Abstract
Microbial penetration of the intestinal epithelial barrier triggers inflammatory responses that include induction of the bactericidal C-type lectin RegIIIγ. Systemic administration of flagellin, a bacterial protein that stimulates Toll-like receptor 5 (TLR5), induces epithelial expression of RegIIIγ and protects mice from intestinal colonization with antibiotic-resistant bacteria. Flagellin-induced RegIIIγ expression is IL-22-dependent, but how TLR signaling leads to IL-22 expression is incompletely defined. Using conditional depletion of lamina propria dendritic cell (LPDC) subsets, we demonstrated that CD103+ CD11b+ LPDCs, but not monocyte-derived CD103− CD11b+ LPDCs, expressed high amounts of IL-23 following bacterial flagellin administration and drove IL-22-dependent RegIIIγ production. Maximal expression of IL-23 subunits IL-23p19 and IL-12p40 occurred within 60 minutes of exposure to flagellin. IL-23 subsequently induced a burst of IL-22 followed by sustained RegIIIγ expression. Thus, CD103+ CD11b+ LPDCs, in addition to promoting long-term tolerance to ingested antigens, also rapidly produce IL-23 in response to detection of flagellin in the lamina propria.
Introduction
The mammalian gastrointestinal microbiota influences mucosal innate and adaptive immune defenses. Commensal microbes provide protection against infection by activating mucosal innate immune defenses (Brandl et al., 2008; Cash et al., 2006) but also induce regulatory cells that prevent detrimental inflammatory responses (Honda and Takeda, 2009; Round and Mazmanian, 2010). The commensal microbe-host relationship has existed throughout vertebrate evolution and, over the course of nearly 500 million years, has resulted in the emergence of unique microbial populations that colonize the mammalian gut (Ley et al., 2008). The introduction of potent antibiotics to medical and agricultural practice has inadvertently disrupted intestinal microbial populations with untoward effects that are becoming increasingly apparent. One of the most important adverse effects of the broad use of antibiotics is the emergence of antibiotic-resistant pathogens, such as vancomycin-resistant Enterococcus (VRE), carbapenem-resistant Klebsiella pneumoniae, and Clostridium difficile. The antibiotic-conditioned gastrointestinal tract has become a major reservoir for multi-drug resistant organisms (Donskey, 2004; Ubeda et al., 2010). Strategies to enhance mucosal innate immune defenses to inhibit pathogen colonization of the intestinal lumen and/or prevent pathogen translocation across mucosal barriers may provide opportunities to complement the shrinking repertoire of antibiotics that are effective against increasingly antibiotic-resistant bacterial pathogens.
A common and clinically important scenario, particularly in antibiotic-treated patients, involves mucosal invasion by otherwise innocuous but highly antibiotic-resistant bacteria following their dense colonization of the intestine. VRE has this potential and thus is a leading cause of bacteremia in hospitalized patients (CDC, 1993; Hidron et al., 2008). Broad-spectrum antibiotic administration kills commensal bacteria in the gut and decreases Toll-like receptor (TLR)-dependent mucosal expression of antimicrobial factors, such as the bactericidal C-type lectin RegIIIγ, leading to a predisposition to VRE colonization (Brandl et al., 2008; Vaishnava et al., 2008). We have shown that exogenous administration of TLR ligands in the setting of antibiotic treatment restores the inflammatory tone of the intestinal mucosal barrier. Lipopolysaccharide (LPS) and flagellin stimulate TLR4 and TLR5, respectively, as well as induce RegIIIγ expression and restrict colonization of the gut by VRE (Brandl et al., 2008; Kinnebrew et al., 2010). Expression of RegIIIγ by intestinal epithelial cells is inducible by both direct TLR signaling in epithelial cells (Brandl et al., 2008; Vaishnava et al., 2008), and TLR signaling in cells of hematopoietic origin (Kinnebrew et al., 2010; Van Maele et al., 2010). Induction of RegIIIγ expression by exogenously administered flagellin is TLR5- and Interleukin-22 (IL-22)-dependent and mediated by hematopoietic cells (Kinnebrew et al., 2010; Van Maele et al., 2010). The identities of the TLR5-expressing cell subset and the in vivo signals involved in flagellin-mediated IL-22 and RegIIIγ induction remain incompletely defined.
IL-22 is critical for early defense against intestinal infections with Citrobacter rodentium and Candida albicans and pulmonary infection with Klebsiella pneumoniae (Aujla et al., 2008; Sonnenberg et al., 2011; Zheng et al., 2008). IL-6, IL-1β, and IL-23 produced by antigen-presenting cells regulate IL-22 expression in innate cells. It has also been suggested that direct stimulation of TLRs on innate immune cells can induce IL-22 expression (Crellin et al., 2010; Martin et al., 2009; Sutton et al., 2009). Although Th17 cells and γδ T cells that express the transcription factors RORγt and the aryl hydrocarbon receptor (AHR) can produce IL-22, the primary source for flagellin-mediated IL-22 expression in the intestine is CD3ε− CD127+ innate lymphoid cells (ILC) (Van Maele et al., 2010). How TLR5-signaling mediates IL-22 secretion by ILCs is not clear.
Tissue-resident lamina propria dendritic cells (LPDCs) are heterogeneous and arise from two principal lineages. LPDCs express Class II MHC and CD11c but can be divided into subsets on the basis of CD103 and CX3CR1 expression. CD103+ CD11b+ LPDCs are derived from the common dendritic cell progenitor (CDP) through Flt3L-dependent pathways (Bogunovic et al., 2009; Varol et al., 2009). In contrast, CX3CR1+ CD103− CD11b+ LPDCs are derived from Ly6chi monocytes through a M-CSF-dependent pathway (Bogunovic et al., 2009; Varol et al., 2009). The relative contributions of these DC subsets to immune tolerance or antimicrobial defense remain controversial. CD103+ CD11b+ LPDCs can up-regulate CCR7 and traffic to mesenteric lymph nodes, where they have been implicated in T regulatory cell and oral tolerance development while CX3CR1+ CD103− CD11b+ LPDCs are non-migratory and pro-inflammatory in the setting of colitis (Coombes et al., 2007). CD103+ CD11b+ LPDCs regulate T cell tolerance, but it is unclear whether they contribute to innate immune surveillance for mucosal invasion by microbial pathogens. Although CD103+ CD11b+ LPDCs are the primary TLR5-expressing cell in the small intestine (Uematsu et al., 2008), it is not known whether these cells contribute to flagellin-induced IL-22 expression.
Although systemic flagellin administration protects mice against intestinal pathogens (Vijay-Kumar et al., 2008), VRE colonization (Kinnebrew et al., 2010), and radiation-induced mortality (Burdelya et al., 2008), the underlying mechanisms of flagellin-mediated protection remain incompletely defined. Herein we show that IL-22 was rapidly induced following flagellin administration and required IL-23 production by TLR5-expressing CD103+ CD11b+ LPDCs. Although CD103+ CD11b+ DCs and CX3CR1+ CD103− CD11b+ DCs express TLR5, CD103+ CD11b+ LPDCs were the major source of IL-23 induced by systemic TLR5 stimulation. The rapidity of IL-23 production by CD103+ CD11b+ LPDCs suggests that this response is principally involved in early innate antimicrobial defense. CD103+ CD11b+ LPDCs, in conjunction with IL-22-producing ILCs, provide a cellular surveillance and effector mechanism to induce antimicrobial defenses in intestinal epithelial cells in the event microbes or microbial molecules enter the lamina propria. Pharmacologic activation of the LPDC and ILC populations involved in this process may provide a novel therapeutic approach to enhance mucosal resistance to bacterial pathogens.
