Abstract
The study of genetic polymorphisms and body mass index (BMI) among African women in Africa and in the United States contributes to our understanding of the genetic and environmental risk factors for hypertension. African American women have the highest prevalence of hypertension and obesity compared to other ethnic groups in the United States. Using a crosssectional research design, we examined the effects of genetic and environmental risks of single nucleotide polymorphisms (SNPs) and BMI on blood pressure (BP) among three generations of West African Dogon women (N = 199). We genotyped six SNPs located in the candidate genes known to be related to hypertension. We tested the associations between these SNPs and systolic BP (SBP) and diastolic BP (DBP) with Fisher’s exact tests, chi-square tests for independence, and multivariable linear mixed models. The SNP rs8179526 (SLC4A5) was significantly associated with SBP adjusted for age, age2, and BMI (p = .02). The “C” allele variant of rs8179526 (allele frequency of 0.445) was associated with higher SBP. This SNP did not deviate from the Hardy-Weinberg equilibrium (HWE) with p value of .772. The SNP × BMI interaction effects associated with SBP and DBP were not significant. rs8179526 is located on the SLC4A5 gene on chromosome 2. SLC4A5 encodes a protein that transports sodium and bicarbonate across cell membranes while regulating cellular pH and contains several SNPs linked to elevated BP. Knowledge of the SNP’s effect on hypertension among West African women can help health practitioners educate their patients about genetic risks of developing hypertension.
Keywords: blood pressure, body mass index, genetic, women, rs8179526, dogon
The study of genetic and body mass index (BMI) risks of increased blood pressure (BP) among African women is important for increasing our understanding of the factors underlying the development of hypertension in women of African descent. Statistically, African American women are more likely to be overweight than those of other ethnic groups (Centers for Disease Control and Prevention [CDC], 2007a), possessing the highest obesity rate among 20- to 30-year-old women (CDC, 2007b). This finding is particularly alarming considering obesity as a leading cause of high BP (Rosamond et al., 2007). Lifestyle behaviors, such as consumption of high-fat foods, a noted trend in minority and poor populations, are risk factors for obesity and related hypertension (Boutin-Foster, Ogedegbe, Ravenell, Robbins, & Charlson, 2007; Douglas et al., 2003). According to the American Heart Association, African American women have the greatest prevalence of hypertension at 44%, while Caucasians have a prevalence rate of 28.1% overall (Roger et al., 2011).
Many African Americans are descendants of slaves brought to the Americas from West Africa during the transatlantic slave trade from 16th to 19th centuries. Among the 10–12 million enslaved Africans who arrived during this period, an estimated 39% came from West Central Africa, including the area now known as Mali (Lovejoy, 2000). The later and larger second Atlantic system of slave trade involved the distribution of Africans to Brazil, Caribbean colonies, and North America. Through this system, slaves from West Africa, the region specific to the present study, were transplanted to the American South (Frey, 2004). Members of the Dogon tribes were among those captured for the West African slave trade (Burnside & Robotham, 1997; Lovejoy, 2000). As late as 1900, nearly 90% of African Americans resided in former slave-holding states (Carney, 1998). Yet, during the Great Migration between 1915 and 1930, many African American slave descendants relocated to industrialized northern cities for better economic and employment opportunities. From 1910 to 1929, Detroit’s Black population increased from 6,000 to 120,000, making Detroit a major northern metropolitan migration destination of Southern Blacks (Gibson, 1998). Today, African Americans comprise the majority of Detroit’s population.
Taylor, Maddox, and Wu (2009) conducted a cross-sectional study examining BMI, physical activity, sodium intake, and DNA samples among African American women in urban Detroit to determine associations with high BP. Investigators selected genes to represent biological pathways or positional candidate genes from systems known to be associated with hypertension and previously linked to hypertension (see Table 1). Of the four single nucleotide polymorphisms (SNPs) on the sodium bicarbonate cotransporter gene (SLC4A5), investigators found three to be significantly related to BP in the Detroit sample (rs8179526, rs10177833, and rs6731545). Given the clear, positive genetic, and lifestyle (marginal physical activity and poor dietary habits) interaction effects on BP among that sample, our purpose in the present study was to replicate the genetics piece of that previous study among a sample of West African women in the rural setting of Dogon villages in Sangha, Mali. We have found no published studies that have explored the main and interaction effects of genetic and environmental factors on hypertension development in West African Dogon women. By examining these genetic factors among these two samples of women, we hope to determine if markers for hypertension differ among women with similar heritage but very different lifestyle and environmental factors.
