Abstract
Background
It is unknown whether women derive comparable benefits and have a similar safety and tolerability profile as men from acamprosate, a widely prescribed drug for the maintenance of abstinence in alcohol dependence. The objective of this study was to assess sex-specific differences in the efficacy, safety and tolerability of acamprosate in the treatment of women and men with alcohol dependence.
Methods
A sex-specific meta-analysis was conducted based on individual patient data (IPD). Data were obtained from double-blind, randomized controlled trials (RCTs) with quantitative drinking measures in patients with alcohol dependence receiving oral acamprosate or placebo. Sources included PubMed, PsychInfo and Cochrane electronic databases; reference lists from retrieved articles and presentations at professional meetings; and direct access to authors and companies who provided IPD.
Results
Individual records were obtained on 1,317 women and 4,794 men who participated in 22 eligible studies conducted in 18 countries. IPD meta-analyses found a significant beneficial effect of acamprosate relative to placebo across all 4 efficacy endpoints: an incremental gain of 10.4% (95% CI 7.1 to 13.7, p<.001) in percent of abstinent days, an incremental gain of 11.0% (7.4 to 14.6, p<.001) in percent of no heavy drinking days, an odds ratio of 1.9 (1.6 to 2.2, p<.001) for rate of complete abstinence and an odds ratio of 1.9 (1.6 to 2.3, p<.001) for rate of no heavy drinking, over the study duration. Acamprosate was also associated with significantly higher rates of treatment completion (p=0.004) and medication compliance (p<.001) than placebo. Men and women did not differ on any measure of acamprosate efficacy, safety or tolerability.
Conclusion
This sex-specific IPD meta-analysis provides evidence that acamprosate has a significant effect compared with placebo in improving rates of abstinence and no heavy drinking in both women and men with alcohol dependence. Further, acamprosate was associated with significantly higher rates of treatment completion and medication compliance than placebo among both women and men, and had a comparable safety and tolerability profile.
Keywords: acamprosate, women, alcoholism treatment, individual patient data, meta-analysis
INTRODUCTION
Alcohol dependence traditionally has been viewed as a man’s disease, and much of our understanding of the disorder derives from studies comprised largely of males (Greenfield, 2002). However, recent epidemiological studies suggest a closing gender gap, with women representing approximately one-third of individuals with alcohol dependence (Grant et al., 2004; Keyes et al., 2008). Furthermore, although 2.3% of American women are estimated to meet criteria for current alcohol dependence overall, the prevalence is more than two-fold greater (5.5%) among women in the child-bearing ages of 18–29 years, with associated risk of fetal alcohol symptoms in their children (Grant et al., 2004). In addition, women generally experience liver damage and other health problems after consuming less alcohol than men, in part because of gender differences in total body water and alcohol metabolism (Bradley et al., 1998; Greenfield, 2002). Among women, chronic consumption of more than two drinks per day is associated with increased risk of mortality, breast cancer, hypertension, stroke, and reproductive problems (Bradley et al., 1998). Binge drinking among women, e.g., consuming four or more drinks in a row, may incur increased risk of accident, rape, assault, and unprotected sex (Wechsler et al., 1995). Comorbid depression and anxiety disorders also are more prevalent among alcoholic women than men, with associated increased risk of suicidality (Conway et al., 2006).
Given the significant disease burden of alcohol dependence in women, early intervention and effective treatment options are clearly needed. One emerging area in the treatment of alcohol dependence is pharmacotherapy. Available medications include disulfiram, naltrexone, and acamprosate. However, relatively little is known about the efficacy of these medications for alcohol dependence in women. This lack of knowledge was highlighted in a pivotal multi-center randomized controlled trial (RCT) showing that the newly approved long-acting injectable form of naltrexone (Vivitrol®) was efficacious for males but not for females (Garbutt et al., 2005). Similarly, a study of oral naltrexone conducted solely in alcoholic women failed to show efficacy relative to placebo (O’Malley et al., 2007).
Acamprosate is an oral prescription medication for the maintenance of abstinence in individuals with alcohol dependence. Its mechanism of action involves normalizing the dysregulation of N-methyl-D-aspartic acid (NMDA)-mediated glutamatergic neurotransmission that occurs in recently abstinent individuals with alcohol dependence, thus attenuating one of the physiological mechanisms that may prompt drinking relapse (Littleton, 2007). Acamprosate generally has been shown to significantly increase the rate of abstinence relative to placebo in non-sex specific trials and to have no known serious adverse events, as demonstrated in more than 6,000 male and female outpatients with alcohol dependence studied in 23 RCTs conducted across 18 countries (See Table 1). Acamprosate is not metabolized in the liver and is not known to interact negatively with alcohol or other illicit or prescribed drugs (Rosenthal et al., 2008). A national audit of retail pharmacies found acamprosate to be the most widely prescribed drug for alcohol dependence in the United States (Market al., 2009).
Table 1.
