Abstract
The ability of osteosarcoma cells to form lung metastases has been inversely correlated to cell surface Fas expression. Down-regulation of Fas allows osteosarcoma cells to circumvent FasL-mediated apoptosis upon entrance into the FasL+ lung microenvironment. However, the mechanism of Fas regulation remains unclear. Here we demonstrate that miRNA plays a role in the down-regulation of Fas expression in osteosarcoma. Expression levels of several members of the miR-17-92 cluster-including miR-20a, and miR-19a-were found to be higher in metastatic low-Fas-expressing LM7 cells than in the parental nonmetastatic high-Fas-expressing SAOS-2 cells. We also found an inverse correlation between Fas and miR-20a expression in all 8 cell lines derived from patient samples. Overexpression of miR-20a consistently resulted in the down-regulation of Fas expression in SAOS-2 cells and thus in decreased sensitivity to FasL. Conversely, inhibiting miR-20a in LM7 cells increased Fas expression and their sensitivity to FasL. Mice injected with LM7 stably transfected with anti-mir-20a had fewer metastases compared to those with control plasmids. Taken together, our findings suggest that miR-20a, encoded by miR-17-92, down-regulates Fas expression in osteosarcoma, thus contributing to the metastatic potential of osteosarcoma cells by altering the phenotype and allowing survival in the FasL+ lung microenvironment.
Keywords: Fas, Osteosarcoma, miR-20a, miR-17-92
Introduction
Osteosarcoma is a primary bone tumor that metastasizes almost exclusively to the lung, one of the four organs in the body where FasL is constitutively expressed. Therefore, Fas+ tumor cells would be expected to be cleared and eliminated by the constitutive FasL upon entrance into the lung. Indeed, we previously demonstrated that the ability of osteosarcoma cells to form lung metastases was inversely correlated to cell surface Fas expression (1–6). Fas− cells formed lung metastases when injected intravenously, whereas Fas+ cells did not. We also demonstrated that while the primary bone tumor was composed of a mixture of Fas− and Fas+ cells, the lung metastases were Fas−. Rapid clearance of Fas+ cells from the lung was seen compared with clearance of Fas− cells. Blocking the Fas signaling pathway or injecting Fas+ cells into FasL-deficient mice resulted in the formation of Fas+ osteosarcoma lung metastases, confirming both the importance of cell surface Fas and the Fas− lung microenvironment (3, 4, 7). In addition, osteosarcoma lung metastases from more than 60 patients were uniformly found to be Fas− (5). Taken together, these data establish the critical role of tumor Fas expression and the metastatic potential of osteosarcoma cells.
Much of the data described above was determined using the metastatic subline LM7, which was derived from SAOS-2 cells by cycling through the mouse 7 times and isolating the lung metastases (6). SAOS-2 cells are highly Fas+ and do not form lung metastases when injected intravenously. By contrast, LM7 cells are Fas− and do form lung metastases. We have demonstrated that the Fas gene was not deleted in LM7 cells but rather its expression down-regulated. Therapeutic intervention using either aerosol interleukin (IL)–12 gene therapy or chemotherapy resulted in the up-regulation of Fas expression on Fas− osteosarcoma lung metastases and tumor regression (8–10). These data indicate that the down-regulation of Fas occurs via an epigenetic mechanism. However, we have already demonstrated that methylation of CpG islands is not responsible for Fas gene silencing (11). Thus, the mechanism of Fas silencing is unclear at this time.
Recently microRNAs (miRNAs) have been shown to post-transcriptionally regulate the expression of protein-encoding genes. These miRNAs are small 21–23 nucleotide noncoding RNAs that suppress translation or directly cleave the target mRNA by binding to complimentary sequences in the three prime untranslated regions (3′-UTRs) or coding region of the mRNA (12, 13). miRNAs have been shown to regulate death receptor signaling by targeting specific proteins such as tumor necrosis factor–α (TNFα), Fas-Associated protein with Death Domain (FADD), Ribosome-inactivating protein (RIP), caspase 3, Bcl-2 interacting mediator of cell death (Bim), and p21 (14–17). Different miRNAs have also been implicated in regulating the expression of Fas, including miR-21 in glioblastoma, miR-200c in NCI60 cells, and miR-146a in mesenchymal cells (18–20).
In the current study, we showed that miR-20a encoded by the miR-17-19 cluster is overexpressed in metastatic osteosarcoma cells and regulates the expression of Fas and metastatic potential to the lung. To our knowledge, this is the first description linking the miR-17-92 cluster to the metastatic potential of tumor cells.
Materials and Methods
Cell lines and cell culture
SAOS-2 cells were obtained from the American Type Culture Collection. The metastatic LM7 cell line was developed by repetitive cycling of SAOS-2 cells through the lungs of nude mice 7 times (6). These cells were cultured in Eagle’s modified essential medium supplemented with 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 1× nonessential amino acids, 2× minimal essential medium vitamin solution, and 10% heat-inactivated (56°C for 30 minutes) fetal bovine serum. Both cell lines were incubated at 37°C in humidified 5% CO2. Peripheral blood mononuclear cells were cultured in RPMI medium (supplemented with 10% fetal bovine serum and 2 mmol/L glutamine).