Table 1.
Single Nucleotide Polymorphisms (SNPs) With a Demonstrated Association With Blood Pressure in African Americans
| SNP | Gene | Chromosome | Reference |
|---|---|---|---|
| rs5049 | AGT | 1 | Gu et al. (2005) and Taylor, Maddox, and Wu (2009) |
| rs5051 | AGT | 1 | Gu et al. (2005) and Taylor et al. (2009) |
| rs672645 | CYP4F12 | 19 |
Cooper et al. (2002) and Taylor et al. (2009) |
| rs10177833 | SLC4A5 | 2 |
Barkley et al. (2004), Cooper et al. (2002), Hunt et al. (2006), Province et al. (2003), and Taylor et al. (2009) |
| rs8179526 | SLC4A5 | 2 |
Barkley et al. (2004), Cooper et al. (2002), Hunt et al. (2006) Province et al. (2003), Taylor et al. (2009) |
| rs679620 | MMP3 | 11 | Taylor, Sun, Chu, Mosley, and Kardia (2008) |
Diet and exercise differ markedly between urban Detroit women and West African Dogon women. Frequent fast-food consumption and lack of physical exercise (Taylor, Maddox, & Wu, 2009) are quite common in Detroit, while processed high-fat foods are nearly nonexistent among the Dogon. The Dogon maintain an agricultural mode of subsistence, cultivating pearl millet, sorghum, and rice, as well as onions, tobacco, peanuts, and other vegetables. The Dogon also raise sheep, goats, and chickens in the region’s villages for sustenance and industry. Women perform the majority of labor in both agricultural endeavors and home maintenance, ensuring that they perform strenuous exercise daily (Strassmann, 1997). In their study of undernutrition in the Dogon region, Schémann et al. (2002) reported that approximately 12.8% of the children were emaciated. Assuming that child weight is a marker for communal nutritional status, some measure of undernutrition probably is present in women of all ages. Because women of the Dogon region have lower body fat ratios and get considerably more physical exercise than urban African American women, it may be that lifestyle factors such as BMI, diet, and physical activity play less of a role in the development of hypertension in the Dogon women. Thus, findings of genetic associations for hypertension among this West African population would help to shed light on the impact heredity and the environment play in hypertension health disparities in Africa and the United States.
Method
Participants
This study was cross sectional in design and included 199 West African women from the Dogon region of Mali. Recruitment strategies commenced after the Institutional Review Board of the University of Michigan and the Mali government granted approval.
The setting for this study was Sangha, Mali, West Africa in the villages of the Dogon people—a rural region where women get daily rigorous physical activity through home and agricultural labor, do not have influences of processed or fast foods, and are typically underweight by U.S. standards. We did not collect data on sodium intake and physical activity, as these variables are naturally controlled by the Dogons’ organic life-style of hunting and gathering.
We (i.e., the investigators and research assistants) used the following procedure to recruit participants: (a) we hired a native Dogon man who was educated as a school teacher and was fluent in Dogon, Bambara, and English as a translator and interpreter to request verbal permission from the chief of each village to recruit women; (b) once we received permission, we set a date and time to visit the village; (c) upon arrival at the village, the translator explained the study to the village women, and (d) asked each woman to give verbal assent to participate, as many women in the tribe were unable to read or write in their native Dogon language or in the national language of Bambara. To meet the inclusion criteria, participants were required to self-identify as West African Dogon and have a living family of at least three female generations to constitute the triad of grandmother–mother–granddaughter participant group. We included only women with living assessable triads and only granddaughters over 2 years of age. The principal investigator trained and assisted research assistants regarding all data collection methods. We compensated study participants with US$1.00 each as honorarium for their participation. As is the Dogon custom, women were allowed to keep the money they received for their participation. At the time of the study, $1.00 was equal to 500 West African CFA currencies. According to the Mali government, anything more than $1.00 each would have been considered excessive and coercive.
