Abstract
An analytical method was developed and validated for the quantitative determination of irinotecan, its active metabolite SN38, and glucuronidated SN38 (SN38-G) in both porcine and human plasma. Calibration curves were linear within the concentration range of 0.5–100 ng/mL for SN38 and SN38-G, and 5–1000 ng/mL for irinotecan. Sample pretreatment involved solid-phase extraction of 0.1 mL aliquots of plasma. Irinotecan, SN38, SN38-G, and the internal standards, irinotecan-d10, tolbutamide, and camptothecin, respectively, were separated on a Waters ACQUITY UPLC™ BEH RP18 column (2.1×50 mm, 1.7 µm), using a mobile phase composed of methanol and 0.1% formic acid. Accuracy of quality control samples in human plasma ranged from 98.5–110.3%, 99.5–101.7% and 96.2–98.9% for irinotecan, SN38, and SN38-G, respectively. Precision of the three analytes in the same order ranged from 0.8–2.8%, 2.4–5.7%, and 2.4–2.8%. All three analytes proved stable in plasma through four freeze/thaw cycles, as well as through six hours in whole blood at room temperature. The method was likewise validated in porcine plasma with comparable accuracies and precisions also within the generally acceptable range. The validated method was applied to both preclinical and clinical trials involving hepatic chemoembolization of irinotecan drug-eluting beads to study the pharmacokinetics of the three analytes.
Keywords: Irinotecan, SN38, camptothecin, mass spectrometry, ultra-high performance liquid chromatography
1. Introduction
Irinotecan (7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin, CPT-11) is a synthetically designed analogue of camptothecin that inhibits DNA topoisomerase [1]. It is a water-soluble prodrug that, upon cleavage of the carbamate bond between the camptothecin moiety and the dipiperidino side chain by carboxyesterase enzymes, forms the more active metabolite SN38 (7-ethyl-10-hydroxycamptothecin). The active metabolite SN38 has been shown to be up to 20,000 times more potent at inhibiting topoisomerase I than irinotecan [2, 3]. Topoisomerase I has also been shown to be a useful pharmacodynamic marker for irinotecan efficacy [4]. Both irinotecan and SN38 undergo spontaneous interconversion between their carboxylate and lactone form, the latter of which is the pharmacologically active form of SN38 [5]. SN38 undergoes glucuronidation by uridine glucuronosyl transferases (UGTs) to form SN38-G, and recently the glucuronidation ratio (SN38-G:SN38) has become a useful pharmacokinetic marker to help define risks to severe side effects [6].
Irinotecan has recently been formulated into drug-eluting beads (DEB) for trans-arterial hepatic chemoembolization (TACE) in order to increase local drug concentration near hepatic metastases from such primary cancers as colorectal [7] and ocular melanoma [8]. Although the DEB-irinotecan formulation has been evaluated in preclinical and clinical studies [9, 10], the pharmacokinetics of irinotecan, SN38 and SN38G following administration of DEB-irinotecan are not well characterized.
There have been numerous analytical methods developed for the determination of irinotecan and its metabolites in various biological matrices (plasma, urine, feces) from numerous species (mice, rat, dog, pig, human). Ramesh et al provides a general overview of the more recent methods [11]. Table 1 lists the analytical methods for irinotecan and metabolites that utilized high performance liquid chromatography with or without mass spectrometry. Only one method in the literature is suitable for simultaneously analyzing irinotecan, SN38, and SN38-G using LC-MS/MS [12]. However, this method had a run time that was 6 minutes and was not applied to samples from TACE with irinotecan DEB. Another method was applicable for TACE with DEB in sheep and human plasma but did not provide for analysis of SN38-G [13]. Thus, there is a need for a simultaneous LC-MS/MS method for irinotecan, SN38, and SN38-G in human plasma. The porcine model is a popular preclinical species for studies on irinotecan DEBs [14, 15], thus there is also a need for validated analytical methods in porcine matrices.
Table 1.
Analytical methods for the analysis of CPT-11 and metabolites in the literature
| Extraction | Analytes (Internal standard) | Assay | Matrix | LLOQa (ng/mL) | Reference |
|---|---|---|---|---|---|
| SPE | CPT-11, SN38 (Camptothecin) | HPLC-FLD | Human plasma | 1 | Barilero et al (1992) [17] |
| PPT | CPT-11, SN38 (Camptothecin) | HPLC-FLD | Human plasma | 10, 2 | Rivory et al (1994) [18] |
| LLE | CPT-11, SN38 (Camptothecin) | HPLC-FLD | Human plasma | 30, 1 | Sumiyoshi et al (1995) [19] |
| PPT | CPT-11, SN38 (Camptothecin) | HPLC-FLD | Human plasma | 2 | de Bruijn et al (1997) [20] |
| PPT | SN38 (none) | HPLC-FLD | Rat plasma | 5 | Kaneda et al (1997) [21] |
| PPT | CPT-11, SN38 (none) | HPLC-FLD | Rat and dog plasma | Chollet et al (1998) [22] | |
| PPT | CPT-11, SN38 SN38-G, APC (none) |
HPLC-FLD | Human plasma, urine, feces |
2, 100, 100 | Sparreboom et al (1998) [23] |
| LLE | SN38 (Camptothecin) | HPLC-FLD | Human plasma | 0.05 | de Bruijn et al (1999) [24] |
| SPE | CPT-11, SN38, SN38-G (Camptothecin) | HPLC-FLD | Rat plasma | 5, 5, 2.5 | Kurita et al (1999) [25] |
| PPT | CPT-11 (Camptothecin) | LC-MS | Human serum | 10 | Ragot et al (1999) [26] |
| LLE | SN38 (Camptothecin) | LC-MS | Human serum | 0.5 | Ragot et al (1999) [26] |
| PPT | CPT-11, SN38 (Camptothecin) | HPLC-FLD | Human plasma | 1, 0.5 | Escoriaza et al (2000) [27] |
| PPT | CPT-11, SN38, SN38-G | HPLC-FLD | Human plasma | 5, 1, 2.5 | Sai et al (2002) [28] |
| APC (none) | 2.5 | ||||
| PPT | CPT-11, SN38 (none) | HPLC-FLD | Human blood, RBCs | 5, 5 | de Jong et al (2003) [29] |
| LLE | SN38 (Camptothecin) | HPLC-FLD | Dog plasma | 1 | Guo et al (2003) [30] |
| PPT | SN38 (Camptothecin) | LC-MS/MS | Human plasma | 0.05 | Khan et al (2003) [31] |
| PPT | CPT-11, SN38, SN38-G | HPLC-FLD | Human plasma | 5, 0.5, 2 | Owens et al (2003) [32] |
| APC | 2 | ||||
| PPT | CPT-11, SN38 (Camptothecin) | LC-MS/MS | Mouse plasma, tissue | 0.5, 0.5 | Bardin et al (2005) [33] |
| PPT | SN38 (Camptothecin) | LC-MS/MS | Mouse plasma, tissue | 0.5, 1 | Khan et al (2005) [34] |
| SPE | CPT-11, SN38, APC (Camptothecin) | LC-MS/MS | Human plasma, liver microsomes | 1.5, 3.1, 0.78 | D’Esposito et al (2008) [35] |
| PPT | CPT-11, SN38 (Camptothecin) | LC-MS/MS | Human plasma | 10, 0.5 | Zhang et al (2009) [36] |
| PPT | CPT-11, SN38, SN38-G | LC-MS/MS | Human plasma | 0.5, 0.2, 0.5 | Corona et al (2010) [12] |
| APC, NPC (Camptothecin) | 0.5, 0.2 |
Abbreviations: LLOQ, lower limit of quantitation; PPT; SPE, solid phase extraction; HPLC-FLD, high performance liquid chromatography with fluorescence detection; LC-MS/MS, liquid chromatography with tandem mass spectrometric detection; SN38-G, glucuronide of SN38; APC, (7-ethyl-10-[4-N-(5-aminopentanoicacid)-1-piperidino]carbonyloxycamptothecin); NPC, (7-ethyl-10-[4-amino-1-piperidino]carbonyloxycamptothecin); RBCs, red blood cells;
None of the previously validated LC-MS/MS methods utilized ultra-high performance liquid chromatography (uHPLC), which affords faster run times with sharper signal peaks. This method will provide the first uHPLC-MS/MS analysis for the simultaneous and sensitive analysis of irinotecan, SN38, and SN38-G in both pig and human plasma.
