Abstract
Prox1, a transcription factor important in regulation and maintenance of lymphatic endothelial phenotype, is consistently expressed in lymphangiomas and Kaposi sarcoma and has also been reported in Kaposiform hemangioendothelioma. However, information on its distribution of vascular tumors, such as angiosarcoma, is limited. In this study we examined selected normal tissues and 314 vascular endothelial and 1086 non-vascular tumors in order to get insight into biology of these tumors and on potential diagnostic use of Prox1 as an immunohistochemical marker. In adult tissues, Prox1 was essentially restricted to lymphatic endothelia, with expression in subsets of pancreatic and gastrointestinal epithelia. However, it was also detected in embryonic liver and heart. Prox1 was consistently expressed in lymphangiomas, venous hemangiomas, Kaposi sarcoma, and endothelia of spindle cell hemangioma, kaposiform hemangioendothelioma, and retiform hemangioendothelioma and half of epithelioid hemangioendotheliomas. It was present in most cutaneous angiosarcomas from different sites, but was less commonly expressed in deep soft tissue and visceral angiosarcomas. In contrast, Prox1 was generally absent in capillary and cavernous hemangiomas. In positive hemangiomas and angiosarcomas it was coexpressed with podoplanin, another marker of lymphatic endothelial phenotype. There was an inverse correlation with CD34 expression. The expression in mesenchymal non-endothelial neoplasm was limited. Prox1 was detected in 5/27 synovial sarcomas, specifically in the epithelia of biphasic tumors. 4/16 Ewing sarcomas and 5/15 paragangliomas were also positive. All melanomas and undifferentiated sarcomas were negative. Among epithelial neoplasms, Prox1 was detected in 18/38 colonic carcinomas and 10/15 cholangiocarcinomas, and in a minority of pulmonary, prostatic, and endometrial adenocarcinomas. The common Prox1 expression in angiosarcoma and its rare presence in non-vascular mesenchymal tumors make this marker suitable in the diagnosis of angiosarcoma and Kaposi sarcoma. However, presence of Prox1 in some malignant epithelial tumors necessitates caution in applying Prox1 as a marker for vascular tumors. Common Prox1-expression in angiosarcoma may reflect lymphatic endothelial phenotype in these tumors. Its patterns of expression in hemangiomas and angiosarcoma may be diagnostically useful and offer a new parameter in biologic classification of vascular tumors.
Keywords: hemangioma, hemangioendothelioma, lymphangioma, angiosarcoma, Prox1, podoplanin, CD34
INTRODUCTION
Classification of vascular endothelial tumors is often problematic and sometimes controversial. This includes subclassification of benign angiomas, as well as determination whether a vascular tumor is benign or malignant. Also, precise identification of angiosarcoma and differentiating it from other malignant tumors can be difficult.
Prox1 (prospero homeobox 1 protein) is an endothelial transcription factor considered a master regulator of lymphatic endothelial differentiation and expressed in the nuclei of the developing and adult lymphatic endothelial cells. 9,10,12,21,30,31 In addition, Prox1 is involved in development of heart, liver, and pancreas. 3,5,6,26
Only limited information of Prox1 expression in vascular tumors exists to date. Kaposi sarcoma expresses Prox1 as a manifestation of lymphatic reprogramming of the neoplastic HHV8-infected endothelia. 11 Kaposiform hemangioendothelioma was recently shown to contain Prox1-positive endothelia, and Prox1 was also implicated in the pathogenesis of its invasive nature. 4,16 One study showed presence of Prox1 in lymphangiomas and general absence in hemangiomas 31, and another one noted its absence in juvenile hemangiomas and lobular capillary hemangiomas. 16 There is no specific information of Prox1 in angiosarcomas.
In this study we evaluated Prox1 expression patterns in 314 vascular tumors to assess its potential of Prox1 as a diagnostic marker. We also correlated it with another lymphatic endothelial marker, podoplanin. 2,13,14 Also examined was CD34, a vascular endothelial marker 27, to obtain an insight into biology of vascular tumors. We also examined 1086 non-vascular tumors to further evaluate specificity of Prox1 for endothelial neoplasms.
MATERIALS AND METHODS
Tissues
Selected normal developing and adult tissues and 314 vascular endothelial tumors were evaluated. Also studied for comparison were 528 non-endothelial mesenchymal tumors, and 558 malignant epithelial neoplasms. Most of these tissues were arranged in multi-tissue blocks containing 5–50 cases. These blocks were generated manually by embedding rectangular tissue pieces into a single block. In the cut slides, these pieces generally yielded tissue profiles measuring 1–2 × 2–4 mm being thus larger than the round tissue profiles of 0.6–1 mm in tumor microarray (TMA) samples and giving more tissue for analysis. A small number of cases were examined using conventional histologic slides (1 case per slide). All tissues were derived from surgical specimens.
All angiosarcomas were verified as CD31-positive and Kaposi sarcomas as HHV8-positive. Previous data on CD31 was available on most hemangioendotheliomas and hemangiomas showing positivity in the neoplastic/lesional endothelial components.