Measures
Accuracy and consistency of the data obtained for BP, height, and weight was assured, as the principal investigator and coinvestigator (both PhD-prepared registered nurse practitioners) took all measurements. The same two research assistants recorded all data, which was checked each day for accuracy, by comparing the two written records of all raw data.
Demographic survey
Participants completed a demographic survey that obtained information regarding age, number of children, village affiliation, and any known family history of hypertension (age at diagnosis, medications, and other treatments). The interpreter read the demographic questions to each woman in the native Dogon language. He orally translated their responses into English, which the investigator and research assistants recorded.
BP, height, and weight
We measured BP using a manual BP manometer with a size-appropriate upper arm cuff using Life-Source blood pressure monitors (model # A&D UA 767PC). Reported BP measurements represent an average of three consecutive seated readings. Procedures for participant preparation for BP measurement were in accordance with JNC-7 recommendations (Chobanian et al., 2003). For children, we defined hypertension as readings above the 95th percentile for age, gender, and height (National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents, 2004). For adults, average BP readings greater than 140/90 were indicative of hypertension (Rosamond et al., 2007).
We measured height using a portable measuring tape and weight using a portable scale that we placed on a level surface and calibrated before use in each village (BWB/807 Tanita, Tokyo, Japan). We calculated BMI from height and weight measurements using the formula weight in pounds divided by the height in inches squared multiplied by 703. BMI of 25–29 kg/m2 is considered overweight and BMI ≥ 30 kg/m2 indicates obesity (CDC, 2008).
Saliva sample and genotyping
We collected saliva samples from all participants using the Oragene DNA sample collection kit. We asked participants not to eat, drink, smoke, or chew gum for 30 min before giving the saliva sample. We instructed participants to spit into the collection tube several times until the amount of liquid saliva reached the fill-line level marked in the tube. The amount of saliva required to reach the fill line of the collection tube was about 2 ml. If participants were unable to produce enough saliva to reach the fill line, we gave them a small amount of plain white sugar or a clear artificially sweetened lollipop that helped to produce more liquid saliva in the mouth. We labeled samples with a participant identification number.
We isolated DNA from the saliva samples using the PureGene DNA Isolation Kit from Gentra Systems (Minneapolis, MN). Genotyping, using polymerase chain reaction (PCR) amplification techniques, was conducted at the University of Michigan Molecular and Behavioral Sciences Laboratory with the TaqMan assay and ABI Prism Sequence Detection System (Applied Biosystems, Foster City, CA). Quality control measures for genotyping assays included robotic liquid handling, separate pre- and post-PCR areas, standard protocols, and quality control analyses, with 5% duplicates, positive and negative controls, computerized sample tracking, and data validity checks (Taylor, Sun, Chu, Mosley, & Kardia, 2008).
Statistical Analysis
We assessed Hardy–Weinberg equilibrium (HWE) in unrelated subsamples using a chi-square test or Fisher’s exact test if a genotype class had less than five individuals (Weir, 1996). For systolic (SBP) and diastolic (DBP) blood pressures, the adjustment variables were age, age2, and BMI. As described in Taylor, Sun, Hunt, and Kardia (2010, p. 152),
[W]e then used linear mixed models (Raudenbush & Bryk, 2002) to test for association between each SNP and the multivariable-adjusted, residual phenotypes to account for the family structure and retain a valid type I error rate (Cupples et al., 2007). Each SNP was tested for additive effects in association with the outcome of interest in a test with one degree of freedom. We also tested the SNP BMI interaction effects of adjusted SBP and DBP using multivariable × linear mixed models with age, age2, BMI, and SNP allele dosage (i.e., additive effect) as covariates. None of the participants were taking antihypertensive medications, so correcting for observed SBP and DBP values was not needed. The genetic additive effects model was used in this study. The additive genetic model of two alleles A and B shows a trend of an increased number of coded alleles. Assuming B is the coded risk allele, among the three genotypes, AA (0 risk allele), AB (1 risk allele), and BB (2 risk alleles), the risk for AB genotypes (r) is half that for BB genotypes (2r). Data management, descriptive statistics for the covariates and outcome variables, and regression analyses were conducted using the statistics software package R in version 2.9.0 (http://www.r-project.org).