Studies Included in the Individual Participant Data Meta-Analysis
| Study, year | Country | Women | Men | X̄ Dose (mg/d) | Study Duration (Months) | Chalmers Scores | ||
|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | |||||
| Lhuintre, 1985 | France | 8 | (11) | 62 | (89) | 1,878 | 3 | 51 |
|
| ||||||||
| Lhuintre, 1990 | France | 103 | (18) | 466 | (82) | 1,708 | 3 | 70 |
|
| ||||||||
| Pelc, 1992 | Belgium | 32 | (31) | 70 | (69) | 1,842 | 6 | 65 |
|
| ||||||||
| Ladewig, 1993 | Switzerland | 14 | (23) | 47 | (77) | 1,838 | 6 | 68 |
|
| ||||||||
| Paille, 1995 | France | 108 | (20) | 430 | (80) | 1,765 | 12 | 80 |
|
| ||||||||
| Roussaux, 1996 | Belgium | 38 | (30) | 89 | (70) | 1,828 | 3 | 65 |
|
| ||||||||
| Sass, 1996 | Germany | 61 | (22) | 211 | (78) | 1,911 | 12 | 80 |
|
| ||||||||
| Whitworth, 1996 | Austria | 95 | (21) | 353 | (79) | 1,911 | 12 | 74 |
|
| ||||||||
| Barrias, 1997 | Portugal | 24 | (08) | 278 | (92) | 1,828 | 12 | 71 |
|
| ||||||||
| Geerlings, 1997 | Belgium, Luxembourg, Netherlands | 63 | (24) | 199 | (76) | 1,875 | 6 | 73 |
|
| ||||||||
| Pelc, 1997 | Belgium, France | 28 | (15) | 160 | (85) | 1,665 | 3 | 74 |
|
| ||||||||
| Poldrugo, 1997 | Italy | 67 | (27) | 179 | (73) | 1,828 | 6 | 79 |
|
| ||||||||
| Besson, 1998 | Switzerland | 22 | (20) | 88 | (80) | 1,878 | 12 | 70 |
|
| ||||||||
| Chick, 2000 | United Kingdom | 96 | (17) | 485 | (83) | 1,998 | 6 | 82 |
|
| ||||||||
| Tempesta, 2000 | Italy | 57 | (17) | 273 | (83) | 1,998 | 6 | 75 |
|
| ||||||||
| Gual and Lehert, 2001 | Spain | 59 | (20) | 229 | (80) | 1,998 | 6 | 81 |
|
| ||||||||
| Kiefer, 2003 | Germany | 23 | (29) | 57 | (71) | 1,864 | 3 | 82 |
|
| ||||||||
| Namkoong, 2003 | South Korea | 6 | (04) | 136 | (96) | 1,642 | 2 | 80 |
|
| ||||||||
| Baltieri, 2004 | Brazil | 0 | (00) | 75 | (100) | 1,998 | 3 | 78 |
|
| ||||||||
| Anton, 2006 | United States | 187 | (31) | 425 | (69) | 3,000 | 4 | 80 |
|
| ||||||||
| Mason, 2006 | United States | 191 | (32) | 401 | (68) | 2,436 | 6 | 85 |
|
| ||||||||
| Morley, 2006 | Australia | 35 | (30) | 81 | (70) | 1,998 | 3 | 80 |
|
| ||||||||
| Total | 1,317 | 4,794 | ||||||
Although acamprosate has relatively widespread use, women represent only 22% of participants in acamprosate RCTs (Table 1) and sex-specific outcome data from RCTs have not been reported. As a result, the differential efficacy and tolerability of this pharmacotherapy in women and men has not been documented through literature-based meta-analysis and it is not known if women derive comparable benefits and have a similar safety and tolerability profile with acamprosate as do men. Our objective was to conduct an individual patient data (IPD) meta-analysis to assess sex-specific differences in acamprosate efficacy, safety and tolerability in a multi-national sample of female and male participants in RCTs of acamprosate treatment of alcohol dependence.
METHODS
Data Sources
PubMed, PsychInfo and Cochrane electronic databases were searched for human studies from inception through February 2011 without date or language restriction to find RCIs using the terms “acamprosate” and “Campral®”, crossed with “alcohol dependence”, “alcoholism”, “randomized controlled trial” and “treatment”. The bibliographies of retrieved articles were examined for other relevant studies, and major scientific meetings were monitored for the results of completed trials that were unpublished at the time of the search.
Study Selection
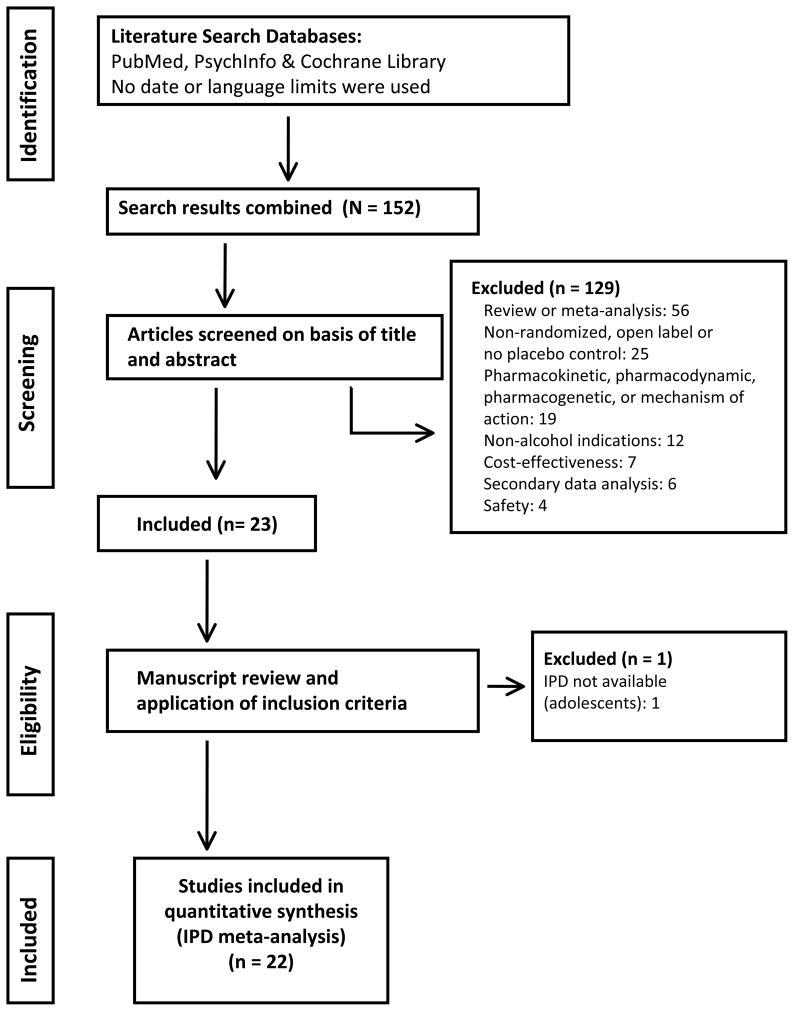
Using the above search criteria, a total of 152 articles were identified (Figure 1). Trials that met the following criteria were included: (1) prospective, placebo-controlled, double-blind parallel groups design, (2) random assignment of patients with current alcohol dependence to either acamprosate or placebo treatment, and (3) at least one quantitative measure of drinking behavior to assess treatment efficacy. One hundred twenty-nine publications were excluded because they were not randomized placebo controlled trials (e.g., review articles or open label studies) or had outcomes other than alcoholism treatment endpoints (endpoints involving biomarkers, cognition, and economic factors). Independent investigators and Lipha s.a., a subsidiary of Merck of Darmstadt, Germany, the developer of acamprosate, were contacted by the authors and asked to provide access to the electronic databases of acamprosate RCTs and an electronic database in the public domain was accessed (http://www.cscc.unc.edu/COMBINE). Of the 23 identified alcoholism RCTs, one small (N=26) study in adolescents that included only 3 females was excluded because IPD were not available (Niederhofer and Staffen, 2003). Thus a total of 22 studies, representing all acamprosate RCTs conducted in adults to date, were included in this IPD meta-analysis, as summarized in Figure 1.
Figure 1.
