Abstract
As the voltage-dependent anion channel (VDAC) forms the interface between mitochondria and the cytosol, its importance in metabolism is well understood. However, research on VDAC’s role in cell death is a rapidly growing field, unfortunately with much confusing and contradictory results. The fact that VDAC plays a role in outer mitochondrial membrane permeabilization is undeniable, however, the mechanisms behind this remain very poorly understood. In this review, we will summarize the studies that show evidence of VDAC playing a role in cell death. To begin, we will discuss the evidence for and against VDAC’s involvement in mitochondrial permeability transition (MPT) and attempt to clarify that VDAC is not an essential component of the MPT pore (MPTP). Next, we will evaluate the remaining literature on VDAC in cell death which can be divided into three models: proapoptotic agents escaping through VDAC, VDAC homo- or hetero-oligomerization, or VDAC closure resulting in outer mitochondrial membrane permeabilization through an unknown pathway. We will then discuss the growing list of modulators of VDAC activity that have been associated with induction/protection against cell death.
Keywords: VDAC, mitochondria, permeability transition, apoptosis, necrosis
1. Introduction
The mitochondrial voltage-dependent anion channel (VDAC), also known as mitochondrial porin, is the most abundant protein in the outer mitochondrial membrane. The VDAC family of proteins consists of three isoforms from three separate genes (VDAC1, VDAC2, and VDAC3). As most studies on VDAC using defined isoforms have been performed with VDAC1, the main focus of this review will be on VDAC1. However, significant findings from the other two isoforms will be noted. For this review, ‘VDAC’ will be used to reference studies of undefined isoforms.
While it has been established that VDACs regulate the ion and metabolite flux between mitochondria and cytosol [1,2,3], most other properties and even the structure of the channels are still highly debated and have been the focus of several past reviews [4,5,6,7,8]. Additionally, up-to-date reviews on VDAC structure, function, and regulation of metabolism can be found within this special issue. The purpose of this review is to highlight the role of VDAC and its regulators in mitochondrial membrane permeabilization and cell death.
2. VDAC and the Mitochondrial Permeability Transition Pore (MPTP)
Mitochondrial permeability transition (MPT) is the sudden permeabilization of the inner mitochondrial membrane in response to a noxious stimulus such as oxidative stress, Ca2+ overload, hypoxia, and cytotoxic drugs [9,10,11,12,13]. The degree of permeabilization is fairly substantial; although not large enough to allow the passage of proteins, solutes and metabolites up to 1.5 kD in size can now freely pass across the normally impermeable inner membrane. This includes protons such that the ΔΨm is dissipated upon MPT, thereby inhibiting ATP synthesis. Moreover, water can now move into the mitochondrial matrix, down its osmotic gradient, causing the mitochondrion to swell and, if left unchecked, rupture completely. MPT appears to play a critical role in cell death, especially necrosis [14], and has been implicated in the development and progression of many diseases including ischemia/reperfusion injury, muscular dystrophy, Alzheimer’s disease, and cardiotoxicity. MPT is believed to be mediated by opening of the MPT pore (MPTP), a highly debated, and undefined protein complex thought to span both the inner and outer mitochondrial membranes at membrane contact sites. Needless to say, due to the important role of MPT in disease pathogenesis there has been a concerted effort to try and elucidate the molecular makeup for the MPTP.
As VDAC is the most abundant protein in the outer mitochondrial membrane, it has long been considered a candidate for the outer membrane component of the MPTP. It was first proposed as an MPTP constituent nearly 20 years ago by Mario Zoratti’s group who put forward that the electrical conductance properties of VDAC were similar to those described for the MPTP [15,16]. Moreover, it was well established that the MPTP was redox, Ca2+, voltage, adenine nucleotide, and pH sensitive [10,11,12,13] – all attributes that applied to VDAC as well. However, what was strange was how this concept of VDAC as the outer membrane component of the MPTP slowly morphed from hypothesis to dogma without much in the way of substantiation in between.
As far as experimental data, initial support for a role for VDAC in MPT came from Crompton’s laboratory, which demonstrated that a GST-CypD fusion protein was able to pull down VDAC along with the adenine nucleotide translocase (ANT), another putative MPTP component from mitochondrial lysates [17]. Reconstitution of this VDAC-ANT-CypD complex then resulted in a Ca2+-dependent, cyclosporine-sensitive channel that was reminiscent of the MPTP [17]. Additional supporting evidence for VDAC’s involvement in MPT came from experiments using putative VDAC inhibitors and anti-VDAC antibodies. Monoclonal antibodies purported to block VDAC’s channel activity were reported to prevent MPT in isolated mitochondria. [18,19]. Yet the specificity of these antibodies for VDAC has been cast into doubt [6]. A study by Cesura et al., also suggested that VDAC1 is the outer membrane component of the MPTP as radioactively labeled MPT inhibitor Ro 68-3400 was shown to bind a protein of ~32 kDa, which was identified as VDAC1 by mass spectrometry [20]. However, the same group has since demonstrated that Ro 68-3400 does not in fact bind to any VDAC isoform [21].
Certainly from a biochemical perspective VDAC would not fit very well into the MPTP paradigm. The “opened” configuration of VDAC is the most conductive, and shows a significant preference for anions, particularly metabolic anions (ATP/ADP) [6]. The “closed” state greatly diminishes, but does not abolish flux of metabolic anions, and now favors conductance of cations. If VDAC were part of the MPTP, it would make sense for VDAC closure to equal MPTP closure. However, Marco Colombini has demonstrated that closure of VDAC actually increases Ca2+ flux, which if anything should actually promote opening of the MPTP [80]. Indeed, Tikunov et al [23] utilized G3139, an 18-mer phosphorothioate blocker of VDAC to cause VDAC closure, and MPT was accelerated. Moreover, even its closed state, the VDAC channel is still large enough to pass solutes up to 1.5 kD in size, i.e., the closed state of VDAC is the same as the open state of the MPTP [6]. Using a similar approach to Crompton’s initial reconstitution of a VDAC-containing MPTP, Halestrap’s group was only able to pulldown ANT (and the mitochondrial phosphate carrier as it later turned out), but not VDAC with GST-CypD [24]. Yet this VDAC-less precipitate was still capable of generating an MPTP-like channel in liposomes [24].