We also examined the distribution of continuous BP measures by age in the sample and in each generation using locally weighted scatterplot smoothing (LOWESS) implemented in R. The LOWESS method fits models to localized subsets of the data to build up a function that describes the deterministic part of the variation in the data, point by point (Cleveland, 1979). LOWESS implemented a function-fitting technique that provides a generally smooth curve, the value of which at a particular location along the x-axis is determined only by the neighboring points. As a result, the LOW-ESS curve minimizes the variance of the residuals or prediction error locally. The method makes no assumptions about the form of the relationship and allows the form to be discovered using the data itself.
Results
Table 2 presents the descriptive statistics for the 199 female West African participants (65 grandmothers, 67 mothers, and 67 daughters) with BP measurements. The mothers had the highest mean BMI, but the mean BMI for all three generational groups indicate normal weight or underweight using U.S. standards. The grandmothers had the highest SBP and DBP compared to the mothers and daughters. A total of 31 women (23 [35.4%] grandmothers, 7 [10.4%] mothers, and 1 [1.5%] daughter) had BP readings diagnostic of hypertension.
Table 2.
Demographic and Blood Pressure Data (M±SD) for Three Generations of West African Dogon Female Participants
| Variable | Grandmothers (n = 65) |
Mothers (n = 67) |
Daughters (n = 67) |
|---|---|---|---|
| Age (years) | 60.2 ± 9.5 | 31.8 ± 7.9 | 7.6 ± 3.5 |
| BMI (kg/m2) | 21.6 ± 3.6 | 22.9 ± 2.8 | 15.1 ± 2.3 |
| SBP (mmHg) | 133.9 ± 18.8 | 118.2 ± 13.1 | 103.3 ± 14.7 |
| DBP (mmHg) | 82.5 ± 11.2 | 78.3 ± 9.7 | 66.2 ± 12.5 |
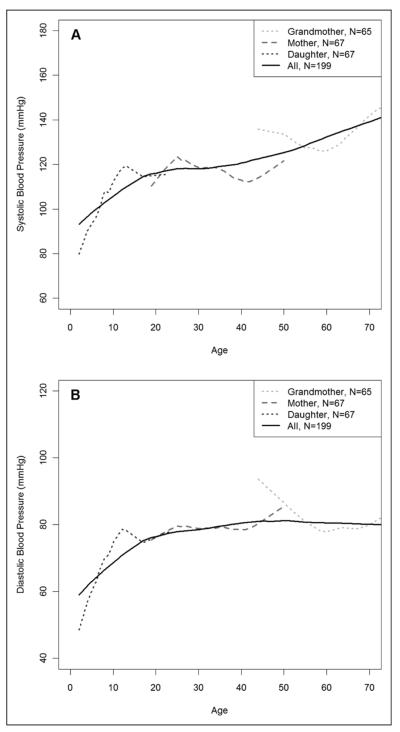
Figure 1 shows the overall trend of BP levels over age. The SBP continuously increased across the age range in all three generations (Figure 1A). The DBP continuously increased before the age of 30 years but were approximately unchanged after the age of 30 in the full sample of West African Dogon women (Figure 1B).
Figure 1.
Mean (A) systolic blood pressure and (B) diastolic blood pressure by age in the sample of three generations of West African Dogon women.