Flow Diagram of the Study Selection Process
Data Extraction
The authors independently assessed the methodological quality of selected RCTs using a validated scale that scores multiple aspects of the experimental design of a trial, including sample size, randomization methods, methods to preserve the double-blind, selection and withdrawal criteria, and statistical analysis (Chalmers et al., 1981). Trials were required to attain a mean Chalmers score of at least 50 of a possible 100 points to be retained in the analysis. The mean Chalmers scores of all studies assessed were within an acceptable range (Table 1).
Study Characteristics
The 22 RCTs selected for inclusion were reasonably homogeneous in terms of admission criteria, outcome variables and provision of concomitant alcoholism counseling. Inclusion criteria typically specified males and females over 18 years of age meeting Diagnostic and Statistical Manual – Third Edition Revised or Fourth Edition (DSM-III R, DSM IV; American Psychiatric Association, 1987, 1994) criteria for current alcohol dependence. Exclusion criteria typically specified pregnancy, lactation or refusal to use reliable birth control in women with child bearing potential1; significant current psychiatric disorders such as depression, anxiety or other substance dependence disorders except nicotine; significant medical disorders such as severe renal failure2; and pharmacological treatments that could confound the results of the medication under study, such as naltrexone. Subjects were required to be abstinent for at least two days prior to randomization in all but three trials (Chick et al., 2000; Mason et al., 2006; Namkoong et al., 2003). Patients generally received the alcoholism counseling offered by the participating site, with manual guided counseling provided in three recent trials (Anton et al., 2006; Mason et al., 2006; Morley et al., 2006). Fourteen trials had durations of at least 6 months, with five studies having duration of one year (Table 1). The earlier trials adjusted dose on the basis of body weight (i.e., 1332 mg/d for subjects weighing <60 kg and 1998 mg/d for ≥ 60 kg). However, dosing by body weight is no longer recommended and 1998 mg per day is the approved standard fixed dose. Two trials (Anton, 2006 and Mason, 2006 in a small experimental group) used a dose of 3,000 mg which represents a 0.5 fold increase over the approved therapeutic dose. In keeping with the standard approach to meta-analysis, treatment conditions are collapsed to active drug versus placebo groups.
Data Synthesis
Efficacy endpoints are: a.) the percent of abstinent days, defined as the total number of abstinent days on study divided by the total potential treatment duration; this outcome evaluates the treatment effect throughout the study duration, as opposed to “time to event” outcomes; b.) the proportion of patients maintaining complete abstinence throughout the treatment duration, which is the outcome most associated with long-term recovery (Dawson et al., 2007); c.) the percent of no heavy drinking days (≥ 4 drinks females, ≥5 drinks males), defined as the total number of no heavy drinking days on study divided by the total potential treatment duration, and d.) the proportion of patients with no heavy drinking days throughout the treatment duration, which is an outcome associated with harm reduction. The Timeline Followback Interview (Sobell and Sobell, 2000) typically was used as the quantitative measure of drinking in the more recent studies (Anton et al., 2006; Baltieri and De Andrade, 2004; Chick et al., 2000; Gual and Lehert, 2001; Kiefer et al., 2003; Mason et al., 2006; Morley et al. 2006; Namkoong et al., 2003; Paille et al., 1995; Pelc et al., 1997). Other studies used protocol-specific interviews, similar to the Timeline Followback Interview, that typically recorded frequency of drinking, quantity of drinks, and frequency of heavy drinking over rating intervals that ranged from 1 week to 1 month. Thus, the percent of abstinent days, rate of complete abstinence, percent of no heavy drinking days and rate of no heavy drinking could be calculated across studies from these data. All of the included trials documented the reason for patients’ premature termination. Early terminations clearly not related to alcoholism, e.g., the patient moved, were not treated as drinking; outcomes in these cases were calculated based on the subject’s actual time on study. Other reasons for early termination, e.g., the patient was lost to follow up, were conservatively attributed to drinking. No other missing data imputation was applied to efficacy endpoints.
The safety and tolerability of acamprosate in women and men were assessed with the rates of: a.) treatment completion; b.) discontinuation due to adverse events; c.) one or more adverse events of moderate or greater severity; d.) one or more digestive system adverse events; and e.) medication compliance. Gastrointestinal adverse events, typically loose stools or diarrhea, are the only side effect to consistently occur with greater frequency in acamprosate than placebo groups across clinical trials (Rosenthal et al., 2008). Therefore adverse events involving the digestive system were specifically chosen for evaluation. Medication compliance was assessed by returned pill counts with verification typically provided by at least one determination of acamprosate in plasma. Missing data for variables not related to efficacy were considered Missing at Random (Little and Rubin, 1987). This supported the use of the systematic Full Information Maximum Likelihood Technique (Anderson, 1957).
Statistical Analysis
Baseline characteristics were compared between men and women using t-test or chi square statistics, as appropriate. All such tests were 2-tailed, with P<0.05 considered significant. The IPD meta-analysis was conducted using mixed linear and non linear models to test the significance of the main effects of treatment and gender and the interaction of treatment and gender, while accounting for study heterogeneity.
To test the continuous efficacy endpoints of percent of abstinent days and percent of no heavy drinking days, we used a multilevel general linear mixed model (Higgins et al., 2001) with treatment considered a random effect, study considered a fixed effect, gender as a main effect, and with a first order interaction of gender and treatment. Percent of abstinent days and percent of no heavy drinking days were calculated with and without arcsine transformation. Similar P-values were obtained for transformed and untransformed data. Thus results are reported for untransformed data to facilitate clinical interpretation. For the binary endpoints of rates of complete abstinence and no heavy drinking, a non linear model was used based on the same assumptions as above. Additionally, traditional meta-analysis using summary data for each study was used to support IPD results and evaluate treatment efficacy for women and men separately, using forest tree diagrams (DerSimonian and Laird, 1986). Safety and tolerability endpoints were evaluated in the IPD by 2-way ANOVA, to test the significance of treatment and gender as main effects and the first order interaction of treatment and gender. All analyses were performed using the R statistical package (Version 2.10.1).
All methods were undertaken according to the 2009 PRISMA guidelines for the conduct and reporting of systematic reviews (Moher et al., 2009).
Bias and sensitivity
Although classical literature-based meta-analyses of aggregated data typically are used for contrasting and comparing results from different studies, those study-level analyses may lead to biased assessments and have limitations in explaining heterogeneity across trials. IPD meta-analyses offer advantages over traditional meta-analyses, including consistency in handling missing data, defining endpoints and analyzing endpoints across studies (Simmons et al., 2005). In this IPD meta-analysis, bias in individual studies and across studies was reduced by 1) consistent handling of missing data, 2) standardizing outcomes, 3) analyses of outcomes in the intent-to-treat population and 4) re-calculating endpoints across studies using the IPD to eliminate bias introduced by studies using different analytic approaches. In addition, trials included in this meta-analysis were required to meet stringent inclusion criteria based on a validated scale for assessing methodological quality.