Immunohistochemistry
A Prox1 polyclonal rabbit antibody from Angiobio (Delmar, CA) was used in a dilution of 1:250. Immunostaining was performed in a Leica Bond-Max automated immunostainer (Leica Microsystems, Bannockburn, IL). Heat-induced epitope retrieval was performed for 30 min using a Bond-Max high-pH epitope retrieval buffer. Primary antibody was applied for 30 minutes, followed by Bond-Max polymer for 15 min. Diaminobenzidine was used as the chromogen, followed by a light hematoxylin counterstain.
The percentage of positive tumor cell nuclei was estimated. Because our preliminary experience showed reduced Prox1-immunoreactivity in old stored slides, the studies were, with few exceptions, done on freshly cut slides.
Only nuclear staining was considered positive. Isolated randomly, often weakly, labeled nuclei (generally <1 %) were not counted as positive, because similar labeling was occasionally seen in negative controls with no primary antibody. The presence of internal control (positive lymphatic endothelia) was considered to validate the immunostaining in any particular tissue. However, this was not universally required as many tissues lacked lymphatics.
Immunostaining for CD34 (QBEND/10 1:150, Leica Microsystems) and podoplanin (D2-40, 1:100, Covance, Princeton, NJ) were performed on vascular endothelial tumors to obtain additional objective parameters of endothelial differentiation for comparison. These studies were performed using similar technology as described above, with the exceptions that a low pH epitope retrieval buffer was used for podoplanin, and epitope retrieval time for both markers was 20 minutes. Each marker was evaluated independently without knowledge of other results.
RESULTS
The results on Prox1-immunoreactivity in 314 vascular endothelial cell tumors, 528 non-endothelial mesenchymal and lymphohematopoietic tumors and 558 malignant epithelial neoplasms, are summarized in Tables 1–3.
Table 1.
Expression of Prox1 in 314 vascular endothelial tumors.
| Tumor type | Positive/total | % positive |
|---|---|---|
| Hemangioma variants | 30/71 | 42 |
| Capillary hemangioma, juvenile | 0/10 | 0 |
| Capillary hemangioma, lobular | 0/7 | 0 |
| Capillary hemangioma, NOS | 3/13 | 23 |
| Cavernous hemangioma | 3/15 | 20 |
| Spindle cell hemangioma | 16/17 | 94 |
| Venous hemangioma | 8/9 | 89 |
| Papillary endothelial hyperplasia | 0/3 | 0 |
| Lymphangioma | 9/9 | 100 |
| Lymphangioendothelioma | 1/1 | 100 |
| Hemangioendotheliomas | 46/81 | 56 |
| Epithelioid hemangioendothelioma | 29/62 | 47 |
| Epithelioid sarcoma-like hemangioendothelioma | 0/1 | 0 |
| Papillary intralymphatic hemangioendothelioma (Dabska tumor) | 1/1 | 100 |
| Kaposiform hemangioendothelioma | 5/5 | 100 |
| Retiform hemangioendothelioma | 11/12 | 92 |
| Angiosarcoma | 53/105 | 48 |
| Scalp and face | 20/23 | 86 |
| Other cutaneous (non-radiation associated) | 3/5 | 75 |
| Deep soft tissue | 4/11 | 36 |
| Radiation associated, chest wall and breast | 3/5 | 60 |
| Postmastectomy angiosarcoma (Stewart-Treves) | 1/2 | 50 |
| Splenic | 1/4 | 25 |
| Visceral/gastrointestinal | 4/13 | 33 |
| Body cavity-associated | 6/11 | 55 |
| Cardiac and pericardial | 3/12 | 20 |
| Other (includes metastases) | 8/19 | 40 |
| Kaposi sarcoma | 43/44 | 98 |
| Total for vascular tumors | 182/314 | 58 |
Table 3.
Prox1 expression in malignant epithelial neoplasms.
| Tumor type | Positive/total | % positive |
|---|---|---|
| Breast, ductal carcinoma | 1/49 | 2 |
| Breast, lobular carcinoma | 0/5 | |
| Larynx, squamous cell carcinoma | 0/34 | |
| Lung, adenocarcinoma | 4/31 | 13 |
| Lung, squamous cell carcinoma | 0/15 | |
| Lung, small cell carcinoma | 5/12 | 42 |
| Malignant mesothelioma | 0/22 | |
| Liver, hepatocellular carcinoma | 4/25 | 16 |
| Liver, cholangiocarcinoma | 10/15 | 67 |
| Pancreas, adenocarcinoma | 0/20 | |
| Pancreas, islet cell tumor | 2/5 | 40 |
| Esophagus, squamous cell carcinoma | 0/26 | |
| Renal cell carcinoma | 0/25 | |
| Small intestine, sarcomatoid carcinoma | 0/12 | |
| Stomach, glandular adenocarcinoma | 3/38 | 8 |
| Stomach, signet ring cell carcinoma | 0/5 | |
| Colon, adenocarcinoma | 18/38 | 47 |
| Prostate, adenocarcinoma | 2/51 | 4 |
| Testis, seminoma | 0/25 | |
| Testis, embryonal carcinoma | 3/10 | 30 |
| Uterine cervix, squamous cell carcinoma | 0/27 | |
| Endometirium, adenocarcinoma | 2/16 | 13 |
| Ovary, adenocarcinoma | 1/33 | 3 |
| Thyroid, papillary carcinoma | 0/19 | |
| Total | 55/558 | 10 |
Developing human tissues
In early 1st trimester embryo, Prox1-immunoreactivity was detected in a large thin-walled body cavity-associated vascular structure consistent with the thoracic duct (Fig. 1A). Vascular positivity was also present in a heart-associated great vessel, but not in other truncal or placental vessels. No vessels were distinguishable in the peripheral mesenchyme. However, isolated Prox1-positive nuclei were detected in the latter. Hepatocytes were moderately positive, and nuclear positivity was present in cardiac muscle (Fig. 1B). Yolk sac epithelia also showed nuclear positivity (Fig. 1C). Truncal paraspinal neural tissue, and pulmonary and intestinal primordia (identified based on the content of TTF1 and CDX2-positive epithelia) were negative.