2. Experimental
2.1. Chemicals and reagents
Irinotecan, tolbutamide, and camptothecin were purchased from Sigma-Aldrich (St. Louis, MO, USA). SN38 and SN38-G were custom synthesized. Irinotecan-d10 was purchased from Toronto Research Chemicals (Toronto, ON, Canada). Formic acid (98%) was obtained from Fluka (via Sigma-Aldrich, St. Louis, MO). Optima grade methanol was purchased from Fisher Scientific (Pittsburgh, PA). Deionized water was generated with a Hydro-Reverse Osmosis system (Durham, NC, USA) connected to a Milli-Q UV Plus purifying system (Billerica, MA, USA). Drug-free heparinized pig plasma was purchased from Innovative Research (Novi, MI). Drug-free heparinized human plasma was obtained from the National Institutes of Health Clinical Center Blood Bank (Bethesda, MD, USA).
2.2. Preparation of stock solutions and standards
Master stock solutions of irinotecan, SN38, and SN38-G were prepared individually in methanol at a concentration of 0.1 mg/mL and stored in glass tubes at −80 °C. From the master stock solution, a working solution containing 25 µg/mL of irinotecan and 2.5 µg/mL of SN38 and SN38-G were prepared in methanol on a monthly basis and stored at −80 °C between use. Serial dilutions were prepared from this working solution for the preparation of calibration and quality control (QC) samples. Master stocks of the internal standards, irinotecan-d10, tolbutamide, and camptothecin were individually prepared in methanol at a concentration of 1.0 mg/mL. From the master stocks, working solutions of the internal standards were prepared by dilution to 50 µg/mL (irinotecan-d10) and 10 µg/mL (tolbutamide and camptothecin) in methanol. Irinotecan-d10 was stored in amber vials due to its susceptibility to photodegradation. All the master and working internal standard solutions were stored at −80 °C.
Final concentrations of calibration standards for SN38 and SN38-G were 0.5, 1, 2, 5, 10, 25, 50 and 100 ng/mL: final irinotecan concentrations were 5, 10, 20, 50, 100, 250, 500 and 1000 ng/mL. QC samples were prepared in batch by adding plasma to the required amount of working solution in a volumetric flask to achieve SN38 and SN38-G concentrations of 1.5, 15, and 75, and irinotecan concentrations of 15, 150, 750 and 5000 (dilution QC) ng/mL. All QCs were aliquoted into cryovials and stored at −80 °C.
2.3. Sample preparation
Calibration standards were prepared by spiking 10 µL of the appropriate working solution (containing all three analytes) into 240 µL of blank human plasma in cryovials, for a total volume of 250 µL. The total amount of methanol added was identical in each sample (4% v/v). After vortexing for 15 sec, 100 µL was added to each of two glass centrifuge tubes (Kimble, Vineland, NJ) per concentration. QC samples were thawed at room temperature, vortex-mixed and then aliquoted into glass centrifuge tubes, with each QC level (low, mid, high, and dilution) containing 5 replicates. Each of the 5000 ng/ml irinotecan-only dilution QC samples were diluted 10-fold by adding 90 µL blank plasma (pig or human) to 10 µL of the dilution QC plasma, prior to addition of the internal standards. Patient and porcine samples were thawed at room temperature, vortex-mixed for 20 sec to ensure uniformity, and a volume of 100 µL of each sample was transferred into a glass centrifuge tube. To each tube, 200 µL of 1% formic acid in H2O was added, which contained 5.0 ng/mL of tolbutamide and camptothecin, and 25 ng/mL irinotecan-d10. Tubes were then vortexed for 30 sec and centrifuged for 5 min at 1000 × g, before the solid-phase extraction (SPE) procedure.
Prior to the SPE procedure, Bond Elute Plexa (Agilent, Santa Clara, CA) (30mg, 1.0 mL) SPE cartridges were conditioned with 1.0 mL methanol followed by 1.0 mL of H2O. The pretreated plasma samples were then loaded onto individual SPE cartridges, allowed to flow through, then rinsed by 1.0 mL of 5% methanol. The analytes and internal standards were eluted with 1.5 mL of methanol and collected into glass drying tubes, which was then evaporated to dryness under desiccated air in a water bath at 40 °C in a Zymark TurboVap LV (Hopkinton, MA, USA). The residue was reconstituted in 600 µL of a mixture of methanol/10 mM ammonium acetate, pH 6.0 (40/60, v/v), and vortex-mixed for 15 s. Finally, each solution was transferred to a glass Waters HPLC vial for injection, where 10 µL was injected into the UPLC™-MS/MS system.
2.4. Equipment
The experiments were conducted on an AB SCIEX 5500 QTRAP (AB SCIEX, Foster City, CA) coupled to a Waters ACQUITY UPLC™ (Waters Corp, Milford, MA) system, which included a binary pump, a refrigerated autosampler, a mobile phase vacuum degassing unit, and a temperature-controlled column compartment. The autosampler was maintained at 4 °C and the column compartment at 45 °C. An ACQUITY UPLC™ BEH RP18 column (2.1× 50 mm, 1.7 µm) was utilized for chromatographic separation and guarded by a VanGuard EBH RP18 pre-column. Samples were eluted using a step-wise gradient at a flow rate of 0.4 mL/min. Mobile phase A was 0.1% formic acid and mobile phase B was Optima grade methanol. The gradient was as follows: the initial condition, 35% B, was gradually increased to 75% within the first 1.5 min, then returned to the initial condition (35% B) at 2.5 min, with a total run time of 3 min. Column eluent was directed into the electrospray ionization source of the mass spectrometer, operated in the positive ion. General mass spectrometer conditions were as follows: curtain gas, 30 psi; ionspray voltage, 5000 V; temperature, 600 °C; gas source 1, 50 psi; gas source 2, 60 psi, and declustering potential, 70 V. Compound-specific mass spectrometer conditions are listed in Table 2. The chromatographic data were acquired and analyzed using the Analyst v1.5.2 and MultiQuant v2.0 software package (AB SCIEX, Foster City, CA).
Table 2.
Compound-specific mass spectrometer MRM settings
| Compound | Precursor ion m/z |
Product ion m/z |
CEa (volts) |
EP (volts) | CXP (volts) |
|---|---|---|---|---|---|
| Irinotecan | 587.3 | 124.2 | 49 | 3.2 | 6.3 |
| Irinotecan-d10 | 597.4 | 133.3 | 48 | 3.2 | 6.3 |
| SN38 | 393.2 | 349.2 | 35 | 3.6 | 10 |
| Tolbutamide | 271.1 | 155.1 | 26 | 10.2 | 5.1 |
| SN38-G | 569.2 | 393.2 | 40 | 5.9 | 15 |
| Camptothecin | 349.1 | 305.2 | 32 | 11 | 5.6 |
Abbreviations: CE, Collision energy; EP, Entrance potential; CXP, collision cell exit potential; MRM, multiple reaction monitoring
2.5. Validation procedures
2.5.1. Calibration
Validation of the method, with respect to accuracy and precision, was carried out according to procedures previously reported in detail by the United States Food and Drug Administration [16] in both drug-free human and porcine plasma. Calibration standards at eight concentration levels were prepared freshly by mixing the working standard solutions of the three analytes and drug-free human plasma. Quality control (QC) samples were prepared in batch at low, middle and high concentrations before aliquotting and storing at −80 °C. Validation runs included blank (zero concentration) and internal standard only samples in duplicate, along with duplicate calibration standards and quintuplet QC samples at each QC level. Higher plasma concentrations were anticipated for the prodrug irinotecan, therefore a 10-fold dilution QC at 5000 ng/mL irinotecan was analyzed to test the accuracy and reproducibility of diluting samples which upon initial analysis are found to exceed the ULOQ of calibration for irinotecan (1000 ng/mL).