Results
Flagellin-Mediated IL-22 Induction Involves the Expression of TLR5 and IL-22 in Distinct Cell Populations
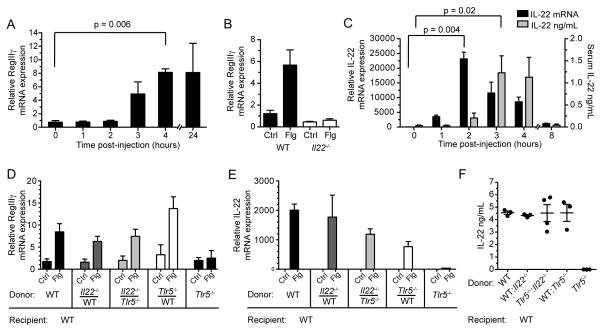
To characterize the response to systemic administration of flagellin, we first analyzed the kinetics of RegIIIγ expression in the small intestine after flagellin administration. RegIIIγ messenger RNA (mRNA) transcript expression was increased in the small intestine of wild-type (WT) mice within 3 hours of flagellin administration and remained elevated for 24 hours post-injection (Figure 1A). Flagellin administration did not induce RegIIIγ expression in IL-22-deficient mice as shown in previous studies (Figure 1B) (Kinnebrew et al., 2010). Induction of IL-22 in the intestinal lamina propria (LP) preceded RegIIIγ induction, with peak IL-22 mRNA transcript and detectable IL-22 protein amounts occurring 2 hours and 3 hours, respectively, following flagellin administration (Figure 1C). We also detected elevated IL-22 mRNA transcripts in the liver, lung, and spleen; however, IL-22 mRNA transcripts were at the highest amounts in the small intestine lamina propria (Figure S1A).
Figure 1. Flagellin-Mediated Upregulation of RegIIIγ Requires TLR5 Signaling and IL-22 Expression in Distinct Cell Populations.
(A) Messenger RNA (mRNA) transcript expression for RegIIIγ in the small intestine (SI) was measured by quantitative PCR (qPCR) at the indicated time periods following intravenous (i.v.) injection flagellin (Flg) or PBS (Ctrl). (n = 3 – 4 mice per time point).
(B) Messenger RNA was extracted from the SI lamina propria of wild-type (WT) or IL-22-deficient (Il22−/−) mice 24 hours following i.v. injection of flagellin (n = 3 mice per group) and quantified by qPCR.
(C) IL-22 messenger RNA expression was measured in the SI lamina propria at the indicated time points following i.v. flagellin administration. IL-22 protein concentrations were detected in the serum by ELISA (n = 3 mice per time point).
(D – E) Mixed bone marrow (BM) chimeric mice reconstituted with a equal ratios of Tlr5−/−, Il22−/−, or WT BM, as indicated on the horizontal axis, were assessed for responsiveness to flagellin by qPCR analysis of RegIIIγ mRNA transcripts in the SI (D) and IL-22 mRNA in the SI lamina propria (E). IL-22 protein concentrations in the serum were measured by ELISA (F) (n = 3 – 4 mice per group). RegIIIγ expression was measured 24 hours after flagellin administration while serum IL-22 protein and lamina propria IL-22 expression were measured 3 hours after flagellin administration. Error bars show ± s.e.m. Data are representative of at least two independent experiments.
IL-22 production by RORγt-dependent innate lymphoid cells (ILC) is critical for defense against the intestinal bacterial pathogen Citrobacter rodentium (Sonnenberg et al., 2011; Zheng et al., 2008). Inoculation of Rag1-deficient mice with flagellin resulted in IL-22 induction, indicating that T cells and B cells were not the primary sources of IL-22 induced by flagellin administration (Figures S1B and S1C). In contrast, IL-22 induction was abrogated in mice lacking both Rag2 and the IL-2R γ chain, suggesting that an innate lymphocyte population was the source of IL-22 (Figures S1B and S1C). Flagellin administration also failed to stimulate IL-22 expression in mice deficient in either Aryl Hydrocarbon Receptor (AHR) or RORγ (Figures S1D – S1F), which is consistent with the previously described role of these transcription factors in development of CD3ε− CD127+ ILCs and expression of IL-22. We characterized the phenotype of IL-22+ lamina propria cells and found that they were similar to previously described immature innate lymphoid cells that lack expression of NK cell receptors and CD4 but were Thy1+, c-kit+, and CD127+ (Figure S1G) (Sanos et al., 2011; Spits and Di Santo, 2010). These results are consistent with the recent demonstration that flagellin stimulates IL-22 production in a population of ILCs (Van Maele et al., 2010); however, it remains unclear how IL-22 production by ILCs is regulated in response to microbial infection or flagellin administration.
To determine if direct TLR5 stimulation of cells capable of producing IL-22 is required for the flagellin response, we generated mixed bone marrow (BM) chimeric mice by transferring a 1:1 mixture of congenically marked TLR5-deficient bone marrow and IL-22-deficient bone marrow into lethally irradiated WT recipient mice. If TLR5 signaling and IL-22 expression occurs in the same cell, flagellin administration should fail to induce IL-22 in these chimeric mice. We, however, found that IL-22 expression following flagellin administration was normal in Tlr5−/−:Il22−/− mixed BM chimeric mice (Figures 1E and 1F). RegIIIγ induction in the small intestine in response to flagellin was also not substantially different among the groups of mixed BM chimeric mice (Figure 1D). Although previously suspected, these experiments demonstrate that TLR5 signaling and IL-22 expression occur in distinct cell populations, with a TLR5-expressing cell population responding to flagellin administration and relaying a second, distinct signal to cells that produce IL-22.
TLR5-Expressing DCs are Required for Flagellin-Mediated Induction of IL-22
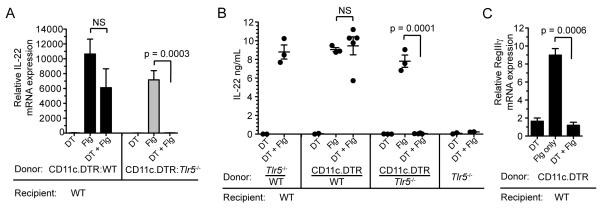
To determine whether dendritic cells (DCs) are required for TLR5-mediated induction of IL-22, we generated mixed BM chimeric mice in which TLR5-expressing DCs could be selectively depleted. Bone marrow cells from TLR5-deficient mice and CD11c.DTR mice were mixed at a 1:1 ratio and used to reconstitute irradiated WT recipient mice. CD11c.DTR mice are transgenic mice that express the diphtheria toxin receptor (DTR) under control of the CD11c promoter. We confirmed that diphtheria toxin (DTX) treatment depleted intestinal MHCII+ CD11c+ lamina propria DCs (LPDCs) derived from CD11c.DTR BM cells (Figure S2A). Other cell populations that may contribute to IL-22 production, including ILCs and NKp46+ cells, were not depleted by DTX treatment (Figure S2B). Specific depletion of DCs capable of expressing TLR5 completely abrogated flagellin-induced IL-22 expression in the lamina propria (Figure 2A). Serum titers of IL-22 were also undetectable following flagellin treatment in these mice (Figure 2B). The lack of IL-22 induction could not be explained by a 50% loss of LPDCs since IL-22 induction was preserved in DTX-treated WT:CD11c.DTR mixed BM chimeric mice (Figures 2A and 2B). We also analyzed flagellin-induced RegIIIγ expression in mice depleted of CD11c+ dendritic cells to verify the role that DCs play in this pathway. RegIIIγ upregulation was completely abrogated in mice that lacked DCs (Figure 2C). These results demonstrate that dendritic cells that express TLR5 rapidly induce IL-22 expression in the lamina propria in response to stimulation with flagellin.