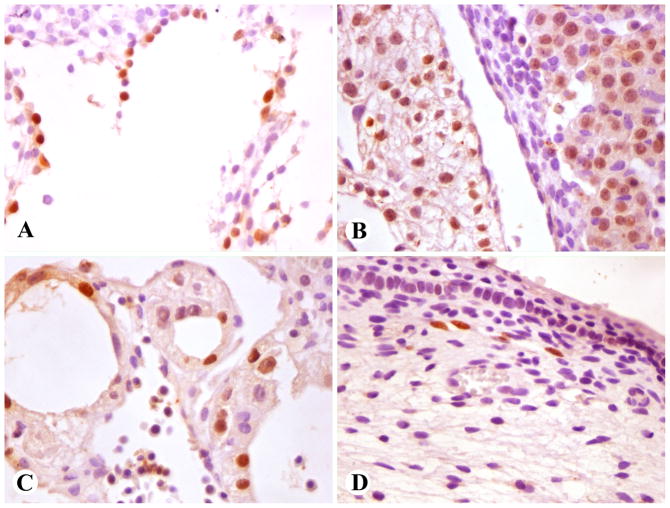
Fig. 1.
Prox1-positive developing tissues (nuclear positivity). A–C: Early first trimester embryo. D. Late first trimester fetus. A. A large vessel with flaccid contours, consistent with thoracic duct. B. Cardiac myocytes (left) and hepatocytes (right). C. Yolk sac epithelia. D. Subdermal lymphatic vessels in a limb.
In a late 1st trimester fetus, Prox1-positive small caliber vessels with attenuated endothelial cells, consistent with lymphatic vessels, were present in soft tissues of limbs (Fig. 1D). No immunoreactivity was detected in liver and kidney (heart unavailable).
Non-neoplastic adult tissues
Lymphatic vascular endothelia in various tissues: skin, subcutaneous and omental fat, tonsil, periphery of lymph nodes, parotid gland periductally, gastrointestinal mucosae, urinary bladder submucosa, and uterine cervix, were highlighted as Prox1-positive. Capillary and larger vessel endothelia, neovascular endothelia of carcinomas, and pericytes were negative.
Other Prox1-positive elements included scattered epithelial cells in gastric mucosa, small numbers of epithelial cells on the intestinal crypt bottom, and pancreatic parenchymal cells. Regenerative bile ducts adjacent to hepatocellular carcinoma were sometimes positive. However, Prox1 was not detected in adult bile ducts or hepatocytes. Hypertrophic cardiac myocyte nuclei in an explanted heart were variably positive.
Lymphoid tissue in lymph nodes and tonsil, squamous cell epithelia, respiratory and glandular epithelia in the nose and gastrointestinal tract, urothelia, renal epithelia, hepatocytes, adipose tissue, vascular and visceral smooth muscle, and skeletal muscle were negative.
Benign vascular endothelial tumors
All lymphangiomas including examples from the skin and subcutis, abdomen, and a cystic lymphangioma from the neck, showed extensive Prox1-positivity in the lining endothelia (Fig. 2A), which were also positive for podoplanin. A cutaneous lymphangioendothelioma showed focal positivity in 15% of tumor endothelia.
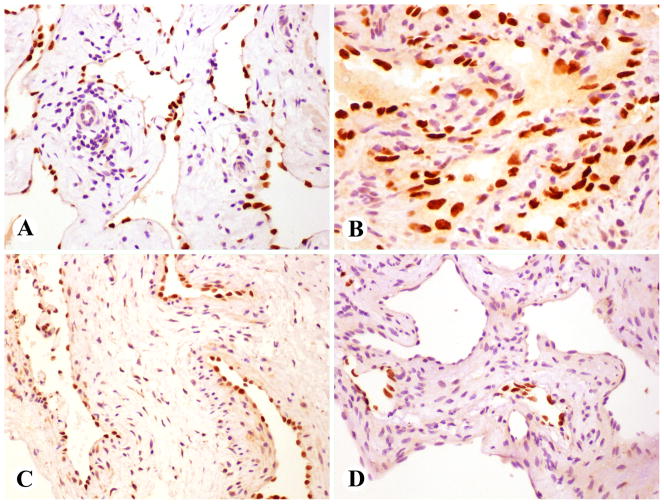
Fig 2.
Prox1 in benign angiomas. A. Endothelial cells of abdominal lymphangioma are Prox1-positive. B. Spindle cell hemangioma endothelia are strongly Prox1-positive, but the interstitial spindle cells are negative. C. Venous hemangioma vessels with irregular muscular walls show endothelial Prox1-positivity. D. Endothelia of hepatic cavernous hemangioma vascular spaces are Prox1-negative, but lymphatics in vessel walls have Prox1-positive endothelia.