Osteosarcoma cells obtained from patient specimens and were designated CCH-OS-C, CCH-OS-D, CCH-OS-G, CCH-OS-K, CCH-OS-M, CCH-OS-O, CCH-OS-R, and CCH-OS-T. All cell lines were derived under IRB-approved protocol LAB04-0361 from patients treated at the Children’s Cancer Hospital at MD Anderson Cancer Center. CCH-OS-C, CCH-OS-D and CCH-OS-O were derived from initial, pretreatment biopsies. CCH-OS-G and CCH-OS-R were derived from post-treatment, resected pulmonary metastases. CCH-OS-K, CCH-OS-M and CCH-OS-T were derived from malignant effusions. Solid tumor tissue was dissected into 1 mm3 or smaller fragments, followed by enzymatic digestion with 1× Collagenase I (Sigma Chemical) at 37°C for 45 minutes. Tumor cells were then harvested by passing them through a 100 μm Cell Strainer, followed by centrifuging (400 g.) to remove all digestion solution. Tumor cells from malignant effusions were isolated by centrifugation. The harvested tumor cells were then cultured in DMEM (supplemented with 10% fetal bovine serum, penicillin, streptomycin and 1% insulin/transferring selenium (Gibco)) for overnight. Non-adherent cells were washed away with PBS and adherent cells cultured in the same media through multiple passages. All lines have been carried >10 passages. Each has a unique signature by DNA microsatellite fingerprinting, with no overlap between the signature of these cells and those of known, described or previously tested cell lines. Fingerprints were performed on 2010 at the University of Texas MD Anderson Cancer Center.
Plasmid construction
Plasmids producing miR-20a, miR-20b, miR-19a, miR-106a, and miR-200b were constructed using BLOCK-iT™ Pol II miR RNAi Expression Vector (Invitrogen Corporation), according to the manufacturer’s instructions. The sequences used were: hsa-miR-19a: UGU GCA AAU CUA UGC AAA ACU GA; hsa-miR-20a:UAA AGU GCU UAU AGU GCA GGU AG; hsa-miR-20b:CAA AGU GCU CAU AGU GCA GGU AG; hsa-miR-106a: AAA GUG CUU ACA GUG CAG GUA G; and hsa-miR-200b: UAA UAC UGC CUG GUA AUG AUG A; All constructs were confirmed by sequencing in the DNA Sequence Core Facility of MD Anderson Cancer Center. pcDNA3.1/V5-His-TOPO-mir17-92 plasmid (21) were obtained from Addgene. The antagomir plasmid Sew-anti-miR-20a and Sew-anti-control were obtained from Dr. Michaela Scherr (Hannover, Germany) (22).
Transfection
SAOS-2 cells were transfected with pcDNA3.1/V5-His-TOPO-mir17-92 plasmids for 48h using Lipofectamine 2000 Reagent (Invitrogen) according to the manufacturer’s instructions. Stable transfected single-cell colonies were selected by incubation of the cells in 500 μg/mL of G418 for 4 weeks. The positive stable transfectants were confirmed by reverse transcription (RT)-PCR. Transfection of the miRNA inhibitors (Ambion, Inc.) was performed using siPORT Neo-FX reagents (Ambion, Inc.) according to the manufacturer’s protocols. Sew-anti-miR-20a and Sew-anti-control lentiviral particles were generated by co-transfection of 293T cells. Lentiviral supernatants were used to transfect LM7 cells. The GFP positive cells were sorted and the stable clones were isolated.
RNA extraction, RT-PCR, and microRNA detection
Total RNA was isolated and purified from cultured cells using the RNeasy Mini Kit (Qiagen, Inc.). RNA concentration was measured by NanoDrop Spectrophotometer (Thermo Scientific, Inc.). A regular reverse transcription was performed using the Reverse Transcription System with oligo-dT primer (Promega Corporation) according to the manufacturer’s instructions. The mRNA level of Fas expression was detected by regular PCR using iTaq DNA polymerase (Bio-Rad Laboratories) or by real-time PCR using iQ SYBR Green Supermix (Bio-Rad Laboratories). PCR primers have been described previously (23, 24). The mRNA level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was detected as an internal control for normalization.
miRNA expression was measured and quantified by using TaqMan MicroRNA Assays (Applied Biosystems) according to the manufacturers’ protocol. Briefly, 10 ng of total RNA was reverse transcribed using specific miRNA primers. The resulting cDNAs were amplified by PCR using TaqMan MicroRNA Assay primers with the TaqMan Universal PCR Master Mix and analyzed with the iQ5 Real-Time PCR detection system (Bio-Rad Laboratories). The relative level of miRNA expression was calculated by the change in cycle threshold method. RNU6, RNU44, or RNU48 levels were also measured as internal controls for normalization.
miRNA microarray analysis
Total RNA samples were isolated from SAOS-2 and LM7 cells and were fluorescently labeled with Cy3 and Cy5 dyes, respectively. MicroRNA microarray assay and data analysis were performed by LC Sciences, LLC.
Western blotting
Cell lysates were prepared using radioimmunoprecipitation assay buffer and total protein concentration was measured using the bicinchoninic acid assay kit (Pierce Biotechnology) with bovine serum albumin as a standard. Western blotting was performed as described previously (24). The levels of the Fas, cleaved caspase-3, α-tubulin, or β-actin, and of total and cleaved Poly (ADP-ribose) polymerase (PARP) were detected by probing the blots with anti-Fas antibody (Alexis Biochemicals), anti-cleaved caspase-3 (Cell Signaling Technology), anti- α-tubulin antibody (Santa Cruz Biotechnology), anti–β-actin (Sigma Aldrich), and anti-total or -cleaved PARP antibody (Cell Signaling Technology), respectively. The second antibody was visualized using an enhanced chemiluminescence detection Western blotting analysis system (Amersham Pharmacia Biotech Inc.). Densitometric analyses were performed to quantify Western blotting signals and normalized against that of α-tubulin.