Calibration curves were calculated by least-squares linear regression analysis of the peak area ratio of analytes and their internal standards versus the drug concentration of the nominal standard. The regression parameters of slope, intercept and correlation coefficient were calculated using a weighting factor of 1/x2 for all three analytes in both human and porcine plasmas. The linearity was evaluated by comparing the correlation coefficient (r2), residuals, and errors between nominal and back-calculated concentrations of calibration standard samples. The zero concentration (blank), and internal standard only sample were used to visually verify the purity of the reagents and the lack of other potentially interfering substances, and were not considered for the regression analysis of standards. This calibration curve was then used to calculate measured QC concentrations, and that of unknown samples, by interpolation.
The lower limit of quantification (LLOQ) of the assay was assessed by determining the concentration of the analytes at which the values for precision and accuracy were less than 20%, and the signal to noise (S/N) was ≥5. At least six different lots of drug-free human plasma were used to assess accuracy and reproducibility at the LLOQ, based on the deviation from nominal concentration. The same LLOQ specificity experiments were not assessed in drug-free porcine plasma due to the relative short supply compared to drug-free human plasma.
2.5.2 Accuracy and Precision
The accuracy and precision of the assay were assessed by the mean relative percentage deviation (DEV) from the nominal concentrations using the following equation:
The between-run precision (BRP), expressed as a percentage relative standard deviation, was defined as:
where n represents the number of replicate observations within each run. For each concentration, the estimate of the within-run precision (WRP) was calculated as:
Estimates of the between-run precision were obtained by one-way analysis of variance (ANOVA) using the run day as the classification variable. The between-groups mean square (MSbet), the within-groups mean square (MSwit), and the grand mean (GM) of the observed concentrations across runs were calculated using Graph Pad Prism. Accuracy and precision of the assay were assessed at three QC levels (n=20 each) in both drug-free human and porcine plasma.
2.5.3. Recovery and Matrix Effects
The overall recovery for irinotecan, SN38, and SN38-G in human plasma, expressed as percentages, was determined at 20 and 500 ng/mL for irinotecan; 2 and 50 ng/mL for both SN38 and SN38-G. Recovery experiments were performed with four replicates and were used to compare samples spiked into drug-free human plasma versus reconstitution solution. Post-column infusion was carried to examine the matrix effect, and different lots of drug-free human plasma were used to prepare QC samples and calibration curves that were analyzed on the same day, in order to assess relative matrix effect. These experiments were not performed in porcine plasma.
2.5.6. Stability
Storage stability of the drug in the reconstitution solution was assessed by reinjection of calibrator and QC samples after remaining in the autosampler (4 °C) for 24 hours following initial injection. The freeze/thaw (F/T) stability of all three analytes in human plasma was evaluated following three F/T cycles, using QC samples at low and high concentrations, in triplicate. Each freeze cycle lasted at least 12 hours at −80 °C and the concentration of the analytes after each storage period were compared to the concentration of freshly prepared samples in the same analytical run. These experiments were not performed in porcine plasma.
Bench-top stability in heparinized drug-free pooled human whole blood was assessed for all three analytes. Low and high concentration calibrators were spiked into 1.0 mL of whole blood and left at room temperature for 1, 2, 4, and 6 hours (chosen to best mimic clinical situations), with each concentration at each time analyzed in triplicate. The concentrations of the analytes after each time point were compared to the concentrations of freshly prepared samples in the same analytical run. These experiments were not performed in porcine blood.
2.6. Method Application
To test the applicability of the method we sampled one preclinical (porcine) cycle (n=1) as a proof-of-concept and three clinical cycles (n=2) following a dose of 100 mg of irinotecan loaded onto 1.0 mL volume of drug-eluting beads (DEBs), then dissolved in 10 mL of deionized water for administration via hepatic chemoembolization. Blood samples were drawn into heparin tubes from the pig through 4 hours, and from humans through 78 hours prior to processing into plasma, and stored at −80 °C until analysis. Due to the complexity of the surgery in order to properly administer the drug-eluting beads, only two subjects were available to analyze clinically.
3. Results and Discussion
3.1. Specificity
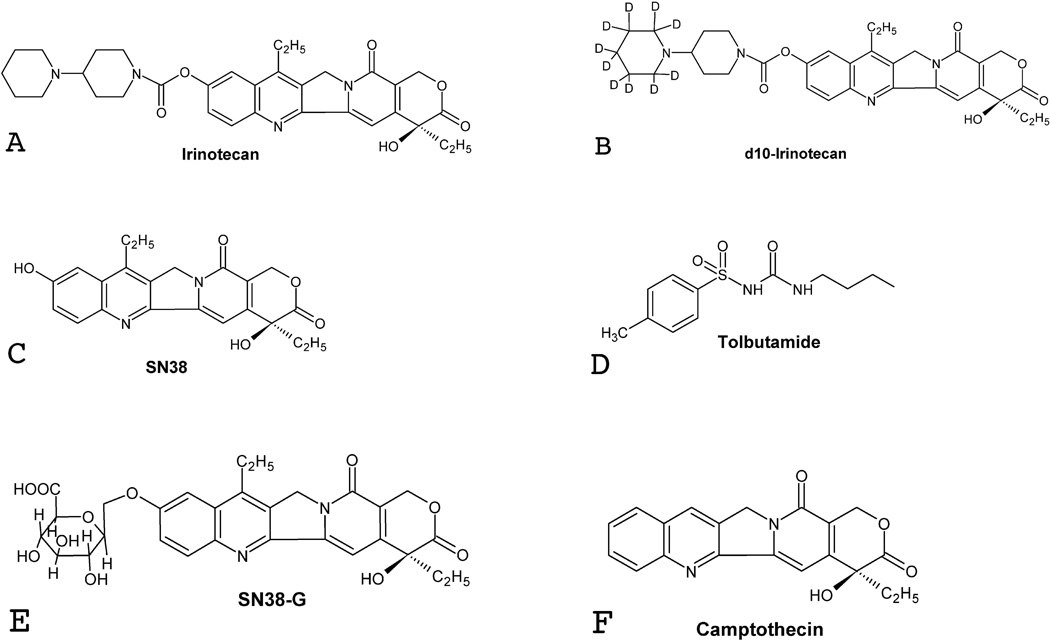
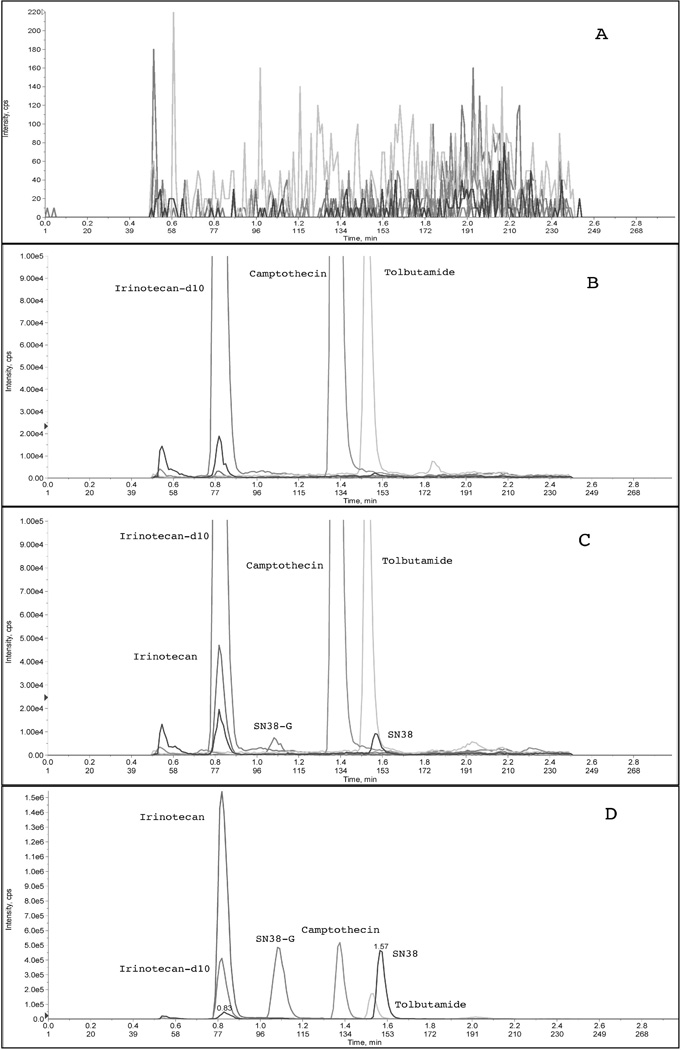
Three different internal standards were chosen during the method development process: irinotecan-d10 for irinotecan, tolbutamide for SN38, and camptothecin for SN38-G (Figure 1). Figure 2 depicts typical chromatograms of a solid-phase extract of a blank human plasma sample (Figure 2A), a solid-phase extract of a plasma sample spiked with the three internal standards (Figure 2B), the lower limit of quantification (LLOQ) sample extract (Figure 2C), and a 4-hr post dose clinical sample extract (Figure 2D). The selectivity of the analysis is shown by symmetrical resolution of the peaks, with no interference around the retention time of the analyte in drug-free plasma obtained from six different individuals. Within the three minute chromatographic run time, retention times were as follows: irinotecan and irinotecan-d10 eluting at tR=0.8 min, SN38-G eluting at tR=1.1 min, camptothecin, at tR=1.4 min, tolbutamide eluting at tR=1.55 min, and SN38 eluting at tR=1.57 min (Figure 2).