Among hemangiomas, most consistent positivity was seen in the endothelia of spindle cell hemangiomas (16/17 cases), whereas the interstitial spindle cells were negative (Fig. 2B). All but 2 of 10 spindle cell hemangiomas also had podoplanin-positive endothelia, and the endothelia were CD34-positive in all cases.
A majority of large caliber vessels of venous hemangioma with irregular smooth muscle cells (6 of 8), were lined by Prox1-positive endothelial cells Fig. 2C). These cases also had CD34-positive endothelia, whereas only 3 of 7 were podoplanin-positive.
While most capillary hemangiomas, other than juvenile and lobular capillary ones, were Prox1-negative, 2 cases showed variable Prox1-positive in the lining endothelia. While all these capillary hemangiomas were CD34-positive, both Prox-1 positive ones were also positive for podoplanin, which also labeled one Prox1-negative example.
Cavernous hemangiomas were generally Prox1-negative. However, in some hepatic examples, Prox1- and podoplanin-positive lymphatic vessels were present within the septa of the cavernous spaces (Fig. 2D). In addition, two cavernous hemangiomas contained 10% and 60% of Prox1-positive endothelia, and one of these also had podoplanin positivity, which was detected in an additional, Prox1-negative case.
All juvenile and lobular capillary hemangiomas were Prox1-negative, with the exception of occasional small caliber lymphatic vessels with positive endothelia.
Hemangioendotheliomas
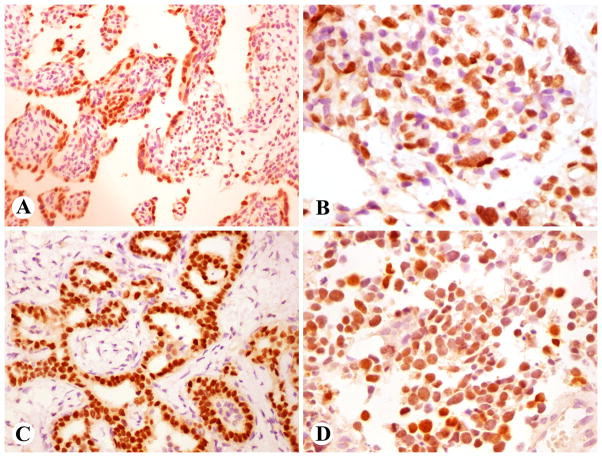
Dabska type angioendothelioma (papillary intralymphatic angioendothelioma) contained Prox1-positive endothelia in the vascular pseudorosette formations (Fig. 3A). Retiform hemangioendotheliomas were almost always strongly Prox1-positive (Fig. 3B). All but two of these cases were also positive for podoplanin. CD34 was detected in 4 of 8 cases studied.
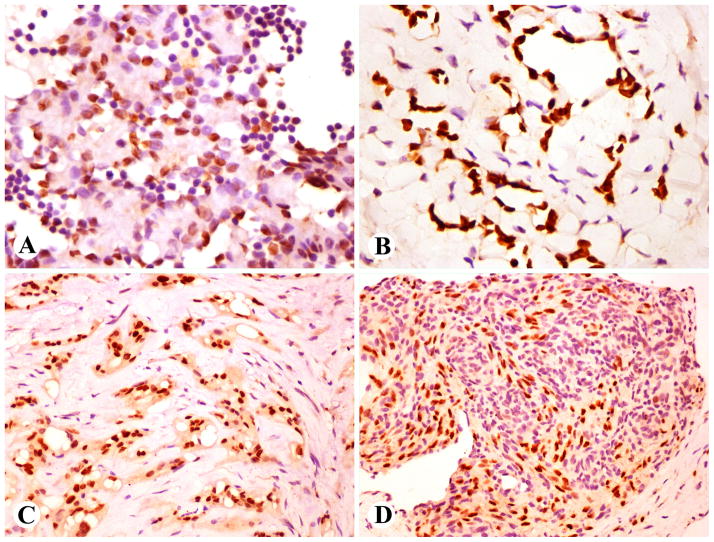
Fig 3.
Prox1 in hemangioendotheliomas. A. Endothelial component in the pseudorosettes of Dabska agngioendothelioma is Prox1-positive. B. Retiform hemangioendotheliomas are almost invariably Prox1-positive. C. Cords of epithelioid hemangioendothelioma are Prox1-positive. D. The endothelial component in Kaposiform hemangioendothelioma is Prox1 positive and the pericytic component is negative.
Epithelioid hemangioendotheliomas (almost all from peripheral soft tissue) were heterogenous: almost half of them (29/62) contained Prox1-positive neoplastic cells with the majority of tumor cells being labeled in the positive cases (Fig. 3C). There was a positive correlation between Prox1- and podoplanin status. Of the Prox1-positive cases 11 of 15 (73%) were also podoplanin-positive, whereas only 3 of 28 (11%) of Prox1-negative cases were podoplanin-positive. However, CD34 positivity was approximately similar in both Prox1-positive and negative cases: 6 of 14 (43%) and 13/24 (54%), respectively. One epithelioid sarcoma-like hemangioendothelioma was Prox1 negative.