RESULTS
Clinical Characteristics of Women and Men with Alcohol Dependence
A total of 1,317 women and 4,794 men participated in the 22 RCTs of acamprosate for alcohol dependence selected for inclusion in the IPD meta-analysis (Table 1).
Women were significantly more likely to be divorced or widowed than men, and had significantly higher rates of suicide attempts, drug abuse, anxiety, depression and non-specific psychiatric problems than men (Table 2). Furthermore, despite having alcoholism of significantly briefer duration than men and lower quantity and frequency of drinking, women showed significantly greater impairment on tests of liver functioning. Women were also more likely to have attended one or more meetings of Alcoholics Anonymous (AA) and to have had prior psychotherapy.
Table 2.
Baseline Characteristics of Women and Men Participants in Randomized Controlled Trials of Acamprosate in Alcohol Dependence
| Women | Men | p-value | |
|---|---|---|---|
| Demographic Variables | 1,317 | 4,794 | |
|
| |||
| Age (years) | 43.3±9.2 | 42.8±9.2 | 0.023 |
|
| |||
| Marital Status | |||
| Single | 18% | 20% | 0.003 |
| Married | 54% | 58% | |
| Divorced | 22% | 18% | |
| Widowed | 6% | 4% | |
|
| |||
|
Alcohol History
| |||
| Days of Abstinence in Prior Year | 85±175 | 106±320 | 0.071 |
|
| |||
| Drinks per Drinking Day | 10.6±4.5 | 12.8±3.9 | <0.001 |
|
| |||
| Drinking Days per Week | 5.9±1.7 | 6.4±1.4 | <0.001 |
|
| |||
| Alcoholism Duration (Years) | 9.7±5.6 | 10.4±5.5 | 0.006 |
|
| |||
| Alcoholism Severity Category | |||
| Mild | 28% | 31% | 0.071 |
| Medium | 31% | 29% | |
| Severe | 41% | 40% | |
|
| |||
| Prior Detoxifications | 61.5% | 64.3% | 0.26 |
|
| |||
| Prior Anti-alcohol Drug | 37.2% | 38.1% | 0.464 |
|
| |||
| Prior AA Attendance | 30.1% | 22.9% | 0.002 |
|
| |||
|
Liver Functioning
| |||
| GGT x ULN | 2.89±4.7 | 2.72±3.3 | 0.018 |
|
| |||
| SGPT x ULN | 1.5±7.2 | 1.3±2.2 | 0.003 |
|
| |||
| SGOT x ULN | 1.2±7.1 | 1.0±1.0 | <0.001 |
|
| |||
|
History of Psychosocial Problems
| |||
| Legal Problems | 45.3% | 45.0% | 0.576 |
|
| |||
| Anxiety Disorder | 45.1% | 31.5% | 0.012 |
|
| |||
| Mood Disorder | 26.3% | 17.4% | 0.019 |
|
| |||
| Non-Specific Psychiatric Problems | 40.0% | 32.5% | 0.048 |
|
| |||
| Suicide Attempt | 21.6% | 13.3% | 0.004 |
|
| |||
| Drug Abuse | 9.5% | 5.5% | 0.044 |
|
| |||
| Psychotherapy | 47.1% | 37.3% | 0.007 |
Values provided are mean ± one standard deviation, or percent (number affected/total number).
Abbreviations: AA=Alcoholics Anonymous, GGT=Gamma-glutamyl transpeptidase, ULN=Upper limit of normal, SGPT=Serum glutamic pyruvic transaminase, SGOT=Serum glutamic oxaloacetic transaminase, MCV=mean cell volume
Efficacy Outcomes
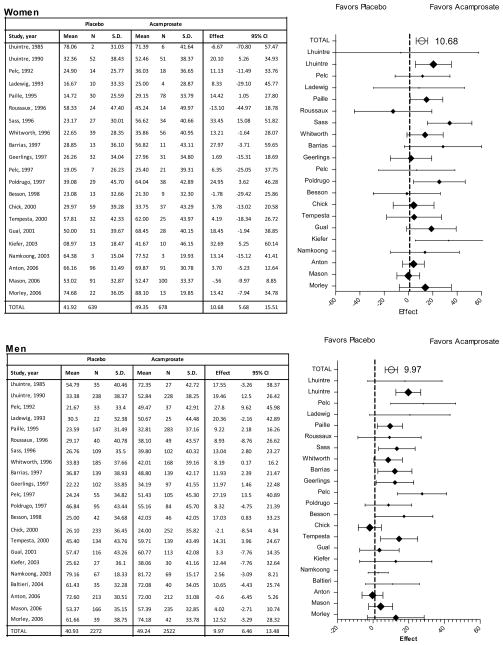
The IPD meta-analysis found a significant treatment effect for percent of abstinent days, with acamprosate showing an incremental gain of 10.38% (95% CI 7.1 to 13.7, p<0.001) relative to placebo (Table 3). The percent of abstinent days did not vary between women (48.02%) and men (47.08%), nor was there an interaction between treatment and gender. Results from traditional meta-analyses, conducted separately for men and women, confirm the significant effect of acamprosate on increase in percent of abstinent days among both women (+10.68%, 5.7 to 15.5, p<.001) and men (+9.97%, 6.5 to 13.5, p<.001) relative to placebo, as shown in Figure 2.
Table 3.
Individual Participant Data (IPD) Meta-Analyses: Efficacy Outcomes
| Mean Percent of Abstinent Days | ||||
| Acamprosate = 51.24 (40.95) | ||||
| Placebo = 42.96 (40.31) | ||||
| Incremental Value | 95% CI | P-value | ||
| Gender | −0.88 | −3.09 | 1.32 | 0.55 |
| Treatment | 10.38 | 7.10 | 13.65 | <.001 |
| Treatment x Gender | 0.12 | −4.27 | 4.12 | 0.95 |
|
| ||||
| Rate of Continuous Abstinence | ||||
| Acamprosate = 28% (45) | ||||
| Placebo = 19% (39) | ||||
| Odds Ratio | 95% CI | P-value | ||
| Gender | 0.83 | 0.66 | 1.04 | 0.11 |
| Treatment | 1.87 | 1.57 | 2.23 | <.001 |
| Treatment x Gender | 1.14 | 0.84 | 1.54 | 0.41 |
|
| ||||
| Mean Percent of No Heavy Drinking Days | ||||
| Acamprosate = 62.78 (39.91) | ||||
| Placebo = 52.95 (40.99) | ||||
| Incremental Value | 95% CI | P-value | ||
| Gender | −0.16 | −3.55 | 3.24 | 0.93 |
| Treatment | 11.03 | 7.45 | 14.62 | <.001 |
| Treatment x Gender | 1.33 | −3.36 | 6.02 | 0.58 |
|
| ||||
| Rate of No Heavy Drinking | ||||
| Acamprosate = 39% (49) | ||||
| Placebo = 28% (45) | ||||
| Odds Ratio | 95% CI | P-value | ||
| Gender | 1.00 | 0.88 | 1.14 | 0.99 |
| Treatment | 1.93 | 1.62 | 2.31 | <.001 |
| Treatment x Gender | 1.11 | 0.84 | 1.34 | 0.49 |
Figure 2.
