Abstract
Melatonin has both neuritogenic and neuroprotective effects in mammalian cell lines such as neuroblastoma cells. The mechanisms of action include receptor-coupled processes, direct binding and modulation of calmodulin and protein kinase C, and direct scavenging of free radicals. While melatonin is produced in invertebrates and has influences on their physiology and behavior, little is known about its mechanisms of action. We studied the influence of melatonin on neuritogenesis in well-differentiated, extensively-arborized crustacean x-organ neurosecretory neurons. Melatonin significantly increased neurite area in the first 24 h of culture. The more physiological concentrations, 1 nM and 1 pM, increased area at 48 h also, whereas the pharmacological 1μM concentration appeared to have desensitizing effects by this time. Luzindole, a vertebrate melatonin receptor antagonist, had surprising and significant agonist-like effects in these invertebrate cells. Melatonin receptors have not yet been studied in invertebrates. However, the presence of membrane-bound receptors in this population of crustacean neurons is indicated by this study. Melatonin also has significant neuroprotective effects, reversing the inhibition of neuritogenesis by 200 and 500 μM hydrogen peroxide. Because this is at least in part a direct action not requiring a receptor, melatonin’s protection from oxidative stress is not surprisingly phylogenetically-conserved.
Keywords: Uca pugilator, crustacean, fiddler crab, melatonin, neuritogenesis, neuroprotection, oxidative stress, x-organ
1. Introduction
The vertebrate pineal hormone melatonin is involved in circadian and seasonal rhythmicity. It is produced during darkness and affects a variety of behavioral and physiological functions such as locomotion, thermoregulation, sleep-wake patterns, and reproduction (Cassone et al., 1993; Golombek et al., 1996; Hyde and Underwood, 1995; Reiter, 1991; Rowe and Kennaway, 1996; Stankov et al., 1991). Many of melatonin’s influences occur through modulation of the nervous system. Some of these effects include the induction of morphological changes and growth in neurons and glial cells (Benítez-King et al., 1990; Bordt et al., 2001; Paulose et al., 2009). Other effects occur through modulation of the neurosecretory activity of the hypothalamic-pituitary axis (Falcón et al., 2007).
Melatonin has neuritogenic effect both in vitro (Benítez-King et al., 1990) and in vivo (Ramirez-Rodriguez et al., 2011) in mouse cell lines. In mouse MDCK and N1E-115 cells, microfilaments and microtubules are involved in development of dome formation and neurite outgrowth. Melatonin increases both processes through activation of cytoskeletal synthesis (Benítez-King et al., 1990; Benítez-King, 2000). This cytoskeletal rearrangement appears to be activated by multiple melatonin-interactive processes: 1) direct inhibition of calmodulin by melatonin (Antón-Tay et al., 1998a; Benítez-King and Antón-Tay, 1993, Benítez-King et al., 1993, 1996; Huerto-Delgadillo et al., 1994); 2) direct stimulation of protein kinase C by melatonin (Antón-Tay et al., 1998b; Bellon et al., 2007; Benítez-King et al., 2001); and 3) the activation of the MT1 melatonin receptor, which signals through two inhibitory G proteins that decrease adenylyl cyclase activity and a G protein that activates phospholipase C (Bordt et al., 2001; Brydon et al., 1999; Witt-Enderby et al., 2000). Melatonin has general and widespread antioxidant actions including the direct scavenging of free radicals and the activation of enzymes in antioxidant pathways (Reiter et al., 2007, 2008). Melatonin directly scavenges hydroxyl radicals, peroxyl radicals, peroxynitrite anions, and singlet oxygen (Reiter et al., 1999, 2007). Melatonin also regulates the activation and expression of antioxidant enzymes such as glutathione peroxidase, superoxide dismutases, and catalase, via the membrane and nuclear receptors previously described (García-Macia et al., 2011; Kotler et al., 1998; Limón-Pacheco and Gonsebatt, 2010; Rodriguez et al., 2004; Tomás-Zapico and Coto-Montes, 2005). Together, these neuroregenerative and neuroprotective effects suggest that melatonin may have therapeutic value in treating neurodegenerative diseases such as Parkinson’s and Alzheimer’s disease (Benítez-King et al., 1990, 2003, 2005, 2010; Benítez-King, 2006; Dabbeni-Sala et al., 2001; González-Burgos et al., 2007; He et al., 2010; Matsubara et al., 2003; Sharma et al., 2006; Singhal et al., 2011; Weishaupt et al., 2006).