Cell survival and apoptosis assays
Cell survival was measured with the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described previously (25). Cell death was measured by identifing nonviable cells with 7-aminoactinomycin D (7-ADD, R&D Systems, Inc) staining. Briefly, Cells were collected by centrifugation at 1,000 rpm for 10 min incubated on ice for 15 min with 5 mg/ml of 7-AAD in flow buffer (PBS containing 2% BSA). Stained cells were washed once, resuspended in flow buffer, and analyzed by flow cytometry. The apoptosis assay was performed using the DeadEnd™ Fluorometric TUNEL System (Promega Corporation) according to the manufacturer’s instructions. Cells were harvested by trypsinization, and the fragmented DNA of apoptotic cells was measured by catalytically incorporating fluorescein-12-dUTP at 3′-OH DNA ends using the enzyme Terminal Deoxynucleotidyl Transferase. Positive cells were visualized by fluorescence microscopy.
In vivo study
Four-week-old female nude (nu/nu) mice were purchased from the National Cancer Institute. The mice were maintained and fed in a specific pathogen-free animal facility approved by the American Association of Laboratory Animal Care in accordance with current regulations and standards of the United States Department of Agriculture, The Department of Health and Human Services, and the National Institutes of Health. LM-7 cells stably transfected with Sew-anti-miR-20a or control plasmid were injected into nude mice through tail vein using a sterile 30-gauge needle with 150 μl cell suspension of 2×106 cells. Mice were sacrificed 10 weeks after tumor cell injection, lungs removed and evaluated by weighing and counting visible nodules. All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee.
Statistical analysis
Analysis of cell survival, numbers of metastatic nodules and lung weight was done using the two-tailed Student’s t test. P values < .05 were considered statistically significant.
For the association analysis between Fas and miRNA, the log10-transformed expression level of Fas as a dependent variable was regressed on the log10-transformed expression level of miR. The multiple correlation coefficient R was estimated, and the null hypothesis was where the slope of regression line was zero. These analyses were performed using GraphPad Prism 5 software (GraphPad Prism Software Inc).
Results
Identification of potential miRNAs that regulate Fas expression
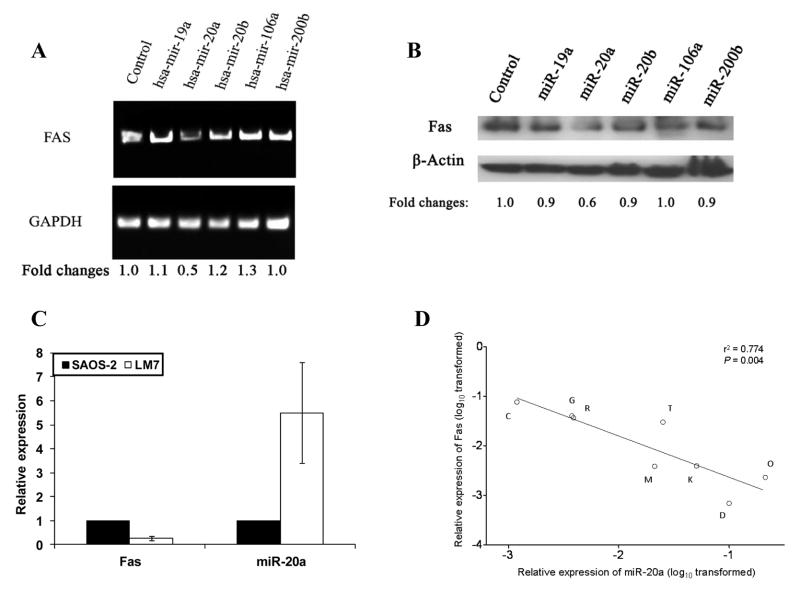
We compared levels of miRNAs in the parental high-Fas-expressing SAOS-2 cells with those of low-Fas-expressing LM7 cells using miRNA microarray. The candidate miRNAs identified were those that were higher in LM7 cells, lower in SAOS-2 cells, and those that had the potential to target Fas mRNA based on an online program (http://www.microrna.org). Five miRNAs were selected: miR-19a, miR-20a, miR-20b, miR-106a, and miR-200b. The raw intensity data for those miRNAs are presented in Table 1 (LM7, column 2; SAOS-2, column 3). Ratio values of LM7 versus SAOS-2 presented in a log2 scale (Table 1, column 3) indicates that the expression of these miRNAs in LM7 cells was up-regulated compared with expression in SAOS-2. To determine whether all or some of these miRNAs could down-regulate Fas expression, we constructed specific plasmids for each individual miRNA and then transfected each individually into the Fas+ SAOS-2 cells. Fas mRNA and protein levels were decreased after transfection with miR-20a (Fig. 1A, B). There was also an inverse correlation between the expression of Fas and miR-20a in both SAOS-2 and LM7 cells (Fig. 1C). Taken together, these data suggest that miR-20a is responsible for regulating Fas expression in osteosarcoma cells.
Table 1.
Candidate miRNAs targeting Fas
| Probe ID | LM7 a | SAOS-2b | log2 (LM-7/SAOS-2)c |
|---|---|---|---|
| hsa-miR-200b | 10,029.67 | 2.46 | 11.99 |
| hsa-miR-20b | 8,217.31 | 443.94 | 4.21 |
| hsa-miR-106a | 16,184.22 | 905.15 | 4.16 |
| hsa-miR-20a | 21,313.67 | 1,431.46 | 3.9 |
| hsa-miR-19a | 860.68 | 85.14 | 3.34 |
NOTE: The intensity values obtained from miRNA microarray for miR-19a, miR-20a, miR-20b, miR-106a and miR-200b.