Figure 2. TLR5-Expressing Dendritic Cells are Required for Flagellin-Mediated IL-22 Expression.
(A – B) Mixed bone marrow (BM) chimeric mice in which the hematopoietic compartment is composed of equal ratios of Tlr5−/−, CD11c.DTR or wild-type (WT) BM cells, in combinations indicated on the horizontal axes, were depleted of WT dendritic cells by intraperitoneal injection of diphtheria toxin. Messenger RNA (mRNA) expression in the small intestine (SI) lamina propria (A) and serum protein amounts (B) of IL-22 in response to flagellin (Flg) administration were assessed 3 hours after injection (n = 2 – 4 mice per group). Control (Ctrl) groups received PBS.
(C) Bone marrow chimeric mice, generated by transferring bone marrow from CD11c.DTR mice into irradiated WT mice, were depleted of DCs using diptheria toxin as described in the Methods section. RegIIIγ mRNA expression in the small intestine was assessed 24 hours following intravenous injection of flagellin. (n = 3 mice per group). Error bars denote ± s.e.m. Data are pooled from two independent experiments.
IL-23 is Rapidly Induced Following Flagellin Administration
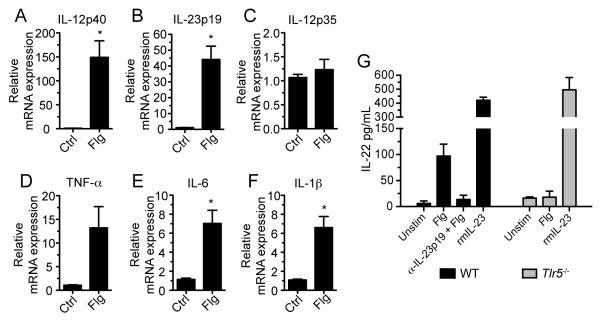
We reasoned that TLR5-signaling in LPDCs stimulates cytokine production that drives IL-22 expression by intestinal ILCs. Soluble factors that mediate IL-22 expression have been identified and may play a role in flagellin-mediated IL-22 expression (Ouyang et al., 2011). Given that IL-22 was induced in the lamina propria within 2 hours of flagellin administration, it seemed likely that TLR5-stimulated DCs express an IL-22-inducing factor at an earlier time point. Therefore, we measured cytokine mRNA transcript expression in the lamina propria 1 hour following flagellin administration. Whereas IL-6 (Figure 3E) and IL-1β (Figure 3F) were upregulated approximately 7-fold in comparison to controls, mRNA transcript expression of IL-12p40 (Figure 3A) and IL-23p19 (Figure 3B) were increased 150-fold and 45-fold, respectively, in flagellin-treated mice. As a heterodimeric protein, IL-23 is composed of two covalently linked subunits IL-12p40 and IL-23p19. IL-12p40 can also join with IL-12p35 to form IL-12. We measured IL-12p35 mRNA transcript expression following flagellin administration, but no increase was detected (Figure 3C). To further characterize the flagellin-induced response, we measured expression of IL-12p40, IL-23p19, and IL-22 in the lamina propria at several early time points following flagellin administration. Messenger RNA transcripts for IL-23p19 and IL12p40 were transiently elevated, peaking at 30 minutes and 1 hour, respectively, whereas IL-22 mRNA transcripts reached the maximum amount at 2 hours post-injection (Figure S3A). In order to ensure that the resolution of IL-23 lamina propria expression to baseline following a peak induction at 30 – 60 minutes is due to the downregulation of the IL-23 and not the migration of IL-23-expressing cells out of the lamina propria or the recruitment of cells to the lamina propria, we analyzed the lamina propria cell composition 2. 5 hours following flagellin administration during which IL-23 mRNA transcript expression returned to baseline. We found that at this early time point there were no significant shifts in DC populations that indicated migration of these cells out of the lamina propria (Figure S3C). We observed recruitment of monocytes and neutrophils to the lamina propria, but these cells composed no more than 10% of total CD45+ cells in the lamina propria (Figure S3D). No changes in T cell populations were detected.
Figure 3. Blockade of IL-23 Signaling Inhibits Flagellin-Mediated Expression of IL-22.
(A – F) Wild-type (WT) mice received flagellin (Flg) or PBS (Ctrl) intravenously. One hour after injection messenger RNA was extracted from the small intestine lamina propria and cytokine expression was assessed by quantitative PCR (n = 3 mice per group; *p < 0.05).
(G) Lamina propria cells were isolated from the small intestine of WT and Tlr5−/− mice and stimulated in vitro with or without flagellin in the presence of IL-23p19 blocking antibody (α-IL-23p19) under the indicated conditions for 24 hours. Recombinant mouse IL-23 (rmIL-23) was used as a positive control. IL-22 protein was quantified in culture supernatants by ELISA. Data are representative of three independent experiments. Error bars denote ± s.e.m.
To determine whether IL-1β, IL-6, or IL-23 contribute to flagellin-mediated IL-22 expression, we stimulated purified lamina propria cells with flagellin in vitro in the presence of soluble IL-1Ra, anti-IL-6R, or anti-IL-23p19 to block cytokine signaling. Only the IL-23p19 antibody blocked flagellin-induced IL-22 production while blockade of IL-1β and IL-6 had no effect (Figures 3G and S3B). Addition of IL-23 to lamina propria cells in the absence of flagellin or TLR5 induced high amounts of IL-22 expression in vitro (Figure 3G), demonstrating that IL-23 alone can induce lamina propria cells to secrete IL-22. These results suggest that rapid and transient expression of IL-23 in response to TLR5 stimulation leads to the upregulation of IL-22 and subsequent production of RegIIIγ.