While melatonin has not been studied extensively in invertebrates, it has been detected in nearly every organism tested, including crustaceans (Balzer et al., 1997; Maciel et al., 2008; Markowska et al., 2009; Tilden et al., 1997, 2001b; Withyachumnarnkul et al., 1992, 1999). The eyestalks of crustaceans contain the optic lobes and the x-organ/sinus gland, a neuropeptide-secreting neurohemal structure that is analogous to the vertebrate hypothalamus-pituitary system in that its many hormone products regulate a variety of functions: molt-inhibiting hormone (MIH), the broadly-acting crustacean hyperglycemic hormone (CHH), red pigment concentrating hormone (RPCH), retinal light-adapting hormone (LAH) and dark-adapting hormone (DAH), crustacean cardioactive peptide (CCAP), methylfarnesoate-inhibiting peptide, and neurodepressing hormone (NDH) (for review: Christie et al., 2010). A variety of neurotransmitters have been shown to affect x-organ/sinus gland activity: The system has inhibitory responses to glutamate and GABA (Duan and Cooke, 2000), and excitatory responses to serotonin (Escamilla-Chimal et al., 2002; Lee et al., 2001), dopamine (Zou et al., 2003), and norepinephrine (Hsieh et al., 2006). Melatonin may interact with this system as well, since melatonin influences x-organ effector responses such as locomotion (Tilden et al., 2003b, a), glucose metabolism (Sainath and Reddy, 2010; Tilden et al., 2001a, 2003a), and limb regeneration (Tilden et al., 1997). Melatonin also influences crustacean ERG rhythms (Balzer et al., 1997; Solís-Chagoyán et al., 2008), potentially via MT2 receptor-type interaction (Mendoza-Vargas et al., 2009). Furthermore, exogenous melatonin has effects on the antioxidant system in crustacean locomotor muscles (Geihs et al., 2010) and gills (Maciel et al., 2010). However, the presence of melatonin receptors in invertebrates has not yet been determined. The x-organ/sinus gland system is a neurohemal organ consisting of approximately 200 somata (the x-organ) clustered on the periphery of the medulla terminalis whose axons terminate in enlarged secretory endings (the sinus gland) on the distal side of the eyestalk. Two size categories of x-organ somata exist, small 15–25 μm and large 30–70 μm cells, which show immunohistological differences in neuropeptide content (Chang et al., 1987; Keller et al., 1985; Mangerich and Keller, 1988): small cells appear to produce RPCH, and large cells appear to produce CHH, MIH, and other peptides. When isolated and cultured, these somata show rapid outgrowth of neurites even under the most basic conditions, which may be due in part to their neurosecretory propensity for trafficking membrane and architectural elements (Cooke et al., 1989).
Crustacean x-organ cells are a potential model system for comparative studies of the role of melatonin in invertebrate neurophysiology. These cells may also serve as an excellent model system for the study of melatonin neuromodulation across phyla: they are functionally analogous to vertebrate neurosecretory hypothalamic cells and are similar in neuromodulatory responsiveness and neuritogenesis to vertebrate hippocampal cells. X-organ cells are functionally and morphologically distinct from the well-studied mouse neuroblastoma cells: neuroblastoma cells are relatively undifferentiated cells that produce approximately 2 to 4 primary neurites with little arborization. X-organ cells, on the other hand, are well-differentiated neurosecretory cells that maintain their neurosecretory function in culture; they are also multipolar with extensive arborization.
In the current study, we explored the influence of both physiological and pharmacological levels of melatonin and a vertebrate melatonin receptor antagonist on crustacean neurite area as a measure of neurite growth. We also studied the neuroprotective effects of melatonin against oxidative stress.
2. Materials and methods
2.1. Crab housing and care
Fiddler crabs (Uca pugilator) were purchased from Gulf Specimen Marine Laboratory (Panacea, FL) and were housed in clear plastic tanks with Coralife® artificial seawater such that both terrestrial and aquatic terrains were available. Crabs were acclimated to a 12L:12D photoperiod and 22 ± 1 °C for at least 2 weeks prior to experimentation; they were fed crushed dry Purina Cat Chow® every other day, and water was changed after every feeding.
2.2. Tissue dissection
Dissection procedures were adapted from Cooke et al. (Cooke et al., 1989). All supplies were purchased from Sigma-Aldrich except where otherwise noted. Eyestalks were removed, rinsed in 70% ethanol, and immediately placed in sterile crab saline (490 mM NaCl, 11 mM KCl, 13 mM CaCl2, 26 mM MgCl2, and 10 mM HEPES, adjusted to pH 7.4) with antibiotics (100 units/mL penicillin, 0.1 mg/mL streptomycin, and 0.2 mg/mL neomycin). Incisions were made through the outer carapace on the lateral sides of each eyestalk up to the cornea, and the top portion of the carapace was lifted to reveal the underlying eyestalk muscular and neural tissue. The neural tissue was dissected away from the eyestalk and examined to locate the light blue, opalescent sinus gland on the medulla terminalis. X-organ-containing tissue on the opposite side of the medulla terminalis was removed and placed in Ca2+- and Mg2+-free saline (529 mM NaCl, 11 mM KCl, and 10 mM HEPES) with 0.1 % trypsin. X-organ tissue was gently stirred in the trypsin solution for 1 h and then rinsed 3 times in normal sterile crab saline.
2.3. Cell culture
Culture medium was adapted from Cooke et al (1989) and consisted of equal volumes of 1.16× concentrated crab saline and Liebovitz L-15 culture medium, with 20 mM HEPES, 0.1 mg/mL gentamicin, 120 mM glucose, and 2 mM L-glutamine. The final culture medium osmolality was 840 mM, equivalent to crab hemolymph osmolality. Each rinsed x-organ tissue was transferred to a 200 μL drop of culture medium in a glass-bottom culture dish, poly-L-lysine-coated, 35 mm, with 1.5 glass thickness (MatTek). The tissue was then triturated with a sterile glass pipette to dissociate cells. Cells were allowed to adhere to the glass-bottom portion of the culture dish for 45 min, and the volume was then brought to 3.6 mL with additional culture medium. The cultures were housed in darkness and high humidity at 22 ± °C. Culture medium was not replaced throughout the duration of an experiment. Cells usually survived for at least 7 days under these conditions.