To understand the genetic effects on SBP and DBP, we genotyped six SNPs located in the candidate genes related to hypertension and tested the associations between these SNPs and SBP and DBP. Among the six SNPs genotyped, rs5051 had a minor allele frequency (MAF) that was less than 0.05. SNP rs673154 also was relatively rare with an MAF of 0.096 (Table 3). Table 4 presents a summary of the results of our association tests for the SNPs and SBP and DBP. SNP rs8179526 (SLC4A5) was significantly associated with SBP adjusted for age, age2, and BMI (p = .02). The “C” allele of rs8179526 (allele frequency of 0.445) was associated with higher SBP. This SNP did not deviate from the HWE with a p value of .772. We also tested the SNP BMI interaction term in the linear regression model and did not × identify any significant association using a p value threshold of .05.
Table 3.
Frequency of Single Nucleotide Polymorphisms (SNPs) Among Sample of 199 West African Dogon Women Across Three Generations
| SNP | Gene | Chromosome | Position | Allele A | Allele B | Allele Frequency A | Allele Frequency B | HWE p Value |
|---|---|---|---|---|---|---|---|---|
| rs5049 | AGT | 1 | 228916956 | C | T | 0.715 | 0.285 | 1.000 |
| rs5051 | AGT | 1 | 228916956 | T | C | 0.979 | 0.021 | 1.000 |
| rs672645 | CYP4F12 | 19 | 15653377 | G | A | 0.713 | 0.287 | 0.859 |
| rs10177833 | SLC4A5 | 2 | 74311476 | C | A | 0.508 | 0.492 | 0.886 |
| rs8179526 | SLC4A5 | 2 | 74323537 | T | C | 0.555 | 0.445 | 0.772 |
| rs679620 | MMP3 | 11 | 102219080 | C | T | 0.662 | 0.338 | 0.869 |
Note. HWE = Hardy–Weinberg equilibrium.
Table 4.
Linear MixedModelAnalysis for SingleNucleotide Polymorphisms (SNPs) on Predicting Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) Among 199 Dogon Women Across Three Generations
| SBP (mmHg) |
DBP (mmHg) |
||||||
|---|---|---|---|---|---|---|---|
| SNP | N | β | SE | p Value | β | SE | p Value |
| rs5049 | 187 | −2.46 | 2.25 | .278 | −2.87 | 1.65 | .084 |
| rs5051 | 190 | 6.78 | 5.31 | .204 | 1.78 | 4.09 | .664 |
| rs672645 | 189 | −7.80 | 4.09 | .059 | −2.74 | 2.88 | .342 |
| rs10177833 | 188 | −0.15 | 2.65 | .955 | −0.28 | 1.92 | .883 |
| rs8179526 | 190 | −6.47 | 2.77 | .021* | −0.13 | 2.04 | .948 |
| rs679620 | 187 | 0.47 | 2.30 | .839 | 0.38 | 1.65 | .821 |
Note. Adjusted by age, age2, and body mass index (BMI).
p < .05.
Discussion
In this study of Dogon women, we found only the SNP rs8179526 to be significantly associated with increases in SBP. These findings are consistent with those of Taylor et al.’s (2009) study of urban African American women, in which investigators found a statistically significant interaction for rs8179526 and BP. rs8179526 is located on the SLC4A5 gene on chromosome 2. SLC4A5 encodes a protein that transports sodium and bicarbonate across cell membranes while regulating cellular pH. The gene contains several SNPs that have been linked to elevated BP.
Although a number of studies have revealed genetic evidence linking one or more loci of chromosome 2 to hypertension and BP (Barkley et al., 2004; Cooper et al., 2002; Gu et al., 2005; Hunt et al., 2006; Province et al., 2003), none have been conducted in a homogenous genetic sample of multigenerational West African women, as in the present study. In one study of SNPs on chromosome 2, investigators found a significant association between one SNP (rs1879282) and elevated BP in African American mothers and daughters in the Hyper-GEN network, part of the large epidemiological sample in the family BP program studies (FBPP; Rao et al., 2003). Of the several SNPs assessed over the past few years, researchers have noted associations between those on the SLC4A5 gene and high BP among African Americans (World Health Organization, 2005), but the rs8179526 SNP on this gene has received little attention.