Figure 1. Structures of analytes and their corresponding internal standards.
The structures of the prodrug irinotecan (A), its deuterated analog and internal standard (d10-irinotecan; B), the active metabolite SN38 (C), its internal standard tolbutaminde (D), glucuronidated SN38 (SN38-G; E), and its internal standard camptothecin (F).
Figure 2.
LC-MS/MS chromatograms of A) drug-free human plasma extract, B) internal standards only (zoomed in to demonstrate lack of analyte peaks), C) the lower limit of quantification (LLOQ) sample containing all analytes and internal standards (zoomed in to demonstrate sufficient peak shape for analytes), and D) a clinical sample at 4 hours post-dose.
3.2. Validation characteristics
The calculated detector response of the irinotecan/irinotecan-d10, SN38/tolbutamide and SN38-G/camptothecin ratio versus the nominal concentration displayed a linear relationship in the tested range of 0.5–100 ng/mL for SN38 and SN38G and 5–1000 ng/mL for irinotecan. Variance increased proportionally with drug concentration, therefore a weighting factor was applied that was inversely proportional to the variance at the given concentration level 1/x2, with x being the nominal analyte concentrations. Using least-squares linear-regression, mean (± standard deviation), correlation coefficients of 0.9992 ± 0.00043 (range: 0.9987–0.9996), 0.9985 ± 0.00057 (range: 0.9978–0.9992), and 0.9973 ± 0.00159 (range: 0.9959–0.9989), were obtained for irinotecan, SN38, and SN38-G, respectively, in human plasma.
In blank human plasma spiked with all three analytes at their LLOQ (0.5 ng/mL for SN38 and SN38-G, 5.0 ng/mL for irinotecan), all of the 8 calibration samples run on four separate days were well below the required ± 20% deviation of the nominal value and had signal-to-noise ratios > 5. The mean percent deviation from nominal value for these eight LLOQ samples was 1.99%, 2.53% and 3.40% for irinotecan, SN38, and SN38-G, respectively (Table 3). Additionally, samples at the LLOQ concentration level were prepared on the same day from five different lots of human plasma and were analyzed as QCs from the same calibration curve, which produced concentrations within 6% deviation of the nominal value for all three analytes.
Table 3.
Back-calculated concentrations from calibration curves run in duplicate on four occasions in human plasma
| Table 3A Irinotecan | |||||
|---|---|---|---|---|---|
| Nominal (ng/mL) |
GMa (ng/mL) |
S.D. (ng/mL) |
DEV(%) | R.S.D.(%) | n |
| 5 | 5.10 | 0.085 | 1.99 | 1.67 | 8 |
| 10 | 9.67 | 0.294 | −3.28 | 3.04 | 8 |
| 20 | 19.6 | 0.598 | −1.81 | 3.04 | 8 |
| 50 | 49.9 | 0.906 | −0.16 | 1.82 | 8 |
| 100 | 101.4 | 3.00 | 1.46 | 2.96 | 8 |
| 250 | 254.4 | 4.29 | 1.76 | 1.69 | 8 |
| 500 | 508.8 | 15.2 | 1.76 | 2.98 | 8 |
| 1000 | 982.8 | 22.9 | −1.72 | 2.33 | 8 |
| Table 3B SN38 | |||||
|
Nominal (ng/mL) |
GMa (ng/mL) |
S.D. (ng/mL) |
DEV(%) | R.S.D.(%) | n |
| 0.5 | 0.51 | 0.013 | 2.53 | 2.63 | 8 |
| 1.0 | 0.97 | 0.021 | −2.60 | 2.14 | 8 |
| 2.0 | 1.93 | 0.034 | −3.33 | 1.77 | 8 |
| 5.0 | 4.79 | 0.143 | −4.24 | 3.00 | 8 |
| 10 | 9.88 | 0.269 | −1.22 | 2.72 | 8 |
| 25 | 25.4 | 1.18 | 1.76 | 4.64 | 8 |
| 50 | 51.6 | 1.84 | 3.14 | 3.57 | 8 |
| 100 | 103.9 | 3.18 | 3.97 | 3.06 | 8 |
| Table 3C SN38-G | |||||
|
Nominal (ng/mL) |
GMa (ng/mL) |
S.D. (ng/mL) |
DEV(%) | R.S.D.(%) | n |
| 0.5 | 0.52 | 0.025 | 3.40 | 4.91 | 8 |
| 1.0 | 0.95 | 0.062 | −5.11 | 6.56 | 8 |
| 2.0 | 1.97 | 0.114 | −1.68 | 5.82 | 8 |
| 5.0 | 4.73 | 0.160 | −5.45 | 3.40 | 8 |
| 10 | 10.02 | 0.528 | −0.21 | 5.27 | 8 |
| 25 | 25.9 | 0.627 | 3.57 | 2.42 | 8 |
| 50 | 51.4 | 1.46 | 2.69 | 2.84 | 8 |
| 100 | 102.4 | 3.35 | 2.38 | 3.27 | 8 |
Abbreviations: GM, grand mean; S.D., standard deviation; DEV, percent deviation from nominal value; R.S.D., relative standard deviation; n, total number of replicate observations within the validation runs.
Validation data for the analytical method in terms of accuracy and precision are summarized in Tables 3 and 4 for human plasma. Table 3 displays the data calculated from duplicate calibration curves on four separate days for all three analytes (n=8), all of which meet standard bioanalytical method requirements regarding accuracy and precision. QC samples run in quintuplicate at each concentration, on each of these same four days (n=20) also demonstrated acceptable accuracy and precision in human plasma (Table 4). Due to the anticipated inter-patient variation in irinotecan plasma levels, a dilution QC at 5000 ng/mL was run for irinotecan only. Values were back-calculated using the calibration curve from the same run. The assay was found to be accurate, within 10.3 % for all three analytes at all concentration levels, and precise with between-run and within-run precision error of less than 5.7 % (Table 4).