2.4. Measurement of neurite area
Cells were viewed with a Zeiss Axiovert 200 microscope with phase contrast; images were collected and analyzed with Zeiss AxioVision software. In the neurite studies, images were collected from the first 10 cells observed in an ordered x-y scanning across each culture dish to provide the highest likelihood that we were measuring the same cells in successive 24-h measures. Cells that were within 200 μm of neighboring cells were excluded from analysis. With AxioVision software, we traced the perimeter of the neurite-encompassing area around each cell. To determine consistency of the neurite area measurement method, 4 people separately analyzed the same 8 unlabeled images. The standard deviation among the 4 observers was not greater than 3.84% of the mean area for any of the images, indicating acceptable precision of the measurement procedure.
2.5. Timecourse of neurite outgrowth
Cells were treated with 0 (control) or 1 μM melatonin (Sigma). Images were taken immediately after cells had adhered to the bottom of the culture dish (approximately 1 h after being placed in culture) and then every 24 h over 96 h. N = 10 cells per culture dish × 4 culture dishes per treatment.
2.6. Melatonin dose-response
Cells were treated with 0 (control), 1 pM, 1 nM, and 1 μM melatonin. Images were taken at 0, 24, and 48 h. N = 10 cells per culture dish × 4 culture dishes per treatment.
2.7. Influence of melatonin antagonist
Cells were treated with 1 μM melatonin, 1 μM melatonin plus 1 μM of the melatonin receptor antagonist luzindole, or no treatment. Images were taken at 0, 24, and 48 h. N = 10 cells per culture dish × 4 culture dishes per treatment.
2.8. Influence of hydrogen peroxide and melatonin
Cell cultures were treated at time zero with 100, 200, 500, and 1000 μM H2O2, with and without 1 μM melatonin (for 200 and 500 μM H2O2 only), versus untreated controls. Images were taken at 60 h. N = 10 cells per culture dish × 4 culture dishes per treatment.
2.9. Statistical analysis
We used a two-way ANOVA to determine differences among culture dishes and among treatments with the Holm-Sidak method for pairwise comparisons. Statistics were performed with SigmaStat software (SPSS, Inc.).
3. Results
3.1. Timecourse of neurite outgrowth
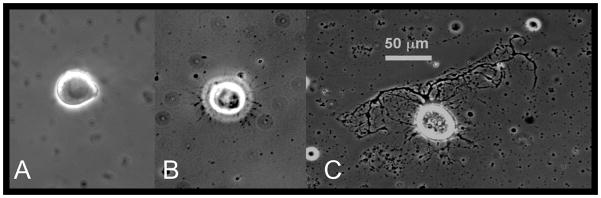
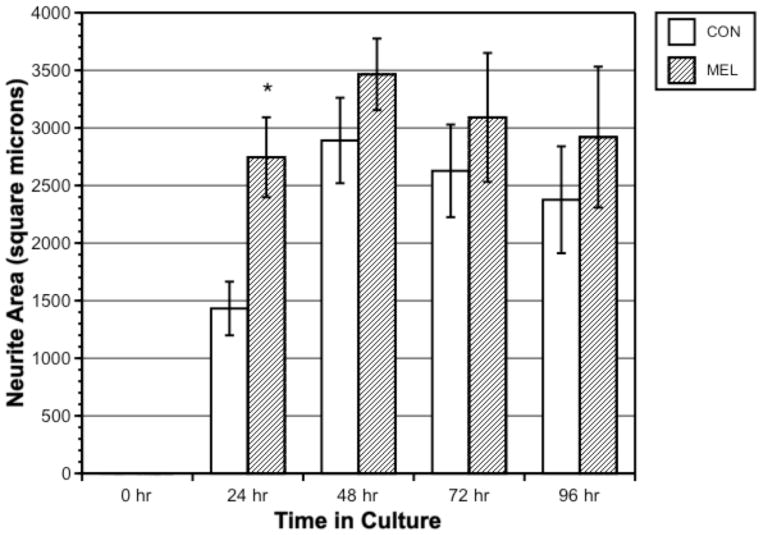
Figure 1 shows a typical x-organ cell with extensive 24-h arborization. Figure 2 shows neurite outgrowth area in microns2 in melatonin-treated (1 μM) vs. control cells, measured every 24 h over 96 h. No cells had neurites at time = 0 h. Melatonin-treated neurite area was significantly greater than controls during the first 24 h of culture (p = 0.007) but was not significantly different from controls thereafter. Overall neurite area was greatest within the first 48 h, with no significant growth or retraction beyond this time over 96 h.
Figure 1.
Cultured crustacean (Uca pugilator) x-organ cell at 400× oil immersion with phase contrast (micron bar applies to all 3 images). Images taken A) within 1 h of culture, B) within 3 h of culture, and C) at 24 h in culture.
Figure 2.
Neurite area (microns2) in control versus 1 μM melatonin-treated crustacean x-organ cells measured every 24 h over 96 h. Bars represent means ± SEM of 40 cells.
3.2. Melatonin dose-response
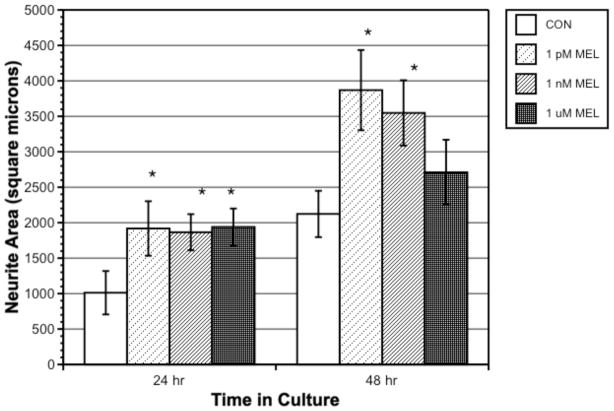
Figure 3 shows neurite growth with 0, 1 pM, 1 nM, and 1 μM melatonin, at 24 and 48 h (area was zero at time = 0). At 24 h, all melatonin concentrations demonstrated significantly greater neurite area than controls. At 48 h, cells treated with 1 nM and 1 pM melatonin showed significantly greater neurite area than controls (p = 0.001 and 0.008, respectively).