Genetic studies have most recently been employed to decipher the role, if any, of VDAC in MPT. Kroemer’s group reported that VDAC-deficient yeast were more resistant to HIV Vpr-induced MPT [25]. A similar loss of MPT response was observed in response to ethanol in ΔVDAC yeast mitochondria [26]. However, the nature of MPT, both in terms of properties and regulation, appears to be inherently different in yeast [27], thus making extrapolations to the mammalian system difficult. In this regard, we have observed an intact MPT response in isolated mouse mitochondria and cells essentially deficient for all three VDAC isoforms, suggesting that VDACs are dispensable for MPT and are not an essential component of the MPTP [28]. Paolo Bernardi’s group has also reported a maintained MPT response in VDAC1−/− [21] and VDAC1/3−/− mitochondria [29]. Thus, in light of both the nonspecific VDAC “blocking” agents used in previous studies and the more recent findings of maintained MPT responses in VDAC-deficient null mitochondria, we must conclude that VDAC is not an essential component of the MPTP. We found that mitochondria and cells lacking VDAC have an exacerbated MPT and death response [28], suggesting that if anything VDAC appears to protect against, rather than contribute to, MPTP. Consistent with this concept, closure of VDAC using Koenig’s polyanion, phosphorothioates, or the anion channel blocker DIDS, actually enhances the MPT response in isolated mitochondria [23,30,31].
3. VDAC and apoptosis
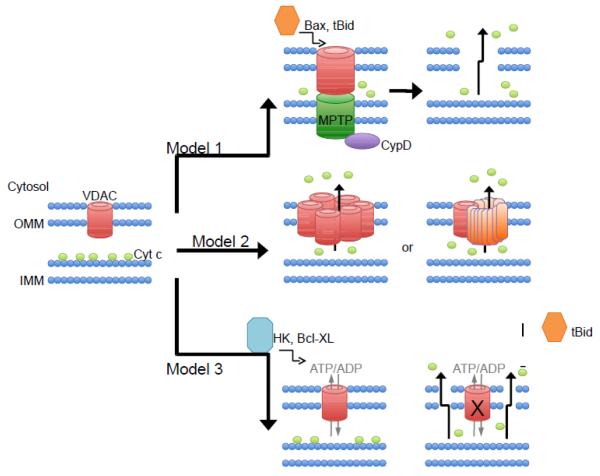
While evidence for a role of VDAC in outer mitochondrial membrane permeabilization and apoptosis persistently accumulates, the mechanisms behind VDAC’s role remain very poorly understood. In fact, it is still debated whether VDAC opening, or closure results in apoptosis [6]. The existing evidence of how VDAC is involved in outer membrane permeability and release of cytochrome c can be grouped into roughly three models: model #1) VDAC is part of the MPTP complex and pro-apoptotic agents therefore induce cytochrome c release indirectly through activation of the MPTP, model #2) VDAC homo- or hetero-oligomerization creating a larger pore capable of releasing cytochrome c, and model #3) VDAC closure resulting in a build up of mitochondrial metabolites, mitochondrial swelling, and either outer membrane permeabilization through an specific undefined mechanism or generalized rupture (Figure 1). In the “VDAC and MPTP” section above, we have already discussed the evidence that largely discredits model #1. We will next discuss the evidence both for and against models #2 and #3 of VDAC-mediated outer membrane permeabilization.
Fig. 1. Proposed models for VDAC’s role in outer mitochondrial membrane permeabilization and induction of apoptosis.
In model #1, the VDAC is a component of the MPTP and therefore causes cytochrome c release indirectly through the swelling and rupture of mitochondria. This model has largely been discredited, as mitochondria lacking VDACs still possess a normal MPT response. Model #2 involves either homo-oligomerization of VDAC channels or hetero-oligomerization with VDAC and Bax/Bak to produce a large channel capable of releasing cytochrome c. However, this model is also highly questionable as cells devoid of all VDACs actually exhibit enhanced cell death in response to apoptotic stimuli, thus suggesting that VDAC plays a protective role against cell death. Evidence of VDAC’s interaction with Bax or Bak is limited and has also been questioned. Model #3 details how anti-apoptotic agents modulate VDAC to keep it in the open state, maintaining adenine nucleotide flux, thus maintaining outer membrane permeability. On the other hand, a growing list of pro-apoptotic modulators of VDAC have been shown to close VDAC, inhibiting adenine nucleotide flux, causing mitochondrial permeability and cell death. This model #3 thus fits with concept of a functional VDAC channel being cytoprotective.
3.1 Model #2 – VDAC homo- or hetero-oligomerization leading to outer mitochondrial membrane permeabilization
Studies in several cell types overexpressing human, murine, yeast, Paralichthys olivaceus, or rice VDAC have observed increased apoptosis [32,33,34,35,36]. Two reports suggest elevated reactive oxygen species (ROS) production leads to the increase in cell death observed with VDAC overexpression [37,38], however, the mechanisms have not been thoroughly investigated. A recent report tested the viability of yeast cells transfected with the three human VDAC isoforms individually [39]. Interestingly, cells that expressed VDAC1 and VDAC2 showed similar levels of ROS and similar viability to wildtype yeast. However, yeast transformed with VDAC3 had higher levels of ROS and decreased viability (increased death) [39]. In contrast, we have shown that murine fibroblasts essentially devoid of all three VDAC isoforms are actually more sensitive to staurosporine-, and TNFα-induced death [28], suggesting that VDAC may actually play an anti-apoptotic, rather than pro-apoptotic, role.
One explanation for VDAC overexpression leading to increased cell death is that higher VDAC expression favors VDAC oligomerization (Figure 1, model #2). Indeed, arrays of VDAC in plant outer mitochondrial membranes were very early observations [40]. It has been suggested that oligomeric VDAC1 mediates the release of cytochrome c [41], since the internal diameter of a single VDAC pore is 2.5-3.0 nm, which is insufficient to pass a folded protein. VDAC1 has been shown to assemble into dimers, trimers, tetramers, and multimers [41,42,43,44,45,46]. Interestingly, overexpression of the anti-apoptotic protein Bcl-xL has been shown to prevent VDAC homo-dimerization [19].
VDAC has also been suggested to assemble into hetero-oligomers with pro- and anti-apoptotic Bcl-2 proteins. There are two models of VDAC associating with Bcl-2 family proteins (Figure 1). The first model involving Bcl-2 proteins (model #2) entails pro-apoptotic Bax binding to VDAC, creating a large VDAC-Bax channel, and causing cytochrome c release [18,26,47,48,49]. Model #2 concludes that, although the channels formed by Bax or VDAC alone are unable to translocate cytochrome c, the new larger Bax-VDAC channel is permeable to cytochrome c [47]. In complete disagreement with this model, mammalian VDAC reconstituted into planar phospholipid membranes has shown Bax to not have any effect on the properties of VDAC channels [50]. While monomeric Bax is found in the cytosol of healthy cells [51,52], tBid triggers Bax oligomerization [53,54] causing Bax to form channels in the outer membrane that allow for cytochrome c release [55,56]. Neither the monomeric nor oligomeric form of Bax showed any interaction with VDAC channels [50]. Additionally, immunoprecipitations have failed to detect Bax-VDAC interaction [57], and the levels of Bax required for killing in VDAC1−/− yeast cells was similar to wild-type cells [58].