Table 4.
Assessment of accuracy and precision from quality control samples in human plasma
| Table 4A Irinotecan | ||||||
|---|---|---|---|---|---|---|
| Nominal (ng/mL) |
GMa (ng/mL) |
S.D. (ng/mL) |
DEV (%) |
BRP (%) |
WRP (%) |
n |
| 15 | 16.54 | 0.381 | 10.3 | - | 2.39 | 20 |
| 150 | 152.7 | 4.65 | 1.83 | 1.50 | 2.74 | 20 |
| 750 | 742.4 | 16.4 | −1.01 | 0.86 | 2.07 | 20 |
| 5000 | 4925 | 176.7 | −1.51 | 2.54 | 2.79 | 20 |
| Table 4B SN38 | ||||||
|
Nominal (ng/mL) |
GMa (ng/mL) |
S.D. (ng/mL) |
DEV (%) |
BRP (%) |
WRP (%) |
n |
| 1.5 | 1.53 | 0.063 | 1.72 | 2.45 | 3.49 | 20 |
| 15 | 14.9 | 0.654 | −0.45 | 3.47 | 3.11 | 20 |
| 75 | 75.4 | 4.26 | −0.51 | 5.74 | 2.42 | 20 |
| Table 4C SN38-G | ||||||
|
Nominal (ng/mL) |
GMa (ng/mL) |
S.D. (ng/mL) |
DEV (%) |
BRP (%) |
WRP (%) |
n |
| 1.5 | 1.48 | 0.053 | −1.06 | 2.47 | 2.83 | 20 |
| 15 | 14.4 | 0.321 | −3.79 | - | 2.26 | 20 |
| 75 | 72.8 | 2.63 | −2.90 | 2.72 | 2.28 | 20 |
Abbreviations: GM, grand mean; S.D., standard deviation; DEV, percent deviation from nominal value; WRP, within-run precision; BRP, between-run precision; n, total number of replicate observations within the validation runs.
A hyphen indicates MSwit>MSbet, thus BRP cannot be calculated and it was concluded that no additional variation was observed as a result of performing the assay in different runs.
Five samples at each concentration were run on four different occasions (n=20).
Tables 5 and 6 summarize the accuracy and precision of the calibrations standards and QCs, respectively, in porcine plasma. Comparable accuracy and precision was obtained in pig plasma that met the generally accepted method validation criteria.
Table 5.
Back-calculated concentrations from calibration curves run in duplicate on four occasions in porcine plasma
| Table 5A Irinotecan | |||||
|---|---|---|---|---|---|
| Nominal (ng/mL) |
GMa (ng/mL) |
S.D. (ng/mL) |
DEV(%) | R.S.D.(%) | n |
| 5 | 5.12 | 0.099 | 2.48 | 1.93 | 8 |
| 10 | 9.64 | 0.330 | −3.63 | 3.42 | 8 |
| 20 | 19.6 | 0.519 | −1.99 | 2.65 | 8 |
| 50 | 49.5 | 1.48 | −1.08 | 2.99 | 8 |
| 100 | 97.8 | 2.95 | −2.25 | 3.02 | 8 |
| 250 | 255.1 | 4.97 | 2.04 | 1.95 | 8 |
| 500 | 514.5 | 12.7 | 2.93 | 2.47 | 8 |
| 1000 | 1015.3 | 38.0 | 1.53 | 3.75 | 8 |
| Table 5B SN38 | |||||
|
Nominal (ng/mL) |
GMa (ng/mL) |
S.D. (ng/mL) |
DEV(%) | R.S.D.(%) | n |
| 0.5 | 0.51 | 0.026 | 2.84 | 5.03 | 8 |
| 1.0 | 0.98 | 0.039 | −2.04 | 3.97 | 8 |
| 2.0 | 1.90 | 0.077 | −4.86 | 4.06 | 8 |
| 5.0 | 4.75 | 0.210 | −4.94 | 4.43 | 8 |
| 10 | 9.74 | 0.228 | −2.56 | 2.34 | 8 |
| 25 | 25.4 | 0.659 | 1.39 | 2.60 | 8 |
| 50 | 52.3 | 1.82 | 4.64 | 3.48 | 8 |
| 100 | 105.6 | 4.79 | 5.55 | 4.55 | 8 |
| Table 5C SN38-G | |||||
|
Nominal (ng/mL) |
GMa (ng/mL) |
S.D. (ng/mL) |
DEV(%) | R.S.D.(%) | n |
| 0.5 | 0.51 | 0.018 | 1.76 | 3.44 | 8 |
| 1.0 | 0.97 | 0.048 | −3.25 | 5.01 | 8 |
| 2.0 | 1.99 | 0.092 | −0.44 | 4.64 | 8 |
| 5.0 | 4.86 | 0.153 | −2.76 | 3.15 | 8 |
| 10 | 10.02 | 0.324 | 0.19 | 3.23 | 8 |
| 25 | 25.7 | 0.426 | 2.86 | 1.66 | 8 |
| 50 | 50.22 | 2.36 | 0.44 | 4.70 | 8 |
| 100 | 101.2 | 6.09 | 1.19 | 6.02 | 8 |
Abbreviations: GM, grand mean; S.D., standard deviation; DEV, percent deviation from nominal value; R.S.D., relative standard deviation; n, total number of replicate observations within the validation runs.
Table 6.
Assessment of accuracy and precision from quality control samples in porcine plasma
| Table 6A Irinotecan | ||||||
|---|---|---|---|---|---|---|
| Nominal (ng/mL) |
GMa (ng/mL) |
S.D. (ng/mL) |
DEV (%) |
BRP (%) |
WRP (%) |
n |
| 15 | 16.7 | 0.487 | 11.4 | - | 3.12 | 20 |
| 150 | 159.5 | 7.14 | 6.32 | - | 4.64 | 20 |
| 750 | 758.8 | 19.5 | 1.17 | 1.54 | 2.18 | 20 |
| 5000 | 5134 | 172.3 | 2.70 | 1.77 | 2.97 | 20 |
| Table 6B SN38 | ||||||
|
Nominal (ng/mL) |
GMa (ng/mL) |
S.D. (ng/mL) |
DEV (%) |
BRP (%) |
WRP (%) |
n |
| 1.5 | 1.54 | 0.080 | 2.53 | 4.07 | 3.82 | 20 |
| 15 | 15.7 | 0.853 | 4.95 | 4.76 | 3.38 | 20 |
| 75 | 78.3 | 2.52 | 4.38 | 2.32 | 2.48 | 20 |
| Table 6C SN38-G | ||||||
|
Nominal (ng/mL) |
GMa (ng/mL) |
S.D. (ng/mL) |
DEV (%) |
BRP (%) |
WRP (%) |
n |
| 1.5 | 1.54 | 0.063 | 2.68 | - | 4.16 | 20 |
| 15 | 14.9 | 0.552 | −0.94 | - | 3.83 | 20 |
| 75 | 75.9 | 3.62 | 1.25 | 3.13 | 3.87 | 20 |
Abbreviations: GM, grand mean; S.D., standard deviation; DEV, percent deviation from nominal value; WRP, within-run precision; BRP, between-run precision; n, total number of replicate observations within the validation runs.
A hyphen indicates MSwit>MSbet, thus BRP cannot be calculated and it was concluded that no additional variation was observed as a result of performing the assay in different runs.
Five samples at each concentration were run on four different occasions (n=20).