Raw intensity values for LM7.
Raw intensity values for SAOS-2.
The ratio of LM7/SAOS-2 presented in a log2 scale.
Fig. 1.
miR-20a down-regulated Fas expression in SAOS-2 cells and miR-20a is inversely correlated to Fas expression in primary osteosarcoma cells. A, B, SAOS-2 cells were transfected with a control plasmid or plasmids encoding for miR-20a, miR-20b, miR-19a, miR-106a, or miR-200b for 48 h. mRNA (A) and protein levels (B) of Fas were quantified using reverse-transcription polymerase chain reaction (PCR) and Western blotting, respectively. Fas expression was normalized to the GAPDH and β-actin control, respectively, and expressed with reference to the control transfected cells. C, The relative expression of Fas mRNA and miR-20a in SAOS-2 and LM7 cells was assayed by real-time PCR. D, Total RNA was extracted from 8 different osteosarcoma cell lines derived from different patients. mRNA levels of Fas and miR-20a were quantified by real-time PCR. The correlation between Fas and miR-20a expression was analyzed by liner regression as described in Materials and Methods.
Inverse correlation between Fas and miR-20a in osteosarcoma patient specimens
To confirm the relationship between Fas and miR-20a in osteosarcoma, an additional 8 osteosarcoma cell lines derived from patient specimens were analyzed. The results showed that there was also an inverse correlation between the expression levels of Fas and miR-20a in all 8 samples (r2 = 0.774, P = 0.004) (Fig. 1D).
miR-20a encoded by miR-17-92 cluster regulates fas expression
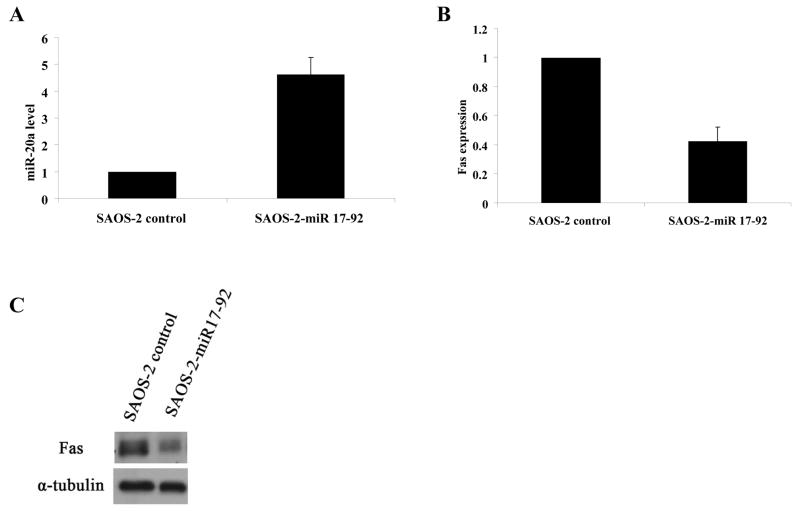
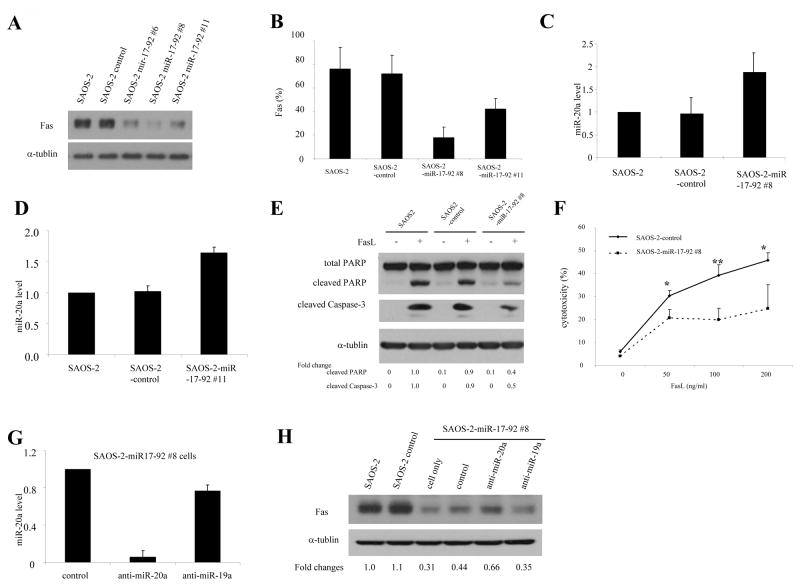
miR-20a is encoded by the miR-17-92 cluster. We therefore next investigated whether transfection of SAOS-2 cells with this cluster decreased Fas expression. SAOS-2 cells were transfected with the plasmid pcDNA3.1/V5-His-TOPO-miR-17-92 (miR-17-92), which encodes for this cluster. After transfection there was an increase in miR-20a and a decrease in Fas mRNA and protein level (Fig. 2A, B, C). Having verified that transfection with miR-17-92 cluster increased miR-20a, we next created SAOS-2 cloned cells stably transfected with miR-17-92. Cells of clones #6, #8, #11 (SAOS-2-miR-17-92 #6, #8 and #11) had decreased Fas protein level by western blotting (Fig. 3A). Cell surface expressions of Fas in SAOS-2-miR-17-92 #8 and #11 were also decreased by flow cytometry performed as described previously (26) (Fig. B). Increased miR-20a levels were also confirmed in SAOS-2-miR-17-92 #8 and #11 cells (Fig. 3C, D). As anticipated, the sensitivity of SAOS-2-miR-17-92 #8 to FasL was decreased, as indicated by PARP cleavage, caspase-3 cleavage, and cell cytotoxicity (Fig. 3E, F). To confirm that this effect on Fas was secondary to miR-20a, SAOS-2-miR-17-92 cells were transfected second time with either miR-20a inhibitor (anti–miR-20a), miR-19a inhibitor (anti–miR-19a), or a control inhibitor (Fig. 3G). Transfection with anti–miR-20a but not the control inhibitor or with anti–miR-19a specifically inhibited the expression of miR-20a and, as anticipated, increased Fas protein (Fig. 3H), confirming that miR-20a regulates Fas expression. Similar results were seen using SAOS-2-miR-17-92 #11 (data not shown).