Percent of Abstinent Days
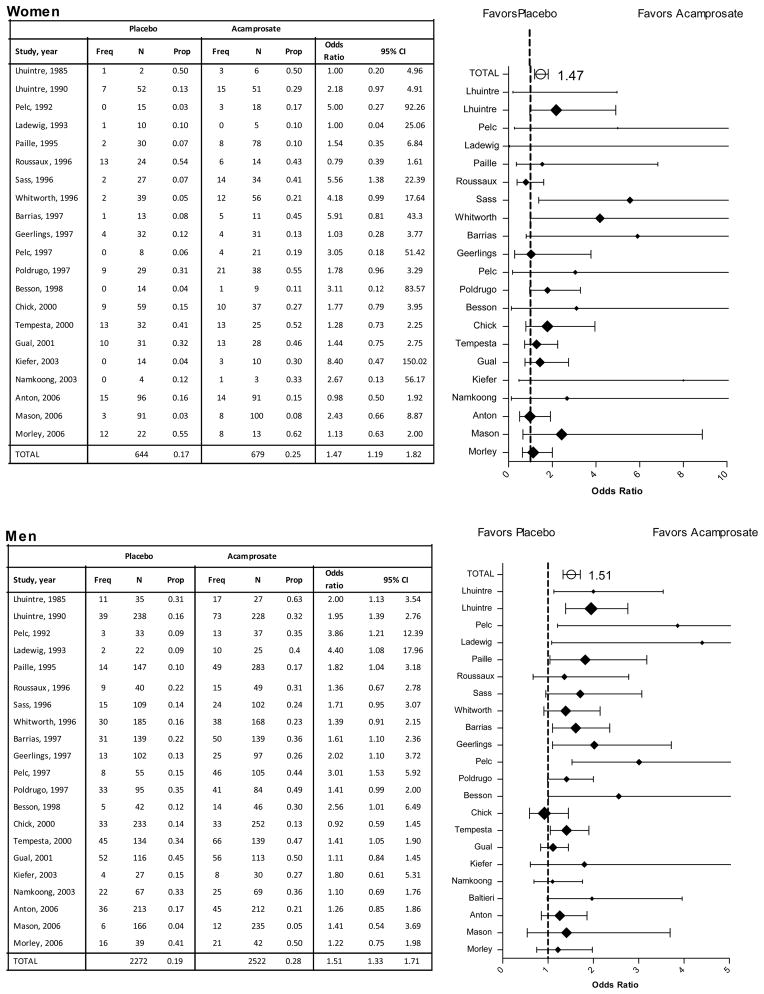
The IPD meta-analysis found a significant effect of acamprosate on the rate of complete abstinence relative to placebo (OR=1.87, 1.6 to 2.2, p<.001; Table 3). The rate of complete abstinence did not differ significantly between men (24.6%) and women (21.3%) and there was no evidence of an interaction between treatment and gender. Traditional meta-analysis and forest diagrams confirm the significant effect of acamprosate for both women (OR=1.47, 1.2 to 1.8, p<.001) and men (OR=1.51, 1.3 to 1.7, p<.001) on rate of complete abstinence relative to placebo (Figure 3).
Figure 3.
Rates of Continuous Abstinence
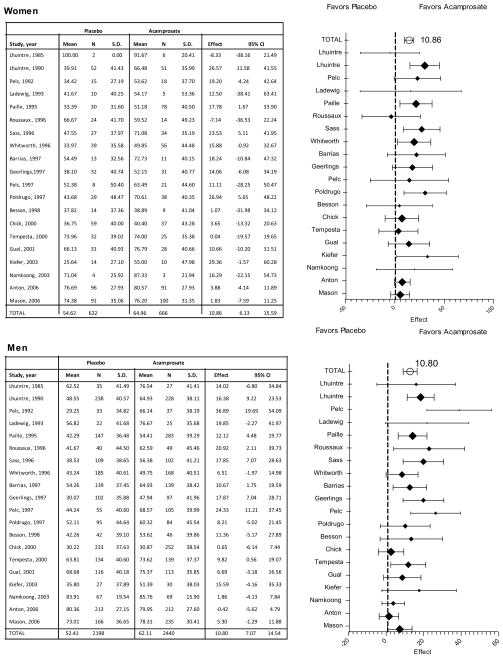
The IPD meta-analysis revealed a significant treatment effect for percent of no heavy drinking days, with acamprosate showing an incremental gain of +11.03% (7.5 to 14.6, p<.001) relative to placebo (Table 3). Results did not vary between women and men, nor was there an interaction between gender and treatment. Results from traditional meta-analyses, conducted separately for men and women, confirmed a significant increase with acamprosate in percent of no heavy drinking days among both women (+10.86%, 6.1 to 15.6, P=0.000) and men (+10.80%, 7.1 to 14.5, P=0.000) relative to placebo (illustrated in Figure 4).
Figure 4.