Flagellin-Mediated Induction of IL-22 Requires IL-23 Expression by Dendritic Cells
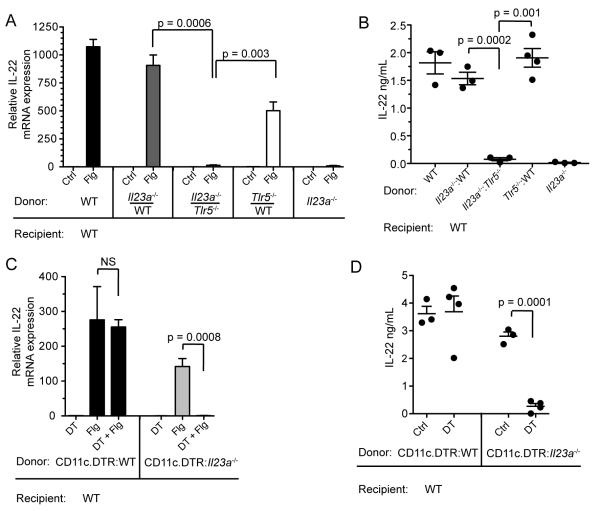
IL-23 has been demonstrated to induce IL-22 expression. IL-23-deficient mice have impaired IL-22 expression during T cell-mediated colitis, Citrobacter rodentium infection, and experimental autoimmune encephalitis (Ahern et al., 2010; Kreymborg et al., 2007; Zheng et al., 2008). However, a role for IL-23 in TLR-mediated IL-22 expression has only been implicated in experiments performed in vitro and by correlative upregulation after in vivo administration of TLR ligands (Takatori et al., 2009; Van Maele et al., 2010). To determine the in vivo role of IL-23 in flagellin-mediated IL-22 expression, we generated mixed BM chimeric mice in which the hematopoietic compartment is composed of both IL-23p19-deficient and TLR5-deficient cells in equal proportions (Figures S4A – S4D). In these mice, flagellin did not induce IL-22 mRNA expression (Figure 4A) or elevate IL-22 protein in the serum (Figure 4B), demonstrating that TLR5-expressing cells must produce IL-23 in order to induce IL-22 expression. This finding was not a consequence of the fact that only half of the hematopoietic compartment was capable of expressing IL-23 since the flagellin response was normal in control mice reconstituted with equal parts WT bone marrow cells and IL-23p19-deficient bone marrow cells (Figures 4A and 4B).
Figure 4. IL-23p19 Expression by TLR5+ Dendritic Cells is Required for Flagellin-Mediated IL-22 Induction.
(A – B) Flagellin (Flg) or PBS (Ctrl) was administered intravenously to mixed bone marrow (BM) chimeric mice reconstituted with equal parts Tlr5−/− and Il23a−/− (gene name for IL-23p19) bone marrow cells, as indicated. Three hours after flagellin administration, lamina propria IL-22 messanger RNA (mRNA) expression was assessed by quantitative PCR (qPCR) (A) and the serum concentration of IL-22 was assessed by ELISA (B) (n = 3 – 4 mice per group).
(C – D) Irradiated mice were reconstituted with a 1:1 mixture of IL-23p19-deficient (Il23a−/−) BM cells and CD11c.DTR BM cells to generate mixed BM chimeric mice, which were subsequently depleted of IL-23p19-sufficient dendritic cells by treatment with diphtheria toxin as described in Methods. Three hours after flagellin administration, lamina propria IL-22 mRNA expression was assessed by qPCR (C) and the serum concentration of IL-22 was assessed by ELISA (D) (n = 3 – 4 mice per group). Error bars denote ± s.e.m. Data are representative of two independent experiments.
To determine whether expression of IL-23 by DCs is required, we again generated mixed BM chimeric mice by transferring a 1:1 mixture of BM cells from IL-23p19-deficient and CD11c.DTR mice into lethally irradiated WT recipients. IL-23p19-deficient DCs efficiently populated the lamina propria in similar numbers compared to TLR5-deficient or WT DCs (Figure S4E). Flagellin failed to induce IL-22 expression in vivo when DTX-sensitive, IL-23p19-sufficient DCs were depleted but DTX-resistant, IL23p19-deficient DCs persisted (Figure 4C and 4D). Mice in which the DC compartment was composed of both DTX-sensitive wild-type DCs and DTX-resistant wild-type DCs responded normally to flagellin administration despite depletion of half of the DC population. Depletion was specific to DCs since we did not observe loss of ILCs or NKp46+ cells (Figure S4F). Collectively, these data demonstrate that IL-23 production by TLR5-expressing lamina propria dendritic cells drives the rapid upregulation of IL-22 in response to stimulation with flagellin.
Flt3L-Dependent Dendritic Cells are Required for Flagellin-Mediated IL-22 Expression
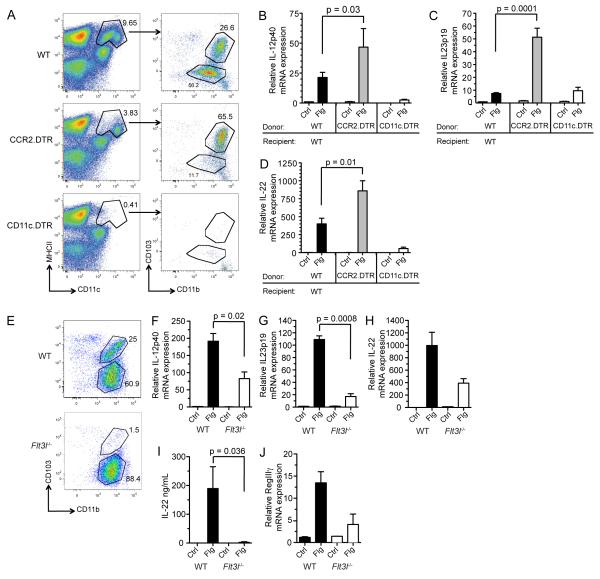
The MHCII+ CD11c+ lamina propria DC (LPDC) population is composed of two major groups, one derived from classic DC precursors and dependent on Flt3L and the other derived from monocyte precursors and dependent on M-CSF (Bogunovic et al., 2009; Varol et al., 2009). The relative functions of these distinct populations remain incompletely defined, and it is not clear whether either, neither, or both subsets produce IL-23. To determine whether these LPDC subsets respond to flagellin administration, we selectively depleted CD103− CD11b+ LPDCs using transgenic mice in which the CCR2 promoter drives expression of the diphtheria toxin receptor (CCR2.DTR) (Hohl et al., 2009). The CCR2 chemokine receptor is selectively expressed by Ly6Chi monocytes, and DTX administration depletes both Ly6Chi monocytes and CD103− CD11b+ LPDCs in CCR2.DTR mice when the toxin is administered over the course of 6 days (Figures S5A – S5E). Ly6Chi monocytes express the CCR2 receptor and thus are depleted in CCR2 mice; however, monocyte-derived CD103− CD11b+ LPDCs do not actively express CCR2 (Figure S5F), but due to the short turnover rate of these cells, elimination of their precursor population (Ly6Chi monocytes) leads to loss of monocyte-derived LPDCs (Figure 5A). While flagellin-mediated IL-12p40 and IL-23p19 induction was completely abrogated in DTX-treated CD11c.DTR mice, we found that 80% depletion of CD103− CD11b+ lamina propria DCs in CCR2.DTR mice resulted in enhanced induction of IL-12p40 (Figure 5B) and IL-23p19 (Figure 5C). Depletion of CD11c+ cells in CD11c.DTR mice did not deplete Ly6Chi monocytes, indicating that Ly6Chi monocytes did not directly drive IL-23 induction (not shown). Consistent with the enhanced induction of IL-23 in mice depleted of CD103− CD11b+ lamina propria DCs, we found that IL-22 expression was also increased compared to the response observed in WT mice (Figure 5D). Enhanced induction of IL-22 was not due to proliferation of CD103+ CD11b+ LPDCs (Figures S5A – S5D). These results suggest that CD103+ CD11b+ DCs orchestrate the IL-22 response while monocyte-derived CD103− CD11b+ lamina propria DCs play a more regulatory role.
Figure 5. Flt3L-Dependent CD103+ CD11b− DCs Not Monocyte-Derived DCs are Required for Flagellin-Mediated IL-23 Expression.