Figure 3.
Dose-response influence of melatonin on neurite area in crustacean x-organ cells measured at 24 and 48 h. Bars represent means ± SEM of 40 cells.
3.3 Influence of melatonin antagonist
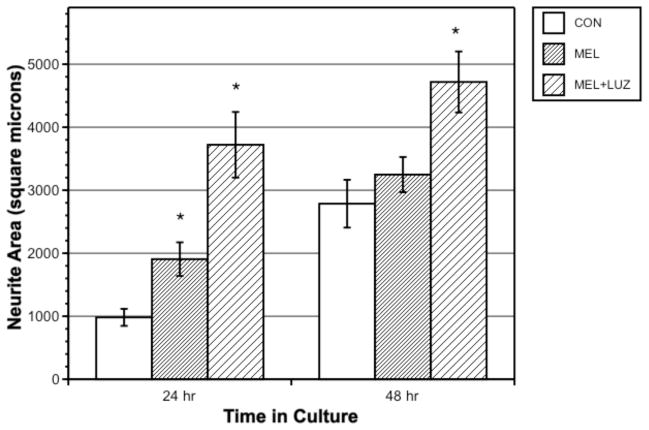
Luzindole + melatonin-treated cells showed significantly greater neurite area than both controls and melatonin-treated cells, at both 24 and 48 h (Fig 4; p < 0.001 for 24 h, p = 0.006 for 48 h for luzindole vs. melatonin).
Figure 4.
Influence of melatonin or melatonin + luzindole on neurite area in crustacean x-organ cells measured at 24 and 48 h. Bars represent means ± SEM of 40 cells.
3.4. Influence of hydrogen peroxide and melatonin
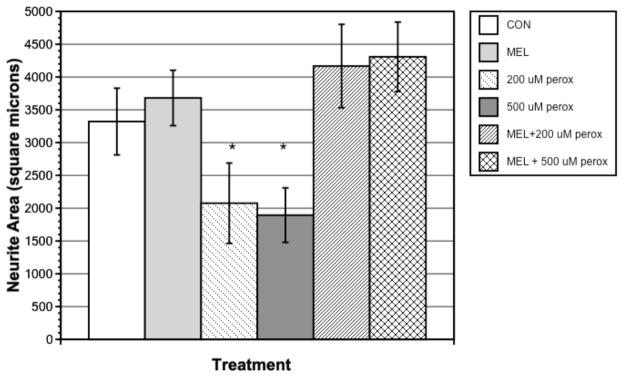
Cells were incubated with 100, 200, 500, and 1000 μM H2O2; no effect on neurite area was seen at 100 μM H2O2, and cells did not survive at 1000 μM H2O2. Since 200 and 500 μM H2O2 had significant neurite-inhibiting effects (p < 0.05 for both) without causing cell death, these two concentrations were used for 1 μM melatonin treatment. H2O2 at 200 and 500 μM significantly reduced neurite outgrowth compared with control and melatonin-treated cells at 60 h (Fig. 5). Melatonin with H2O2 treatment significantly reversed the effects of H2O2 alone for both 200 and 500 μM H2O2 (p < 0.01 for both).
Figure 5.
Influence of peroxide or peroxide and 1 μM melatonin on neurite area in crustacean x-organ cells measured at 60 h. Bars represent means ± SEM of 40 cells.
4. Discussion
Melatonin at 1 μM, a pharmacological concentration, caused a significant increase in neurite area within the first 24 h of culture; neurite area reached a maximum at 48 h in both controls and melatonin-treated cells and was sustained for 96 h in static cell cultures. Further growth may have occurred if cultures had been regularly perfused with fresh medium; furthermore, melatonin receptors – if present - were likely desensitized by pharmacological levels of melatonin beyond 24 h.
A dose-response study of melatonin showed that all tested concentrations of melatonin, from physiological (1 pM and 1 nM) to pharmacological (1 μM), increased neurite area in the first 24 h to an equal extent, nearly doubling area in comparison to controls. At 48 h, physiological concentrations had a greater effect on area than the higher pharmacological concentration, suggesting a receptor-mediated response of these cells to melatonin and further suggesting receptor desensitization by 48 h with higher concentrations of melatonin (Figs. 1 and 2).
The MT1 receptor was previously implicated in the neuritogenic effects of melatonin in mammalian cells (Bordt et al., 2001; Witt-Enderby et al., 2000). Luzindole is an MT1 and MT2 receptor antagonist but is more highly selective for the MT2 receptor. Treatment of crustacean x-organ cells with luzindole actually enhanced the effects of melatonin at both 24 and 48 h. Three hypotheses regarding this effect are: A) Crustaceans may have an as-yet unidentified subtype of membrane-bound melatonin receptor for which luzindole acts as an agonist instead of an antagonist. B) Luzindole may prevent the desensitization of MT1 receptors by melatonin or may enhance MT1 expression. C) Luzindole may have a direct, non-receptor-mediated effect similar to melatonin’s direct inhibition of calmodulin. Luzindole is structurally similar to melatonin (Dubocovich, 1988) and therefore may also be a ligand to calmodulin. A similar MT1-activating effect of luzindole was seen in cell lines expressing human MT1 receptors but with eventual receptor desensitization by melatonin (Kokkola et al., 2007). This agonist-type effect of luzindole was also seen in a mouse model of pain response (Ray et al., 2004). The results of the current study indicate that at least one type of membrane-bound melatonin receptor exists in crustacean x-organ cells. However, melatonin’s actions are likely more complex, involving the previously-described direct calmodulin and protein kinase C interactions. A dose-response study of luzindole should be undertaken. Melatonin prevented the H2O2-induced inhibition of neuritogenesis, as seen in mouse N1E-115 cells (Benítez-King et al., 2005). We examined cells at 60 h, after the initial 24-h neuritogenesis-enhancing effects of melatonin had ceased, since those effects are separate from the receptor-independent neuroprotective effects of melatonin (Reiter et al., 1999). Thus some of the growth-enhancing effect of melatonin in this portion of the study was likely due to its direct and indirect protective effects against oxidative stress.