The VDAC2 isoform has been shown to bind the pro-apoptotic Bak, however, its role is still controversial. The first report by Cheng et al. [59] suggested that VDAC2, but not VDAC1, binds inactive Bak in normal healthy cells. Treatment with tBid released Bak from VDAC2, causing activation of Bak and apoptosis. In this study, VDAC2−/− cells had greater activation (oligomerization) of Bak, and increased sensitivity to apoptotic stimuli [59]. Bax was not shown to make complexes with VDAC2 even in apoptotic situations, which argues against model #2. Interestingly, in Bax-deficient cells, the VDAC2-Bak interaction is no longer observed with or without apoptotic stimulation [60]. A more recent study showed that VDAC2 is required for the formation of the inactive Bak complex, and that the Bak transmembrane anchor is required for interacting with VDAC2 [61]. Furthermore, another study suggests that VDAC2 is required for tBid-induced Bak activation and apoptosis [62]. This study showed that although VDAC1−/−, VDAC3−/−, and VDAC1/3−/− fibroblasts respond normally to tBid, VDAC2−/− fibroblasts are virtually insensitive to tBid-induced outer membrane permeabilization and apoptosis [62]. Additionally, VDAC2 may be required for Bax-induced outer membrane permeability and apoptosis in Bak−/− cells [63]. Thus, these studies suggest VDAC2 interacts with Bak and mediates Bak-activated apoptosis in a Bax- and tBid-dependent manner [60,62,64].
3.2 Model #3 – Proteins and metabolites alter VDAC conductance, resulting in outer membrane permeabilization through unknown mechanisms
In a new model (Figure 1, model #3), tBid was shown to induce VDAC closure, reducing adenine nucleotide exchange between mitochondria and cytosol, thus creating mitochondrial dysfunction [50]. Conversely, but also along the lines of model #3, the anti-apoptotic Bcl-xL has been shown to bind VDAC [44] and promote its open configuration and maintain ATP/ADP exchange [65]. Thus, model#3 describes how proteins and metabolites modulate VDAC conductance. VDAC closure prevents the exchange of ATP/ADP and all other larger respiratory metabolites, which then leads to outer membrane permeabilization and release of proteins from the intermembrane space [65,66]. It is unknown how the permeabilization occurs, either through undefined pathways or via swelling and rupture of the outer membrane. It is also not known how or if tBid and VDAC physically interact.
Although the mechanism(s) are unclear, it is apparent that VDACs modulate outer membrane permeability and therefore the release of intermembrane proteins and apoptosis. The next section of this review will detail several metabolites and non-Bcl-2 family proteins that have been shown to modulate VDAC permeability.
4. Regulators of VDAC permeability and their role in cell death
To date, a number of metabolites and proteins have been shown to bind and modulate VDAC conductance. However, the effects of altered VDAC permeability on cell death are still unclear. While ATP/ADP [67,68], glutamate [69], ROS [70], the 18 kDa translocator protein [71], and hexokinase [72] have all been suggested to modulate MPT, it is easy to propose that these agents are working through VDAC to affect MPT (Model #1). However, as VDACs are dispensable for MPT [28,29], these agents must be working through another mechanism, perhaps by altering VDAC structure and preventing Bcl-2 protein interaction (Model #2) or by altering VDAC conductance and creating outer mitochondrial membrane permeability through an unknown mechanism or outer membrane rupture (Model #3) as discussed above.
4.1 Adenine nucleotides
As the main purpose of VDAC is to shuttle adenine nucleotides between the mitochondria and cytosol, it only makes sense that adenine nucleotides can modulate VDAC behavior. The discovery of at least two nucleotide-binding sites on VDAC [73,74,75] explains how NAD(P)H, ATP, and ADP can decrease VDAC channel conductance [3,76]. Indeed, ATP bound to the nucleotide-binding site on VDAC is suggested to sterically block VDAC [3,76]. Mechanistically, this interaction of VDAC with ATP/ADP and NAD(P)H explains how energetic pathway intermediates can modulate outer mitochondrial membrane permeability and respiration rates to adjust to cellular energy requirements.
The effect of adenine nucleotides on mitochondrial permeability (and subsequent cell death) has been thoroughly studied. Yehezkel et al [75] showed that T-Rex-293 cells expressing VDAC1 with a mutation in the nucleotide-binding site had severely diminished ATP synthesis and ATP levels. NAD(P)H, ADP, and particularly ATP inhibit the MPTP [67]. During ischemia, ATP and ADP are degraded to nucleosides and bases, and thus the inhibition of the MPTP is lost. Therefore, ischemia or other noxious stimuli that decrease the exchange of adenine nucleotides through VDAC can cause MPT and subsequently, cell death. Thus, excess adenine nucleotides may decrease VDAC conductance, cause outer membrane permeabilization, resulting in cell death, which fits model #3 (Figure 1).
4.2 Glutamate
Much of a cells glutamate is formed in the mitochondria through the action of glutamate dehydrogenase on the TCA cycle intermediate α-ketoglutarate. The glutamate formed in the mitochondria can then be exported to the cytosol, crossing the outer membrane through VDAC. Studies suggest that VDAC contains a glutamate-binding site, as L-glutamate has been shown to cause VDAC to oscillate between the stable (more closed) and open conformations decreasing Ca2+ transport [69,77,78]. The decreased Ca2+ import caused by L-glutamate has been shown to inhibit MPTP opening, decrease mitochondrial swelling, as well as decrease cytochrome c release from mitochondria [69]. Thus, it appears that glutamate is protective against cell death by inhibiting mitochondrial Ca2+ import through VDAC. The decreased VDAC conductance caused by glutamate clearly fits with model #3 in that decreased VDAC conductance can result in outer membrane permeabilization.
4.3 Tubulin
Tubulin, the heterodimeric subunit of microtubules has been shown to bind mitochondria via VDAC [79,80]. Dimeric tubulin closes VDAC channels reconstituted into planar phospholipid membranes [81]. Tubulin decreased outer mitochondrial membrane permeability to adenine nucleotides in isolated brain mitochondria and permeabilized cardiomyocytes [81,82]. Recently, the microtubule destabilizing compounds rotenone, colchicine, and nocodazole were shown to increase free tubulin and decrease the mitochondrial membrane potential; conversely, the microtubule stabilizer paclitaxel decreased free tubulin and hyperpolarized mitochondria of HepG2 cells (cancerous hepatoma cells) and primary rat hepatocytes [83]. Thus, free tubulin can decrease VDAC conductance and ultimately lead to outer mitochondrial membrane permeabilization and cell death, which fits model #3. This inhibition of VDAC by free tubulin limits mitochondrial respiration, and may explain the Warburg effect in cancer cells, which by nature require excess tubulin to support rapid division. Only time will tell if this interaction can be utilized to control cancer cell death in vivo.