Although BMI was low in the present sample, risk of hypertension continues to be a problem among West African Dogon women. Aggregated survey data indicate that the prevalence of hypertension by 65 years of age is estimated to be 30–40% in rural West Africa for both men and women (Schémann et al., 2002). Our finding in the present study that BP increased with age in this population is consistent with previous research (Addo, Smeeth, & Leon, 2007; Opie & Seedat, 2005). In addition, increases in BMI are a risk factor for the development of hypertension in this population (Mensah, 2008). The prevalence in urban- and rural-dwelling women in West Africa of BMI > 25 kg/m2 was 17.6% and of BMI > 30 kg/m2 was 5.2%. The prevalence of BMI > 25% was higher in women in urban areas (30.4%) when compared to those living in rural areas (10%). The women in the present study were rural dwelling, and the prevalence of BMI > 25% that we found was consistent with previous research. Given that the percentage of urbanization in Mali is 30%, overweight and obesity is of concern (World Health Organization, 2006; World Health Organization Public Health and the Environment, 2009). In addition, the prevalence of overweight and obesity increases with age among Mali women and is the greatest in the 40- to 49-year-old age group who are also most affected by hypertension (Mensah, 2008). The women in the present study with the highest BMI were approximately 24–40 years of age.
Our study had several limitations. First was the language barrier. We had to rely on the translator to communicate with the participants. All translations were done orally because most of the participants were illiterate. The investigator and/or research assistants recorded the oral translations provided by the translator. Second, study participation was restricted to West African Dogon women and girls residing in a primitive rural tribal area. Because of a tribal rule prohibiting marriage with members of tribes outside of the seven Dogon tribes, our sample is genetically more homogeneous than the African American sample in the previous study. These results may thus not be generalizable to other ethnic groups or those living in suburban or urban areas. Third, the associations we examined in this study involved a small set of biological or positional candidate genes and not genome-wide association study SNPs. The approach was based on the premise that susceptibility alleles for common diseases were not under strong negative selection and that common variants contribute to common disease traits (i.e., the “common disease-common variant” hypothesis; Reich & Lander, 2001). However, the allelic spectrum for genes associated with complex quantitative traits, such as BP, has not been fully delineated. It is possible that multiple rare polymorphisms in the biological and positional candidate genes we studied could influence BP. However, due to lack of statistical power, identifying associations with BP using such alleles would not be possible with the approaches we employed in this study. Fourth, the findings may not be generalizable to individuals who are younger than those in the present study, of other ethnic groups, or men.
Finally, sample size was another potential limitation. Genetic variants usually have small effects compared to nongenetic risk factors. The sample size in this study (n = 199) could have been underpowered for detecting the SNP effect on SBP and DBP. The poor power for detecting the SNP BMI interaction may explain the lack of significant results×for SBP and DBP. The study needs to be replicated in a larger sample from the same population, but when we conducted the study, there was no such comparable sample available. The sample comprised triads of females related across three generations. To avoid further reducing sample size by stratified analysis within each generation, we applied linear mixed model techniques to adjust for relatedness in the pooled sample. In spite of these limitations, the approach we employed in the present study illustrates the use of SNPs in candidate genes to construct a more complete picture of the genetic architecture of complex traits such as BP.