The mean overall recoveries for all three analytes, estimated by comparing the mass spectrometric signal response of the analyte spiked into human plasma versus reconstitution solution, were approximately 76%, 90% and 75% for irinotecan, SN38, and SN38-G, respectively, independent of the spiked concentration. Minimal matrix effect was observed via post-column infusion. QC samples prepared using different lots of human plasma from the calibration curve did not show additional plasma-to-plasma variation.
3.3. Stability
The 24-hour reinjection measurements were consistent with the initial run, allowing samples extracted from human plasma to be reanalyzed on the following day when necessary (for example, in the case of machine failure). For the F/T stability test, back-calculated values at both low and high QC concentrations after each freeze-thaw cycle were well below 10% of the nominal values, indicating no degradation in human plasma. Bench-top stability in human whole blood at room temperature was assessed up to 6 hours. All three analytes (irinotecan, SN38, SN38-G) demonstrated stability through 4 hours (<15% difference from freshly extracted and prepared). At 6 hours, irinotecan and SN38-G both demonstrated increased signal response, where the increases were approximately 20% different from fresh (Table 7). It was recommended to obtain plasma from whole blood containing the three analytes after withdrawn from clinical subjects within 4 hours at room temperature.
Table 7.
Assessment of benchtop room temperature stability in human whole blood up to 6 hours
| Table 7A Irinotecan | ||
|---|---|---|
| 10 ng/mL | 500 ng/mL | |
| Hours at room temp |
DEVa (%) from Fresh |
DEV(%) from Fresh |
| 0 (fresh) | - | - |
| 1 | 2.35 | −7.82 |
| 2 | 6.32 | 4.10 |
| 4 | 6.04 | 5.15 |
| 6 | 15.9 | 20.5 |
| Table 7B SN38 | ||
| 1.0 ng/mL | 50 ng/mL | |
|
Hours at room temp |
DEV(%) from Fresh |
DEV(%) from Fresh |
| 0 (fresh) | - | - |
| 1 | 0.69 | −0.96 |
| 2 | 4.86 | 1.75 |
| 4 | −4.17 | 1.80 |
| 6 | 9.95 | 13.06 |
| Table 7C SN38-G | ||
| 1.0 ng/mL | 50 ng/mL | |
|
Hours at room temp |
DEV(%) from Fresh |
DEV(%) from Fresh |
| 0 (fresh) | - | - |
| 1 | 1.71 | 1.97 |
| 2 | 0.64 | 5.84 |
| 4 | −4.49 | 8.27 |
| 6 | 11.32 | 20.85 |
Abbreviations: DEV, percent deviation from nominal value
3.4. Application
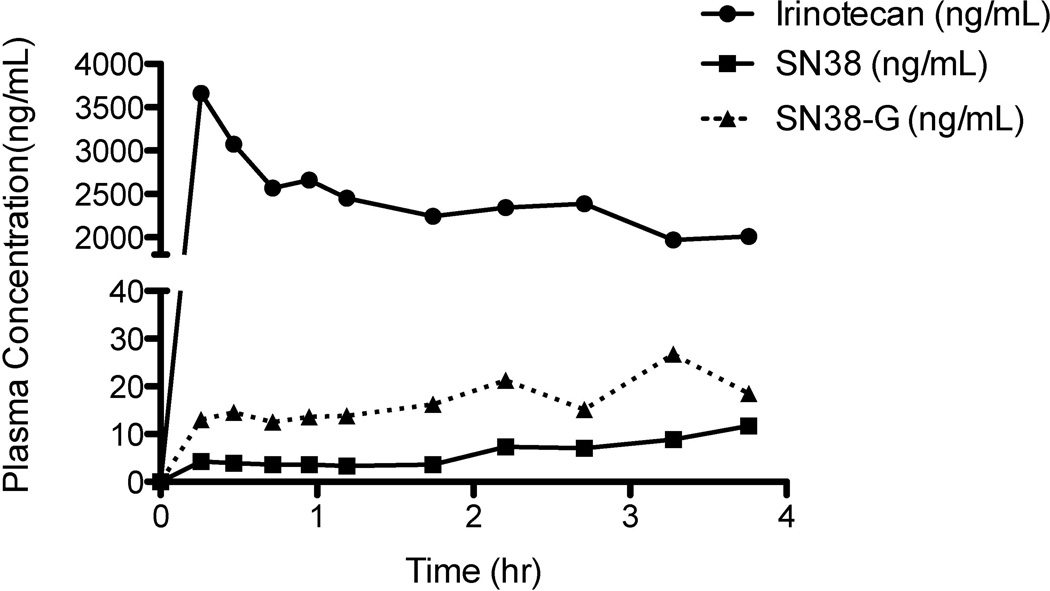
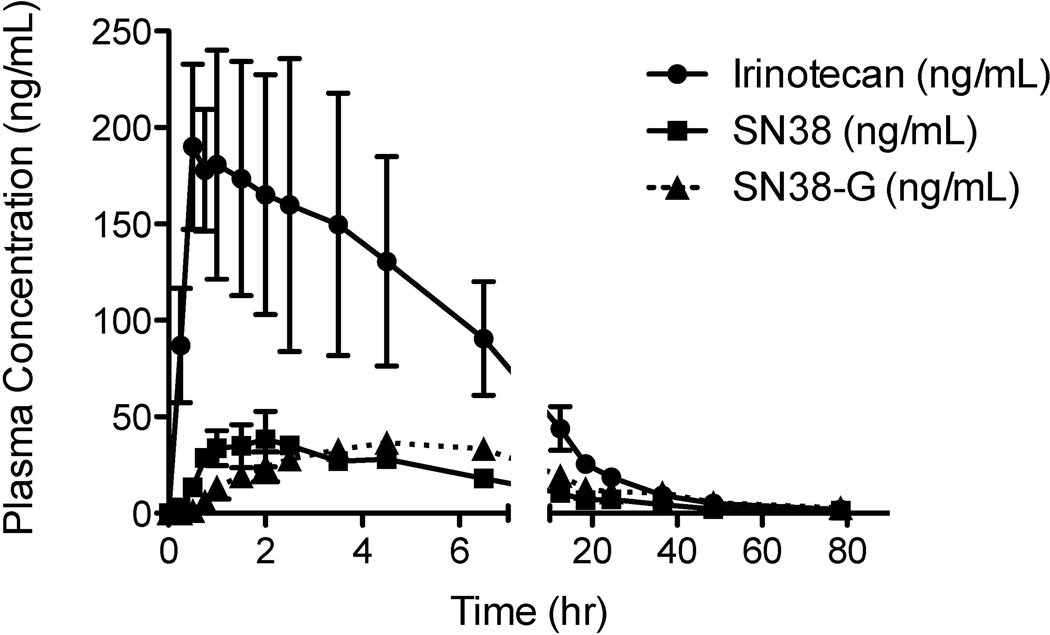
The validated method was first applied to study the preclinical pharmacokinetics of irinotecan, SN38, and SN38-G in pigs, before studying clinical pharmacokinetics on patients in a NCI-initiated phase I clinical trial. A typical chromatogram of a human patient sample is presented in Figure 2D, with calculated plasma concentrations of 168.9, 26.4, and 38.7 ng/mL for irinotecan, SN38, and SN38-G, respectively. Figure 3 depicts preclinical plasma concentration versus time curves up to 4 hours in a porcine model. Irinotecan plasma levels were above the upper limit of quantification (ULOQ) of 1000 ng/mL, thus the dilution QC at 5000 ng/mL was used to validate the dilution of these samples. Figure 4 depicts clinical plasma concentration versus time curves for each analyte up to 78 hours post dose in humans. The prodrug irinotecan demonstrated an approximately 10-fold higher maximum plasma concentration (CMAX), compared to the active metabolite (SN38) and its glucuronidated metabolite (SN38-G). This was expected and validated for by using a 10-fold higher calibration range for irinotecan compared to SN38 and SN38-G. Furthermore, it is evident that the time to CMAX (i.e. tMAX) for SN38-G occurs later than the tMAX for SN38, which is logical in that SN38 must first be formed from irinotecan before SN38-G can be formed from SN38.