(A) Wild-type (WT), CD11c.DTR, or CCR2.DTR bone marrow chimeric mice received 200 ng of diphtheria toxin by intraperitoneal injection every other day for 6 days. Mice then received 1 μg of flagellin (Flg) or PBS (Ctrl) by intravenous injection and were sacrificed 2 hours later. FACS analysis of purified small intestine (SI) lamina propria cells, gated on CD45+ cells, demonstrates depletion of dendritic cell populations following diphtheria toxin treatment. Numbers on dot plots indicate the percentage of cells within the gate out of the parent population.
(B – D) Quantitative PCR (qPCR) was performed on SI lamina propria tissue from WT, CCR2.DTR, and CD11c.DTR mice to determine the relative expression of IL-12p40 (B), IL-23p19 (C), and IL-22 (D) (n = 3 mice per group). Data are representative of two independent experiments.
(E – G) Flagellin or PBS was administered by i.v. injection to Flt3L-deficient (Flt3−/−) mice or WT control mice, and mice were sacrificed 1 hour following injection. Lamina propria cells were isolated for FACS analysis of dendritic cell populations. Dot plots show cells that were gated on CD45+ MHCII+ CD11c+ cells. The numbers on the dot plot indicate the percentage of cells out of this population within the gate. SI lamina propria tissue was harvested for analysis of IL-12p40 (F) and IL-23p19 (G) mRNA transcripts by qPCR.
(H) To assess IL-22 mRNA transcript expression in the lamina propria, Flt3l−/− mice were sacrificed 2 hours post-injection with flagellin and processed for analysis by qPCR.
(I) To assess secretion of IL-22, Flt3l−/− mice were sacrificed three hours following flagellin administration and blood was collected for analysis of IL-22 protein concentration by ELISA.
(J) Flagellin-induced RegIIIγ expression in the small intestine in Flt3l−/− mice was evaluated 24 hours following flagellin injection by qPCR (n = 3 mice per group). The data shown is representative of two independent experiments. Error bars denote ± s.e.m.
To determine whether CD103+ CD11b+ LPDCs contribute to flagellin-mediated induction of IL-23 in the lamina propria, we evaluated flagellin responses in mice that lack Flt3L, which is required for the development of CD103+ CD11b+ DCs but not monocyte-derived DCs (Figure 5E). Consistent with a role for CD103+ CD11b+ LPDCs in the flagellin-induced response, we found that IL12p40 (Figure 5F) and IL23p19 (Figure 5G) induction were significantly reduced in Flt3L-deficient mice. In Flt3L-deficient mice, we also observed impaired flagellin-induced IL-22 and RegIIIγ expression indicating that CD103+ DCs play a critical role in initiating IL-22-dependent antimicrobial defense (Figures 5H – 5J). This result was not a consequence of impaired development of innate lymphoid cells in the absence of Flt3L since ILC populations in the lamina propria were found intact in Flt3L-deficient mice (Figures S5H and S5I). Although the degree of IL-23 induction was reduced in Flt3L-deficient mice, an over 90% reduction in CD103+ CD11b+ LPDCs only reduced IL12p40 and IL23p19 by 60 and 80%, respectively. This result suggests that Flt3L-independent cell populations, particularly in the setting of congenital Flt3L deficiency, may also contribute to the production of IL-23 following flagellin administration.
IL-10 production from monocyte-derived DCs and macrophages have been shown to limit inflammatory responses in the intestinal lamina propria (Takeda et al., 1999). To determine if IL-10 expression by monocytes or monocyte-derived DCs restricts the responsiveness of CD103+ CD11b+ DCs to TLR stimulation, we generated mixed BM chimeric mice in which CCR2.DTR bone marrow cells were combined in equal parts with either WT bone marrow cells or IL-10-deficient bone marrow cells and transferred into lethally irradiated WT recipients. Depletion of WT but not IL-10 monocyte-derived DCs did not affect the flagellin-mediated IL-22 induction compared to control mice (Figure S5G). These data suggest that the hyperresponsiveness of CD103+ CD11b+ LPDCs that we observed is not due to the loss of IL-10 produced by monocyte-derived DCs and their precursors.
TLR5-Expressing CD103+ CD11b+ LPDCs Upregulate IL-23 in Response to Stimulation with Flagellin
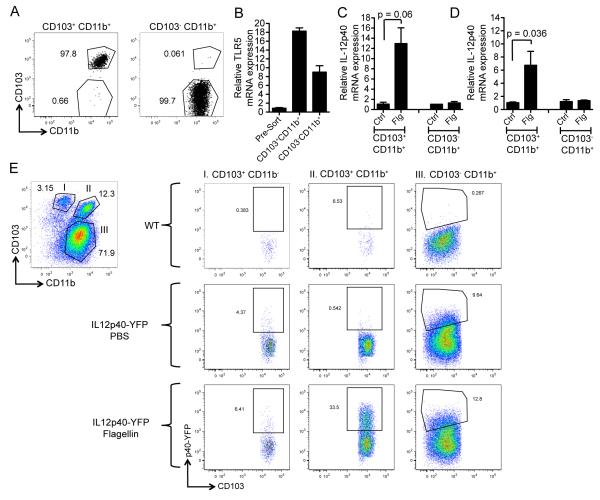
To determine if TLR5 is differentially expressed by lamina propria DC subsets, we sorted CD103+ CD11b+ LPDCs and CD103− CD11b+ LPDCs (Figure 6A) and quantified TLR5 mRNA transcripts in each population by qPCR (Figure 6B). Both DC subsets expressed high amounts of TLR5 compared to the entire population of lamina propria cells, but we detected more TLR5 mRNA transcripts in CD103+ CD11b+ DCs compared to CD103− CD11b+ DCs as previously demonstrated (Uematsu et al., 2008). To further evaluate IL-23 expression by lamina propria DCs, we individually stimulated sorted populations of CD103+ CD11b+ LPDCs and CD103− CD11b+ LPDCs with flagellin in vitro. Flagellin induced the expression of IL12p40 (Figure 6C) and IL-23p19 (Figure 6D) mRNA transcripts in CD103+ CD11b+ DCs but not CD103− CD11b+ DCs.
Figure 6. CD103+ CD11b+ DCs but not CD103− CD11b+ DCs Express IL-23p19 and IL-12p40 in Response to TLR5 Stimulation.
(A) Small intestinal lamina propria cells were isolated from six mice and pooled for FACS sorting. DAPI− CD45+ CD11c+ MHCII+ lamina propria cells were FACS-sorted into CD103+ CD11b+ or CD103− CD11b+ groups and individually assessed for purity by FACS analysis.
(B) TLR5 mRNA expression by quantitative PCR.
(C – D) Fold induction of messenger RNA (mRNA) encoding for IL-23 protein subunits IL-12p40 (C) and IL-23p19 (D) after 2 hours of in vitro incubation with flagellin. Data is representative of 1 out of 4 independent experiments. Error bars denote ± s.e.m.
(E) IL-12p40-YFP reporter mice received 1 μg of flagellin intravenously. Three hours after flagellin injection, mice were sacrificed and lamina propria cells were harvested for FACS analysis. Dot plots show cells gated by CD45+ CD11c+ MHCII+. DC subsets were analyzed for YFP expression in the following three groups: CD103+ CD11b−, CD103+ CD11b+, and CD103− CD11b+ LPDCs. Numbers are percentage of gated cells out of the parent population (n = 3 mice per group).