The role of rs8179526 and its link to hypertension susceptibility has been understudied. The current study and Taylor et al.’s (2009) earlier study add to the small body of evidence suggesting the SNP’s relationship to high BP in African American and African women and girls. Knowledge of rs8179526’s effect on SBP in African American and West African women could help health practitioners educate their patients about a potential increased risk of developing high BP based on their individual genotypes. Further, gene–environment interaction research is a necessary part of increasing our understanding of hypertension and its disproportionate effect on Americans of African descent. Accordingly, we are currently engaged in an examination of rs8179526 and other SNPs associated with BP using a genome-wide approach in larger similar samples. Although hypertension is a complex disease, understanding the contribution of a single SNP is an important step toward increasing our ability to assess individual and population risk factors and designing appropriate interventions to reduce the incidence of the disease.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Funding for this research was provided in part by the University of Michigan Office of the Vice Provost and University of Michigan School of Nursing Office of the Dean to Jacquelyn Taylor.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Addo J, Smeeth L, Leon DA. Hypertension in sub-Saharan Africa: A systematic review. Hypertension. 2007;50:1012–1018. doi: 10.1161/HYPERTENSIONAHA.107.093336. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Chakravarti A, Cooper RS, Ellison RC, Hunt SC, Province MA, Family Blood Pressure Program Positional identification of hypertension susceptibility genes on chromosome 2. Hypertension. 2004;43:477–482. doi: 10.1161/01.HYP.0000111585.76299.f7. [DOI] [PubMed] [Google Scholar]
- Boutin-Foster C, Ogedegbe G, Ravenell JE, Robbins L, Charlson ME. Ascribing meaning to hypertension: A qualitative study among African Americans with uncontrolled hypertension. Ethnicity & Disease. 2007;17:29–34. [PubMed] [Google Scholar]
- Burnside M, Robotham R. Spirits of the passage: The transatlantic slave trade in the seventeenth century. Simon and Schuster; New York, NY: 1997. [Google Scholar]
- Carney JA. The role of African rice and slaves in the history of rice cultivation in the Americas. Human Ecology. 1998;26:525–545. [Google Scholar]
- Centers for Disease Control and Prevention. National Center for Health Statistics Childhood overweight and obesity: Obesity prevalence. 2007a Retrieved June 28, 2011, from http://www.cdc.gov/obesity/childhood/index.html.
- Centers for Disease Control and Prevention. National Center for Health Statistics National health and nutrition examination survey. 2007b Retrieved June 28, 2011, from http://www.cdc.gov/nchs/data/hestat/obesity_adult_07_08/obesity_adult_07_08.htm.
- Centers for Disease Control and Prevention. Retrieved February 11, 2008, from http://www.cdc.gov/nccdphp/dnpa/bmi/adult_BMI/about_adult_BMI.htm About BMI for adults. 2008 http://www.cdc.gov/nccdphp/dnpa/bmi/adult_BMI/about_adult_BMI.htm
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., National High Blood Pressure Education Program Coordinating Committee The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. Journal of the American Medical Association. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Cleveland WS. Robust locally weighted regression and smoothing scatterplots. Journal of the American Statistical Association. 1979;74:829–836. [Google Scholar]
- Cooper RS, Luke A, Zhu X, Kan D, Adeyemo A, Rotimi C, Ward R. Genome scan among Nigerians linking blood pressure to chromosomes 2, 3 and 19. Hypertension. 2002;40:629–633. doi: 10.1161/01.hyp.0000035708.02789.39. [DOI] [PubMed] [Google Scholar]
- Cupples LA, Arruda HT, Benjamin EJ, D’Augustino RB, Demissie S, DeStefano AL, Atwood LD. The Framingham heart study 100K SNP genome-wide association study resource: Overview of 17 phenotype working group reports. BMC Medical Genetics. 2007;8:1–19. doi: 10.1186/1471-2350-8-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas JG, Bakris GL, Epstein M, Ferdinand KC, Ferrario C, Flack JM, Hypertension in African Americans Working Group Management of high blood pressure in African Americans: Consensus statement of the hypertension in African Americans working group of the international society on hypertension in blacks. Archives of Internal Medicine. 2003;163:525–541. doi: 10.1001/archinte.163.5.525. [DOI] [PubMed] [Google Scholar]