Kaposiform hemangioendotheliomas contained a prominent Prox1-positive endothelial component, whereas the spindle cells corresponding with the pericytic population were negative (Fig. 3D). The endothelial component further examined in 3 cases was also positive for both CD34 and podoplanin.
Angiosarcomas and Kaposi sarcoma
The results are summarized in Table 1. Angiosarcomas showed variable Prox1 nuclear labeling, and overall 53 of 105 cases (50%) were positive. All but 4 cases had a fraction of 20% or more tumor cells positive. A great majority of cutaneous angiosarcomas from the head and neck and extremities were positive (23/28, 82%), as were the majority of radiation-induced angiosarcomas of chest wall and breast. Less commonly positive were angiosarcomas of deep tissue and viscera. Prox1 was equally detected in vasoformative and poorly differentiated solid angiosarcomas. As noted with other transcription factors, mitotic nuclei were generally negative (Fig. 4A–C). Of the epithelioid angiosarcomas, 7 of 12 (58%) were Prox1-positive.
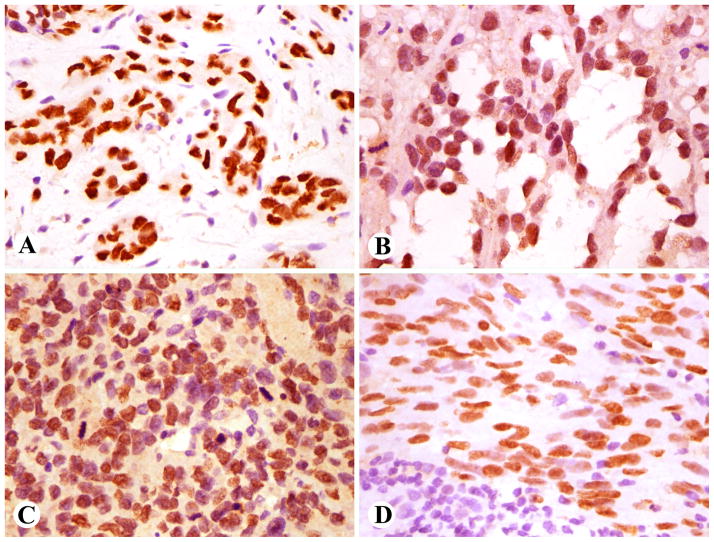
Fig. 4.
Prox1 positive angiosarcoma and Kaposi sarcoma. A. Vasoformative cutaneous angiosarcoma. B. Post-radiation angiosarcoma involving mediastinum. C. Solid poorly differentiated angiosarcoma involving skeletal muscle. Note that mitotic nuclei are negative. D. Cutaneous Kaposi sarcoma cells are positive but lymphoid cells negative.
There was an inverse correlation between Prox1-positivity and CD34-status: Of Prox1-positive angiosarcomas 20 of 46 (43%) were CD34-positive, whereas Prox1-negative angiosarcomas were nearly twice as often CD34-positive: 39 of 52 (75%).
There was a positive correlation between Prox1 and podoplanin-immunoreactivity. Most Prox1-positive angiosarcomas were also positive for podoplanin (43 of 52, 83%), whereas only few Prox1-negative angiosarcomas were positive (11 of 56, 20%). However, due to extensive non-endothelial labeling of podoplanin in submesothelial and stromal elements, some angiosarcomas were uninterpretable for podoplanin. Compared with Prox1, podoplanin identified a greater percentage of tumor cells in some angiosarcomas; only 1 positive case showed <50% of tumor cells positive, and overall 55 angiosarcomas were podoplanin-positive. All but one Kaposi sarcomas were Prox1-positive (Fig 4D).
Non-endothelial mesenchymal and hematopoietic tumors
All non-endothelial vascular tumors including soft tissue hemangiopericytomas, glomus tumors and cerebellar hemangioblastomas were negative for Prox1 (Table 2).
Table 2.
Prox 1 expression in non-endothelial mesenchymal tumors.
| Tumor type | Positive/total | % positive |
|---|---|---|
| Angiomatoid FH | 0/14 | |
| Cardiac myxoma | 0/20 | |
| Chordoma | 0/12 | |
| Clear cell sarcoma | 0/5 | |
| Dendritic reticulum cell sarcoma | 0/1 | |
| Epithelioid sarcoma | 0/17 | |
| Ewing sarcoma | 4/16 | 25 |
| Gastrointestinal stromal tumor, gastric | 0/59 | |
| Gastrointestinal stromal tumor, intestinal | 0/23 | |
| Glomus tumor | 0/14 | |
| Hemangioblastoma of cerebellum | 0/3 | |
| Leiomyosarcoma | 0/21 | |
| Lymphoma, small B-cell | 0/32 | |
| Lymphoma, large B-cell | 0/41 | |
| Extramedullary myeloid tumor, blastic | 0/10 | |
| Malignant peripheral nerve sheath tumor | 0/16 | |
| Meningioma | 0/30 | |
| Metastatic melanoma | 0/38 | |
| Nodular fasciitis | 0/29 | |
| Paraganglioma, retroperitoneal | 5/15 | 33 |
| Schwannoma | 0/15 | |
| Solitary fibrous tumor/hemangiopericytoma | 0/35 | |
| Synovial sarcoma | 5/27 | 19 |
| Undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma | 0/26 | |
| Unclassified spindle cell sarcoma | 0/9 | |
| Total | 14/528 | 2.6 |
Among mesenchymal non-endothelial tumors, Prox1-positivity was detected in the epithelia of some biphasic synovial sarcomas (Fig. 5A), whereas monophasic tumors were negative. Four of 16 Ewing sarcomas and 5 of 15 retroperitoneal paragangliomas also contained Prox1-positive tumor cells (Fig. 5B). Prox1 was not detected in any undifferentiated sarcomas, chordomas, gastrointestinal stromal tumors, leiomyosarcomas, malignant peripheral nerve sheath tumors, melanomas, and small and large cell B-cell lymphomas.