Fig. 2.
Transfection of the miR-17-92 gene increases miR-20a and decreases Fas expression. SAOS-2 cells were transfected with a control plasmid or one encoding for miR-17-92 cluster for 48 h. A, B, miR-20a (A) and Fas mRNA levels (B) were then quantified by real-time PCR. C, Expression of the Fas protein and α-tubulin (internal control) were measured by Western blotting.
Fig. 3.
Expression of miR-20a influences both Fas expression and sensitivity to FasL. SAOS-2 cells were transfected with a control plasmid or one encoding for miR 17-92 cluster for 48 h. Stable colonies were selected by culturing the cells with 500 μg/mL G418 for 4 weeks. A, B, The protein levels (A) and cell surface expression (B) of Fas were determined by Western blotting and flow cytometry, respectively. C, D, Expression of miR-20a in clones #8 (C) and #11 (D) was quantified by real-time PCR. E, F, SAOS-2, SAOS-2 control transfected, and SAOS-2-miR17-92 clone #8 cells were incubated with FasL for 24 hours. Total and cleaved PARP, and cleaved caspase-3 were then measured by Western blotting (E). α-tubulin levels served as internal controls. Cell cytotoxicity was measured by staining cells with 7-aminoactinomycin D (7-AAD) and analyzed by flow cytometry (F) (*, P < 0.05; **, P < 0.01). G, H, SAOS-2-miR-17-92 cluster #8 cells were transfected with anti–miR-20a, anti–miR-19a, or control for 6 days (twice, 3 days apart). miR-20a levels were then quantified by real-time PCR (G). Fas expression and α-tubulin (internal control) were measured by Western blotting (H).
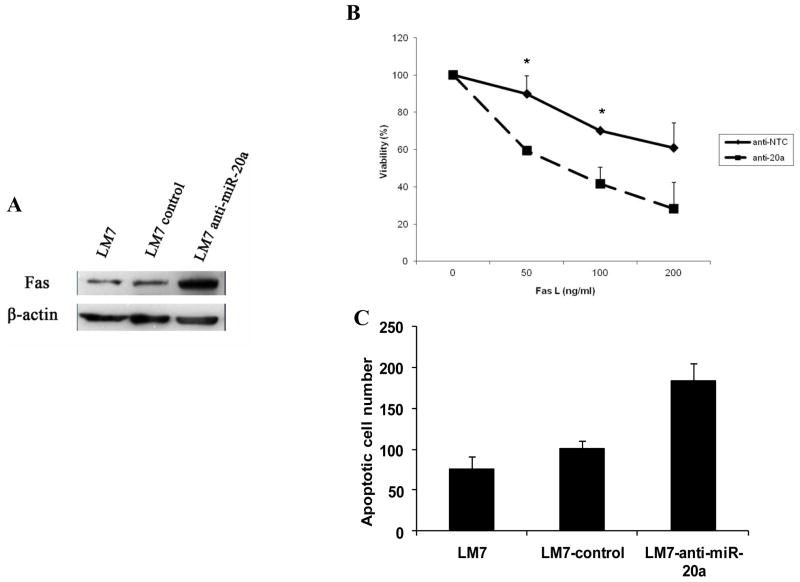
To further confirm the effect of miR-20a inhibition on Fas expression, low-Fas-expressing LM7 cells were transfected with anti-miR-20a. After transfection, Fas protein was up-regulated (Fig. 4A). This resulted in a significant increase in FasL-induced apoptosis (Fig. 4B, C). Taken together, these results indicate that the inhibition of miR-20a up-regulates Fas expression and that the up-regulation of miR-20a may be responsible for the low levels of Fas expression on LM7 cells. The mechanism for increased miR-20a in LM7 cells compared to the parental cells is unclear and not due to DNA copy number changes at the miR-17-92 cluster. The miR-17-92 DNA copy number as assessed by real-time PCR was the same in SAOS2, LM7 and normal peripheral blood mononuclear cells (data not shown).
Fig. 4.
Inhibition of miR-20a increased Fas expression and sensitivity to FasL. A, LM7 cells were transfected with anti–miR-20a or control for 72 hours. Fas expression and β-actin were then measured by Western blotting. B,C, LM7 control and anti–miR-20a transfected cells were incubated with FasL or medium for 24 hours. Cell survival was measured by MTT assay (B) (*, P < 0.05). Apoptotic cells (average from 5 high-power microscope fields) were quantified by TUNEL assay (C).