In order to detect IL-23-expressing DC subsets in vivo, we evaluated the flagellin response in reporter mice that express YFP under control of the endogenous IL-12p40 promoter (Reinhardt et al., 2006). Because IL-12p35 is not upregulated during the time frame of the experiment, IL-12p40-driven YFP expression predominantly reflects induction of IL-23. We detected no increase in YFP+ CD103+ CD11b− LPDCs following flagellin administration (Figure 6E). Consistent with the previously presented data of sorted LPDCs stimulated in vitro, the percentage of YFP+ CD103+ CD11b+ LPDCs dramatically increased from 0.5% to 34% following stimulation of TLR5 in vivo (Figure 6E). In contrast, the percentage YFP+ CD103− CD11b+ LPDCs only increased by three percentage points following flagellin administration; however, 10% of these cells were YFP+ under steady-state conditions, indicating that this population constitutively expresses IL-12p40 as previously described (Becker et al., 2003). Collectively, these data demonstrate that CD103+ CD11b+ LPDCs are the primary source of flagellin-mediated IL-23 expression and, therefore, play critical role in inducible IL-22 expression in the intestinal lamina propria (Figure S6).
Discussion
The luminal contents of the intestine are separated from the vasculature, connective tissue and immune cells of the lamina propria by a single layer of epithelial cells. Interruption of this barrier represents a threat to the mammalian host and results in rapid and robust inflammatory responses that prevent systemic dissemination of intestinal bacteria. Upon introduction of bacterial flagellin into the lamina propria, CD103+ CD11b+ LPDCs activate antimicrobial inflammatory responses by rapidly producing IL-23, thereby stimulating ILCs to produce IL-22, which promotes epithelial cell recovery and induces expression of RegIIIγ. Induction of IL-23 in CD103+ CD11b+ LPDCs occurs within 15 to 30 minutes exposure of the lamina propria to flagellin and is transient. Thus, it is unlikely that this early and transient wave of IL-23 induction directly influences subsequent CD4 T cell differentiation into Th17 cells. Instead, TLR5-dependent production of IL-23 by CD103+ CD11b+ LPDCs represents a local, expeditious response that informs ILCs that the epithelial barrier has been disrupted and that epithelial repair and the production of antimicrobial factors must be initiated.
We have previously manipulated the IL-22-RegIIIγ pathway for therapeutic purposes by administering flagellin systemically to antibiotic-treated mice in order to restore intestinal epithelial defenses against colonization with multi-drug resistant bacteria (Kinnebrew et al., 2010). Stimulation of TLR5 by flagellin induces intestinal RegIIIγ expression and subsequently limits intestinal colonization with vancomycin-resistant Enterococcus (VRE). In patients susceptible to infections with antibiotic-resistant organisms, intestinal colonization has been shown to precede blood infection; therefore, efforts to reduce or prevent colonization with these pathogens may provide an important prophylactic therapy (Ubeda et al., 2010). In the current study, we have elucidated a previously unappreciated role for IL-23 and CD103+ CD11b+ dendritic cells in the regulation of TLR-induced RegIIIγ expression in the small intestine. A clear understanding of the innate immune pathways that regulate intestinal mucosal defenses may lead to the development of therapeutics which broadly target antibiotic-resistant pathogens that colonize the gastrointestinal system.
Prior studies have shown that homeostatic RegIIIγ expression requires direct activation of TLR-MyD88-mediated signaling pathways in intestinal epithelial cells by microbial products shed by the intestinal microbiota (Brandl et al., 2008; Vaishnava et al., 2008). However, during active inflammation, IL-22 is required to stimulate RegIIIγ expression in intestinal epithelial cells (Kinnebrew et al., 2010; Zheng et al., 2008). Thus, it is possible that IL-22-mediated RegIIIγ induction provides an independent mechanism by which immune cells can regulate antimicrobial expression under threat of invasion by enteric pathogen. However, while the intestinal epithelial barrier is intact, microbial products from the intestinal microbiota provide signals directly to IECs leading to cell-intrinsic MyD88-mediated RegIIIγ expression. Yet there is some evidence that these pathways are not completely independent avenues for regulating RegIIIγ expression. Antibody blockade of IL-22 signaling leads to the downregulation of RegIIIγ expression in normal mice under steady-state conditions (Sanos et al., 2009). In addition, RegIIIγ expression is markedly diminished in IL-22-deficient mice under steady-state conditions, and is not further reduced by antibiotic-mediated depletion of the microbial flora (Kinnebrew et al., 2010). These observations suggest that MyD88 and IL-22 are necessary components of the pathway that regulates homeostatic RegIIIγ expression. The precise nature of the interaction between MyD88 expression by intestinal epithelial cells and IL-22 expression by immune cells and is unclear and requires further research.
A rapid, innate inflammatory role for CD103+ CD11b+ LPDCs contrasts with the more widely appreciated tolerance-inducing role attributed to this DC subset. CD103+ CD11b+ LPDCs induce tolerogenic T cell responses by producing TGFβ and retinoic acid (Coombes et al., 2007). Signals from the intestinal tissue microenvironment, including epithelial cell-derived thymic stromal lyphopoietin (TSLP) and bile acid retinoids, condition CD103+ CD11b+ LPDCs to preferentially generate T regulatory cells once these DCs have trafficked to the mesenteric lymph nodes (Manicassamy and Pulendran, 2011). However, in the setting of chronic colitis, CD103+ CD11b+ LPDCs are activated and acquire a pro-inflammatory phenotype (Laffont et al., 2010). Our experiments demonstrate that in healthy mice under steady-state conditions, CD103+ CD11b+ LPDCs can rapidly produce IL-23 in response to microbial products, despite homeostatic conditioning towards a tolerizing state by the tissue microenvironment.
Determining the distinct functions of the different populations of lamina propria DCs is challenging because LPDCs are difficult to harvest from the network of connective tissue that forms the lamina propria. Most experiments characterizing the functions of LPDC subsets have been performed in vitro, requiring isolation of LPDCs. After lengthy incubation periods and mechanical and proteolytic disruption of the lamina propria extracellular matrix and exposure to luminal bacteria, “freshly” isolated LPDCs have almost certainly been altered. Procedural and microbiota differences, therefore, might explain differences in the functions assigned to distinct LPDC populations by different laboratories (Pabst and Bernhardt, 2010; Varol et al., 2010). We demonstrate here that IL-23 is required for flagellin-mediated IL-22 expression in vivo. Uematsu and colleagues characterized TLR5-expressing CD103+ CD11b+ LPDCs from the lamina propria and found that they specifically promote IgA production and Th17 cell differentiation (Uematsu et al., 2008). The Th17 response appears to be dependent on the ability of these TLR5-expressing DCs to produce IL-6. In contrast, IL-23 was not detected in the supernatants of flagellin-stimulated CD103+ CD11b+ DCs. The differences in results may be due to the DC isolation technique and whether in vitro or in vivo experiments were performed.
Because IL-23 can drive the development and persistence of colitis, mechanisms to limit IL-23 expression in the intestine are critical for intestinal homeostasis (Hue et al., 2006). How IL-23 responses are controlled is not completely understood. We show that CD103+ CD11b+ LPDCs rapidly produce IL-23 upon TLR stimulation. Specific depletion of monocytes and monocyte-derived DCs leads to enhanced flagellin-mediated IL-23 and IL-22 expression, suggesting that these cells play a role in limiting the responsiveness of CD103+ CD11b+ LPDCs to TLR5 stimulation. However, the regulatory role of monocytes and monocyte-derived DCs was not due to their ability to secrete IL-10. Whether monocytes or monocyte-derived dendritic cells directly inhibit IL-23 expression in CD103+ DCs via inhibitory cytokines or indirectly through stimulation of another cell type, such as T regulatory cells, requires further investigation.