Fig. 5.
Prox1 in mesenchymal and epithelial neoplasms. A. Biphasic synovial sarcoma epithelium is positive. B. Prox1 labeling in paraganglioma cells. C. Cholangio-carcinoma with strong Prox1-immunoreactivity. D. Nuclear Prox1 positivity in small cell carcinoma of the lung.
Malignant epithelial neoplasms
Prox1-positive tumor cells were detected in 55/558 carcinomas of various types (Table 3). Most commonly positive were intrahepatic cholangiocarcinoma (10/15) (Fig. 5C), colorectal carcinoma (18/38), and pulmonary small cell carcinoma (5/12) (Fig. 5D), each of which typically showed heterogenous positivity. Less commonly Prox1-positivity was detected in hepatocellular carcinoma (4 of 25), pulmonary adenocarcinoma (4 of 31), prostate carcinoma (2/51), and ductal carcinoma of the breast (1 of 49). No Prox1-positive tumor cells were detected in papillary thyroid carcinomas, squamous cell carcinomas, pancreatic adenocarcinomas, renal cell carcinomas, and seminomas.
DISCUSSION
In this study we immunohistochemically examined the expression of Prox1 protein, encoded by the corresponding PROX1 (prospero homeobox 1) gene in a broad range of tumors, especially the vascular ones. Prox1 is known to be expressed in lymphatic endothelium and is important in the programming and maintenance its differentiation. 9,10,30,31 Its haploinsufficiency promotes lymphatic vascular defects and is associated with adult-onset obesity. 8 Prox1 is also expressed in some epithelial tissues, notably liver and pancreas, especially during development.5,6 In this study, we were able to replicate previously known patterns of Prox1 expression in normal tissues, indicating that sufficient proficiency of Prox1 detection can be obtained in routinely-processed formalin-fixed and paraffin embedded tissue with the antibody used.
Prox1 is consistently expressed in lymphatic endothelium and lymphangiomas, in agreement with previous observations. 31 In general, Prox1 is not present neither in juvenile nor adult capillary or cavernous hemangiomas, and its expression correlates fairly well with that of podoplanin. Previous studies have also demonstrated lack of LYVE-1, another lymphatic endothelial marker, in juvenile hemangiomas.20
However, a small subset of capillary and cavernous hemangiomas is Prox1-positive. Venous hemangioma consisting of larger vascular profiles with irregular smooth muscle lining, often shows endothelial Prox1 expression. This could possibly be related to the observation that subsets of lymphatics originate from veins. 31 Further studies are needed to determine whether there is any clinical correlation to this observation. It remains to be determined whether Prox1 contributes to clinicopathologic distinction of hemangioma vs. vascular malformation, a prominent trend in pediatric surgery and pathology.17 In this study, lack of clinical correlation did not allow further examination of this issue.
Spindle cell hemangioma is an unusual hemangioma variant usually occurring in peripheral extremity sites, sometimes associated with vascular syndromes, such as Klipper-Trenaunau syndrome and Maffucci syndrome.24 In this study we demonstrated consistent Prox1-expression in endothelia, paralleling podoplanin expression and indicating a lymphatic endothelial-like phenotype.
Retiform hemangioendothelioma, a low-grade cutaneous-subcutaneous vascular tumor was consistently positive for Prox1. These tumors also expressed podoplanin, another lymphoid vascular determinant, indicating lymphatic-like vascular differentiation. Podoplanin was previously reported in retiform hemangioendothelioma.18 Similarly, Prox1 is also expressed in papillary intralymphatic angioendothelioma (Dabska tumor), previously known to express vascular endothelial growth factor 3, another lymphatic vascular determinant. 7
Epithelioid hemangioendotheliomas (EHEs) were heterogeneous for Prox1 expression, with approximately half of them being positive. The positive cases were typically also positive for podoplanin, supporting lymphatic endothelial-like differentiation. It remains to be seen whether clinicopathologic studies reveal any correlation with the Prox1-status in these tumors.
Angiosarcomas commonly express Prox1, and this is especially true for cutaneous angiosarcomas. Great majority of angiosarcomas of the scalp and face and peripheral cutaneous sites were positive, along with a majority of radiation-associated cutaneous angiosarcomas of the chest wall and breast. Prox1-positivity correlated strongly with podoplanin-positivity, supporting lymphatic vessel-like phenotypic features, as also previously shown based on podoplanin 13 and vascular endothelial growth factor receptor 3 expression 23 in a subset of angiosarcomas. The common expression of Prox1 indicates that this marker is useful in the identification of angiosarcomas. However, Prox1 does not separate benign and malignant vascular tumors, as subsets of both can be positive.