4.4 Reactive oxygen species
Mitochondrially produced ROS has been shown to be involved in cell death, and Ca2+ accumulation by mitochondria is associated with mitochondrial ROS generation by mechanism(s) that are poorly understood [84,85]. Several studies suggest that VDAC plays a role in this mechanism. Mitochondrial-generated ROS has been shown to induce cytochrome c release, which is inhibited by VDAC blockers or anti-VDAC antibody [70,86]. Interestingly, the same effect is not seen with the MPTP inhibitor cyclosporine A [86]. ROS caused cardiolipin peroxidation, which was interpreted as the means by which cytochrome c was released from its cardiolipin-mediated attachment to the inner mitochondrial membrane [70]. However, how the cytochrome c is released and the role of VDAC in its release remains unknown. As discussed earlier, cytochrome c is too large to be released through monomeric VDAC. Possible mechanisms include oligomeric VDAC forming a larger channel [41] (model #2), or that mitochondrial membrane lipid peroxidation impairs membrane function and alters VDAC properties, resulting in outer mitochondrial membrane permeabilization (model #3). Indeed, the gating properties of VDAC channels reconstituted into lipid bilayers were significantly affected by lipid composition [87].
4.5 Peripheral Benzodiazepine Receptor (PBR) or 18kDa translocator protein (TSPO)
The peripheral benzodiazepine receptor (PBR), now known as the 18kDa translocator protein (TSPO), is expressed on the outer mitochondrial membrane and mediates cholesterol import into the mitochondria for steroidogenesis [88]. TSPO interacts with VDAC on the outer membrane, and may alter its properties [89]. Importantly, the TSPO has been implicated in ROS production in models of neurodegeneration and cancer [90,91]. The proapoptotic agent erucylphosphohomcholine (ErPC3) was shown to increase ROS generation and cause oxidation of cardiolipin on the inner mitochondrial membrane, while the TSPO ligand PK11195 prevented this ROS generation and induction of apoptosis [71]. Interestingly, electron microscopy studies have found large groupings of the TSPO around VDAC channels [92], potentially increasing ROS concentrations in the proximity of VDAC. Therefore, the 18kDa TSPO likely affects VDAC conductance by production of ROS, causing outer membrane permeabilization (model #3).
4.6 Hexokinases
Perhaps the most studied regulator of VDAC function is the glycolytic enzyme hexokinase. A 21-amino-acid sequence in the N-termini of hexokinase isoforms 1 (HK1) and 2 (HK2) are predicted to form a hydrophobic α-helix, which is essential and sufficient for binding to mitochondria [93,94]. Interaction with VDAC is believed to cause the specificity of hexokinases binding to the outer mitochondrial membrane [95,96]. Binding of HK2 to isolated mitochondria or overexpression of HK2 in HeLa cells has been shown to inhibit the mitochondrial translocation of Bax and the release of cytochrome c [97]. Majewski et al [98] confirmed that mitochondrial HK inhibits cytochrome c release and apoptosis; however, this was also true in Bax- and Bak-deficient cells. This study suggested that mitochondrial hexokinase prevented VDAC closure. Thus, this study is in agreement with Vander Heiden et al [65,66] in that VDAC closure (due to HK detachment) leads to mitochondrial swelling and consequently to cell death via an undefined pathway. HK binding and inhibiting closure of VDAC therefore fits into model #3 and explains how mitochondrial-bound HK protects against outer mitochondrial membrane permeabilization and cell death.
Conversely, a direct inhibitory effect of HK1 has been reported on VDAC channels reconstituted into planar membranes [72]. This study showed that HK1 induced VDAC closure, and that addition of hexokinase’s reaction product glucose-6-phosphate released HK from VDAC and allowed VDAC reopening [72]. These authors suggest that HK1-induced closure of VDAC inhibits MPTP opening, which is not in agreement with VDACs being inessential for MPT [28,29]. Biochemically, HK1-mediated closure of VDAC does not make sense, as VDAC closure would inhibit nucleotide exchange and hinder the ATP supply by which HK1 depends on for its enzymatic activity. In support of this notion, hexokinases preferentially utilize intramitochondrially generated ATP from oxidative phosphorylation [99]. In fact, in most cells, mitochondrial respiration increases dramatically after addition of glucose, a phenomenon termed the Pasteur effect [100]. Therefore, HK1-mediated closure of VDAC causing decreased MPT argues against not only the biochemical activity of HK, but also does not fit with model #3. While this finding should be highly scrutinized, it does imply that the mechanism of how HK protects against cell death and what role, if any, VDAC plays in this mechanism still remains to be definitely described.
5. Questions raised by genetic models of VDAC deficiency/overexpression
One conundrum raised by the genetic ablation of VDAC isoforms, is how do mitochondria devoid of VDAC still function metabolically, and perhaps more importantly, what has changed? The same argument can be made for overexpression of VDAC isoforms. Certainly cells lacking essentially all 3 VDAC isoforms still seem to exist in culture relatively easily, although, as mentioned above and later on, they are more susceptible to death stimuli than their wildtype counterparts [6]. It is conceivable that other outer membrane anion channels such as the peripheral benzodiazepine receptor could compensate electrically and/or metabolically for the loss of VDACs, indicating that adaptations occur that could also influence cell death end-points. However, VDAC1−/− cells have enlarged mitochondria [6,101], and VDAC1−/− and VDAC1/VDAC3−/− muscle mitochondria have substantially less HK activity [102], suggesting that substantial mitochondrial alterations can occur. Thus, the question that arises is this: are the effects (or lack thereof in the case of the MPTP) of VDAC depletion indicative of a primary role for VDAC in that process, or are they due to secondary changes in mitochondrial structure and/or function caused by the general impairment of metabolite transport across the outer membrane? Can these even be separated? For example, model #3 suggests that the role of VDAC in modulating cell death is entirely due to its ability to greatly influence metabolite flux across the outer membrane.
Another question that is raised is the concept of redundant versus non-redundant functions of each VDAC isoform. For the most part, one mammalian VDAC seems to be able to compensate for the lack of another [103]. This of course brings up the issue of a negative phenotype simply being due to redundancy, and that is certainly why we, and others, attempt to ablate all 3 VDAC isoforms in a cell at a given time. However, it is becoming more apparent that there are some functional differences between isoforms especially with regards to tissue-specificity. For instance, VDAC1 deletion affects mitochondrial respiration similarly in both heart and skeletal muscles, whereas VDAC3 ablation primarily only affects the mixed glycolytic/oxidative skeletal muscle [101,104]. The relative expression of each VDAC isoform also differs between tissues [105], which in turn could also influence mitochondrial function and, ultimately, cell death progression in a tissue-specific manner.
These are all issues that need to be considered when using any genetic model, but especially when there are several isoforms involved, both with regards to the designing and interpretation of experiments. The refinement of genetic approaches (tissue-specificity, temporal induction, reconstitution of selective isofoms etc.) should hopefully help address these issues.