Inhibition of miR-20a decreased metastatic potential of LM7 in vivo
To determine whether altering miR-20a expression affect the metastatic potential of osteosarcoma cells, LM-7 cells stably transfected with Sew-anti-miR-20a or control plasmids were injected into nude mice. The stable transfected cell line LM7-Sew-anti-miR-20a was confirmed to express lower mir-20a and higher Fas expression compared to LM7-Sew-anti-control (data not shown). All mice were sacrificed at 10 weeks after tumor cell injection to evaluate lung metastases. The numbers of visible lung nodules as well as lung weight were significantly decreased in mice injected with Sew-anti-miR-20a compared to those injected with control plasmids (Table 2, Fig. 5). These findings demonstrate that altering mir-20a impacts the metastatic potential of LM-7 cells.
Table 2.
Effect of miR-20a inhibition on metastatic potential of LM7 in vivo
| Mouse group | LM7-Sew-controla | LM7-Sew-anti-miR-20aa | P valuec |
|---|---|---|---|
| Average lung weight | 0.45g ± 0.24b | 0.26g ± 0.12b | P < 0.05 |
| Average number of visible tumor nodules | 40.6 ± 23.3b | 15.6 ± 18.6b | P < 0.05 |
LM7 cells were transfected with either Sew anti-control or Sew-anti-miR-20a vector. Cloned cells were isolated, verified for the level of miR-20a and then injected into nude mice as described in Materials and Methods. Mice were sacrificed 10 weeks after tumor cell injection, and lungs removed and assessed for the presence of tumor nodules.
Mean ± SD
P value using Student’s t test
Fig. 5.
Inhibition of miR-20a decreased the metastatic potential of LM7 in vivo. LM-7 cells stably transfected with Sew-anti-miR-20a or control plasmid were injected into nude mice. Mice were sacrificed 10 weeks after tumor cell injection and lungs removed and analyzed for the presence of visible nodules.
Discussion
The data presented here demonstrate that miR-20a encoded by the miRNA-17-92 cluster regulates Fas expression in osteosarcoma cells and plays a critical role in the metastatic process of osteosarcoma to the lung. The human miR-17-92 cluster encodes 6 miRNAs (miR-17, miR-18a, miR-19a, miR-20a, miR-19b, and miR-92). It is located in the third intron of chromosome 13, C13orf25 (27). We found higher expression levels of several members of the miR-17-92 cluster in our metastatic LM7 cells than in the parental nonmetastatic SAOS-2 cells. These included miR-20a, and miR-19a. We went on to show an inverse correlation between Fas and miR-20a expression in all 8 cell lines derived from patient samples. Overexpression of miR-20a consistently resulted in the down-regulation of Fas expression in SAOS-2 cells, resulting in decreased sensitivity to FasL. Conversely, inhibiting miR-20a in LM7 cells increased Fas expression and their sensitivity to FasL. Altering miR-20a expression also changed the ability of osteosarcoma cells to form lung metastases following intravenous injection. LM7 cells transfected with anti-miR-20a had decreased levels of miR-20a, increased Fas and induced significantly fewer lung metastases than control-transfected LM7 cells. Based on these findings, we conclude that miR-20a encoded by the miR-17-92 cluster increases the metastatic potential of osteosarcoma cells by regulating Fas expression.
Our findings add to the growing number of studies showing a critical role for miRNAs, specifically the miR-17-92 cluster, in tumorigenicity. Overexpression of miR-17-92 has been shown to promote cell cycle progression and proliferation (28), inhibit apoptosis (29, 30), induce angiogenesis (31), and inhibit oncogene-induced senescence (32). Our studies are the first to show that in addition to the anti-apoptotic effect and its well-defined role in tumorigenesis, the miR-17-92 cluster, specifically miR-20a, may play a role in the metastatic process and the potential of osteosarcoma cells to form lung metastases by regulating tumor cell Fas expression. Because FasL is constitutively expressed by the lung epithelium, cells with Fas on their cell surface are cleared from the lung by interaction of the ligand with the receptor and the subsequent initiation of tumor cell apoptosis. We previously demonstrated that both the expression of Fas on osteosarcoma cells and an intact functional Fas signaling pathway results in the rapid clearance of osteosarcoma cells from the lung after intravenous injection (3, 4). Cells with low or no Fas expression are not removed by this mechanism and thus can survive and go on to grow, proliferate, and form lung metastases. We also demonstrated that FasL in the lung microenvironment is required for the clearance of Fas+ tumor cells. In the present study, increased expression of miR-20a and the miR-17-92 cluster has been shown to contribute to the metastatic potential of osteosarcoma cells by altering tumor cell biology to a phenotype that is more favorable to survival in the lung microenvironment.
The role of Fas-FasL signaling in tumor promotion vs inhibition is controversial and different depending on the tumor type. It may not only act as a signal for apoptosis but may in some instances promote cell proliferation through a nonapoptotic pathway (33, 34). However, cell proliferation by Fas-FasL interaction has been described mostly in epithelial cancers such as ovarian cancer, renal cell carcinoma, breast and liver cancer. We have previously shown that Fas-FasL interaction is essential for the regression of ostersarcoma lung metastases (3–5). Therefore, osteosarcoma cells differ from epithelial cancer cells in this regard.
It has also been suggested that certain tumors that express FasL on the cell surface operate a “counter attack” towards Fas+ immune cells resulting in tumor growth (35). This is once again not the case with osteosarcoma. We have previously demonstrated that IL-12 up-regulates Fas expression on osteosarcoma lung metastases and that when IL-12 is administered to mice with osteosarcoma lung metastases in combination with a chemotherapeutic agent that up-regulates FasL, the therapeutic efficacy was superior to that seen by either agent alone (9, 36). These data demonstrated that upregulating both Fas and FasL on the osteosarcoma cell surface was advantageous and increased cell killing rather than promoting tumor growth. Furthermore these data suggest that interfering with immune cell function is not a concern in this disease setting.