Kaposi sarcomas are also consistently Prox1 positive, and therefore additional markers, such as HHV8, and observation of morphologic differences are necessary to separate it from angiosarcoma. Prox1 expression in Kaposi sarcoma is also paralleled by expression of other lymphatic endothelial markers, such as podoplanin and vascular endothelial growth factor receptor 3. 29
The relative rarity of Prox1 expression in mesenchymal mimics of angiosarcoma, such as metastatic melanoma, undifferentiated sarcomas, and sarcomatoid carcinomas, makes Prox1 a fairly specific endothelial marker. However, Prox1 expression in synovial sarcoma, Ewing sarcoma, paraganglioma, and a significant subset of carcinomas, especially cholangiocarcinoma, intestinal carcinomas, and neuroendocrine carcinomas and to a lesser degree, other carcinomas, has to be considered in the differential diagnosis.
The expression of Prox1 in cholangiocarcinomas, hepatocellular carcinomas, and pancreatic endocrine tumors seems to parallel the Prox1-expression in developing liver and pancreas indicating oncofetal-like expression. Further studies are necessary to determine whether the Prox-1-positive carcinomas of GI-tract and liver and elsewhere are clinicopathologically distinctive. The same applies to Prox1-positive subsets of paragangliomas, Ewing sarcomas, and synovial sarcomas. The biologic role of Prox1 in carcinomas seems to be complex. Two studies indicate that Prox1 may be a tumor suppressor protein specifically lost in breast cancer and cholangiocarcinoma progression by genetic or epigenetic mechanisms. 15,28 On the other hand, Prox1 has been also identified as an oncogenic factor related to progression of colon cancer. 25
Compared with pan-endothelial markers CD31, ERG, and claudin-5 Prox1 is less sensitive in comprehensive identification of angiosarcomas, due to its restricted reactivity with lymphatic-like endothelial phenotype.19,20 However, compared with podoplanin, another marker of the lymphatic endothelial phenotype, Prox1 expression is sometimes easier to interpret, as podoplanin immunoreacitivity is often additionally present in non-endothelial components, such as mesothelial, submesothelial and some non-endothelial mesenchymal cells. 14 However, Prox1 may be slightly less sensitive than podoplanin, as it identifies a smaller number of tumors and often also a smaller fraction of cells in vascular tumors, especially angiosarcomas.
In conclusion, we examined Prox1 expression in a large number of vascular endothelial and non-endothelial neoplasms. Prox1 is consistently present in lymphangiomas, spindle cell and venous hemangiomas, retiform and kaposiform hemangioendothelioma, and Kaposi sarcoma. In addition, it is expressed in a majority of cutaneous angiosarcomas while being less commonly expressed in visceral angiosarcomas, and in a half of epithelioid hemangioendotheliomas. Prox1 expression correlates strongly with podoplanin expression and represents a marker of lymphatic endothelial-like differentiation. Prox1 is a useful marker in identification of subsets of vascular endothelial tumors and studying biology of vascular tumors. However, Prox1 expression in some carcinomas and sarcomas, and paragangliomas has to be considered when applying it as an endothelial marker.
Acknowledgments
This work was supported as a part of NCI’s intramural research program.
References
- 1.Arai E, Kuramochi A, Tsuchida T, et al. Usefulness of D2-40 immunohistochemistry for differentiation between kaposiform hemangioendothelioma and tufted angioma. J Cutan Pathol. 2006;33:492–497. doi: 10.1111/j.1600-0560.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 2.Breiteneder-Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burke Z, Oliver G. Prox1 is an early specific marker for the developing liver and pancreas in the mammalian foregut endoderm. Mech Dev. 2002;118:147–155. doi: 10.1016/s0925-4773(02)00240-x. [DOI] [PubMed] [Google Scholar]
- 4.Dadras SS, Skrzypek A, Nguyen L, et al. Prox-1promotes invasion of kaposiform hemangioendotheliomas. J Invest Dermatol. 2008;128:2798–2806. doi: 10.1038/jid.2008.176. [DOI] [PubMed] [Google Scholar]
- 5.Dudas J, Papoutsi M, Hecht M, et al. The homeobox transcription factor Prox1 is highly conserved in embryonic hepatoblasts and in adult and transformed hepatocytes, but is absent from bile duct epithelium. Anat Embryol (Berl) 2004;208:359–366. doi: 10.1007/s00429-004-0403-4. [DOI] [PubMed] [Google Scholar]
- 6.Dudas J, Elmaouhoub A, Masuroglu T, et al. Prospero-related homeobox 1 (Prox1) is a stable hepatocyte marker during liver development, injury and regeneration, and is absent from “oval cells”. Histochem Cell Biol. 2006;126:549–562. doi: 10.1007/s00418-006-0191-4. [DOI] [PubMed] [Google Scholar]