6. Conclusions
Although VDAC undoubtedly plays a role in outer mitochondrial membrane permeabilization and induction of cell death, the mechanisms remain poorly understood. As we have discussed, VDAC on its own cannot induce apoptosis. The diameter of the VDAC pore cannot be drastically increased to allow passage of proapoptotic proteins. Likewise, while VDAC overexpression may increase VDAC homo-oligomerization, we have shown that mitochondria devoid of all VDACs have an increased MPT response, portraying not a pro-death role, but rather a pro-survival role for VDAC. Lastly, modulation of VDAC by cytosolic mediators is the most definitive mechanism for causing outer membrane permeabilization. Future research is needed to further characterize the mechanisms of these VDAC-modulating agents.
Highlights.
Review the evidence both for and against a role for VDACs in mitochondrial permeability transition
Review the literature regarding the role of VDACs in mitochondrial-dependent apoptosis
Discuss the factors that regulate VDAC permeability and how they influence cell death
Acknowledgements
The authors research is sponsored by National Institutes of Health grant HL094404 (to CPB) and by an American Heart Association Predoctoral grant (to KSM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bathori G, Sahin-Toth M, Fonyo A, Ligeti E. Transport properties and inhibitor sensitivity of isolated and reconstituted porin differ from those of intact mitochondria. Biochim. Biophys. Acta. 1993;1145:168–176. doi: 10.1016/0005-2736(93)90394-f. [DOI] [PubMed] [Google Scholar]
- [2].Lee AC, Xu X, Colombini M. The role of pyridine dinucleotides in regulating the permeability of the mitochondrial outer membranes. J. Biol. Chem. 1996;271:26724–26731. doi: 10.1074/jbc.271.43.26724. [DOI] [PubMed] [Google Scholar]
- [3].Rostovtseva TK, Komarov A, Bezukov SM, Colombini M. Dynamics of nucleotides in VDAC channels: structure-specific noise generation. Biophys. J. 2002;82:193–205. doi: 10.1016/S0006-3495(02)75386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].De Pinto V, Reina S, Guarino F, Messina A. Structure of the voltage dependent anion channel: state of the art. J. Bioenerg. Biomembr. 2008;40:139–147. doi: 10.1007/s10863-008-9140-3. [DOI] [PubMed] [Google Scholar]
- [5].Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator-thinking outside the box. Biochemica et Biophysica Acta. 2006;1762:181–190. doi: 10.1016/j.bbadis.2005.10.006. [DOI] [PubMed] [Google Scholar]
- [6].Rostovtseva TK, Tan W, Colombini M. On the role of VDAC in apoptosis: fact and fiction. J. Bioenerg. Biomembr. 2005;37:129–142. doi: 10.1007/s10863-005-6566-8. [DOI] [PubMed] [Google Scholar]
- [7].Shoshan-Barmatz V, Keinan N, Zaid H. Uncovering the role of VDAC in the regulation of cell life and death. J. Bioenerg. Biomembr. 2008;40:183–191. doi: 10.1007/s10863-008-9147-9. [DOI] [PubMed] [Google Scholar]
- [8].Zeth K, Thein M. Porins in prokaryotes and eukaryotes: common themes and variations. Biochem. J. 2010;431:13–22. doi: 10.1042/BJ20100371. [DOI] [PubMed] [Google Scholar]
- [9].Baines CP. The cardiac mitochondrion: nexus of stress. Annu. Rev. Physiol. 2010;72:61–80. doi: 10.1146/annurev-physiol-021909-135929. [DOI] [PubMed] [Google Scholar]
- [10].Di Lisa F, Canton M, Menabo R, Kaludercic N, Bernardi P. Mitochondria and cardioprotection. Heart Fail. Rev. 2007;12:249–260. doi: 10.1007/s10741-007-9028-z. [DOI] [PubMed] [Google Scholar]
- [11].Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion-a target for cardioprotection. Cardiovasc. Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- [12].Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ. Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- [14].Baines CP. The mitochondrial permeability transition pore and the cardiac necrotic program. Pediatr. Cardiol. 2011;32:258–262. doi: 10.1007/s00246-010-9880-9. [DOI] [PubMed] [Google Scholar]
- [15].Szabó I, Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules. I. Binary structure and voltage dependence of the pore. FEBS Lett. 1993;330:201–205. doi: 10.1016/0014-5793(93)80273-w. [DOI] [PubMed] [Google Scholar]
- [16].Szabó I, De Pinto V, Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules. II. The electrophysiological properties of VDAC are compatible with those of the mitochondrial megachannel. FEBS Lett. 1993;330:206–210. doi: 10.1016/0014-5793(93)80274-x. [DOI] [PubMed] [Google Scholar]
- [17].Crompton M, Virji S, Ward JM. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur. J. Biochem. 1998;258:729–735. doi: 10.1046/j.1432-1327.1998.2580729.x. [DOI] [PubMed] [Google Scholar]
- [18].Shimizu S, Matsuoka Y, Shinohara Y, Yoneda Y, Tsujimoto Y. Essential role of voltage-dependent anion channel in various forms of apoptosis in mammalian cells. J. Cell Biol. 2001;152:237–250. doi: 10.1083/jcb.152.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zheng Y, Shi Y, Tian C, Jiang C, Jin H, Chen J, Almasan A, Tang H, Chen Q. Essential role of the voltage-dependent anion channel (VDAC) in mitochondrial permeability transition pore opening and cytochrome c release by arsenic trioxide. Oncogene. 2004;23:1239–1247. doi: 10.1038/sj.onc.1207205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cesura AM, Pinard E, Schubenel R, Goetschy V, Friedlein A, Langen H, Polcic P, Forte MA, Bernardi P, Kemp JA. The voltage-dependent anion channel in the target for a new class of inhibitors of the mitochondrial permeability transition pore. J. Biol. Chem. 2003;278:49812–49818. doi: 10.1074/jbc.M304748200. [DOI] [PubMed] [Google Scholar]