IL-22 has been implicated as an effector cytokine in defense against mucosal pathogens, but the signals and cells that initiate IL-22 expression are currently unknown. During Citrobacter rodentium infection of the gut, IL-23-dependent IL-22 expression by ILCs is required for clearance of the pathogen (Sonnenberg et al., 2011; Zheng et al., 2008). These previous studies, while demonstrating that IL-22 or IL-23 deficiency result in enhanced in vivo growth of C. rodentium over the course of one to two weeks, did not identify the cell populations that produce IL-23 during infection. Control of Candida albicans infection of gastrointestinal tract has also been shown to require early activation of the IL-23-IL-22 axis (De Luca et al., 2010). Further research is needed to determine the cells responsible for IL-23 expression and whether TLR signaling is required for IL-22 mediated defense in these infection models.
Flagellin is a potential immunomodulatory agent that activates innate mucosal defense. TLR5 is restricted to certain tissues, with the highest expression occurring in the small intestine (Uematsu et al., 2006). Although TLR5 expression on the basolateral side of intestinal epithelial cells has been reported, CD103+ CD11b+ LPDCs express the highest amounts of TLR5 (Uematsu et al., 2008). Subepithelial sensing of flagellin by TLR5-expressing CD103+ CD11b+ DCs likely plays an important role in keeping commensal intestinal bacteria at bay by forming an important layer of defense when microperforation of the intestinal epithelium occurs. Commensal microbes colonizing the intestinal lumen are a major source of TLR ligands, including flagellin. Crohn’s disease patients, for example, suffer from frequent damage to the intestinal epithelium, and the dominant antigen against which mucosal adaptive responses are directed is flagellin derived from commensal bacteria residing in the cecum (Lodes et al., 2004). The roles of IL-22 in wound healing (Pickert et al., 2009) and RegIIIγ in maintaining the spatial segregation of gut microbes from the intestinal epithelial cells (Vaishnava et al., 2011) support the idea that the rapid response of CD103+ CD11b+ DCs to TLR stimulation is important for the repair of the intestinal epithelial barrier.
Our present work demonstrates that TLR5 signaling in immune cells stimulates RegIIIγ expression in intestinal epithelial cells through a multicellular model in which TLR5-stimulated dendritic cells produce IL-23 and ILCs respond by secreting IL-22. This indirect mechanism of inducing RegIIIγ in intestinal epithelial cells is in contrast to previous work demonstrating that direct, microbiota-driven activation of TLR-MyD88 signaling pathways within intestinal epithelial cells drives RegIIIγ under steady-state conditions (Brandl et al., 2008; Vaishnava et al., 2008). Identifying the microbial molecules, innate immune receptors and signaling pathways that drive RegIIIγ expression during homeostasis is an important topic for future research.
Methods
Mice and Reagents
Wild-type C57BL/6 mice were purchased from Jackson Labs. TLR5-deficient, IL-22-deficient, AHR-deficient and IL-23p19-deficient mice were obtained as previously described (Fernandez-Salguero et al., 1995; Ivanov et al., 2009; Kinnebrew et al., 2010; Zenewicz et al., 2007). Flt3L-deficient mice were purchased from Taconic Farms. IL-12p40-YFP reporter mice and IL-10-deficient mice were purchased from Jackson Laboratories. TLR5-deficient mice were crossed to the Ly5.1+ (CD45.1) C57BL/6 background. The generation of CCR2.DTR mice has been previously described.(Hohl et al., 2009) Mice were maintained in a specific pathogen-free barrier facility at Memorial Sloan-Kettering Cancer Institute and experiments were performed according institution-approved animal protocols. In experiments where the flagellin response was assessed in vivo, 1 μg of “ultrapure” flagellin (Invivogen), derived from Salmonella typhimurium, was injected intravenously through the tail vein. Control mice received PBS. In vitro stimulation of single-cell suspensions from the lamina propria was performed using a concentration of 200 ng/mL of flagellin. Diphtheria toxin (List Biological Laboratories) was administered every other day at a dose of 10 ng/g starting 6 days prior to injection of flagellin. Mice received a total of 3 doses.
Generation of Bone Marrow Chimeric Mice
Six-week old C57BL/6 mice were lethally irradiated with 950 cGy using a 137Cs source. Within 24 hours of irradiation, mice received an intravenous injection of a 1:1 mixture of 2.5 × 106 congenically marked bone marrow cells harvested from the femurs and tibias of wild-type C57BL/6, TLR5-deficient, IL-22-deficient, IL-23p19-deficient, IL-10-deficient, CD11c.DTR, or CCR2.DTR mice as indicated in the figures. Mice were allowed to rest for at least 7 weeks following irradiation to allow for reconstitution of the hematopoietic compartment before being used for experiments.
Real-Time PCR Analysis
For measurement of RegIIIγ expression in the proximal small intestine, a 2 cm segment located 1 cm distal to the pylorus was excised and preserved in RNAlater (Ambion) at −20°C. For cytokine expression in lamina propria tissue, a 2 cm segment of the proximal small intestine was isolated. Peyer’s patches were excised from this segment and the remaining tissue was washed with PBS and incubated for 10 minutes in a 10 mM buffer of EDTA in HBSS at 37°C to dissociate the epithelial cells from the lamina propria. The lamina propria tissue was pulse-vortexed and washed two times in PBS. Tissues were homogenized in 1 mL of Trizol (Invitrogen) for 2:15 minutes using the Mini-Beadbeater-16 (Biospec). The manufacturer’s protocol was followed for RNA extraction from tissues homogenized in Trizol. RNA was isolated from single-cell suspensions using the RNAeasy Mini Kit (Qiagen). Isolated RNA was reverse-transcribed using the Quantitect Reverse Transcription Kit (Qiagen). Gene expression was assessed by quantitative real-time PCR using Taqman Expression Assay pre-designed probes (Applied Biosystems) and the Step One Plus Real Time PCR system (Applied Biosystems). Signals were normalized to Hprt1 mRNA expression. Normalized values were used to calculate relative expression with ΔΔCt analysis. Taqman Expression Assay IDs are listed in Table S1.
Detection of Cytokines by ELISA
Mice were anesthetized with isofluorane. Blood was collected retro-orbitally using a glass Pasteur pipet. Blood was stored at 4°C overnight to allow for clotting. IL-22 protein was measured in blood serum or lamina propria cell culture supernatants using an IL-22 ELISA Construction Kit (Antigenix).
Isolation of Lamina Propria Cells
The small intestine was carefully separated from the mesentery. Peyer’s patches were excised, and the intestine was opened longitudinally and washed twice in PBS. Epithelial cells were separated from the underlying lamina propria by incubation in HBSS containing 10 mM EDTA for 10 min. at 37°C with gentle rotation in a water bath shaker (125 rpm). Lamina propria tissue was pulse-vortexed and washed two times in PBS. The remaining tissue was finely chopped with a razor blade and digested in a solution of 0.5 mg/mL Collagenase type IV (Worthington), 40 μg/mL DNase I, and 8% fetal calf serum in HBSS for 20 min at 37°C with gentle rotation in a shaker (125 rpm). Tissue digestion was repeated two times. Leukocytes were isolated from the supernatant using a Percoll (MP Biomedicals) gradient separation method in which the cells were resuspended in 40% Percoll and underlayered with 80% Percoll followed by centrifugation at 2500 RPM for 20 min. The interface was collected for FACS analysis or FACS sorting. Cells numbers were determined using a Z2 Coulter counter (Beckman Instruments).