Percent of No Heavy Drinking Days
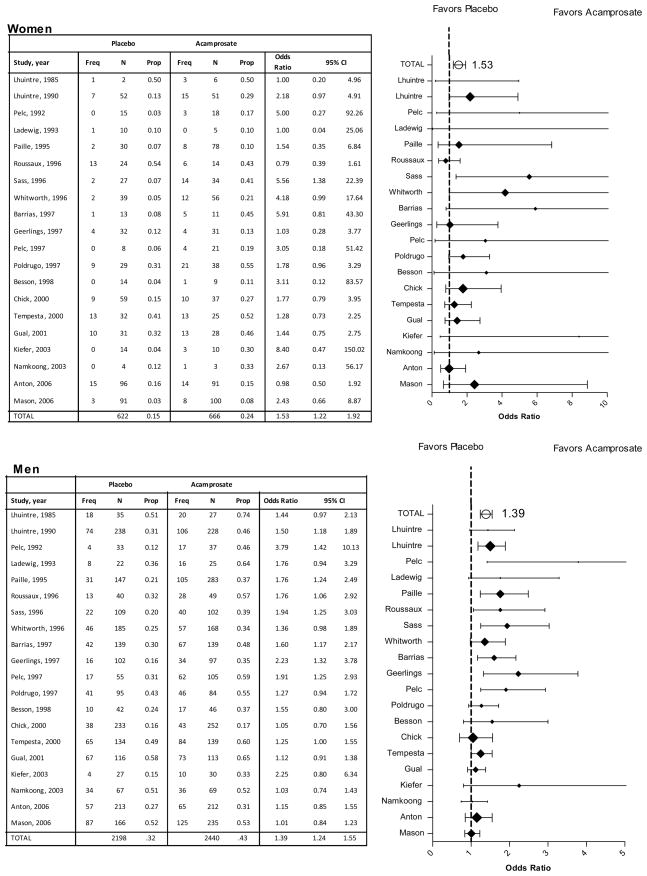
IPD meta-analysis revealed a significant effect of acamprosate on the rate of no heavy drinking relative to placebo (OR=1.93, 1.6 to 2.3, p<.001). The rate of no heavy drinking did not vary between women (33.8%) and men (34.3%), nor was there evidence of an interaction between treatment and gender (Table 3). Traditional meta-analysis and forest diagrams (Figure 5) confirmed a significant effect of acamprosate relative to placebo for rate of no heavy drinking among both women (OR=1.53, 1.2 to 1.9, p<.001) and men (OR=1.39, 1.2 to 1.6, p<.001).
Figure 5.
Rates of No Heavy Drinking
The IPD and traditional meta-analyses and forest diagrams indicate heterogeneity across studies, such that the treatment effect is greater in some studies and smaller in others, particularly among men with regard to the percent of abstinent days (Figure 2), percent of no heavy drinking days (Figure 4), and rate of no heavy drinking (Figure 5).
Safety and Tolerability
Acamprosate was associated with significantly higher rates of treatment completion (A=56.3%, P=52.1%, p=0.004) and medication compliance (A=76%, P=72%, p<.001) than placebo, among both men and women (Table 4). Treatment groups did not differ in the rate of discontinuation due to adverse events, the rate of one or more adverse events at a moderate level of severity or greater, or the rate of one or more digestive system adverse events. However, women complained of significantly more adverse events of at least a moderate level of severity or greater than did men (27.6% vs. 19.8%, p<.001), regardless of treatment assignment. There were no interactions between gender and treatment on any of the safety and tolerability measures.
Table 4.
Safety and Tolerability of Acamprosate
| Women | Men | ||||||
|---|---|---|---|---|---|---|---|
| N=1,317 | N=4,794 | ||||||
| Acamprosate | Placebo | Acamprosate | Placebo | Treatment | Gender | Interaction | |
| N=678 | N=639 | N=2,522 | N=2,272 | P-value | P-value | P-value | |
| Treatment completion | 57.8% | 52.7% | 55.9% | 51.9% | 0.004 | 0.37 | 0.72 |
| Discontinuation due to AE | 5.2% | 4.8% | 5.3% | 4.8% | 0.51 | 0.99 | 0.93 |
| ≥1 AE of ≥ moderate severity | 28.1% | 27.1% | 20.2% | 19.3% | 0.16 | <.001 | 0.86 |
| ≥1 Digestive AE | 5.6% | 6.3% | 5.7% | 4.4% | 0.63 | 0.20 | 0.14 |
| Medication compliance | 77.0% | 73.0% | 75.0% | 72.0% | <.001 | 0.28 | 0.78 |
Abbreviation: AE, adverse event.
DISCUSSION
Statement of Principal Findings
This IPD meta-analysis is the first comprehensive sex-specific evaluation of acamprosate efficacy and safety and tolerability in a multi-national sample of women and men with alcohol dependence. IPD from 1,317 women and 4,794 men participating in 22 multi-national RCTs of acamprosate showed a significant benefit of acamprosate on all four efficacy endpoints relative to placebo, including the percent of abstinent days, rate of complete abstinence, percent of no heavy drinking days, and rate of no heavy drinking, over the study duration. The effect size of acamprosate in women was comparable to that of men, and comparable to or better than effect sizes reported in non sex-specific literature-based meta-analyses (Mann et al., 2004), thus supporting the efficacy of acamprosate in the treatment of women with alcohol dependence. Furthermore, despite a history of significantly more anxiety, depression, suicide attempts, drug abuse, interpersonal loss and greater liver impairment at baseline than men, women responded comparably well to alcoholism treatment. The side effect and tolerability profile of acamprosate was comparable between women and men. Importantly, acamprosate was associated with significantly higher rates of treatment completion and medication compliance than placebo among both women and men.
Strengths and Weaknesses of the Study
The external validity of this report is supported by similarities between the included trials and clinical practice: all but four trials(Anton et al., 2006; Kiefer et al., 2003; Mason et al., 2006; Morley et al., 2006) used the counseling that was routinely provided on site, instead of a standardized manual-guided therapy for all subjects, which tends to inflate response in studies of substance dependence (Nunes and Levin, 2004); most studies recruited from established practices and not through advertisement and did not pay subjects for participation; admission criteria was relatively liberal and appropriate for acamprosate, but did exclude concomitant use of drugs that could affect outcome. Internal validity is supported by the use of raw IPD to re-calculate pre-treatment and outcome measures uniformly across trials. Additionally, the trials included here met rigorous a priori defined methodological criteria, including adequate sample size, research design, and study duration.
Strengths and Weaknesses in Relation to Other Studies
Our IPD meta-analysis differs from previous analyses in several ways that should increase its scientific value. It is the first sex-specific IPD meta-analysis from acamprosate RCTs in alcohol dependence. Second, it is large and comprehensive: the data comprise 1,317 women and 4,794 men participants in 22 acamprosate RCTs conducted in 18 countries, and is the largest dataset representing women seeking treatment for alcohol dependence studied to date. A limitation of this report relative to that of a RCT is that it is an IPD meta-analysis and not a prospective study. The meta-analyses and forest diagrams indicate considerable heterogeneity in effect sizes across trials. Methodological differences across studies may contribute to heterogeneity in effect sizes. For example, Roussaux et al. 1996 was the only study to show a negative effect of acamprosate across all four efficacy endpoints in women (Figures 2–5). This study had a relatively high proportion of women (30%) and was the only trial to admit patients with either alcohol abuse or dependence. Only with alcohol dependence would the neuroadaptions occur that acamprosate is hypothesized to normalize; in alcohol abuse there would be no neuropharmacological target for acamprosate to treat (Littleton, 2007). Similarly, Chick et al. 2000 was the only trial to show negative effects of acamprosate across three efficacy endpoints in men (Figures 2, 3, 5). This is one of the few trials to begin treatment in non abstinent participants and the negative findings likewise may be related to the mechanism of action of acamprosate, which is believed to normalize hyperactivity in the glutamate system in the brain after chronic heavy alcohol use and withdrawal (Littleton, 2007). This system theoretically would not become destabilized or hyperactive in an alcoholic who continues to drink. Another source of heterogeneity may be the contribution of a patient’s baseline characteristics to treatment outcome. Future analyses may investigate patients’ baseline characteristics as predictors of treatment response.