In conclusion, melatonin had neuritogenic and neuroprotective effects in well-differentiated, highly arborized crustacean neurosecretory cells that were similar to its effects in less differentiated mammalian neuroblastoma cells. We observed effects that may be due to both receptor interactions and direct intracellular regulation of enzymes and free radicals. Unusual receptor interactions were seen with the agonistic effects of luzindole, suggesting some receptor-level differences between mammalian and crustacean cells. The phylogenetically-conserved roles of melatonin have not been studied extensively; cellular-level studies of melatonin in invertebrates are very few, particularly of receptor-mediated activities. The ease of culture, prolific neurite outgrowth, and functional integrity of these cells make them a good comparative model system for the study of cellular-level growth in well-differentiated neuronal systems.
Acknowledgments
The project described was supported by: a) Award Number P20RR016463 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health from the INBRE Program of the National Center for Research Resources; b) the Clare Boothe Luce Program of the Henry Luce Foundation; c) a Mount Desert Island New Investigator Award. We thank Dr. David Barnes and Dr. David Towle (in memoriam), Mount Desert Island Biological Laboratory, and Dr. Ian Cooke, University of Hawaii, for guidance and technical assistance with this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antón-Tay F, Martínez I, Tovar R, Benítez-King G. Modulation of the subcellular distribution of calmodulin by melatonin in MDCK cells. J Pineal Res. 1998a;24:35–42. doi: 10.1111/j.1600-079x.1998.tb00363.x. [DOI] [PubMed] [Google Scholar]
- Antón-Tay F, Ramírez G, Martínez I, Benítez-King G. In vitro stimulation of protein kinase C by melatonin. Neurochem Res. 1998b;23:601–606. doi: 10.1023/a:1022474402458. [DOI] [PubMed] [Google Scholar]
- Balzer I, Espinola IR, Fuentes-Pardo B. Daily variations of immunoreactive melatonin in the visual system of crayfish. Biol Cell. 1997;89:539–543. [Google Scholar]
- Bellon A, Ortíz-López L, Ramírez-Rodríguez G, Antón-Tay F, Benítez-King G. Melatonin induces neuritogenesis at early stages in N1E-115 cells through actin rearrangements via activation of protein kinase C and Rho-associated kinase. J Pineal Res. 2007;42:214–221. doi: 10.1111/j.1600-079X.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- Benítez-King G. PKC activation by melatonin modulates vimentin intermediate filament organization in N1E-115 cells. J Pineal Res. 2000;29:8–14. doi: 10.1034/j.1600-079x.2000.290102.x. [DOI] [PubMed] [Google Scholar]
- Benítez-King G. Melatonin as a cytoskeletal modulator: implications for cell physiology and disease. J Pineal Res. 2006;40:1–9. doi: 10.1111/j.1600-079X.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- Benítez-King G, Antón-Tay F. Calmodulin mediates melatonin cytoskeletal effects. Experientia. 1993a;49:635–641. doi: 10.1007/BF01923944. [DOI] [PubMed] [Google Scholar]
- Benítez-King G, Hernández ME, Tovar R, Ramírez G. Melatonin activates PKC-alpha but not PKC-epsilon in N1E-115 cells. Neurochem Int. 2001;39:95–102. doi: 10.1016/s0197-0186(01)00021-3. [DOI] [PubMed] [Google Scholar]
- Benítez-King G, Huerto-Delgadillo L, Antón-Tay F. Melatonin effects on the cytoskeletal organization of MDCK and neuroblastoma N1E-115 cells. J Pineal Res. 1990;9:209–220. doi: 10.1111/j.1600-079x.1990.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Benítez-King G, Huerto-Delgadillo L, Antón-Tay F. Binding of 3H-melatonin to calmodulin. Life Sci. 1993b;53:201–207. doi: 10.1016/0024-3205(93)90670-x. [DOI] [PubMed] [Google Scholar]
- Benítez-King G, Ortíz-López L, Jiménez-Rubio G, Ramírez-Rodríguez G. Haloperidol causes cytoskeletal collapse in N1E-115 cells through tau hyperphosphorylation induced by oxidative stress: Implications for neurodevelopment. Eur J Pharmacol. 2010;644:24–31. doi: 10.1016/j.ejphar.2010.06.057. [DOI] [PubMed] [Google Scholar]
- Benítez-King G, Ortiz-López L, Jiménez G. Melatonin precludes cytoskeletal collapse caused by hydrogen peroxide: participation of protein kinase C. Therapy. 2005;2:767–778. [Google Scholar]
- Benítez-King G, Ríos A, Martínez A, Antón-Tay F. In vitro inhibition of Ca2+/calmodulin-dependent kinase II activity by melatonin. Biochim Biophys Acta. 1996;1290:191–196. doi: 10.1016/0304-4165(96)00025-6. [DOI] [PubMed] [Google Scholar]
- Benítez-King G, Túnez I, Bellon A, Ortíz GG, Antón-Tay F. Melatonin prevents cytoskeletal alterations and oxidative stress induced by okadaic acid in N1E-115 cells. Exp Neurol. 2003;182:151–159. doi: 10.1016/s0014-4886(03)00085-2. [DOI] [PubMed] [Google Scholar]