Transgenic mice with moderate overexpression of miR-17-92 in lymphocytes develop a lymphoproliferative disease (30). Fas was first identified on T-cells and functions as a regulator the inflammatory response. Our data showing an inverse correlation between Fas expression and miR-17-92 offers a mechanistic explanation for the pathology seen in these transgenic mice. Lymphocytes that overexpress miR-17-92 would be expected to have decreased Fas and thus be less sensitive to FasL-induced cell death, allowing these cells to escape control and overproliferate. It has also been demonstrated that deletion of miR-17-92 in B-cells disrupts normal B-cell development as a result of premature cell death (37). Based on our data showing a link between Fas and miR-17-92, deletion of miR-17-92 would result in increased Fas expression and an increased sensitivity to FasL-mediated apoptosis, which may explain the premature cell death in these B-cells.
In summary, based on the data presented here, we conclude that Fas expression in osteosarcoma cells is regulated by a transcriptional or post-transcriptional mechanism that involves the down-regulation of cell surface Fas expression by miRNA. We have demonstrated increased expression of several miRNAs encoded by the miR-17-92 cluster and that, specifically, miR-20a expression inversely correlates with cell surface Fas expression in osteosarcoma cell lines and patient specimens. Higher miR-20a expression was also correlated with increased metastatic potential. The down-regulation of Fas allows osteosarcoma cells to circumvent FasL-mediated apoptosis upon entrance into the lung. By down-regulating Fas, miR-17-92 and its miR-20a component contribute to the metastatic potential of osteosarcoma cells by allowing them to evade the clearance mechanism in the lung mediated by the constitutive FasL. These studies identify a new target of an miRNA component of the miR-17-92 cluster and are the first to correlate the expression of miR-17-92 with a metastatic phenotype.
Acknowledgments
We thank Dr. Deepa Sampath for expert advice on this project and Dr. Michaela Scherr for supplying antagomir-plasmids.
Financial support: This work was supported by National Cancer Institute grant R01 CA 42992 (to E.S.K.) and CA 16672 Cancer Center Support (Core) Grant.
Footnotes
Disclosure of Potential Conflicts of Interest: No conflicts of interest exist.
References
- 1.Lafleur EA, Koshkina NV, Stewart J, Jia SF, Worth LL, Duan X, et al. Increased Fas expression reduces the metastatic potential of human osteosarcoma cells. Clin Cancer Res. 2004;10:8114–9. doi: 10.1158/1078-0432.CCR-04-0353. [DOI] [PubMed] [Google Scholar]
- 2.Worth LL, Lafleur EA, Jia SF, Kleinerman ES. Fas expression inversely correlates with metastatic potential in osteosarcoma cells. Oncol Rep. 2002;9:823–7. [PubMed] [Google Scholar]
- 3.Gordon N, Koshkina NV, Jia SF, Khanna C, Mendoza A, Worth LL, et al. Corruption of the Fas pathway delays the pulmonary clearance of murine osteosarcoma cells, enhances their metastatic potential, and reduces the effect of aerosol gemcitabine. Clin Cancer Res. 2007;13:4503–10. doi: 10.1158/1078-0432.CCR-07-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koshkina NV, Khanna C, Mendoza A, Guan H, DeLauter L, Kleinerman ES. Fas-negative osteosarcoma tumor cells are selected during metastasis to the lungs: the role of the Fas pathway in the metastatic process of osteosarcoma. Mol Cancer Res. 2007;5:991–9. doi: 10.1158/1541-7786.MCR-07-0007. [DOI] [PubMed] [Google Scholar]
- 5.Gordon N, Arndt CA, Hawkins DS, Doherty DK, Inwards CY, Munsell MF, et al. Fas expression in lung metastasis from osteosarcoma patients. J Pediatr Hematol Oncol. 2005;27:611–5. doi: 10.1097/01.mph.0000188112.42576.df. [DOI] [PubMed] [Google Scholar]
- 6.Jia SF, Worth LL, Kleinerman ES. A nude mouse model of human osteosarcoma lung metastases for evaluating new therapeutic strategies. Clin Exp Metastasis. 1999;17:501–6. doi: 10.1023/a:1006623001465. [DOI] [PubMed] [Google Scholar]
- 7.Khanna C, Prehn J, Yeung C, Caylor J, Tsokos M, Helman L. An orthotopic model of murine osteosarcoma with clonally related variants differing in pulmonary metastatic potential. Clin Exp Metastasis. 2000;18:261–71. doi: 10.1023/a:1006767007547. [DOI] [PubMed] [Google Scholar]
- 8.Koshkina NV, Kleinerman ES. Aerosol gemcitabine inhibits the growth of primary osteosarcoma and osteosarcoma lung metastases. Int J Cancer. 2005;116:458–63. doi: 10.1002/ijc.21011. [DOI] [PubMed] [Google Scholar]
- 9.Duan X, Jia SF, Koshkina N, Kleinerman ES. Intranasal interleukin-12 gene therapy enhanced the activity of ifosfamide against osteosarcoma lung metastases. Cancer. 2006;106:1382–8. doi: 10.1002/cncr.21744. [DOI] [PubMed] [Google Scholar]
- 10.Koshkina NV, Kleinerman ES, Waidrep C, Jia SF, Worth LL, Gilbert BE, et al. 9-Nitrocamptothecin liposome aerosol treatment of melanoma and osteosarcoma lung metastases in mice. Clin Cancer Res. 2000;6:2876–80. [PubMed] [Google Scholar]