Future Research Considerations
Women were more likely to have side effect complaints of greater severity than men, with either acamprosate or placebo treatment. This greater level of physical discomfort may relate to women’s higher rates of emotional distress, as evidenced by higher lifetime rates of suicide attempts, depression, anxiety and non specific psychiatric complaints. Thus, future studies of acamprosate in patients with alcohol dependence and comorbid psychiatric disorders may be especially relevant for women. Recent findings of a beneficial effect of acamprosate on sleep (Staner et al., 2006) may be particularly germaine to comorbid disorders in which sleep disruption is a factor, e.g., depression, anxiety and post traumatic stress disorder. Importantly, acamprosate is not metabolized in the liver and has been found to raise no safety concerns when used in combination with drugs prescribed to treat comorbid psychiatric disorders (Rosenthal et al., 2008).
Clinical Implications of the Study
Results from this IPD meta-analysis indicate that acamprosate has a significant effect compared with placebo in improving rates of abstinence and no heavy drinking in both women and men with alcohol dependence. Further, acamprosate was associated with significantly higher rates of treatment completion and medication compliance than placebo among both women and men. Women and men treated with acamprosate shared similar tolerability profiles in terms of rates of discontinuation due to adverse events, rates of adverse events of moderate severity or greater, and rates of digestive system adverse events, and this profile did not differ from that of men and women treated with placebo. This evidence base suggests that treatment providers may routinely consider acamprosate for treating alcohol dependence in both women and men, taking into account the patient’s treatment goals and preferences as well as specific safety considerations.
Acknowledgments
Appreciation is expressed to Falk Kiefer, M.D., Kirsten C. Morley, Ph.D., M.P.H., Kee Namkoong, M.D., the COMBINE study and Merck Sante for providing access to electronic datasets used in this project. We also thank Karyn Coveney, B.A. and Allison Versaggi, A.A. for manuscript preparation and Vivian Goodell, M.P.H., John Light, Ph.D., and Charles Heyser, Ph.D., for their editorial comments.
Footnotes
Acamprosate is Category C in pregnancy.
Acamprosate is not metabolized in the liver and is excreted unchanged via the kidneys thus severe renal failure (creatinine > 35) but not liver disease is contraindicated.
Financial Disclosure: Dr. Mason has served as a scientific consultant for several pharmaceutical companies, including Merck Sante and Forest Laboratories. Dr. Lehert has served as a statistical consultant for several pharmaceutical companies, including Merck Sante and Forest Laboratories, Inc.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Press; 1987. rev. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Anderson TW. Maximum likelihood estimates for a multivariate normal distribution when some observations are missing. J Am Stat Assoc. 1957;52:200–203. [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. COMBINE Study Research Group: Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Baltieri DA, De Andrade AG. Acamprosate in alcohol dependence: a randomized controlled efficacy study in a standard clinical setting. J Stud Alcohol. 2004;65:136–139. doi: 10.15288/jsa.2004.65.136. [DOI] [PubMed] [Google Scholar]
- Barrias JA, Chabac S, Ferreira L, Fonte A, Potgieter AS, Teixeira de Sousa E. Acamprosate: multicenter Portuguese efficacy and tolerance evaluation study. Psiquiatria Clinica. 1997;18:149–160. [Google Scholar]
- Besson J, Aeby F, Kasas A, Lehert P, Potgieter A. Combined efficacy of acamprosate and disulfiram in the treatment of alcoholism: a controlled study. Alcohol Clin Exp Res. 1998;22:573–579. doi: 10.1111/j.1530-0277.1998.tb04295.x. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Badrinath S, Bush K, Boyd-Wickizer J, Anawalt B. Medical risks for women who drink alcohol. J Gen Intern Med. 1998;13:627–639. doi: 10.1046/j.1525-1497.1998.cr187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers TC, Smith H, Jr, Blackburn B, Silverman B, Schroeder B, Reitman D, Ambroz A. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981;2:31–49. doi: 10.1016/0197-2456(81)90056-8. [DOI] [PubMed] [Google Scholar]
- Chick J, Howlett H, Morgan MY, Ritson B. United Kingdom Multicentre Acamprosate Study (UKMAS): a 6-month prospective study of acamprosate versus placebo in preventing relapse after withdrawal from alcohol. Alcohol Alcohol. 2000;35:176–187. doi: 10.1093/alcalc/35.2.176. [DOI] [PubMed] [Google Scholar]
- Combining Medications and behavioral interventions [CSCC COMBINE website] [Accessed December 3, 2010];2008 September 29; Available at: http://www.cscc.unc.edu/COMBINE.