- [21].Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Properties of the permeability transition in VDAC1(−/−) mitochondria. Biochim. Biophys. Acta. 2006;1757:590–595. doi: 10.1016/j.bbabio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- [22].Tan W, Colombini M. VDAC closure increases calcium ion flux. Biochim. Biophys. Acta. 2007;1768:2510–2515. doi: 10.1016/j.bbamem.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tikunov A, Johnson CB, Pediaditakis P, Markevich N, Macdonald JM, Lemasters JJ, Holmuhamedov E. Closure of VDAC causes oxidative stress and accelerates the Ca2+-induced mitochondrial permeability transition in rat liver mitochondria. Arch. Biochem. Biophys. 2010;495:174–181. doi: 10.1016/j.abb.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Woodfield K, Ruck A, Brdiczka D, Halestrap AP. Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem. J. 1998;336:287–290. doi: 10.1042/bj3360287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jacotot E, Ravagnan L, Loeffler M, Ferri KF, Vieira HL, Zamzami N, Costantini P, Druillennec S, Hoebeke J, Briand JP, Irinopoulou T, Daugas E, Susin SA, Cointe D, Xie ZH, Reed JC, Roques BP, Kroemer G. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med. 2000;191:33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shimizu S, Shinohara Y, Tsujimoto Y. Bax and Bcl-xL independently regulate apoptotic changes of yeast mitochondria that require VDAC but not the adenine nucleotide translocator. Oncogene. 2000;19:4309–4318. doi: 10.1038/sj.onc.1203788. [DOI] [PubMed] [Google Scholar]
- [27].Uribe-Carvajal S, Luévano-Martínez LA, Guerrero-Castillo S, Cabrera-Orefice A, Corona-de-la-Peña NA, Gutiérrez-Aguilar M. Mitochondrial Unselective Channels throughout the eukaryotic domain. Mitochondrion. 2011;11:382–390. doi: 10.1016/j.mito.2011.02.004. [DOI] [PubMed] [Google Scholar]
- [28].Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chiara F, Castellaro D, Marin O, Petronilli V, Brusilow WS, Juhaszova M, Sollott SJ, Forte M, Bernardi P, Rasola A. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS ONE. 2008;3:e1852. doi: 10.1371/journal.pone.0001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bernardes CF, Meyer-Fernandes JR, Basseres DS, Castilho RF, Vercesi AE. Ca2+-dependent permeabilization of the inner mitochondrial membrane by 4,4′- diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) Biochim. Biophys. Acta. 1994;1188:93–100. doi: 10.1016/0005-2728(94)90026-4. [DOI] [PubMed] [Google Scholar]
- [31].Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J. Neurochem. 2002;80:207–218. doi: 10.1046/j.0022-3042.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- [32].Abu-Hamad S, Zaid H, Israelson A, Nahon E, Shoshan-Barmatz V. Hexokinase-I protection against apoptotic cell death is mediated via interaction with the voltage-dependent anion channel-1: mapping the site of binding. J. Biol. Chem. 2008;283:13482–13490. doi: 10.1074/jbc.M708216200. [DOI] [PubMed] [Google Scholar]
- [33].Ghosh T, Pandey N, Maitra A, Brahmachari SK, Pillai B. Role for voltage-dependent anion channel VDAC1 in polyglutamine-mediated neuronal cell death. PLoS ONE. 2007;2:e1170. doi: 10.1371/journal.pone.0001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Godbole A, Varghese J, Sarin A, Mathew MK. VDAC is a conserved element of death pathways in plant and animal systems, Biochim. Biophys. Acta. 2003;1642:87–96. doi: 10.1016/s0167-4889(03)00102-2. [DOI] [PubMed] [Google Scholar]
- [35].Lu AJ, Dong CW, Du CS, Zhang QY. Characterization and expression analysis of Paralichthys olivaceus voltage-dependent anion channel (VDAC) gene in response to virus infection. Fish Shellfish Immunol. 2007;23:601–613. doi: 10.1016/j.fsi.2007.01.007. [DOI] [PubMed] [Google Scholar]
- [36].Zaid H, Abu-Hamad S, Israelson A, Nathan I, Barmatz V. Shoshan. The voltage-dependent anion channel-1 modulates apoptotic cell death. Cell Death Differ. 2005;12:751–760. doi: 10.1038/sj.cdd.4401599. [DOI] [PubMed] [Google Scholar]
- [37].Simamura E, Hirai K, Shimada H, Koyama J, Niwa Y, Shimizu S. Furanonaphthoquinones cause apoptosis of cancer cells by inducing the production of reactive oxygen species by the mitochondrial voltage-dependent anion channel. Cancer Biol. Ther. 2006;5:1523–1529. doi: 10.4161/cbt.5.11.3302. [DOI] [PubMed] [Google Scholar]
- [38].Yuan S, Fu Y, Wang X, Shi H, Huang Y, Song X, Li L, Song N, Luo Y. Voltage-dependent anion channel 1 is involved in endostatin-induced endothelial cell apoptosis. FASEB J. 2008;22:2809–2820. doi: 10.1096/fj.08-107417. [DOI] [PubMed] [Google Scholar]
- [39].De Pinto V, Guarino F, Guarnera A, Messina A, Reina S, Tomasello FM, Palermo V, Mazzoni C. Characterization of human VDAC isoforms: A peculiar function for VDAC3? Biochim. Biophys. Acta. 2010;1797:1268–1275. doi: 10.1016/j.bbabio.2010.01.031. [DOI] [PubMed] [Google Scholar]
- [40].Parsons DF, Bonner WD, Verboon JG. Electron microscopy of isolated plant mitochondria and plastids using both the thin-section and negative-staining techniques. Can. J. Bot. 1965;43:647–655. [Google Scholar]
- [41].Zalk R, Israelson A, Garty ES, Azoulay-Zohar H, Shoshan-Barmatz V. Oligomeric states of the voltage-dependent anion channel and cytochrome c release from mitochondria. Biochem. J. 2005;386:73–83. doi: 10.1042/BJ20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gonclaves RP, Buzhynskyy N, Prima V, Sturgis JN, Scheuring S. Supramolecular assembly of VDAC in native mitochondrial outer membranes. J. Mol. Biol. 2007;369:413–418. doi: 10.1016/j.jmb.2007.03.063. [DOI] [PubMed] [Google Scholar]
- [43].Hoogenboom BW, Suda K, Engel A, Fotadis D. The supramolecular assemblies of voltage-dependent anion channels in the native membrane. J. Mol. Biol. 2007;370:246–255. doi: 10.1016/j.jmb.2007.04.073. [DOI] [PubMed] [Google Scholar]
- [44].Malia TJ, Wagner G. NMR structural investigation of the mitochondrial outer membrane protein VDAC and its interaction with antiapoptotic Bcl-xL. Biochemistry. 2007;46:514–525. doi: 10.1021/bi061577h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shi Y, Jiang C, Chen Q, Tang H. One-step on-column affinity refolding purification and functional analysis of recombinant human VDAC1. Biochem. Biophys. Res. Commun. 2003;303:475–482. doi: 10.1016/s0006-291x(03)00359-0. [DOI] [PubMed] [Google Scholar]
- [46].Shoshan-Barmatz V, Zalk R, Gincel D, Vardi N. Subcellular localization of VDAC in mitochondria and ER in the cerebellum. Biochim. Biophys. Acta. 2004;1657:105–114. doi: 10.1016/j.bbabio.2004.02.009. [DOI] [PubMed] [Google Scholar]
- [47].Shimizu S, Ide T, Yanagida T, Tsujimoto Y. Electrophysiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. J. Biol. Chem. 2000;275:12321–12325. doi: 10.1074/jbc.275.16.12321. [DOI] [PubMed] [Google Scholar]