- 7.Fanburg-Smith JF, Michal M, Partanen TA, et al. Papillary intralymphatic angioendothelioma (PILA): A report of 12 cases of a distinctive vascular tumor with phenotypic features of lymphatic vessels. Am J Surg Pathol. 1999;23:1004–1010. doi: 10.1097/00000478-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Harvey NL, Srinivasan RS, Dillard ME, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency caused adult-onset obesity. Nat Genet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 9.Hirakawa S, Hong YK, Harvey N, et al. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162:575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong YK, Detmar M. Prox1, master regulator of the lymphatic vasculature phenotype. Cell Tissue Res. 2003;314:85–92. doi: 10.1007/s00441-003-0747-8. [DOI] [PubMed] [Google Scholar]
- 11.Hong YK, Foreman K, Shin JW, et al. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat Genet. 2004;36:683–685. doi: 10.1038/ng1383. [DOI] [PubMed] [Google Scholar]
- 12.Johnson NC, Dillard ME, Baluk P, et al. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 2008;22:3282–3291. doi: 10.1101/gad.1727208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40 a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434–440. doi: 10.1038/modpathol.3880543. [DOI] [PubMed] [Google Scholar]
- 14.Kalof AN, Cooper K. D2-40 immunohistochemistry – so far. Adv Anat Pathol. 2009;16:62–64. doi: 10.1097/PAP.0b013e3181915e94. [DOI] [PubMed] [Google Scholar]
- 15.Laerm A, Helmbold P, Goldberg M, et al. Prospero-related homeobox 1 (PROX1) is frequently inactivated by genetic deletions and epigenetic silencing in carcinomas of the biliary system. J Hepatol. 2007;46:89–97. doi: 10.1016/j.jhep.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Le Huu AR, Jokinen CH, Rubin BP, et al. Expression of Prox1, lymphatic endothelial nuclear transcription factor, in Kaposiform hemangioendothelioma and tufted angioma. Am J Surg Pathol. 2010;34:1563–1573. doi: 10.1097/PAS.0b013e3181f6076f. [DOI] [PubMed] [Google Scholar]
- 17.Marler JJ, Mulliken JB. Vascular anomalies – classification, diagnosis, and natural history. Review Facial Plast Surg Clin North Am. 2001;9:495–504. [PubMed] [Google Scholar]
- 18.Mentzel T, Kuzner H. Tumors of the lymphatic vessels of the skin and soft tissue. Pathologe. 2002;23:118–127. doi: 10.1007/s00292-001-0498-9. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen M, Miettinen M, Wang ZF, et al. ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol. 2011;35:432–441. doi: 10.1097/PAS.0b013e318206b67b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miettinen M, Sarlomo-Rikala M, Wang ZF. Claudin 5 as immunohistochemical marker for angiosarcoma and hemangioendotheliomas. Am J Surg Pathol. doi: 10.1097/PAS.0b013e318229a401. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishima K, Watabe T, Saito A, et al. Prox1 induces lymphatic endothelial differentiation via integrin alpha-9 and other signaling cascades. Mol Biol Cell. 2007;18:1421–1429. doi: 10.1091/mbc.E06-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen VA, Kutzner H, Fürhapter C, et al. Infantile hemangioma is a proliferation of LYVE-1 negative blood endothelial cells without lymphatic competence. Mod Pathol. 2006;19:291–298. doi: 10.1038/modpathol.3800537. [DOI] [PubMed] [Google Scholar]
- 23.Partanen TA, Alitalo K, Miettinen M. Lack of lymphatic vascular specificity of vascular endothelial growth factor 3 in 185 vascular tumors. Cancer. 1999;86:2406–2412. [PubMed] [Google Scholar]
- 24.Perkins P, Weiss SW. Spindle cell hemangioendothelioma. An analysis of 78 cases with reassessment of its pathogenesis and biologic behavior. Am J Surg Pathol. 1996;20:1196–1204. doi: 10.1097/00000478-199610000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Petrova TV, Nykanen A, Norrmen C, et al. Transcription factor PROX1 induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype. Cancer Cell. 2008;13:407–419. doi: 10.1016/j.ccr.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Riseboro CA, Searles RG, Melville AA, et al. Prox1 maintains muscle structure and growth in the developing heart. Development. 2009;136:495–505. doi: 10.1242/dev.030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traweek ST, Kandalaft PL, Mehta P, et al. The human hematopoietic progenitor cell antigen (CD34) in vascular neoplasia. Am J Clin Pathol. 1991;96:25–31. doi: 10.1093/ajcp/96.1.25. [DOI] [PubMed] [Google Scholar]
- 28.Versmold G, Felsberg J, Mikeska T, et al. Epigenetic silencing of the candidate tumor suppressor gene PROX1 in sporadic breast cancer. Int J Cancer. 2007;121:547–554. doi: 10.1002/ijc.22705. [DOI] [PubMed] [Google Scholar]
- 29.Weninger W, Partanen TA, Breiteneder-Geleff S, et al. Expression of vascular endothelial growth factor receptor-3 and podoplanin suggests a lymphatic endothelial cell origin of Kaposi’s sarcoma tumor cells. Lab Invest. 1999;79:243–251. [PubMed] [Google Scholar]
- 30.Wigle JT, Harvey N, Detmar M, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;7:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilting J, Papoutsi M, Christ B, et al. The transcription factor Prox1 is a marker for lymphatic endothelial cells in normal and diseased human tissues. FASEB J. 2002;16:1271–1273. doi: 10.1096/fj.01-1010fje. [DOI] [PubMed] [Google Scholar]