- Dawson DA, Goldstein RB, Grant BF. Rates and correlates of relapse among individuals in remission from DSM-IV alcohol dependence: a 3-year follow-up. Alcohol Clin Exp Res. 2007;31:2036–2045. doi: 10.1111/j.1530-0277.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman MD, Loewy JW, Ehrich EW. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: A randomized controlled trial. JAMA. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Geerlings PJ, Ansoms C, van den Brink W. Acamprosate and prevention of relapse in alcoholics. Eur Addict Res. 1997;3:129–137. [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Greenfield SF. Women and alcohol use disorders. Harv Rev Psychiatry. 2002;10:76–85. doi: 10.1080/10673220216212. [DOI] [PubMed] [Google Scholar]
- Gual A, Lehert P. Acamprosate during and after acute alcohol withdrawal: a double-blind placebo- controlled study in Spain. Alcohol Alcohol. 2001;36:413–418. doi: 10.1093/alcalc/36.5.413. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Whitehead A, Turner RM, Omar RZ, Thompson SG. Meta analysis of continuous outcome data from individual patients. Stat Med. 2001;20:2219–2241. doi: 10.1002/sim.918. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend. 2008;93:21–29. doi: 10.1016/j.drugalcdep.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Tarnaske T, Helwig H, Briken P, Holzbach R, Kampf P, Stracke R, Baehr M, Naber D, Wiedemann K. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2003;60:92–99. doi: 10.1001/archpsyc.60.1.92. [DOI] [PubMed] [Google Scholar]
- Ladewig D, Knecht T, Leher P, Fendl A. Acamprosate—a stabilizing factor in long-term withdrawal of alcoholic patients. Ther Umsch. 1993;50:182–188. [PubMed] [Google Scholar]
- Lhuintre JP, Daoust M, Moore ND, Chretien P, Saligaut C, Tran G, Bosimare F, Hillemand B. Ability of calcium bis acetyl homotaurine, a GABA agonist, to prevent relapse in weaned alcoholics. Lancet. 1985;1:1014–1016. doi: 10.1016/s0140-6736(85)91615-0. [DOI] [PubMed] [Google Scholar]
- Lhuintre JP, Moore N, Tran G, Steru L, Langrenon S, Daoust M, Parot P, Ladure P, Libert C, Boismare F, Hillemand B. Acamprosate appears to decrease alcohol intake in weaned alcoholics. Alcohol Alcohol. 1990;25:613–622. doi: 10.1093/oxfordjournals.alcalc.a045057. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. John A. Wiley & Sons, Inc; New York: 1987. [Google Scholar]
- Littleton JM. Acamprosate in alcohol dependence: implications of a unique mechanism of action. J Addict Med. 2007;1:115–125. doi: 10.1097/ADM.0b013e318156c26f. [DOI] [PubMed] [Google Scholar]
- Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28:51–63. doi: 10.1097/01.ALC.0000108656.81563.05. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Goodman AM, Chabac S, Lehert P. Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double-blind, placebo-controlled trial: the role of patient motivation. J Psychiatr Res. 2006;40:383–393. doi: 10.1016/j.jpsychires.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Morley KC, Teesson M, Reid SC, Sannibale C, Thomson C, Phung N, Weltman M, Bell JR, Richardson K, Haber PS. Naltrexone versus acamprosate in the treatment of alcohol dependence: a multi-centre, randomized, double-blind, placebo-controlled trial. Addiction. 2006;101:1451–1462. doi: 10.1111/j.1360-0443.2006.01555.x. [DOI] [PubMed] [Google Scholar]
- Namkoong K, Lee BO, Lee PG, Choi MJ, Lee E. Acamprosate in Korean alcohol-dependent patients: a multi-centre, randomized, double-blind, placebo-controlled study. Alcohol Alcohol. 2003;38:135–141. doi: 10.1093/alcalc/agg038. [DOI] [PubMed] [Google Scholar]
- Niederhofer H, Staffen W. Acamprosate and its efficacy in treating alcohol dependent adolescents. Eur Child Adolesc Psychiatry. 2003;12:144–148. doi: 10.1007/s00787-003-0327-1. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291:1887–1896. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Sinha R, Grilo CM, Capone C, Farren CK, McKee SA, Rounsaville BJ, Wu R. Naltrexone and cognitive behavioral coping skills therapy for the treatment of alcohol drinking and eating disorder features in alcohol-dependent women: a randomized controlled trial. Alcohol Clin Exp Res. 2007;31:625–34. doi: 10.1111/j.1530-0277.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Paille FM, Guelfi JD, Perkins AC, Royer RJ, Steru L, Parot P. Double-blind randomized multicentre trial of acamprosate in maintaining abstinence from alcohol. Alcohol Alcohol. 1995;30:239–247. [PubMed] [Google Scholar]
- Pelc I, Le Bon O, Verbanck P, Lehert PH, Opsomer L. Calcium-acetylhomotaurinate for maintaining abstinence in weaned alcoholic patients: a placebo-controlled double-blind multi-centre study. In: Naranjo CA, Sellers EM, editors. Novel Pharmacological Interventions for Alcoholism. Springer-Verlag; New York: 1992. pp. 348–352. [Google Scholar]
- Pelc I, Verbanck P, Le Bon O, Gavrilovic M, Lion K, Lehert P. Efficacy and safety of acamprosate in the treatment of detoxified alcohol-dependent patients. A 90-day placebo-controlled dose-finding study. Br J Psychiatry. 1997;171:73–77. doi: 10.1192/bjp.171.1.73. [DOI] [PubMed] [Google Scholar]
- Poldrugo F. Acamprosate treatment in a long-term community-based alcohol rehabilitation programme. Addiction. 1997;92:1537–1546. [PubMed] [Google Scholar]
- Rosenthal RN, Gage A, Perhach JL, Goodman AM. Acamprosate: safety and tolerability in the treatment of alcohol dependence. J Addict Med. 2008;2:40–50. doi: 10.1097/ADM.0b013e31816319fd. [DOI] [PubMed] [Google Scholar]
- Roussaux JP, Hers D, Ferauge M. Does acamprosate diminish the appetite for alcohol in weaned alcoholics? J Pharm Belg. 1996;51:65–68. [PubMed] [Google Scholar]
- Sass H, Soyka M, Mann K, Zieglgansberger W. Relapse prevention by acamprosate. Results from a placebo-controlled study on alcohol dependence. Arch Gen Psychiatry. 1996;53:673–680. doi: 10.1001/archpsyc.1996.01830080023006. [DOI] [PubMed] [Google Scholar]
- Simmons M, Higgins J, Stewart L, Tierney J, Clark M, Thomson S. Meta-analysis of IDP data from randomized trials: a review of methods used in practice. Clin Trials. 2005;2:209–207. doi: 10.1191/1740774505cn087oa. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Alcohol Timeline Followback (TFLB) Handbook of Psychiatric Measures. 2000:477–479. [Google Scholar]
- Staner L, Boeijinga P, Danel T, Gendre I, Muzet M, Landron F. Effects of acamprosate on sleep during alcohol withdrawal: a double-blind placebo-controlled polysomnographic study in alcohol-dependent subjects. Alcohol Clin Exp Res. 2006;30:1492–99. doi: 10.1111/j.1530-0277.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- Tempesta E, Janiri L, Bignamini A, Chabac S, Potgieter A. Acamprosate and relapse prevention in the treatment of alcohol dependence: a placebo-controlled study. Alcohol Alcohol. 2000;35:202–209. doi: 10.1093/alcalc/35.2.202. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Dowdall GW, Davenport A, Rimm EB. A gender-specific measure of binge drinking among college students. Am J Public Health. 1995;85:982–985. doi: 10.2105/ajph.85.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth AB, Fischer F, Lesch OM, Nimmerrichter A, Oberbauer H, Platz T, Potgieter A, Walter H, Fleischhacker WW. Comparison of acamprosate and placebo in long-term treatment of alcohol dependence. Lancet. 1996;347:1438–1442. doi: 10.1016/s0140-6736(96)91682-7. [DOI] [PubMed] [Google Scholar]