Figure 3.
Preclinical plasma concentration versus time curves for irinotecan, SN38, and SN38-G from preclinical TACE experiments performed on a pig. The porcine model demonstrates a very high irinotecan maximum plasma concentration (Cmax), much higher than the upper limit of quantification for irinotecan (1000 ng/mL). This validates the need for the dilution QC at 5000 ng/mL for irinotecan only.
Figure 4.
Clinical plasma concentration versus time curves for irinotecan, SN38, and SN38-G from three separate regimens on two subjects. The irinotecan prodrug demonstrated a higher maximum plasma concentration (Cmax), as expected and validated for by the 10-fold higher calibration curve range from the metabolites SN38 and SN38-G. Also as expected, SN38 levels rise prior to SN38-G.
4. Conclusion
In conclusion, we present a validated and novel ultra high performance chromatographic method with tandem mass-spectrometric detection for the rapid quantitative determination of irinotecan, SN38, and SN38-G in human and porcine plasma. This method is specific, accurate, and precise, and can be easily implemented into routine preclinical or clinical practice. Compared to the method of Corona [12], our method is twice as fast (3 min vs 6 min) and has concentration ranges that are more applicable to DEB TACE pharmacokinetic studies. The resulting preclinical and clinical application further support the usefulness of this method.
Highlights.
We developed and validated a novel uHPLC-MS/MS assay for the simultaneous quantification of irinotecan, SN38, and SN38-G in both pig and human plasma> Clinically-relevant calibration ranges for irinotecan (5–1000 ng/mL), its metabolites (0.5–100 ng/mL) were used> Successfully applied to both preclinical and clinical studies undergoing hepatic chemoembolization.
Acknowledgements
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, as well as the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. Some authors are affiliated with the SAIC Frederick, Inc, with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E.
Abbreviations
- uHPLC
Ultra-high performance liquid chromatography
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- QC
Quality control
- LLOQ
Lower limit of quantification
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government. The views in this manuscript are those of the authors and may not necessarily reflect NIH policy. No official endorsement is intended nor should be inferred. SAIC-Frederick is specifically and solely a government contractor for the NCI. There is no competing interest.
References
- 1.Sawada S, Okajima S, Aiyama R, Nokata K, Furuta T, Yokokura T, Sugino E, Yamaguchi K, Miyasaka T. Synthesis and antitumor activity of 20(S)-camptothecin derivatives: carbamate-linked, water-soluble derivatives of 7-ethyl-10-hydroxycamptothecin. Chem Pharm Bull (Tokyo) 1991;39:1446–1450. doi: 10.1248/cpb.39.1446. [DOI] [PubMed] [Google Scholar]
- 2.Jansen WJ, Zwart B, Hulscher ST, Giaccone G, Pinedo HM, Boven E. CPT-11 in human colon-cancer cell lines and xenografts: characterization of cellular sensitivity determinants. Int J Cancer. 1997;70:335–340. doi: 10.1002/(sici)1097-0215(19970127)70:3<335::aid-ijc15>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.van Ark-Otte J, Kedde MA, van der Vijgh WJ, Dingemans AM, Jansen WJ, Pinedo HM, Boven E, Giaccone G. Determinants of CPT-11 and SN-38 activities in human lung cancer cells. Br J Cancer. 1998;77:2171–2176. doi: 10.1038/bjc.1998.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikeguchi M, Arai Y, Maeta Y, Ashida K, Katano K, Wakatsuki T. Topoisomerase I expression in tumors as a biological marker for CPT-11 chemosensitivity in patients with colorectal cancer. Surg Today. 2011;41:1196–1199. doi: 10.1007/s00595-011-4546-7. [DOI] [PubMed] [Google Scholar]
- 5.Gelderblom HA, DEJ MJ, Sparreboom A, Verweij J. Oral topoisomerase 1 inhibitors in adult patients: present and future. Invest New Drugs. 1999;17:401–415. doi: 10.1023/a:1006394610219. [DOI] [PubMed] [Google Scholar]
- 6.Di Paolo A, Bocci G, Polillo M, Del Re M, Di Desidero T, Lastella M, Danesi R. Pharmacokinetic and Pharmacogenetic Predictive Markers of Irinotecan Activity and Toxicity. Curr Drug Metab. 2011;12:932–943. doi: 10.2174/138920011798062283. [DOI] [PubMed] [Google Scholar]
- 7.Fiorentini G, Aliberti C, Benea G, Montagnani F, Mambrini A, Ballardini PL, Cantore M. TACE of liver metastases from colorectal cancer adopting irinotecan-eluting beads: beneficial effect of palliative intra-arterial lidocaine and post-procedure supportive therapy on the control of side effects. Hepatogastroenterology. 2008;55:2077–2082. [PubMed] [Google Scholar]
- 8.Fiorentini G, Aliberti C, Del Conte A, Tilli M, Rossi S, Ballardini P, Turrisi G, Benea G. Intra-arterial hepatic chemoembolization (TACE) of liver metastases from ocular melanoma with slow-release irinotecan-eluting beads. Early results of a phase II clinical study. In Vivo. 2009;23:131–137. [PubMed] [Google Scholar]
- 9.Fiorentini G, Aliberti C, Turrisi G, Del Conte A, Rossi S, Benea G, Giovanis P. Intraarterial hepatic chemoembolization of liver metastases from colorectal cancer adopting irinotecan-eluting beads: results of a phase II clinical study. In Vivo. 2007;21:1085–1091. [PubMed] [Google Scholar]
- 10.Eyol E, Boleij A, Taylor RR, Lewis AL, Berger MR. Chemoembolisation of rat colorectal liver metastases with drug eluting beads loaded with irinotecan or doxorubicin. Clin Exp Metastasis. 2008;25:273–282. doi: 10.1007/s10585-008-9142-x. [DOI] [PubMed] [Google Scholar]
- 11.Ramesh M, Ahlawat P, Srinivas NR. Irinotecan and its active metabolite, SN-38: review of bioanalytical methods and recent update from clinical pharmacology perspectives. Biomed Chromatogr. 2010;24:104–123. doi: 10.1002/bmc.1345. [DOI] [PubMed] [Google Scholar]
- 12.Corona G, Elia C, Casetta B, Toffoli G. Fast liquid chromatography-tandem mass spectrometry method for routine assessment of irinotecan metabolic phenotype. Ther Drug Monit. 2010;32:638–646. doi: 10.1097/FTD.0b013e3181ec3bf5. [DOI] [PubMed] [Google Scholar]
- 13.Baylatry MT, Joly AC, Pelage JP, Bengrine-Lefevre L, Prugnaud JL, Laurent A, Fernandez C. Simple liquid chromatography method for the quantification of irinotecan and SN38 in sheep plasma: application to in vivo pharmacokinetics after pulmonary artery chemoembolization using drug eluting beads. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:738–742. doi: 10.1016/j.jchromb.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Tang Y, Taylor RR, Gonzalez MV, Lewis AL, Stratford PW. Evaluation of irinotecan drug-eluting beads: a new drug-device combination product for the chemoembolization of hepatic metastases. J Control Release. 2006;116:e55–e56. doi: 10.1016/j.jconrel.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 15.Taylor RR, Tang Y, Gonzalez MV, Stratford PW, Lewis AL. Irinotecan drug eluting beads for use in chemoembolization: in vitro and in vivo evaluation of drug release properties. Eur J Pharm Sci. 2007;30:7–14. doi: 10.1016/j.ejps.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration. Guidelines for Industry: Bioanalytical Methods Validation. 2001