- Bordt SL, McKeon RM, Li PK, Witt-Enderby PA, Melan MA. N1E-115 mouse neuroblastoma cells express MT1 melatonin receptors and produce neurites in response to melatonin. Biochim Biophys Acta. 2001;1499:257–264. doi: 10.1016/s0167-4889(00)00127-0. [DOI] [PubMed] [Google Scholar]
- Brydon L, Roka F, Petit L, de Coppet P, Tissot M, Barrett P, Morgan PJ, Nanoff C, Strosberg AD, Jockers R. Dual signaling of human Mel1a melatonin receptors via G(i2), G(i3), and G(q/11) proteins. Mol Endocrinol. 1999;13:2025–2038. doi: 10.1210/mend.13.12.0390. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Warren WS, Brooks DS, Lu J. Melatonin, the pineal gland, and circadian rhythms. J Biol Rhythms. 1993;8:S73–S81. [PubMed] [Google Scholar]
- Chang ES, Bruce MJ, Newcomb RW. Purification and amino acid composition of a peptide with molt-inhibiting activity from the lobster, Homarus americanus. Gen Comp Endocrinol. 1987;65:56–64. doi: 10.1016/0016-6480(87)90222-x. [DOI] [PubMed] [Google Scholar]
- Christie AE, Stemmler EA, Dickinson PS. Crustacean neuropeptides. Cell Mol Life Sci. 2010;67:4135–4169. doi: 10.1007/s00018-010-0482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke I, Graf R, Grau S, Haylett B, Meyers D, Ruben P. Crustacean peptidergic neurons in culture show immediate outgrowth in simple medium. Proc Natl Acad Sci U S A. 1989;86:402–406. doi: 10.1073/pnas.86.1.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbeni-Sala F, Di Santo S, Franceschini D, Skaper SD, Giusti P. Melatonin protects against 6-OHDA-induced neurotoxicity in rats: a role for mitochondrial complex I activity. FASEB J. 2001:15. doi: 10.1096/fj.00-0129com. [DOI] [PubMed] [Google Scholar]
- Duan S, Cooke IM. Glutamate and GABA activate different receptors and Cl(-) conductances in crab peptide-secretory neurons. J Neurophysiol. 2000;83:31–37. doi: 10.1152/jn.2000.83.1.31. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML. Luzindole (N-0774): a novel melatonin receptor agonist. J Pharmacol. 1988;246:902–910. [PubMed] [Google Scholar]
- Escamilla-Chimal EG, Hiriart M, Sánchez-Soto MC, Fanjul-Moles ML. Serotonin modulation of CHH secretion by isolated cells of the crayfish retina and optic lobe. Gen Comp Endocrinol. 2002;125:283–290. doi: 10.1006/gcen.2001.7752. [DOI] [PubMed] [Google Scholar]
- Falcón J, Besseau L, Sauzet S, Boeuf G. Melatonin effects on the hypothalamo-pituitary axis in fish. Trends Endocrinol Metab. 2007;18:81–88. doi: 10.1016/j.tem.2007.01.002. [DOI] [PubMed] [Google Scholar]
- García-Macia M, Vega-Naredo I, De Gonzalo-Calvo D, Rodríguez-González SM, Camello PJ, Camello-Almaraz C, Martín-Cano FE, Rodríguez-Colunga MJ, Pozo MJ, Coto-Montes AM. Melatonin induces neural SOD2 expression independent of the NF-kappaB pathway and improves the mitochondrial population and function in old mice. J Pineal Res. 2011;50:54–63. doi: 10.1111/j.1600-079X.2010.00809.x. [DOI] [PubMed] [Google Scholar]
- Geihs MA, Vargas MA, Maciel FE, Caldas SS, Cruz BP, Primel EG, Monserrat JM, Nery LE. Effect of melatonin in the antioxidant defense system in the locomotor muscles of the estuarine crab Neohelice granulata (Decapoda, Brachyura) Gen Comp Endocrinol. 2010;166:72–82. doi: 10.1016/j.ygcen.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Pévet P, Cardinali DP. Melatonin effects on behavior: possible mediation by the central GABAergic system. Neurosci Biobehav Rev. 1996;20:403–412. doi: 10.1016/0149-7634(95)00052-6. [DOI] [PubMed] [Google Scholar]
- González-Burgos I, Letechipía-Vallejo G, López-Loeza E, Moralí G, Cervantes M. Long-term study of dendritic spines from hippocampal CA1 pyramidal cells, after neuroprotective melatonin treatment following global cerebral ischemia in rats. Neurosci Lett. 2007;423:162–166. doi: 10.1016/j.neulet.2007.06.050. [DOI] [PubMed] [Google Scholar]
- He H, Dong W, Huang F. Anti-amyloidogenic and anti-apoptotic role of melatonin in Alzheimer disease. Curr Neuropharmacol. 2010;8:211–217. doi: 10.2174/157015910792246137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh SL, Chen SM, Yang YH, Kuo CM. Involvement of norepinephrine in the hyperglycemic responses of the freshwater giant prawn, Macrobrachium rosenbergii, under cold shock. Comp Biochem Physiol A. 2006;143:254–263. doi: 10.1016/j.cbpa.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Huerto-Delgadillo L, Antón-Tay F, Benítez-King G. Effects of melatonin on microtubule assembly depend on hormone concentration: role of melatonin as a calmodulin antagonist. J Pineal Res. 1994;17:55–62. doi: 10.1111/j.1600-079x.1994.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Hyde LL, Underwood H. Daily melatonin infusions entrain the locomotor activity of pinealectomized lizards. Physiol Behav. 1995;58:943–951. doi: 10.1016/0031-9384(95)00157-e. [DOI] [PubMed] [Google Scholar]