- [48].Shimizu S, Konishi A, Kodama T, Tsujimoto Y. BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc. Natl. Acad. Sci. USA. 2000;97:3100–3105. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- [50].Rostovtseva TK, Antonsson B, Suzuki M, Youle RJ, Colombini M, Bezrukov SM. Bid, but not Bax, regulates VDAC channels. J. Biol. Chem. 2004;279:13575–13583. doi: 10.1074/jbc.M310593200. [DOI] [PubMed] [Google Scholar]
- [51].Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem. J. 2000;345:271–278. [PMC free article] [PubMed] [Google Scholar]
- [52].Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- [53].Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBid, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- [55].Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou JC. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- [56].Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G, Korsmeyer SJ. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc. Natl. Acad. Sci. USA. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mikhailov V, Mikhailova M, Pulkrabek DJ, Dong Z, Venkatachalam MA, Saikumar P. Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J. Biol. Chem. 2001;276:18361–18374. doi: 10.1074/jbc.M100655200. [DOI] [PubMed] [Google Scholar]
- [58].Polcic P, Forte M. Response of yeast to the regulated expression of proteins in the Bcl-2 family. Biochem. J. 2003;374:393–402. doi: 10.1042/BJ20030690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Cheng EHY, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits Bak activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- [60].Chandra D, Choy G, Daniel PT, Tang DG. Bax-dependent regulation of Bak by voltage-dependent anion channel 2. J. Biol. Chem. 2005;280:19051–19061. doi: 10.1074/jbc.M501391200. [DOI] [PubMed] [Google Scholar]
- [61].Lazarou M, Stojanovski D, Frazier AE, Kotevski A, Dewson G, Craigen WJ, Kluck RM, Vaux DL, Ryan MT. Inhibition of Bak activation by VDAC2 is dependent on the Bak transmembrane anchor. J. Biol. Chem. 2010;285:36876–36883. doi: 10.1074/jbc.M110.159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Roy SS, Ehrlich AM, Craigen WJ, Hajnoczky G. VDAC2 is required for truncated BID-induced mitochondrial apoptosis by recruiting Bak to the mitochondria. EMBO Rep. 2009;10:1341–1347. doi: 10.1038/embor.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yamagata H, Shimizu S, Nishida Y, Watanabe Y, Craigen WJ, Tsujimoto Y. Requirement of voltage-dependent anion channel 2 for pro-apoptotic activity of Bax. Oncogene. 2009;28:3563–3572. doi: 10.1038/onc.2009.213. [DOI] [PubMed] [Google Scholar]
- [64].Setoguchi K, Otera H, Mihara K. Cytosolic factor- and TOM-independent import of C-tail-anchored mitochondrial outer membrane proteins. EMBO J. 2006;25:5635–5647. doi: 10.1038/sj.emboj.7601438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Vander Heiden MG, Li XX, Gottleib E, Hill RB, Thompson CB, Colombini M. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J. Biol. Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- [66].Vander Heiden MG, Chandel NS, Li XX, Schumacher PT, Colombini M, Thompson CB. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc. Natl. Acad. Sci. USA. 2000;97:4666–4671. doi: 10.1073/pnas.090082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- [68].Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim. Biophys. Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- [69].Gincel D, Shoshan-Barmatz V. Glutamate interacts with VDAC and modulates opening of the mitochondrial permeability transition pore. J. Bioenerg. Biomembr. 2004;36:179–186. doi: 10.1023/b:jobb.0000023621.72873.9e. [DOI] [PubMed] [Google Scholar]
- [70].Petrosillo G, Ruggiero FM, Pistolese M, Paradies G. Ca2+-induced reactive oxygen species production promotes cytochrome c release from rat liver mitochondria via mitochondrial permeability transition (MPT)-dependent and MPT-independent mechanisms: role of cardiolipin. J. Biol. Chem. 2004;279:53103–53108. doi: 10.1074/jbc.M407500200. [DOI] [PubMed] [Google Scholar]
- [71].Veenman L, Shandalov Y, Gavish M. VDAC activation by the 18 kDa translocator protein (TSPO), implications for apoptosis. J. Bioenerg. Biomembr. 2008;40:199–205. doi: 10.1007/s10863-008-9142-1. [DOI] [PubMed] [Google Scholar]
- [72].Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V. In self-defense: hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem. J. 2004;377:347–355. doi: 10.1042/BJ20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Florke H, Thinnes FP, Winkelbach H, Stadtmuller U, Paetzold G, Morys-Wortmann C, Hesse D, Sternbach H, Zimmermann B, Kaufmann-Kolle P. Channel active mammalian porin, purified from crude membrane fractions of human B lymphocytes and bovine skeletal muscle, reversibly binds adenosine triphosphate (ATP) Biol. Chem. Hoppe Seyler. 1994;375:513–520. doi: 10.1515/bchm3.1994.375.8.513. [DOI] [PubMed] [Google Scholar]
- [74].Yehezkel G, Hadad N, Zaid H, Sivan S, Shoshan-Barmatz V. Nucleotide-binding sites in the voltage-dependent anion channel: characterization and localization. J. Biol. Chem. 2006;281:5938–5946. doi: 10.1074/jbc.M510104200. [DOI] [PubMed] [Google Scholar]
- [75].Yehezkel G, Abu-Hamad S, Shoshan-Barmatz V. An N-terminal nucleotide-binding site in VDAC1: involvement in regulating mitochondrial function. J. Cell Physiol. 2007;212:551–561. doi: 10.1002/jcp.21048. [DOI] [PubMed] [Google Scholar]
- [76].Rostovtseva TK, Bezrukoz SM. Atp transport through a single mitochondrial channel, VDAC, studied by current fluctuation analysis. Biophys. J. 1998;74:2365–2373. doi: 10.1016/S0006-3495(98)77945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Deniaud A, Rossi C, Berquand A, Homand J, Campagna S, Knoll W, Brenner C, Chopineau J. Voltage-dependent anion channel transports calcium ions through biomimetic membranes. Langmuir. 2007;23:3898–3905. doi: 10.1021/la063105+. [DOI] [PubMed] [Google Scholar]
- [78].Gincel D, Silberberg SD, Shoshan-Barmatz V. Modulation of the voltage-dependent anion channel (VDAC) by glutamate. J. Bioenerg. Biomembr. 2000;32:571–583. doi: 10.1023/a:1005670527340. [DOI] [PubMed] [Google Scholar]