- 17.Barilero I, Gandia D, Armand JP, Mathieu-Boue A, Re M, Gouyette A, Chabot GG. Simultaneous determination of the camptothecin analogue CPT-11 and its active metabolite SN-38 by high-performance liquid chromatography: application to plasma pharmacokinetic studies in cancer patients. J Chromatogr. 1992;575:275–280. doi: 10.1016/0378-4347(92)80156-k. [DOI] [PubMed] [Google Scholar]
- 18.Rivory LP, Robert J. Reversed-phase high-performance liquid chromatographic method for the simultaneous quantitation of the carboxylate and lactone forms of the camptothecin derivative irinotecan, CPT-11, and its metabolite SN-38 in plasma. J Chromatogr B Biomed Appl. 1994;661:133–141. doi: 10.1016/0378-4347(94)00340-8. [DOI] [PubMed] [Google Scholar]
- 19.Sumiyoshi H, Fujiwara Y, Ohune T, Yamaoka N, Tamura K, Yamakido M. High-performance liquid chromatographic determination of irinotecan (CPT-11) and its active metabolite (SN-38) in human plasma. J Chromatogr B Biomed Appl. 1995;670:309–316. doi: 10.1016/0378-4347(95)00130-1. [DOI] [PubMed] [Google Scholar]
- 20.de Bruijn P, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A. Determination of irinotecan (CPT-11) and its active metabolite SN-38 in human plasma by reversed-phase high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl. 1997;698:277–285. doi: 10.1016/s0378-4347(97)00290-9. [DOI] [PubMed] [Google Scholar]
- 21.Kaneda N, Hosokawa Y, Yokokura T. Simultaneous determination of the lactone and carboxylate forms of 7-ethyl-10-hydroxycamptothecin (SN-38), the active metabolite of irinotecan (CPT-11), in rat plasma by high performance liquid chromatography. Biol Pharm Bull. 1997;20:815–819. doi: 10.1248/bpb.20.815. [DOI] [PubMed] [Google Scholar]
- 22.Chollet DF, Goumaz L, Renard A, Montay G, Vernillet L, Arnera V, Mazzo DJ. Simultaneous determination of the lactone and carboxylate forms of the camptothecin derivative CPT-11 and its metabolite SN-38 in plasma by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1998;718:163–175. doi: 10.1016/s0378-4347(98)00270-9. [DOI] [PubMed] [Google Scholar]
- 23.Sparreboom A, de Bruijn P, de Jonge MJ, Loos WJ, Stoter G, Verweij J, Nooter K. Liquid chromatographic determination of irinotecan and three major metabolites in human plasma, urine and feces. J Chromatogr B Biomed Sci Appl. 1998;712:225–235. doi: 10.1016/s0378-4347(98)00147-9. [DOI] [PubMed] [Google Scholar]
- 24.de Bruijn P, de Jonge MJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A. Femtomole quantitation of 7-ethyl-10-hydroxycamptothecine (SN-38) in plasma samples by reversed-phase high-performance liquid chromatography. Anal Biochem. 1999;269:174–178. doi: 10.1006/abio.1999.4001. [DOI] [PubMed] [Google Scholar]
- 25.Kurita A, Kaneda N. High-performance liquid chromatographic method for the simultaneous determination of the camptothecin derivative irinotecan hydrochloride, CPT-11, and its metabolites SN-38 and SN-38 glucuronide in rat plasma with a fully automated on-line solid-phase extraction system, PROSPEKT. J Chromatogr B Biomed Sci Appl. 1999;724:335–344. doi: 10.1016/s0378-4347(98)00554-4. [DOI] [PubMed] [Google Scholar]
- 26.Ragot S, Marquet P, Lachatre F, Rousseau A, Lacassie E, Gaulier JM, Dupuy JL, Lachatre G. Sensitive determination of irinotecan (CPT-11) and its active metabolite SN-38 in human serum using liquid chromatography-electrospray mass spectrometry. J Chromatogr B Biomed Sci Appl. 1999;736:175–184. doi: 10.1016/s0378-4347(99)00452-1. [DOI] [PubMed] [Google Scholar]
- 27.Escoriaza J, Aldaz A, Castellanos C, Calvo E, Giraldez J. Simple and rapid determination of irinotecan and its metabolite SN-38 in plasma by high-performance liquid-chromatography: application to clinical pharmacokinetic studies. J Chromatogr B Biomed Sci Appl. 2000;740:159–168. doi: 10.1016/s0378-4347(00)00048-7. [DOI] [PubMed] [Google Scholar]
- 28.Sai K, Kaniwa N, Ozawa S, Sawada J. An analytical method for irinotecan (CPT-11), and its metabolites using a high-performance liquid chromatography: parallel detection with fluorescence and mass spectrometry. Biomed Chromatogr. 2002;16:209–218. doi: 10.1002/bmc.137. [DOI] [PubMed] [Google Scholar]
- 29.de Jong FA, Mathijssen RH, de Bruijn P, Loos WJ, Verweij J, Sparreboom A. Determination of irinotecan (CPT-11) and SN-38 in human whole blood and red blood cells by liquid chromatography with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;795:383–388. doi: 10.1016/s1570-0232(03)00574-9. [DOI] [PubMed] [Google Scholar]
- 30.Guo W, Ahmad A, Khan S, Dahhani F, Wang YF, Ahmad I. Determination by liquid chromatography with fluorescence detection of total 7-ethyl-10-hydroxy-camptothecin (SN-38) in beagle dog plasma after intravenous administration of liposome-based SN-38 (LE-SN38) J Chromatogr B Analyt Technol Biomed Life Sci. 2003;791:85–92. doi: 10.1016/s1570-0232(03)00210-1. [DOI] [PubMed] [Google Scholar]
- 31.Khan S, Ahmad A, Ahmad I. A sensitive and rapid liquid chromatography tandem mass spectrometry method for quantitative determination of 7-ethyl-10-hydroxycamptothecin (SN-38) in human plasma containing liposome-based SN-38 (LE-SN38) Biomed Chromatogr. 2003;17:493–499. doi: 10.1002/bmc.257. [DOI] [PubMed] [Google Scholar]
- 32.Owens TS, Dodds H, Fricke K, Hanna SK, Crews KR. High-performance liquid chromatographic assay with fluorescence detection for the simultaneous measurement of carboxylate and lactone forms of irinotecan and three metabolites in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;788:65–74. doi: 10.1016/s1570-0232(02)01016-4. [DOI] [PubMed] [Google Scholar]
- 33.Bardin S, Guo W, Johnson JL, Khan S, Ahmad A, Duggan JX, Ayoub J, Ahmad I. Liquid chromatographic-tandem mass spectrometric assay for the simultaneous quantification of Camptosar and its metabolite SN-38 in mouse plasma and tissues. J Chromatogr A. 2005;1073:249–255. doi: 10.1016/j.chroma.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 34.Khan S, Ahmad A, Guo W, Wang YF, Abu-Qare A, Ahmad I. A simple and sensitive LC/MS/MS assay for 7-ethyl-10-hydroxycamptothecin (SN-38) in mouse plasma and tissues: application to pharmacokinetic study of liposome entrapped SN-38 (LE-SN38) J Pharm Biomed Anal. 2005;37:135–142. doi: 10.1016/j.jpba.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 35.D'Esposito F, Tattam BN, Ramzan I, Murray M. A liquid chromatography/electrospray ionization mass spectrometry (LC-MS/MS) assay for the determination of irinotecan (CPT-11) and its two major metabolites in human liver microsomal incubations and human plasma samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;875:522–530. doi: 10.1016/j.jchromb.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Dutschman GE, Li X, Ye M, Cheng YC. Quantitation of Irinotecan and its two major metabolites using a liquid chromatography-electrospray ionization tandem mass spectrometric. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3038–3044. doi: 10.1016/j.jchromb.2009.07.025. [DOI] [PubMed] [Google Scholar]