- Keller R, Jaros PP, Kegel G. Crustacean hyperglycemic neuropeptides. Am Zool. 1985;25:207–221. [Google Scholar]
- Kokkola T, Vaittinen M, Laitinen JT. Inverse agonist exposure enhances ligand binding and G protein activation of the human MT1 melatonin receptor, but leads to receptor down-regulation. J Pineal Res. 2007;43:255–262. doi: 10.1111/j.1600-079X.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- Kotler M, Rodríguez C, Sáinz RM, Antolín I, Menéndez-Peláez A. Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J Pineal Res. 1998;24:83–89. doi: 10.1111/j.1600-079x.1998.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Lee CY, Yang PF, Zou HS. Serotonergic regulation of crustacean hyperglycemic hormone secretion in the crayfish, Procambarus clarkii. Physiol Biochem Zool. 2001;74:376–382. doi: 10.1086/320430. [DOI] [PubMed] [Google Scholar]
- Limón-Pacheco JH, Gonsebatt ME. The glutathione system and its regulation by neurohormone melatonin in the central nervous system. Cent Nerv Syst Agents Med Chem. 2010;10:287–297. doi: 10.2174/187152410793429683. [DOI] [PubMed] [Google Scholar]
- Maciel FE, Geihs MA, Vargas MA, Cruz BP, Ramos BP, Vakkuri O, Meyer-Rochow VB, Maia Nery LE, Allodi S. Daily variation of melatonin content in the optic lobes of the crab Neohelice granulata. Comp Biochem Physiol A. 2008;149:162–166. doi: 10.1016/j.cbpa.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Maciel FE, Ramos BP, Geihs MA, Vargas MA, Cruz BP, Meyer-Rochow VB, Vakkuri O, Allodi S, Monserrat JM, Nery LE. Effects of melatonin in connection with the antioxidant defense system in the gills of the estuarine crab Neohelice granulata. Gen Comp Endocrinol. 2010;165:229–236. doi: 10.1016/j.ygcen.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Mangerich S, Keller R. Localization of pigment-dispersing hormone (PDH) immunoreactivity in the central nervous system of Carcinus maenas and Orconectes limosus (Crustacea), with reference to FMRFamide immunoreactivity in O. limosus. Cell Tissue Res. 1988;253:199–208. doi: 10.1007/BF00221755. [DOI] [PubMed] [Google Scholar]
- Markowska M, Bentkowski P, Kloc M, Pijanowska J. Presence of melatonin in Daphnia magna. J Pineal Res. 2009;46:242–244. doi: 10.1111/j.1600-079X.2008.00642.x. [DOI] [PubMed] [Google Scholar]
- Matsubara E, Bryant-Thomas T, Pacheco Quinto J, Henry TL, Poeggeler B, Herbert D, Cruz-Sanchez F, Chyan YJ, Smith MA, Perry G, Shoji M, Abe K, Leone A, Grundke-Ikbal I, Wilson GL, Ghiso J, Williams C, Refolo LM, Pappolla MA, Chain DG, Neria E. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer's disease. J Neurochem. 2003;85:1101–1108. doi: 10.1046/j.1471-4159.2003.01654.x. [DOI] [PubMed] [Google Scholar]
- Mendoza-Vargas L, Solís-Chagoyán H, Benítez-King G, Fuentes-Pardo B. MT2-like melatonin receptor modulates amplitude receptor potential in visual cells of crayfish during a 24-hour cycle. Comp Biochem Physiol A. 2009;154:486–492. doi: 10.1016/j.cbpa.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Paulose JK, Peters JL, Karaganis SP, Cassone VM. Pineal melatonin acts as a circadian zeitgeber and growth factor in chick astrocytes. J Pineal Res. 2009;46:286–294. doi: 10.1111/j.1600-079X.2008.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Rodriguez G, Ortíz-López L, Domínguez-Alonso A, Benítez-King GA, Kempermann G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J Pineal Res. 2011;50:29–37. doi: 10.1111/j.1600-079X.2010.00802.x. [DOI] [PubMed] [Google Scholar]
- Ray M, Mediratta PK, Mahajan P, Sharma KK. Evaluation of the role of melatonin in formalin-induced pain response in mice. Indian J Med Sci. 2004;58:122–130. [PubMed] [Google Scholar]
- Reiter RJ. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol. 1991;79:C153–C158. doi: 10.1016/0303-7207(91)90087-9. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Paredes SD, Korkmaz A, Jou MJ, Tan DX. Melatonin combats molecular terrorism at the mitochondrial level. Interdiscip Toxicol. 2008;1:137–149. doi: 10.2478/v10102-010-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Cabrera J, D'Arpa D. Melatonin and tryptophan derivatives as free radical scavengers and antioxidants. Adv Exp Med Biol. 1999;467:379–387. doi: 10.1007/978-1-4615-4709-9_48. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Terron MP, Flores LJ, Czarnocki Z. Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim Pol. 2007;54:1–9. [PubMed] [Google Scholar]
- Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- Rowe SA, Kennaway DJ. Effect of NMDA receptor blockade on melatonin and activity rhythm responses to a light pulse in rats. Brain Res Bull. 1996;41:351–358. doi: 10.1016/s0361-9230(96)00189-x. [DOI] [PubMed] [Google Scholar]
- Sainath SB, Reddy PS. Melatonergic regulation of hemolymph sugar levels in the freshwater edible crab, Oziotelphusa senex senex. J Exp Zool A. 2010;313:201–208. doi: 10.1002/jez.594. [DOI] [PubMed] [Google Scholar]
- Sharma R, McMillan CR, Tenn CC, Niles LP. Physiological neuroprotection by melatonin in a 6-hydroxydopamine model of Parkinson's disease. Brain Res. 2006;1068:230–236. doi: 10.1016/j.brainres.2005.10.084. [DOI] [PubMed] [Google Scholar]
- Singhal NK, Srivastava G, Patel DK, Jain SK, Singh MP. Melatonin or silymarin reduces maneb- and paraquat-induced Parkinson's disease phenotype in the mouse. J Pineal Res. 2011;50:97–109. doi: 10.1111/j.1600-079X.2010.00819.x. [DOI] [PubMed] [Google Scholar]
- Solís-Chagoyán H, Mendoza-Vargas L, Fuentes-Pardo B. Melatonin modulates the ERG circadian rhythm in crayfish. Comp Biochem Physiol A. 2008;149:373–379. doi: 10.1016/j.cbpa.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Stankov B, Fraschini F, Reiter RJ. Melatonin binding sites in the central nervous system. Brain Res Rev. 1991;16:245–256. doi: 10.1016/0165-0173(91)90008-v. [DOI] [PubMed] [Google Scholar]
- Tilden AR, McGann L, Schwartz J, Bowe A, Salazar C. Effect of melatonin on hemolymph glucose and lactate levels in the fiddler crab Uca pugilator. J Exp Zool. 2001a;290:379–383. doi: 10.1002/jez.1078. [DOI] [PubMed] [Google Scholar]
- Tilden AR, Alt J, Brummer K, Groth R, Herwig K, Wilson A, Wilson S. Influence of photoperiod on N-acetyltransferase activity and melatonin in the fiddler crab Uca pugilator. Gen Comp Endocrinol. 2001b;122:233–237. doi: 10.1006/gcen.2001.7641. [DOI] [PubMed] [Google Scholar]
- Tilden AR, Brauch R, Ball R, Janze AM, Ghaffari AH, Sweeney CT, Yurek JC, Cooper RL. Modulatory effects of melatonin on behavior, hemolymph metabolites, and neurotransmitter release in crayfish. Brain Res. 2003a;992:252–262. doi: 10.1016/j.brainres.2003.08.053. [DOI] [PubMed] [Google Scholar]
- Tilden AR, Rasmussen P, Awantang RM, Furlan S, Goldstein J, Palsgrove M, Sauer A. Melatonin cycle in the fiddler crab Uca pugilator and influence of melatonin on limb regeneration. J Pineal Res. 1997;23:142–147. doi: 10.1111/j.1600-079x.1997.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Tilden AR, Shanahan JK, Khilji ZS, Owen JG, Sterio TW, Thurston KT. Melatonin and locomotor activity in the fiddler crab Uca pugilator. J Exp Zool. 2003b;297:80–87. doi: 10.1002/jez.a.10230. [DOI] [PubMed] [Google Scholar]
- Tomás-Zapico C, Coto-Montes A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J Pineal Res. 2005;39:99–104. doi: 10.1111/j.1600-079X.2005.00248.x. [DOI] [PubMed] [Google Scholar]
- Weishaupt JH, Bartels C, Pölking E, Dietrich J, Rohde G, Poeggeler B, Mertens N, Sperling S, Bohn M, Hüther G, Schneider A, Bach A, Sirén AL, Hardeland R, Bähr M, Nave KA, Ehrenreich H. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J Pineal Res. 2006;41:313–323. doi: 10.1111/j.1600-079X.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- Withyachumnarnkul B, Ajpru S, Rachawong S, Pongsa-Asawapaiboon A, Sumridthong A. Sexual dimorphism in N-acetyltransferase and melatonin levels in the giant freshwater prawn Macrobrachium rosenbergii de Man. J Pineal Res. 1999;26:174–177. doi: 10.1111/j.1600-079x.1999.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Withyachumnarnkul B, Buppaniroj K, Pongsa-Asawapaiboon A. N-acetyltransferase and melatonin levels in the optic lobe of giant freshwater prawns, Macrobrachium rosenbergii De Man. Comp Biochem Physiol A. 1992;102:703–707. [Google Scholar]
- Witt-Enderby PA, MacKenzie RS, McKeon RM, Carroll EA, Bordt SL, Melan MA. Melatonin induction of filamentous structures in non-neuronal cells that is dependent on expression of the human mt1 melatonin receptor. Cell Motil Cytoskeleton. 2000;46:28–42. doi: 10.1002/(SICI)1097-0169(200005)46:1<28::AID-CM4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Zou HS, Juan CC, Chen SC, Wang HY, Lee CY. Dopaminergic regulation of crustacean hyperglycemic hormone and glucose levels in the hemolymph of the crayfish Procambarus clarkii. J Exp Zool. 2003;298:44–52. doi: 10.1002/jez.a.10273. [DOI] [PubMed] [Google Scholar]