- Frey WH. The new great migration: Black Americans’ return to the South, 1965-2000. Brookings Institution. 2004:1–3. Retrieved from http://www.brookings.edu/reports/2004/05demographics_frey.aspx. [Google Scholar]
- Gibson C. Population of the 100 largest cities and other urban places in the United States: 1790 to 1990. U.S. Bureau of the Census, Population Division; Washington, DC: 1998. Population Division Working Paper. [Google Scholar]
- Gu CC, Chang YC, Hunt SC, Schwander K, Arnett D, Djousse L, Rao DC. Haplotype association analysis of AGT variants with hypertension-related traits: The HyperGEN study. Human Heredity. 2005;60:164–174. doi: 10.1159/000090118. [DOI] [PubMed] [Google Scholar]
- Hunt SC, Xin Y, Wu LL, Cawthon RM, Coon H, Hasstedt SJ, Hopkins P. Sodium bicarbonate cotransporter polymorphisms are associated with baseline and 10 year follow up blood pressure. Hypertension. 2006;47:532–536. doi: 10.1161/01.HYP.0000196949.26088.3c. [DOI] [PubMed] [Google Scholar]
- Lovejoy P. Transformations in slavery. Cambridge University Press; Cambridge, UK: 2000. [Google Scholar]
- Mensah G. Epidemiology of stroke and high blood pressure in Africa. Heart. 2008;94:697–705. doi: 10.1136/hrt.2007.127753. [DOI] [PubMed] [Google Scholar]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- Opie L, Seedat Y. Hypertension in sub-Saharan African populations. Circulation. 2005;112:3562–3568. doi: 10.1161/CIRCULATIONAHA.105.539569. [DOI] [PubMed] [Google Scholar]
- Province MA, Kardia SL, Ranade K, Rao DC, Thiel BA, Cooper RS, Boerwinkle E. A meta-analysis of genome-wide linkage scans for hypertension: The national heart, lung and blood institute family blood pressure program. American Journal of Hypertension. 2003;16:144–147. doi: 10.1016/s0895-7061(02)03248-x. [DOI] [PubMed] [Google Scholar]
- Rao DC, Province MA, Leppert MF, Oberman A, Heiss G, Ellison RC, HyperGEN Network A genome-wide affected sibpair linkage analysis of hypertension: The HyperGEN network. American Journal of Hypertension. 2003;16:148–150. doi: 10.1016/s0895-7061(02)03247-8. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd ed. Sage; Thousand Oaks, CA: 2002. [Google Scholar]
- Reich DE, Lander ES. On the allelic spectrum of human disease. Trends in Genetics. 2001;17:502–510. doi: 10.1016/s0168-9525(01)02410-6. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K. Heart disease and stroke statistics—2007 update: A report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. [DOI] [PubMed] [Google Scholar]
- Schémann JF, Banou AA, Guindo A, Joret V, Traore L, Malvy D. Prevalence of undernutrition and vitamin A deficiency in the Dogon region, Mali. Journal of the American College of Nutrition. 2002;21:381–387. doi: 10.1080/07315724.2002.10719239. [DOI] [PubMed] [Google Scholar]
- Strassmann B. Life-history theory, fertility and reproductive success in humans. Biological Sciences. 1997;269:553–562. doi: 10.1098/rspb.2001.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JY, Maddox R, Wu CY. Genetic and environmental risks for high blood pressure among African American mothers and daughters. Biological Research for Nursing. 2009;11:53–65. doi: 10.1177/1099800409334817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JY, Sun Y, Chu J, Mosley T, Kardia S. Interactions between metallopeptidase 3 polymorphisms rs679620 and BMI in predicting blood pressure in African American women with hypertension. Journal of Hypertension. 2008;26:2312–2318. doi: 10.1097/HJH.0b013e3283110402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JY, Sun Y, Hunt S, Kardia SLK. Gene-environment interaction for blood pressure among African American women across generations. Biological Research for Nursing. 2010;12:149–155. doi: 10.1177/1099800410371225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir B. Genetic data analysis II. Sinauer Associates; Boston, MA: 1996. [Google Scholar]
- World Health Organization . Cardiovascular diseasesinthe African region: Current situation and perspectives (AFR/RC55/12) Regional office for Africa; 2005. Retrieved June 28, 2011, from http://www.afro.who.int/en/clusters-a-programmes/dpc/non-communicable-diseasesmanagementndm/npc-publications.html. [Google Scholar]
- World Health Organization Enquête démographique et de Santé du Mali in 2006. 2006 Retrieved June 28, 2011, from www. measuredhs.com/pubs/pdf/FR199/FR199.pdf.
- World Health Organization. Public Health and the Environment Country profile of environmental burden of disease: Mali. 2009 Retrieved September 30, 2009, from http://www.who.int/quantifying_ehimpacts/national/countryprofile/mali.pdf.