknowledgements
The authors thank Dr David Foureau for his invaluable advice with data analysis and interpretation. This work was funded in part by grants from the NIH (AA014891 (LWS) & AA016858 (IHM)).
Footnotes
Disclosures: None of the authors have any potential conflicts of interest to disclose.
REFERENCES
- Albano E, French SW, Ingelman-Sundberg M. Hydroxyethyl radicals in ethanol hepatotoxicity. Front Biosci. 1999;4:D533–40. doi: 10.2741/albano. [DOI] [PubMed] [Google Scholar]
- Altekruse S, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clinic Oncology. 2009;27(9):1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird M, Karavitis J, Kovacs EJ. Sex differences and estrogen modulation of the cellular immune response after injury. Cell Immuno. 2008;252:57–67. doi: 10.1016/j.cellimm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon-Warner E, Sugg JA, Schrum LW, McKillop IH. Silibinin inhibits ethanol metabolism and ethanol-dependent cell proliferation in an in vitro model of hepatocellular carcinoma. Cancer Lett. 2010;291(1):120–9. doi: 10.1016/j.canlet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda F, Kinne RKH. Cytotoxicity of millimolar concentrations of ethanol on HepG2 human tumor cell line compared to normal rat hepatocytes in vitro. Journal of Cancer Research and Clinical Oncology. 2000;126(9):503–510. doi: 10.1007/s004320000119. [DOI] [PubMed] [Google Scholar]
- Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166(7):4312–8. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- Crabb DW, Liangpunsakul S. Acetaldehyde generating enzyme systems: roles of alcohol dehydrogenase, CYP2E1 and catalase, and speculations on the role of other enzymes and processes. Novartis Found Symp. 2007;285:4–16. doi: 10.1002/9780470511848.ch2. discussion 16-22, 198-9. [DOI] [PubMed] [Google Scholar]
- Ekstrom G, Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1) Biochem Pharmacol. 1989;38(8):1313–9. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- El-Serag H, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- Enomoto N, Schemmer P, Ikejima K, Takei Y, Sato N, Brenner DA, Thurman RG. Long-term alcohol exposure changes sensitivity of rat Kupffer cells to lipopolysaccharide. Alcohol Clin Exp Res. 2001;25(9):1360–7. [PubMed] [Google Scholar]
- Essers J, Theil AF, Baldeyron C, van Cappellen WA, Houtsmuller AB, Kanaar R, Vermeulen W. Nuclear dynamics of PCNA in DNA replication and repair. Molec and Cell Biology. 2005;25(21):9350–9359. doi: 10.1128/MCB.25.21.9350-9359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N, Campbell JS. Mouse models of hepatocellular carcinoma. Semin Liver Dis. 2010;30(01):87–98. doi: 10.1055/s-0030-1247135. [DOI] [PubMed] [Google Scholar]
- Goldfarb S, Pugh TD, Koen H, He YZ. Preneoplastic and neoplastic progression during hepatocarcinogenesis in mice injected with diethylnitrosamine in infancy. Environ Health Perspect. 1983;50:149–61. doi: 10.1289/ehp.8350149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsworthy T, Fransson-Steen R. Quantification of the cancer process in C57BL/6J, B6C3F1and C3H/HeJ mice. Toxicol Pathol. 2002;30:97–105. doi: 10.1080/01926230252824770. [DOI] [PubMed] [Google Scholar]
- Gramenzi A, Caputo F, Biselli M, Kuria F, Loggi E, Andreone P, Bernardi M. Review article: alcoholic liver disease--pathophysiological aspects and risk factors. Aliment Pharmacol Ther. 2006;24(8):1151–61. doi: 10.1111/j.1365-2036.2006.03110.x. [DOI] [PubMed] [Google Scholar]
- Harada S, Tachiyashiki K, Imaizumi K. Effect of sex hormones on rat liver cytosolic alcohol dehydrogenase activity. J Nutr Sci Vitaminol (Tokyo) 1998;44(5):625–39. doi: 10.3177/jnsv.44.625. [DOI] [PubMed] [Google Scholar]
- Hoek J, Pastorino JG. Ethanol oxidative stress and cytokine induced liver injury. Alcohol. 2002;27:63–68. doi: 10.1016/s0741-8329(02)00215-x. [DOI] [PubMed] [Google Scholar]
- Jirillo E, Caccavo D, Magrone T, Piccigallo E, Amati L, Lembo A, Kalis C, Gumenscheimer M. The role of the liver in the response to LPS: experimental and clinical findings. J Endotoxin Res. 2002;8(5):319–327. doi: 10.1179/096805102125000641. [DOI] [PubMed] [Google Scholar]
- Kang J, Wanibuchi H, Morimura K, Gonzalez FJ, Fusushima S. Role of CYP2E1 in diethylnitrosamine-induced hepatocarcinogenesis in vivo. Cancer Research. 2007;67:11141–11146. doi: 10.1158/0008-5472.CAN-07-1369. [DOI] [PubMed] [Google Scholar]