- [79].Bernier-Valentin F, Rousset B. Interaction of tubulin with rat liver mitochondria. J. Biol. Chem. 1982;257:7092–7099. [PubMed] [Google Scholar]
- [80].Carre M, Andre N, Carles G, Borghi H, Brichese L, Briand C, Braguer D. Tubulin is an inherent component of mitochondrial membranes that interacts with the voltage-dependent anion channel. J. Biol. Chem. 2002;277:33664–33669. doi: 10.1074/jbc.M203834200. [DOI] [PubMed] [Google Scholar]
- [81].Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, Sackett DL. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc. Natl. Acad. Sci. USA. 2008;105:18746–18751. doi: 10.1073/pnas.0806303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Timohhina N, Guzman R, Tepp K, Monge C, Varikmaa M, Vija H, Sikk P, Kaambre T, Sackett D, Saks V. Direct measurement of energy fluxes from mitochondria into cytoplasm in permeabilized cardiac cells in situ: some evidence for mitochondrial interactosome. J. Bioenerg. Biomembr. 2009;41:259–75. doi: 10.1007/s10863-009-9224-8. [DOI] [PubMed] [Google Scholar]
- [83].Maldonaldo EN, Patnaik J, Mullins MR, Lemasters JJ. Free tubulin modulates mitochondrial membrane potential in cancer cells. Cancer Res. 2010;70:10192–10201. doi: 10.1158/0008-5472.CAN-10-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kowaltowski AJ, Castilho RF, Vercesi AE. Ca(2+)-induced mitochondrial membrane permeabilization: role of coenzyme Q redox state. Am. J. Physiol. Cell Physiol. 1995;269:C141–147. doi: 10.1152/ajpcell.1995.269.1.C141. [DOI] [PubMed] [Google Scholar]
- [85].Starkov AA, Polster BM, Fiskum G. Regulation of hydrogen peroxide production by brain mitochondria by calcium and Bax. J. Neurochem. 2002;83:220–228. doi: 10.1046/j.1471-4159.2002.01153.x. [DOI] [PubMed] [Google Scholar]
- [86].Madesh M, Hajnoczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J. Cell Biol. 2001;155:1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Rostovtseva TK, Kazemi N, Weinrich M, Bezrukov SM. Voltage gating of VDAC is regulated by nonlamellar lipids of mitochondrial membranes. J. Biol. Chem. 2005;281:37496–37506. doi: 10.1074/jbc.M602548200. [DOI] [PubMed] [Google Scholar]
- [88].Li H, Papadopoulos V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology. 1998;139:4991–4997. doi: 10.1210/endo.139.12.6390. [DOI] [PubMed] [Google Scholar]
- [89].McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc. Natl. Acad. Sci. USA. 1992;89:3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- [91].Veenman L, Gavish M. The peripheral-type benzodiazepine receptor and the cardiovascular system. Implications for drug development. Pharmacol. Ther. 2006;110:503–524. doi: 10.1016/j.pharmthera.2005.09.007. [DOI] [PubMed] [Google Scholar]
- [92].Papadopoulos V, Boujrad N, Ikonomovic MD, Ferrara P, Vidic B. Topography of the Leydig cell mitochondrial peripheral-type benzodiazepine receptor. Mol. Cell Endocrinol. 1994;104:R5–9. doi: 10.1016/0303-7207(94)90061-2. [DOI] [PubMed] [Google Scholar]
- [93].Xie JC, Wilson JE. Rat brain hexokinase: the hydrophobic N-terminus of the mitochondrially bound enzyme is inserted in the lipid bilayer. Arch. Biochem. Biophys. 1988;267:803–810. doi: 10.1016/0003-9861(88)90090-2. [DOI] [PubMed] [Google Scholar]
- [94].Sui D, Wilson JE. Structural determinants for the intracellular localization of the isozymes of mammalian hexokinase: intracellular localization of fusion constructs incorporating structural elements from the hexokinase isozymes and the green fluorescent protein. Arch. Biochem. Biophys. 1997;345:111–125. doi: 10.1006/abbi.1997.0241. [DOI] [PubMed] [Google Scholar]
- [95].Fiek C, Benz R, Roos N, Brdiczka D. Evidence for identity between the hexokinase-binding protein and the mitochondrial porin in the outer membrane of rat liver mitochondria. Biochem. Biophys. Acta. 1982;688:429–440. doi: 10.1016/0005-2736(82)90354-6. [DOI] [PubMed] [Google Scholar]
- [96].Linden M, Gellerfors P, Nelson BD. Pore protein and the hexokinase binding protein from the outer membrane of rat liver mitochondria are identical. FEBS Lett. 1982;141:189–192. doi: 10.1016/0014-5793(82)80044-6. [DOI] [PubMed] [Google Scholar]
- [97].Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosos. J. Biol. Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- [98].Majewski N, Nogueira V, Bhasker P, Coy PE, Skeen JE, Gottlob K, Chandel NS, Thompson CB, Robey RB, Hay N. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol. Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- [99].Arora KK, Pederson PL. Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J. Biol. Chem. 1988;263:17422–17428. [PubMed] [Google Scholar]
- [100].Racker E. History of the Pasteur effect and its pathobiology. Mol. Cell Biochem. 1974;5:17–23. doi: 10.1007/BF01874168. [DOI] [PubMed] [Google Scholar]
- [101].Anflous K, Armstrong DD, Craigen WJ. Altered mitochondrial sensitivity for ADP and maintenance of creatine-stimulated respiration in oxidative striated muscles from VDAC1-deficient mice. J. Biol. Chem. 2001;276:1954–1960. doi: 10.1074/jbc.M006587200. [DOI] [PubMed] [Google Scholar]
- [102].Anflous-Pharayra K, Cai ZJ, Craigen WJ. VDAC1 serves as a mitochondrial binding site for hexokinase in oxidative muscles. Biochim. Biophys. Acta. 2007;1767:136–142. doi: 10.1016/j.bbabio.2006.11.013. [DOI] [PubMed] [Google Scholar]
- [103].Wu S, Sampson MJ, Decker WK, Craigen WJ. Each mammalian mitochondrial outer membrane porin protein is dispensable: effects on cellular respiration. Biochim. Biophys. Acta. 1999;1452:68–78. doi: 10.1016/s0167-4889(99)00120-2. [DOI] [PubMed] [Google Scholar]
- [104].Anflous-Pharayra K, Lee N, Armstrong DL, Craigen WJ. VDAC3 has differing mitochondrial functions in two types of striated muscles. Biochim. Biophys. Acta. 2011;1807:150–156. doi: 10.1016/j.bbabio.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Yamamoto T, Yamada A, Watanabe N, Yoshimura Y, Yamazaki N, Yoshimura Y, Yamauchi T, Kataoka M, Nagata T, Terada H, Shinohara Y. VDAC1, having a shorter N-terminus than VDAC2 but showing the same migration in an SDS-polyacrylamide gel, is the predominant form expressed in mitochondria of various tissues. J. Proteome Res. 2006;5:3336–3344. doi: 10.1021/pr060291w. [DOI] [PubMed] [Google Scholar]