- 11.Huang G, Koshkina NV, Kleinerman ES. Fas expression in metastatic osteosarcoma cells is not regulated by CpG island methylation. Oncol Res. 2009;18:31–9. doi: 10.3727/096504009789745638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 13.Shivdasani RA. MicroRNAs: regulators of gene expression and cell differentiation. Blood. 2006;108:3646–53. doi: 10.1182/blood-2006-01-030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082–9. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 15.Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I, et al. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272–86. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Inomata M, Tagawa H, Guo YM, Kameoka Y, Takahashi N, Sawada K. MicroRNA-17-92 down-regulates expression of distinct targets in different B-cell lymphoma subtypes. Blood. 2009;113:396–402. doi: 10.1182/blood-2008-07-163907. [DOI] [PubMed] [Google Scholar]
- 17.Ovcharenko D, Kelnar K, Johnson C, Leng N, Brown D. Genome-scale microRNA and small interfering RNA screens identify small RNA modulators of TRAIL-induced apoptosis pathway. Cancer Res. 2007;67:10782–8. doi: 10.1158/0008-5472.CAN-07-1484. [DOI] [PubMed] [Google Scholar]
- 18.Papagiannakopoulos T, Shapiro A, Kosik KS. MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res. 2008;68:8164–72. doi: 10.1158/0008-5472.CAN-08-1305. [DOI] [PubMed] [Google Scholar]
- 19.Schickel R, Park SM, Murmann AE, Peter ME. miR-200c regulates induction of apoptosis through CD95 by targeting FAP-1. Mol Cell. 38:908–15. doi: 10.1016/j.molcel.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki Y, Kim HW, Ashraf M, Haider H. Diazoxide potentiates mesenchymal stem cell survival via NF-kappaB-dependent miR-146a expression by targeting Fas. Am J Physiol Heart Circ Physiol. 299:H1077–82. doi: 10.1152/ajpheart.00212.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–43. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 22.Scherr M, Venturini L, Battmer K, Schaller-Schoenitz M, Schaefer D, Dallmann I, et al. Lentivirus-mediated antagomir expression for specific inhibition of miRNA function. Nucleic Acids Res. 2007;35:e149. doi: 10.1093/nar/gkm971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Z, Lafleur EA, Koshkina NV, Worth LL, Lester MS, Kleinerman ES. Interleukin-12 up-regulates Fas expression in human osteosarcoma and Ewing’s sarcoma cells by enhancing its promoter activity. Mol Cancer Res. 2005;3:685–91. doi: 10.1158/1541-7786.MCR-05-0092. [DOI] [PubMed] [Google Scholar]
- 24.Huang G, Mills L, Worth LL. Expression of human glutathione S-transferase P1 mediates the chemosensitivity of osteosarcoma cells. Mol Cancer Ther. 2007;6:1610–9. doi: 10.1158/1535-7163.MCT-06-0580. [DOI] [PubMed] [Google Scholar]
- 25.Jia SF, Worth LL, Turan M, Duan Xp XP, Kleinerman ES. Eradication of osteosarcoma lung metastasis using intranasal gemcitabine. Anticancer Drugs. 2002;13:155–61. doi: 10.1097/00001813-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Koshkina NV, Rao-Bindal K, Kleinerman ES. Effect of the histone deacetylase inhibitor SNDX-275 on Fas signaling in osteosarcoma cells and the feasibility of its topical application for the treatment of osteosarcoma lung metastases. Cancer. 117:3457–67. doi: 10.1002/cncr.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ota A, Tagawa H, Karnan S, Tsuzuki S, Karpas A, Kira S, et al. Identification and characterization of a novel gene, C13orf25, as a target for 13q31–q32 amplification in malignant lymphoma. Cancer Res. 2004;64:3087–95. doi: 10.1158/0008-5472.can-03-3773. [DOI] [PubMed] [Google Scholar]
- 28.Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, et al. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–32. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 29.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–33. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–86. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38:1060–5. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong L, Lai M, Chen M, Xie C, Liao R, Kang YJ, et al. The miR-17-92 cluster of microRNAs confers tumorigenicity by inhibiting oncogene-induced senescence. Cancer Res. 70:8547–57. doi: 10.1158/0008-5472.CAN-10-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peter ME, Budd RC, Desbarats J, Hedrick SM, Hueber AO, Newell MK, et al. The CD95 receptor: apoptosis revisited. Cell. 2007;129:447–50. doi: 10.1016/j.cell.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Park SM, Tumanov AV, Hau A, Sawada K, Feig C, et al. CD95 promotes tumour growth. Nature. 2010;465:492–6. doi: 10.1038/nature09075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green DR, Ferguson TA. The role of Fas ligand in immune privilege. Nat Rev Mol Cell Biol. 2001;2:917–24. doi: 10.1038/35103104. [DOI] [PubMed] [Google Scholar]
- 36.Duan X, Zhou Z, Jia SF, Colvin M, Lafleur EA, Kleinerman ES. Interleukin-12 enhances the sensitivity of human osteosarcoma cells to 4-hydroperoxycyclophosphamide by a mechanism involving the Fas/Fas-ligand pathway. Clin Cancer Res. 2004;10:777–83. doi: 10.1158/1078-0432.ccr-1245-02. [DOI] [PubMed] [Google Scholar]
- 37.Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9:405–14. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]