- Karin M. The I[kappa]B kinase - a bridge between inflammation and cancer. Cell Res. 2008;18(3):334–342. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- Kitano M, Ichihara T, Matsuda T, Wanibuchi H, Tamano S, Hagiwara A, Imaoka S, Funae Y, Shirai T, Fukushima S. Presence of a threshold for promoting effects of phenobarbital on diethylnitrosamine-induced hepatic foci in the rat. Carcinogenesis. 1998;19(8):1475–1480. doi: 10.1093/carcin/19.8.1475. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev. 1997;77(2):517–44. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- Lin W-W, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. The Journal of Clinical Investigation. 2007;117(5):1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30(1-2):42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl J, Banhegyi G, Garzo T, Lapis K, Antoni F, Schaff Z. Ethanol treatment inhibits the devlopment of diethylnitrosamine-induced tumors in rats. J Exp Pathol. 1989;4:227–235. [PubMed] [Google Scholar]
- Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50(6):1258–66. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel PY, Kuipers H, Boyman O, Rhyner C, Ouaked N, Ruckert B, Karagiannidis C, Lambrecht BN, Hendriks RW, Crameri R, Akdis CA, Blaser K, Schmidt-Weber CB. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5(12):e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32(5):1008–17. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- McKillop I, Moran DM, Jin X, Koniaris LG. Molecular pathogenesis of hepatocellular carcinoma. Journal of Surgical Research. 2006 doi: 10.1016/j.jss.2006.04.013. [DOI] [PubMed] [Google Scholar]
- McKillop IH, Schrum LW. Alcohol and liver cancer. Alcohol. 2005;35(3):195–203. doi: 10.1016/j.alcohol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- McKillop IH, Schrum LW. Role of alcohol in liver carcinogenesis. Semin Liver Dis. 2009;29(2):222–32. doi: 10.1055/s-0029-1214377. [DOI] [PubMed] [Google Scholar]
- Morgan K, French SW, Morgan TR. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology. 2002;36(1):122–134. doi: 10.1053/jhep.2002.33720. [DOI] [PubMed] [Google Scholar]
- Muller C. Liver, alcohol and gender. Wien Med Wochenschr. 2006;156(19-20):523–6. doi: 10.1007/s10354-006-0348-8. [DOI] [PubMed] [Google Scholar]
- Nanji AA, French SW. Animal models of alcoholic liver disease--focus on the intragastric feeding model. Alcohol Res Health. 2003;27(4):325–30. [PMC free article] [PubMed] [Google Scholar]
- Naugler W, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317 doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Buchmann A, Wiebeck G, Kunz W. Effect of ethanol on early stages in nitrosamine carcinogenesis in rat liver. Cancer Lett. 1983;20:305–312. doi: 10.1016/0304-3835(83)90029-0. [DOI] [PubMed] [Google Scholar]
- Seki E, Park E, Fujimoto J. Toll-like receptor signaling in liver regeneration, fibrosis and carcinogenesis. Hepatology Research. 2011;41(7):597–610. doi: 10.1111/j.1872-034X.2011.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Liang X, Rogers CQ, Rideout D, You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298(3):G364–74. doi: 10.1152/ajpgi.00456.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundrud MS, Nolan MA. Synergistic and combinatorial control of T cell activation and differentiation by transcription factors. Curr Opin Immunol. 2010;22(3):286–92. doi: 10.1016/j.coi.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. Animal models in alcohol research. Alcohol Res Health. 2000;24(2):77–84. [PMC free article] [PubMed] [Google Scholar]
- Takada A, Nei J, Takase S, Matsuda Y. Effects of ethanol on experimental hepatocarcinogenesis. Hepatology. 1986;6(1):65–72. doi: 10.1002/hep.1840060113. [DOI] [PubMed] [Google Scholar]
- Yan L, Stanley SL., Jr. Blockade of caspases inhibits amebic liver abscess formation in a mouse model of disease. Infect Immun. 2001;69(12):7911–4. doi: 10.1128/IAI.69.12.7911-7914.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7(8):448–58. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M, Rogers CQ. Adiponectin: a key adipokine in alcoholic fatty liver. Exp Biol Med (Maywood) 2009;234(8):850–9. doi: 10.3181/0902-MR-61. [DOI] [PubMed] [Google Scholar]
- Yu J, Wei M, Becknell B, Trotta R, Liu S, Boyd Z, Jaung MS, Blaser BW, Sun J, Benson DM, Jr., Mao H, Yokohama A, Bhatt D, Shen L, Davuluri R, Weinstein M, Marcucci G, Caligiuri MA. Pro- and antiinflammatory cytokine signaling: reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity. 2006;24(5):575–90. doi: 10.1016/j.immuni.2006.03.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.