Antibodies and Flow Cytometry
The following antibodies were purchased from BD Biosciences: anti-CD45.2 (104, Alexa700 or PerCP Cy5.5); anti-CD45.1 (A20, PE or APC); anti-CD11c (HL3, APC or PE-Cy7); anti-CD11b (M1/70, PerCP-Cy5.5); anti-CD45 (30-F11, PE-Cy7); anti-THY1 (53-2.1, APC); anti-CD4 (RM4-5, Pacific Blue). The following antibodies were purchased from eBioscience: MHC class II (M5/114.15.2, Alexa700; eBioscience); anti-CD103 (OX62, PE); anti-CD45 (30-F11, Pacific Blue); anti-NKp46 (29A1.4, PE); anti-CD3ε (145-2C11, PE-Cy7); anti-IL-22 (IL22JOP, APC). A BD LSR II flow cytometer was used for FACS analysis. Lamina propria dendritic cell subsets were sorted with a FACSAria (BD Biosciences) to 95 – 99% purity. DAPI (Sigma) was used to distinguish live cells from dead cells during cell sorting. Purified, carrier-free anti-IL-6R (10 μg/mL, BD Pharmingen), IL-1Ra (10 μg/mL, R&D), anti-IL-23p19 (10 μg/mL, eBioscience) in the presence or absence of flagellin were used to block cytokine signaling in overnight cell cultures of cells purified from the lamina propria. Recombinant mouse IL-23 (rmIL-23) (40 ng/mL, eBioscience) was used to stimulate IL-22 secretion by lamina propria cells in vitro. Intracellular cytokine staining for IL-22 was preformed on freshly isolated lamina propria cells that were stimulated in vitro with rmIL-23 (40 ng/mL, eBioscience) for 2.5 hours in the presence of Brefeldin A.
Statistical Tests
Statistical analysis was conducted using GraphPad Prism software. Unless otherwise noted, the student’s unpaired t-test was used as statistical test for significance. P-values < 0.05 were considered significant. Error bars denote ± s.e.m.
Supplementary Material
Acknowledgements
This work was supported by NIH grants RO1-AI042135, R37-AI039031 and PO1-CA023766 to E.G.P. NIH Medical Scientist Training Program grant GM07739 to Cornell/RU/MSKCC Tri-Institutional MD-PhD Program supported M.A.K. and C.G.B. NIH K08 Fellowship AI07998 supported T.A.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahern PP, Schiering C, Buonocore S, Mcgeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, Mcallister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Wirtz S, Blessing M, Pirhonen J, Strand D, Bechthold O, Frick J, Galle PR, Autenrieth I, Neurath MF. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J. Clin. Invest. 2003;112:693–706. doi: 10.1172/JCI17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455:804–807. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Didonato JA, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. C.f.D.C.a.P. Nosocomial enterococci resistant to vancomycin--United States, 1989-1993. MMWR Morbidity and mortality weekly report. 1993;42:597–599. [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun C-M, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crellin NK, Trifari S, Kaplan CD, Satoh-Takayama N, Di Santo JP, Spits H. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity. 2010;33:752–764. doi: 10.1016/j.immuni.2010.10.012. [DOI] [PubMed] [Google Scholar]
- De Luca A, Zelante T, D’angelo C, Zagarella S, Fallarino F, Spreca A, Iannitti RG, Bonifazi P, Renauld JC, Bistoni F, et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010;3:361–373. doi: 10.1038/mi.2010.22. [DOI] [PubMed] [Google Scholar]
- Donskey CJ. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin. Infect. Dis. 2004;39:219–226. doi: 10.1086/422002. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- Hidron Alicia I., Edwards Jonathan R., Patel J, Horan Teresa C., Sievert Dawn M., Pollock Daniel A., Fridkin Scott K. NHSN Annual Update:Antimicrobial Resistant Pathogens Associated With Healthcare Associated Infections: Annual Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6:470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Takeda K. Regulatory mechanisms of immune responses to intestinal bacteria. Mucosal Immunol. 2009;2:187–196. doi: 10.1038/mi.2009.8. [DOI] [PubMed] [Google Scholar]
- Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J. Exp. Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnebrew MA, Ubeda C, Zenewicz LA, Smith N, Flavell RA, Pamer EG. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J. Infect. Dis. 2010;201:534–543. doi: 10.1086/650203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreymborg K, Etzensperger R, Dumoutier L, Haak S, Rebollo A, Buch T, Heppner FL, Renauld J-C, Becher B. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J. Immunol. 2007;179:8098–8104. doi: 10.4049/jimmunol.179.12.8098. [DOI] [PubMed] [Google Scholar]
- Laffont S, Siddiqui KRR, Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur. J. Immunol. 2010;40:1877–1883. doi: 10.1002/eji.200939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat. Rev. Micro. 2008;6:776–788. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J. Clin. Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol. Rev. 2011;241:206–227. doi: 10.1111/j.1600-065X.2011.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- Pabst O, Bernhardt G. The puzzle of intestinal lamina propria dendritic cells and macrophages. Eur. J. Immunol. 2010;40:2107–2111. doi: 10.1002/eji.201040557. [DOI] [PubMed] [Google Scholar]
- Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr H-A, Hirth S, Weigmann B, Wirtz S, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt RL, Hong S, Kang SJ, Wang ZE, Locksley RM. Visualization of IL-12/23p40 in vivo reveals immunostimulatory dendritic cell migrants that promote Th1 differentiation. J. Immunol. 2006;177:1618–1627. doi: 10.4049/jimmunol.177.3.1618. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanos SL, Vonarbourg C, Mortha A, Diefenbach A. Control of epithelial cell function by interleukin-22-producing ROγt+ innate lymphoid cells. Immunology. 2011;132:453–465. doi: 10.1111/j.1365-2567.2011.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 2010:1–7. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KHG. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O’Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Förster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MRM, Kamboj M, Pamer EG. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J. Clin. Invest. 2010;120:4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu S, Fujimoto K, Jang MH, Yang B-G, Jung Y-J, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat. Immunol. 2006;7:868–874. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- Vaishnava S, Behrendt C, Ismail A, Eckmann L, Hooper L. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. USA. 2008 doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Maele L, Carnoy C, Cayet D, Songhet P, Dumoutier L, Ferrero I, Janot L, Erard F, Bertout J, Leger H, et al. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3(neg)CD127+ immune cells in spleen and mucosa. J. Immunol. 2010;185:1177–1185. doi: 10.4049/jimmunol.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt W-D, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Varol C, Zigmond E, Jung S. Securing the immune tightrope: mononuclear phagocytes in the intestinal lamina propria. Nat. Rev. Immunol. 2010;10:415–426. doi: 10.1038/nri2778. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Sanders CJ, Frias A, Sloane VM, Xu J, Neish AS, Rojas M, Gewirtz AT. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J. Immunol. 2008;180:8280–8285. doi: 10.4049/jimmunol.180.12.8280. [DOI] [PubMed] [Google Scholar]
- Zenewicz L, Yancopoulos G, Valenzuela D, Murphy A, Karow M, Flavell R. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, De Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.